Abstract
The mitigation and reduction of carbon footprint is nowadays one of the most pressing challenges covering the most diverse fields of civil activity and industrial production, to meet the climate neutrality targets of the Paris protocol by 2050. However, the intermittency of renewable sources necessitates diverse technical solutions for energy storage. An attractive peculiarity of NH3 as an energy vector stems in its double possibility of being used both as a source of H2 and directly as a green fuel. Intriguingly, an aspect common to most scientific publications on the subject is the limited attention to safety and risk problems connected with the use of NH3. This paper intended to fill a gap pertaining to the emerging risks associated with the use of ammonia as an energy vector and to provide experimental and theoretical investigations on liquid spray curtains as an effective mitigation technique for accidental releases of ammonia in air.
1. Introduction
1.1. Ammonia’s Energy Potential
Climate changes are one of the more evident consequences of an uncontrolled environmental pollution, involving air, soil, and water [1,2,3,4,5]. As reported by Mikulčić et al. [1], fossil fuel production and use strongly contribute to the anthropogenic emissions of methane, which is the second most significant contributor in terms of greenhouse gas emissions. Given this evidence, the reduction in fossil fuel usage combined with the exploitation of renewable energy sources is recognized as an effective action toward sustainability. Electrical energy can be stored as mechanical, potential, or chemical energy, the latter relying upon the bonding energy contained in specific compounds synthesized in chemical or electrochemical processes. In the former case, Jankowski et al. [6] proposed a very exhaustive survey on compressed air energy storage (CAES) technology, which has currently reached a technical maturity, even at medium and large scale, with installed power up to 290 MW. In the second case, pumped hydro energy storage systems [7] have been advantageously linked to solar and wind facilities, but the applicability of this strategy largely depends on installation costs and the orographic characteristics of the area. The case of chemical energy storage deserves a separate discussion, owing to the fact that, over the past few years, it has diversified into many more branches and variants than the two previously mentioned techniques. As is well known, electrochemical energy storage relies upon the use of rechargeable batteries [8], the development and refinement of which in recent years has also been boosted by the anti-pollution regulations on motor vehicles [9]. On a larger scale, the attention has been focused on chemical species like green energy vectors, namely on substances capable of transferring energy directly by chemical reactions, such as combustion, or indirectly, by generating other energy vectors, without releasing greenhouse gases (GHG) or other compounds capable of altering the planet’s climate balance. The energy transition era is giving further amplification to the important role that ammonia has always held in the chemical and process industry [10]. Furthermore, the implementation of renewable energy sources as substitutes for fossil fuels is looking to the immense ecological potential of hydrogen to respond to the increasing energy demand and to reduce greenhouse gas emissions at the same time. Despite low-carbon hydrogen being recognized as one of the most promising opportunities to build a carbon-neutral society [11], several technological challenges still need to be addressed along the whole supply chain [12]. Indeed, the extremely low critical temperature of hydrogen and its low specific energy per unit volume pose serious limits to its use as an energy carrier for long distances, including maritime transport [13]. Osman et al. [14] analyzed the environmental impact of four different techniques for H2 production by means of a life cycle assessment (LCA) and pointed out the need for a “cradle-to-grave” analysis for balanced decision making when choosing an appropriate synthesis process, possibly starting at the conceptualization stage of the process [15]. To overcome these technical drawbacks, many researchers focused on hydrogen carriers based on organic [16] and inorganic structures [17]. In the former subset, methane [18], methanol, dimethyl ether, formic acid, aliphatic, and aromatic hydrocarbons [19] have been proposed and experienced with different results, often strictly related to the relevant plant scale. The best representatives of inorganic H2 carriers are metal hydrides and ammonia (NH3). In this context, ammonia is an efficient hydrogen carrier, offering higher hydrogen density than liquid hydrogen per unit volume, allowing significantly lower transportation and development costs [20], and, also considering diffuse production, displaying better commercial feasibility and handling [21]. Ammonia can be obtained, though indirectly, from both fossil fuels and renewable sources and is fully recyclable, being made from water and nitrogen as starting materials [22].
1.2. The Need of Risk Mitigation Research
On the one hand, ammonia represents the only carbon-free strategy whose technological maturity enables scaling-up from MWh to TWh, making green ammonia commercially attractive as an energy carrier [23]. On the other hand, ammonia is corrosive to copper, brass, and zinc alloys [21]; ammonia–air mixtures are explosive in the range of 15.5–27% by volume [24], and it is potentially life-threatening [25]. Until now, NH3 has been produced at a large scale by the Haber–Bosch (HB) process, which is traditionally based on CH4 steam reforming (SMR) followed by a water gas shift (WGS) process in order to produce H2 necessary for further ammonia catalytic synthesis. Under these conditions, as pointed out by Wang et al. [26], NH3 synthesis is highly environmentally critical, producing even 1% of all planetary GHG emissions. As is widely known, ammonia’s strong polar characteristics make it highly soluble in water, with important consequences for human health and materials employed in the relevant storage or combustion facilities, owing to its irritating and corrosive properties. Ammonia toxicity is one of the main safety concerns. Human exposure limits of ammonia are set between 25 and 50 ppm [21], and general discomfort can be experienced at concentrations higher than 39.5 ppm [27], while severe consequences can occur by exposure to concentrations above 300 ppm [21], including pronounced irritation (between 400 and 700 ppm) and instant death at 5000 ppm [27]. Notably, an 8 h Time-Weighted Average (TWA) of 25 ppm is indicated by the Health and Safety Executive (HSE) and the short-term exposure limit (STEL) is 35 ppm, while for Europe, these values are established at 20 ppm (TWA) and 50 ppm (STEL) [21]. Existing short-term exposure in connection with accidental releases and emergency actions are provided by the American Industrial Hygiene Association (AIHA) under the Emergency Response Planning Guidelines (ERPGs) and established as follows: ERPG-1: 25 ppm; ERPG-2: 200 ppm; ERPG-3: 1000 ppm. Additionally, being liquefied under pressure, ammonia expands 850 times when released into ambient air, generating large vapor clouds [28], due to its relative density of 0.589 compared to dry air. It is worth noting that ammonia is classified as a non-flammable gas by the United Nations, but it is flammable within the concentration range 15.5–27% v/v with autoignition temperature AIT = 651 °C. Even though the LFL (lower flammable limit) is relatively high, it represents a peculiar hazard under totally or semi-confined geometries, where can give rise to hazardous build-up [29].
Due to ammonia’s peculiar properties, its widespread exploitation in the energy sector is expected in the future. Nevertheless, new mitigation strategies should accompany the spread of this new energy vector. For this reason, this work aims to investigate an enhanced system to face the accidental release of ammonia. The novelty of this study lies in the novel approach adopted, based on the use of a reactive curtain to enhance the ammonia abatement associated with its accidental release and theoretical model development.
