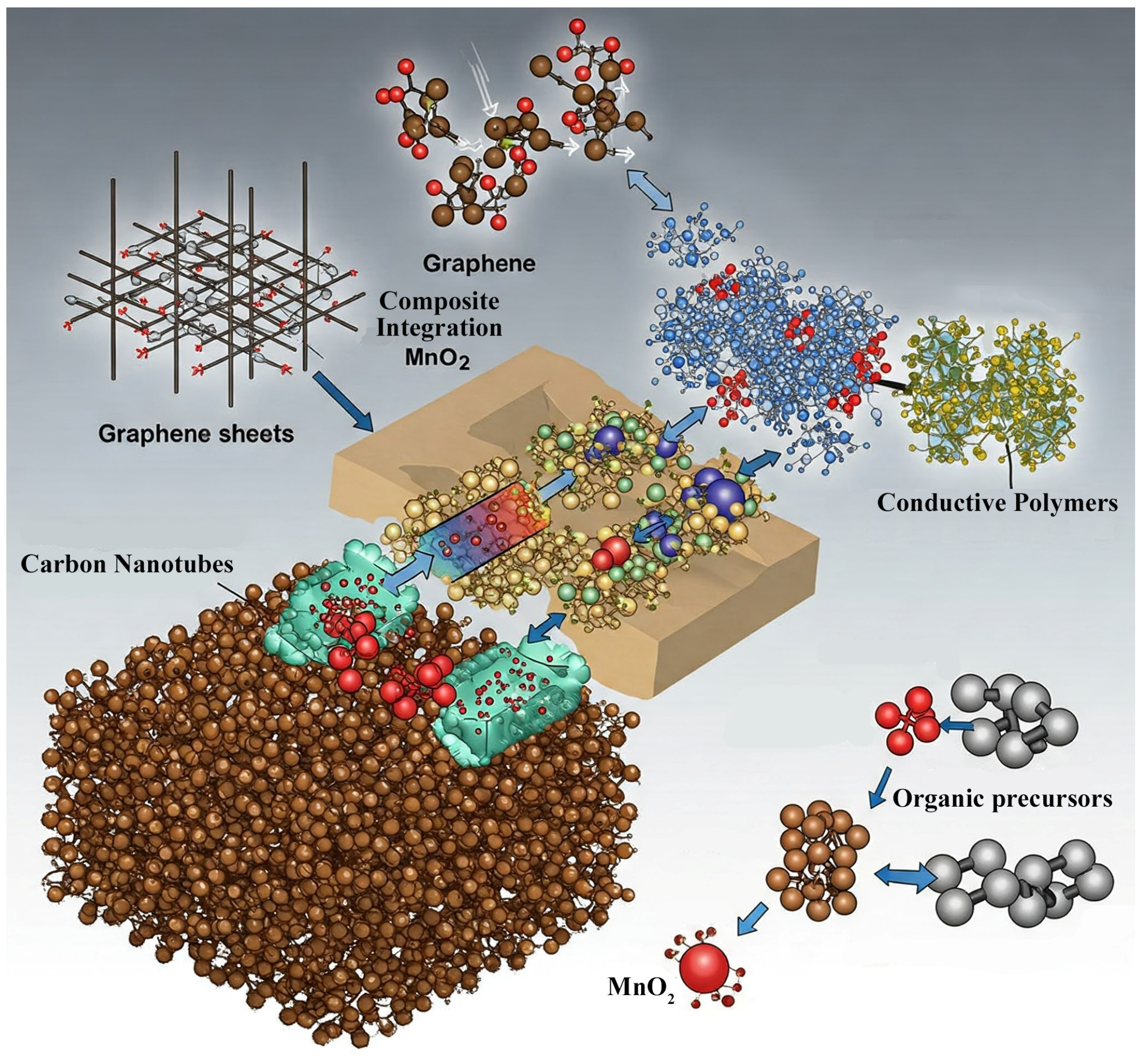
2.2.1. Incorporation with Conductive Materials
The enhancement of MnO
2’s poor conductivity can be conducted via its combination with carbon-based materials such as graphene, carbon nanotubes, and activated carbon. For instance, electrochemical redox deposition of MnO
2 onto carbon nanotubes (CNTs) and carbon nanofibers (CNFs) yields composite materials with enhanced electrical conductivity. In order to improve the mechanical properties, thermal stability, and electrical conductivity of CNTs, they have been electrospun and incorporated into polymer nanofibers. The constructed CNT-embedded CNF hybrid material resulted in uniform deposition of MnO
2, while the final material showed improved electrochemical performance of MnO
2 at high mass loading. The MnO
2 deposits incorporated onto the CNTs-embedded CNFs network by electrodeposition resulted in a capacitance of 374 F g
−1 and a rate capability of 53.4% in comparison to the specific capacitance of MnO
2/CNFs composite electrode at 329 F g
−1 [
21].
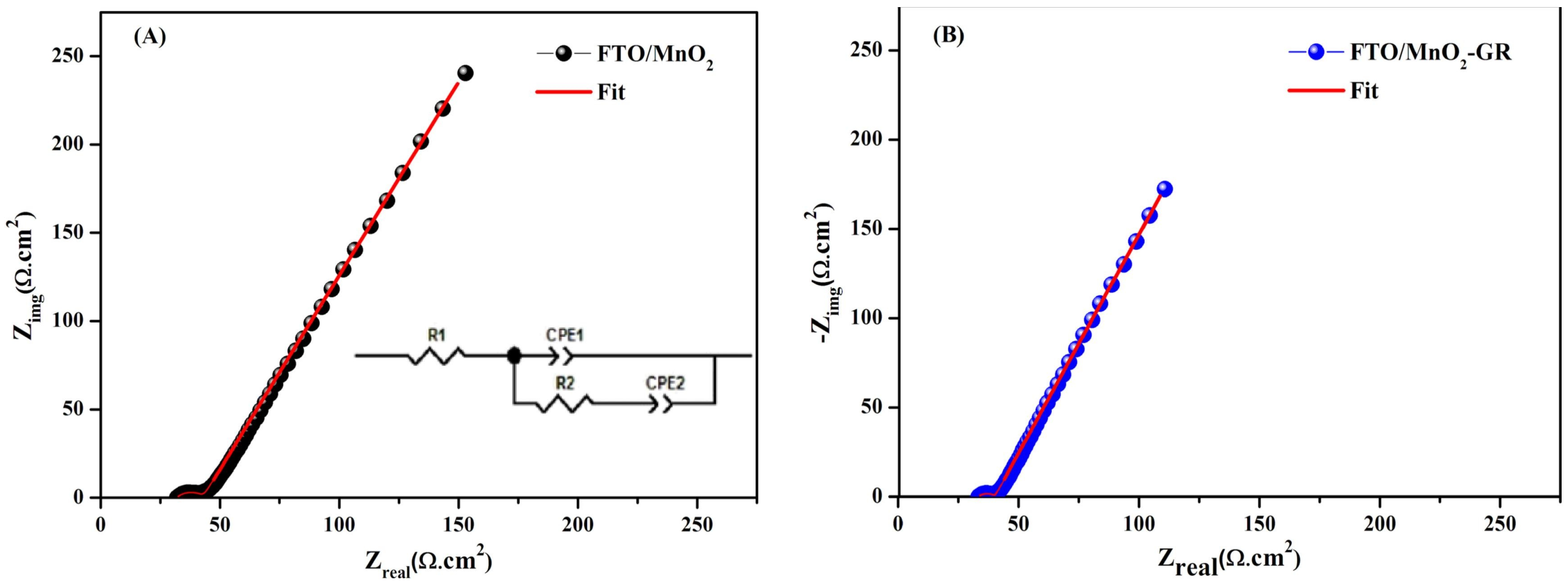
An FTO/MnO
2–graphene composite was synthesized through a one-step electrochemical method using chronoamperometry (SnO
2 doped in Fluorine: FTO). This composite film revealed significant potential for use in supercapacitor electrodes and energy storage devices due to its improved electrochemical properties, low cost, availability, and simple application. In asymmetric supercapacitor tests, the FTO/MnO
2–graphene composite exhibited excellent reversible performance, with an energy density of 3 W h kg
−1 at a specific power of 25 W kg
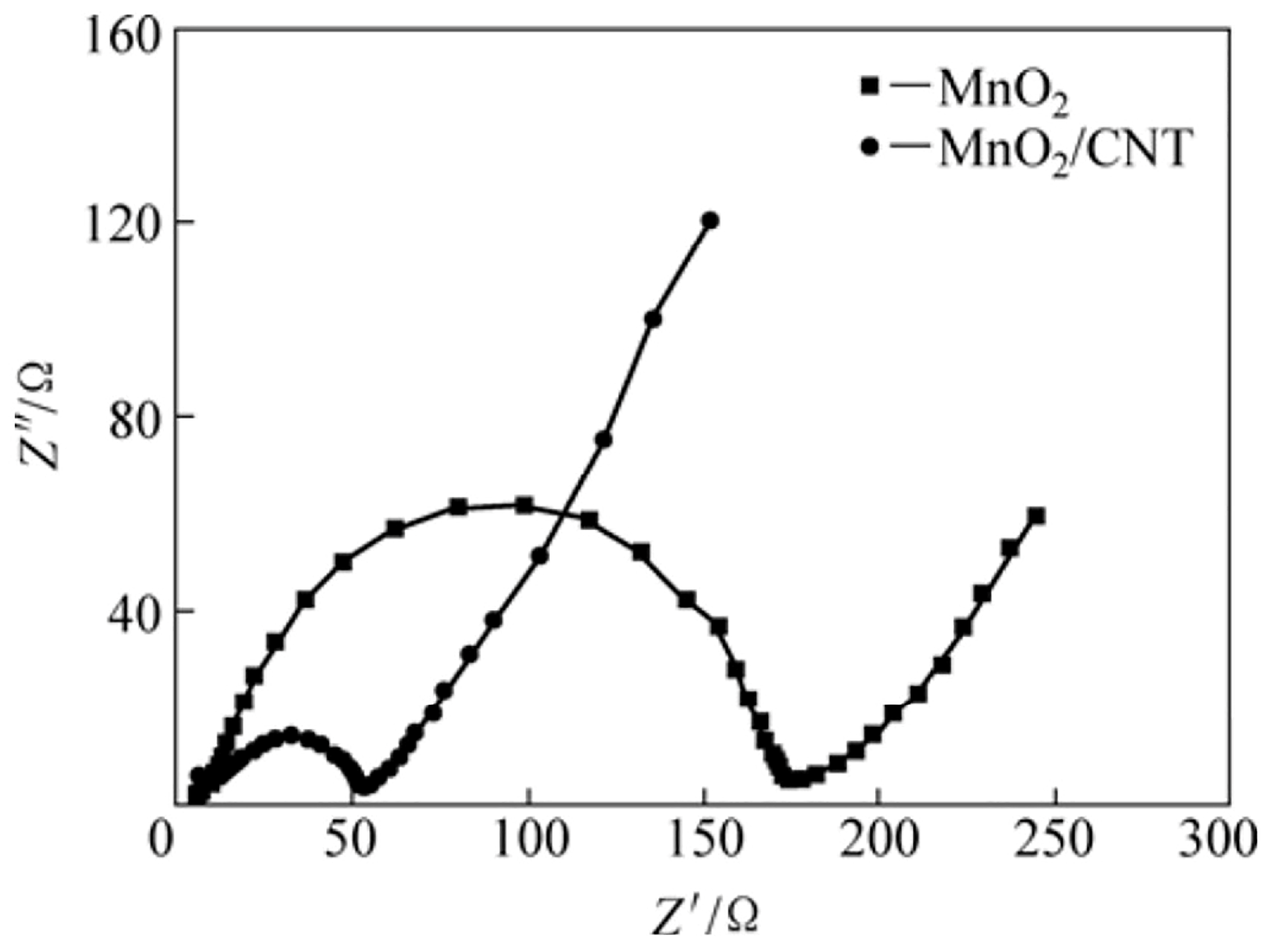
