Direct Air Capture Using Pyrolysis and Gasification Chars: Key Findings and Future Research Needs
Abstract
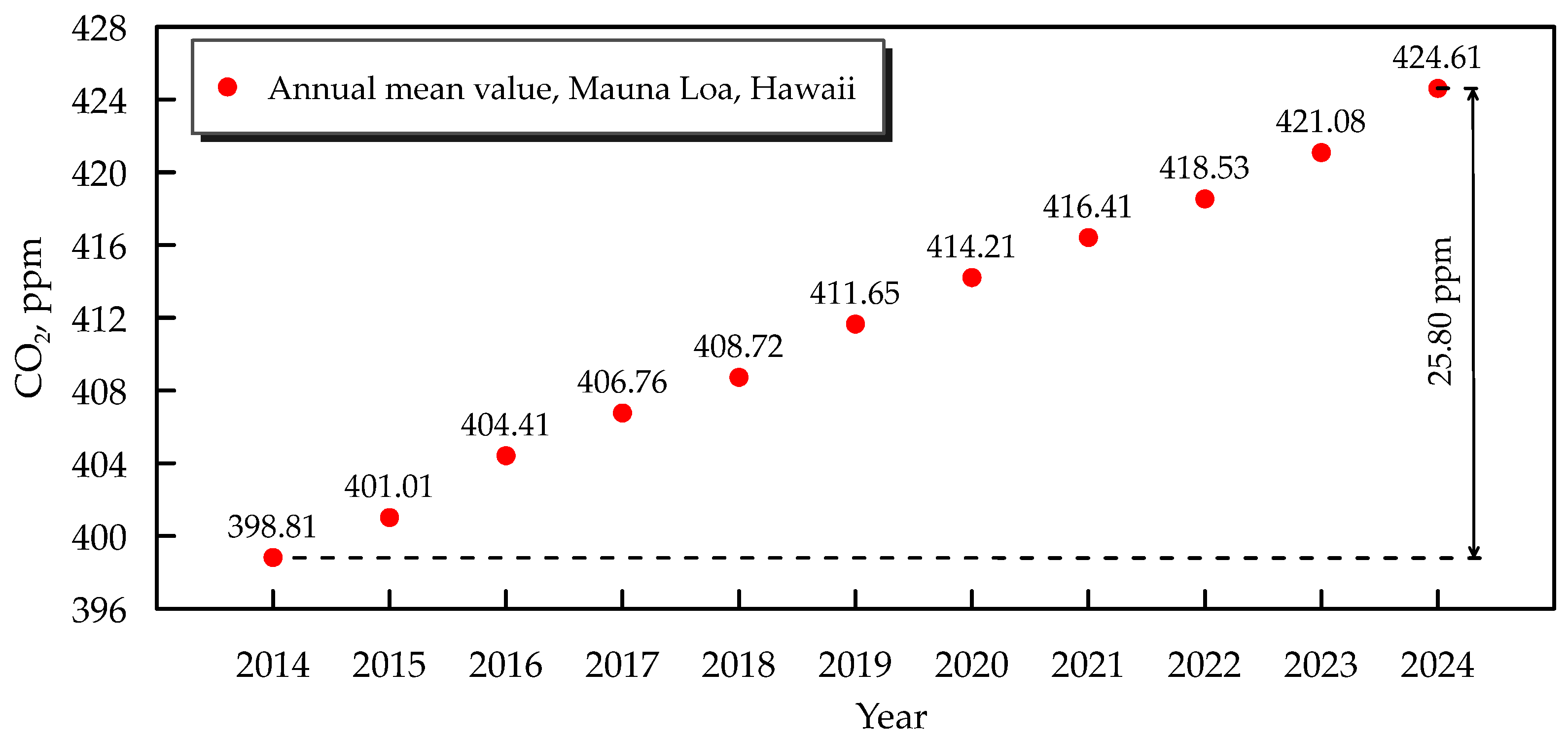
1. Background and Importance of Atmospheric CO2 Mitigation
- (1)
- What are the key physicochemical properties and modification strategies that enhance the CO2 adsorption capacity of the pyrolysis and gasification chars?
- (2)
- What are the major knowledge gaps and future research needs that must be addressed to enable the large-scale implementation of char-based materials in direct air capture systems?
2. Overview of Direct Air Capture Technologies and Materials
2.1. Liquid-Based Absorption
2.2. Solid-Based Absorption
2.3. Solid-Based Adsorption
2.4. Electrochemically (Liquid and Solid Sorbent)
2.5. Hybrid Systems
2.6. Relevance of Carbon-Based Solid Adsorbents for DAC
3. Pyrolysis and Gasification Chars as CO2 Sorbents
3.1. Physicochemical Properties
3.1.1. Specific Surface Area
| Biomass Types | Pyrolysis | Activation | BET SSA, m2/g | Refs. | ||||
|---|---|---|---|---|---|---|---|---|
| T, °C | RT, min | Method | T, °C | RT, min | C:A (a) | |||
| 70% Pine Wood- 30% Sewage Sludge | 600 | 240 | Raw | - | - | - | 182 | [33] |
| 700 | 240 | 223 | ||||||
| 800 | 240 | 150 | ||||||
| 300 | 240 | KOH | 600 | 240 | 1:1 | 703 | ||
| 300 | 240 | KOH | 700 | 240 | 1:1 | 2623 | ||
| 300 | 240 | KOH | 800 | 240 | 1:1 | 2047 | ||
| Bamboo impregnated with H2SO4 | 350 | 120 | - | - | - | - | 89 | [42] |
| 550 | 120 | 140 | ||||||
| 750 | 120 | 229 | ||||||
| 950 | 60 | 310 | ||||||
| 950 | 120 | 350 | ||||||
| 950 | 180 | 346 | ||||||
| 950 | 240 | 314 | ||||||
| Garlic Peel | 400 | 120 | Raw | - | - | - | 306 | [43] |
| KOH | 600 | 60 | 1:2 | 947 | ||||
| KOH | 700 | 60 | 1:2 | 1179 | ||||
| KOH | 800 | 60 | 1:2 | 1262 | ||||
| Whitewood | 500 | - | Steam | 700 | 85 | 1:0.94 | 840 | [44] |
| CO2 | 890 | 100 | 1:8.7 | 820 | ||||
| KOH | 775 | 120 | 1:1.23 | 1400 | ||||
| Pine Sawdust | 800 | 5 | Raw | - | - | - | 368 | [45] |
| Steam | 850 | 25 | 1:0.4 | 701 | ||||
| KOH | 850 | 60 | 1:3 | 1375 | ||||
| Wood Pellet | 1200 | - | Raw | - | - | - | 161 | [48] |
| Steam | 550 | 60 | 1:2.5 | 307 | ||||
| CO2 | 550 | 60 | 1:16.7 | 287 | ||||
| ZnCl2 | 550 | 60 | 1:1 | 4.56 | ||||
| H3PO4 | 550 | 60 | 1:1 | 3.19 | ||||
| KOH | 550 | 60 | 1:1 | 439 | ||||
| Solid digestate | 600 | 9 | Raw | - | - | - | 6 | [49] |
| 700 | 5 | |||||||
| 800 | 16 | |||||||
| 900 | 63 | |||||||
3.1.2. Pore Size Distribution
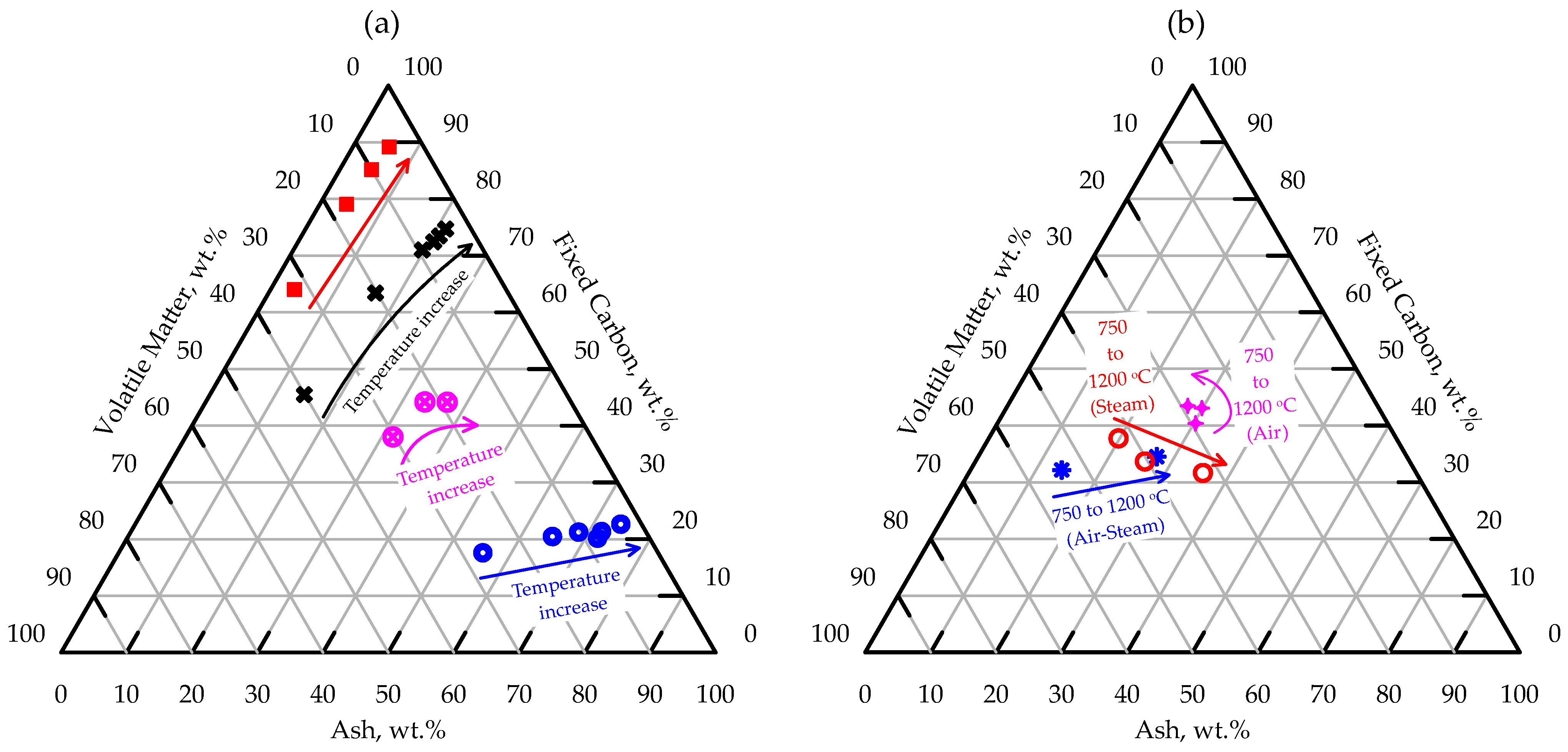
3.1.3. Ash, Fixed Carbon, and Volatile Matter
3.1.4. Hydrogen-to-Carbon and Oxygen-to-Carbon Ratios
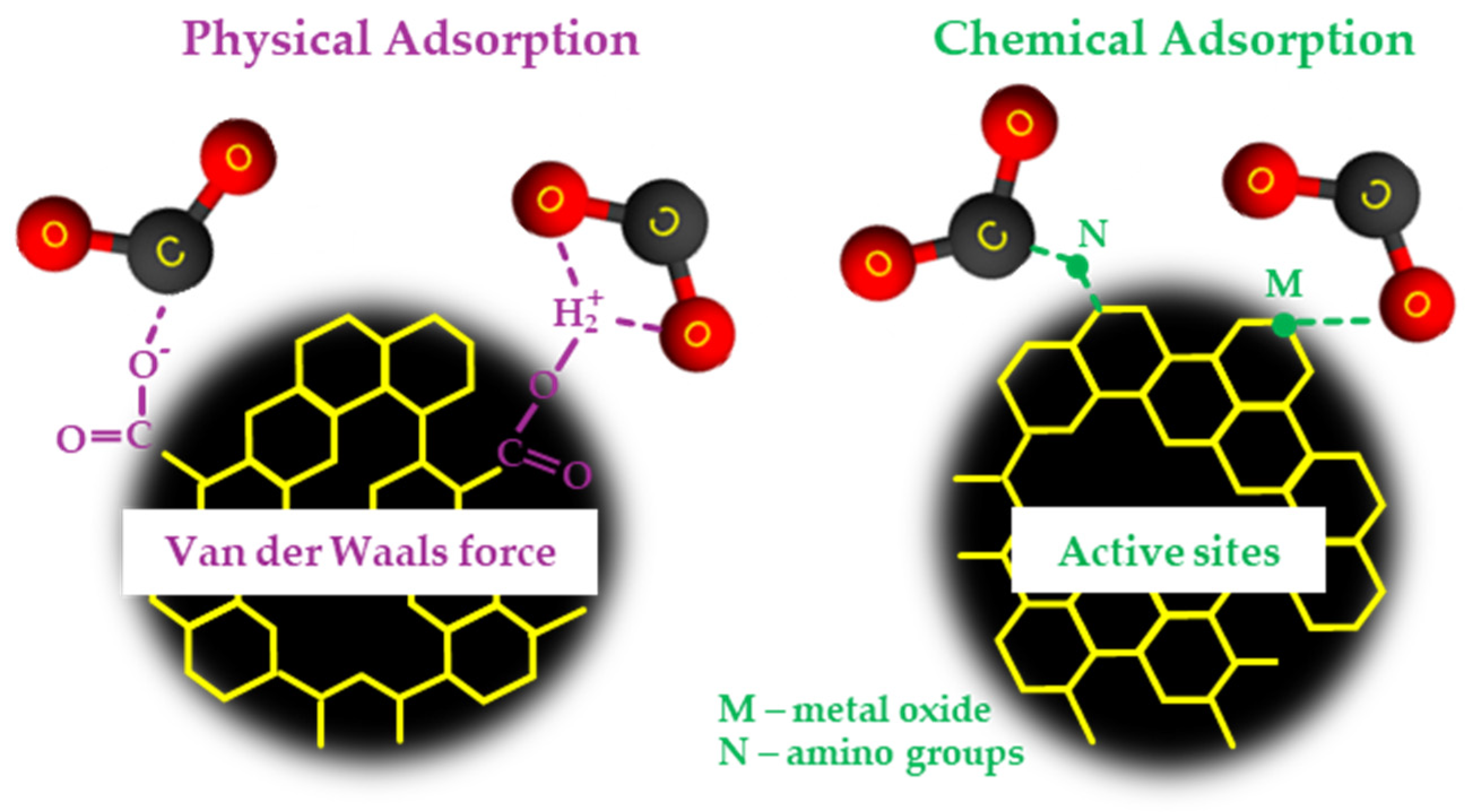
3.2. Adsorption Mechanisms
3.2.1. Surface Chemistry
