The Study of Phase Behavior of Multi-Component Alkane–Flue Gas Systems Under High-Temperature Conditions Based on Molecular Dynamics Simulations
Abstract
1. Introduction
2. Methods
2.1. Phase Behavior Analysis Model
2.2. Interface Parametric Model
3. Results and Discussion
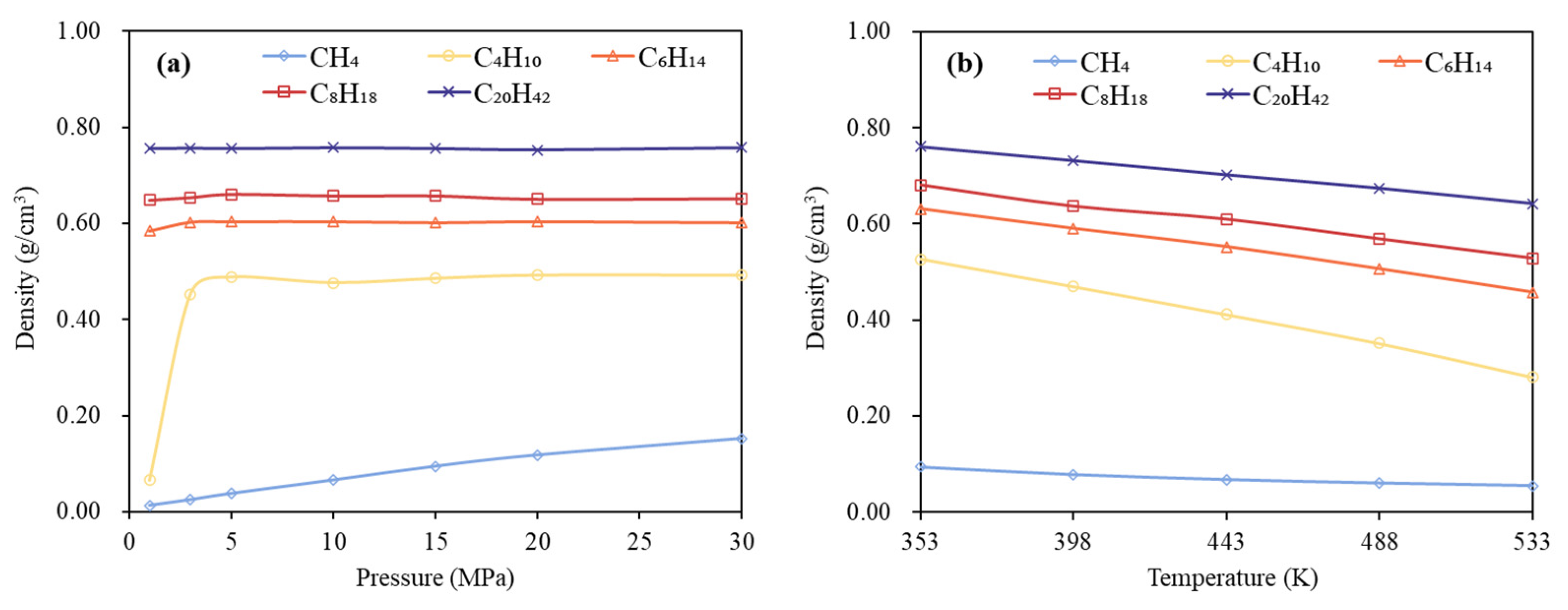
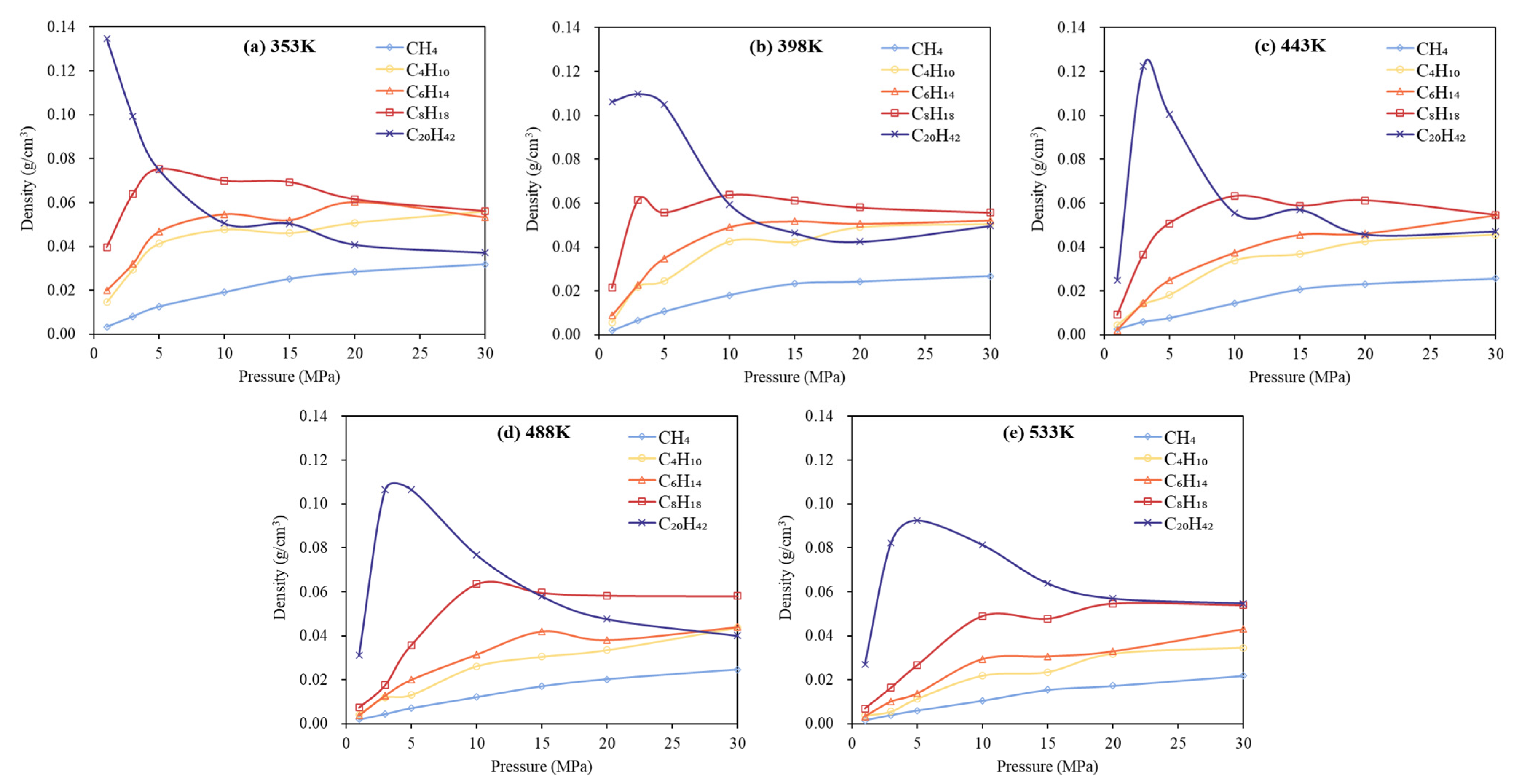
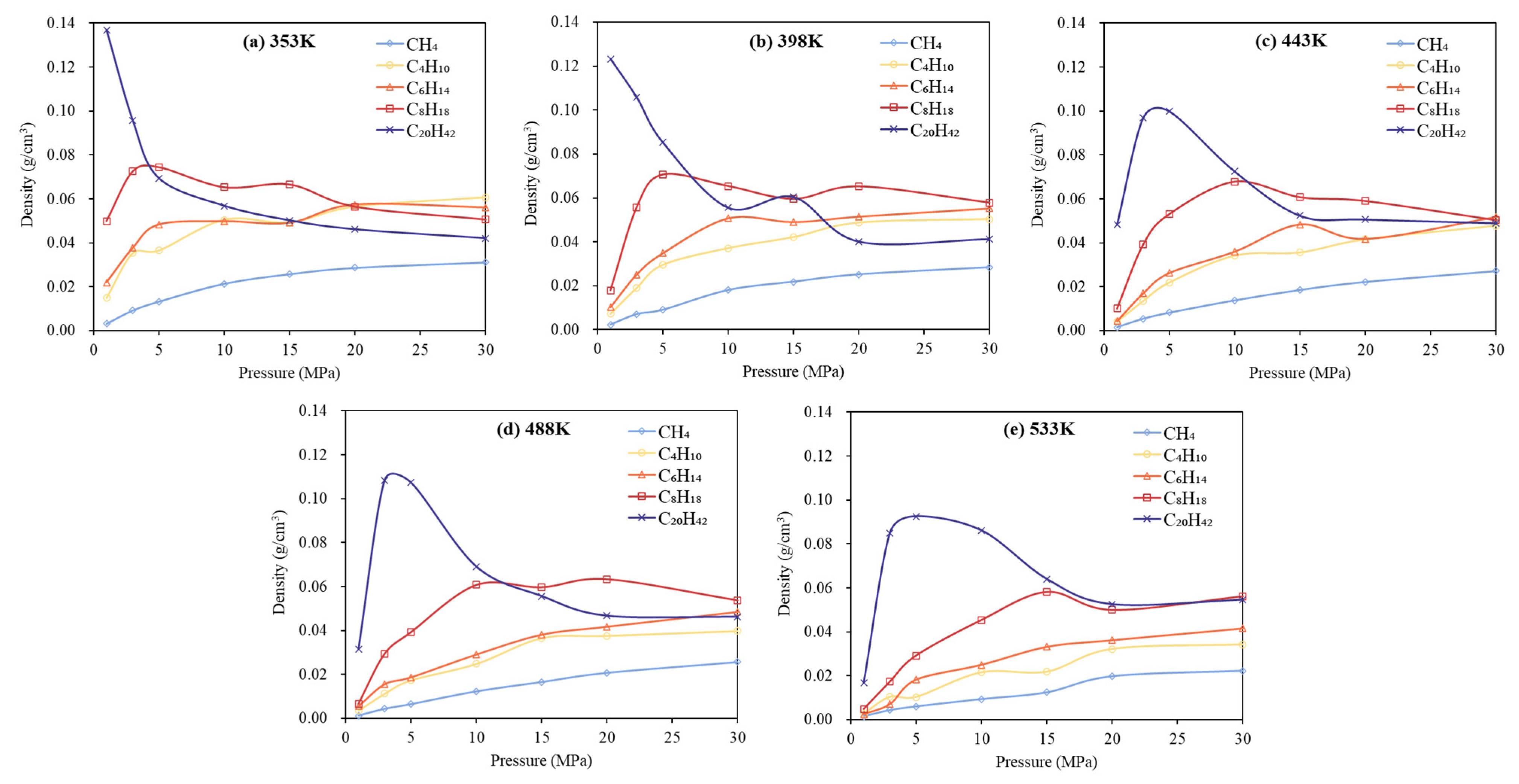
3.1. Phase Behavior Analysis of Single-Component Alkane System
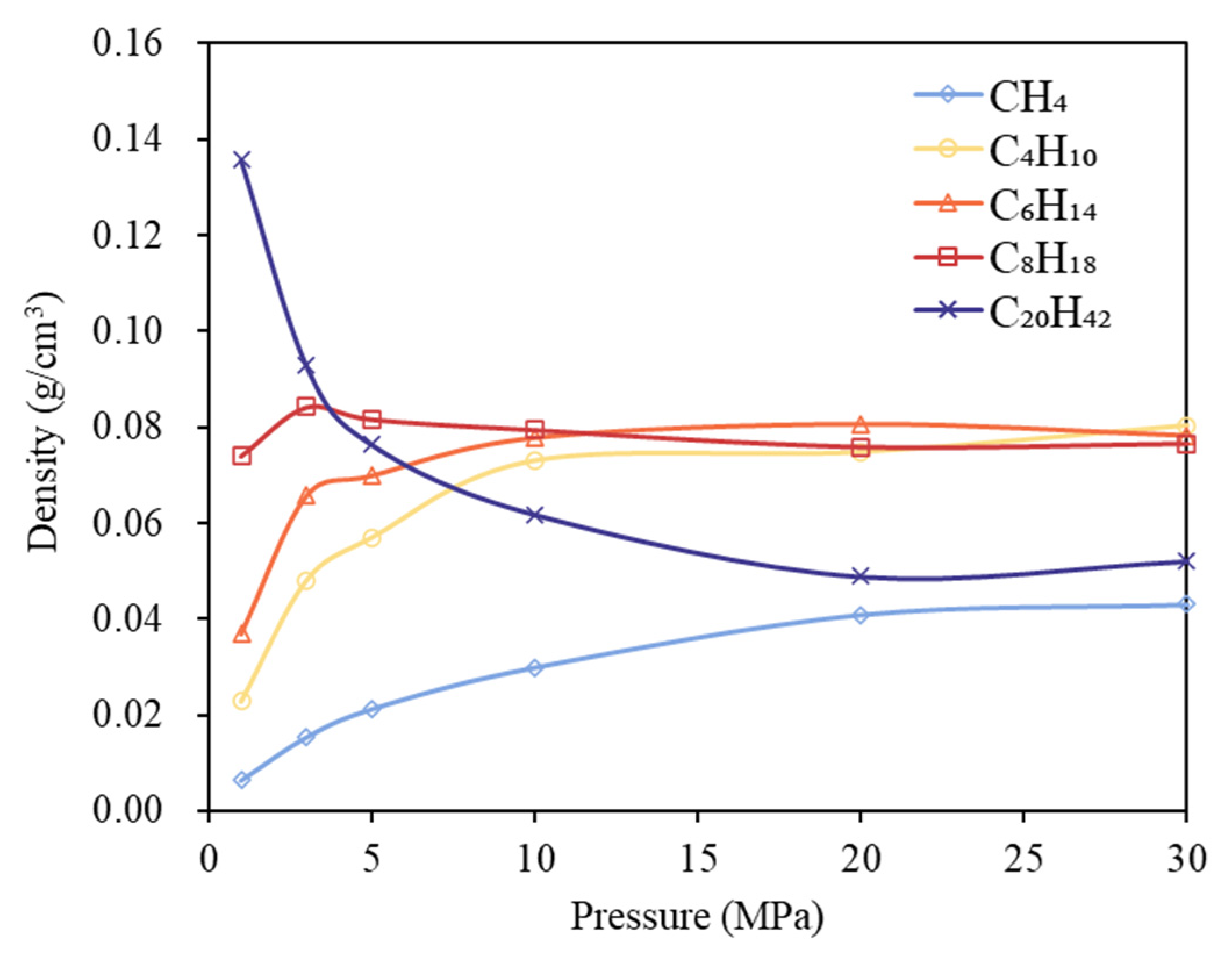