The remainder of this paper is as follows. Section 2 presents recent trends and challenges related to ammonia production and use as an energy vector. Section 3 reports a statistical analysis of ammonia-related accidents over an extended time span, ranging from 1973 to 2022; Section 4 details the material and methods utilized in the experimental phase of the study and the theoretical approach. Results are presented and critically discussed in Section 5, while Section 6 draws conclusions and further research perspectives.
2. Ammonia Use Evolution and Safety Implications
Typically, in the scientific community, NH3 is labelled as brown, gray, blue, and green NH3 according to the growing environmental friendliness of the synthesis technique, where the latter refers to NH3 produced by H2 of green origin, namely deriving from water electrolysis driven by renewable sources like solar, wind, tidal, and sea wave energy. In this context, studies have been carried out to ascertain the best operative conditions in case of off-shore [30] or on-shore NH3 production, as electric energy transfer to the end-use destination may represent a key factor in some situations. Compared to brown/gray ammonia, green ammonia aims to address the environmental concerns associated with conventional production processes, but introduces new challenges related to cost, infrastructure, water exploitation, and potential environmental impacts. Moreover, additional hazards can be ascribed to hydrogen production (electrolysis) and the potential for increased explosion risks due to higher hydrogen partial pressures. The most recent trends for NH3 synthesis are oriented towards a progressive substitution/elimination of the traditional HB process, but the path is slow and there are many obstacles to a short-term goal. In fact, many alternative synthesis techniques have been proposed, but none of them seem to have yet been implemented at the same scale as the HB plant. Among them, thermal [31] or non-thermal plasma synthesis [32], electrochemical synthesis [33], electrocatalytic synthesis [34], chemical looping ammonia synthesis (CLAS), and relying upon metal nitride dissociation by hydrolysis or under H2 stream [35] are currently object of intense investigations. Spatolisano et al. [36] exhaustively reviewed many different NH3 splitting techniques for H2 release, and they pointed out that most technologies for NH3 cracking suffer from scalability problems, due to the stability and high costs of the relevant catalysts and electrocatalysts that are thereby required. Adopting NH3 as a fuel is not a recent novelty. However, when used alone with air as a comburent, its limitations lie in its slow combustion kinetics compared to hydrocarbons, small calorific power with respect to traditional fuels, relatively high autoignition temperature, and finally its low flame stability and temperature [37]. These important drawbacks made the use of NH3 somewhat problematic in turbine combustors and in internal combustion engines, whether they are spark-ignition or compression-ignition type [25]. These disadvantages have been partially overcome by co-firing strategies, namely by mixing NH3 with H2 [38] or with other combustibles acting as a “fuel enhancer” like CH4 [39], biogas [40], n-heptane [41], and dimethyl-ether [42]. The dual-fuel operation can dramatically reduce carbon-based emissions by burning ammonia alongside diesel, biodiesel, or another fuel at a lower auto-ignition temperature, even though concerns remain about ammonia’s safety and efficacy [43].
Recently, the classification society American Bureau of Shipping (ABS) [44] has issued the maritime industry’s first guidance focused on ammonia bunkering, emphasizing the need of risk assessment, safety procedures, and thoroughly developed bunkering plans, including advanced control systems, emergency shutdown mechanisms, mitigation by technical and managerial barriers to protect personnel, and ensuring safe bunkering capabilities, whether by truck, ship, or in land storage terminals. Ammonia offers great flexibility across storage, supply, and consumer technologies so that the hierarchy of controls onboard should firstly focus on safer options rather than on mitigative barriers, crew training, or personal protective equipment (PPE), which are substantially emphasized in the existing regulations and literature [45]. However, it should be noted that in the world’s major bunkering hubs it is foreseeable a noteworthy development as a future marine fuel of ammonia storage and handling, in large volumes. Due to several hazards related to the employment of ammonia, effective methods are needed to control the spread of large ammonia clouds and mitigate its environmental/toxic effects when considering port or industrial storage. Although ammonia is characterized by a strong odor, easily detectable even at low concentrations (5 ppm) [46], gaseous ammonia is colorless and its release in the atmosphere can form dense gas clouds and consequently it is desirable to increase their natural dispersion by enhancing the dilution rate. Spray curtains can act on a gas cloud to mitigate the consequences of its release by working as a barrier to the passage of gas, by dispersing and diluting through air entrainment, by imparting upward momentum, by heating or cooling a gas, or by physical or chemical absorption [47]. The interception of the released ammonia can be obtained by using water curtains or water–air spray systems. The addition of a strong acid at low concentration to the water can enhance the abatement efficiency through reactive absorption [28]. This approach is successfully used for several processes for nitrogen oxide removal, desulphurization, and carbon capture [48]. Palazzi et al. [49,50] evaluated reacting curtains using alkaline solutions for the mitigation of chlorine release in a detailed study at a laboratory scale and the development of a mathematical model of a two-phase jet to observe the entrained air rate in connection with the liquid flow rate. In the present work, which finds its grounding in preliminary work [51], a thorough wind tunnel experimental study was performed for the abatement of different ammonia releases, both in water and in reacting solutions, and, taking inspiration from the model obtained by Palazzi et al. [50], a short-cut design tool is presented.
3. Statistical Analysis of Accidents
The scientific community has contributed to systematic research into the safe use of ammonia for onshore industries, while the emerging trend of utilizing ammonia as a marine fuel was faced only recently in 2020 with risk assessment papers. Additionally, the scientific literature concerning NH3 accident statistics and theoretical insights on them is still limited. Several accidents were recorded involving ammonia gas dispersion leading to serious injuries and impact on the environment. Accident analysis during transport, limited to USA, was performed by Crolius et al. [52] over the time span 1971–2019, collecting figures of 797, 2301 and 14 events, respectively, with reference to road, rail, and waterway transportation. In a recent paper, Tan et al. [27] performed a CFD simulation and experimental test in a wind tunnel [53] to study the leakage and the consequences of dispersion of ammonia used as refrigerant in a food factory in Tianjin, China. They found that the concentration of ammonia near the source increased with the increase in pressure, while the dispersion of ammonia far from the source is mainly influenced by the wind field. However, the lethal concentration and explosion range were limited near the release source.
In this section, a statistical analysis is performed collecting data from different databases, namely FACTS (Failure and Accidents Technical information System), managed by the Unified Industrial & Harbour Fire Department in Rotterdam-Rozenburg (NL), which was the primary source of data, eMARS [54], and IChemE [55], globally considering the time span from 1973 to 2022.
