Abstract
Piston engines used for powering automobiles as well as machinery and equipment have traditionally relied on petroleum-derived fuels. Subsequently, renewable fuels began to be used in an effort to reduce the combustion of hydrocarbon-based fuels and the associated greenhouse effect. Researchers are currently developing technologies aimed at eliminating fuels containing carbon in their molecular structure, which would effectively minimize the emission of carbon oxides into the atmosphere. Ammonia is considered a highly promising carbon-free fuel with broad applicability in energy systems. It serves as an excellent hydrogen carrier (NH3), free from many of the storage and transportation limitations associated with pure hydrogen. Safety concerns regarding the storage and transport of hydrogen make ammonia an increasingly important fuel also due to its larger hydrogen storage capacity. This manuscript investigates the use of ammonia for powering a dual-fuel engine. The results indicate that the addition of ammonia improves engine performance; however, it may also lead to an increase in NOx emissions. Due to the limitations of ammonia as a fuel, approximately 40% of the energy input must still be provided by diesel fuel to achieve optimal engine performance and acceptable NOx emission levels. The presented research findings highlight the significant potential of NH3 as an alternative fuel for compression-ignition engines. Proper control of the injection strategy or the adoption of alternative combustion systems may offer a promising approach to reducing greenhouse gas emissions while maintaining satisfactory engine performance parameters.
1. Introduction
We are in times of intensive energy transformation driven by concerns about air quality. Climate change, caused by excessive CO2 emissions, contributes to numerous problems such as droughts and floods in areas where they were rare, as well as changes in flora [1]. Legal regulations enforce the reduction of fossil fuel use in favor of renewable energy sources. Internal combustion engines, due to their high efficiency in converting chemical energy from fuel into mechanical energy (for large industrial engines, it is over 50%), are still widely used in transportation, machinery propulsion, and distributed energy systems [2,3]. Decarbonization of IC engines can be achieved by using carbon-free fuels. For many years, renewable fuels such as biodiesel, alcohol fuels, biogases, and hydrogen have been used to power piston engines [4,5,6,7]. Among these fuels, only hydrogen is a clean fuel because it does not contain carbon and, thus, does not contribute to CO2 emissions. It is important to note the technology used to produce hydrogen, as it may contribute to CO2 emissions even though the hydrogen itself does not. Only hydrogen produced using renewable energy sources such as solar or wind energy can be considered truly clean [8,9,10]. There are regions in the world where renewable energy can be easily obtained and used to power hydrogen production facilities. Unfortunately, these regions are often far from the target locations where the hydrogen is needed. Thus, the hydrogen must be stored and transported to the target locations by creating a distribution network. The primary technology for producing hydrogen is the electrolysis of water. This process is quite energy-intensive but yields the purest hydrogen [11]. Hydrogen is difficult to store and transport due to its low liquefaction temperature (−240.18 °C), low density, wide flammability limits in air, and tendency to leak through container walls [12]. Storing or transporting hydrogen in liquid form requires expensive and energy-intensive cryogenic technologies. These characteristics significantly limit the widespread use of hydrogen. In recent years, attention has turned to ammonia (NH3) as a hydrogen carrier with high energy density [13]. The volumetric energy density of ammonia compressed to 1 MPa is 13.6 GJ/m3, higher than that of methane compressed to 25 MPa (10.4 GJ/m3). The hydrogen content per unit mass in liquid ammonia is over four times greater than in hydrogen stored under pressure in the form of hydrides. Ammonia can be compressed and liquefied at much lower parameters than hydrogen. Ammonia liquefies at −33 °C. Increasing its pressure by about 10 times allows it to liquefy at room temperature. Ammonia production technology and distribution networks are well-developed worldwide. A disadvantage of using NH3 as a fuel is its toxic properties and its corrosive effect on various construction materials [14]. Its main benefit lies in being a zero-carbon fuel, which allows it to be categorized as green ammonia. Figure 1 presents the main advantages of ammonia as a fuel.
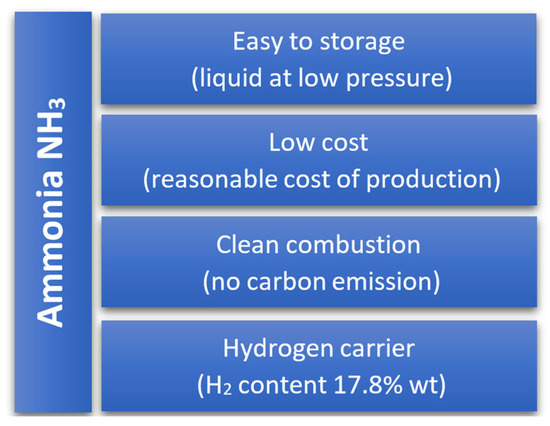
Figure 1.
Properties of ammonia as a fuel.
Ammonia is composed of one nitrogen atom and three hydrogen atoms, forming a molecule with a trigonal pyramidal geometry. It is a colorless gas with a characteristic sharp odor. The density of ammonia under normal conditions is 0.73 kg/m3, its calorific value is 18.8 MJ/kg, and its auto-ignition temperature is 651 °C. The global production of ammonia is steadily increasing, with 230 million tons produced in 2018, 240.38 million tons in 2023, and an estimated production of nearly 290 million tons by 2030 [15,16]. In Poland, approximately 2.6 million tons of ammonia is produced annually. Poland is the third-largest producer of ammonia in Europe. This provides a strong foundation for the development of technologies based on this chemical compound, which is regarded as a promising energy source for applications in the energy sector. Traditionally, the primary consumer of ammonia has been the chemical industry, but recently, the broader energy sector has increasingly seen ammonia as a promising energy storage medium.
According to Kojima [17], three types of efficient hydrogen energy carriers are currently being considered in the energy sector: ammonia, liquid hydrogen, and liquid organic hydrogen carriers (LOHCs) such as methylcyclohexane, methanol, formic acid, and formaldehyde. Among these, ammonia exhibits the highest volumetric hydrogen density, amounting to 12.1 kg H2/100 L at 240 K. The volumetric energy density of ammonia storage tanks is approximately twice as high as that of liquid hydrogen tanks. Because of its comparatively high boiling point of 240 K at 0.1 MPa, ammonia can be stored over long periods. Moreover, the cost of converting gaseous hydrogen into ammonia is lower compared to both the liquefaction of hydrogen and the conversion of hydrogen into LOHCs. According to Dinesh and Kumar [18], ammonia is more readily available and offers simpler storage and transport compared to hydrogen, primarily due to the already established infrastructure. Ammonia occupies only 30% of the storage volume and necessitates a storage pressure that is 87.5 times lower compared to hydrogen. As a consequence, ammonia offers notably lower storage and transport expenses. The main challenges associated with the use of ammonia in internal combustion engines are primarily its high ignition temperature and low combustion velocity, which hinder effective ignition and stable combustion. These factors lead to reduced engine efficiency, especially under low load conditions. Additionally, the combustion of ammonia is accompanied by elevated NOx emissions. Improvements in ignition and combustion can be achieved through the addition of highly reactive fuels such as hydrogen [19,20], increasing the engine’s compression ratio [21], and the use of intake air preheating systems [22]. To reduce NOx emissions, established emission control technologies such as SCR [23] or EGR [24] may be employed. Another practical limitation in the application of ammonia is related to its storage and transport. Ammonia is stored in liquid form under a pressure of approximately 10 bar, and due to its toxic and irritating nature, it requires special procedures for storage, transportation, and refueling, including sealed tanks, leak detection sensors, and neutralization systems. Furthermore, ammonia exhibits corrosive effects on certain engine components, necessitating the use of special alloys and protective coatings, particularly in the fuel and exhaust systems [25].
Both spark-ignition [26,27,28] and compression-ignition engines can operate using ammonia as a fuel [29,30,31]. In a spark-ignition engine powered solely by NH3, a large ignition energy and a high compression ratio are required. To eliminate this limitation, hydrogen can be used as an additive to facilitate the combustion process, improve flame propagation speed, and reduce the ignition energy requirements [32,33,34]. A beneficial application of ammonia is its combustion in a compression-ignition engine alongside diesel or biodiesel. The injected pilot dose of diesel fuel allows for efficient ignition and control of the combustion process [35,36,37].
This paper reviews and evaluates the use of ammonia as a fuel for powering compression-ignition piston engines. The growing interest in this fuel, manifested in a large number of published scientific works and information on applications in transportation and industry, motivates a summary and focus on both the advantages and disadvantages of these applications. The presented case studies often provide different perspectives on the issue of ammonia-fueled engines in terms of both engine performance and GHG emissions.
2. Ammonia as a Fuel for Piston Engines
Internal combustion engines, both spark-ignition (SI) and compression-ignition (CI), convert the chemical energy contained in the fuel into mechanical energy. The oxidation of the fuel occurs in the engine cylinder. Fuel delivery to the engine is executed in various ways depending on the combustion system employed. The method of fuel delivery is also influenced by its physical properties such as density, viscosity, liquid or gaseous phase, and energy density. To release the chemical energy from the fuel, combustion initiation is required, either by a spark in SI engines or by auto-ignition in CI engines. In a CI engine, it is necessary to achieve a sufficiently high temperature in the cylinder at the end of the compression stroke for auto-ignition to occur. Engine fuels have widely varying auto-ignition temperatures, which can sometimes pose challenges for their application in CI engines. The greatest challenge facing compression-ignition engines is meeting stringent exhaust emission standards (Euro 6 and 7) concerning nitrogen oxides and particulate matter, as well as carbon monoxide and carbon dioxide emissions. Meeting emissions standards often requires costly exhaust aftertreatment systems, such as SCR, diesel particulate filters, and EGR systems. However, this approach is associated with increased production and maintenance costs. The basic properties of fuels are presented in Table 1.

Table 1.
Fuel specifications [35,38,39,40].