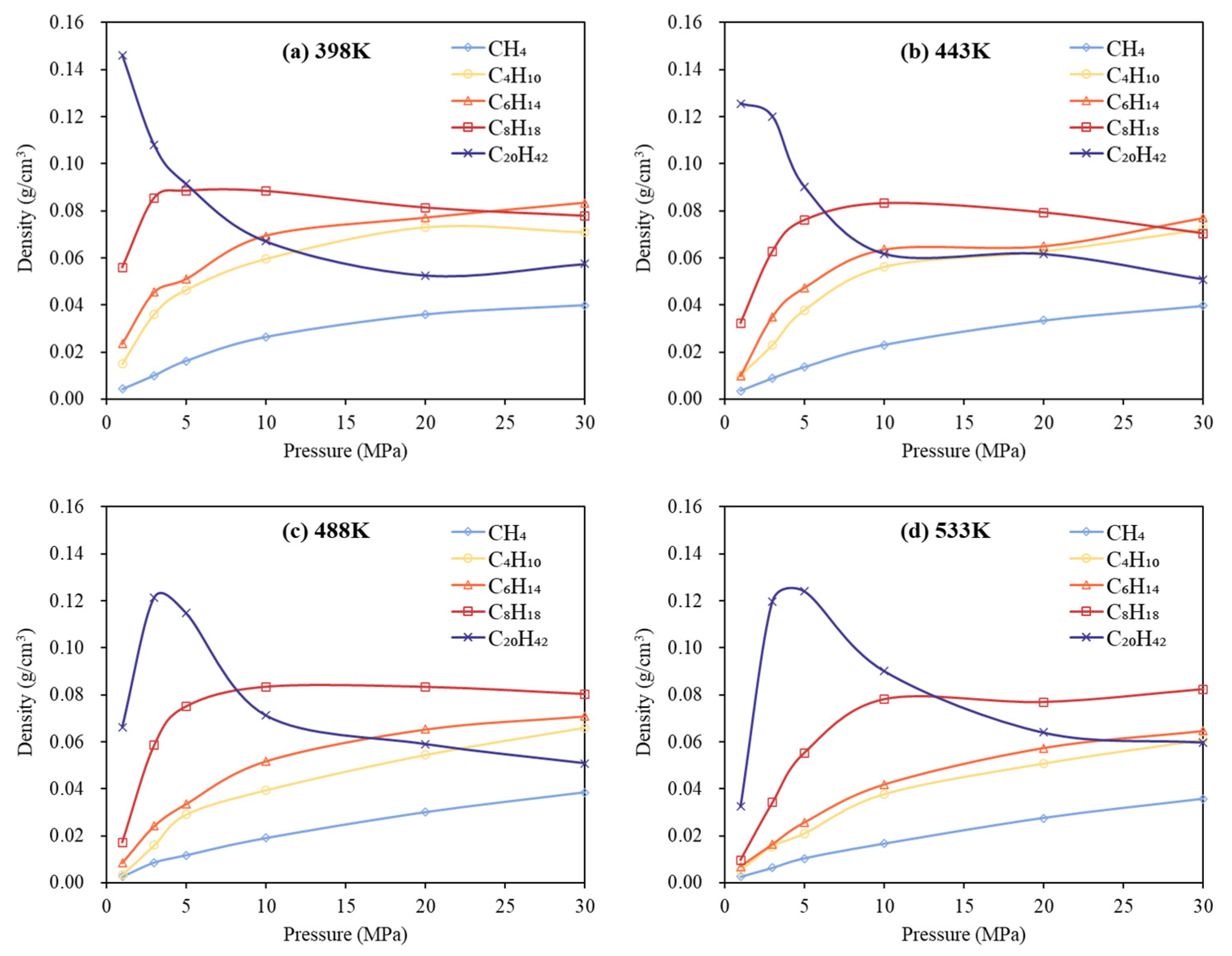
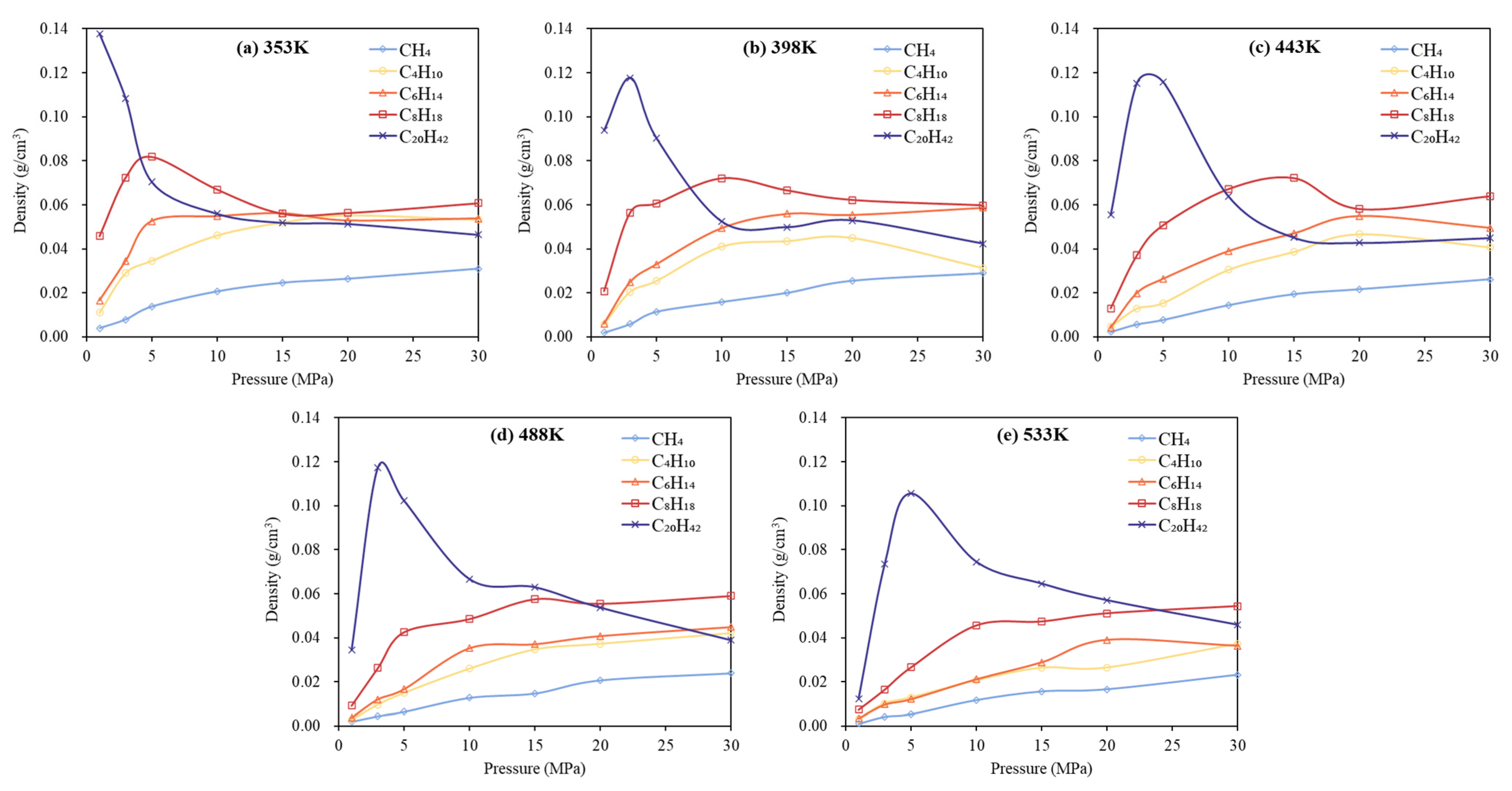
3.2. Phase Behavior Analysis of Multi-Component Alkane System
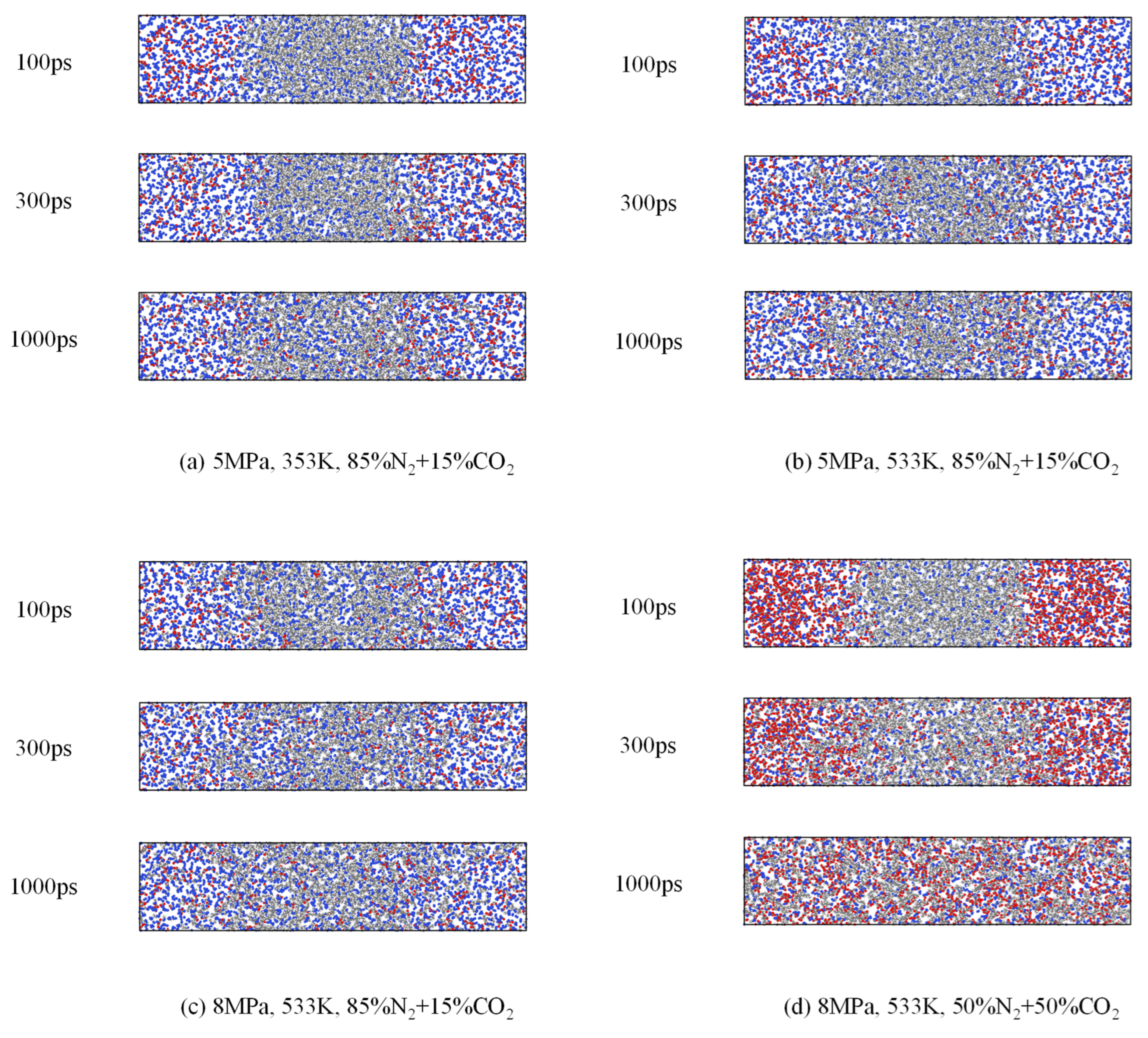
3.3. Phase Behavior Analysis of Multi-Component Alkane–Flue Gas System
3.4. Analysis of Physical Properties of Multi-Component Alkane-Flue Gas Interface Parameters
- (1)
- Density distribution.
- (2)
- Diffusion coefficient.
4. Conclusions
- (1)
- Low-carbon alkanes, due to their weaker intermolecular forces, exhibit greater sensitivity to temperature changes, resulting in more rapid density decreases. In contrast, high-carbon alkanes, characterized by longer molecular chains and stronger intermolecular interactions, show limited thermal expansion and relatively stable density profiles. Pressure has a more significant influence on the phase behavior of low-carbon alkanes than on that of high-carbon alkanes.
- (2)
- In multicomponent alkane systems, those with a higher proportion of light components are less prone to phase transitions. In multicomponent alkane–flue gas systems, increasing pressure leads to higher alkane densities, while increasing temperature reduces density and gradually diminishes or eliminates density peaks in the low-pressure region. Additionally, the influence of increased CO2 content on the system’s phase behavior becomes progressively less significant.
- (3)
- At a given temperature, when the system pressure exceeds a certain threshold, CO2 becomes miscible with alkanes in the multicomponent system, while N2 diffuses into the alkane phase, causing system expansion and density reduction. The previously well-defined interface between the alkane mixture and flue gas becomes progressively diffuse and eventually disappears, indicating the onset of a fully miscible state. The corresponding pressure at which this occurs is defined as the miscibility pressure at that temperature.