3.2.2. Nitrogen Function Groups
3.2.3. CO2 Adsorption Capacity
3.2.4. Regeneration Energy, Cyclic Stability, and Environmental Degradation Risks
3.3. Spectroscopic Characterization
3.3.1. Fourier Transform Infrared Spectroscopy
3.3.2. Carbon-13 Nuclear Magnetic Resonance
3.3.3. X-Ray Photoelectron Spectroscopy
3.3.4. Raman Spectroscopy
4. Optimization Strategies and Char-Based Adsorbents
4.1. Structural and Chemical Modifications
4.2. Influence of Pyrolysis Atmosphere on CO2 Capture Efficiency
4.3. Coupling Pyrolysis/Gasification with Carbon Capture and Utilization
4.4. Integration of CO2 Recycling in Gasification Processes
4.5. Technological Advances in Char Modification and Activation
5. Key Challenges and Limitations
- (a)
- The just energy transition between the energy and industrial sectors can be better smoothed;
- (b)
- Socioenvironmental benefits can be supported with more everyday practices in the population’s lives;
- (c)
- Socioenvironmental and economic aspects, such as detrimental factors, can be considerably mitigated through the adoption of public policies;
- (d)
- The high capital costs of implementing DACs can be addressed more specifically and quantified;
- (e)
- The problems in developing DACs from a commercial perspective can be addressed through bilateral agreements between countries interested in this technology;
- (f)
- Successful strategies for implementing DACs can be more widely quantified and disseminated.
- (a)
- Hybrid SST—dehumidification system: develop projects that integrate supersaturated steam treatment (SST) technologies with dehumidification systems to minimize the influence of moisture on process efficiency;
- (b)
- (a)
- Residual biomass supply chains: establish policy frameworks to ensure compliance with regulations such as RED II (Renewable Energy Directive II) and promote sustainability in the residual biomass supply chain;
- (b)
- Biomass characterization: perform detailed analyses of biomass to understand better its physicochemical properties, morphostructural characteristics, and thermal behavior, enabling process adjustments to optimize efficiency [238].
- (a)
- Coal reactivation energy: conduct technical-economic optimization studies to minimize the energy required for coal reactivation and reduce operating costs.
- (b)
- Life cycle analysis: conduct life cycle analyses to assess the environmental and economic impact of different design and operation options [25].
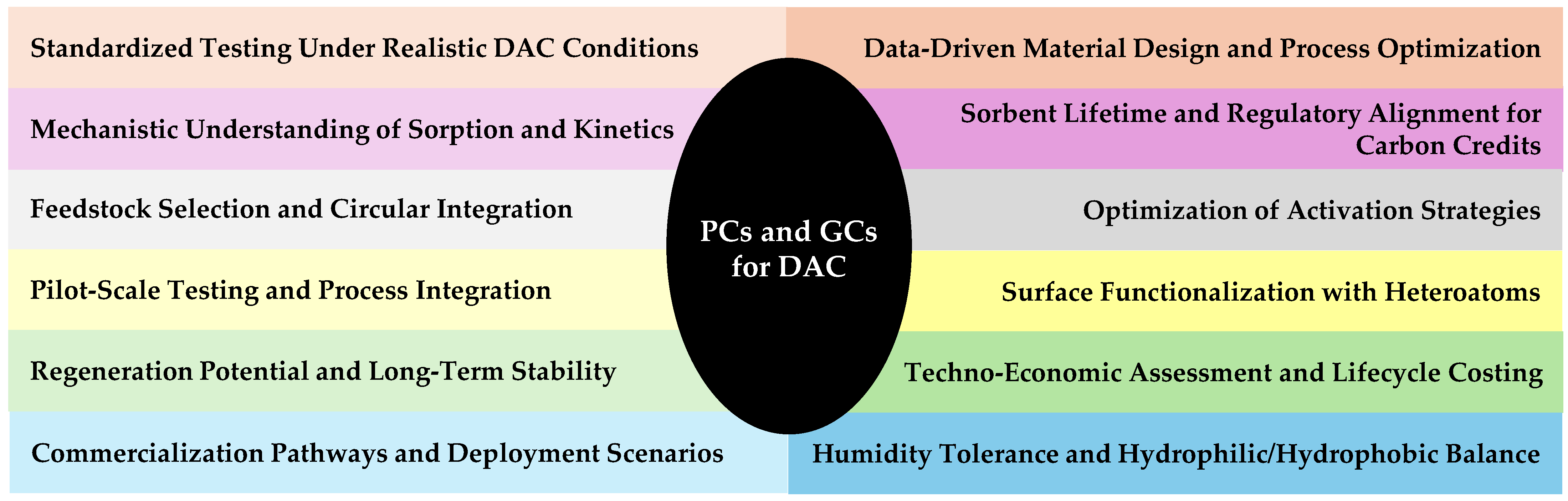
6. Research Outlook and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agancy (IEA). Global Energy Review 2025, Paris. Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 14 April 2025).