In a CI engine, liquid fuel is delivered through direct injection into the cylinder, where it undergoes various physico-chemical processes preparing it for auto-ignition. The primary fuel is, of course, diesel, which is a hydrocarbon. In the era of decarbonization, extensive research and development efforts are being made to eliminate carbon-based fuels. In addressing the concept of decarbonizing transport and various industrial sectors, achieving climate neutrality requires implementing solutions that regulate CO2 emissions. Piston engines operate based on the combustion of fuels. Fuels containing carbon in their molecular structure inevitably contribute to the emission of carbon oxides. Replacing carbon-based fuels with carbon-free alternatives will reduce these emissions but does not fully resolve all the emission challenges faced by compression-ignition engines. Despite technological advancements—such as advanced injection systems and turbocharging—CI engines still struggle to completely eliminate NOx and particulate matter emissions under real-world operating conditions. Ammonia is considered a potential substitute for hydrocarbon fuels. It does not contain carbon and has a relatively high calorific value (18.8 MJ/kg). A significant feature of ammonia is its low oxygen requirement; the stoichiometric air–fuel ratio is three times lower than for diesel fuel.
However, ammonia cannot be the sole fuel for CI engines due to its limitations in auto-ignition, such as its high auto-ignition temperature (651 °C), low cetane number (CN), and high heat of vaporization. This does not mean that ammonia cannot be a good fuel for CI engines. There are many advanced combustion technologies for fuels with challenging properties. RCCI, HCCI, and dual-fuel engine combustion systems can be utilized here [41,42,43,44].
These combustion systems allow the application of fuels that are not compatible with conventional CI engine technology and generally provide better operational performance and reduced exhaust emissions. HCCI technology ensures ignition throughout the entire combustion chamber volume, which accelerates the combustion process of the air–fuel mixture, ensures high combustion efficiency, and, thus, results in exhaust gases free of many harmful components [45,46,47]. Combustion efficiency is expressed as the ratio of the thermal energy actually obtained from the burned fuel to the chemical energy contained in that fuel. In a typical internal combustion engine, combustion efficiency ranges from 95% to 99%, meaning that almost the entire fuel is combusted. In an HCCI engine, the fuel–air mixture combusts simultaneously throughout the entire cylinder volume, approaching an ideal Otto cycle. Multiple ignition points within the combustion chamber promote nearly complete fuel burn, resulting in a combustion efficiency exceeding 99%. In the RCCI combustion system, two fuels with different reactivity’s are mixed. The low-reactivity fuel forms a homogeneous mixture, and ignition is controlled by the injection of the high-reactivity fuel [48,49]. The simplest form of combustion for so-called challenging fuels in CI engines is dual-fuel technology. Fuel that does not auto-ignite is delivered either into the intake manifold during the intake stroke or directly injected into the cylinder, allowing enough time for thorough mixing with air. This mixture is ignited by the injection of a pilot dose of, for example, diesel fuel [50,51]. These combustion systems can be used for burning ammonia. Ammonia exhibits a low tendency to ignite and is characterized by very slow combustion kinetics. Its application in piston engines is, therefore, challenging, as it requires high compression ratios to generate the elevated pressure and temperature within the cylinder necessary for effective ignition and combustion. According to Hu et al. [21], ammonia combustion can be significantly enhanced at increased intake temperatures and pressures as well as higher compression ratios, resulting in higher peak pressures, accelerated flame propagation, and reduced time of combustion. Under such conditions, the engine’s indicated power output can be enhanced.
Ammonia is characterized by a low laminar flame speed (LFS 0.07 m/s at ER 1.1). Laminar burning velocity is the speed at which an undisturbed premixed fuel–air mixture propagates relative to a stationary flame front under laminar flow conditions, typically expressed in meters per second (m/s). It is a characteristic property of a given fuel–air mixture and depends on factors such as fuel type, equivalence ratio, initial temperature, and pressure of the mixture. Laminar burning velocity is measured under conditions where the flame is not distorted by turbulence, vortices, or other flow disturbances. As shown in Figure 2, like other fuels, LFS depends on the air–fuel ratio and reaches its maximum for slightly rich mixtures. As shown in Figure 2, the addition of hydrogen can increase the combustion speed, which is often used in engine applications.
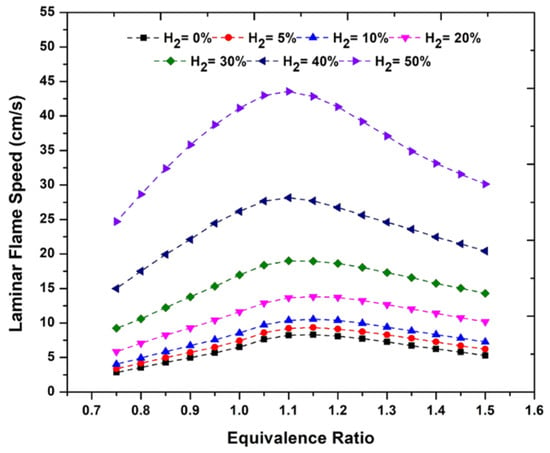
Figure 2.
LFS of ammonia and ammonia–hydrogen mixture as a function of equivalence ratio (at 1 atm and 298 K) [52].
The addition of H2 is a factor that significantly affects the LFS of the mixture with NH3. It can be seen here that with the increase of the H2 share in the mixture, the influence of the excess air factor on the LFS increases. The effect presented in Figure 2 of enriching the NH3–air mixture with hydrogen at 1 atm pressure and 298 K temperature indicates that as the hydrogen fraction increases, the flame speed rises significantly but nonlinearly. This is due to the higher reactivity and flame speed of H2 compared to NH3. With increasing hydrogen content, the share of NH3 decreases, and hydrogen begins to play the dominant role in the combustion process. Shah et al. [52] recommend avoiding excessively high hydrogen concentrations due to the risk of flame flashback. The peak burning velocity is observed at an equivalence ratio (ER) ranging from 1.1 to 1.15. For hydrogen contents of 40% and 50%, the burning velocities are approximately 29 cm/s and 44 cm/s, respectively. Under these conditions, the LFS of methane is around 37 cm/s. In summary, to achieve combustion characteristics similar to those of an engine fueled with CH4 at ER 1.1–1.15, it is suggested to use a mixture containing approximately 40% hydrogen. The high reactivity of hydrogen can promote ammonia ignition, and its high flame speed facilitates the fuel oxidation reactions, shortens combustion duration, decreases heat transfer to the cylinder walls, thereby improving engine efficiency. According to Dinesh and Kumar [18], pure ammonia is challenging to ignite and sustain combustion because of its low heating value, slow laminar flame speed, and high ignition temperature and energy. In contrast, hydrogen serves as an effective combustion promoter, improving engine performance and stability, particularly during the initial combustion phase.
The strong molecular diffusivity of hydrogen increases mixture homogeneity, which, in turn, positively affects combustion efficiency. Furthermore, hydrogen is a beneficial additive to ammonia as it mitigates cold start issues by improving the ignition and combustion of ammonia.
The burning velocity of the mixture must be considered in the engine control system. For high-speed engines, where the available combustion time is short, a high laminar flame speed is desirable. In contrast, for industrial low-speed engines, a lower LFS may be acceptable because the engine allows a longer combustion duration. The efficiency of combustion is also impacted by the shape of the combustion chamber and its aerodynamic properties, as well as the shape and penetration of the high-reactivity fuel jet used for ignition.
The reduced burning velocity characteristic of ammonia limits the flame front penetration into the combustion chamber space, which can result in unburned areas and lead to ammonia emissions [53,54]. The basic combustion reaction of ammonia in air is [55]
Therefore, theoretically, the resulting products from combustion of ammonia are H2O and N2.
In conditions of excess air, NH3 combustion takes place in the reaction
However, as research results show, an increased proportion of ammonia in the combustion process contributes to a reduction in combustion efficiency [56,57,58]. According to Tornatore et al. [20], compared to pure diesel, ammonia results in a decrease in combustion efficiency. The combustion mechanisms of NH3, as compared to diesel fuel, explain this tendency. Indeed, the diluted mixture conditions commonly found in compression-ignition engines hinder the propagation of the premixed flame, leading to decreased combustion efficiency. Experimental studies have shown that the combustion of pure ammonia results in a thermal efficiency that is 14% lower (24%) compared to that of pure diesel combustion (38%). A disadvantage of using ammonia as a fuel is its flammability limits in air; a minimum of 15% by volume of ammonia is required to create a flammable mixture. The minimum volumetric concentration of ammonia in an air mixture that enables ignition and combustion ranges between 15% and 28%. The corresponding range for hydrogen is 4.7% to 75%, for diesel fuel 0.46% to 0.6%, and for gasoline, 0.6% to 0.8%. The values corresponding to NH3, H2, and diesel fuel are shown in Table 1. Another characteristic of ammonia as a fuel is its high heat of vaporization, which is 1370 kJ/kg, more than 5.6 times greater than that of diesel fuel. Evaporating ammonia lowers the temperature of the fresh charge in the engine cylinder, which, on one hand, is advantageous as it directs the combustion process closer to the LTC concept. In contrast, it reduces the engine combustion chamber’s potential for autoignition. When using alternative fuels to power internal combustion engines, attention must be paid to their impact on greenhouse gas emissions (GHG). Ammonia (NH3), containing nitrogen in its molecular structure, interacts with the formation of NOx differently than hydrocarbon fuels. NOx formation in the engine is mainly controlled by two mechanisms, one of which is the thermal mechanism, known as the Zeldovich mechanism, which occurs where there is access to nitrogen, oxygen, and sufficiently high temperatures; the fuel mechanism is associated with the fuel composition [59,60]. When ammonia is the fuel, there is significant access to nitrogen, which intensifies the fuel mechanism of nitrogen oxide formation. In the dominant fuel–NO mechanism inside the engine cylinder, NH3 undergoes dehydrogenation to form NH2 radicals through reactions with OH, H, and O radicals, or via thermal decomposition [20,61]. At temperatures ranging from 450 to 925 K, nitrogen radicals NH2 react with oxidized forms (mainly HO2) to form H2NO, as described by the reaction [20]
NH2 + HO2 → H2NO + OH
Subsequently, nitroxyl radicals (HNO) are produced through the reaction
H2NO + O2 → HNO + HO2
In the final step of the fuel-NO mechanism, nitroxyl radicals (HNO) combine with oxygen (O2) to form nitrogen oxide (NO):
HNO + O2 → NO + HO2
Within the temperature range of 1100–1400 K, chemical reduction of NO (chemical de-NOx) occurs through interactions between NH2 radicals and NO, proceeding through two distinct reactions:
NH2 + NO → N2 + H2O
NH2 + NO → NNH + OH
Next, NNH breaks down into nitrogen and hydrogen through the reaction
NNH → N2 + H
Simultaneously, NNH combines with oxygen to form nitrogen oxide NO via the following reaction:
NNH + O → NH + NO
At temperatures exceeding 1400 K, some NH2 radicals undergo further dehydrogenation to form NH, which can reduce NO and lead to the formation of N2O via the following reactions:
NH + NO → N2O + H
NH + NO → N2 + OH
NH + NO → N + N + OH
These reactions occur most intensively under fuel-rich (pyrolytic) conditions and elevated pressure. At temperatures above 1600 K, the resulting N and N2 radicals further participate in the Zeldovich thermal–NO mechanism. During fuel combustion in the cylinder of a reciprocating engine, chemical reactions take place that result in the formation of NO. Once emitted with the exhaust gases, NO undergoes further oxidation to form NOx. The rate of NO formation is influenced by fuel properties, oxygen concentration (air–fuel equivalence ratio), cylinder temperature, and the residence time of the gas mixture in high-temperature regions [62,63,64].