In Figure 1, the classification of the primary causes is analyzed by considering environmental, technical, organizational, and human factors, whose subclassification codes are reported in Table 1. Data analysis revealed an annual average of 6.6 ± 9.4 accidents, with a non-negligible injury count (108 ± 470 per year), which included an accident of exceptional severity occurring in 2001 in Toulouse (FR), involving 2442 individuals affected by the accidental explosion of ammonium nitrate in a fertilizer plant. All collected and validated cases belong to the chemical industry, a sector characterized by a well-developed safety culture, thanks to the awareness gained over time of hazards related to manipulation of chemicals, which have led to consolidated safety protocols and a high level of education of the personnel involved. Liang et al. [56], in a study on occupational health services in China, reports ammonia among the three top causes of acute occupational poisoning and, together with carbon monoxide and hydrogen sulfide, it accounted for about 50% of the total deaths of acute poisoning. It is worth mentioning that an extensive study on ammonia-related refrigeration accidents in China from 2010 to 2020 [57], reviewed a total of 82 cases, which led to 189 deaths and 1081 injured.
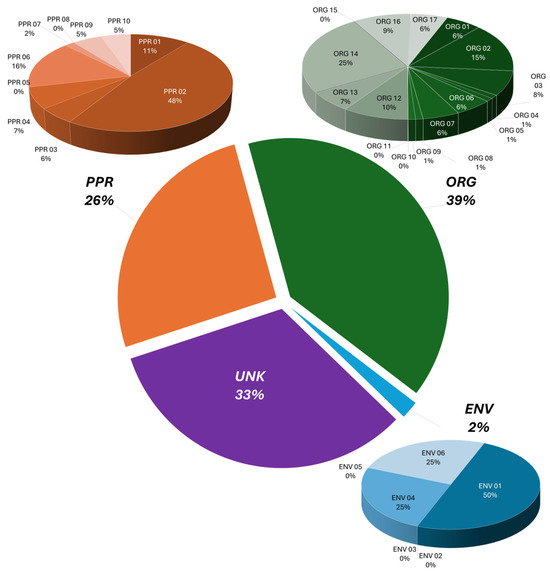
Figure 1.
Classification of accident causes into three macro-areas, namely process/plant causes (PPR), environmental causes (ENV), and human factor/organizational causes (ORG). UNK = unknown.

Table 1.
Macro area classification of identified immediate causes for ammonia accidents.
Referring to the shipping context, the ad hoc developed database accounts for one leakage event during NH3 cargo transport plus three events connected to NH3 refrigeration system [58]. Due to the high pressure to achieve energy transition goals in a short timeframe, it is expected that there will be a higher probability of underestimating the risk associated with the management of new energy vectors by industrial sectors which are not accustomed to handling them, or under unusual conditions, whose hazards have not yet been properly considered. In this regard, industrial activities involving hazardous cargo of new energy carriers like ammonia and hydrogen pose significant risks to port infrastructure, potentially leading to extensive damage, destruction, and extended disruption of port logistics. These events may become more critical when considering ports with both industrial and tourist functions, where the storage and handling of hazardous chemicals and new fuels potentially introduces new hazards to the system. Nevertheless, there is still a lack of international regulations regarding the use of ammonia as a fuel, and only the International Code of Safety for Ships Using Gases or Other Low-Flashpoint Fuels [59] and Interim Guidelines for Ships Using Ammonia as Fuel [60] are available, together with a few specific codes like the International Code for the Construction and Equipment of Ships Carrying Dangerous Chemicals in Bulk [61], which is applicable for aqueous ammonia, while the International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk [62] is dedicated to anhydrous ammonia, among other liquefied gases.
A recent study displayed evidence that bunkering ammonia stored in fully refrigerated tanks as an atmospheric pressure saturated liquid is the safest and that severity can be mitigated by release duration and transfer flow rate reduction [63]. For these reasons, research on and the development of new mitigating strategies can play a pivotal role in preventing the escalation of undesired events towards worse consequences. Among them, efforts in enhancing the mitigation action by barriers could be essential to reduce the level of risk associated with the accidental release of toxic compounds like ammonia.
4. Materials and Methods
4.1. Experimental Apparatus and Release Tests
Experimental tests were performed inside a glass laboratory wind tunnel, shown in Figure 2, with a rectangular section of 0.9 × 0.9 m and 5 m of length. A total of 19 AISI 316 stainless steel sprayers (C.B.N. S.n.c., Milan, Italy) were used to form the liquid curtain composed of pure water or HCl solution (0–0.55 kmol·m−3), as a compromise between the need to provide a reactive effect to the curtain, while avoiding generating another hazardous stream for equipment and operators. Series of replicated measures of ammonia concentration were carried out at two sampling points, located, respectively, at a distance of 0.70 m (upwind the barrier) and 1.50 m (downwind the barrier) from the release. The optimal operating conditions adopted are summarized in Table 2. Such conditions were selected based on previous results obtained by the authors and described in Palazzi et al. [49,50] and on preliminary fluid-dynamic and absorption runs simulating a continuous release with a constant release rate, in order to define the air entrainment and the release dilution.
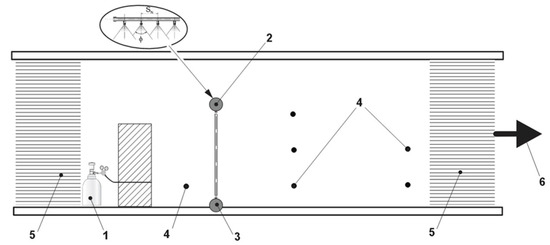
Figure 2.
Experimental set-up: 1. Ammonia source; 2. Downward liquid curtain; 3. Upward liquid curtain; 4. Sampling points; 5. Honeycomb flow rectifiers; 6. Stainless steel centrifugal fan.

Table 2.
Range and reference values of experimental parameters.
4.2. Analytical Measures
Ammonia concentration was measured by bubbling air samples through an acidic water trap (pH = 4.0). A UV-vis spectrophotometer (model Lambda 25, Perkin Elmer, Wellesley, MA, USA) was employed for ammonia determination in the samples using the Nessler reagent. Briefly, in an Erlenmeyer flask, one drop of EDTA was poured in about 50 mL of sample followed by 2 mL of Nessler reagent. After 20 min, sample absorbance was read at the wavelength of 420 nm. Analytical measurements were performed in triplicate with average error lower than 8%.
4.3. Theoretical Model
The adopted model was already reported in [51]. Figure 3 summarizes the logic behind the model and the main hypotheses. Briefly, it is based on the description of the air entrainment rate into the curtain, on dilution of NH3 into the circulating air, and on NH3 absorption in the liquid phase, respectively.