- Lan, X.; Tans, P.; Thoning, K.W. Trends in Globally-Averaged CO2 Determined from NOAA Global Monitoring Laboratory Measurements. Available online: https://gml.noaa.gov/ccgg/trends/global.html?doi=10.15138/9n0h-zh07 (accessed on 5 May 2025). [CrossRef]
- Wu, C.; Huang, Q.; Xu, Z.; Sipra, A.T.; Gao, N.; Vandenberghe, L.P.d.S.; Vieira, S.; Soccol, C.R.; Zhao, R.; Deng, S.; et al. A Comprehensive Review of Carbon Capture Science and Technologies. Carbon Capture Sci. Technol. 2024, 11, 100178. [Google Scholar] [CrossRef]
- Ghaffari, S.; Gutierrez, M.F.; Seidel-Morgenstern, A.; Lorenz, H.; Schulze, P. Sodium Hydroxide-Based CO2 Direct Air Capture for Soda Ash Production-Fundamentals for Process Engineering. Ind. Eng. Chem. Res. 2023, 62, 7566–7579. [Google Scholar] [CrossRef]
- Zolfaghari, Z.; Aslani, A.; Zahedi, R.; Kazzazi, S. Simulation of Carbon Dioxide Direct Air Capture Plant Using Potassium Hydroxide Aqueous Solution: Energy Optimization and CO2 Purity Enhancement. Energy Convers. Manag. X 2024, 21, 100489. [Google Scholar] [CrossRef]
- An, K.; Li, K.; Yang, C.M.; Brechtl, J.; Stamberga, D.; Zhang, M.; Nawaz, K. Direct Air Capture with Amino Acid Solvent: Operational Optimization Using a Crossflow Air-Liquid Contactor. AIChE J. 2024, 70, e18429. [Google Scholar] [CrossRef]
- Hua, J.; Shen, X.; Jiao, X.; Lin, H.; Li, G.; Sun, X.; Yan, F.; Wu, H.; Zhang, Z. Direct Air Capture of CO2 by Amine-Impregnated Resin: The Effect of Resin Pore Structure and Humid Conditions. Carbon Capture Sci. Technol. 2024, 12, 100237. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Rim, G.; Rosu, C.; Song, M.G.; Jones, C.W. Direct Air Capture of CO2 Using Amine/Alumina Sorbents at Cold Temperature. ACS Environ. Au 2023, 3, 295–307. [Google Scholar] [CrossRef]
- Schellevis, H.M.; Brilman, D.W.F. Experimental Study of CO2 Capture from Air via Steam-Assisted Temperature-Vacuum Swing Adsorption with a Compact Kg-Scale Pilot Unit. React. Chem. Eng. 2024, 9, 910–924. [Google Scholar] [CrossRef]
- Shi, W.K.; Zhang, X.J.; Liu, X.; Wei, S.; Shi, X.; Wu, C.; Jiang, L. Temperature-Vacuum Swing Adsorption for Direct Air Capture by Using Low-Grade Heat. J. Clean. Prod. 2023, 414, 137731. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Liang, C.; Fang, K.; Li, S.; Guo, X.; Wang, T.; Fang, M. Direct Air Capture of CO2 Using Biochar Prepared from Sewage Sludge: Adsorption Capacity and Kinetics. Sci. Total Environ. 2024, 948, 174887. [Google Scholar] [CrossRef]
- Li, L.; Xiao, Z.; Xu, C.; Zhou, Y.; Li, Z. The Utility of MOF-Based Materials in Direct Air Capture (DAC) Application to Ppm-Level CO2. Environ. Res. 2024, 262, 119985. [Google Scholar] [CrossRef]
- Xiang, X.; Guo, T.; Yin, Y.; Gao, Z.; Wang, Y.; Wang, R.; An, M.; Guo, Q.; Hu, X. High Adsorption Capacity Fe@13X Zeolite for Direct Air CO2 Capture. Ind. Eng. Chem. Res. 2023, 62, 5420–5429. [Google Scholar] [CrossRef]
- Seo, H.; Hatton, T.A. Electrochemical Direct Air Capture of CO2 Using Neutral Red as Reversible Redox-Active Material. Nat. Commun. 2023, 14, 313. [Google Scholar] [CrossRef]
- Chae, J.E.; Choi, J.; Lee, D.; Lee, S.; Kim, S. Development of Anion Exchange Membrane-Based Electrochemical CO2 Separation Cells for Direct Air Capture. J. Ind. Eng. Chem. 2024, 145, 543–550. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, W.; Yong, J.Y.; Zhang, X.J.; Jiang, L. Techno-Economic Analysis on Temperature Vacuum Swing Adsorption System Integrated with Pre-Dehumidification for Direct Air Capture. Carbon Capture Sci. Technol. 2024, 12, 100199. [Google Scholar] [CrossRef]
- Grossmann, Q.; Stampi-Bombelli, V.; Yakimov, A.; Docherty, S.; Copéret, C.; Mazzotti, M. Developing Versatile Contactors for Direct Air Capture of CO2 through Amine Grafting onto Alumina Pellets and Alumina Wash-Coated Monoliths. Ind. Eng. Chem. Res. 2023, 62, 13594–13611. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, F.; Mehta, M.; Grimm, A.; Gazzani, M.; Gallucci, F.; Kramer, G.J.; Van Sint Annaland, M. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2020, 59, 7007–7020. [Google Scholar] [CrossRef]
- Barahimi, V.; Ho, M.; Croiset, E. From Lab to Fab: Development and Deployment of Direct Air Capture of CO2. Energies 2023, 16, 6385. [Google Scholar] [CrossRef]
- Kiani, A.; Conway, W.; Abdellah, M.H.; Puxty, G.; Minor, A.J.; Kluivers, G.; Bennett, R.; Feron, P. A Study on Degradation and CO2 Capture Performance of Aqueous Amino Acid Salts for Direct Air Capture Applications. Greenh. Gases Sci. Technol. 2024, 870, 859–870. [Google Scholar] [CrossRef]
- Ali, K.; Mohamed, H.A.; Ali, P.; Paul, F. Amine Based Liquid Capture Technology for Direct Air Capture of CO2—An Overview on Technology Development. Aust. Energy Prod. J. 2025, 65, EP24224. [Google Scholar] [CrossRef]
- Wang, E.; Luo, L.; Wang, J.; Dai, J.; Li, S.; Chen, L.; Li, J. A Dataset for Investigations of Amine-Impregnated Solid Adsorbent for Direct Air Capture. Sci. Data 2025, 12, 724. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, H.; Liu, Y.; An, H.; Lee, J.S. Optimizing Amine-Based Adsorbents for Direct Air Capture: A Comprehensive Review of Performance under Diverse Climatic Conditions. Renew. Sustain. Energy Rev. 2025, 217, 115782. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Zentou, H.; Hoque, B.; Abdalla, M.A.; Saber, A.F.; Abdelaziz, O.Y.; Aliyu, M.; Alkhedhair, A.M.; Alabduly, A.J.; Abdelnaby, M.M. Recent Advances and Challenges in Solid Sorbents for CO2 Capture. Carbon Capture Sci. Technol. 2025, 15, 100386. [Google Scholar] [CrossRef]
- Stampi-Bombelli, V.; Storione, A.; Grossmann, Q.; Mazzotti, M. On Comparing Packed Beds and Monoliths for CO2 Capture from Air Through Experiments, Theory, and Modeling. Ind. Eng. Chem. Res. 2024, 63, 11637–11653. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.H.; Marek, E.J. Kinetics of CO2 Capture with Calcium Oxide during Direct Air Capture in a Fluidized Bed. Energy Fuels 2024, 38, 22290–22297. [Google Scholar] [CrossRef] [PubMed]
- Europian Commission. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable; Europian Commission: Brussels, Belgium, 2018. [Google Scholar]
- Laeim, H.; Molahalli, V.; Prajongthat, P.; Pattanaporkratana, A.; Pathak, G.; Phettong, B.; Hongkarnjanakul, N.; Chattham, N. Porosity Tunable Metal-Organic Framework (MOF)-Based Composites for Energy Storage Applications: Recent Progress. Polymers 2025, 17, 130. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Ozden, A. CO2 Capture via Electrochemical PH-Mediated Systems. ACS Energy Lett. 2025, 10, 1550–1576. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable Porous Carbons with a Superior Performance for CO2 Capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Li, K.; Zhang, D.; Niu, X.; Guo, H.; Yu, Y.; Tang, Z.; Lin, Z.; Fu, M. Insights into CO2 Adsorption on KOH-Activated Biochars Derived from the Mixed Sewage Sludge and Pine Sawdust. Sci. Total Environ. 2022, 826, 154133. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal Properties of Biochars Derived from Waste Biomass Generated by Agricultural and Forestry Sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Miskolczi, N.; Gao, N.; Quan, C.; Laszlo, A.T. CO2 Reduction by Chars Obtained by Pyrolysis of Real Wastes: Low Temperature Adsorption and High Temperature CO2 Capture. Carbon Capture Sci. Technol. 2025, 14, 100332. [Google Scholar] [CrossRef]
- Brebu, M.; Ioniță, D.; Stoleru, E. Thermal Behavior and Conversion of Agriculture Biomass Residues by Torrefaction and Pyrolysis. Sci. Rep. 2025, 15, 11505. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Song, H. Critical Review on Catalytic Biomass Gasification: State-of-Art Progress, Technical Challenges, and Perspectives in Future Development. J. Clean. Prod. 2024, 408, 137224. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Varsha Vuppaladadiyam, S.S.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; S, M.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.Y. A Critical Review on Biomass Pyrolysis: Reaction Mechanisms, Process Modeling and Potential Challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The Effect of Pyrolysis Temperature and Feedstock on Biochar Agronomic Properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and Pyrolysis Conditions Affect Suitability of Biochar for Various Sustainable Energy and Environmental Applications. J. Anal. Appl. Pyrolysis 2023, 170, 105881. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar Production under Different Pyrolysis Temperatures with Different Types of Agricultural Wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Mackey, H.R.; Mariyam, S.; Zuhara, S.; Al-Ansari, T.; McKay, G. Char Products From Bamboo Waste Pyrolysis and Acid Activation. Front. Mater. 2021, 7, 624791. [Google Scholar] [CrossRef]
- Huang, G.G.; Liu, Y.F.; Wu, X.X.; Cai, J.J. Activated Carbons Prepared by the KOH Activation of a Hydrochar from Garlic Peel and Their CO2 Adsorption Performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Shahkarami, S.; Azargohar, R.; Dalai, A.K.; Soltan, J. Breakthrough CO2 Adsorption in Bio-Based Activated Carbons. J. Environ. Sci. 2015, 34, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jerzak, W.; Mlonka-Mędrala, A.; Gao, N.; Magdziarz, A. Potential of Products from High-Temperature Pyrolysis of Biomass and Refuse-Derived Fuel Pellets. Biomass Bioenergy 2024, 183, 107159. [Google Scholar] [CrossRef]
- Wang, K.; Xu, S. Preparation of High Specific Surface Area Activated Carbon from Petroleum Coke by KOH Activation in a Rotary Kiln. Processes 2024, 12, 241. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated Bio-Chars Derived from Rice Husk via One- and Two-Step KOH-Catalyzed Pyrolysis for Phenol Adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, Y.; Li, C.; Zhang, Y.; Sun, S.; Xu, Y.; Jiang, L.; Wu, C. The Application of Biochar for CO2 Capture: Influence of Biochar Preparation and CO2 Capture Reactors. Ind. Eng. Chem. Res. 2023, 62, 17168–17181. [Google Scholar] [CrossRef]