In internal combustion engines, there are three main mechanisms of NO formation: the prompt–NO mechanism, the fuel–NO mechanism, and the thermal–NO mechanism (Zeldovich mechanism). Prompt–NO arises from the reaction of hydrocarbon radicals derived from the fuel with atmospheric nitrogen under fuel-rich conditions. Initially formed nitrogenous intermediates such as NH3 and HCN are subsequently oxidized to NO in the lean flame zone. Prompt–NO contributes minimally to the total NOx emissions. Fuel–NO results from the oxidation of nitrogen bound in the fuel and can be neglected for fuels that do not contain elemental nitrogen.
The predominant mechanism of NO formation in internal combustion engines is thermal–NO, in which nitric oxide is generated through the reaction of atmospheric nitrogen and oxygen at elevated temperatures. Because breaking the strong triple bond in the nitrogen molecule requires substantial energy, significant production of thermal NO occurs only at temperatures exceeding 1600 K. The Zeldovich mechanism involves reactions in which oxygen radicals (O) react with molecular nitrogen (N2), followed by nitrogen radicals (N) reacting with oxygen molecules (O2) to form NO. The amount of NO generated depends on the quantities of N2 and O2 present in the combustion environment as well as the combustion temperature.
The three fundamental reactions of the Zeldovich mechanism are as follows [20]:
N2 + O = N + NO
N + O2 = O + NO
N + OH = H + NO
Selective catalytic reduction, also known as ‘thermal de-NOx’ [65], is an effective and widely implemented method for NOx mitigation. SCR is an advanced active emission control technology that converts NOx into diatomic nitrogen and H2O in the presence of a catalyst. This conversion requires a reducing agent, typically either an ammonia solution or pure ammonia. The primary NOx reduction reactions occurring in SCR systems are as follows [66]:
4NH3 + 4NO + O2 → 4N2 + 6H2O
2NH3 + NO + NO2 → 2N2 + 3H2O
4NH3 + 3NO2 → 3.5N2 + 6H2O
Another effective strategy for reducing NOx emissions from internal combustion engines is EGR, which operates by reintroducing a portion of the exhaust gases into the engine’s intake stream. As noted by Farzam et al. [67], EGR reduces NOx formation through two primary mechanisms: First, it dilutes the intake charge by substituting a portion of fresh air with exhaust components such as CO2 and H2O, which possess a higher heat capacity. This increases the mixture’s ability to absorb combustion heat, thereby lowering the peak combustion temperature for a given fuel quantity. Second, EGR reduces the oxygen concentration in the combustion chamber—a key parameter in the formation of nitrogen oxides.
Taking into consideration alternative fuels, such as ammonia, one must also consider economic and safety aspects. The costs of fuel include not only its production but also transportation and storage. Due to its LHV, ammonia requires larger volumes to provide the same energy value as conventional fuels, thus increasing transportation and storage costs. However, compared to hydrogen, it is much cheaper to store over long periods and transport [68,69].
Ammonia is highly toxic to humans and other living organisms. Its toxicity is significantly higher than that of conventional fuels. It is an alkaline chemical compound that can lead to suffocation. The effects of human exposure to ammonia depend on its concentration and duration of exposure. At high concentrations, ammonia can cause burns to the respiratory tract and eyes. Its corrosive action can lead to skin burns, eye damage, and lung injury. Therefore, careful handling of ammonia is required, along with the use of equipment to monitor its concentration in environments where people are present [70,71].
3. Compression-Ignition Engine Powered by Ammonia
Ammonia as a fuel with properties that strongly limit its use as the sole fuel for compression ignition (CI) piston engines encourages the adoption of alternative combustion systems. The concept of dual-fuel engines has long been known and performs well for co-firing fuels with different properties [72,73]. This combustion technology is utilized for co-firing both liquid fuels [74,75] and gases [76,77]. It requires intervention in the engine’s fuel supply system and control system. The conventional CI engine fuel supply system, such as direct injection of diesel, biodiesel, or their blends, is used to deliver the igniting dose of the alternative fuel–air mixture filling the engine’s combustion chamber. Typically, the alternative fuel is introduced into the intake manifold during the engine’s intake [78,79], or in more advanced technologies, directly into the engine’s combustion chamber [80,81]. In solutions where the fuel is delivered to the intake manifold, the cylinder is filled with a fuel–air mixture, which may result in emissions of unburned fuel due to the crevice effect. The mixture during the compression stroke is pushed into crevices where combustion does not occur [82,83]. The dual-fuel injection system can mitigate the crevice effect. The properties of ammonia as a fuel encourage the use of dual-fuel technology for its combustion in piston engines. Hydrogen is most commonly used as an assisting fuel to enhance flame propagation in the combustion chamber, but there are also other proposals for partially combusted fuels with ammonia.
3.1. Ammonia in Dual-Fuel Engines
Due to the properties of ammonia outlined in Chapter 1, for a CI engine to operate solely on ammonia, it requires a very high compression ratio. Initial experiments with this fuel concept revealed that the engine needed a compression ratio of 35:1, and additionally, the engine had to be warmed up using another fuel source [84]. Attempts to replace fossil fuels with ammonia in CI engines, primarily driven by military applications, yielded unpromising results, and the literature on this subject remains limited. The requirement for very high compression ratios in CI engines for ammonia combustion deterred widespread adoption of this CI engine solution. Promising results were achieved using PFI for ammonia fueling, prompting focused development on this combustion technology. For dual-fuel technology, compression ratios as low as 15:1, similar to those for conventional CI engines, are sufficient, significantly influencing engine design. The compression ratio required for dual-fuel technology depends on the self-ignition properties of the directly injected fuel serving as the igniting dose into the combustion chamber. The most basic system for co-firing ammonia with diesel involves injecting ammonia into the intake manifold and igniting a dose of diesel fuel directly into the engine’s combustion chamber (Figure 3a).
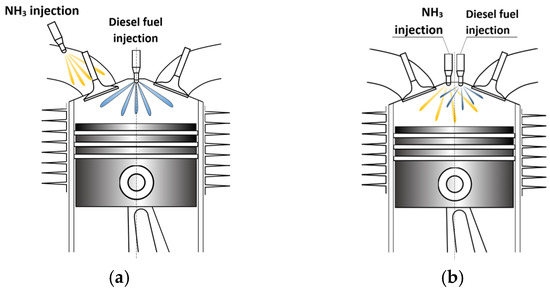
Figure 3.
Dual-fuel engine supply system with PFI (a) and DI (b) for ammonia.
In such a combustion system, ammonia serves as the primary fuel, providing energy for engine operation, while diesel acts merely as the ignition source. A more advanced dual-fuel engine supply system involves direct injection of both fuels (Figure 3b).
3.2. Engine Powered by Ammonia in Port Fuel Injection System
The most common design solution for a dual-fuel engine involves injecting the alternative fuel into the intake manifold during the cylinder filling stroke [85]. Port fuel injection is a low-pressure injection method suitable for both liquid and gaseous fuels. The ignition fuel dose is injected directly into the engine combustion chamber. This technique is also used for ammonia fueling in CI engines. Nadimi et al. [86] presented results on partial replacement of diesel oil with ammonia in a dual-fuel compression-ignition engine. They found that the alternative energy fraction (AEF) could reach up to 85% while maintaining engine performance parameters (ITE). Another significant finding was that AEF changes the combustion process character in the dual-fuel engine, decreasing diffusive combustion and favoring kinetic combustion with increasing AEF. Ignition delay decreased by 6.8 degrees and combustion duration shortened by 32 degrees. Regarding greenhouse gas emissions (GHG), AEF reduced CO2, CO, and soot emissions but increased NOx emissions. Unfortunately, significant unburned ammonia emissions were noted (14,800 ppm). Cai et al. [87] highlighted the unregulated legal emissions of HCN from a dual-fuel engine fueled by diesel oil and ammonia (PFI). In contrast to Nadimi et al. [86], they observed increased ignition delay and combustion duration. The use of a pilot dose of diesel oil improved NH3 combustion efficiency from 74% to 89%. However, this pilot dose led to increased emissions of CO, THC, NOx, N2O, and HCN. It was noted that the highest HCN emissions occur at temperatures between 1000 and 1750 K and with a high air–fuel ratio. H2CN plays a significant role in HCN formation mechanisms. Mi et al. [88] studied the impact of AEF on the performance of a dual-fuel engine across a wide range of rotational speeds. They found that as engine speed increased, AEF should decrease to maintain engine operational indicators. As AEF increased, unburned ammonia emissions increased while NOx emissions decreased. Wang et al. [89] presented a comprehensive analysis of the injection strategy: pilot dose (timing and quantity), main injection, and injection pressure on the performance of a dual-fuel engine fueled by diesel oil and ammonia. They found that the main injection dose determines cylinder pressure and emissions of CO, HC, and unburned NH3. Increasing the pilot injection timing advance causes an increase in COVIMEP. An increase in the pilot dose relative to the main injection worsened ammonia combustion processes. A comparison of the results of ammonia and diesel fuel co-combustion in such a fueling system is presented in Table 2.

