Abstract
Benefiting from their good mechanical and electrical properties, epoxy resin materials are widely utilized in the field of high-voltage electrical insulation devices. However, with the increase in voltage levels of equipment, the epoxy resin materials used for insulating pull rods in high-voltage electrical equipment are facing increasingly severe challenges. This study enhanced the mechanical and insulating properties of epoxy resin materials by molecular structure regulation, composite incorporation and formula optimization. The tensile strength, bending strength and impact strength of the epoxy resin materials with molecular structure regulation increased by 20.6%, 8.5% and 42.1%. The breakdown strength successfully increased from 27.6 kV/mm to 29.9 kV/mm. After combining with the modified Al2O3 nanofillers, the breakdown strength, surface resistivity and volumetric resistivity of the composite further improved to 35.8 kV/mm, 2.7 × 1016 Ω and 5.8 × 1017 Ω·cm. The insulating pull rod prepared by this method achieved a flashover voltage of 18.5 kV, meeting the requirements for both insulating and mechanical performance of a prototype of 200 kV high-voltage direct current floor tank-type high-speed mechanical switch. This study can provide important support for the optimization of epoxy resin material formulation design and the development of epoxy-resin-insulating pull rods.
1. Introduction
Direct current high-speed mechanical switches are usually required to withstand high-voltage electric fields, complex electromagnetic environments and long-term mechanical stress during operation. Properties including the thermal stability, breakdown strength and mechanical strength of the insulating pull rod in direct current high-speed mechanical switches, which serve as one of the most significant devices, significantly affect the operational performances of high-speed switches. Given the merits of epoxy resins (EP), including their excellent dielectric properties, adhesivity and thermal stability, epoxy resin composites have attracted attention in the fabrication of insulating components for high-voltage electrical equipment [1,2,3,4,5,6]. However, the mechanical and dielectric properties of insulating pull rods based on epoxy resins inevitably face harsh operating conditions and ever-rising performance requirements with the incessant growth in transmission distance and voltage levels [7,8,9,10,11]. Notably, complex or extreme operational conditions, combined with thermal damage factors, tend to accelerate the performance degradation of epoxy composites, ultimately leading to device failure [12,13,14,15,16,17]. Therefore, the necessity has arisen to explore advanced modification strategies for epoxy resin materials to enhance their comprehensive properties and safeguard their reliability in practical engineering applications.
Generally, epoxy resins could be classified as cycloaliphatic, glycidyl ether, and glycidyl amine types according to their molecular structures [18,19,20,21,22]. Especially, bisphenol A-based epoxy resins (BPA-EP) have emerged as typical materials in high-voltage electrical equipment primarily due to their cost-effectiveness and abundance [23,24,25]. Nevertheless, they exhibit inherent drawbacks in mechanical properties, weather resistance and stability. Recent studies have adopted modification approaches including graft copolymerization and blending to optimize the performances of BPA-EP systems [26,27,28]. For instance, Shih et al. synthesized high-molecular-weight epoxy resins, which demonstrated good mechanical and thermal properties by reacting bisphenol A resin with bisphenol derivatives containing different aromatic ring structures [29]. Zhang et al. investigated the toughening effects and dielectric property modifications of various additives in BPA-EP [30]. Zhao et al. conducted a chain extension modification on low-molecular-weight epoxy resin using methyl tetrahydrophthalic anhydride (Me-THPA), and prepared modified insulating composite materials. The breakdown voltage of the composite increased by 11.2%, and the flashover voltage along the surface improved by 6% [31].
In addition to modifying the epoxy resin matrix, researchers often use inorganic fillers such as SiO2 and boron nitride to enhance the material performances. The addition of fillers introduces new bonding and interfacial structures into the composite material, which can alter the trap level and trap density. This modification effectively restricts charge migration and significantly improves the breakdown strength [32,33,34,35]. Castellon et al. incorporated SiO2 particles with different size scales into the epoxy matrix and investigated the space charge behaviors. Their work revealed a significant reduction in space charge density in micro-/nano-SiO2/epoxy composites compared to unmodified epoxy resins, which led to a remarkable improvement in insulating performance [36]. Yang et al. prepared nano-boron-nitride/epoxy composite materials and investigated their thermal and electrical properties. The results showed that the breakdown strength of the boron nitride/epoxy composite increased by approximately 11%. The thermal conductivity of the composite material also showed an improvement [37]. Cheng et al. prepared insulating composite materials using BPA-EP E51 as the matrix and SiO2/Al2O3 as the nanofillers. Tests revealed that the flashover voltage and breakdown strength of the composite material were enhanced by 20.9% and 23.1%, respectively [38].
Although previous studies have paid attention to the modification of mechanical strength, dielectric properties, and thermal performance of epoxy resins, challenges still remain in achieving synergistic enhancements on both electrical and mechanical properties. Herein, an epoxy resin composite was fabricated by regulating the molecular structure of epoxy resins and incorporating with modified Al2O3 nanoparticles. The effects of molecular structure regulation and inorganic filler incorporation on the mechanical and electrical properties of this composite were systematically studied. Furthermore, this modified Al2O3/epoxy resin nanocomposite-based insulating pull rod with the optimal formulation design was applied in the direct current high-speed mechanical switch. The insulating and mechanical performances of the switch prototype were successfully verified. We believe that this work will provide inspiration for the optimization of formulation design on epoxy resin composites and the development of epoxy-resin-based insulating pull rods.
2. Material Preparation and Characterization
2.1. Raw Materials
The raw epoxy resins consisted of bisphenol A-type epoxy resin and cycloaliphatic epoxy resin. Phthalic anhydride was used as the curing agent. Al2O3 was chosen as the inorganic filler, and the silane coupling agent γ-aminopropyl triethoxysilane was used in the surface treatment for fillers. Bisphenol A-type chain extender and p-Cumylphenol typed capping agent were utilized for the chain extension reaction and regulation of crosslinking structures. The parameters of materials used in this work are shown in Table 1.

Table 1.
Material parameters used in the preparation of epoxy composites.
2.2. Material Preparation
For the preparation of dual-crosslinked epoxy resins, firstly, cycloaliphatic epoxy resin HE-179 was added to the bisphenol A-type epoxy resin solution at 130 °C under the mass ratios of 0, 10%, 20%, and 30%, respectively. After stirring for 2 h, a curing agent of 38% mass was added and continuously stirred for 15 min. The mixture was then subjected to vacuum degassing at 130 °C for 10 min in a vacuum oven. The degassed mixture was poured into preheated molds and underwent a secondary vacuum degassing process. Finally, the molds were placed in a vacuum oven for curing following the conditions of 130 °C and 40 h to obtain the dual-crosslinked epoxy resin materials.