- Aissaoui, M.H.; Hertzog, J.; Sambusiti, C.; Gauthier-Maradei, P.; Pons, M.N.; Carré, V.; Brech, Y.L.; Dufour, A. Thermochemical Conversion of Solid Digestates: Effects of Temperature and Fluidizing Gas on Products Composition. J. Anal. Appl. Pyrolysis 2025, 186, 106928. [Google Scholar] [CrossRef]
- Yue, Y.; Jin, X.; Deng, L. Experimental Study on Properties of Syngas, Tar, and Biochar Derived from Different Gasification Methods. Appl. Sci. 2023, 13, 11490. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification Biochar as a Valuable By-Product for Carbon Sequestration and Soil Amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef]
- Sieradzka, M.; Mlonka, -M.A.; Kalemba-Rec, I.; Reinmöller, M.; Kuster, F.; Kalawa, W.; Magdziarz, A. Evaluation of Physical and Chemical Properties of Residue from Gasification of Biomass Wastes. Energies 2022, 15, 3539. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Catalyst Properties and Catalytic Performance of Char from Biomass Gasification. Ind. Eng. Chem. Res. 2012, 51, 13113–13122. [Google Scholar] [CrossRef]
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 Adsorption Study on Pure and Chemically Activated Chars Derived from Commercial Biomass Gasifiers. J. CO2 Util. 2019, 33, 46–54. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable Gasification Biochar as a High Efficiency Adsorbent for CO2 Capture: A Facile Method to Designer Biochar Fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Q.; Xie, G.; Ye, Z.; Zhu, Z.; Ye, C. Effect of Air Equivalence Ratio on the Characteristics of Biomass Partial Gasification for Syngas and Biochar Co-Production in the Fluidized Bed. Renew. Energy 2024, 222, 119881. [Google Scholar] [CrossRef]
- Edeh, I.G.; Masek, O.; Fusseis, F. 4D Structural Changes and Pore Network Model of Biomass during Pyrolysis. Sci. Rep. 2023, 13, 22863. [Google Scholar] [CrossRef]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.W.; Sajdak, M. Various Biomass Pyrolysis Conditions Influence the Porosity and Pore Size Distribution of Biochar. Energy 2023, 263, 126128. [Google Scholar] [CrossRef]
- Ding, S.; Kantarelis, E.; Engvall, K. Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures. Energies 2020, 13, 5004. [Google Scholar] [CrossRef]
- Wu, R.; Beutler, J.; Price, C.; Baxter, L.L. Biomass Char Particle Surface Area and Porosity Dynamics during Gasification. Fuel 2020, 264, 116833. [Google Scholar] [CrossRef]
- Surup, G.R.; Trubetskaya, A.; Tangstad, M. Charcoal as an Alternative Reductant in Ferroalloy Production: A Review. Processes 2020, 8, 1432. [Google Scholar] [CrossRef]
- Abdelaal, A.; Benedetti, V.; Villot, A.; Patuzzi, F.; Gerente, C.; Baratieri, M. Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review. Energies 2023, 16, 4175. [Google Scholar] [CrossRef]
- Chen, J.; Ding, L.; Wang, P.; Zhang, W.; Li, J.; Mohamed, B.A.; Chen, J.; Leng, S.; Liu, T.; Leng, L.; et al. The Estimation of the Higher Heating Value of Biochar by Data-Driven Modeling. J. Renew. Mater. 2022, 10, 1555–1574. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of Biochars to Evaluate Recalcitrance and Agronomic Performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Ahmad, M.A.; Md Ali, U.F.; Ken, K. Gasification Char Residues Management: Assessing the Characteristics for Adsorption Application. Arab. J. Chem. 2023, 16, 104993. [Google Scholar] [CrossRef]
- Hernández, J.J.; Lapuerta, M.; Monedero, E. Characterisation of Residual Char from Biomass Gasification: Effect of the Gasifier Operating Conditions. J. Clean. Prod. 2016, 138, 83–93. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.M.; Ahmad, M.M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar Production from Date Palm Waste: Charring Temperature Induced Changes in Composition and Surface Chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Wanignon Ferdinand, F.; Van De Steene, L.; Kamenan Blaise, K.; Siaka, T. Prediction of Pyrolysis Oils Higher Heating Value with Gas Chromatography-Mass Spectrometry. Fuel 2012, 96, 141–145. [Google Scholar] [CrossRef]
- Suresh Babu, K.K.B.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of Biochar from Waste Biomass Using Slow Pyrolysis: Studies of the Effect of Pyrolysis Temperature and Holding Time on Biochar Yield and Properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Adhikari, S.; Moon, E.; Paz-Ferreiro, J.; Timms, W. Comparative Analysis of Biochar Carbon Stability Methods and Implications for Carbon Credits. Sci. Total Environ. 2024, 914, 169607. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Petersen, H.I.; Sanei, H. The H/C Molar Ratio and Its Potential Pitfalls for Determining Biochar’s Permanence. GCB Bioenergy 2025, 17, e70049. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zheng, W.; Leng, S.; Ai, Z.; Zhang, W.; Yang, Z.; Yang, J.; Xu, Z.; Cao, J.; et al. A Complete Review on the Oxygen-Containing Functional Groups of Biochar: Formation Mechanisms, Detection Methods, Engineering, and Applications. Sci. Total Environ. 2024, 946, 174081. [Google Scholar] [CrossRef]
- Sharma, T.; Hakeem, I.G.; Gupta, A.B.; Joshi, J.; Shah, K.; Vuppaladadiyam, A.K.; Sharma, A. Parametric Influence of Process Conditions on Thermochemical Techniques for Biochar Production: A State-of-the-Art Review. J. Energy Inst. 2024, 113, 101559. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen Containing Functional Groups of Biochar: An Overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Masutani, S.M.; Ishimura, D.M.; Turn, S.Q.; Kinoshita, C.M. Release of Fuel-Bound Nitrogen during Biomass Gasification. Ind. Eng. Chem. Res. 2000, 39, 626–634. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 Capture by Absorption and Adsorption: A Comprehensive Review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents. Carbon Capture Sci. Technol. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous Materials as Adsorbents for CO2 Capture: Synthesis and Modification. Carbon Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of Spent Coffee Grounds as CO2 Adsorbents for Postcombustion Capture Applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 Capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a Dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons Prepared from Hydrothermally Carbonized Waste Biomass Used as Adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Yang, H.; Gong, M.; Chen, Y. Preparation of Activated Carbons and Their Adsorption Properties for Greenhouse Gases: CH4 and CO2. J. Nat. Gas Chem. 2011, 20, 460–464. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.; Luo, J.; Tian, Z.; Zhang, J.; Sun, S.; Shen, Y.; Ma, R. Enhanced CO2 Capture Performance of N, S Co-Doped Biochar Prepared by Microwave Pyrolysis: Synergistic Modulation of Microporous Structure and Functional Groups. Fuel 2025, 379, 132987. [Google Scholar] [CrossRef]
- Shao, S.; Wang, Y.; Ma, L.; Huang, Z.; Li, X. Sustainable Preparation of Hierarchical Porous Carbon from Discarded Shells of Crustaceans for Efficient CO2 Capture. Fuel 2024, 355, 129287. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Fu, N.; Chen, H.; Lin, H.; Han, S. Biomass-Derived Nitrogen-Doped Porous Carbon with Superior Capacitive Performance and High CO2 Capture Capacity. Electrochim. Acta 2018, 266, 161–169. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 Capture Capacity of Nitrogen-Doped Biomass-Derived Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Li, D.; Tian, Y.; Li, L.; Li, J.; Zhang, H. Production of Highly Microporous Carbons with Large CO2 Uptakes at Atmospheric Pressure by KOH Activation of Peanut Shell Char. J. Porous Mater. 2015, 22, 1581–1588. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-Doped Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Xu, J.; Shi, J.; Cui, H.; Yan, N.; Liu, Y. Preparation of Nitrogen Doped Carbon from Tree Leaves as Efficient CO2 Adsorbent. Chem. Phys. Lett. 2018, 711, 107–112. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable Biomass-Based Carbon Adsorbents for Post-Combustion CO2 Capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Khuong, D.A.; Tsubota, T. Experimental Investigation of CO2 Adsorption Using Adsorbents Derived from Residual Char of Agricultural Waste Gasification. Therm. Sci. Eng. Prog. 2024, 49, 102446. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Production of Palm Kernel Shell-Based Activated Carbon by Direct Physical Activation for Carbon Dioxide Adsorption. Environ. Sci. Pollut. Res. 2019, 26, 33732–33746. [Google Scholar] [CrossRef] [PubMed]
- Khosrowshahi, M.S.; Mashhadimoslem, H.; Shayesteh, H.; Singh, G.; Khakpour, E.; Guan, X.; Rahimi, M.; Maleki, F.; Kumar, P.; Vinu, A. Natural Products Derived Porous Carbons for CO2 Capture. Adv. Sci. 2023, 10, 2304289. [Google Scholar] [CrossRef]
- Wiegner, J.F.; Grimm, A.; Weimann, L.; Gazzani, M. Optimal Design and Operation of Solid Sorbent Direct Air Capture Processes at Varying Ambient Conditions. Ind. Eng. Chem. Res. 2022, 61, 12649–12667. [Google Scholar] [CrossRef]
- Jamdade, S.; Cai, X.; Sholl, D.S. Assessment of Long-Term Degradation of Adsorbents for Direct Air Capture by Ozonolysis. J. Phys. Chem. C 2025, 129, 899–909. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Lo, S.L.; Kumar, S. A Green Approach towards Sorption of CO2 on Waste Derived Biochar. Environ. Res. 2022, 214, 113954. [Google Scholar] [CrossRef]
- Grams, J. Surface Analysis of Solid Products of Thermal Treatment of Lignocellulosic Biomass. J. Anal. Appl. Pyrolysis 2022, 161, 105429. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Zhu, B. Feedstock and Pyrolysis Temperature Influence Biochar Properties and Its Interactions with Soil Substances: Insights from a DFT Calculation. Sci. Total Environ. 2024, 922, 171259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Liang, X.; Huang, L.; Wei, L.; Zheng, X.; Albert, H.A.; Huang, Q.; Liu, Z.; Li, Z. Characterization of Biochars from Woody Agricultural Wastes and Sorption Behavior Comparison of Cadmium and Atrazine. Biochar 2022, 4, 27. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; Kang, R.; Bin, F.; Dou, B. Precise In-Situ Infrared Spectra and Kinetic Analysis of Gasification under the H2O or CO2 Atmospheres. Int. J. Hydrogen Energy 2024, 52, 46–57. [Google Scholar] [CrossRef]