Table 2.
Results of port fuel injection engines.
In comparative analyses of selected studies on ammonia use in port fuel injection (PFI) engines, the primary limitation identified is the presence of unburned ammonia in the exhaust gases [53,86,93]. It can also be stated that replacing fossil fuels with ammonia contributes to reducing greenhouse gas emissions (GHG) [53,86,90,91]. This reduction is primarily due to the absence of combustion of carbon-containing fuel molecules, resulting in virtually no emissions of CO and CO2. Regarding the emission of one of the GHG components, NOx, the results are not consistent across studies; some show a reduction in emissions [61], while others indicate an increase [91,93]. The mechanism of nitrogen oxides formation in this context is quite complex. Nitrogen present in the fuel intensifies the fuel-NO mechanism alongside the thermal–NO mechanism. However, the processes of nitrogen oxides formation and decomposition are bidirectional, involving the creation of free radicals and cyanides, which alters the dynamics of NOx formation (basic information included in Section 2).
3.3. Engine Powered by Ammonia in the Direct Injection System
Ammonia injected into the intake manifold creates an air mixture that is nearly homogeneous, which fills the cylinder space and the combustion chamber. Before ignition, this mixture is pushed into all crevices within the combustion chamber, influencing the subsequent emission of unburned NH3 in the exhaust gases. A potential solution to this issue is the direct injection of ammonia into the combustion chamber, allowing it to ignite in a manner similar to that of conventional dual-fuel engine systems. Direct injection provides significantly greater control over the ammonia dosage and the spatial distribution within the combustion chamber. A schematic of such a fueling system is shown in Figure 3b. Bjørgen et al. [96] presented results from a study of a dual-fuel engine powered by ammonia and diesel fuel, where both fuels were directly injected into the combustion chamber. Ammonia was injected using a GDI injector at a pressure of 18 MPa, with energy contributions of 40%, 50%, and 60%. They argued that early injection of ammonia (80 deg bTDC) resulted in high emissions of unburned NH3. Delaying the injection of NH3 to 15 deg before diesel injection caused a significant increase in ignition delay due, in part, to the high heat of vaporization of NH3. The best engine performance parameters were achieved when the injection timings of NH3 and diesel fuel overlapped. They noted a high combustion stability when ammonia burned during the diffusive combustion phase of diesel fuel. For this injection strategy, the highest combustion efficiency was achieved with high emissions of NOx and low emissions of N2O. Lewandowski et al. [97] presented results on the cooling effect of ammonia injected directly into the engine cylinder and the formation of a mixture with air using CFD modeling methods (Figure 4). It was observed that direct injection of ammonia decreased the temperature inside the combustion chamber by approximately 130 K, resulting in a pressure decrease of 3 bar. Injected ammonia at 40 deg bTDC completely evaporated before the injection of diesel fuel at 15 deg bTDC. The heat release rate is notably affected by changes in the NH3 injection angle (Figure 4b).
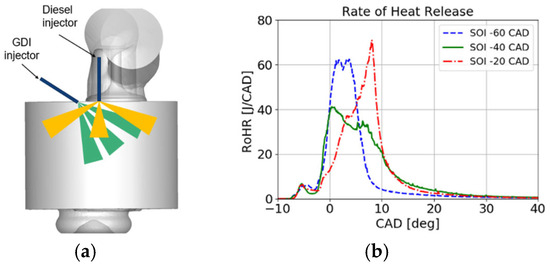
Figure 4.
Direct injection both diesel fuel and NH3: (a) injection diagram, (b) influence of NH3 injection start angle on HRR [97].
Similar to Bjørgen et al. [96], they found that overly early injection of NH3 contributed to increased ammonia emissions in the exhaust gases. The heat release curve can be shaped by the NH3 injection start angle. As shown in Figure 5, the injection phases of both fuels are a very important aspect, as they significantly affect each other. Due to its high heat of vaporization, ammonia as a fuel injected directly before the dose of diesel oil can make it difficult to evaporate and worsen the conditions for its self-ignition.
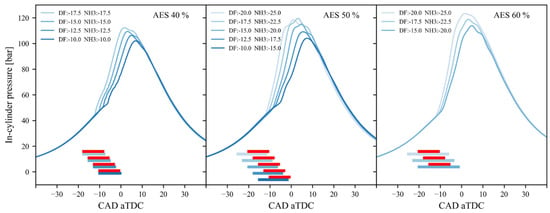
Figure 5.
In-cylinder pressure curves for different combustion phasings at three ammonia energy fractions (AEF) of 40%, 50%, and 60%. Red lines represent the diesel fuel injection timing, while blue lines indicate the NH3 injection timing [96].
In Figure 5, the authors of [86] used the term AES (ammonia energetic share) to denote the ammonia fraction, which is synonymous with the term AEF used in this work. Drazdauskas et al. [98] demonstrated the impact evaluation results of direct ammonia injection strategies on pmax and Tmax values with minimal interference in the engine design. In their studies, liquid-phase ammonia was delivered to the combustion chamber with an AEF of 95% and injection pressures ranging from 50 to 200 MPa. An increase in NH3 injection pressure shifted the heat release process to a single kinetic phase, shortened the ignition delay, and increased ITE by nearly 5%. They found that the optimal injection pressure for ammonia was 100 MPa considering engine performance indicators and GHG emissions. Increasing the injection pressure from 50 to 100 MPa resulted in a 24% decrease in CO2 emissions.
Figure 6 presents the simulation study results of the impact of ammonia share AEF 0–80% and injection strategy [99]. Figure 6a shows the influence of AEF at a constant start of injection angle of the diesel pilot injection. It was found that up to 40% NH3 share resulted in an increase in pmax pressure, while for higher NH3 shares, pmax pressure began to decline. Lower NH3 shares shifted the diffusive combustion phase to kinetic combustion, generating greater pressure increases, whereas for higher AFRs, above 40%, the dominant phenomenon was the cooling effect of ammonia. A higher NH3 share also corresponded to a lower diesel fuel dosage, emphasizing the dominance of ammonia combustion, which has a low LFS value, thereby slowing down the combustion process.
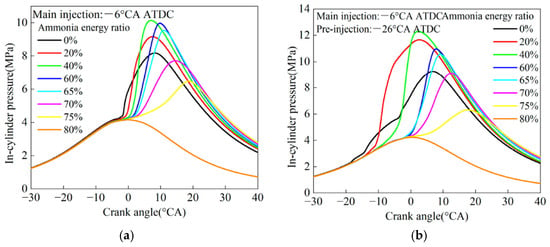
Figure 6.
Pressure profiles in the cylinder of an ammonia-powered engine with direct injection of diesel fuel with a single dose (a) and pilot injection (b) [99].
In Figure 6b, a similar analysis is shown, but the diesel fuel injection was divided into two doses: a main dose injected at the same timing as in the case without dose splitting, and a pilot dose injected 20 degrees before the main dose. Pilot injection resulted in shortened combustion duration, with the greatest effect observed for an ammonia energy fraction (AEF) of 70%. Pilot injection enhances the reactivity of the diesel fuel and ammonia mixture during the main injection, improving ignition properties and shortening the combustion duration [99].
The comparison of co-combustion results of ammonia and diesel fuel delivered by direct injection into the engine is presented in Table 3.
Figure 7 shows two direct injection systems into the engine combustion chamber (a) and (b) and the full engine fuel system (c). Fueling the engine with direct NH3 injection is a more technically difficult issue than for PFI fueling. An easier solution here is to equip the engine with two separate injection systems for diesel oil and ammonia separately (Figure 7a). Technically, this is often problematic due to the space in the engine head that allows for the installation of an additional injector. There are also technical solutions that allow for the supply of both fuels using one injector equipped with two separate fuel systems. This solution is easier to implement due to the design of the engine, because one hole in the engine head is used (Figure 7b). As shown in Figure 7c, an engine fueled with two fuels must be equipped with two separate fuel tanks and separate fuel pumps and injection control systems.
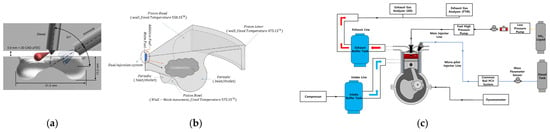
Figure 7.
Direct ammonia injection: (a) injector location in the combustion chamber—fuels supplied by separate injectors, (b) fuel injection using a single injector, (c) complete engine fuel supply system [96,100,101].

Table 3.
Results of direct injection powering of dual fuel engine.
Table 3.