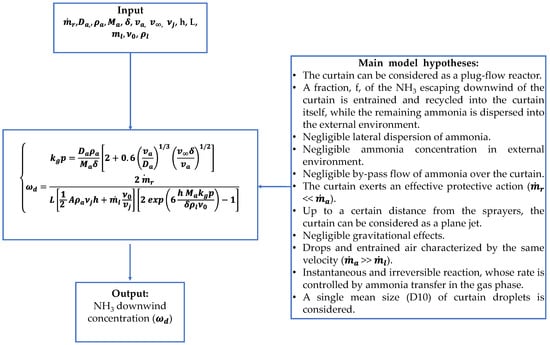
Figure 3.
Summary of model hypotheses and logic.
Equation (1) shows the mass reduction of the released compound, by means of physical and/or chemical absorption:
where is the NH3 release mass flow rate after curtain absorption (kg/s), is the absorption mass flow rate (kg/s), and the absorption efficiency () is defined as given in Equation (2):
By defining the dilution efficiency ( as reported in Equation (3), the mechanism of concentration reduction of NH3 in downwind adjacency by mixing effect with air (, kg/s) was taken into account.
The concentration of released substance downwind of the curtain ( ppm) may be expressed as in Equations (4)–(7), where and are the mass flow rate of air entrained and induced by the curtain, respectively, and are the spray exit velocity and the velocity at the end of the jet phase (m/s), A is the entrainment constant (-), and is the density of the gas phase (kg/m3).
In Equation (8), the chemical–physical absorption of NH3 into the fluid curtain is considered:
where Xg was the intrinsic single-pass absorption efficiency of ammonia either in water or in HCl solution, defined in Equation (9):
The mass transfer coefficient in the gas phase ( was estimated starting from Foust et al. [64] (Equation (10)):
where is the terminal velocity of liquid phase (m).
Combining the previous equations, the NH3 concentration was expressed as reported in Equation (11):
As detailed in the experimental section, the model was validated by means of replicated experimental runs in the wind tunnel under different windy conditions.
5. Results and Discussion
5.1. Experimental Results and Theoretical Model
Experimental data simulating the release of ammonia in the wind tunnel are shown in Figure 4, where the NH3 concentration upwind (Figure 4a,b) and downwind (Figure 4c,d) are reported as functions of the wind velocity and release flow rates using a water barrier (Figure 4a,c) and a reactive barrier composed of dilute HCl solution (Figure 4b,d). According to the physical model of the barrier, the air flow rate induced by the curtain ( and the air flow rate entrained by the curtain () were both nearly 0.45 kg s−1. The reactive system can be modeled as two in-series phenomena, i.e., absorption of the ammonia from the gaseous phase and ammonia reaction within the liquid phase. This in-series system implies that only the absorbed ammonia is involved in the reaction, which, consuming this reagent, increases the driving force between the gaseous phase and the liquid phase, promoting further absorption of ammonia.
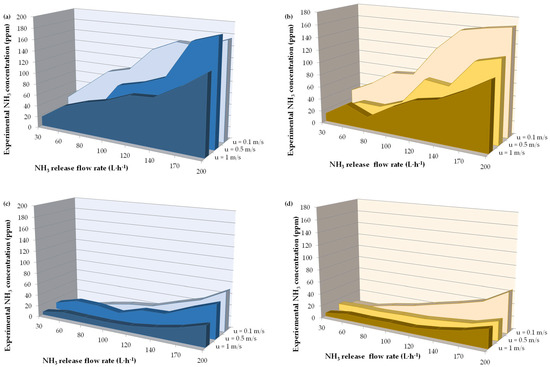
Figure 4.
HCl curtains reduce downwind concentration at high release rate. Experimental NH3 concentrations as a function of wind velocities (u, m/s) and flow rates of NH3 release under the following conditions: (a) upwind with a water barrier configuration, (b) upwind with a HCl solution (HCl concentration = 0.55 kmol m−3) barrier configuration, (c) downwind with a water barrier configuration, (d) downwind with a HCl solution (HCl concentration = 0.55 kmol m−3) barrier configuration.
In this study, only scenarios involving low wind velocities were experimentally analyzed, representing the most hazardous scenario due to the week compound atmospheric diffusion characterizing stable conditions. Conditions of still air were not considered since they represent an uncommon situation, especially in port areas where NH3 is gaining greater appeal for use as an energy vector and alternative maritime fuel. However, wind absence implies a higher concentration in the release point proximity, while it can represent an underestimation of risk for targets at longer distances. Table 3 reports the results obtained by the application of the model for the cases of pure physical and physical-chemical absorption.

Table 3.
Application of the model: physical absorption and physical–chemical absorption.
The whole experimental dataset was utilized as input for a scatter plot comparing the downwind ammonia concentrations ωd obtained at the different operating conditions utilized, as shown in Figure 5, where the y-axis shows the experimentally measured values and the x-axis displays the corresponding simulation results under the various tested conditions. Considering the whole range of performed runs, pertaining to the water barrier configuration shown in Figure 5a, it resulted R2 = 0.878, while for the reacting HCl solution barrier of Figure 5b it resulted R2 = 0.871, showing a fairly good agreement between theoretical prediction and experimental data, even though it should be noted that some tested conditions were affected by a significant error.
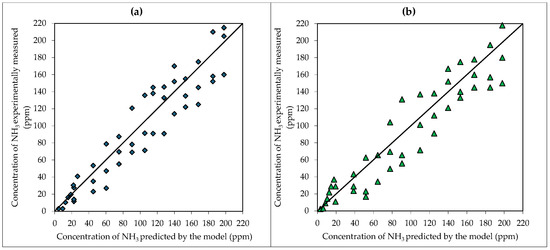
Figure 5.
Correlation between data predicted by the model and experimental data about NH3 concentration with (a) a water barrier and (b) a reacting HCl solution barrier. Solid line represents perfect agreement.