- Brewer, C.E.; Unger, R.; Schmidt-Rohr, K.; Brown, R.C. Criteria to Select Biochars for Field Studies Based on Biochar Chemical Properties. Bioenergy Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
- Li, B.; Liu, D.; Lin, D.; Xie, X.; Wang, S.; Xu, H.; Wang, J.; Huang, Y.; Zhang, S.; Hu, X. Changes in Biochar Functional Groups and Its Reactivity after Volatile-Char Interactions during Biomass Pyrolysis. Energy Fuels 2020, 34, 14291–14299. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental Investigation into the Effect of Pyrolysis on Chemical Forms of Heavy Metals in Sewage Sludge Biochar (SSB), with Brief Ecological Risk Assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. CO2 Gasification Reactivity of Biomass Char: Catalytic Influence of Alkali, Alkaline Earth and Transition Metal Salts. Bioresour. Technol. 2013, 144, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Paredes, P.; Mood, S.H. Biomass Thermo-Chemical Products; Garcia-Perez, M., Chejne-Janna, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2025; ISBN 9780323955515. [Google Scholar]
- Jha, S.; Pattnaik, F.; Zapata, O.; Acharya, B.; Dalai, A.K. KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater. Sustainability 2025, 17, 2170. [Google Scholar] [CrossRef]
- Romero, C.M.; Redman, A.A.P.H.; Terry, S.A.; Hazendonk, P.; Hao, X.; McAllister, T.A.; Okine, E. Molecular Speciation and Aromaticity of Biochar-Manure: Insights from Elemental, Stable Isotope and Solid-State DPMAS 13C NMR Analyses. J. Environ. Manag. 2021, 280, 111705. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Kuang, X.; Xu, Z.; Li, D.; Li, Y.; Zhang, Y. Adsorption of Cadmium and Lead Capacity and Environmental Stability of Magnesium-Modified High-Sulfur Hydrochar: Greenly Utilizing Chicken Feather. Toxics 2024, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Banik, C.; Bakshi, S.; Andersen, D.S.; Cady, S.D.; Smith, R.G.; Brown, R.C. Does Biochar Stabilize the Bioreactive Carbon Fractions of Swine Manure? ACS ES&T Eng. 2023, 3, 1212–1226. [Google Scholar] [CrossRef]
- Bin Mobarak, M.; Pinky, N.S.; Mustafi, S.; Chowdhury, F.; Nahar, A.; Akhtar, U.S.; Quddus, M.S.; Yasmin, S.; Alam, M.A. Unveiling the Reactor Effect: A Comprehensive Characterization of Biochar Derived from Rubber Seed Shell via Pyrolysis and in-House Reactor. RSC Adv. 2024, 14, 29848–29859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jia, X.; Wang, X.; Chen, J.; Cheng, C.; Jia, X.; Hu, H. Using the Conditional Process Analysis Model to Characterize the Evolution of Carbon Structure in Taxodium Ascendens Biochar with Varied Pyrolysis Temperature and Holding Time. Plants 2024, 13, 460. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Y.; Zhang, H.; Wang, W.; Zhu, J.; Chen, Y.; Zhang, S.; Chen, H. Effect Mechanism of Phosphorous-Containing Additives on Carbon Structure Evolution and Biochar Stability Enhancement. Biochar 2024, 6, 39. [Google Scholar] [CrossRef]
- Hwang, H.; Sahin, O.; Choi, J.W. Manufacturing a Super-Active Carbon Using Fast Pyrolysis Char from Biomass and Correlation Study on Structural Features and Phenol Adsorption. RSC Adv. 2017, 7, 42192–42202. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, D.; Cao, B.; Qian, L.; Hu, Y.; Liu, L.; Yuan, C.; Abomohra, A.E.F.; He, Z.; Wang, Q.; et al. Bio-Char and Bio-Oil Characteristics Produced from the Interaction of Enteromorpha Clathrate Volatiles and Rice Husk Bio-Char during Co-Pyrolysis in a Sectional Pyrolysis Furnace: A Complementary Study. J. Anal. Appl. Pyrolysis 2018, 135, 219–230. [Google Scholar] [CrossRef]
- Wang, S.; Wu, L.; Hu, X.; Zhang, L.; O’Donnell, K.M.; Buckley, C.E.; Li, C.Z. An X-Ray Photoelectron Spectroscopic Perspective for the Evolution of O-Containing Structures in Char during Gasification. Fuel Process. Technol. 2018, 172, 209–215. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M.; Nawaz Khan, S.; Liu, Y.; Zhao, S.; Dong, W.; Song, Q.; Wang, C. Deeper Insights into the Devolatilization Mechanism of Biomass Fixed-Bed Gasification under Various Atmospheres. Energy Convers. Manag. 2024, 322, 119113. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Wang, Z.; Zhao, R.; He, J.; Wu, J.; Qin, J. Oxygen-Enriched Gasification of Lignocellulosic Biomass: Syngas Analysis, Physicochemical Characteristics of the Carbon-Rich Material and Its Utilization as an Anode in Lithium Ion Battery. Energy 2020, 212, 118771. [Google Scholar] [CrossRef]
- Del Grosso, M.; Cutz, L.; Tiringer, U.; Tsekos, C.; Taheri, P.; de Jong, W. Influence of Indirectly Heated Steam-Blown Gasification Process Conditions on Biochar Physico-Chemical Properties. Fuel Process. Technol. 2022, 235, 107347. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Asadullah, M.; Zhang, S.; Li, C.Z. Evaluation of Structural Features of Chars from Pyrolysis of Biomass of Different Particle Sizes. Fuel Process. Technol. 2010, 91, 877–881. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Ling, P.; Zhang, X.; Xu, K.; He, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman Spectroscopy of Biochar from the Pyrolysis of Three Typical Chinese Biomasses: A Novel Method for Rapidly Evaluating the Biochar Property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kozinski, J.A.; Dalai, A.K.; Sutarto, R. Effects of Temperature on the Physicochemical Characteristics of Fast Pyrolysis Bio-Chars Derived from Canadian Waste Biomass. Fuel 2014, 125, 90–100. [Google Scholar] [CrossRef]
- Naim, W.; Treu, P.; Dohrn, M.; Saraçi, E.; Grunwaldt, J.D.; Fendt, S.; Spliethoff, H. Structure-Reactivity- and Modelling-Relationships during Thermal Annealing in Biomass Entrained-Flow Gasification: The Effect of Temperature and Residence Time. Fuel 2025, 383, 133848. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Medina-Martos, E.; Gálvez-Martos, J.L.; Almarza, J.; Lirio, C.; Iribarren, D.; Valente, A.; Dufour, J. Environmental and Economic Performance of Carbon Capture with Sodium Hydroxide. J. CO2 Util. 2022, 60, 101991. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.J. Tunable Nitrogen-Doped Microporous Carbons: Delineating the Role of Optimum Pore Size for Enhanced CO2 Adsorption. Chem. Eng. J. 2019, 362, 731–742. [Google Scholar] [CrossRef]
- Su, G.; Yang, S.; Jiang, Y.; Li, J.; Li, S.; Ren, J.C.; Liu, W. Modeling Chemical Reactions on Surfaces: The Roles of Chemical Bonding and van Der Waals Interactions. Prog. Surf. Sci. 2019, 94, 100561. [Google Scholar] [CrossRef]
- Yu, S.; Bo, J.; Fengli, L.; Jiegang, L. Structure and Fractal Characteristic of Micro- and Meso-Pores in Low, Middle-Rank Tectonic Deformed Coals by CO2 and N2 Adsorption. Microporous Mesoporous Mater. 2017, 253, 191–202. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical Role of Small Micropores in High CO2 Uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef]
- Bell, J.G.; Benham, M.J.; Thomas, K.M. Adsorption of Carbon Dioxide, Water Vapor, Nitrogen, and Sulfur Dioxide on Activated Carbon for Capture from Flue Gases: Competitive Adsorption and Selectivity Aspects. Energy Fuels 2021, 35, 8102–8116. [Google Scholar] [CrossRef]
- Vorokhta, M.; Kusdhany, M.I.M.; Švábová, M.; Nishihara, M.; Sasaki, K.; Lyth, S.M. Hierarchically Porous Carbon Foams Coated with Carbon Nitride: Insights into Adsorbents for Pre-Combustion and Post-Combustion CO2 Separation. Sep. Purif. Technol. 2025, 354 Pt 5, 129054. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Fan, J.; Zhu, Y.B.; Wang, F.; Wu, H. Transport of Shale Gas in Microporous/Nanoporous Media: Molecular to Pore-Scale Simulations. Energy Fuels 2021, 35, 911–943. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous Materials for Energy Conversion and Storage Devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Hijazi, N.; Bavykina, A.; Yarulina, I.; Shoinkhorova, T.; Ramos-Fernandez, E.V.; Gascon, J. Chemical Engineering of Zeolites: Alleviating Transport Limitations through Hierarchical Design and Shaping. Chem. Soc. Rev. 2025, 54, 6335–6384. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of Hierarchically Structured Porous Materials from Energy Storage and Conversion, Catalysis, Photocatalysis, Adsorption, Separation, and Sensing to Biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of Surface Modification on Selective CO2 Adsorption: A Technical Review on Mechanisms and Methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Sun, G.D.; Cao, Y.N.; Hu, M.Z.; Liang, X.H.; Wang, Z.; Cai, Z.J.; Shen, F.Y.; He, H.; Wang, Z.X.; Zhou, K. Bin Pyrrolic N-Doped Carbon Catalysts for Highly Efficient Electrocatalytic Reduction of CO2 with Superior CO Selectivity over a Wide Potential Window. Carbon 2023, 214, 118320. [Google Scholar] [CrossRef]
- Guo, J.; Lu, D.; Chen, P.; Zhu, H.; Li, B.; Li, S.; Zhang, C.; Dong, Z.; Cong, Y.; Li, X. Evolution of Sulfur Chemical Morphology of Petroleum Pitches on Their Mesophase Transformation Behaviors and Microcrystalline Structure of the Derived Artificial Graphite. J. Anal. Appl. Pyrolysis 2025, 191, 107189. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Gourdin, G.; Foster, M.; Qu, D. Carbon Surface Functionalities and SEI Formation during Li Intercalation. Carbon 2015, 92, 193–244. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic π-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Kobaisi, M.A.; Bhosale, S.V.S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional Naphthalene Diimides: Synthesis, Properties, and Applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Sagboye, P.A.; Micheal, K.T.; Micheal, T.T.; Saliu, O.D.; James, R. Biomass-Derived Activated Carbon Monoliths: A Review of Production Routes, Performance, and Commercialization Potential. J. Clean. Prod. 2023, 423, 138711. [Google Scholar] [CrossRef]