−1. After 1000 cycles, the composite maintained 99% of its capacitance. The inclusion of graphene enhances ion diffusion and electron transport, improving the electrolyte–electrode interaction and therefore, the overall performance. Cyclic voltammetry tests demonstrated that the addition of graphene increased the capacitance of the thin films from 73.5 F g
−1 for pure MnO
2 to 192.3 F g
−1 for MnO
2–graphene composites. Additionally, electrochemical impedance spectroscopy (EIS) highlighted the positive impact of graphene on charge transfer and capacitance behavior. The diameter of the semicircles was found to decrease with increasing graphene content in the MnO
2 matrix. The charge transfer resistance values were 11.5 Ω·cm
2 for the FTO/MnO
2 sample and 6.57 Ω·cm
2 for the FTO/MnO
2-GR sample, highlighting the enhanced conductivity of the oxide upon graphene incorporation (
Figure 4) [
10].
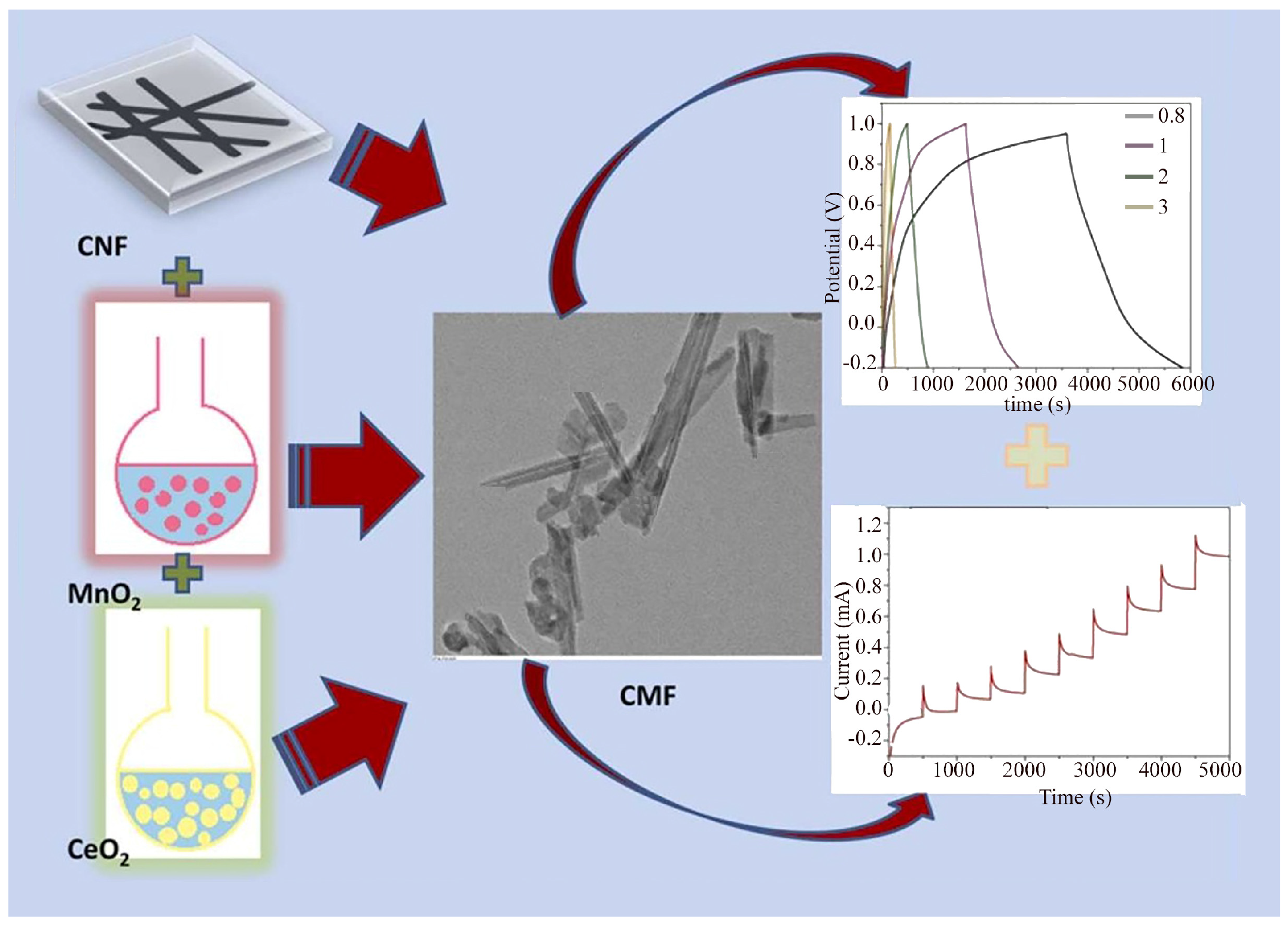
Another hybrid material with a wide range of applications due to the synergistic effect of its components was a CeO
2-MnO
2/CNF composite, which was produced hydrothermally. The produced material consisted of fragmented CeO
2 grown with MnO
2 (crystalline phase) on the surface of carbon nanofibers. The specific nanocomposite was used in the manufacture of supercapacitors with maximum electrochemical capacitance of 1453 F g
−1 (scan rate—10 mV s
−1) and 1498 F g
−1 (at current density of 0.8 A g
−1). Due to the flexibility and porosity of CNF with CeO
2-MnO
2, the composite was also used as an H
2O
2 sensor with a high sensitivity of 612 μA cm
−2 and a detection limit of 0.1 μm (
Figure 5) [
22].