The reactive curtain provided a significant mitigation effect (∆X = 29%; ∆ηabs = 23%) but, to enhance the absorption rate, HCl concentration must exceed the stoichiometric ratio, with value of the order of 2% w/w. Comparing the results with those reported in Palazzi et al. [50,51], it should be noted that higher single pass efficiency (67%) and higher absorption efficiency enhancement (46%) were achieved in the case of chlorine release mitigation by adopting an alkaline reacting curtain. However, the chemical absorption of chlorine in NaOH solution occurred by a non-reversible and instantaneous reaction, while the mechanism of the reaction appears rather different for NH3, as shown by the negligible absorption enhancement obtained in runs carried out with 0.5 and 1% (w/w) HCl solutions. Whereas higher values of the HCl concentration could improve chemical absorption rate, on the other hand it could give rise to environmental problems connected to potential acid release into the atmosphere, as well as to technical and economic constraints due to potential corrosion phenomena on the equipment materials [65,66] and on the working safety device, related, for instance, to the solution viscosity and fouling. In addition, it was previously reported [67] that, even if water barriers are effective in diluting NH3 vapor cloud, downwind concentrations may still exceed toxic thresholds under specific conditions. Therefore, a compromise can be obtained in improving the downwind mitigation by employing a reacting curtain composed of a 2% w/w HCl water solution, suitable to increase the NH3 dissolution with a negligible additional environmental and economic impact. An alternative solution could consider other organic acids with a lower extent of potential equipment corrosion [68] and environmental impact, but their effective performances on NH3 abatement should be evaluated in depth evaluated. Moreover, the application of some plant-based corrosion inhibitors in the circulating solution may be considered as an option [69,70,71,72]. Comparing the performance with a previous study involving the reactive absorption of HCl by a reactive curtain [73], a higher impact of the reactive effect can be observed. Concerning HCl, its solubility in water is limited; thus, saturation occurs quickly [74,75]. When a reaction occurs in the water environment, HCl, as reagent involved in the reaction, is fast consumed, making it possible for chlorine to be further absorbed. For this reason, the efficiency of the reactive curtain in the case study involving chlorine is remarkably increased compared to the curtain working on physical absorption only. Conversely, ammonia shows a good solubility in water so the extent of the enhancement due to the reactive action has less impact on the overall efficiency when compared to the case involving chlorine.
5.2. Curtain Design and Operation
The containment effectiveness represents the essential requirement of a curtain addressing human health and environmental protection from toxic releases by acting as a mitigating barrier. The release can escape the obstacle represented by the curtain according to different mechanisms, such as lateral dispersion, direct horizontal crossing, and bypass. An effective curtain should minimize the above-mentioned phenomena. The reduction in lateral dispersion can be reasonably achieved by adequate arrangement of the curtain, also in connection with site and plant layout. The minimization of direct crossing requires designing ad hoc the mitigation device (nozzle type and pinch, liquid flow rate, etc.) and limiting its application to low–medium wind speed conditions (vw < critical velocity). The bypass by a toxic release can be controlled by adequately selecting the height of the curtain and its distance from possible obstacles near the release source. Furthermore, as the release overcoming was experimentally verified in several tests, this feature can be discussed further. Both under free and forced dispersion conditions, the overcoming is directly connected to the features of the windward recirculation zone, upstream of the curtain. The dimension of the vortex induced by the sprays is comparable to the curtain height. Even in the absence of convective transport from upwind region to downwind region (wind absence and complete geometric/dynamic symmetry of upwind and downwind regions), a limited transport of the released gas occurs above the curtain, due to turbulent diffusion. Under windy conditions, convective transport and turbulent diffusion also take place, becoming more and more relevant as wind speed increases. Nevertheless, the preliminary experimental phase evidenced that the overcoming effects are partially counterbalanced by different concurrent phenomena, in its turn of increasing importance as wind speed increases. First of all, a progressive vortex height reduction was verified in the upstream region, at height lower than h, and then a corresponding reduction in the toxic gas transported over the curtain. In addition, it was possible to experimentally verify the entrainment into the curtain of increasing amounts of the bypass flux, which improves the absorption efficiency of the safety device. Finally, the enhancement of the dilution effect, due to the wind increasing, reduces the bypass flow above the curtain, with respect to the overall flow. As a design rule of thumb, a water curtain more than twice the height of the gas cloud can be recommended to guarantee limited values of by-pass flow, in conditions of low and medium wind speed [76]. The experimental observations performed in the present wind tunnel study revealed that this condition is not completely effective in all tested situations. Consequently, in the presence of obstacles characterized by significant geometrical dimensions (e.g., storage tanks), the fluid-dynamic behavior of the upwind region can be significantly affected, so that the circulating vortex increases in size, far above the curtain height. It is then foreseeable that in this case, the toxic gas bypassing the barrier can increase by one order of magnitude. During the experimental phase, this phenomenon was verified in the configuration with curtain height corresponding to 0.61 and 0.67 m, where a deformation of the upwind vortex was observed. According to the condition tested in the wind tunnel, the transition from non-interaction to interaction, between obstacle and vortex, was verified in the range 1.30 < x/h < 1.45. Thus, as a conservative approach, for the attainment of negligible vortex interactions and to minimize the toxic release bypass, under situations characterized by wind speed lower than the critical one, the conditions summarized in Table 4 should be met; this will also work to enhance the reliability of the mitigation system to reduce risks. Due to the several simplifying hypotheses (summarized in Figure 3), the approach discussed here should be regarded as a short-cut design tool for a reacting barrier. As a further refinement, the model should take into account other aspects, including, e.g., high pressure release, scenarios with obstacles, etc., so the actual design requires a deep evaluation of the location of the protected target, the expected spill based on the storage volume, and the discharge rate, utilizing CFD modeling to increase precision and establish consequence for the scenario [77]. Further theoretical and experimental studies should be devoted to more refined approaches to addressing this challenge.

Table 4.
Optimal design values for the curtain under wind speed lower than the critical value.
Finally, it should be remarked that given the slightly corrosive character of the hydrochloric solution at the explored concentrations, the piping should be cleaned after an emergency activation, analogously to what is suggested for different reacting curtains based on inorganic salt solutions [78].
6. Conclusions
The main purpose of this paper is to analyze safety issues related to the utilization of ammonia as an energy vector and fuel, highlighting the new risks introduced by ammonia regarding public safety. The absorption and dispersion of accidental releases of NH3 in air was investigated in a wind tunnel to experimentally verify the accuracy of the model developed to describe the rate of air entrainment into the curtain, the dilution of the chlorine into the circulating air, and its physical and chemical absorption in the liquid phase. For this reason, different curtain configurations and environmental conditions were explored and the abatement of a wide range of ammonia releases was performed in the wind tunnel using a spray curtain, either with tap water or with hydrochloric acid solution feed. The experimental results indicated that the curtain mode accurately describes the abatement of ammonia release and showed that the use of a reactive solution may increase the efficiency of the ammonia release mitigation with potential environmental trade-offs, even though the percentage abatement enhancement is lower than the values obtained with gases characterized by low water solubility. To limit the drawbacks possibly connected to environmental impact, the adoption of alternative environmentally friendly curtain agents is currently under development.
Author Contributions
Conceptualization, B.F.; methodology, B.F.; software, F.C., M.P. and B.F.; validation, M.P., A.P.R. and B.F.; formal analysis, M.P. and F.C.; investigation, F.C. and M.P.; data curation, M.P., A.P.R. and B.F.; writing—original draft preparation, M.P., F.C. and B.F.; writing—review and editing, M.P., F.C., A.P.R. and B.F.; visualization, F.C. and A.P.R.; supervision, B.F., project administration, B.F. and M.P.; funding acquisition, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by the European Union—NextGenerationEU and by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.5, project “RAISE—Robotics and AI for Socio-economic Empowerment” (ECS00000035). Bruno Fabiano is part of RAISE Innovation Ecosystem.