- (4)
- With increasing temperature and pressure, the diffusion coefficients of N2 and CO2 rise, accelerating the transport of flue gas into the alkane phase and promoting faster disappearance of the interface. The transition zone required to achieve miscibility broadens under these conditions, indicating that elevated temperature and pressure facilitate miscibility between the alkane and flue gas phases. The diffusion coefficients of N2 and CO2 are not affected by their relative proportions in the flue gas mixture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersen, P.Ø.; Brattekås, B.; Zhou, Y.; Nadeau, P.; Nermoen, A.; Yu, Z.; Fjelde, I.; Oelkers, E. Carbon capture utilization and storage (CCUS) in tight gas and oil reservoirs. J. Nat. Gas Sci. Eng. 2020, 81, 103458. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Li, Q.; Wang, F.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Sensitivity Analysis. Nat. Resour. Res. 2025, 34, 1667–1699. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Guo, P.; Wang, J. Molecular insights into CO2-EOR/CCUS: Nanoconfinement effects on multi-component diffusion and miscibility in shale reservoirs. Fuel 2025, 395, 135238. [Google Scholar] [CrossRef]
- Deng, Q.; Ling, X.; Zhang, K.; Tan, L.; Qi, G.; Zhang, J. CCS and CCUS technologies: Giving the oil and gas industry a green future. Front. Energy Res. 2022, 10, 919330. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Ning, X.; Wang, Y.; Bai, B. Settling behavior and mechanism analysis of kaolinite as a fracture proppant of hydrocarbon reservoirs in CO2 fracturing fluid. Colloid. Surf. A 2025, 724, 137463. [Google Scholar] [CrossRef]
- Wang, F.; Liao, G.; Su, C.; Wang, F.; Ma, J.; Yang, Y. Carbon Emission Reduction Accounting Method for a CCUS-EOR Project. Pet. Explor. Dev. 2023, 50, 989–1000. [Google Scholar] [CrossRef]
- Miao, L.; Feng, L.; Ma, Y. Comprehensive Evaluation of CCUS Technology: A Case Study of China’s First Million-Tonne CCUS-EOR Project. Environ. Impact Assess. Rev. J. 2025, 110, 107684. [Google Scholar] [CrossRef]
- Edouard, M.N.; Okere, C.J.; Ejike, C.; Dong, P.; Suliman, M.A. Comparative Numerical Study on the Co-Optimization of CO2 Storage and Utilization in EOR, EGR, And EWR: Implications for CCUS Project Development. Appl. Energy 2023, 347, 121448. [Google Scholar] [CrossRef]
- Lv, Q.; Zheng, R.; Zhou, T.; Guo, X.; Wang, W.; Li, J.; Liu, Z. Visualization Study of CO2-EOR in Carbonate Reservoirs Using 2.5 D Heterogeneous Micromodels for CCUS. Fuel 2022, 330, 125533. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, C.; Li, C.; Yao, E.; Yan, W. Characterization of 13Cr Steel Corrosion in Simulated EOR-CCUS Environment with Flue Gas Impurities. Process Saf. Environ. Prot. 2020, 140, 124–136. [Google Scholar] [CrossRef]
- Subramanian, N.; Madejski, P. Analysis of CO2 Capture Process from Flue-Gases in Combined Cycle Gas Turbine Power Plant Using Post-Combustion Capture Technology. Energy 2023, 282, 128311. [Google Scholar] [CrossRef]
- Liu, D.; Li, W. Flue Gas Enhanced Oil Recovery (EOR) As a High Efficient Development Technology for Offshore Heavy Oil in China. J. Pet. Gas Eng. 2013, 4, 127–142. [Google Scholar]
- Chen, H.; Wang, Z.; Wang, K.; Li, Z.; Li, S. Investigation of EOR Mechanism for Flue Gas Assisted SAGD. J. Pet. Sci. Eng. 2020, 193, 107420. [Google Scholar] [CrossRef]
- Nassabeh, M.; You, Z.; Keshavarz, A.; Iglauer, S. Hybrid EOR Performance Optimization through Flue Gas-Water Alternating Gas (WAG) Injection: Investigating the Synergistic Effects of Water Salinity and Flue Gas Incorporation. Energy Fuels 2024, 38, 13956–13973. [Google Scholar] [CrossRef]
- Sofla, M.J.D.; Farahani, Z.D.; Ghorbanizadeh, S.; Namdar, H. Experimental Study of Asphaltene Deposition during CO2 and Flue Gas Injection EOR Methods Employing a Long Core. Sci. Rep. 2024, 14, 3772. [Google Scholar] [CrossRef] [PubMed]
- Díaz, O.C.; Modaresghazani, J.; Satyro, M.A.; Yarranton, H. Modeling the Phase Behavior of Heavy Oil and Solvent Mixtures. Fluid Phase Equilib. 2011, 304, 74–85. [Google Scholar] [CrossRef]
- Li, X.; Han, H.; Yang, D.; Liu, X.; Qin, J. Phase Behavior of C3H8-CO2-Heavy Oil Systems in the Presence of Aqueous Phase under Reservoir Conditions. Fuel 2017, 209, 358–370. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D. Experimental and Theoretical Quantification of Nonequilibrium Phase Behavior and Physical Properties of Foamy Oil under Reservoir Conditions. J. Energy Resour. Technol. 2017, 139, 062902. [Google Scholar] [CrossRef]
- Sun, X.-F.; Song, Z.-Y.; Cai, L.-F.; Zhang, Y.-Y.; Li, P. Phase Behavior of Heavy Oil-Solvent Mixture Systems under Reservoir Conditions. Pet. Sci. 2020, 17, 1683–1698. [Google Scholar] [CrossRef]