Another study by Guo and co-workers focused on enhancing the capacitance of textile-based electrodes by depositing MnO
2 onto graphene/polyester composite fabrics using a facile hydrothermal method. Adjusting the reaction time impacts the electrochemical performance by controlling the morphology of MnO
2. The composite electrode achieved a specific capacitance of 332 F g
−1 (scan rate of 2 mV s
−1), demonstrating excellent cycling stability. Notably, the material maintained stable electrochemical performance even under mechanical bending and stretching. The integration of graphene enhanced the conductivity and MnO
2 growth, providing a 3D conductive network that contributed to the superior capacitive properties, along with the synergistic effect of the materials combined. This approach offered a scalable method for fabricating flexible electrode materials for energy storage devices [
23].
Furthermore, a study investigated the development of hybrid supercapacitor–battery devices using flexible MnO
2 nanoparticle-coated air-oxidized carbon nanotube (MnO
2/aCNT) electrodes, through a redox reaction of KMnO
4 and aCNTs. The MnO
2 nanoparticles (≈10 nm in diameter) are uniformly attached to the aCNTs through Mn–O–C linkages, which create strong chemical interactions. This structure allowed the aCNT network to mitigate the strain caused by MnO
2 volume changes during rapid charge/discharge cycles, maintaining electrode integrity. The network also ensured efficient electron flow and lithium-ion diffusion, thereby enhancing ultrafast, reversible lithium storage, a feature desired in electric vehicle applications. The MnO
2/aCNT electrodes showed impressive high-current performance, with capacities of 395.8 mAh g
−1 at a current density of 10 A g
−1 and 630.2 mAh g
−1 after 150 cycles at 2 A g
−1. Their low-capacity fading rates over 1000 cycles further highlight their potential for high-performance energy storage devices. This combination of high energy density and quick charge/discharge times positions MnO
2/aCNT electrodes as promising candidates for hybrid supercapacitor–battery applications [
24]. The incorporation of MnO
2 onto carbon structures produces materials with improved structural, electrochemical, or mechanical properties. Carbon nanostructures such as CNTs and carbon incorporated onto synthetic fabrics (graphene/polyester fabric) provide flexibility of the end product, ideal for applications in wearable technology and smart devices. The porous, crystalline structure and synergistic oxide interactions of the CeO
2-MnO
2/CNF composite cause the most impressive maximum electrochemical capacitance of 1498 F g
−1. On the other hand, graphene, as a composite component, produced materials with better energy density retention (99% after 1000 cycles).
Amjad from the Polytechnic University of Tehran and his coworkers proposed a method to improve the conductivity of polyaniline (PAni) fibers with the assistance of MnO
2 during the polymerization. Using MnO
2 as seeds during interfacial polymerization results in a product with higher conductivity and greater capacity compared to PAni nanofibers, as observed by electrochemical capacitance measurements. The constructed composites made by rapid mixing, interfacial polymerization of Pani, and using MnO
2 as seeds were characterized by SEM, Fourier transform infrared (FT-IR) spectroscopy, and UV-vis spectroscopy, and demonstrated a uniform structure compared to other MnO
2-PAni nanofibers. The manufactured material was evaluated as a fitting electrode component in energy storage systems [
25].
A different composite material of polyaniline (PAni-MnO
2) was synthesized through the in situ chemical oxidative polymerization of aniline in an acidic aqueous solution (MnO
2 sourced from the reaction of MnSO
4 with KMnO
4). Examination of the structure of PAni and PAni-MnO
2 composites using FT-IR, SEM, and thermogravimetric analysis (TGA) confirms the formation of these materials with spherical MnO
2 particles distributed within the PAni matrix. Supercapacitor performance was evaluated using cyclic voltammetry and chronopotentiometry in a 0.5 M Na
2SO
4 solution, with the PAni-MnO
2 showing a high specific capacitance (Cs) of 242 F g
−1, which remained stable over 1000 cycles (at a current density of 0.25 A g
−1). The specific capacitance of the constructed materials grows gradually from PAni to PM5 composite, ranging from 99 to 242 F g
−1 at 0.1 A g
−1. These results demonstrate that PAni-MnO
2 composites possess excellent electrochemical properties, making them promising candidates for supercapacitor applications [
26].
Poly ortho-aminophenol (POAP) is also a conducting polymer, which is proven to improve its conductive performance with the presence of mesoporous MnO
2@Zeolite-Y (MOZ) synthesized by the ultrasonic method. Researchers used electro-polymerization to produce a POAP/MnO
2@Zeolite-Y (MOZ/POAP) composite on the zeolite electrode surface. The desired structure of the composite was evaluated through X-ray diffraction (XRD), SEM, Energy Dispersive X-ray elemental analysis (EDS), FT-IR, and Brunauer–Emmett-Teller (BET) analysis. The electrochemical analysis showed a specific capacitance of 422 F g
−1 at 1 A g
−1 and the best rate at 16 A g
−1 with 97.22% cyclic stability over 10,000 cycles. The astonishing results can be attributed to the presence of N and O in the matrix of POAP, as well as the synergistic effect of Mn (MnO
2@Zeolite-Y), enhancing the pseudocapacitance behavior. The synthesized (MOZ/POAP) shows great cycle life, high power density, and specific capacitance, making it an ideal component in energy storage applications [
27].
Examining the synergistic effect that both conductive polymers and carbon structures have on MnO
2 is essential to this research. Researchers, led by A. Rehman, created a composite material from active carbon cloth (ACC) layered with 3,4-ethylene dioxythiophene (PEDOT) and MnO
2. The experimental synthesis of ACC@MnO
2@PEDOT composite initiated by the electrodeposition of MnO
2 on ACC and was followed by a second electrodeposition of the PEDOT layer on the prepared ACC@MnO
2. The produced electrode exhibited excellent capacitance performance, 1882.5 mF cm
−2, with a discharge current density of 1 mA cm
−2. When combined with a negative ACC electrode, the produced SC device shows an areal capacitance of 368.05 mF cm
−2 at a scan rate of 1 mV cm
−2 with a voltage window of 1.8 V and a cycling stability of 94.6% after 10,000 GCD cycles. These results demonstrate that the constructed material has great cyclic performance, making it a suitable application for high-performance devices [
28].
Metals such as Ag, Ni, Co, Cu, and others also have conductive properties and can further enhance electron mobility when combined with conductive polymer materials. Researchers from the School of Chemistry and Chemical Engineering in Guangzhou developed an advanced asymmetric supercapacitor using hierarchical polypyrrole (PPy)-based composites as both the anode and cathode. The anode consists of ultrathin PPy and pedal-like MnO
2 coating onto 3D-Ni (3D snuzzle-like Ni@PPy@MnO
2 structure) with great charge storage capability. The cathode features a 3D cochleate-like Ni@MnO
2@PPy structure with enhanced energy storage and fast ion transport abilities. The unique 3D structures create storage chambers and efficient ion pathways. The cathode, in particular, offers excellent pseudocapacitance and contributes to a wide voltage window of 1.3–1.5 V, with high energy (59.8 Wh kg
−1) and power (7500 W kg
−1) densities. The supercapacitor demonstrates superior performance, including impressive cycling stability over 3000 cycles, and shows promise for future asymmetric supercapacitor designs. This work marks the first use of hierarchical PPy-based composites for both electrodes in asymmetric supercapacitors [
29]. Both polyaniline composites showcase improved conductivity compared to plain PAni, but did not outperform the rest of the composite materials. Performance metrics indicate that the inclusion of secondary structures such as zeolites, carbon cloth, and 3D nickel substrates acted synergistically with the polymer materials and contributed significantly to enhanced ion/electron transport and mechanical stability.
In an attempt to overcome the dissolution problems and volume fluctuations of MnO
2 as a cathode material, Sho Li and Junsheng Zhu composed a material based on coconut activated carbon, MnO
2 nanorods, and cobalt. A simple one-step oil bath method results in uniform deposition of Co-doped MnO
2 onto the CAC surface, revealing their synergistic effect and enhancing the H
+ and Zn
2+ ion diffusion kinetics when used as a cathode in Zinc-ion batteries. The capacity of Co-βMnO
2/CAC composite increases from 224.7 mAh g
−1 to 436.7 mAh g
−1 after 120 cycles at 0.2 A g
−1, while also displaying a high reversible capacity of 233.2 mAh g
−1 after 900 cycles at 0.5 A g
−1. According to
Table 1, which tabulates the fitted EIS parameters of the produced materials, it can be clearly seen that the charge transfer resistance (R
ct) of β-MO (30.9 Ω) is higher than that of Co-β-MO (21.2 Ω) and Co-β-MO/CAC (19.4 Ω), demonstrating that cobalt doping and CAC addition effectively lower the electrochemical reaction resistance in the Co-β-MO/CAC system. The electrochemical tests (EIS) and the galvanostatic intermittent titration technique) reveal that the end cathode material shows improved performance compared to a non-doped material due to the combined effect of the components [
12].