Data Availability Statement
The raw data supporting the conclusions are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A | entrainment constant |
| CAES | compressed air energy storage |
| CHCl | concentration of the absorbing solution |
| CLAS | chemical looping ammonia synthesis |
| Da | diffusivity of gas in liquid |
| EDTA | ethylenediaminetetraacetic acid |
| GHG | greenhouse gases |
| h | curtain height |
| HB | Haber–Bosch process |
| hr | release height |
| HSE | Health and Safety Executive |
| kgp | mass transfer coefficient in the gas phase |
| L | curtain length |
| Ma | mean molar mass of the gas phase (NH3) |
| mass flow rate of the release in downwind immediacy by mixing effect with air | |
| absorption flow rate | |
| air flow rate entrained by the curtain | |
| air flow rate induced by the curtain | |
| ammonia release flow rate after curtain absorption | |
| curtain flow rate | |
| release flow rate | |
| N | number of nozzles |
| r | Pearson coefficient |
| SMR | steam reforming |
| SN | nozzle pitch |
| STEL | short-term exposure limit |
| T | temperature |
| tr | release duration |
| TWA | time-weighted average |
| v0 | spray exit velocity |
| vw | mean wind velocity |
| vw | wind velocity |
| WGS | water gas shift |
| Xg | intrinsic single-pass absorption efficiency of ammonia |
| δ | mean diameter of drops |
| ϕ | spray angle |
| ηabs | absorption efficiency |
| ηdil | dilution efficiency |
| ν∞ | terminal velocity of liquid phase |
| νj | spray velocity at the end of the jet phase |
| ρa | density of the gas phase |
| ρl | density of the liquid phase |
| ωd | concentration of released substance downwind the curtain |
References
- Mikulčić, H.; Baleta, J.; Klemeš, J.J.; Wang, X. Energy Transition and the Role of System Integration of the Energy, Water and Environmental Systems. J. Clean. Prod. 2021, 292, 126027. [Google Scholar] [CrossRef]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A Review of Trends and Drivers of Greenhouse Gas Emissions by Sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Marazziti, D.; Cianconi, P.; Mucci, F.; Foresi, L.; Chiarantini, I.; Della Vecchia, A. Climate Change, Environment Pollution, COVID-19 Pandemic and Mental Health. Sci. Total Environ. 2021, 773, 145182. [Google Scholar] [CrossRef]
- Pettinato, M.; Mukherjee, D.; Andreoli, S.; Minardi, E.R.; Calabro, V.; Curcio, S.; Chakraborty, S. Industrial Waste-an Economical Approach for Adsorption of Heavy Metals from Ground Water. Am. J. Eng. Appl. Sci. 2015, 8, 48–56. [Google Scholar] [CrossRef][Green Version]
- Biswas, B.; Qi, F.; Biswas, J.K.; Wijayawardena, A.; Khan, M.A.I.; Naidu, R. The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review. Soil Syst. 2018, 2, 51. [Google Scholar] [CrossRef]
- Jankowski, M.; Pałac, A.; Sornek, K.; Goryl, W.; Żołądek, M.; Homa, M.; Filipowicz, M. Status and Development Perspectives of the Compressed Air Energy Storage (CAES) Technologies—A Literature Review. Energies 2024, 17, 2064. [Google Scholar] [CrossRef]
- Javed, M.S.; Ma, T.; Jurasz, J.; Amin, M.Y. Solar and Wind Power Generation Systems with Pumped Hydro Storage: Review and Future Perspectives. Renew. Energy 2020, 148, 176–192. [Google Scholar] [CrossRef]
- Fortunato, M.; Reverberi, A.P.; Fabiano, B.; Cardinale, A.M. Thermal Evolution of NiFe-NO3 LDH and Its Application in Energy Storage Systems. Energies 2024, 17, 1035. [Google Scholar] [CrossRef]
- Mao, R.; Lei, Z.; Di, J.; Shang, Y.; Bai, R.; Yan, C. Composite Structural Battery: A Review. J. Electrochem. Energy Convers. Storage 2025, 22, 010801. [Google Scholar] [CrossRef]
- Pasman, H.; Sripaul, E.; Khan, F.; Fabiano, B. Energy Transition Technology Comes with New Process Safety Challenges and Risks—What Does It Mean? Process Saf. Prog. 2024, 43, 226–230. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in Energy Transition: A Review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Zanobetti, F.; Pio, G.; Jafarzadeh, S.; Ortiz, M.M.; Cozzani, V. Inherent Safety of Clean Fuels for Maritime Transport. Process Saf. Environ. Prot. 2023, 174, 1044–1055. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 20, ISBN 0123456789. [Google Scholar]
- Bassani, A.; Vianello, C.; Mocellin, P.; Dell’Angelo, A.; Spigno, G.; Fabiano, B.; Maschio, G.; Manenti, F. Aprioristic Integration of Process Operations and Risk Analysis: Definition of the Weighted F&EI-Based Concept and Application to AG2S Technology. Ind. Eng. Chem. Res. 2023, 62, 500–510. [Google Scholar] [CrossRef]
- Sage, V.; Patel, J.; Hazewinkel, P.; Yasin, Q.U.A.; Wang, F.; Yang, Y.; Kozielski, K.; Li, C. Recent Progress and Techno-Economic Analysis of Liquid Organic Hydrogen Carriers for Australian Renewable Energy Export—A Critical Review. Int. J. Hydrogen Energy 2024, 56, 1419–1434. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen Storage Materials for Hydrogen and Energy Carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue Hydrogen Production from Natural Gas Reservoirs: A Review of Application and Feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Clematis, D.; Bellotti, D.; Rivarolo, M.; Magistri, L.; Barbucci, A. Hydrogen Carriers: Scientific Limits and Challenges for the Supply Chain, and Key Factors for Techno-Economic Analysis. Energies 2023, 15, 6035. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A Review on Ammonia, Ammonia-Hydrogen and Ammonia-Methane Fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using Ammonia as a Sustainable Fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Faria, J.A. Renaissance of Ammonia Synthesis for Sustainable Production of Energy and Fertilizers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100466. [Google Scholar] [CrossRef]
- Griffiths, R.F.; Kaiser, G.D. Production of Dense Gas Mixtures from Ammonia Releases—A Review. J. Hazard. Mater. 1982, 6, 197–212. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an Energy Vector: Current and Future Prospects for Low-Carbon Fuel Applications in Internal Combustion Engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Wang, M.; Khan, M.A.; Mohsin, I.; Wicks, J.; Ip, A.H.; Sumon, K.Z.; Dinh, C.T.; Sargent, E.H.; Gates, I.D.; Kibria, M.G. Can Sustainable Ammonia Synthesis Pathways Compete with Fossil-Fuel Based Haber-Bosch Processes? Energy Environ. Sci. 2021, 14, 2535–2548. [Google Scholar] [CrossRef]