- Kundu, S.; Khandaker, T.; Anik, M.A.A.M.; Hasan, M.K.; Dhar, P.K.; Dutta, S.K.; Latif, M.A.; Hossain, M.S. A Comprehensive Review of Enhanced CO2 Capture Using Activated Carbon Derived from Biomass Feedstock. RSC Adv. 2024, 14, 29693–29736. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill Leachate: Sources, Nature, Organic Composition, and Treatment: An Environmental Overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Caliman, F.A.; Robu, B.M.; Smaranda, C.; Pavel, V.L.; Gavrilescu, M. Soil and Groundwater Cleanup: Benefits and Limits of Emerging Technologies. Clean Technol. Environ. Policy 2011, 13, 241–268. [Google Scholar] [CrossRef]
- Alammar, A.Y.; Choi, S.H.; Buonomenna, M.G. Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration. Processes 2024, 12, 563. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef] [PubMed]
- Pet, I.; Sanad, M.N.; Farouz, M.; ElFaham, M.M.; El-Hussein, A.; El-sadek, M.S.A.; Althobiti, R.A.; Ioanid, A. Review: Recent Developments in the Implementation of Activated Carbon as Heavy Metal Removal Management. Water Conserv. Sci. Eng. 2024, 9, 62. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Lei, X.; Zhang, W.; Ai, Z.; Yang, Z.; Zhan, H.; Yang, J.; Yuan, X.; Peng, H.; et al. Machine Learning Predicting and Engineering the Yield, N Content, and Specific Surface Area of Biochar Derived from Pyrolysis of Biomass. Biochar 2022, 4, 63. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shao, S.; Cai, Y. Machine Learning Prediction of Physical Properties and Nitrogen Content of Porous Carbon from Agricultural Wastes: Effects of Activation and Doping Process. Fuel 2024, 356, 129623. [Google Scholar] [CrossRef]
- Liu, D.J.; Garcia, A.; Wang, J.; Ackerman, D.M.; Wang, C.J.; Evans, J.W. Kinetic Monte Carlo Simulation of Statistical Mechanical Models and Coarse-Grained Mesoscale Descriptions of Catalytic Reaction-Diffusion Processes: 1D Nanoporous and 2D Surface Systems. Chem. Rev. 2015, 115, 5979–6050. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, A.; Bell, R.G.; Mellot-Draznieks, C. Predicting the Impact of Functionalized Ligands on CO2 Adsorption in MOFs: A Combined DFT and Grand Canonical Monte Carlo Study. Microporous Mesoporous Mater. 2013, 168, 225–238. [Google Scholar] [CrossRef]
- Habibur Rahman Sobuz, M.; Khan, M.H.; Kawsarul Islam Kabbo, M.; Alhamami, A.H.; Aditto, F.S.; Saziduzzaman Sajib, M.; Johnson Alengaram, U.; Mansour, W.; Hasan, N.M.S.; Datta, S.D.; et al. Assessment of Mechanical Properties with Machine Learning Modeling and Durability, and Microstructural Characteristics of a Biochar-Cement Mortar Composite. Constr. Build. Mater. 2024, 411, 134281. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Suvarna, M.; Tong, Y.W.; Wang, X. Fuel Properties of Hydrochar and Pyrochar: Prediction and Exploration with Machine Learning. Appl. Energy 2020, 269, 115166. [Google Scholar] [CrossRef]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial Assembly and Applications of Functional Mesoporous Materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Zaid, M.; Dutta, J.; Parvin, M.; Martha, S.K. Soft Carbon in Non-Aqueous Rechargeable Batteries: A Review of Its Synthesis, Carbonization Mechanism, Characterization, and Multifarious Applications. Energy Adv. 2024, 3, 1167–1195. [Google Scholar] [CrossRef]
- Mochida, I.; Fujimoto, K.; Oyama, T. Chemistry in the Production and Utilization of Needle Coke. In Chemistry & Physics of Carbon; CRC Press: Boca Raton, FL, USA, 1993; pp. 111–212. ISBN 9781003418184. [Google Scholar]
- Cho, D.W.; Kwon, E.E.; Song, H. Use of Carbon Dioxide as a Reaction Medium in the Thermo-Chemical Process for the Enhanced Generation of Syngas and Tuning Adsorption Ability of Biochar. Energy Convers. Manag. 2016, 117, 106–114. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, H.; Chen, S.; Jiang, B. A Microwave-Assisted Boudouard Reaction: A Highly Effective Reduction of the Greenhouse Gas CO2 to Useful Co Feedstock with Semi-Coke. Molecules 2021, 26, 1507. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-Specific Enhancement of the Carbon−Carbon Dioxide (Boudouard) Reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Singh, R.; Wang, L.; Ostrikov, K.; Huang, J. Designing Carbon-Based Porous Materials for Carbon Dioxide Capture. Adv. Mater. Interfaces 2024, 11, 2202290. [Google Scholar] [CrossRef]
- Vidal-Vidal, Á.; Faza, O.N.; Silva López, C. CO2 Complexes with Five-Membered Heterocycles: Structure, Topology, and Spectroscopic Characterization. J. Phys. Chem. A 2017, 121, 9118–9130. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, R.; Nahil, M.A.; Williams, P.T. Hydrogen Production by Three-Stage (i) Pyrolysis, (ii) Catalytic Steam Reforming, and (iii) Water Gas Shift Processing of Waste Plastic. Energy Fuels 2023, 37, 3894–3907. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, F.; Rezaei, M. High Temperature Water Gas Shift Reaction over Promoted Iron Based Catalysts Prepared by Pyrolysis Method. Int. J. Hydrogen Energy 2014, 39, 16318–16328. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Herreyre, S.; Papin, A.; Desprez, P.; Lecocq, A. New Insight on the Risk Profile Pertaining to Lithium-Ion Batteries under Thermal Runaway as Affected by System Modularity and Subsequent Oxidation Regime. J. Energy Storage 2022, 52 Pt B, 104790. [Google Scholar] [CrossRef]
- Jagtap, S.; Handore, K.; Adhav, P.; Deshpande, P.; Bhopale, A.; Khaladkar, M.; Khandagale, P.; Chabukswar, V.V. Room Temperature Operating, Fast and Reusable Polyaniline Sensor Synthesized Ultrasonically Using Organic and Inorganic Acid Dopants. J. Macromol. Sci. Part B Phys. 2022, 61, 942–957. [Google Scholar] [CrossRef]
- Lakhal, R.; Almatar, M.; Alkalaf, T.; Albarri, O. Transcriptome-Based Analysis of the Oxidative Response of Thermotoga Maritima to the O2 Stress. Comb. Chem. High Throughput Screen. 2025, in press. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. Catalytic Conversion of CO2 to Chemicals and Fuels: The Collective Thermocatalytic/Photocatalytic/Electrocatalytic Approach with Graphitic Carbon Nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Kuang, H.Y.; Lin, Y.X.; Li, X.H.; Chen, J.S. Chemical Fixation of CO2 on Nanocarbons and Hybrids. J. Mater. Chem. A 2021, 9, 20857–20873. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst Deactivation and Its Mitigation during Catalytic Conversions of Biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Pota, F.; Costa de Oliveira, M.; Schröder, C.; Brunet Cabré, M.; Nolan, H.; Rafferty, A.; Jeannin, O.; Camerel, F.; Behan, J.; Barrière, F.; et al. Porous N-doped Carbon-encapsulated Iron as a Novel Catalyst Architecture for the Electrocatalytic Hydrogenation of Benzaldehyde. ChemSusChem 2024, 18, e202400546. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Li, G. Recent Advances in Carbonaceous Sustainable Nanomaterials for Wastewater Treatments. Sustain. Mater. Technol. 2022, 32, e00406. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Singh, G.; Gopalan, A.-I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2021, 5, 2000169. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Molina, A.; Mondragón, F. Reactivity of Coal Gasification with Steam and CO2. Fuel 1998, 77, 1831–1839. [Google Scholar] [CrossRef]
- Lakzian, E.; Yazdani, S.; Salmani, F.; Mahian, O.; Kim, H.D.; Ghalambaz, M.; Ding, H.; Yang, Y.; Li, B.; Wen, C. Supersonic Separation towards Sustainable Gas Removal and Carbon Capture. Prog. Energy Combust. Sci. 2024, 103, 101158. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Barone, L.; Papa, E.; Materazzi, S. On-Line Thermally Induced Evolved Gas Analysis: An Update—Part 1: EGA-MS. Molecules 2022, 27, 3518. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.Y.A.; Kumarachari, R.K.; Bukke, S.P.N.; Neerugatti, D.; Mekasha, Y.T.; Bandarapalle, K. Plasma Catalysis for Sustainable Industry: Lab-Scale Studies and Pathways to Upscaling. Discov. Appl. Sci. 2025, 7, 271. [Google Scholar] [CrossRef]
- Mondal, S.; Ruidas, S.; Chongdar, S.; Saha, B.; Bhaumik, A. Sustainable Porous Heterogeneous Catalysts for the Conversion of Biomass into Renewable Energy Products. ACS Sustain. Resour. Manag. 2024, 1, 1672–1704. [Google Scholar] [CrossRef]
- Xiao, N.; Kong, L.; Wei, M.; Hu, X.; Li, O. Innovations in Food Waste Management: From Resource Recovery to Sustainable Solutions. Waste Dispos. Sustain. Energy 2024, 6, 401–417. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, D.; Wang, X.; Qin, Y.; Hu, L.; Zhang, Y. Sustainable CO2 Management through Integrated CO2 Capture and Conversion. J. CO2 Util. 2023, 72, 102493. [Google Scholar] [CrossRef]
- Razzak, S.A. Municipal Solid and Plastic Waste Co-Pyrolysis Towards Sustainable Renewable Fuel and Carbon Materials: A Comprehensive Review. Chem. Asian J. 2024, 19, e202400307. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent Advancements and Challenges in Emerging Applications of Biochar-Based Catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef] [PubMed]
- Chidhambaram, N.; Kay, S.J.J.; Priyadharshini, S.; Meenakshi, R.; Sakthivel, P.; Dhanbalan, S.; Shanavas, S.; Kamaraj, S.K.; Thirumurugan, A. Magnetic Nanomaterials as Catalysts for Syngas Production and Conversion. Catalysts 2023, 13, 440. [Google Scholar] [CrossRef]
- Ansari, M.N.M.; Sayem, M.A. Microwave-Assisted Activated Carbon: A Promising Class of Materials for a Wide Range of Applications. In Radiation Technologies and Applications in Materials Science; Chowdhury, S.R., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–38. ISBN 9788490225370. [Google Scholar]
- Zhang, J.; Sewell, C.D.; Huang, H.; Lin, Z. Closing the Anthropogenic Chemical Carbon Cycle toward a Sustainable Future via CO2 Valorization. Adv. Energy Mater. 2021, 11, 2102767. [Google Scholar] [CrossRef]
- Mahmoudi Kouhi, R.; Jebrailvand Moghaddam, M.M.; Doulati Ardejani, F.; Mirheydari, A.; Maghsoudy, S.; Gholizadeh, F.; Ghobadipour, B. Carbon Utilization Technologies & Methods. In Carbon Capture, Utilization, and Storage Technologies Towards More Sustainable Cities; Ahmadian, A., Elkamel, A., Almansoori, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–50. ISBN 978-3-031-46590-1. [Google Scholar]
- Chinenye Divine, D.; Hubert, S.; Epelle, E.I.; Ojo, A.U.; Adeleke, A.A.; Ogbaga, C.C.; Akande, O.; Okoye, P.U.; Giwa, A.; Okolie, J.A. Enhancing Biomass Pyrolysis: Predictive Insights from Process Simulation Integrated with Interpretable Machine Learning Models. Fuel 2024, 366, 131346. [Google Scholar] [CrossRef]