- Bang, V.S.; Pope, G.A.; Sharma, M.M. Phase-behavior Study of Hydrocarbon/Water/Methanol Mixtures at Reservoir Conditions. SPE J. 2010, 15, 952–962. [Google Scholar] [CrossRef]
- Anselme, M.J.; Gude, M.; Teja, A.S. The Critical Temperatures and Densities of the n-Alkanes from Pentane to Octadecane. Fluid Phase Equilib. 1990, 57, 317–326. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Predicting Multicomponent Phase Equilibria and Free Energies of Transfer for Alkanes by Molecular Simulation. J. Am. Chem. Soc. 1997, 119, 8921–8924. [Google Scholar] [CrossRef]
- Errington, J.R.; Boulougouris, G.C.; Economou, I.G.; Panagiotopoulos, A.Z.; Theodorou, D.N. Molecular Simulation of Phase Equilibria for Water-Methane and Water-Ethane Mixtures. J. Phys. Chem. B 1998, 102, 8865–8873. [Google Scholar] [CrossRef]
- Boulougouris, G.C.; Errington, J.R.; Economou, I.G.; Panagiotopoulos, A.Z.; Theodorou, D.N. Molecular Simulation of Phase Equilibria for Water-n-Butane and Water-n-Hexane Mixtures. J. Phys. Chem. B 2000, 104, 4958–4963. [Google Scholar] [CrossRef]
- Zhang, L.; Siepmann, J.I. Pressure Dependence of the Vapor-Liquid-Liquid Phase Behavior in Ternary Mixtures Consisting of n-Alkanes, n-Perfluoroalkanes, and Carbon Dioxide. J. Phys. Chem. B 2005, 109, 2911–2919. [Google Scholar] [CrossRef]
- Nikolaidis, I.K.; Poursaeidesfahani, A.; Csaszar, Z.; Ramdin, M.; Vlugt, T.J.H.; Economou, I.G.; Moultos, O.A. Modeling the Phase Equilibria of Asymmetric Hydrocarbon Mixtures Using Molecular Simulation and Equations of State. AIChE J. 2019, 65, 792–803. [Google Scholar] [CrossRef]
- Moultos, O.A.; Tsimpanogiannis, I.N.; Panagiotopoulos, A.Z.; Trusler, J.M.; Economou, I.G. Atomistic Molecular Dynamics Simulations of Carbon Dioxide Diffusivity in n-Hexane, n-Decane, n-Hexadecane, Cyclohexane, and Squalane. J. Phys. Chem. B 2016, 120, 12890–12900. [Google Scholar] [CrossRef]
- Neyt, J.-C.; Wender, A.; Lachet, V.; Malfreyt, P. Modeling the Pressure Dependence of Acid Gas+n-Alkane Interfacial Tensions Using Atomistic Monte Carlo Simulations. J. Phys. Chem. C 2012, 116, 10563–10572. [Google Scholar] [CrossRef]
- Müller, E.A.; Mejía, A. Interfacial Properties of Selected Binary Mixtures Containing n-Alkanes. Fluid Phase Equilib. 2009, 282, 68–81. [Google Scholar] [CrossRef]
- Zabala, D.; Nieto-Draghi, C.; de Hemptinne, J.C.; de Ramos, A.L.L. Diffusion Coefficients in CO2/n-Alkane Binary Liquid Mixtures by Molecular Simulation. J. Phys. Chem. B 2008, 112, 16610–16618. [Google Scholar] [CrossRef]
- Wang, R.; Bi, S.; Guo, Z.; Feng, G. Molecular Insight into Replacement Dynamics of CO2 Enhanced Oil Recovery in Nanopores. Chem. Eng. J. 2022, 440, 135796. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Liu, K.; Burke, N. Molecular Simulation of CO2 Solubility and Its Effect on Octane Swelling. Energy Fuels 2013, 27, 2741–2747. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Qi, C.; Li, X.; Mai, T.; Zhang, J. Mechanism of Asphaltene Aggregation Induced by Supercritical CO2: Insights from molecular dynamics simulation. RSC Adv. 2017, 7, 50786–50793. [Google Scholar] [CrossRef]
- Lansangan, R.M.; Smith, J.L. Viscosity, Density, and Composition Measurements of CO2/West Texas Oil Systems. SPE Reserv. Eng. 1993, 8, 175–182. [Google Scholar] [CrossRef]
- Ahmed, T.; Nasrabadi, H.; Firoozabadi, A. Complex Flow and Composition Path in CO2 Injection Schemes from Density Effects. Energy Fuels 2012, 26, 4590–4598. [Google Scholar] [CrossRef]
- Kariznovi, M.; Nourozieh, H.; Abedi, J. Phase Composition and Saturated Liquid Properties in Binary and Ternary Systems Containing Carbon Dioxide, n-Decane, and n-Tetradecane. J. Chem. Thermodyn. 2013, 57, 189–196. [Google Scholar] [CrossRef]
- Nourozieh, H.; Kariznovi, M.; Abedi, J. Measurement and Correlation of Saturated Liquid Properties and Gas Solubility for Decane, Tetradecane and Their Binary Mixtures Saturated with Carbon Dioxide. Fluid Phase Equilib. 2013, 337, 246–254. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. A Review of the Current Progress of CO2 Injection EOR and Carbon Storage in Shale Oil Reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Alfarge, D.; Wei, M.; Bai, B. Factors Affecting CO2-EOR in Shale-Oil Reservoirs: Numerical Simulation Study and Pilot Tests. Energy Fuels 2017, 31, 8462–8480. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Fu, W.; Lu, X.; Chen, Z.; Qi, S.; Wang, Y.; Yang, W.; Lu, J. Extraction of Shale Oil with Supercritical CO2: Effects of Number of Fractures and Injection Pressure. Fuel 2021, 285, 118977. [Google Scholar] [CrossRef]
- Shokir, E.M.E.-M. CO2-Oil Minimum Miscibility Pressure Model for Impure and Pure CO2 Streams. J. Pet. Sci. Eng. 2007, 58, 173–185. [Google Scholar] [CrossRef]