Results of direct injection powering of dual fuel engine.
| Ref. | Engine Type | Fueling Type | Operating Parameters | Emissions |
|---|---|---|---|---|
| Bjørgen et al. [96] (Exp.) | 1-cylinder, CR 17.5:1, 1500 rpm | DI, ammonia injection strategy, AEF 40, 50, and 60% | Early IT of NH3 cause premixed combustion, best when NH3 and diesel are injected at the same time | Higher NH3 emission, NOx and N2O emissions have opposite trends |
| Lewandowski et al. [97] (CFD) | 1-cylinder, CR 21.5:1 | DI, ammonia injection up to 200 MPa, variable IT | Decrease in Tmax of 130K and pmax of 3 bar, best performance for injector coverage | N2O decreased, higher NOx emission |
| Drazdauskas et al. [98] (CFD) | Wartsila 6L46 diesel engine, 500 rpm | DI, AEF 95%, Liquid ammonia injection of 50 to 200 MPa | ITE increase of 5% | Injection 100 MPa causes decrease in CO2 of 24%, GHG emissions were also reduced by 45% |
| Guo et al. [99] (CFD) | 1-cylinder, CR 19:1, 1200 rpm | DI, AEF up to 70%, NH3 injection 150 and 60 deg bTDC at 60 MPa, pre-injection | pmax increases and then decreases with AEF, ID increase | GHG decrease of 41%, 98.13% and 99.6% decrease in soot with single and pre-injection |
| Nadimi et al. [102] (CFD) | 1-cylinder, CR 16.45:1, 1500 rpm | DI, AEF 50%, NH3 injection 10 MPa | ITE increases with 3 injector holes | Reduction in 3-injector holes causes 29.2% reduction in NH3 and CO |
| Shin et al. [103] (CFD) | 1-cylinder, CR 16.25:1, 910 rpm | DI, AEF 95, 97, and 99%, injection strategy | ITE increased by 8%, AEF lowering in-cylinder temperature | NO decreased by up to 13.5%, GHG reduced by 91% |
| Park et al. [101] (Exp.) | 1-cylinder, CR13-14, 900 rpm | DI, pilot injection | Lower CR causes unstable combustion, increase in CR causes increase ITE | decrease in unburned ammonia |
As shown in Table 3, the vast majority of studies evaluating combustion processes and GHG emissions in the concept of direct injection (DI) of both fuels were conducted using CFD modeling. The complexity of this process, including the mixing and interaction of two fuel injections with different properties, necessitates the use of advanced models. Through modeling, insights into the combustion process within the combustion chamber can be obtained, which is crucial for such a complex process [104]. In most of the presented studies, it was indicated that this combustion technology can effectively reduce GHG emissions, particularly unburned NH3 emissions, which remains a challenge in PFI technology. Research indicates that the timing of ammonia injection should correspond with diesel fuel injection timing and pressure [98]. Attention was drawn to the cooling effect of the ammonia jet injected directly into the combustion zone in CI engine chambers, which affects the combustion phases, especially ignition delay time.
As shown in the example of selected works on the use of direct injection of both fuels, the vast majority of works are carried out using CFD modeling. There is a clear lack of experimental work, although with the growing interest in NH3 as a fuel, such work is already appearing in scientific databases. The direct injection of both fuels is difficult to implement and a very interesting issue, especially in terms of the interaction of both fuel streams meeting in the combustion chamber. On the one hand, diesel oil as a highly reactive fuel, responsible for initiating the combustion process, should have gas-thermodynamic conditions for this initiation, and on the other hand, NH3 as a fuel with very limited self-ignition properties and a high vaporization heat value disturbs these conditions. As shown by the results of the research by Bjørgen et al. [96], anticipating the diesel oil dose with ammonia injection can disturb the diesel oil ignition process.
3.4. Ammonia Co-Burned with Other Fuels in a Dual-Fuel Engine
The concept of a dual-fuel engine allows for the combustion of ammonia while achieving satisfactory operational parameters and reduced GHG emissions. As indicated by the results presented in Section 3.1, there remains an issue in engines due to the low combustion velocity of ammonia (0.07 m/s), which sometimes leads to significant emissions of unburned ammonia into the atmosphere [86]. Combustion of so-called “difficult fuels,” such as ammonia, can be assisted by adding a fuel with higher reactivity, which improves ignition capabilities and flame propagation [105]. According to the data presented in Table 1, hydrogen has a laminar burning velocity over 50 times greater than that of ammonia. Even a small amount of hydrogen added significantly enhances flame propagation speed in the combustion chamber of a dual-fuel engine. Additionally, hydrogen has a beneficial effect on reducing soot emissions, further enhancing the environmental efficiency of such engines [106]. The literature includes studies on the use of fuels such as hydrogen, DME, biogas, or methane co-fired with ammonia in dual-fuel engines.
Zhang et al. [107] conducted research on the impact of ammonia and hydrogen content on combustion characteristics and exhaust emissions of a dual-fuel engine. They found that increasing the ammonia content co-firing with diesel reduces emissions of NOx, CO2, and soot but, unfortunately, it increases unburned NH3 emissions. Adding hydrogen, even around 4%, to the mixture of ammonia co-fired with diesel effectively reduces unburned NH3 emissions. Paterson et al. [108] investigated the effect of hydrogen on engine performance parameters, GHG emissions, and unburned NH3. They demonstrated that hydrogen addition (H2O2) in the mixture with ammonia in a dual-fuel engine decreased emission of unburned NH3. Wang et al. [109] studied the impact of ammonia and hydrogen injection strategies on engine performance, GHG emissions, and NH3 emissions. This engine fueling technology can reduce N2O emissions, which are nearly 200 times more harmful to the environment than CO2.
As shown in Figure 8 at too low CR, NH3 may not ignite, but adding hydrogen as a highly reactive fuel improves the properties of the combustible mixture and, as shown, a 20% share of H2 allowed the combustion process to be initiated. Only a 30% share of H2 proved to be good enough in terms of the generated pressure and HRR.
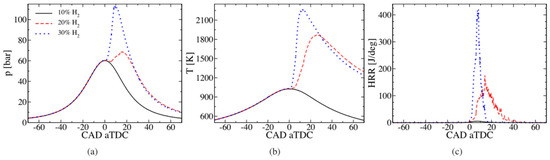
Figure 8.
Influence of the share of H2 (10%, 20%, and 30%) co-combusted with NH3 on the course of changes in pressure (a), temperature (b), and heat release rate (c) [108].
Wang et al. [110] used ammonia as a fuel to control knocking combustion by reducing the combustion rate in a CI engine fueled with natural gas. The mixture of natural gas and ammonia was ignited with a dose of diesel. The best economic properties were achieved with 60% ammonia fraction in the natural gas blend, along with the lowest GHG emissions. The authors noted that as the ammonia concentration in the natural gas mixture rose, unburned NH3 emissions also increased, a phenomenon referred to as the ‘slip effect’. Bayramoğlu et al. [111] presented results from a CI engine powered by three fuels: methane, hydrogen, and ammonia. They conducted combustion studies with methane and hydrogen, finding that hydrogen addition increases the combustion temperature in the engine; adding 15% H2 raised the temperature by 100 K. Adding 15% ammonia to this mixture lowered the temperature by 200 K. The authors concluded that co-firing methane with hydrogen and ammonia can significantly reduce GHG emissions.
3.5. Ammonia Combustion in Alternative Combustion Systems
Several studies in the literature address the combustion characteristics of NH3 using alternative combustion systems such as HCCI, RCCI, and dual-chamber combustion engines. The concept of combustion based on RCCI presents a promising approach to overcoming the drawbacks of CI engines, such as high NOx and soot emissions. Fakhari et al. [112] conducted simulation studies on an NH3-fueled engine operating in RCCI mode. Increasing the NH3 share to 70% resulted in a rise in IMEP with low emissions of CO, THC, NH3, and N2O. The findings indicate that this engine mode achieves better performance and reduced GHG emissions relative to dual-fuel engine operation. Elumalai et al. [113] used RCCI technology as an LTC combustion concept, where they supplied NH3 to the intake manifold in amounts of 20–50% energy share with direct injection of biodiesel derived from algae. They achieved significant reductions in exhaust emissions, THC by 44%, CO by 32%, CO2 by 48%, NOx by 55%, and soot by 66%, compared to a conventional engine. In the studies, an NH3 share of up to 50% was achieved due to the low cylinder temperature significantly limiting ignition. Elumalai et al. [114] studied an engine fueled with NH3 introduced through the intake system operating in RCCI mode with a fuel share split of 40/60. They obtained an increase in BTE and a decrease in BSEC. They demonstrated that the use of a split injection of a highly reactive fuel, here, biodiesel, positively impacts not only engine performance but also GHG emissions.
The concept of HCCI combustion, developed over many years, promises low emissions of harmful substances in exhaust gases due to its volumetric combustion process. Research is ongoing into using this combustion system for engines powered by NH3. Xu et al. [115] conducted studies using an NH3/H2 mixture to achieve controlled ignition kinetics combustion without carbon-based fuels. They found that increasing the proportion of H2 accelerated ignition and increased the heat release rate. However, they observed increased NO emissions mainly due to increased radicals (O, H, and OH) promoting NO formation. Hydrogen (H2) is a natural fuel that improves the ignition limits of NH3 [116]. The addition of hydrogen has been used to facilitate better ignition of NH3 in HCCI engine applications [117]. Wang et al. [117], additionally, conducted simulation studies replacing the nitrogen in air with argon, an inert gas. As a result, there was a notable reduction in ignition delay and an increase in combustion efficiency. Using a mixture of 79% argon and 21% oxygen, they reduced the ignition delay time from 700 ms to 0.31 ms. In the presence of hydrogen, the ignition delay was reduced by about 90%. They concluded that the optimal hydrogen content in this HCCI engine concept is 5%. Similar to with other engine technologies, researchers recommend using H2 as a fuel to improve ignition and stabilize the combustion process in HCCI engines.
Pochet et al. [118] studied the effect of the NH3/H2 ratio on combustion in an HCCI engine. The goal was to achieve stable combustion with a high NH3 content while minimizing NOx emissions. They achieved stable combustion with 70% NH3 content but required an intake manifold pressure of 1.5 bar. However, they encountered issues with N2O emissions, which were high. Shafiq et al. [119] used hydrogen peroxide (H2O2) as an ignition promoter for NH3 in an HCCI engine. Their findings indicate that H2O2 facilitates ignition of the NH3/air mixture without the necessity of preheating during cylinder filling. Increasing H2O2 content improved engine performance but also increased NOx emissions.