- Sirohi, R.; Kumar, M.; Vivekanand, V.; Shakya, A.; Tarafdar, A.; Singh, R.; Sawarkar, A.D.; Hoang, A.T.; Pandey, A. Integrating Biochar in Anaerobic Digestion: Insights into Diverse Feedstocks and Algal Biochar. Environ. Technol. Innov. 2024, 36, 103814. [Google Scholar] [CrossRef]
- Olivier, A.; Desgagnés, A.; Mercier, E.; Iliuta, M.C. New Insights on Catalytic Valorization of Carbon Dioxide by Conventional and Intensified Processes. Ind. Eng. Chem. Res. 2023, 62, 5714–5749. [Google Scholar] [CrossRef]
- Wang, Q.; Han, L. Hydrogen Production. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 1855–1900. ISBN 9783319144092. [Google Scholar]
- Goel, A.; Moghaddam, E.M.; Liu, W.; He, C.; Konttinen, J. Biomass Chemical Looping Gasification for High-Quality Syngas: A Critical Review and Technological Outlooks. Energy Convers. Manag. 2022, 268, 116020. [Google Scholar] [CrossRef]
- Lott, P.; Deutschmann, O. Heterogeneous Chemical Reactions—A Cornerstone in Emission Reduction of Local Pollutants and Greenhouse Gases. Proc. Combust. Inst. 2023, 39, 3183–3215. [Google Scholar] [CrossRef]
- Shadle, L.J.; Indrawan, N.; Breault, R.W.; Bennett, J. Gasification Technology. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Baharak, S., Wei-Yin, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 653–741. ISBN 978-3-030-72578-5. [Google Scholar]
- El-Fawal, E.M.; El Naggar, A.M.A.; El-Zahhar, A.A.; Alghandi, M.M.; Morshedy, A.S.; El Sayed, H.A.; Mohammed, A.e.M.E. Biofuel Production from Waste Residuals: Comprehensive Insights into Biomass Conversion Technologies and Engineered Biochar Applications. RSC Adv. 2025, 15, 11942–11974. [Google Scholar] [CrossRef]
- Kasipandi, S.; Bae, J.W. Recent Advances in Direct Synthesis of Value-Added Aromatic Chemicals from Syngas by Cascade Reactions over Bifunctional Catalysts. Adv. Mater. 2019, 31, 1803390. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.G.d.; Alencar, A.C. Biomass-Derived Syngas Production via Gasification Process and Its Catalytic Conversion into Fuels by Fischer Tropsch Synthesis: A Review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Gao, S.; Song, Z.; Sun, H.; Zhang, Y.Y.; Ma, J.; Zhang, Y.Y. Progress of Oxygen Generation Technologies in High-Altitude and Emergency Rescue Conditions. Asia-Pac. J. Chem. Eng. 2025, e70040. [Google Scholar] [CrossRef]
- Ghasemi, A.; Nikafshan Rad, H.; Izadyar, N.; Marefati, M. Optimizing Industrial Energy: An Eco-Efficient System for Integrated Power, Oxygen, and Methanol Production Using Coke Plant Waste Heat and Electrolysis. Energy Convers. Manag. X 2024, 22, 100571. [Google Scholar] [CrossRef]
- Bhatia, M.; Gugnani, R.; Yaqub, M.Z.; Agarwal, V. Drivers and Challenges in Achieving Corporate Carbon Neutrality—Qualitative Investigation of Carbon Capture, Utilization, and Storage Technologies. Bus. Strateg. Environ. 2024, 34, 1771–1791. [Google Scholar] [CrossRef]
- Liu, Y.; Paskevicius, M.; Sofianos, M.V.; Parkinson, G.; Wang, S.; Li, C.Z. A SAXS Study of the Pore Structure Evolution in Biochar during Gasification in H2O, CO2 and H2O/CO2. Fuel 2021, 292, 120384. [Google Scholar] [CrossRef]
- Ravenni, G.; Elhami, O.H.; Ahrenfeldt, J.; Henriksen, U.B.; Neubauer, Y. Adsorption and Decomposition of Tar Model Compounds over the Surface of Gasification Char and Active Carbon within the Temperature Range 250–800 °C. Appl. Energy 2019, 241, 139–151. [Google Scholar] [CrossRef]
- Sneddon, G.; Greenaway, A.; Yiu, H.H.P. The Potential Applications of Nanoporous Materials for the Adsorption, Separation, and Catalytic Conversion of Carbon Dioxide. Adv. Energy Mater. 2014, 4, 1301873. [Google Scholar] [CrossRef]
- Ciccone, B.; Murena, F.; Ruoppolo, G.; Urciuolo, M.; Brachi, P. Methanation of Syngas from Biomass Gasification: Small-Scale Plant Design in Aspen Plus. Appl. Therm. Eng. 2024, 246, 122901. [Google Scholar] [CrossRef]
- Gao, K.; Chen, G.; Yan, B.; Ti, S.; Wang, H.; Si, G.; Qi, T. Modeling of Biomass Thermal Decomposition/Gasification in a Downdraft Gasifier under Low Pressure by Aspen Plus. Therm. Sci. Eng. Prog. 2025, 59, 103229. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Tar Evolution in a Two Stage Fluid Bed-Plasma Gasification Process for Waste Valorization. Fuel Process. Technol. 2014, 128, 146–157. [Google Scholar] [CrossRef]
- Cinti, G.; Baldinelli, A.; Di Michele, A.; Desideri, U. Integration of Solid Oxide Electrolyzer and Fischer-Tropsch: A Sustainable Pathway for Synthetic Fuel. Appl. Energy 2016, 162, 308–320. [Google Scholar] [CrossRef]
- Alsunousi, M.; Kayabasi, E. The Role of Hydrogen in Synthetic Fuel Production Strategies. Int. J. Hydrogen Energy 2024, 54, 1169–1178. [Google Scholar] [CrossRef]
- Galán-Martín, Á.; Tulus, V.; Díaz, I.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Sustainability Footprints of a Renewable Carbon Transition for the Petrochemical Sector within Planetary Boundaries. One Earth 2021, 4, 565–583. [Google Scholar] [CrossRef]
- Prifti, K.; Lechtenberg, F.; Manenti, F.; Espuña, A.; Graells, M. Comparing the Climate Impact of Methanol Production in Europe: Steam Methane Reforming vs. Plastic Waste Gasification Processes. Resour. Conserv. Recycl. 2024, 208, 107653. [Google Scholar] [CrossRef]
- Faheem, M.; Azher Hassan, M.; Du, J.; Wang, B. Harnessing Potential of Smart and Conventional Spent Adsorbents: Global Practices and Sustainable Approaches through Regeneration and Tailored Recycling. Sep. Purif. Technol. 2025, 354 Pt 3, 128907. [Google Scholar] [CrossRef]
- Patra, N.; Ramesh, P.; Țălu, S. Advancements in Cellulose-Based Materials for CO2 Capture and Conversion. Polymers 2025, 17, 848. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Rahaman, M.; Hassan, A.; Parvez, M.A.; Chandan, M.R. Biomass-Based Sustainable Graphene for Advanced Electronic Technology: A Review. Chem. Asian J. 2025, 20, e202500128. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Hao, R.; Wang, Y. Synthesis and Applications of Biomass-Derived Porous Carbon Materials in Energy Utilization and Environmental Remediation. Chemosphere 2023, 339, 139635. [Google Scholar] [CrossRef] [PubMed]
- Beak, S.; Kim, S.; Oh, S.; Bae, J. Lignin-Based Porous Carbon for Efficient Hydrogen Storage. J. Environ. Chem. Eng. 2025, 13, 116086. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, B.; Wang, Z.; Hu, Y.; Du, M. Pore Engineering in Biomass-Derived Carbon Materials for Enhanced Energy, Catalysis, and Environmental Applications. Molecules 2024, 29, 5172. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ghosh, M. Post-Combustion Capture of Carbon Dioxide by Natural and Synthetic Organic Polymers. Polysaccharides 2023, 4, 156–175. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Molecular Simulation Studies of CO2 Adsorption by Carbon Model Compounds for Carbon Capture and Sequestration Applications. Environ. Sci. Technol. 2013, 47, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, I.; Sharma, P.; Nebhani, L. Polybenzoxazine—An Enticing Precursor for Engineering Heteroatom-Doped Porous Carbon Materials with Applications beyond Energy, Environment and Catalysis. Mater. Today Chem. 2022, 23, 100734. [Google Scholar] [CrossRef]
- Etim, U.J.; Zhang, C.; Zhong, Z. Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials 2021, 11, 3265. [Google Scholar] [CrossRef]
- López-Periago, A.M.; Domingo, C. Features of Supercritical CO2 in the Delicate World of the Nanopores. J. Supercrit. Fluids 2018, 134, 204–213. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and Structural Changes in Shale Associated with Supercritical CO2 Exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Zhang, Z.; Cano, Z.P.; Luo, D.; Dou, H.; Yu, A.; Chen, Z. Rational Design of Tailored Porous Carbon-Based Materials for CO2 Capture. J. Mater. Chem. A 2019, 7, 20985–21003. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Dasgupta Ghosh, B.; Goh, K.L.; Sharma, K.; Chang, Y.W. From Hazardous Waste to Green Applications: Selective Surface Functionalization of Waste Cigarette Filters for High-Performance Robust Triboelectric Nanogenerators and CO2 Adsorbents. ACS Appl. Mater. Interfaces 2022, 14, 31973–31985. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Lindbråthen, A.; Waris, Z.; Deng, L. High Capacity and Robust Moisture-Swing CO2 Adsorption for Direct Air Capture by Functionalized Cellulose Aerogels. Chem. Eng. J. 2025, 512, 162377. [Google Scholar] [CrossRef]
- Hardiagon, A.; Coudert, F.X. Multiscale Modeling of Physical Properties of Nanoporous Frameworks: Predicting Mechanical, Thermal, and Adsorption Behavior. Acc. Chem. Res. 2024, 57, 1620–1632. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A Comparative Energy and Costs Assessment and Optimization for Direct Air Capture Technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Realmonte, G.; Drouet, L.; Gambhir, A.; Glynn, J.; Hawkes, A.; Köberle, A.C.; Tavoni, M. An Inter-Model Assessment of the Role of Direct Air Capture in Deep Mitigation Pathways. Nat. Commun. 2019, 10, 3277. [Google Scholar] [CrossRef]
- Zolfaghari, Z.; Aslani, A.; Moshari, A.; Malekli, M. Direct Air Capture from Demonstration to Commercialization Stage: A Bibliometric Analysis. Int. J. Energy Res. 2022, 46, 383–396. [Google Scholar] [CrossRef]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xu, W.; Lindbråthen, A.; Cheng, X.; Chen, X.; Zhu, L.; Deng, L. Development of High Capacity Moisture-Swing DAC Sorbent for Direct Air Capture of CO2. Sep. Purif. Technol. 2023, 324, 124489. [Google Scholar] [CrossRef]
- Bouter, A.; Hurtig, O.; Besseau, R.; Buffi, M.; Kulisic, B.; Scarlat, N. Updating the Greenhouse Gas Emissions of Liquid Biofuels from Annex V of the Renewable Energy Directive II (RED II): An Overview. Biomass Bioenergy 2025, 199, 107886. [Google Scholar] [CrossRef]