Research conducted by the Department of Nanoscience and Engineering at Inje University focused on the development of nickel (Ni)-doped λ-MnO
2 used as anode materials for lithium-ion (Li-ion) batteries. The composite was synthesized using a facile and cost-effective method and has a hierarchical hollow porous structure, providing high surface area, loading capacity, and low density to the anode composite. The Ni-doping method was employed to tailor the physical and chemical properties of MnO
2, enhancing its electrochemical performance. The Ni-doped MnO
2 demonstrated excellent cycling stability, rate capability, and enhanced diffusion properties when used as an anode in Li-ion batteries. Additionally, the material was tested in a Li-ion hybrid capacitor, where it exhibited superior energy and power performance, achieving a specific capacity of 25 mAh g
−1 at a high current density of 5 A g
−1, with a power density of 29 W kg
−1 and an energy density of 30 Wh kg
−1. These results highlight Ni-doped MnO
2 as a promising candidate for energy storage applications, with potential for other metal oxide systems. The study opens up avenues for creating advanced nanostructured materials for various energy storage devices [
30].
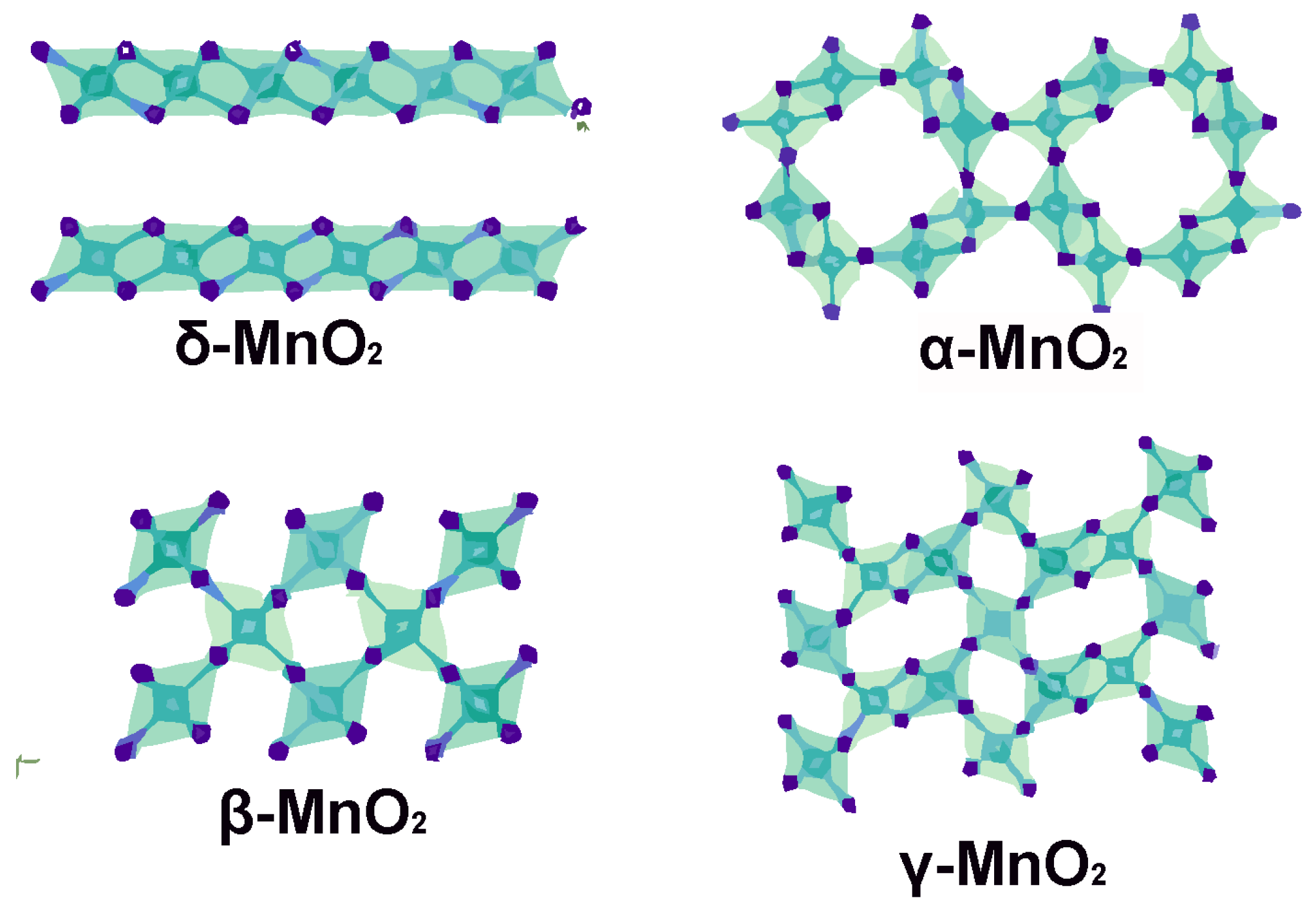
One additional doping method promising to improve the low conductivity and instability of δ-MnO
2 produces a copper-doped Cu
0.05K
0.11Mn
0.84O
2•0.54H
2O (Cu
2-KMO, copper-potassium manganese oxide) cathode. The cathode material is produced via a hydrothermal method and has a 3D pedal-shaped mesoporous structure, which promotes electron transfer and hastens reaction kinetics. Due to the double electron transfer between Mn
4+ and Mn
2+ in the Cu
2-KMO cathode, which converts into alkaline zinc sulphate nanosheets, the Zn//Cu
2-KMO battery displays high performance capacity. Partial capacity for the ZIBs is also provided by Mn
2+ in the mixed electrolyte. After electrochemical tests, the battery with Cu
2-KMO used as the cathode material showed a specific capacity of 600 mAh g
−1 at 0.1 A g
−1 after 75 cycles, shedding light on the beneficial effect of Cu doping on manganese dioxide base materials [
31].
Another composite nanostructure highlighting the synergistic effect of its components consists of 2D MXene, 1D tubular MnO
2, and Ag nanospheres, forming one electrode with a triple heterogeneous multi-level structure. The MXene nanosheets were produced using a water intercalation method, while the MnO
2 nanotubes were synthesized hydrothermally. The end composite was prepared through sonication and magnetic stirring of the components, resulting in an anode material with excellent properties. More specifically, the MXene/MnO
2/Ag anode achieved a specific capacitance of 880.8 F g
−1 at 2 A g
−1, while the assembled MXene/MnO
2/Ag//AC asymmetric supercapacitor displayed a specific capacitance of 112.5 F g
−1 at 3 A g
−1 and maintained 85% of its capacitance after 5000 cycles. These notable results can be attributed to the synergistic effect of the components, as the MXene sheets promote rapid electron transfer due to the expanded interlayer caused by the integration of MnO
2 and Ag between the nanosheets. This three-dimensional structure also shortens the electron transmission distance and increases the available active sites of the material, improving the overall electrochemical performance. In conclusion, this novel nanoarchitectural MXene/MnO
2/Ag anode material is promising to upgrade the technology of energy storage supercapacitors [
32].
While metallic Li is considered to be an excellent anode material, the growth of Li dendrites and volume fluctuations pose an obstacle to its applications in modern-day electronics. With the hope of overcoming these difficulties, Derong Liu, along with a team of researchers, developed a carbon cloth (CC) modified with Ag-doped MnO
2 nanosheets as a 3D host for Li metal anode (LMA). The Ag-doped MnO
2 vertically aligned nanosheets decorate the CC through a hydrothermal process, producing the final anode (Ag/MnO
2@CC). The existing pathways in the 3D structure allow for sufficient spaces that can accommodate volume fluctuation, enhancing the electrochemical performance. The restriction of dendrite growth was attributed to the unique nanostructure, which homogenizes Li-ion flux and reduces local current density. Density functional theory (DFT) calculations confirmed that the MnO
2 and Ag presented better lithiophilicity (−4.31 and −2.34 eV) than C (−1.65 eV). Ag/MnO
2@CC electrodeposited with Li showcased a Coulombic efficiency of 99.9% (after 300 cycles), while the full cell using LiFePO
4 as a cathode, exhibited excellent capacity of 105.5 mAh g
−1 after 400 cycles (at 1 C). Therefore, the constructed 3D structure could play a promising role in lithium metal battery applications in modern technology [
33]. As previously mentioned, multicomponent synergistic designs, such as Ag/MnO
2@CC and MXene/MnO
2/Ag, seem to maximize capacitance. The use of 3D porous layers in the structure also promotes electron transferability and is ideal for supercapacitor applications.