Molecular-structure-regulated epoxy resin materials were prepared by using chain extenders and capping agents. Initially, the bisphenol A-based epoxy resin solution and HE-179 were mixed in a reactor at a mass ratio of 10:2. Subsequently, chain extenders (0%, 18%, 25%, 30% mass) and capping agents (0%, 6% mass) were added to the mixture, which was then stirred at 130 °C for 4 h. Thereafter, the curing agent equivalent to 38% of total resin mass was added to the solution and the solution was continuously stirred for 15 min. The mixture was then subjected to vacuum degassing at 130 °C in a vacuum oven for 10 min. Subsequently, the mixture was injected into preheated molds for secondary vacuum degassing. Finally, the molds were placed in a vacuum oven for curing under the conditions of 130 °C and 40 h to obtain the regulated epoxy resin materials.
Dual-crosslinked epoxy resin samples incorporating capping agents and chain extenders with different ratios were labeled as EP-0, EP-1, EP-2, EP-3 and EP-4. Corresponding ratios and curing conditions are shown in Table 2.

Table 2.
The formulas and curing conditions of epoxy resin samples with different mass ratios of chain extender and end-capping agent.
Surface modification treatment of Al2O3 nanofillers was conducted to improve the dispersion and combination of nanofillers within the epoxy resin matrix. Firstly, Al2O3 nanoparticles were treated by the plasma surface treatment instrument and stirred in the ethanol/water mixture for 2 h. Then KH550 silane-coupling agent of 20% by mass was added into the mixture and stirred at 50 °C for 3 h to integrate with nanoparticles. After the mixture centrifuging and precipitation collecting, these nanoparticles were dried at 90 °C for 12 h to obtain the modified Al2O3 nanoparticles.
To prepare the modified Al2O3/epoxy resin nanocomposites, modified Al2O3 nanoparticles were incorporated into the regulated epoxy resin solution with mass ratios of 10%, 20% and 30%. The mixture was subjected to mechanical blending at 130 °C for 2 h, following by the addition of curing agent equivalent to 38% of the total resin mass and continuous stirring for 15 min. The mixture was then vacuum degassed in a vacuum oven at 130 °C for 10 min. Subsequently, the degassed mixture was injected into preheated molds for secondary vacuum degassing. Finally, the molds were placed in a vacuum oven under a curing condition of 130 °C and 40 h to obtain the ultimate Al2O3/epoxy resin nanocomposites. The fabrication processes of materials are illustrated in Figure 1.
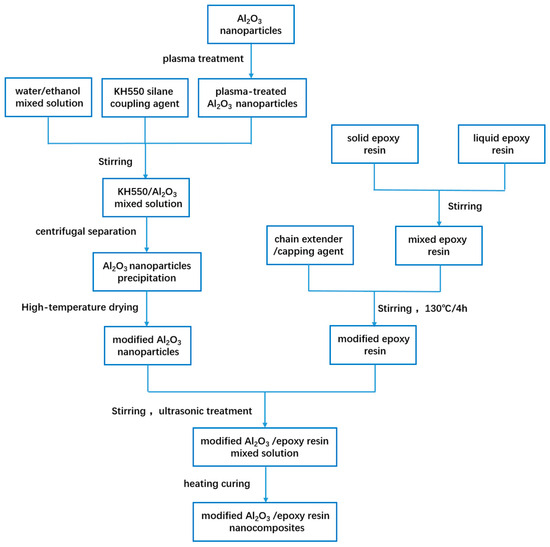
Figure 1.
The fabrication process of the materials.
2.3. Testing and Characterization
The glass transition temperatures (Tg) of the epoxy resin materials were tested by using a differential scanning calorimeter (DSC8500, PerkinElmer, Waltham, MA, USA) according to Recommended National Standard of the People’s Republic of China (GB/T 40396-2021) [39]. The combination of nanofillers with the epoxy resins under different surface treatment conditions was investigated by Fourier transform infrared spectrometer (FTIR) (Nicolet iS50, Thermo Fisher, Waltham, MA, USA) complying with GB/T 6040-2019 [40]. The tensile and bending strengths of different epoxy resin materials were tested using a mechanical universal testing machine (CMT4204, MTS, Eden Prairie, MN, USA) on the basis of GB/T 1040.1-2018 [41] and GB/T 9341-2008 [42], respectively. The impact strengths of different epoxy resin materials were tested by a film impact tester (EZ83, Vice, Hangzhou, China) according to GB/T 1843-2008 [43]. The breakdown strengths of samples were investigated by a voltmeter (RK7110, Rek, Shenzhen, China) according to GB/T 11408.1-2016 [44]. The laser particle size analyzer (Bettersize2600, Dongjiang Bettersize, Dandong, China) was used to investigate the dispersion of nanoparticles in the matrix according to GB/T 41949-2022 [45]. Corona charging experiments were conducted through a surface potential measurement system including an electrostatic voltmeter (Trek 341B, Trek, Cambridge, MA, USA). The surface and volume resistivity were tested by using a resistivity tester (SCX7680/SCX7660, JUNDA, Guangzhou, China) in accordance with GB/T 31838.3-2019 [46]. A transmission electron microscope (TEM) (JEM F200, JEOL, Tokyo, Japan) equipped with an energy-dispersive spectroscopy (EDS) and scanning electron microscope (SEM) (Verios G4 UC, Thermo Scientific, Waltham, MA, USA) were used to investigate the morphology and element distribution of materials and samples.
3. Results and Discussion
3.1. Molecular Structure Modification of Epoxy Resin Materials
The bisphenol A-type epoxy resin has a relatively lower crosslinking density, indicating fewer crosslinking nodes within epoxy resin networks and longer molecular chains. This structural configuration facilitates the rotational and translational mobility of polymer chain segments upon heating, thereby enabling the initiation of cooperative segmental motion at relatively lower temperatures and a relatively lower Tg. The appropriate incorporation of cycloaliphatic epoxy resin with a higher crosslinking density will provide more crosslinking nodes and shorten the average length of molecular chains in the epoxy resin composite, thus restricting the rotational and translational mobility of polymer chain segments and resulting in a higher Tg of epoxy resin composite which facilitates the structural stability and the mechanical and electrical properties at higher temperatures. Therefore, crosslinking modification was performed by introducing cycloaliphatic epoxy resin HE-179 into the BPA-EP matrix with a molecular structure, as shown in Figure 2. The Tg of materials with different mass ratios were measured and the results are shown in Figure 3. The Tg of the modified epoxy resin increased progressively with increasing the mass ratio of HE-179 resin. When the mass ratio of HE-179 reached 20%, Tg of the polymer reached 134 °C. The high Tg can prevent the polymer from transforming into a highly elastic state and improve the performances of insulating devices. For prevention of the further increase in fabrication cost and brittleness of materials, the following studies were conducted using 20% HE-179 containing dual-crosslinked epoxy resins.