In piston engines burning challenging fuels, a pre-chamber design is often employed, where the main chamber holds a difficult-to-ignite mixture, while the pre-chamber contains a readily ignitable mixture. Ignition of the main chamber is triggered by the flame jet from the pre-chamber, allowing combustion to proceed [120]. This combustion system concept has also been applied to NH3-powered engines. Liu et al. [121] achieved ignition in a hydrogen-powered combustion chamber, with the flame jet entering the NH3-powered main chamber, enabling ignition. The primary advantage of this combustion system was very low emissions of unburned NH3.
These studies highlight the ongoing efforts to optimize NH3 combustion in various alternative combustion systems, aiming to achieve efficient and low-emission power generation while addressing the challenges associated with NH3 as a fuel.
3.6. Challenges for Ammonia-Powered Engines
Ammonia can be a valuable alternative fuel for powering both spark-ignition and compression-ignition engines. As a carbon-free fuel, it becomes an environmentally neutral energy carrier. However, as discussed in the section on ammonia as a fuel, it also presents significant limitations in its application. In reciprocating internal combustion engines, besides its performance, its exhaust emissions are the most crucial aspect. When powered by ammonia, other hazardous substances are formed in the exhaust gases, which, when fueled by hydrocarbon fuels, were either negligibly small or not considered in emission control. The upcoming Euro 7 emission standard expands the spectrum of controlled exhaust components, such as NH3 and N2O. This will require not only engines that meet these standards but also precise measuring equipment for their control. In port fuel injected (PFI) engines fueled by ammonia, the emission of unburned NH3 generated by the so-called crevice effect is practically unavoidable. The NH3/air mixture is forced into crevices during the compression stroke and a significant portion of it remains unburned, escaping into the exhaust gases during the expansion stroke and subsequently leaving the engine cylinder. The complexity of the nitrogen oxides formation process, where fuel-NO dominates in such applications, poses a challenge for N2O emissions. The direct injection of NH3 into the combustion chamber can be a way to reduce NH3 emissions significantly, thereby mitigating the crevice effect.
Another challenge for ammonia-powered engines is the difficulty in cold engine starting. This is a similar drawback to engines fueled by alcohols, where the high heat of vaporization lowers the charge temperature, potentially worsening ignition capabilities or sometimes making it impossible, especially in cold ambient conditions. For dual-fuel compression-ignition engines, this issue can be overcome by adjusting the fuel proportions.
The challenges faced by ammonia-powered engines are as follows:
- Safety aspects: Ammonia is harmful to health and requires safety precautions during transport, storage, and usage. It forms an explosive mixture in air within a volume concentration range of 15 to 33.6%.
- Infrastructure: Currently, there is inadequate infrastructure for storing and distributing NH3 as a widely used fuel. This necessitates significant financial and technological investments. For ammonia to be considered a zero-emission fuel, it should be produced using renewable energy, which also encourages the development of this technology.
- Energy value: The LHV of ammonia is 18.8 MJ/kg, more than two times lower than that of hydrocarbon fuels. This requires a correspondingly higher mass flow of NH3 to achieve the same energy output compared to conventional fuels. It also necessitates the use of larger storage tanks, creating logistical challenges in transportation due to larger volumes.
- Combustion technologies: Efficient combustion of ammonia is not straightforward, as indicated by selected studies on this topic. Despite increasing scientific research on ammonia combustion in ICE, there are still many unresolved aspects. Challenges include the ignition difficulties of the NH3/air mixture and the emission of pollutants, particularly unburned NH3 and N2O.
- Corrosion and material degradation: Ammonia adversely affects engine construction materials and fuel system components, necessitating the use of materials such as nickel alloys or specialized protective coatings.
- Economic and social aspects: For NH3 to be widely adopted as a fuel, it must be economically viable. Currently, the cost of ammonia is higher than that of traditional fuels. Concerns regarding safety may influence the societal acceptance of ammonia-powered engines.
Ammonia, from a scientific perspective, is a very promising decarbonization fuel for compression-ignition (CI) reciprocating engines. In CI engine applications, where ignition is initiated by a dose of diesel fuel or another high-reactivity fuel, ammonia does not pose major ignition problems. However, when used in port fuel injection (PFI), there is a challenge with excessive emissions of unburned NH3, primarily due to the phenomenon known as crevice effect. This drawback of ammonia fueling is largely mitigated by the direct injection of ammonia into the combustion chamber.
The results obtained do not account for the variable operating conditions of the engines used to power the vehicles. There are technical solutions that utilize ammonia as an engine fuel in vehicles, where the variability of engine operating conditions has been minimized through the hybrid drive concept. In the study by Ezzat and Dincer [122], two propulsion systems employing ammonia in automotive applications were presented and analyzed.
The first system consists of a liquefied ammonia and hydrogen storage tank, and a spark-ignition internal combustion engine, which serves as the primary propulsion unit, generating a traction power output of 118 kW. The internal combustion engine was fueled with an ammonia–hydrogen mixture in an 80/20 ratio.
The second system is a hybrid configuration comprising a liquefied ammonia storage tank, a dissociation and separation unit (DSC), a small spark-ignition engine, and a fuel cell system. In this configuration, the fuel cell serves as the main power unit, with the internal combustion engine functioning as a supplementary source. It has been demonstrated that the integration of fuel cells with the internal combustion engine enhances the efficiency of the vehicle’s power system compared to a vehicle powered solely by an internal combustion engine.
4. Conclusions
Replacing hydrocarbon fuels with alternative fuels can give ICEs a “second life”. In industries and transportation, reciprocating engines are often the primary source of power for machines and electric power generators. Legal regulations and increasing environmental awareness demand reductions in GHG emissions. Ammonia, as a zero-carbon fuel, is currently seen as a future-oriented fuel for CI engines. Due to limitations, ammonia cannot be the sole fuel for CI engines and requires ignition assistance from a high-reactivity fuel. Two ammonia fueling systems are distinguished: port fuel injection (PFI) and direct injection of ammonia (DI).
The key features of NH3-fueled CI engines with a PFI system are as follows:
- −
- Easier to implement technically; simple control system.
- −
- High emissions of unburned NH3—crevice effect.
The features of NH3-fueled CI engines with a DI system are as follows:
- −
- Require additional injectors in the engine head; advanced control system.
- −
- Potential for achieving low NH3 emissions.
The characteristics of a CI engine fueled with ammonia are as follows:
- −
- The use of PFI technology contributes to an increase in unburned NH3 emissions in the exhaust gases, primarily due to the crevice effect, with ammonia emissions potentially exceeding 10,000 ppm. Direct injection of ammonia exhibits better characteristics in this regard.
- −
- As the NH3 share increases, ignition delay and combustion duration also increase; this effect can be mitigated by appropriate control of the injection of the highly reactive fuel.
- −
- The use of ammonia as a fuel can lead to excessive NOx emissions as well as the release of N2O, a highly harmful compound to the environment. Unburned NH3 can intensify deNOx reactions, thereby reducing nitrogen oxide emissions.
- −
- In CI engines fueled with ammonia, increasing the compression ratio improves combustion stability and engine efficiency.
- −
- To enhance the combustion properties of the NH3–air mixture, hydrogen can be used as a fuel additive that increases flame propagation speed; a 20% hydrogen share doubles the laminar flame speed (LFS).
- −
- Many researchers indicate that an optimal NH3 share in a dual-fuel engine is around 40% (AEF).
Both combustion systems are burdened with increased NOx and N2O emissions, imposing high demands on exhaust gas aftertreatment systems. Ammonia, as a decarbonization fuel, should find application in CI engine fueling because it effectively reduces particulate emissions, a major constraint for such engines. However, the use of such fuel presents many challenges related to its toxic properties and aggressive impact on engine materials. The literature does not adequately address the durability issues of NH3-fueled engines. This is a critical aspect for every user. For newly designed engines, resistant materials can be used to withstand the aggressive nature of NH3. For engines adapted to such fueling, it is important to have information on durability risks.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AEF | ammonia energetic fraction |
| aTDC | after top dead center |
| BTE | brake thermal efficiency |
| BSEC | brake specific energy consumption |
| CFD | computational fluid dynamics |
| CI | compression ignition |
| CN | cetane number |
| CR | compression ratio |
| DI | direct injection |
| GHG | greenhouse gas |
| HCCI | homogeneous charge compression ignition |
| IC | internal combustion |
| IMEP | indicated mean effective pressure |
| ITE | indicated thermal efficiency |
| IT | injection time |
| LFS | laminar flame speed |
| LHV | lower heating value |
| WC | water cooled |
| PFI | port fuel injection |
| RCCI | reactivity controlled compression ignition |
| rpm | rotation per minute |
| SoI | start of injection |
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Ovaere, M.; Proost, S. Cost-effective reduction of fossil energy use in the European transport sector: An assessment of the Fit for 55 Package. Energy Policy 2022, 168, 113085. [Google Scholar] [CrossRef]
- Venkata Sundar Rao, K.; Kurbet, S.N.; Kuppast, V.V. A Review on Performance of the IC Engine Using Alternative Fuels. Mater. Today Proc. 2018, 5, 1989–1996. [Google Scholar] [CrossRef]
- Ergen, G. Comprehensive analysis of the effects of alternative fuels on diesel engine performance combustion and exhaust emissions: Role of biodiesel, diethyl ether, and EGR. Therm. Sci. Eng. Prog. 2024, 47, 102307. [Google Scholar] [CrossRef]
- Geng, P.; Cao, E.; Tan, Q.; Wei, L. Effects of alternative fuels on the combustion characteristics and emission products from diesel engines: A review. Renew. Sustain. Energy Rev. 2017, 71, 523–534. [Google Scholar] [CrossRef]
- Othman, M.F.; Adam, A.; Najafi, G.; Mamat, R. Green fuel as alternative fuel for diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 80, 694–709. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Zhang, Z.; Li, W.; Yuan, T.; Tan, D.; Duan, L.; Yang, G. A comprehensive review on combustion, performance and emission aspects of higher alcohols and its additive effect on the diesel engine. Fuel 2023, 335, 127011. [Google Scholar] [CrossRef]
- Jin, C.; Xiao, J.; Hou, J.; Wu, X.; Zhang, J.; Du, E. Flexibility improvement evaluation of hydrogen storage based on electricity–hydrogen coupled energy model. Glob. Energy Interconnect. 2012, 4, 371–383. [Google Scholar] [CrossRef]
- Maka, A.O.M.; Ghalut, T.; Elsaye, E. The pathway towards decarbonisation and net-zero emissions by 2050: The role of solar energy technology. Green Technol. Sustain. 2024, 2, 100107. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Ham, K.; Bae, S.; Lee, J. Classification and technical target of water electrolysis for hydrogen production. J. Energy Chem. 2024, 95, 554–576. [Google Scholar] [CrossRef]
- Zhang, Z.; Turap, Y.; Wang, Y.; Wang, Y.; Wang, Z.; Wang, W. Study on the impact of hydrogen storage temperature on iron-based thermochemical hydrogen storage technology. Chem. Eng. J. 2024, 490, 151536. [Google Scholar] [CrossRef]
- Gopinathan, R.L.; Ibrahim, M.M. Ammonia as a sustainable fuel for diesel engines: Exploring advanced combustion strategies for green transportation. J. Energy Inst. 2015, 121, 102159. [Google Scholar] [CrossRef]
- Jafar, U.; Nuhu, U.; Khan, W.U.; Hossain, M.M. A review on green ammonia as a potential CO2 free fuel. Int. J. Hydrogen Energy 2024, 71, 857–876. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/ (accessed on 20 May 2025).