2.2.2. Structural Engineering
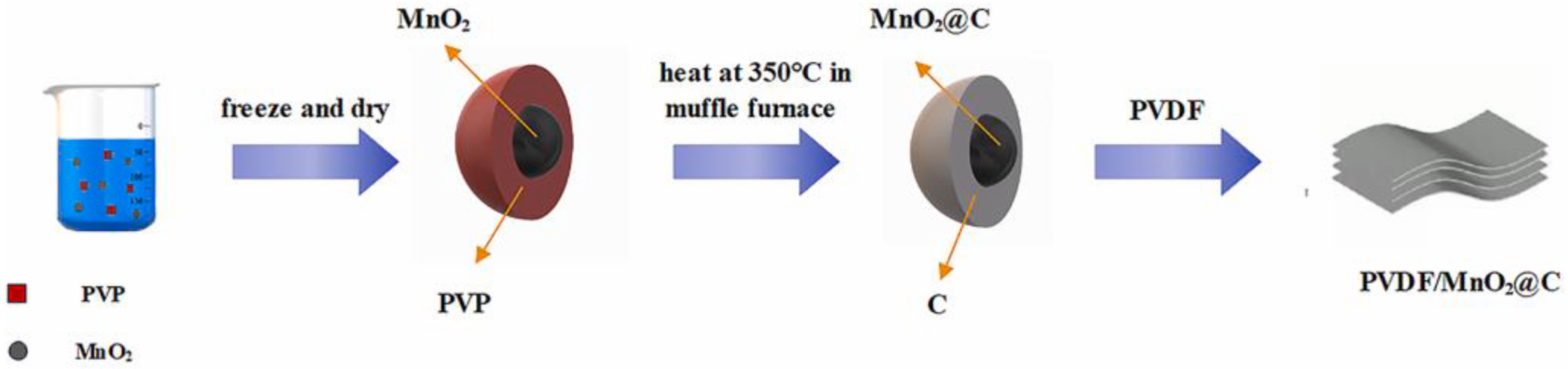
The deliberate design and manipulation of a material on a micro- or nano-scale is another challenge for researchers in the development of high-performing composites. The combination of materials with differences in morphology, porosity, and hierarchical organization results in products with enhanced electrochemical properties ideal for various energy storage applications. Some of the methods employed by researchers include the fabrication of core–shell architectures, hollow nanostructures, and layered frameworks, which improve mechanical resistance and electron mobility. A constructed material suggested by the Harbin University of Science and Technology is based on PVDF films doped with MnO
2@carbon core–shell particles. The process followed starts with the formation of the C-shell around the MnO
2 nanoparticles, yielding MnO
2@C, and ends with combining it with the polymer by solution bending. The existing C layer promotes electron transfer while enhancing interfacial polarization, while the produced composite film shows a resemblance to the dielectric properties of conductive fillers and complies with the percolation theory. Compared to the dielectric constant of PVDF at 100 Hz (7.68), the constructed MnO
2@carbon composite is 3 times more (24.4). With an energy storage capacity of PVDF/MnO
2@C, which is 4.95 J cm
−3, the material can be used as a semiconductor and as a component of high-energy storage capacitors (
Figure 6) [
34].
The design of MnO
2@amorphous carbon core–shell using a hard template method was also studied as a proposed cathode material in aqueous ZIBs with improved electron transfer properties. It was proved that the nano dimensions of MnO
2 rods contribute to the insertion reactions of Zn in active sites, thereby shortening the ion diffusion path and therefore improving the electrochemical performance. The porous structure of MnO
2@C also limited the phenomenon of capacity degradation caused by MnO
2 distortion, as it allowed a necessary room for volume expansion. After a series of tests, the MnO
2@C displayed a capacity of 102 mAh g
−1 at 0.8 A g
−1, maintaining 89.5% of its capacity after 600 cycles at 0.8 A g
−1. This research offered an effective method for developing high-performance manganese-based cathode materials for zinc-ion batteries, while also showcasing a pathway to enhance electrode materials [
35].
One additional material used in zinc-ion batteries is a MnO
2@polyaniline core–shell nanowire film, produced by an in situ interfacial method. The polyaniline nanoshells coated the MnO
2 cores uniformly and formed the films using a simple vacuum filtration technique. This composite showcased high flexibility, increased reaction kinetics between electrons, and impressive stability due to the conducting PAni, increasing the battery’s cycle life (2000-cycling lifetime). The MnO
2@PAni core–shell nanowire films reach a maximum specific capacity of 342 mAh g
−1 (at 0.2 A g) and a high capacity of 100 mAh g
−1 at 3 A g
−1. One remarkable application of the constructed material consists of batteries used in portable and wearable electronic devices, leading the path for the development of advanced technology in this field [
36].
Researchers Du Huang and his co-workers from the School of Materials Science and Engineering at South China University of Technology developed another more complex material, based on Ti foil, as an electrode component. The core–shell nanostructure consists of 3D TiO
2 nanoflowers (NFs) on activated Ti foil coated uniformly by Au film and MnO
2 nanowires. The process follows a three-step reaction method of hydrothermal treatment, gold-spraying, and electrodeposition. The structure of TiO
2 nanoflowers improves electron transfer and hastens reaction rate due to the larger surface area provided, while the Au film increases conductivity and adherence between the TiO
2 scaffold and MnO
2. The TiO
2 NFs@Au@MnO
2 based supercapacitor shows maximum specific capacitance of 223.75 F g
−1 (0.5 A g), an energy density of 19.87 Wh kg (0.2 Kw kg
−1), and a cycling stability of 85.8% after 2000 cycles. Therefore, this specific nanostructure, combined with other carbon-based materials, could be widely used in the manufacture of flexible asymmetric supercapacitors [
37].
Another core–shell and spherical structured Fe
3O
4@MnO
2 nanostructure is proposed by Jieting Ding (and others) who followed an affordable two-step hydrothermal method via a facile. In order to form the core–shell structure, δ-MnO
2 is grown on the surface of Fe
3O
4 nanospheres uniformly. The BET surface area of Fe
3O
4@MnO
2, which is 81.17 m
2 g
−1, was twice the size of Fe
3O
4 due to the nanosized layer of δ-MnO
2. The constructed composite as an electrode shows a specific capacitance of 243.7 F g
−1 (with a current density of 0.1 A g) and a capacitance retention of nearly 100% after 3000 cycles. This cycling stability and affordability of the core–shell structured Fe
3O
4@MnO
2 material could make it ideal for practical applications in pseudo capacitors [
38].
Through research, Jhankal and co-workers synthesized a nanocomposite bonding MnO
2 in nanoneedle form with reduced graphene oxide (rGO) by facile synthesis. The composed material acts as the positive electrode in an ion-quasi-solid-state asymmetric supercapacitor, showcasing great pseudo-capacitive electrochemical behavior with potential ranging from 0 to 1.8 V and a maximum capacity of 216 F g
−1 at 1 A g
−1 current density. The deposition of nanoneedles onto graphene oxide was executed uniformly, causing an interconnected structure that promotes ion exchange during electrochemical processes. After the execution of electrochemical tests, the asymmetric supercapacitor (rGO/MnO
2–GO) device demonstrated a maximum energy density of 24.5 Wh kg
−1, a power density of 900 W kg
−1, and a retention of 90% following 6000 cycles. This research paves the way for electrochemical investigations of the newly created nano-structured composite material combined with various polymer electrolytes, while also exploring differing composite morphologies for other energy storage uses [
39].
Researchers from the Hunan University of Science and Engineering in China proposed the structural alignment of Chinese fir (pine tree) scraps for energy storage applications. The anode consisted of aligned carbon nanotube arrays chemically deposited in carbonized wood tracheids, while a manganese dioxide-activated wood carbon (MnO
2@AWC) acts as a cathode material. Achieving high energy density and cycle stability in electrodes was a challenge for the researchers, who used chemical vapor deposition to align CNTs within the tracheids of Chinese fir in order to boost conductivity and surface area. The electrochemical deposition of MnO
2 nanosheets onto the inner surface of the tracheids improved the specific capacitance to 652.0 F g
−1 at 10 mA cm
−2. The resulting all-solid-state asymmetric supercapacitor achieved an energy density of 48.6 Wh kg
−1 (twice that of supercapacitors made from other biomass materials) and maintained 93% capacitance retention after 10,000 cycles. This approach, combining CNTs and MnO
2, creates an electrode with excellent stability, high energy density, and long-term cycle performance, offering a promising path for future wood-based carbon electrode development in energy storage systems [
40].
In order to improve the specific capacitance and enhance the environmental profile of MnO
2, researchers M. Mohsen and N. Moosavi propose the combination of MnO
2 with a 3D carbon substrate. Targeting the low conductivity of MnO
2, the researchers introduced metal ions into the structure, enhancing the electron transfer phenomenon with the deposition of Mg-, Zn-, and Mg-Zn-doped MnO
2 nanostructures onto CC hydrothermally. Amongst the three, Zn-MnO
2@CC showcased the best performance, while the fabricated flexible solid-state symmetric supercapacitor (with Zn-MnO
2@CC used both as an anode and cathode) showcased a maximum energy density of 0.197 mWh cm
−2 (at a current density of 2 mA cm
−2) and a power density of 2378 MW cm
−2. In addition, the device maintained 83% of its capacitance following 4000 charge-discharge cycles. Finally, in another series of practical tests, the supercapacitor provided electricity for 3 LEDs and a fan motor. Therefore, according to the research team, the obtained results could be considered as a novel technology, providing a low-cost, high-performance material with useful applications in modern-day technology [
41].