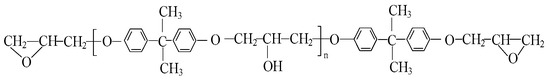
Figure 2.
The molecular structure of bisphenol A-type epoxy resin.
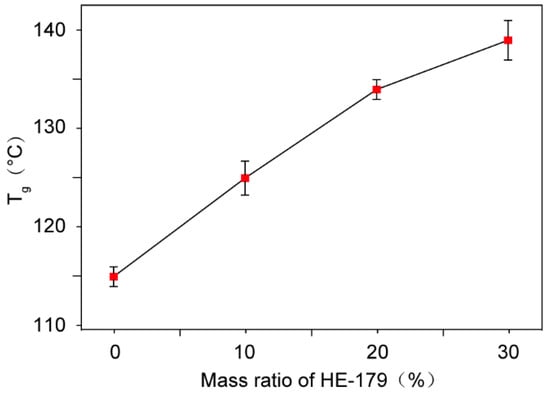
Figure 3.
The glass transition temperature of dual-crosslinked epoxy resins with different HE-179 contents.
It is known that the functional groups of the extender can react with the terminal groups of epoxy resins to realize the extension or crosslinking of molecular chains, enhancing the molecular weight, stiffness and impact strength of epoxy resins. The functional groups of the capping agent can temporarily passivate the reactivity by masking terminal groups of epoxy resins or curing agents, thereby delaying cure initiation and lowering the crosslinking density. Owing to same targets of the terminal groups, the extenders and capping agents show an antagonistic effect while the integrated utilization in the curing process, and cause a trade-off between epoxy resin’s molecular weight and pot life, influencing the crosslinking structure with a controllable molecular weight, and regulating the mechanical and thermal properties of epoxy resins. Thus, the above-prepared dual-crosslinked epoxy resin material was modified by chain extenders and capping agents to simultaneously enhance the molecular weight with expanding the crosslinked networks and loosen the crosslinked structure as well as dissipate the curing stress with reducing the growth ratio and speed of molecular chains. The reaction mechanism is shown in Figure 4.
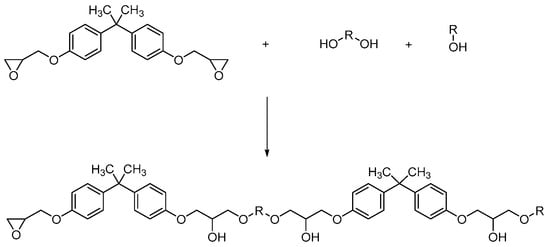
Figure 4.
The modification mechanism of resin chain extender and end-capping agent on epoxy resins.
As shown in Table 3, by comparing the test results of EP-0 and EP-1, it can be found that the introduction of the capping agent partially alleviated the curing-induced internal stress, reduced defect sites and increased the tensile, bending and impact strengths, as well as the breakdown strength. By comparing the test results of EP-1, 2, 3 and 4, it can be found that the mechanical properties of polymers continuously improved when increasing the mass ratio of the chain extender due to the enhanced crosslinking density. However, the breakdown strength first increased and then decreased. The breakdown strength of crosslinked epoxy resin reached a maximum of 29.9 kV/mm at chain extender of 25% coupling with 6% capping agent, which corresponded to 20.6%, 8.5% and 42.1% improvements in tensile, bending and impact strengths compared with the samples without the addition of chain extender and capping agent. Further increase in the chain extender content led to a decline in breakdown strength to 29.6 kV/mm. This could be at-tributed to the insufficient capping agent to counteract the increased crosslinking density caused by excessive chain extender addition. These results confirm the effectiveness of molecular structure regulation methods in enhancing both mechanical and electrical properties of the epoxy resin matrix.

Table 3.
Mechanical strengths and breakdown strengths of different epoxy resin samples.
3.2. Preparation and Performances of Al2O3/Epoxy Nanocomposite
Nanoparticles are widely used in the composition of epoxy resins. The selection of nanoparticles for epoxy resin composites must adhere to several critical principles to ensure mechanical and thermal performances under high-voltage service conditions. Primarily, the nanoparticles must possess excellent inherent dielectric properties and chemical inertness to avoid compromising the insulating capability of epoxy matrix. Nanoparticles usually need surface modification, achieving uniform dispersion to prevent agglomerations that act as defect sites which initiate electrical trees. Strong interfacial adhesion between the particle surface and the epoxy matrix is essential for effective stress transfer and minimizing interfacial voids or polarization. Al2O3 nanoparticles offer significant advantages aligning with these principles and are typically used as a nanoscale-reinforcing phase. They can integrate with the crosslinked molecular chains of epoxy matrix to stabilize the cured network structures, restrict the migration of molecular chain and effectively suppress the growth of electrical trees during partial discharge, thereby achieving enhancement simultaneously in mechanical properties and electrical strength [47,48]. However, the high surface energy and hydrophilicity of Al2O3 nanoparticles are liable to cause severe agglomeration in polymer matrix, resulting in severe performance degradation until failure [49]. Consequently, ensuring uniform dispersion of nanofillers in the matrix becomes critical to unlock their full potential in composite materials.
Surface modification with organo-silanes replaces surface hydroxyl groups with organofunctional moieties, which can significantly enhance hydrophobicity, improve the interfacial compatibility with epoxy networks and establish covalent bonds through reactions with resins. Consequently, modified fillers achieve uniform dispersion stability via electrostatic repulsion, eliminating agglomeration induced defects. Crucially, the reinforced interface enables efficient stress transfer, augmenting mechanical strength and crack resistance, while minimizing interfacial void content and charge trapping sites. This stabilizes the dielectric behavior by suppressing interfacial polarization and moisture ingress. By utilizing surface plasma treatment technique combined with silane coupling agent KH550 coating, surface modification of Al2O3 nanoparticles was performed. As shown in Figure 5, KH550-modified Al2O3 samples exhibited new characteristic peaks at 1418 cm−1 and 1552 cm−1, corresponding to the stretching vibrations of -NH groups and Si-O bonds, respectively. This confirms the successful coating of a silane coupling agent on Al2O3 nanoparticles and efficient modification of surface nanostructures. TEM and EDS mapping images of Al2O3 nanoparticles before and after surface modification are presented in Figure 6. The size of unmodified and modified Al2O3 nanoparticles are around 15 nm. Si and Al elements show nearly conformal distribution after coating with silane coupling agent, and it can be observed that the Si element of the modified Al2O3 nanoparticles with surface plasma treatment show a more even distribution, suggesting a better combination of the silane coupling agent and Al2O3 nanoparticles.