- Zhou, H.; Chen, Z.; Meng, W.; Yang, S. Design, global energy integration, and sustainability analyses of a process coupling renewable energy water electrolysis for hydrogen production with ammonia synthesis. J. Environ. Chem. Eng. 2024, 12, 112892. [Google Scholar] [CrossRef]
- Kojima, Y. Safety of ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2024, 50, 732–739. [Google Scholar] [CrossRef]
- Dinesh, M.H.; Kumar, G.N. Effects of compression and mixing ratio on NH3/H2 fueled Si engine performance, combustion stability, and emission. Energy Convers. Manag. X 2022, 15, 100269. [Google Scholar] [CrossRef]
- Zhang, R.; Shu, G.; Zhao, H.; Chen, L.; Wei, H.; Pan, J. A comparative study on NH3/H2 and NH3/CH3OH combustion and emission in an optical SI engine. Fuel 2024, 369, 131731. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as green fuel in internal combustion engines: State-of-the-art and future perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Hu, X.; Pan, J.; Zhang, R.; Li, J.; Li, W.; Wei, H. Effects of intake parameters and compression ratio on ammonia combustion and emissions in SI engines. Fuel 2023, 354, 129382. [Google Scholar] [CrossRef]
- Wen, H.; Li, J.; Li, J.; Xu, C. Effect of intake air conditions on combustion and emission performance of ammonia-diesel dual fuel engine. J. Energy Inst. 2025, 118, 101938. [Google Scholar] [CrossRef]
- Raza, H.; Woo, S.; Kim, H. Investigation of an ammonium carbamate–based SCR system for NOx reduction in diesel engines under transient conditions. Energy 2022, 251, 123918. [Google Scholar] [CrossRef]
- Nie, X.; Bi, Y.; Shen, L.; Lei, J.; Wan, M.; Xiao, Y.; Chen, G. Experimental study for optimizing EGR strategy in an ammonia-diesel dual-fuel engine under different altitudes. Energy 2024, 313, 133953. [Google Scholar] [CrossRef]
- Salcedo, A.; Caputo, S.; Loehlé, S.; Steinmann, S.N.; Michel, C. Molecular modeling of the diffusion of ammonia through corrosion inhibitor films on copper. Corros. Sci. 2024, 240, 112491. [Google Scholar] [CrossRef]
- Jespersen, M.C.; Rasmussen, T.Ø.H.; Ivarsson, A. Widening the operation limits of a SI engine running on neat ammonia. Fuel 2024, 358, 130159. [Google Scholar] [CrossRef]
- Zhu, T.; Fan, Y.; Zhu, L.; Huang, Z. Research on the spark-integrated auto ignition in ammonia/polyoxymethylene dimethyl ether engine. Fuel 2025, 387, 134376. [Google Scholar] [CrossRef]
- Uddeen, K.; Tang, Q.; Shi, H.; Turner, J.W.G. Ammonia-methanol and ammonia-ethanol dual-fuel combustion in an optical spark-ignition engine: A multiple flame generation approach. Appl. Therm. Eng. 2025, 265, 125544. [Google Scholar] [CrossRef]
- Sonthalia, A.; Varuvel, E.G.; Subramanian, T.; Femilda Josephin, J.S.; Alahmadi, T.A.; Pugazhendhi, A. Comparative analysis to reduce greenhouse gas (GHG) emission in CI engine fuelled with sweet almond oil using ammonia/after treatment system. Fuel 2024, 371, 131865. [Google Scholar] [CrossRef]
- Cheng, H.; Tang, Q.; Uddeen, K.; Huang, L.; Zheng, Z.; Turner, J.; Yao, M. The ignition mechanisms and chemical reaction kinetics of nitrogen oxides of ammonia/diesel dual-fuel engine combustion. Appl. Therm. Eng. 2025, 262, 125287. [Google Scholar] [CrossRef]
- Alvarez, L.F.; Ulishney, C.J.; Askari, O.; Dumitrescu, C.E. Dumitrescu, Neat ammonia use in a heavy-duty diesel engine converted to spark ignition focused on lean operation. Fuel 2025, 382, 133786. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]
- Fakhari, A.H.; Gharehghani, A.; Salahi, M.M.; Amin Andwari, M. Numerical investigation of the hydrogen-enriched ammonia-diesel RCCI combustion engine. Fuel 2024, 375, 132579. [Google Scholar] [CrossRef]
- Sarıtaş, M.; Kül, V.S.; Akansu, S.O.; Sinkala, H. Compression ignition engine performance and emissions: An experimental study on the impact of aqueous ammonia, hydrogen, and diesel fuel blends. Int. J. Hydrogen Energy 2025, 137, 679–688. [Google Scholar] [CrossRef]
- Scharl, V.; Sattelmayer, T. Ignition and combustion characteristics of diesel piloted ammonia injections. Fuel Commun. 2022, 11, 100068. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Guo, L.; Zhang, H.; Zeng, W.; Lin, S.; Zhu, G.; Jiang, M.; Ma, X. Study on effects of EGR and injection strategies on the combustion and emission characteristics of ammonia/diesel dual-fuel engine. Energy 2025, 315, 134391. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Zhou, X.; Huang, S.; Chen, R.; Yi, P.; Lv, Y.; Wang, Y.; Rao, H.; Liu, Y.; et al. Reductions in GHG and unburned ammonia of the pilot diesel-ignited ammonia engines by diesel injection strategies. Appl. Therm. Eng. 2025, 260, 124967. [Google Scholar] [CrossRef]
- Szabados, G.; Bereczky, Á.; Ajtai, T.; Bozóki, Z. Evaluation analysis of particulate relevant emission of a diesel engine running on fossil diesel and different biofuels. Energy 2018, 161, 1139–1153. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jakubowski, M.; Jaworski, A.; Lubas, J.; Krzysztof Balawender, K. Effect of temperature on tribological properties of 1-butanol–diesel fuel blends—Preliminary experimental study using the HFRR method. Fuel 2021, 296, 120700. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental investigation of the effect of ambient gas temperature on the autoignition properties of ethanol–diesel fuel blends. Fuel 2018, 214, 26–38. [Google Scholar] [CrossRef]
- Feroskhan, M.; Gobinath, N. Experimental study of butanol blending in biogas-biodiesel fueled CI engine under dual fuel, RCCI and HCCI combustion modes. Case Stud. Therm. Eng. 2024, 58, 104349. [Google Scholar] [CrossRef]
- Paykani, A.; Garcia, A.; Shahbakhti, M.; Rahnama, P.; Reitz, R.G. Reactivity controlled compression ignition engine: Pathways towards commercial viability. Applied Energy 2021, 282, 116174. [Google Scholar] [CrossRef]
- Splitter, D.A.; Reitz, R.D. Fuel reactivity effects on the efficiency and operational window of dual-fuel compression ignition engines. Fuel 2014, 118, 163–175. [Google Scholar] [CrossRef]
- Reitz, R.D.; Duraisamy, G. Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines. Prog. Energy Combust. Sci. 2015, 46, 12–71. [Google Scholar] [CrossRef]
- Duan, X.; Lai, M.-C.; Jansons, M.; Guo, G.; Liu, J. A review of controlling strategies of the ignition timing and combustion phase in homogeneous charge compression ignition (HCCI) engine. Fuel 2021, 285, 119142. [Google Scholar] [CrossRef]
- Verma, S.K.; Gaur, S.; Akram, T.; Gautam, S.; Kumar, A. Emissions from homogeneous charge compression ignition (HCCI) engine using different fuels: A review. Environ. Sci. Pollut. Res. 2022, 29, 50960–50969. [Google Scholar] [CrossRef]
- Ali, K.; Kyritsis, D.C.; Ali, M.H. Computational investigation of combustion and emission characteristics of HCCI engine fueled with Carbon-Free NH3–H2O2 blend. Energy Convers. Manag. 2024, 322, 119105. [Google Scholar] [CrossRef]
- Krishnan, M.G.; Rajkumar, S.; Devarajan, Y.; Rajiv, A. A comprehensive review on advancement and challenges of renewable biofueled reactivity controlled compression ignition (RCCI) engine. J. Energy Inst. 2024, 113, 101540. [Google Scholar] [CrossRef]
- Saxena, S.; Bedoya, I.D. Fundamental phenomena affecting low temperature combustion and HCCI engines, high load limits and strategies for extending these limits. Prog. Energy Combust. Sci. 2013, 39, 457–488. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Gnatowska, R. Combustion of different reactivity fuel mixture in a dual fuel engine. Therm. Sci. 2018, 22, 1285–1297. [Google Scholar] [CrossRef]
- Tutak, W. Bioethanol E85 as a fuel for dual fuel diesel engine. Energy Convers. Manag. 2014, 86, 39–48. [Google Scholar] [CrossRef]
- Shah, Z.A.; Marseglia, G.; De Giorgi, M.G. Predictive models of laminar flame speed in NH3/H2/O3/air mixtures using multi-gene genetic programming under varied fuelling conditions. Fuel 2024, 368, 131652. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Bai, X.-S.; Repo, J.A.; Hautala, S.; Hyvönen, J. Performance and emission characteristics of an ammonia/diesel dual-fuel marine engine. Renew. Sustain. Energy Rev. 2023, 185, 113631. [Google Scholar] [CrossRef]