Utilizing a simple one-pot method, Jianghua Wu and co-workers synthesized potassium(K)-doped MnO
2 nanowires with promising qualities backed up by experimental tests. The addition of potassium causes the MnO
2 to change from its a- to its δ-form, increasing lattice defects and improving the material’s conductivity. There was a notably higher percentage of active sites in the composite, enhancing its capacitive performance. The composed K-doped MnO
2 showed enhanced morphology, electrochemical properties, and stability due to the presence of KOH and its ions (OH
− and K
+) in the lattice structure. The K0.35MnO
2/CC electrode showcased a maximum capacity of 405 F g
−1 (with a current density of 1 A g
−1) while the asymmetric supercapacitor K0.35MnO
2/AC displayed a maximum energy density of 71.5 Wh kg
−1. After 8000 cycles, the device maintained 92.7% of its capacitance (at 20 A g
−1). Through this research, the electrode K0.35MnO
2/CC could be considered as a novel and defective material for various energy storage applications with remarkable performance [
42].
Following a two-step method, Rahul S. Ingole, with a team of researchers, deposited rGO sheets onto unique 3D-hierarchical MnO
2 nanorods, using CC as a base. The process involveδ a hydrothermal deposition of MnO
2 nanorods onto CC, and afterwards, an ex-situ decoration with rGO. By modifying the concentration of GO each time, the researchers can note its impact on the physical and electrochemical properties of MnO
2-rGO electrodes. The addition of rGO sheets created a mesoporous structure, promoting the interfacial electron transfer phenomenon and improving the overall electrochemical performance. The rGO-decorated MnO
2 electrode (concentration of rGO was 20 mg/100 mL) achieved a specific capacitance of 1072.28 F g
−1 at 5mV sec
−1 in a 1 M Na
2SO
4 electrolyte and exhibited great cycle stability, as it retained 87% of its capacitance after 2000 cycles. This innovative material showed great morphological, electrochemical, and structural characteristics, ideal for wearable technology and other advanced applications [
43]. Superior capacitive behavior is attributed to materials with controlled nanostructured morphology and porous design. More specifically, MnO
2-rGO decorated nanorods showcased outstanding specific capacity (1072.28 F g
−1) while Fe
3O
4@MnO
2 and MnO
2–nanoneedle/rGO had incredible cycle stability, 100% and 90%, respectively. Core–shell structures also seem to improve electron transfer and structural stability as the core–shell architectures in MnO
2@carbon, MnO
2@polyaniline, and Fe
3O
4@MnO
2 enhance conductivity, accommodate volume changes, and boost cycling stability.
2.2.3. Doping and Functionalization
Two pivotal strategies in advancing the performance of MnO
2-based composites for energy storage applications are doping and functionalization. Doping involves the incorporation of foreign atoms or ions into the matrix of the MnO
2 lattice, which can significantly enhance electronic conductivity, modify the crystal structure, and improve the overall electrochemical performance [
44]. Functionalization, on the other hand, refers to the modification of MnO
2 surfaces or interfaces, which can strengthen interfacial interactions, facilitate charge transfer, and further optimize the material’s capacitive behavior; often through the integration of conductive materials or chemical groups (e.g., -OH, -COOH) [
45,
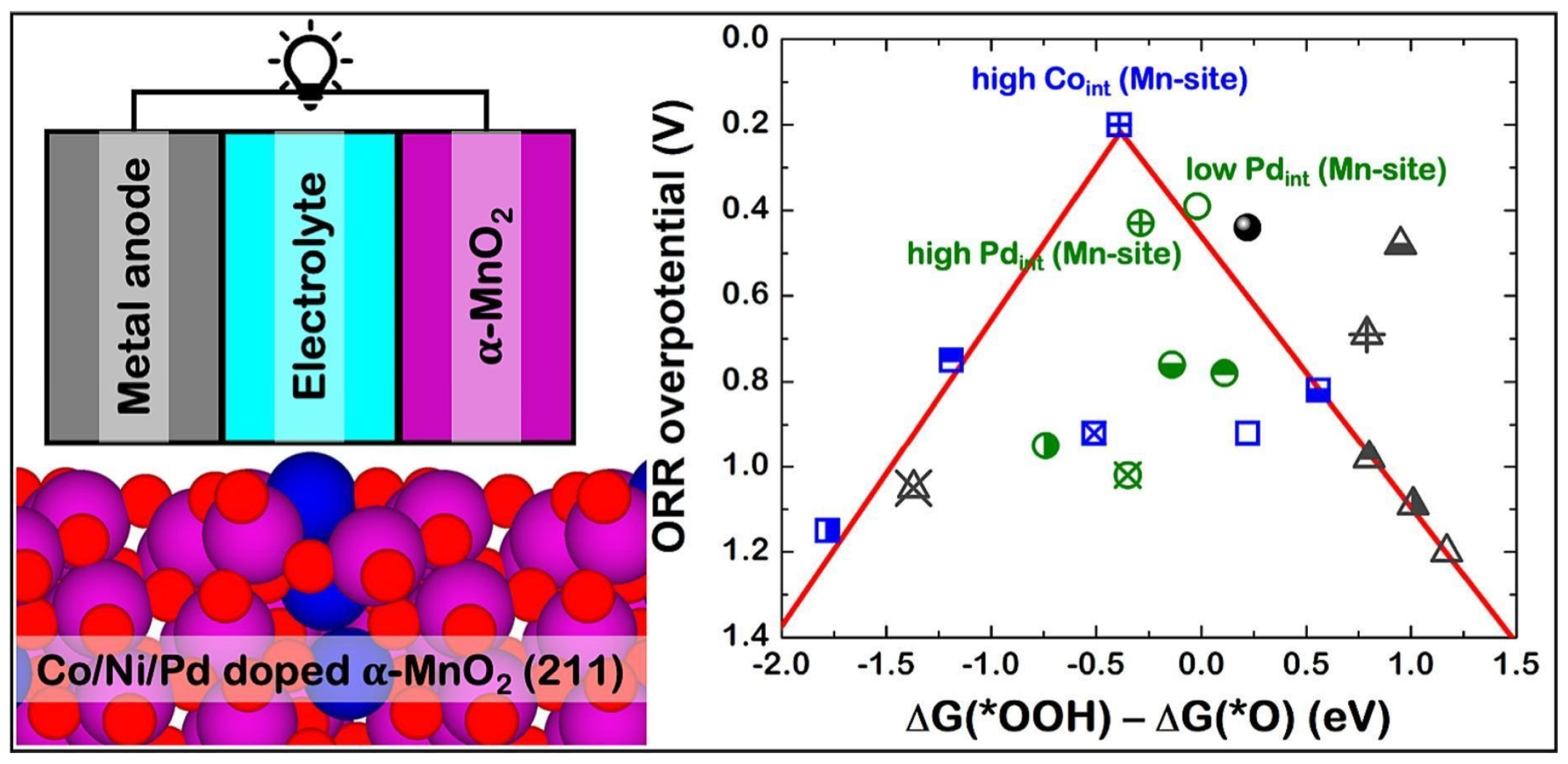
46]. Meena Rittiruam and researchers examined the effects of metal doping (Co, Ni, and Pd) on α-MnO
2 for enhancing oxygen reduction reaction (ORR) performance in metal–air batteries (MABs), utilizing the DFT and computational hydrogen electrode (CHE) calculations. After examination, it has been proven that the doping of metals modifies the adsorption strength of surface species during ORR by inducing electron accumulation at the Mn site and altering the d-band centre. More specifically, Co and Pd doping improve ORR activity, acting as promoters, while Ni doping negatively affects certain ORR steps, lowering activity. The obtained results revealed that enhanced catalytic performance occurred when Co and Pd were doped at interstitial sites, rather than substituting for Mn. These findings underscore the importance of controlling dopant concentration and atomic dispersion at interstitial sites to optimize electrocatalytic activity for MABs. Bulk calculations suggested that doping transitions α-MnO
2 from semiconductor to metallic behaviour, enhancing conductivity. The study provided valuable insights into how doping affects electronic properties and surface interactions, offering guidance for improving the performance of MAB cathodes (
Figure 7) [
14].