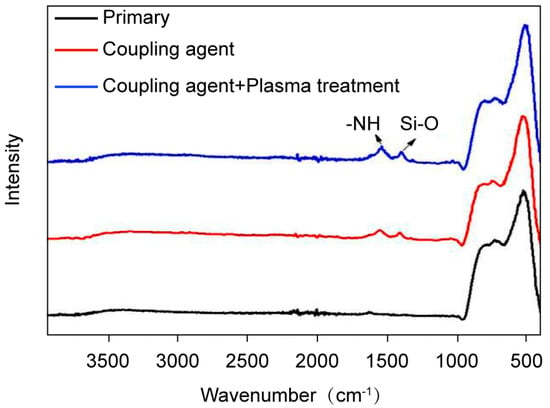
Figure 5.
FTIR spectra of the samples before and after modification.
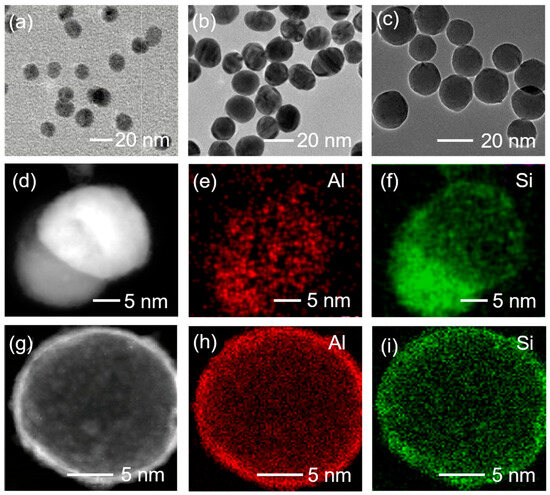
Figure 6.
TEM images of (a) primary Al2O3 nanoparticles, (b) modified Al2O3 nanoparticles without plasma treatment and (c) modified Al2O3 nanoparticles with plasma treatment. (d–f) TEM image of a modified Al2O3 nanoparticle without plasma treatment and EDS mapping images of Al and Si. (g–i) TEM images of a modified Al2O3 nanoparticle with plasma treatment and its EDS mapping images of Al and Si.
The size distribution of agglomerating Al2O3 particles in organic media was determined using a laser diffraction particle size analyzer to verify particle dispersion quality. As shown in Figure 7, the unmodified Al2O3 nanoparticles exhibited the widest particle size distribution. KH550-modified Al2O3 nanoparticles showed a narrower distribution, while Al2O3 nanoparticles with plasma treatment and silane coupling agent grafting displayed the narrowest particle size distribution. Notably, the combined modification approach resulted in significantly improved dispersion of Al2O3 nanoparticles in organic matrices.
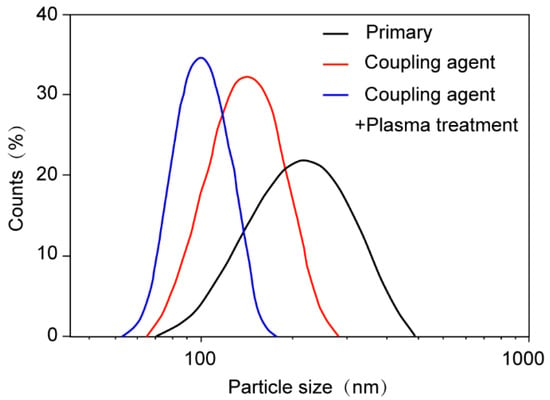
Figure 7.
Particle size distribution diagrams of nanofillers before and after surface modification.
To further investigate the influence of Al2O3 nanofillers on the mechanical and dielectric properties of epoxy resins, the tensile, bending and impact strengths of Al2O3/epoxy composites with varying Al2O3 mass fractions (1%, 3% and 5%) were evaluated. As depicted in Figure 8, all Al2O3-reinforced composites demonstrated an increase in tensile and impact strength compared with the neat epoxy resin matrix, and the bending strength of all samples exceeded 140 MPa, confirming the effective enhancement of mechanical properties through Al2O3 nanofillers reinforcement. The introduced Al2O3 nanoparticles can act as physical crosslinking sites within the epoxy network, restricting molecular chain mobility through adsorption and mechanical interlocking, and improving thermal stability during operation. Concurrently, the nanoparticles establish a reinforced percolation network that impedes crack propagation. SEM images of the regulated epoxy resin and modified Al2O3/epoxy resin nanocomposite are presented in Figure 9. The unmodified epoxy displayed a smooth surface in Figure 9a. Figure 9b shows that the Al2O3 nanoparticles of modified Al2O3/epoxy resin nanocomposites were uniformly distributed in the polymer matrix without obvious agglomeration.
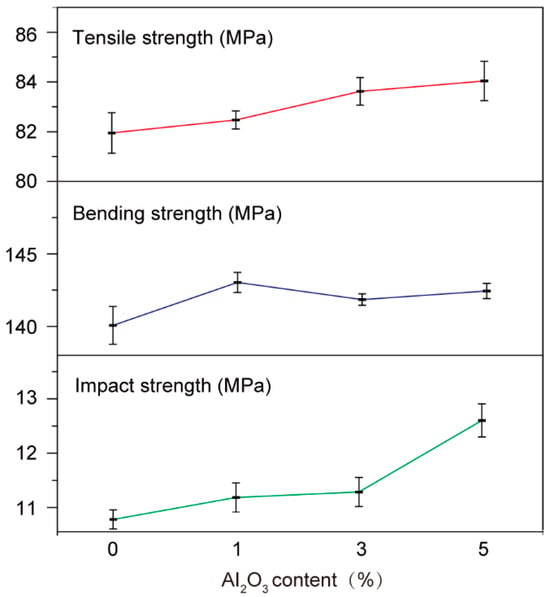
Figure 8.
The influence of Al2O3 content on the mechanical properties of epoxy resin composites.
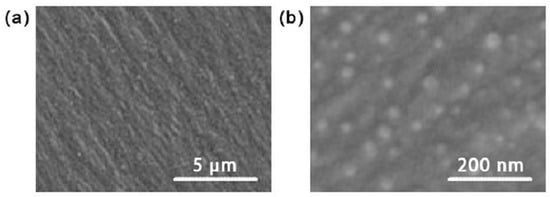
Figure 9.
SEM images of the (a) regulated epoxy resin and (b) modified Al2O3/epoxy resin nanocomposite.