- Tan, W.; Lv, D.; Guo, X.; Du, H.; Liu, L.; Wang, Y. Accident Consequence Calculation of Ammonia Dispersion in Factory Area. J. Loss Prev. Process Ind. 2020, 67, 104271. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Ponte, M.; Arena, U. Design of Mitigation Systems for Indoor and Outdoor Ammonia Releases. J. Loss Prev. Process Ind. 2003, 16, 93–101. [Google Scholar] [CrossRef]
- Palazzi, E.; Currò, F.; Fabiano, B. Low Rate Releases of Hazardous Light Gases under Semi-Confined Geometry: A Consequence Based Approach and Case-Study Application. J. Loss Prev. Process Ind. 2020, 63, 104038. [Google Scholar] [CrossRef]
- Driscoll, H.; Salmon, N.; Bañares-Alcántara, R. Technoeconomic Evaluation of Offshore Green Ammonia Production Using Tidal and Wind Energy: A Case Study. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 7222–7244. [Google Scholar] [CrossRef]
- Van Duc Long, N.; Pourali, N.; Lamichhane, P.; Mohsen Sarafraz, M.; Nghiep Tran, N.; Rebrov, E.; Kim, H.H.; Hessel, V. Catalytic Ammonia Formation in a Microreaction Chamber with Electrically Intensified Arc Plasma. ChemCatChem 2024, 16, e202400005. [Google Scholar] [CrossRef]
- Shahed Gharahshiran, V.; Zheng, Y. Sustainable Ammonia Synthesis: An in-Depth Review of Non-Thermal Plasma Technologies. J. Energy Chem. 2024, 96, 1–38. [Google Scholar] [CrossRef]
- Xu, H.; Ithisuphalap, K.; Li, Y.; Mukherjee, S.; Lattimer, J.; Soloveichik, G.; Wu, G. Electrochemical Ammonia Synthesis through N2 and H2O under Ambient Conditions: Theory, Practices, and Challenges for Catalysts and Electrolytes. Nano Energy 2020, 69, 104469. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S. Recent Advances in Ammonia Synthesis Technologies: Toward Future Zero Carbon Emissions. Int. J. Hydrogen Energy 2023, 48, 11237–11273. [Google Scholar] [CrossRef]
- Brown, S.; Hu, J. Review of Chemical Looping Ammonia Synthesis Materials. Chem. Eng. Sci. 2023, 280, 119063. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Ammonia as a Carbon-Free Energy Carrier: NH3 Cracking to H2. Ind. Eng. Chem. Res. 2023, 62, 10813–10827. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; De Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Chen, X.; Guivarch, T.; Lulic, H.; Hasse, C.; Chen, Z.; Ferraro, F.; Scholtissek, A. Evaluation of Hydrogen/Ammonia Substitute Fuel Mixtures for Methane: Effect of Differential Diffusion. Int. J. Hydrogen Energy 2024, 69, 1056–1068. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sorrentino, G.; Ragucci, R.; de Joannon, M.; Sabia, P. Ammonia/Methane Combustion: Stability and NOx Emissions. Combust. Flame 2022, 241, 112071. [Google Scholar] [CrossRef]
- Mong, G.R.; Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Tran, M.V.; Lee, K.M.; Samiran, N.A.; Wong, K.Y.; Valera-Medina, A. Fuel-Lean Ammonia/Biogas Combustion Characteristics under the Reacting Swirl Flow Conditions. Fuel 2023, 331, 125983. [Google Scholar] [CrossRef]
- Xu, L.; Chang, Y.; Treacy, M.; Zhou, Y.; Jia, M.; Bai, X.S. A Skeletal Chemical Kinetic Mechanism for Ammonia/n-Heptane Combustion. Fuel 2023, 331, 125830. [Google Scholar] [CrossRef]
- Guan, W.; Abdelsamie, A.; Chi, C.; He, Z.; Thévenin, D. A Dedicated Reduced Kinetic Model for Ammonia/Dimethyl-Ether Turbulent Premixed Flames. Combust. Flame 2023, 257, 113002. [Google Scholar] [CrossRef]
- Jayabal, R. Ammonia as a Potential Green Dual Fuel in Diesel Engines: A Review. Process Saf. Environ. Prot. 2024, 188, 1346–1354. [Google Scholar] [CrossRef]
- American Bureau of Shipping (ABS). Ammonia Bunkering: Technical and Operational Advisory; American Bureau of Shipping: Spring, TX, USA, 2024. [Google Scholar]
- Abubakirov, R.; Yang, M.; Scarponi, G.E.; Moreno, V.C.; Reniers, G. Towards Risk-Informed Design and Operation of Ammonia-Powered Ships: Critical Aspects and Prospective Solutions. Ocean. Eng. 2024, 314, 119753. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green Ammonia as a Spatial Energy Vector: A Review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Schoten, H.H.; Molag, M.; Duffield, J.S.; Powell-Price, M. Use of Fluid Curtains for Post-Release Mitigation of Gas Dispersion. Inst. Chem. Eng. Symp. Ser. 2000, 147, 287–298. [Google Scholar]
- Yildirim, Ö.; Kiss, A.A.; Hüser, N.; Leßmann, K.; Kenig, E.Y. Reactive Absorption in Chemical Process Industry: A Review on Current Activities. Chem. Eng. J. 2012, 213, 371–391. [Google Scholar] [CrossRef]
- Palazzi, E.; Curro, F.; Fabiano, B. Mathematical Modeling of Fluid Spray Curtains for Mitigation of Accidental Releases. Chem. Eng. Commun. 2007, 194, 446–463. [Google Scholar] [CrossRef]
- Palazzi, E.; Curro, F.; Fabiano, B. N-Compartment Mathematical Model for Transient Evaluation of Fluid Curtains in Mitigating Chlorine Releases. J. Loss Prev. Process Ind. 2007, 20, 135–143. [Google Scholar] [CrossRef]
- Palazzi, E.; Curro’, F.; Pastorino, R.; Fabiano, B. Effectiveness of Reacting Spray Curtains Mitigating Toxic Releases of High Solubility Gases. In Chemical Engineering Transactions 11, Proceedings of the 8th International Conference on Chemical & Process Engineering, Ischia (NA), Italy, 24-27 June 2007; Pierucci, S., Ed.; AIDIC: Milano, Italy, 2007; pp. 407–412. [Google Scholar]
- Crolius, S.; Pugh, D.; Morris, S.; Velera-Medina, A. Safety Aspects. Techno-Economic Challenges of Green Ammonia as an Energy Vector; Elsevier Inc.: London, UK, 2021. [Google Scholar]
- Tan, W.; Du, H.; Liu, L.; Su, T.; Liu, X. Experimental and Numerical Study of Ammonia Leakage and Dispersion in a Food Factory. J Loss Prev Process Ind 2017, 47, 129–139. [Google Scholar] [CrossRef]
- European Major Accident Hazards Bureau. 2023. Available online: https://emars.jrc.ec.europa.eu/en/emars/accident/search (accessed on 17 June 2025).