In order to improve the poor conductivity and overall performance of MnO
2, a suggested approach by Rathod, Vishal. T et al. involved doping the surface with Ni ions (or various metals), which enhances the charge storage capacity, especially in lithium-ion batteries. In this study, pristine and Ni-doped MnO
2 nanocrystals were synthesized using a triethanolamine (TEA)–ethoxylate-assisted hydrothermal method. The results of electrochemical testing on the final composite showed that the 4% Ni-doped MnO
2 electrodes exhibited the highest specific capacitance of 432.5 F g
−1 at a scan rate of 5 mV s
−1 and maintained 96.56% stability after 2000 cycles at a high current density. The Ni doping enhanced the overall electrical conductivity while lowering the electrolyte and charge-transfer resistance of MnO
2. Additionally, an asymmetric supercapacitor using 4% Ni-doped MnO
2 as the positive electrode and activated charcoal as the negative electrode achieved a maximum specific capacitance of 136.6 F g
−1. Based on the results, the researchers stated that Ni-doped MnO
2 can act as a high-performance electrode material for supercapacitors and advanced energy storage systems [
47]. It should be mentioned that in the work of Rathod, Vishal. T et al., the incorporation of Ni positively influenced the final product, whereas in the work of M. Rittiruam et al., the addition of Ni negatively affected the final electrode.
Researchers from the Department of Physics of Annamalai University in India focused on the effects of cobalt doping on α-MnO
2 nanoparticles, which were synthesized using a co-precipitation technique. The electrochemical properties of the Co-doped α-MnO
2 electrode were assessed through cyclic voltammetry, galvanostatic charge–discharge measurements, and electrochemical impedance spectroscopy. The results revealed that the Co-doped α-MnO
2 electrode exhibited pseudocapacitive behavior and significantly higher capacitance values compared to pure α-MnO
2. Specifically, the Co-doped α-MnO
2 demonstrated a capacitance of 1015 F g
−1 at 10 mV s
−1, compared to 901 F g
−1 for pure α-MnO
2. Furthermore, charge–discharge measurements showed a capacitance of 996 F g
−1 at 1 A g
−1 for Co-doped α-MnO
2, outperforming pure α-MnO
2, which had a capacitance of 876 F g
−1. The Co-doped α-MnO
2 also exhibited a lower charge transfer resistance, indicating improved supercapacitor performance. Phase and morphological analyses indicated that the Co-doped α-MnO
2 nanoparticles have a tetragonal structure with smaller crystallite sizes and a rod-like shape due to the Co doping, as confirmed by SEM and TEM imaging [
48].
According to the research of N.D. Raskar and co-workers, following a hydrothermal method, α-MnO
2 nanocomposites, rGO-based α-MnO
2 composites, and Fe-doped α-MnO
2 composites were fabricated. They proceeded to evaluate the applicability of the composites in supercapacitors and photocatalytic dye degradation devices through the examination of the phase transition from α-MnO
2 to the cubic phase of α-Mn
2O
3, using XRD analysis. Incorporating rGO and Fe doping significantly enhanced the materials’ electrochemical performance and photocatalytic activity. Specifically, the rGO-based 5% Fe-doped MnO
2 (GFMO
5) exhibited a specific capacitance of 683.35 F/g and achieved a 90% degradation of methylene blue dye under visible light. The XRD, SEM, and X-ray photoelectron spectroscopy (XPS) analyses confirmed the phase transition and morphology changes due to Fe substitution in the MnO
2 lattice, enhancing the material’s performance. The results showed that Fe doping and rGO incorporation improved the MnO
2 capabilities, making it suitable for both energy storage and environmental applications. The rGO-based 5% Fe-doped MnO
2 nanocomposite demonstrated the best overall performance, highlighting its potential as both a photocatalyst and a high-performance supercapacitor [
49].
Researchers from the Wuhan Polytechnic University in Hubei synthesized mesoporous Ag-doped γ-MnO
2 nanowires at room temperature, for the first time, following an Ag
+ activated persulfate process. This budget-friendly method results in nanopowder materials with uniformly distributed nanoparticles, aiming to enhance the performance of cathodes in aqueous ZIBs. The resulting Ag-doped γ-MnO
2 electrodes showed remarkable electrochemical performance, achieving a discharge capacity of 978 mAh g
−1 after 65 cycles at a battery current rate of 0.1 C, stabilizing at 804 mAh g
−1 after 150 cycles. The Ag@γ-MnO
2 electrode showcased excellent rate capability, as the retention of the specific capacity using differing C-rates was calculated to be more than 100% due to the predominant surface capacitive-controlled process. This Ag doping improved both oxygen defects and the stability of the material, contributing to superior electrochemical performance. The electrodes delivered a capacity of 320 mAh g
−1 at a current density of 1540 mAh g
−1 and exhibited outstanding cycling stability, highlighting the potential of Ag-doped γ-MnO
2 for high-performance aqueous ZIBs [
50].
As mentioned, MnO
2 is a promising cathode material for aqueous zinc-ion batteries but faces challenges with cycling stability due to the instability of its structure and the dissolution of Mn
2+ ions. To address this, amino-functionalized multi-wall carbon nanotubes (MWCNTs-NH
2) were introduced by the group of Huang Du in order to create a nanocomposite (MWCNTs-NH
2/γ-MnO
2) that significantly improved the stability of MnO
2. The MWCNTs-NH
2 showed enhanced structural integrity and conductivity due to their three-dimensional structure, which created pathways enabling electron movement. The integration of amino groups (groups with high nucleophilicity) helps in the storage of Zn
2+/H
+ ions in the material and the deposition of Mn
2+ to the cathode, preventing the loss of active material. This leads to a high capacity of 417.9 mAh g
−1 (at a current density of 0.2 A g
−1) and excellent cycling stability, with 91.4% capacity retention after 3500 cycles. Therefore, it was proven that the presence of amino-doped carbon nanotubes increased the electrochemical performance of γ-MnO
2, offering a strategy to improve the long-term performance of MnO
2-based cathodes in zinc-ion batteries [
13].
In another study, researchers Dipanwita Majumdar and Swapan Kumar Bhattacharya utilized hydroxyl groups to improve the electrochemical performance of a graphene–MnO
2 nanocomposite. The hydroxy-functionalized graphene/MnO
2 (hGO-MnO
2) material was synthesized through a one-pot sonochemical method in order to anchor MnO
2 nanosheets onto hGO, optimizing oxygen content on graphene, something not successfully achieved through other techniques. Both of the components’ optimum properties combine in this composite, exploiting their synergistic effect, resulting in electrodes with high specific capacitance (376.7 F g
−1) and stable cycling performance (91% after 1000 cycles, compared to 60% for the non-MnO
2–graphene composite). The (hGO-MnO
2) electrode material exhibits great electrochemical performance and uses a simple, low-cost, environmentally friendly method, making it a great candidate for energy storage applications [
51].
Researchers from Daegu University in South Korea examined the effect of B
2O
3 on the structure of multilayer carbon nanofiber/MnO
2 composites. Utilizing sequential electrospinning, they composed a tri-layered boron-MnO
2/CNF composite (PPMnB) and studied its applicability as a supercapacitor material based on the characteristics. Each of the composite components acted individually to enhance the electrochemical performance as it exhibits a specific capacitance of 215 F g
−1 at 1 mA cm
−2, a rate capability of 83% capacity retention at 20 mA cm
−2, and a great cycling stability (6% loss after 10,000 cycles at 1 mA cm
−2). The B-related functional groups exist independently between the nanosheets within the 3D interconnected CNF network while optimizing the pseudocapacitance of the material. Specifically, the first PAN-based CNF layer consists of micropores enhancing the materials’ capacitance. The second layer showed a quasi-capacitance behavior due to the MnO
2 particles and numerous pores, leading to a high-rate response and a stable cycle life. The third layer was more hydrophilic and had high electrical conductivity due to the B-related functional groups, promoting electron transfer. Therefore, the composed PPMnB material was mentioned as a promising component in high-power, high-energy, high-capacity supercapacitors [
52].
Pseudocapacitive materials with ion exchange sites on the surface are popular for asymmetric capacitors due to their electrochemical properties. Using activated CC decorated with featherlike MnO
2 nanoparticles, researchers manage to compose oxygen-enriched MnO
2 with a sheetlike structure via electrochemical oxidation. Because of the abundant oxidative functional groups on the surface of the composite, great conductivity and a capacitance of 3160 mF cm
−2 at 1 mA cm
−2 were exhibited. The anode of the capacitor consisted of activated carbon with an organic framework via the hydrothermal method, which is additionally doped with N in the AC lattice using annealing. N-doping further increased the functional groups and disorders on the surface, improving the overall performance. The complete device showed a maximum energy density of 8723 mWh cm
−3 at a power density of 14,248 mWh cm
−3, with a capacitance of 95.5% after 10,000 cycles, while it maintained 4548 mWh cm
−3 at 148.835 mW cm
−3. This research proposes an excellent product with applications in modern-day manufacture of energy storage devices [
53].
Therefore, based on these studies, it is indicated that Co and Pd doping significantly promote the oxygen reduction reaction (ORR) in metal–air batteries by modulating the d-band center and electronic density on Mn sites. Nickel, on the other hand, while it improves conductivity and capacitance in MnO2 used in supercapacitors, it hinders ORR activity when introduced inappropriately, highlighting the importance of site-specific doping and application context. The most impressive specific capacitance results are exhibited by the Co-doped α-MnO2 and the Ag-doped γ-MnO2 composites at 1015 F g−1 and 978 mAh g−1 (capacity), selectively. In the cobalt composite, smaller crystallite size and morphological control improve electrochemical performance in most applications, while noble metals perform incredibly for AIZBs in specialized systems. Two of the carbon-functionalized products, hGO-MnO2 and MWCNTs-NH2/γ-MnO2, exhibit remarkable cycling performance at ≈91% after 1000–3500 cycles.