The interface interaction zone between nanofillers and the polymer matrix is a critical region influencing charge transport in materials. Corona-charging experiments presented in Figure 10 were conducted through a surface potential measurement system including an electrostatic voltmeter to investigate the charge transport mechanism. The results are shown in Figure 11a. By analyzing the surface potential decay kinetics, the relative relationship between trap level and trap density was investigated (Figure 11b) [50]. The static shielding layer surrounding nanoparticles creates a high potential barrier, while surface functional groups can trap charge carriers to achieve the electrostatic equilibrium, thereby inhibiting surface charge migration. By continuously adding Al2O3 nanoparticles, the trap level and trap density first increase and then decrease. At an Al2O3 mass fraction of 3%, trap level and trap density reach their maximum values, indicating the enhanced capability of charge restriction in the interfacial region. The subsequent decline of trap level can result from the excessive Al2O3 introduction and insufficient interfacial bonding between fillers and polymer matrix at higher filler loadings, leading to more defects which weaken the effect of charge capture [51,52].
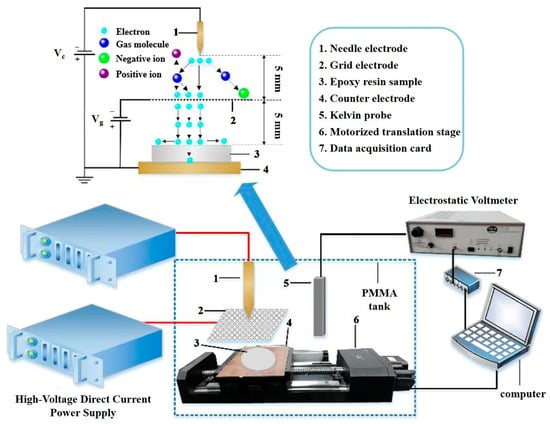
Figure 10.
Illustration of the corona-charging experiment.
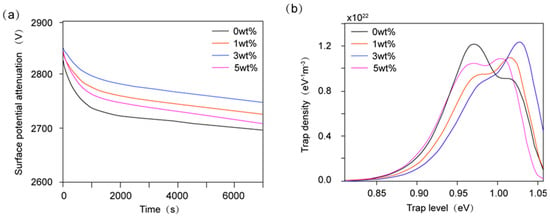
Figure 11.
(a) The surface potential attenuation curves and (b) relationship between the trap level and trap density of epoxy resin composites with different Al2O3 contents.
The resistivity variations in the composite materials demonstrated in Figure 12 corroborate the results above. When Al2O3 content reached 3%, the surface resistivity and volume resistivity of Al2O3/epoxy composite achieved maximum values of 2.7 × 1016 Ω and 5.8 × 1017 Ω·cm, respectively. Volume resistivity is fundamental to quantifying the inherent resistance of materials due to its direct correlation with electrical insulation integrity under high-voltage induced stress. The high-volume resistivity of the Al2O3/epoxy composite signifies minimal charge migration through the bulk material, thereby suppressing leakage currents that cause energy loss, Joule heating and accelerated electrochemical degradation. It is critical for composite to maintain dielectric isolation between conductive components, preventing flashovers and short circuits.
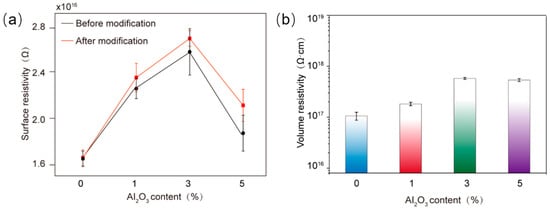
Figure 12.
The influence of different Al2O3 contents on the surface resistivity (a) and volume resistivity (b) of epoxy resin composites.
Optimal structural integrity hinges on homogeneous particle distribution. Agglomeration-free dispersion is achieved through surface modification minimizes stress concentration resulting from interfacial defects under electromechanical stress. The refined microstructure can ensure dense polymer–filler interfaces with covalent bonds, substantially reducing interfacial voids and moisture-permeable pathways. Consequently, the composite develops a hierarchically reinforced architecture which constrains molecular dynamics and forms energy dissipating zones that blunt advancing cracks at high voltage or stress. The resultant structure can synergistically mitigate partial discharge erosion and suppress electrical tree initiation. As shown in Figure 13a, the breakdown strength of Al2O3/epoxy composite initially enhances and then declines with increasing Al2O3 filler content. Notably, composite samples exhibited superior breakdown strength compared to the neat epoxy resin matrix. The composite with Al2O3 of 3% showed the most optimized dielectric properties and achieved the highest breakdown strength of 35.8 kV/mm, demonstrating a significant performance enhancement in this composite system. As depicted in Figure 13b, compared with previous studies, the modified Al2O3/epoxy resin nanocomposite developed in our work demonstrates great advantages in simultaneously improving the mechanical and dielectric properties [53,54,55,56]. However, the excessive Al2O3 introduction at a high loading of over 3% may passivate the dispersion of nanoparticles and their interactions with epoxy resins, which lead to insufficient interfacial contact and bonding between fillers and polymer matrix, weakening the charge capture capacity of composites. Thus, the surface resistivity, volume resistivity and breakdown strength of the Al2O3/epoxy composite with Al2O3 of 5% will decrease.
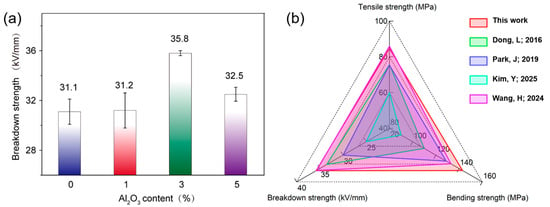
Figure 13.
(a) The influence of Al2O3 content on the breakdown strength of epoxy resin composites and (b) performances comparison of the modified Al2O3/epoxy nanocomposite and other epoxy resin-based materials reported in the literature [53,54,55,56].
Based on the modified Al2O3/epoxy composites, we fabricated insulating pull rods for high-voltage electrical equipment. The inner and outer sleeves are shown in Figure 14a,b. Figure 14c summarizes the flashover voltages of samples before and after modified Al2O3 doping modification. The average flashover voltage of the unmodified material was 14.7 kV, while that of the modified material increased to 18.5 kV, representing 26% enhancement, which demonstrates the significant application advantages of the modified Al2O3/epoxy composite.
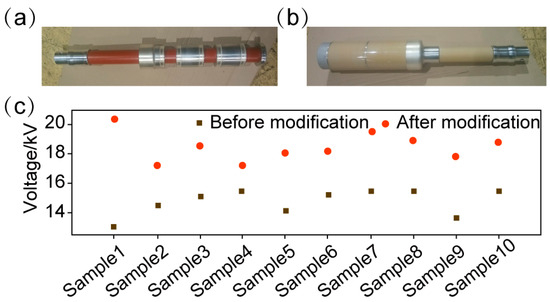
Figure 14.