- Institution of Chemical Engineers. 2000. Available online: https://www.icheme.org/knowledge-networks/knowledge-resources/safety-centre/resources/accident-data/ (accessed on 17 June 2025).
- Liang, Y.; Xiang, Q. Occupational Health Services in PR China. Toxicology 2004, 198, 45–54. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, Y.; Xu, K. Study on the Regularity of Ammonia-Related Refrigeration Accidents in China from 2010 to 2020. Int. J. Environ. Res. Public Health 2022, 19, 8230. [Google Scholar] [CrossRef] [PubMed]
- Special Report. Burns, Blindness and Agonising Deaths: Is It Safe to Ship Hydrogen-Derived Ammonia Around the World? Recharge. Available online: https://www.rechargenews.com/energy-transition/special-report-burns-blindness-and-agonising-deaths-is-it-safe-to-ship-hydrogen-derived-ammonia-around-the-world-/2-1-1267513 (accessed on 17 April 2025).
- International Maritime Organization Resolution MSC.391(95) (Adopted on 11 June 2015) Adoption of the International Code of Safety for Ships Using Gases or Other Low-Flashpoint Fuels (IGF Code). 2015. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MSCResolutions/MSC.391(95).pdf (accessed on 17 June 2025).
- International Maritime Organization Interim Guidelines for the Safety of Ships Using Ammonia as Fuel. 2025. Available online: https://www.bimco.org/media/bxvcygg1/msc1-circ1687-interim-guidelines-for-the-safety-of-ships-using-ammonia-as-fuel-secretariat.pdf (accessed on 17 June 2025).
- International Maritime Organization Amendments to the International Code for the Construction and Equipment of Ships Carrying Dangerous Chemicals in Bulk (IBC Code). 2004. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MEPCDocuments/MEPC.119(52).pdf (accessed on 17 June 2025).
- International Maritime Organization Resolution MSC.370(93) (Adopted on 22 May 2014) Amendments to the International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (IGC Code). 2014. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MSCResolutions/MSC.370(93).pdf (accessed on 17 June 2025).
- Ng, C.K.L.; Liu, M.; Lam, J.S.L.; Yang, M. Accidental Release of Ammonia during Ammonia Bunkering: Dispersion Behaviour under the Influence of Operational and Weather Conditions in Singapore. J. Hazard. Mater. 2023, 452, 131281. [Google Scholar] [CrossRef] [PubMed]
- Foust, A.S. Principles of Unit Operations, 2nd ed.; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Azim, A.A.A.; Sanad, S.H. Effects of Acid Concentration, C-Content and Temperature on the Corrosion Rate of Steel in HCI; Pergamon Press: Oxford, UK, 1972; Volume 12. [Google Scholar]
- Noor, E.A.; Al-Moubaraki, A.H. Corrosion Behavior of Mild Steel in Hydrochloric Acid Solutions. Int. J. Electrochem. Sci. 2008, 3, 806–818. [Google Scholar] [CrossRef]
- Dandrieux, A.; Dusserre, G.; Ollivier, J.; Fournet, H. Effectiveness of Water Curtains to Protect Firemen in Case of an Accidental Release of Ammonia: Comparison of the Effectiveness for Two Different Release Rates of Ammonia. J. Loss Prev. Process Ind. 2001, 14, 349–355. [Google Scholar] [CrossRef]
- Abbas, A.; Adesina, A.Y.; Suleiman, R.K. Influence of Organic Acids and Related Organic Compounds on Corrosion Behavior of Stainless Steel—A Critical Review. Metals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Medupin, R.O.; Ukoba, K.O.; Yoro, K.O.; Jen, T.C. Sustainable Approach for Corrosion Control in Mild Steel Using Plant-Based Inhibitors: A Review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Parangusan, H.; Sliem, M.H.; Abdullah, A.M.; Elhaddad, M.; Al-Thani, N.; Bhadra, J. Plant Extract as Green Corrosion Inhibitors for Carbon Steel Substrate in Different Environments: A Systematic Review. Int. J. Electrochem. Sci. 2025, 20, 100919. [Google Scholar] [CrossRef]
- Casanova, L.; Ceriani, F.; Messinese, E.; Paterlini, L.; Beretta, S.; Bolzoni, F.M.; Brenna, A.; Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Recent Advances in the Use of Green Corrosion Inhibitors to Prevent Chloride-Induced Corrosion in Reinforced Concrete. Materials 2023, 16, 7462. [Google Scholar] [CrossRef]
- Sabiha, M.; Kerroum, Y.; El Hawary, M.; Boudalia, M.; Bellaouchou, A.; Hammani, O.; Amin, H.M.A. Investigating the Adsorption and Corrosion Protection Efficacy and Mechanism of Marjoram Extract on Mild Steel in HCl Medium. Molecules 2025, 30, 272. [Google Scholar] [CrossRef]
- Fabiano, B.; Currò, F.; Reverberi, A.; Palazzi, E. Generalized Mathematical Modelling of Spray Barriers. Chem. Eng. J. 2019, 377, 120108. [Google Scholar] [CrossRef]
- Roscoe, H.E.; Dittimar, W. On the Absorption of Hydrochloric Acid and Ammonia in Water. Q. J. Chem. Soc. Lond. 1860, 12, 128–151. [Google Scholar] [CrossRef]
- Kojima, Y. Safety of Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2024, 50, 732–739. [Google Scholar] [CrossRef]
- Buchlin, J.M. Mitigation of Problem Clouds. J. Loss Prev. Process Ind. 1994, 7, 167–174. [Google Scholar] [CrossRef]
- Min, D.S.; Choi, S.; Oh, E.Y.; Lee, J.; Lee, C.G.; Choi, K.Y.; Jung, S. Numerical Modelling for Effect of Water Curtain in Mitigating Toxic Gas Release. J. Loss Prev. Process Ind. 2020, 63, 103972. [Google Scholar] [CrossRef]
- Hua, M.; Shen, X.; Zhang, J.; Pan, X. Protective Water Curtain Ammonia Absorption Efficiency Enhancement by Inorganic and Surfactant Additives. Process Saf. Environ. Prot. 2018, 116, 737–744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).