2.2.4. Hybridization with Synergistic Materials
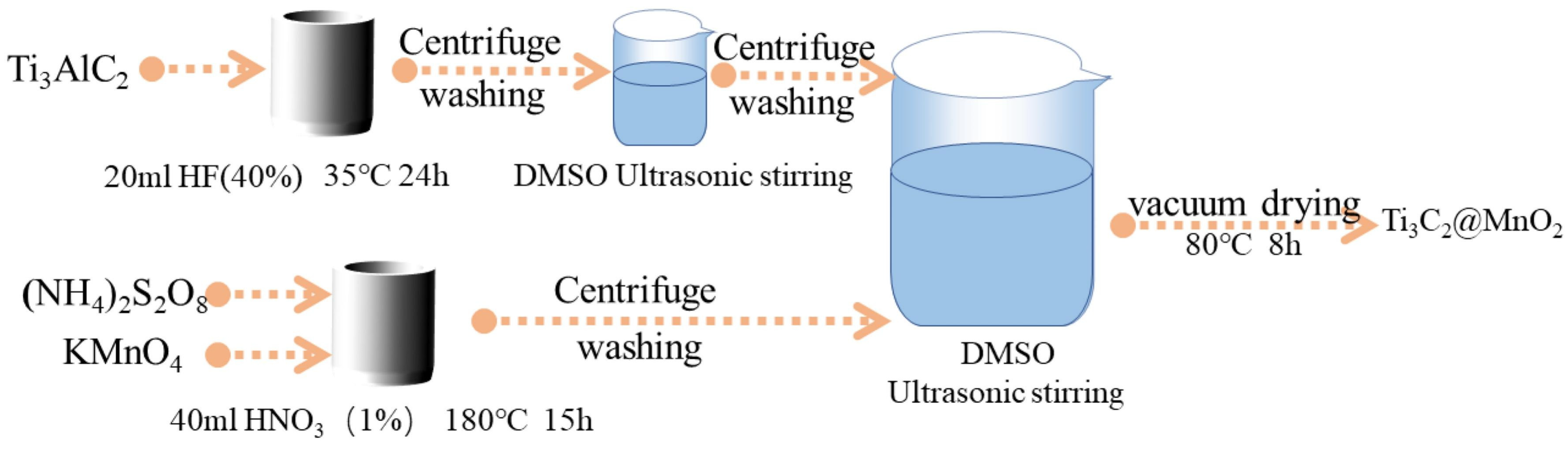
The synergistic interactions between MnO
2 and complementary materials such as conductive polymers, carbon-based materials, or other metal oxides are critical to overcome the poor electrical conductivity and slow reaction kinetics of MnO
2. Utilizing the synergistic effect of MnO
2 and Ti
3C
2 on MgH
2 can improve the performance of Mg/MgH
2 as a hydrogen storage material by constructing a MnO
2-doped Ti
3C
2 composite. By depositing MnO
2 onto Mxene, the original flat structure of the material was maintained while showing lower hydrogen absorption/desorption temperatures and faster reaction rates. The investigated composite exhibited numerous pathways for hydrogen atoms and promoted H diffusion due to the coexistence of Mg/Ti
3C
2, Mg/TiO
2, Mg/MnO
2, Mg/MnO, and Mg/Mn multiphase interfaces in the MgH
2+Ti
3C
2@MnO
2 system. The present study carves the path for novel materials useful in hydrogen storage applications, increasing in popularity in recent years (
Figure 8) [
54].
Neha Kanaujia and coworkers reported the synthesis of flower-like MoS
2, spherical MnO
2, and their MnO
2/MoS
2 nanocomposite using a hydrothermal method in order to bind the MnO
2 nanoparticles and MoS
2 nanosheets together. Specifically, MoS
2 demonstrated higher ionic conductivity than metal oxides, had a wide surface area, and exhibited high specific capacitance due to ion diffusion through its layers. The electrochemical properties of nanocomposites, with differing ratios, were evaluated using a three-electrode setup in a 2M KOH electrolyte at room temperature. The MnO
2@MoS
2 nanocomposite with a 3:1 molar ratio exhibited superior performance, with a specific capacitance of 352 F g
−1 at 1 A g
−1, 72% capacity retention after 2000 cycles at 3 A g
−1, and 88% coulombic efficiency. This sample also demonstrated enhanced ion transport with a smaller relaxation time constant (150 ms) and a higher diffusion coefficient. The high surface area, mesopore structure, and optimal pore size distribution contributed to its exceptional electrochemical properties, offering valuable insights for the development of high-performance energy storage devices [
55].
Scientists from the National Center of Nanoscience and Technology and the Institute of Physics in Beijing fabricated a coral-like porous MnO
2 nanostructure film on stainless steel using a simple ZnO-nanorod-array-mediated hydrothermal method. The resulting electrode materials exhibited impressive electrochemical performance, with a specific capacitance of 221 F g
−1 at a current density of 0.5 A g
−1 and 86% capacitance retention after 3000 cycles at 5 A g
−1. The high performance was attributed to the porous, hierarchical MnO
2 nanostructure formed on the conductive stainless-steel substrate and the existence of ZnO, a semiconductive material that is easily dissolved and purchased. The ZnO nanowires served as a template, directing the formation of disordered birnessite-MnO
2 nanocrystals, enhancing the overall stability and efficiency of the supercapacitor. This unique structure offered great potential for use in low-cost, high-performance supercapacitor applications [
56].
A study by Godlaveti and co-workers introduced a new binary composite of copper sulfide and manganese oxide designed to improve supercapacitor performance. Generally, introducing metal oxides, metal sulfides, and their derivatives as electrode material has been proven to enhance the device’s electrochemical behavior. The synthesized composite consisted of 80% CuS and 20% MnO
2, as this ratio shows optimized performance. The resulting high-purity nanoparticles and nanorods showed promising properties, with the CuS/MnO
2 composite demonstrating excellent charge storage capabilities. The material exhibited a specific capacitance of 451 F g
−1, low resistance, and remarkable cyclic stability (98.33% after 2000 cycles). Additionally, the composite achieved a high-power density of 1997 W kg
−1 and an energy density of 28.19 Wh kg
−1. Structural analysis confirmed the successful synthesis of CuS and MnO
2, and the composite outperformed bare CuS electrodes in electrochemical tests, highlighting the synergistic effects between CuS and MnO
2. These results suggested that CuS/MnO
2 composites could be promising for advanced energy storage applications [
57].
A Sm-doped δ-MnO
2/Carbon aerogel (SDM/CA) composite was synthesized using a hydrothermal method to enhance the electrical conductivity and structural stability of δ-MnO
2 for supercapacitors. The carbon aerogel acted synergistically due to its 3d porous structure providing more redox reaction sites and electron transferability, while the presence of Sm
3+ improved electron diffusion. The SDM/CA material, rich in oxygen vacancies, exhibited improved conductivity, reduced charge transfer resistance, and increased surface area compared to pure δ-MnO
2. As a result, it demonstrated excellent cycling stability with 94% retention after 10,000 cycles at 10 A g
−1. The assembled asymmetric supercapacitor using SDM/CA as the positive electrode showed a specific capacitance of 31.28 F g
−1 and 73.75% capacity retention after 10,000 cycles. Overall, the enhanced performance was attributed to the synergistic effect of Sm-doping and carbon aerogels, improving charge accumulation and reducing polarization [
58]. Synergistic hybridization greatly improves performance, as demonstrated in all studies. The hybridization of MnO
2 with synergistic materials—such as MXenes (Ti
3C
2), MoS
2, carbon aerogels, metal sulfides, semiconductors, and templates—enhances capacitance, energy/power density, H
2 kinetics, and long-term cycling stability (
Table 2).
The goal of functionalization and doping is to enhance the capacity performance of MnO
2 in composite materials, utilizing its synergistic effect with various components. Metal doping, for instance, promotes electron mobility while non-metal doping boosts pseudo-capacitance through surface redox reactions. The integration of conductive materials, such as graphene, CNTs, conductive polymers, with manganese oxide also boosts conductivity and leads to higher specific capacitance. Additionally, modifying composites at the nanoscale (nano structuring) utilizing nanomaterials for deposition and synthesis targets the low theoretical capacitance of such materials [
21,
59,
60]. The phenomenon of structural breaking can be addressed by incorporating the metal oxide onto flexible substrates comprised of nanofibers or polymer binders with reduced mechanical stress. Core–shell structures can also enhance the durability of the material, maintaining its integrity during expansion/contraction [
35,
61,
62].