The (a) inner and (b) outer sleeves of the insulating pull rod based on modified Al2O3/epoxy composites, and (c) the flashover voltages of the materials before and after modification.
We further applied this composite to a prototype of high-voltage (200 kV) direct current floor tank-type high-speed mechanical switch (Figure 15). The modified Al2O3/epoxy composite-based insulating pull rod successfully met the requirements for both insulation and mechanical performances in the direct current high-speed switch. During the dynamic insulation test, no breakdowns occurred after 15 repeated operations under applied voltage of ±300 kV. In the static insulation test, the direct current withstand voltage reached 230 kV, with rated lightning impulse withstand voltages of 410/640 kV (breaker gap/to ground) and rated switching impulse withstand voltages of 320/320 kV (breaker gap/to ground). After 2000 cycles, the opening stroke was 30.8 mm, with an opening time of 2.1 ms and opening speed of 9.8 m/s, while the closing stroke was 31.35 mm, with a closing time of 7.5 ms and closing speed of 4.18 m/s, which all meet the performance requirement for high-speed mechanical switches. These results successfully validate the engineering effectiveness of our key technologies and provide theoretical foundations and technical supports for developing 200~500 kV high-voltage direct current high-speed mechanical switches.
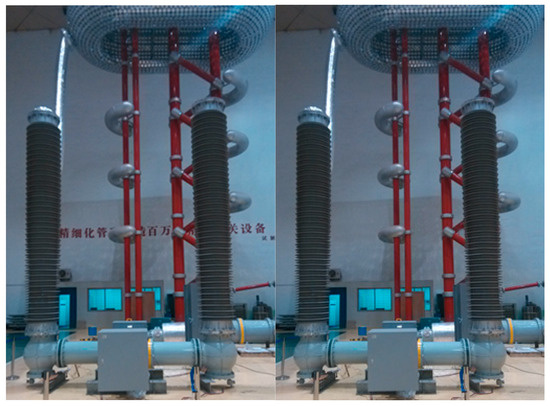
Figure 15.
The direct current withstand voltage test diagram of high-voltage direct current high-speed mechanical switch.
4. Conclusions
In this work, a dual-crosslinked epoxy resin material with excellent dielectric and mechanical properties was obtained through molecular structure regulation by using bisphenol A typed and cycloaliphatic epoxy resins, chain extenders and capping agents as the raw materials, achieving a breakdown strength of 29.9 kV/mm with tensile, bending and impact strength increase of 20.6%, 8.5% and 42.1%, respectively. Plasma surface treatment and silane coupling agent modification of Al2O3 nanoparticles, as well as their combination with the regulated epoxy resin, significantly improved the dispersion and bonding of nanofillers and epoxy matrix. This led to remarkable enhancement of mechanical properties. Simultaneously, the trap level and trap density of the composites increased resulting from the significantly suppressed charge migration. The surface and volume resistivity, as well as breakdown strength, were effectively enhanced to 2.7 × 1016 Ω, 5.8 × 1017 Ω·cm and 35.8 kV/mm.
The flashover voltage of the insulating pull rod prepared by this composite could reach 18.5 kV. The insulating pull rod was then applied in the prototype of high-voltage (200 kV) direct current floor tank type high-speed mechanical switches and successfully met the requirement of insulating and mechanical performances. This research provides an inspiration to the development of 200–500 kV high-voltage direct current high-speed mechanical switches and realized significant enhancement on the mechanical and insulating properties of epoxy resin materials used in high-voltage electrical engineering.
Author Contributions
J.Z. and X.L. supervised the project. Y.Z. (Youpeng Zhang), L.H. and Y.Z. (Yue Zhai) conceived the idea. Y.Z. (Youpeng Zhang), Y.W. and M.S. prepared the materials and samples. Y.Z. (Youpeng Zhang), Y.W., Y.Z. (Ye Zhao) and Y.Z. (Yuanyuan Zhang) performed the structural and performance characterizations as well as data analysis. Y.Z. (Youpeng Zhang), L.H., Y.Z. (Yue Zhai) and D.Y. carried out the data discussed and wrote the manuscript. Y.Z. (Youpeng Zhang) is the first author of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data in this article were collected from tests conducted during the product development process. Due to the involvement of confidential corporate product information, they are not being made public.
Acknowledgments
The authors sincerely appreciate all the efforts and supports from the collaborators and collaborating organizations.
Conflicts of Interest
Author Y. P. Zhang is a Ph.D. candidate at Shenyang University of Technology and was employed by Pinggao Group Co., Ltd. Author X. Lin is a professor at Shenyang University of Technology. Authors L. C. Hao, Y. Zhai, D. P. Yuan, Y. X. Wang, Y. Zhao, Y. Y. Zhang and M. J. Sun were employed by Pinggao Group Co., Ltd. Author J. Y. Zhong was employed by the company China Electric Equipment Group Science and Technology Research Institute Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kumer, V. Assessment of impact of impurities in epoxy-anhydride vacuum impregnation (vi) resin system. IEEE Trans. 2011, 18, 1947–1954. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Xie, Q.; Xia, G.W.; Zhang, Y.P.; Gao, F. Constructing aramid nanofibers self-assembly structure on fiber surface with electric field-assisted enhances insulation performance of aramid fiber reinforced epoxy resin. Appl. Surf. Sci. 2025, 702, 163341. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, G.; Liu, Z.; Tao, Y.; Yang, B.; Wu, K.; Shi, J.; Fu, Y. High-performance epoxy thermosets with excellent low dielectric, flame-retardant and antibacterial properties based on bio-based derived active ester curing agents. Chem. Eng. J. 2025, 509, 161189. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, J.; Shi, X.; Li, X. Surface flashover characteristics of epoxy resin composites in SF6/CF4 gas mixture with DC voltage. Energies 2022, 15, 4675. [Google Scholar] [CrossRef]