- Zaker, A.; ben Hammouda, S.; Sun, J.; Wang, X.; Li, X.; Chen, Z. Carbon-Based Materials for CO2 Capture: Their Production, Modification and Performance. J. Environ. Chem. Eng. 2023, 11, 109741. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, S.; He, S.; Wu, C. Direct Air Capture of CO2 by KOH-Activated Bamboo Biochar. J. Energy Inst. 2022, 105, 399–405. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Gao, N.; Yang, T.; Wang, J.; Wu, C. Direct CO2 Capture from Air Using Char from Pyrolysis of Digestate Solid. Biomass Bioenergy 2023, 175, 106891. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Alonso, M.; Stoica, A.I. Investigation of Effectiveness of KOH-Activated Olive Pomace Biochar for Efficient Direct Air Capture of CO2. Sep. Purif. Technol. 2025, 352, 127997. [Google Scholar] [CrossRef]
- Podder, S.; Jungi, H.; Mitra, J. In Pursuit of Carbon Neutrality: Progresses and Innovations in Sorbents for Direct Air Capture of CO2. Chem. Eur. J. 2025, 31, e202500865. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Xie, Z.; Zhang, X.; Sui, X.; Li, J.-R. Porous Sorbents for Direct Capture of Carbon Dioxide from Ambient Air. Chin. Chem. Lett. 2024, 36, 109676. [Google Scholar] [CrossRef]
- Okonkwo, E.C.; AlNouss, A.; Shahbaz, M.; Al-Ansari, T. Developing Integrated Direct Air Capture and Bioenergy with Carbon Capture and Storage Systems: Progress towards 2 °C and 1.5 °C Climate Goals. Energy Convers. Manag. 2023, 296, 117687. [Google Scholar] [CrossRef]
- Küng, L.; Aeschlimann, S.; Charalambous, C.; McIlwaine, F.; Young, J.; Shannon, N.; Strassel, K.; Maesano, C.N.; Kahsar, R.; Pike, D.; et al. A Roadmap for Achieving Scalable, Safe, and Low-Cost Direct Air Carbon Capture and Storage. Energy Environ. Sci. 2023, 16, 4280–4304. [Google Scholar] [CrossRef]
- Izikowitz, D. Carbon Purchase Agreements, Dactories, and Supply-Chain Innovation: What Will It Take to Scale-Up Modular Direct Air Capture Technology to a Gigatonne Scale. Front. Clim. 2021, 3, 636657. [Google Scholar] [CrossRef]

| Type | Technology | Mechanism and Key Features | Refs. |
|---|---|---|---|
| Liquid-based | Alkaline Solvents (KOH, NaOH) | CO2 reacts with an alkaline solution to form carbonates; regenerated by calcination. | [4,5] |
| Liquid-based | Amino Acid Solution | CO2 forms bicarbonates; regeneration through mild heating; low volatility and energy demand. | [6] |
| Solid-based | Amine-Functionalized Solids | CO2 chemically absorbed by amines on supports (e.g., alumina, resins). | [7,8] |
| Solid-based | Temperature-Vacuum Swing Adsorption (TVSA) | CO2 is physically adsorbed at room temperature and is desorbed by heating and vacuum. | [9,10] |
| Chars/Activated Carbons | Porous carbon materials (e.g., doped chars); adsorption via surface area and functionality. | [11] | |
| Metal-Organic Frameworks (MOFs) | Crystalline porous materials with a tunable structure and high CO2 selectivity. | [12] | |
| Zeolites | Selective CO2 separation using microporous crystalline structures. | [13] | |
| Electrochemical | Electro-Swing Adsorption | Redox-active materials bind/release CO2 via voltage control under mild conditions. | [14] |
| Electrochemical Cells | CO2 capture and conversion using ion transport through electrochemical cells | [15] | |
| Hybrid | TVSA + Dehumidification | The pre-drying air improves the CO2 capture efficiency in TVSA systems. | [16] |
| Passive Air Contactors | Wind-driven flow over solid sorbents (e.g., amine-grafted monoliths); low energy demand. | [17] |
| Biomass Types | Gasification | Activation | BET SSA, m2/g | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|
| T, °C | Atmosphere | RT, min | Method | T, °C | RT, min | C:A (a) | |||
| Solid digestate | 600 | N2/Steam | 9 | Raw | - | - | - | 11 | [49] |
| 700 | 337 | ||||||||
| 800 | 461 | ||||||||
| 900 | 374 | ||||||||
| Corn stalk | 600 | CO2 | 25 | Raw | - | - | - | 452 (b) | [50] |
| 700 | 481 (b) | ||||||||
| 800 | 512 (b) | ||||||||
| 900 | 526 (b) | ||||||||
| 1000 | 490 (b) | ||||||||
| 600 | 312 (c) | ||||||||
| 700 | 408 (c) | ||||||||
| 800 | 481 (c) | ||||||||
| 900 | 503 (c) | ||||||||
| 1000 | 423 (c) | ||||||||
| Straw Wood Wood | 725–750 | Air | - | Raw | - | - | - | 75 (d) | [51] |
| 426 (e) | |||||||||
| 1027 (f) | |||||||||
| Wheat straw | 800 | CO2 | 30 | Raw | - | - | - | 470 | [52] |
| 900 | 892 | ||||||||
| 1000 | 624 | ||||||||
| Hay | 800 | CO2 | 30 | Raw | - | - | - | 256 | [52] |
| 900 | 419 | ||||||||
| 1000 | 269 | ||||||||
| Poplar wood | 750 | N2/Steam | 30 | Raw | - | - | - | 429 | [53] |
| 750 | N2/Steam | 60 | 621 | ||||||
| 750 | N2/CO2 | 30 | 435 | ||||||
| 920 | N2/CO2 | 30 | 687 | ||||||
| Wood chips | 900 | - | - | Raw | - | - | - | 603 | [54] |
| KOH | 600 | 60 | 1:1 | 774 | |||||
| ZnCl2 | 600 | 60 | 1:1 | 739 | |||||
| Wood | 800–1000 | Air | 180 | Raw | - | - | - | 126 | [55] |
| KOH | 850 | 120 | 1:1 | 1282 | |||||
| KOH/CO2 | 850/550 | 120/60 | 1:1/1:100 | 1013 | |||||
| 70% Wood 30% Manure | 800–1000 | Air | 180 | Raw | - | - | - | 256 | [55] |
| KOH | 850 | 120 | 1:1 | 1409 | |||||
| KOH/CO2 | 850/550 | 120/60 | 1:1/1:100 | 1404 | |||||
| PC | ||||
|---|---|---|---|---|
| Feedstock | T, °C | Dominant Pore Types | Main Observations | Ref. |
| Wheat straw | >350 | Macropores (>75 µm) | Larger macropore volumes; suitable for improving soil structure and supporting larger organisms (e.g., nematodes, protists). | [57] |
| Rice husk | <550 | Mesopores (30–75 µm) | Higher mesopore volume; beneficial for increasing plant-available water. | [57] |
| Miscanthus straw | <350 | Micropores (5–30 µm) | More micropores; may serve as habitat for bacteria and fungal hyphae. | [57] |
| Wheat Straw | 500–700 | Micropores (44–75%) | Micropores increase with temperature; short residence time at 700 °C yields the highest microporosity (75%). The mesopore content declines and the macropores remain minimal. | [58] |
| GC | ||||
| Poplar Wood | 1150 | Micropores (constant), mesopores (minor), macropores (vascular channels) | Despite > 90% char conversion, the micropores remain unchanged. Pore volume increases due to vascular channel wall thinning and macropore growth. | [60] |
| Pine wood | 700, 750, 800 | Micropores (<2 nm) | Micropores dominate the surface area, but mesopores grow faster initially. Beyond 70% conversion, macropores increase while mesopores decrease, indicating progressive pore widening. | [59] |
| PC | ||||||||
|---|---|---|---|---|---|---|---|---|
| Feedstocks | Activation | Doping Element | CO2 Concentration, % | CO2 ads. Capacity in 1st Cycle (b), mg/g | CO2 ads. Capacity After 10th Cycle (b), mg/g | Refs. | ||
| Methods | T, °C | C:A (a) | ||||||
| Eucalyptus sawdust | KOH | 600; 650; 700; 800 600; 700; 800 | 1:2 1:2 1:4 1:4 | - | 100 100 100 100 | 212; 206; 190; 170 128; 128; 130 | - | [32] |
| 70% Pine wood 30% Sewage sludge | Raw | - | - | - | 100 | 43 | - | [33] |
| KOH | 600; 700; 800 | 1:1 | - | 100 | 137; 187; 142 | -; 181 138 | [33] | |
| Garlic peel | Raw | - | - | - | 100 | 73 | - | [43] |
| KOH | 600; 700; 800 600 | 1:2 1:2 1:4 | - | 100 100 100 | 186; 176; 124 125 | - | [43] | |
| Whitewood | Steam CO2 KOH | 700 890 775 | 1:0.94 1:8.7 1:1.23 | - | 30 30 30 | 59 63 78 | 51 63 77 | [44] |
| Pine sawdust | Steam KOH | 850 850 | 1:0.4 1:3 | - | 100 100 | 104 156 | - | [45] |
| Wood pellet | Raw Steam CO2 ZnCl2 H3PO4 KOH | - 550 550 550 550 550 | - 1:2.5 1:16.7 1:1 1:1 1:1 | - | 15 15 15 15 15 15 | 168 175 175 178 172 180 | - | [48] |
| Coffee grounds | KOH | 600 | 1:2 1:3 | - | 100 100 | 132 132 | - | [80] |
| Pomegranate peels Carrot peels Fern leaves | KOH KOH KOH | 700 700 700 | 1:1 1:1 1:1 | - - - | 100 100 100% | 181 184 181 | - | [81] |
| Rice husk | CO2 | 900 | - | - | 100 | 136 | - | [82] |
| Bamboo | KOH | 500; 600; 700; 800; 850 | 1:3 | - | 100 | 187; 308; 308; 279; 242 | - | [83] |
| Grass cuttings | CO2 | 800 | - | - | 100 | 119 | - | [84] |
| Coconut shell | Raw H3PO4 KOH | - 600 800 | - 1:2 1:4 | - | 100 | 60 75 50 | - | [85] |
| Enteromorpha | KOH | 700; 800; 900 | 1:3 | - | 100 | 125 (d); 126 (d); 127 (d) | - | [86] |
| Enteromorpha | KOH KOH KOH | 700 800 900 | 1:3 1:3 1:3 | N, S (c) N, S (c) N, S (c) | 100 100 100 | 123 (d); 121 (d); 119 (d) 121 (d); 130 (d); 121 (c) 122 (d); 121 (d); 120 (d) | - | [86] |
| Greasyback shrimp shell | KOH | 800 | 1:1 1:2 1:3 | N | 100 | 190 157 155 | - | [87] |
| Water chestnut | KOH | 600; 650; 700; 800 | 1:3 | N | 158; 180; 207; 141 | [88] | ||
| Coconut shell | KOH | 600; 650; 700 | 1:2 1:3 1:4 | N | 100 | 180; 180; 180 176; 211; 207 189; 189; 194 | - | [89] |
| Peanut shell | KOH | 680; 730; 780 | 1:2 | N | 100 | 194; 186; 172 | - | [90] |
| Sugarcane bagasse | KOH | 500; 600; 700; 800 | 1:2 | N | 100 | 158; 211; 208; 190 | - | [91] |
| Camphor leaves | KOH | 500; 600; 700; 800 | 1:2 | N | 100 | 122; 165; 124; 107 | - | [92] |
| GC | ||||||||
| Wood | Raw KOH KOH/CO2 | - 850 850/550 | - 1:1 1:1/1:100 | - | 100 | 27 33 37 | - - ~37 | [55] |
| 70% Wood 30% Manure | Raw KOH KOH/CO2 | - 850 850/550 | - 1:1 1:1/1:100 | - | 100 | 22 31 29 | - - ~29 | [55] |
| Olive stones Almond shell | Raw Raw | - - | - - | - - | 100 | 106–136 (e) 101–119 (e) | - | [93] |
| Bagasse Macadamia nut shell Rice straw | Raw Raw Raw | - | - | - | 100 100 100 | 106 123 53 | 106 123 53 | [94] |
| Palm shell | Raw | - | - | - | 100 | 96 | - | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzak, W.; Li, B.; Silva, D.C.d.; Cruz, G. Direct Air Capture Using Pyrolysis and Gasification Chars: Key Findings and Future Research Needs. Energies 2025, 18, 4120. https://doi.org/10.3390/en18154120
Jerzak W, Li B, Silva DCd, Cruz G. Direct Air Capture Using Pyrolysis and Gasification Chars: Key Findings and Future Research Needs. Energies. 2025; 18(15):4120. https://doi.org/10.3390/en18154120
Chicago/Turabian StyleJerzak, Wojciech, Bin Li, Dennys Correia da Silva, and Glauber Cruz. 2025. "Direct Air Capture Using Pyrolysis and Gasification Chars: Key Findings and Future Research Needs" Energies 18, no. 15: 4120. https://doi.org/10.3390/en18154120
APA StyleJerzak, W., Li, B., Silva, D. C. d., & Cruz, G. (2025). Direct Air Capture Using Pyrolysis and Gasification Chars: Key Findings and Future Research Needs. Energies, 18(15), 4120. https://doi.org/10.3390/en18154120