- Mosavat, N.; Abedini, A.; Torabi, F. Phase Behaviour of CO2-Brine and CO2-Oil Systems for CO2 Storage and Enhanced Oil Recovery: Experimental Studies. Energy Procedia 2014, 63, 5631–5645. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Z.; Sun, L.; Pan, X.; Liu, Y.; Xu, Z.; Zheng, X.; Wang, Y.; Liu, P. Study on the Minimum Miscibility Pressure and Phase Behavior of CO2-Shale Oil in Nanopores. Chem. Eng. J. 2024, 497, 154493. [Google Scholar] [CrossRef]
- Mehana, M.; Fahes, M.; Huang, L. The Density of Oil/Gas Mixtures: Insights from Molecular Simulations. SPE J. 2018, 23, 1798–1808. [Google Scholar] [CrossRef]
- Fang, T.; Li, S.; Zhang, Y.; Su, Y.; Yan, Y.; Zhang, J. How the Oil Recovery in Deep Oil Reservoirs Is Affected by Injected Gas Types: A Molecular Dynamics Simulation Study. Chem. Eng. J. 2021, 231, 116286. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, X.; Xi, C.; Wang, B.; Zhao, F.; Zhao, Z. Air Injection into Condensate Reservoirs in the Middle and Late Stages Increases Recovery with Thermal Miscible Mechanism. SPE J. 2024, 29, 6375–6388. [Google Scholar] [CrossRef]
- Jorgensen, W.; David, S.; Maxwell, J.T. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Jeffry, D.; Carol, J. Optimized intermolecular potential functions forliquid hydrocarbons. J. Am. Chem. Soc. 1984, 106, 6638–6646. [Google Scholar] [CrossRef]
- Siu, S.; Kristyna, P.; Rainer, A. Optimization of the OPLS-AA Force Field for Long Hydrocarbons. J. Chem. Theory Comput. 2012, 8, 1459–1470. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Development of the transferable potentials for phase equilibria model for hydrogen sulfide. J. Phys. Chem. B. 2015, 119, 7041–7052. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Liu, S.; Zhang, Y.; Song, Y. Investigation of the effect of CO2 on asphaltene deposition and flow mechanism under nano-confined environment. J. Mol. Liq. 2024, 396, 124092. [Google Scholar] [CrossRef]
- Wang, F.; Meng, X.; Xu, H.; Quan, H.; Liu, Y.; Jia, W.; Liu, L. Study on the Influence of Wettability and Displacement Pressure on CO2 Storage Mechanisms under Nano-confinement: Insights from molecular dynamics simulations. Colloid. Surf. A 2025, 726, 137751. [Google Scholar] [CrossRef]
- Li, J.; Lang, Y.; Li, B.; Liu, Y.; Pan, Z.; Rahman, S.S. N2 influences on CH4 accumulation and displacement in shale by molecular dynamics. Sci. Rep. 2025, 15, 1833. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, C.; Chen, Z.; He, Y.; Wang, S. Recovery mechanisms of shale oil by CO2 injection in organic and inorganic nanopores from molecular perspective. J. Mol. Liq. 2024, 398, 124276. [Google Scholar] [CrossRef]



| CH4 | C4H10 | C6H14 | C8H18 | C20H42 | CO2 | N2 | |
|---|---|---|---|---|---|---|---|
| Basial model | 0.500 | 0.150 | 0.130 | 0.120 | 0.100 | / | / |
| Basial model + 85% N2 + 15% CO2 | 0.300 | 0.090 | 0.078 | 0.072 | 0.060 | 0.060 | 0.340 |
| Comparative model + 65% N2 + 35% CO2 | 0.300 | 0.090 | 0.078 | 0.072 | 0.060 | 0.140 | 0.260 |
| Comparative model + 50% N2 + 50% CO2 | 0.300 | 0.090 | 0.078 | 0.072 | 0.060 | 0.200 | 0.200 |
| Comparative model + 35% N2 + 65% CO2 | 0.300 | 0.090 | 0.078 | 0.072 | 0.060 | 0.260 | 0.140 |
| N2 (10−9 m2/s) | Simulation Error (10−9 m2/s) | CO2 (10−9 m2/s) | Simulation Error (10−9 m2/s) | |
|---|---|---|---|---|
| 5 MPa, 533 K 85% N2 + 15% CO2 | 103.6 | 10.1 | 108.5 | 8.1 |
| 5 MPa, 353 K 85% N2 + 15% CO2 | 98.1 | 15.8 | 76.1 | 3.8 |
| 8 MPa, 533 K 85% N2 + 15% CO2 | 106.0 | 6.7 | 111.2 | 3.4 |
| 8 MPa, 533 K 50% N2 + 50% CO2 | 106.1 | 7.8 | 110.8 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, J.; Qi, Z.; Liu, S.; Xi, C.; Zhao, F.; Hu, P.; Zhou, H.; Wang, C.; Wang, B. The Study of Phase Behavior of Multi-Component Alkane–Flue Gas Systems Under High-Temperature Conditions Based on Molecular Dynamics Simulations. Energies 2025, 18, 4169. https://doi.org/10.3390/en18154169
Zhang X, Tang J, Qi Z, Liu S, Xi C, Zhao F, Hu P, Zhou H, Wang C, Wang B. The Study of Phase Behavior of Multi-Component Alkane–Flue Gas Systems Under High-Temperature Conditions Based on Molecular Dynamics Simulations. Energies. 2025; 18(15):4169. https://doi.org/10.3390/en18154169
Chicago/Turabian StyleZhang, Xiaokun, Jiagao Tang, Zongyao Qi, Suo Liu, Changfeng Xi, Fang Zhao, Ping Hu, Hongyun Zhou, Chao Wang, and Bojun Wang. 2025. "The Study of Phase Behavior of Multi-Component Alkane–Flue Gas Systems Under High-Temperature Conditions Based on Molecular Dynamics Simulations" Energies 18, no. 15: 4169. https://doi.org/10.3390/en18154169
APA StyleZhang, X., Tang, J., Qi, Z., Liu, S., Xi, C., Zhao, F., Hu, P., Zhou, H., Wang, C., & Wang, B. (2025). The Study of Phase Behavior of Multi-Component Alkane–Flue Gas Systems Under High-Temperature Conditions Based on Molecular Dynamics Simulations. Energies, 18(15), 4169. https://doi.org/10.3390/en18154169