- Chen, C.; Sun, Q.; Wang, C.; Bue, Y.; Zhang, J.; Peng, Z. Dielectric relaxation characteristics of epoxy resin modified with hydroxyl-terminated nitrile rubber. Molecules 2020, 25, 4128. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Ran, Z.; Yao, H.; Du, B.; Takada, T. Molecular structure modulated trap distribution and carrier migration in fluorinated epoxy resin. Molecules 2020, 25, 3071. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.L.; Yang, Z.F.; Xia, G.W.; Zhang, J.T.; Zhan, Z.Y.; Xin, W.F.; Wang, Q.F.; Xu, B.B.; Zhang, Y.J.; Xie, J. Enhance the surface insulation properties of EP materials via plasma and fluorine-containing coupling agent co-fluorinated graphene. Nanomaterials 2024, 14, 2009. [Google Scholar] [CrossRef]
- Xia, G.W.; Duan, Q.J.; Yin, G.H.; Li, J.W.; Wan, Z.J.; Yan, J.Y.; Xie, Q. Enhancing surface insulation of epoxy composite by constructing nanofiller-skeleton charge channel. Polym. Compos. 2022, 43, 6234–6243. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yao, L.X.F.; Bu, Y.; Sun, Q. Synergistical performance modification of epoxy resin by nanofillers and carboxyl-terminated liquid nitrile-butadiene rubber. Materials 2021, 14, 4601. [Google Scholar] [CrossRef]
- He, S.; Zheng, Y.; Lin, C.J.; Sun, Z.C.; Tu, Y.P.; He, J.L. Effect of alumina doping content on VFTO tolerance of epoxy resin cone-type insulators. High Volt. Eng. 2020, 46, 4006–4013. (In Chinese) [Google Scholar] [CrossRef]
- Du, B.X.; Li, A.; Li, J. Effects of AC and pulse voltage combination on surface charge accumulation and decay of epoxy resin. IEEE 2016, 23, 2368–2376. [Google Scholar] [CrossRef]
- Hussain, G.A.; Hassan, W.; Mahmood, F.; Shafiq, M.; Rehman, H.; Kay, J.A. Review on partial discharge diagnostic techniques for high voltage equipment in power systems. IEEE Access 2023, 11, 51382–51394. [Google Scholar] [CrossRef]
- Huang, Y.W.; Huang, Z.X.; Li, X.; Ji, Y.Q.; Yang, Z.; Yin, T.; Long, J.Y. High-temperature electrical insulation degradation mechanism in TiN/Ti (C, N)/Al2O3 composite ceramic films. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Sun, S.Y.; Fan, K.; Yang, J.; Liu, J.X.; Li, X.; Zhao, L.H.; He, X.; Liu, X.Y.; Jia, S.L.; Li, Q. Surface modification engineering on polymer materials toward multilevel insulation properties and subsequent dielectric energy storage. Mater. Today 2024, 80, 758–823. [Google Scholar] [CrossRef]
- Mohamed, H.; Lazaridis, P.; Mather, P.; Tachtatzis, C.; Judd, M.; Akinson, R.; Glover, I.A. Partial discharge detection and localization: Using software defined radio. IEEE Ind. Electron. Mag. 2019, 13, 77–85. [Google Scholar] [CrossRef]
- Bilal, I.A.; Zhang, L.; Wang, G.; Wang, Y.W.; Zhou, S.R. Molecular dynamics and finite element analysis of partial discharge mechanisms in polyimide under high-frequency electric stress. Polym. Degrad. Stabil. 2025, 234, 111252. [Google Scholar] [CrossRef]
- Chen, C.X.; Li, Y.F.; Hao, L.C. Simulation study on stress distribution of 1100 kV GIS insulator during curing. Proc. CSEE 2022, 42, 4992. (In Chinese) [Google Scholar] [CrossRef]
- El-Aouni, N.; Dagdag, O.; Amri, A.E.; Kim, H.; Haldhar, R.; Kim, S.; Dkhireche, N.; Bachiri, A.E.; Berisha, A.; Rafik, M. Synthesis, structural characterization and anticorrosion properties of a new hexafunctional epoxy prepolymer based on urea and phosphorus trichloride for E24 carbon steel in 1.0 M HCl. Colloids Surf. A 2024, 682, 132963. [Google Scholar] [CrossRef]
- Xing, A.; Gao, C.; Yuan, P.; Qiao, Y.; Guo, L.; Li, X.; Yang, W. A novel glycidyl ether type tetrafunctional epoxy resin: Synthesis, cure kinetic and properties. Pure Appl. Chem. 2024, 61, 117–130. [Google Scholar] [CrossRef]
- Swan, S.R.; Creighton, C.; Griffin, J.M.; Gashi, B.V.; Varley, R.J. Aromatic tetra-glycidyl ether versus tetra-glycidyl amine epoxy networks: Influence of monomer structure and epoxide conversion. Polymer 2022, 239, 124401. [Google Scholar] [CrossRef]
- Xie, J.; Hu, Y.; Gao, Y.; Sun, F. Synthesis and properties of trifluoromethyl organosilicon cycloaliphatic epoxy monomers for cationic photopolymerization. J. Appl. Polym. Sci. 2023, 140, e53987. [Google Scholar] [CrossRef]
- Wei, M.; Wang, B.; Zhang, X.; Wei, W.; Li, X. Cycloaliphatic epoxy-functionalized polydimethylsiloxanes for comprehensive modifications of epoxy thermosets. Eur. Polym. J. 2024, 202, 112656. [Google Scholar] [CrossRef]
- Zhao, X.M.; Babu, H.V.; Llorca, J.; Wang, D.Y. Impact of halogen-free flame retardant with varied phosphorus’s chemical surrounding on the properties of diglycidyl ether of bisphenol-A type epoxy resin: Synthesis, fire behaviour, flame-retardant mechanism and mechanical properties. RSC Adv. 2016, 6, 59226–59236. [Google Scholar] [CrossRef]
- Hatano, R.; Tominaga, Y.; Imai, Y.; Nakano, K. Preparation process for biomass nanofiber/bisphenol A-type epoxy resin composites with superior mechanical and thermal properties. Cellulose 2025, 32, 3189–3206. [Google Scholar] [CrossRef]
- Isik, M.; Ahmetli, G. Nanocomposites based on MWCNT and nano clay: Effect of acrylated epoxidized soybean oil on curing and composite properties. Ind. Crop. Prod. 2024, 221, 119421. [Google Scholar] [CrossRef]
- Li, D.; Gui, Y.W.; Chun, P.H.; Shu, S.W. Preparation and characterization of polysiloxane-modified epoxy resin aqueous dispersions and their films. J. Appl. Polym. Sci. 2005, 98, 880–885. [Google Scholar] [CrossRef]
- Kocaman, S.; Ahmetl, G.; Temiz, M. Newly epoxy resin synthesis from citric acid and the effects of modified almond shell waste with different natural acids on the creation of bio-based composites. Ind. Crop. Prod. 2024, 220, 119106. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, C.S.; Cao, J.T.; He, S.; Huang, Z.F.; Shen, N.L.; Zhu, Z.M.; Zhao, H.B.; Rao, W.H. Efficient hyperbranched flame retardant derived from quercetin for use in epoxy resin with well-balanced comprehensive performance. ACS Sustain. Chem. Eng. 2025, 13, 661. [Google Scholar] [CrossRef]
- Shih, W.C.; MA, C.M. Synthesis and characterization of phenoxy resins prepared from diglycidyl ether of bisphenol A and various aromatic dihydroxyl monomers. J. Appl. Polym. Sci. 1999, 73, 2369–2376. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, X.C.; Men, W.W. The effect of toughening agents on heat-resistance mechanical and dielectric properties of resins used in radome materials. In Proceedings of the 2016 CIE International Conference on Radar (RADAR), Guangzhou, China, 10–13 October 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Zhao, Y.S.; He, Y.H.; Yang, K.R.; Wang, X.P.; Zhang, S. Insulation performance of Me-THPA chain-extended epoxy Resin cured products. Mater. Rep. 2020, 35, 311. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y.H.; Tian, X.Y.; Cao, X.L.; Wang, Q.; Wang, J.K.; Xu, Y.G.; Luo, M.; Wang, Z.D. Enhanced thermal conductivity and electrical insulation properties of liquid crystalline epoxy composites by using optimized alumina hybrid fillers. Mater. Today Phys. 2025, 54, 101719. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmad, H.; Yap, H.; Ismail, H.A. Investigation of electrical properties of TiO2 nanocomposite based polymer. J. Phys. Conf. Ser. 2021, 15, 2–15. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Tian, X.Y.; Cao, X.L.; Wang, Q.; Wang, J.K.; Xu, Y.G.; Luo, M.; Wang, Z.D. Effects of methyl and carbon-carbon double bond in anhydride molecule on dielectric properties of epoxy/Al2O3 composite. IEEE Xplore 2021, 28, 1531–1538. [Google Scholar] [CrossRef]
- Lv, G.; Li, K.; Shi, Y.; Zhang, R.; Tang, H.; Tang, C. Effect of aminosilane coupling agent-modified nano-SiO2 particles on thermodynamic properties of epoxy resin composites. Process 2021, 9, 771. [Google Scholar] [CrossRef]
- Castellon, J.; Nguyen, H.N.; Agnel, S.; Toureille, A. Electrical properties analysis of micro and nano composite epoxy resin materials. IEEE Trans. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Z.; Zhang, Y.F.; Li, J.; Shang, K.; Wang, H.H. Thermal conductivity of nano-boron nitride composite Film/Liquid crystalline epoxy fiber. High Volt. Eng. 2022, 48, 3551. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, X.; Li, W.B.; Chen, S.; Yang, Z.; Han, S.; Ge, G.W. Effect of Nano-SiO2/Al2O3 on the insulation properties of epoxy resin. Polym. Mater. Sci. Eng. 2020, 36, 86. (In Chinese) [Google Scholar] [CrossRef]
- GB/T 40396-2021; Test Method for Glass Transition Temperature of Polymer Matrix Composites-Dynamic Mechanical Analysis (DMA). Standardization Administration of China (SAC): Beijing, China, 2021.
- GB/T 6040-2019; General Rules for Infrared Analysis. Standardization Administration of China (SAC): Beijing, China, 2019.
- GB/T 1040.1-2018; Plastics-Determination of Tensile Properties-Part 1: General Principles. Standardization Administration of China (SAC): Beijing, China, 2018.
- GB/T 9341-2008; Plastics-Determination of Flexural Properties. Standardization Administration of China (SAC): Beijing, China, 2008.
- GB/T 1843-2008; Plastics-Determination of Izod Impact Strength. Standardization Administration of China (SAC): Beijing, China, 2008.
- GB/T 11408.1-2016; Insulating Materials-Test Methods for Electric Strength-Part 1: Test at Power Frequencies. Standardization Administration of China (SAC): Beijing, China, 2016.
- GB/T 41949-2022; Particle-Laser particle size analyser-Technical requirements. Standardization Administration of China (SAC): Beijing, China, 2022.
- GB/T 31838.3-2019; Solid insulating materials-Dielectric and resistive properties-Part 3: Resistive properties (DC methods)-Surface resistance and surface resistivity. Standardization Administration of China (SAC): Beijing, China, 2019.
- Lau, K.Y.; Vaughan, A.S.; Chen, G. Nanodielectrics: Opportunities and challenges. IEEE Electrical Insulation Magazine. 17 June 2015, pp. 45–54. Available online: https://ieeexplore.ieee.org/document/7126073 (accessed on 17 June 2015).
- Štefan, H.; Jozef, K.; Anton, B.; Pavel, T.; Ondrej, M.; Adam, T.; Tomasz, K.; Alena, K.; Tomáš, D.; Jaroslav, H. Fabrication and Broadband Dielectric Study of Properties of Nanocomposites Materials Based on Polyurethane. IEEE Access 2024, 12, 114227–114241. [Google Scholar]
- Khan, M.Z.; Wang, F.; He, L.; Shen, Z.; Huang, Z.; Mehmood, M. Influence of treated nano-alumina and gas-phase fluorination on the dielectric properties of epoxy resin/alumina nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 410–417. [Google Scholar] [CrossRef]
- Li, Z.L.; Du, B.X.; Yang, Z.R.; Li, J. Effects of crystal morphology on Space charge transportation and dissipation of SiC/Silicone rubber composites. IEEE Trans. 2017, 24, 2616. [Google Scholar] [CrossRef]
- Rui, G.; Jerzy, B.; Zhang, S.; Zhang, Q. Dilute nanocomposites: Tuning polymer chain local nanostructures to enhance dielectric responses. Adv. Mater. 2024, 36, 2311739. [Google Scholar] [CrossRef]
- Fan, X.; Ding, X.; Wang, P.; Li, Z.; Cheng, Y.; Liu, J.; Yu, J.; Zhai, J.; Pan, Z.; Li, W. Ultra-low loading fillers induced excellent capacitive performance in polymer-based multilayer nanocomposites under harsh environments. Small 2024, 20, 2405786. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhou, W.; Sui, X.; Wang, Z.; Cai, H.; Wu, P.; Zhang, Y.; Zhou, A. Mechanical and electrical properties of aluminum/epoxy nanocomposites. J. Electron. Mater. 2016, 45, 5885–5894. [Google Scholar] [CrossRef]
- Park, J. Electrical insulation and mechanical properties of epoxy/micro-silica (MS)/micro-alumina (MA) composites. Trans. Electr. Electron. Mater. 2019, 20, 46–51. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, H.; Yoon, S.; Choi, Y.; Shim, J.; Zha, J.; Wie, J.; Kim, J.; Jung, Y. Eco-friendly hybrid composite fillers for high-performance epoxy insulators: Advancing thermal and electrical durability. ACS Appl. Eng. Mater. 2025, 3, 938. [Google Scholar] [CrossRef]
- Wang, H.; Gong, W.; Yuan, Y.; Wu, J.; Wen, S.; Liu, X. Design of hBN/nano Al2O3 epoxy composites with enhanced thermally conductive and dielectric properties. J. Mater. Sci. Mater. Electron. 2024, 35, 1775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).