- Han, L.; Gong, Y.; Qian, D.; Liu, M.; Ma, H.; Xie, F. Optical investigation of the influence of passive pre-chamber turbulent jet ignition on the combustion characteristics of carbon-free ammonia-hydrogen engines. Appl. Therm. Eng. 2025, 278 Pt A, 127200. [Google Scholar] [CrossRef]
- Hroch, E. Ammonia—A fuel for motor buses. J. Inst. Pet 1945, 31, 213–223. [Google Scholar]
- Sehili, Y.; Loubar, K.; Tarabet, L.; Cerdoun, M.; Lacroix, C. Computational Investigation of the Influence of Combustion Chamber Characteristics on a Heavy-Duty Ammonia Diesel Dual Fuel Engine. Energies 2024, 17, 1231. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Yang, L.; Chen, G.; He, S. Intelligent optimization of diesel engine Selective catalytic reduction urea injection based on multi-model state estimation to reduce NH3 slip and NOx emission. Fuel 2024, 365, 131188. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Yu, J.; Hong, J.; Lee, Y. Parametric evaluation of the operating conditions for NO reduction in flameless combustion of an H2/NH3/N2 fuel mixture. Int. J. Hydrogen Energy 2025, 74, 404–413. [Google Scholar] [CrossRef]
- Lu, M.; Long, W.; Wei, F.; Dong, D.; Cong, L.; Dong, P.; Tian, H.; Chen, X.; Chen, S.; Wang, Y.; et al. Assessment of carbon-free fuel ammonia combustion with low methanol blends in reducing GHG emissions including N2O. J. Clean. Prod. 2024, 463, 142755. [Google Scholar] [CrossRef]
- Zheng, Z.; Xia, M.; Liu, H.; Shang, R.; Ma, G.; Yao, M. Experimental study on combustion and emissions of n-butanol/biodiesel under both blended fuel mode and dual fuel RCCI mode. Fuel 2018, 226, 240–251. [Google Scholar] [CrossRef]
- Yang, R.; Liu, J.; Liu, J. Investigation of the formation mechanisms of nitrogen-based pollutants in ammonia-diesel dual-fuel engines by decoupling dilution, thermal, and kinetic effects. J. Energy Inst. 2025, 120, 102125. [Google Scholar] [CrossRef]
- Imtenan, S.; Masjuki, H.H.; Varman, M.; Rizwanul Fattah, I.M.; Sajjad, H.; Arbab, M.I. Effect of n-butanol and diethyl ether as oxygenated additives on combustion–emission-performance characteristics of a multiple cylinder diesel engine fuelled with diesel–jatropha biodiesel blend. Energy Convers. Manag. 2015, 94, 84–94. [Google Scholar] [CrossRef]
- Börnhorst, M.; Deutschmann, O. Advances and challenges of ammonia delivery by urea-water sprays in SCR systems. Prog. Energy Combust. Sci. 2021, 87, 100949. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, F.; Li, S.; Wang, R.; Yang, X. Combustion performance and emission characteristics of a diesel engine burning biodiesel blended with n-butanol. Fuel 2019, 258, 115887. [Google Scholar] [CrossRef]
- Farzam, R.; Liko, B.; Bebar, A.; Dev, S.; Stevenson, D.; Guo, H.; McTaggart-Cowan, G. Performance and emissions characteristics of hydrogen-diesel heavy-duty engines: The influence of engine control parameters. Int. J. Hydrogen Energy 2025, 109, 1325–1340. [Google Scholar] [CrossRef]
- Bartels, J.R. A Feasibility Study of Implementing an Ammonia Economy. Master’s Thesis, Iowa State University, Ames, IA, USA, 2008. [Google Scholar]
- Zhao, F.; Wang, Z.; Dong, B.; Li, M.; Ji, Y.; Han, F. Comprehensive life cycle cost analysis of ammonia-based hydrogen transportation scenarios for offshore wind energy utilization. J. Clean. Prod. 2023, 429, 139616. [Google Scholar] [CrossRef]
- Jang, H.; Mujeeb-Ahmed, M.P.; Wang, H.; Park, C.; Hwang, I.; Jeong, B.; Zhou, P.; Mickeviciene, R. Regulatory gap analysis for risk assessment of ammonia-fuelled ships. Ocean Eng. 2023, 287, 115751. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Wang, W. Effect of ammonia co-firing on heat transfer, safety, and economy of coal-fired boilers. Fuel 2023, 334, 126649. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. Co-Combustion of Hydrogen with Diesel and Biodiesel (RME) in a Dual-Fuel Compression-Ignition Engine. Energies 2023, 16, 4892. [Google Scholar] [CrossRef]
- Paykani, A.; Kakaee, A.H.; Rahnama, P.; Reitz, R.D. Effects of diesel injection strategy on natural gas/diesel reactivity controlled compression ignition combustion. Energy 2015, 90, 814–826. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Grab-Rogaliński, K. Effects of Propanol on the Performance and Emissions of a Dual-Fuel Industrial Diesel Engine. Appl. Sci. 2022, 12, 5674. [Google Scholar] [CrossRef]
- Yang, S.; Wan, M.; Shen, L.; Wang, Z.; Huang, F.; Ma, Y.; Xiao, Y. Investigation of the impacts of regeneration temperature and methanol substitution rate on the active regeneration of diesel particulate filter in a diesel-methanol dual-fuel engine. Energy 2024, 301, 131657. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Grab-Rogaliński, K. Experimental study on ammonia-diesel co-combustion in a dual-fuel compression ignition engine. J. Energy Inst. 2024, 115, 101711. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Tsolakis, A.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Pan, J.; Peng, W.; Tabatabaei, M.; Aghbashlo, M. Use of hydrogen in dual-fuel diesel engines. Prog. Energy Combust. Sci. 2023, 98, 101100. [Google Scholar] [CrossRef]
- Tutak, W.; Lukács, K.; Szwaja, S.; Bereczky, Á. Alcohol–diesel fuel combustion in the compression ignition engine. Fuel 2015, 154, 196–206. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Chen, H.; Ji, Z.; Ma, Y.; Sun, F. A review on performance, combustion and emission of diesel and alcohols in a dual fuel engine. J. Energy Inst. 2024, 116, 101760. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Jia, M.; Wang, Y.; Su, X.; Li, L. A full-parameter computational optimization of both injection parameters and injector layouts for a methanol/diesel dual-fuel direct injection compression ignition engine. Fuel 2024, 369, 131733. [Google Scholar] [CrossRef]
- Li, T.; Zhao, P.; He, H.; Wang, C.; Zhang, H.; Chen, Z.; Chen, H. Dual-fuel dual-direct injection: An efficient and clean combustion technology for diesel engines. J. Energy Inst. 2025, 119, 102006. [Google Scholar] [CrossRef]
- Chintala, V. Influence of flame quenching and crevice gas on hydrocarbon emission formation in an enriched biogas dual-fuel engine—An experimental and theoretical investigation. Fuel 2020, 277, 118084. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, X.; Raman, V.; Shi, H.; Chang, J.; Im, H.G.; Johansson, B. Effects of fuel trapping in piston crevice on unburned hydrocarbon emissions in early-injection compression ignition engines. Combust. Flame 2021, 231, 111496. [Google Scholar] [CrossRef]
- Grimes, P. Energy Depot Fuel Production and Utilization. SAE Tech. Pap. 1965, 650051. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
- Cai, K.; Liu, Y.; Chen, Q.; Qi, Y.; Li, L.; Wang, Z. Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine. Energies 2023, 16, 7004. [Google Scholar] [CrossRef]
- Mi, S.; Shi, Z.; Wu, H.; Zheng, L.; Zhao, W.; Qian, Y.; Lu, X. Expanding high ammonia energy ratios in an ammonia-diesel dual-fuel engine across wide-range rotational speeds. Appl. Therm. Eng. 2024, 251, 123608. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhao, W.; Ji, Q.; Li, Z.; Xiang, P.; Wang, X. Experimental investigation on in-cylinder working process and thermal efficiency of ammonia/diesel low-carbon engine with pre-injection strategy. Appl. Therm. Eng. 2024, 248, 123272. [Google Scholar] [CrossRef]
- Elumalai, R.; Sumathy, S.; Ravi, K.; Akhtar, M.N.; Elumalai, P.V.; Khan, S.A.; Gupta, M.S.; Asif, M. Experimental investigation and gray relational optimization of engine parameters to improve the output characteristics of an ammonia biodiesel powered dual fuel combustion engine. Case Stud. Therm. Eng. 2024, 56, 104197. [Google Scholar] [CrossRef]
- Lasocki, J.; Bednarski, M.; Sikora, M. Simulation of ammonia combustion in dual-fuel compression-ignition engine. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012081. [Google Scholar] [CrossRef]
- Cheng, C.; Cordtz, R.F.; Førby, N.L.; Schramm, J. Experimental and simulation investigation of n-heptane/ammonia dual fuel on a light-duty compression ignition engine. Int. J. Hydrogen Energy 2024, 57, 1339–1353. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Lang, M.; Su, Y.; Wang, Y.; Zhang, Y.; Wang, B.; Chen, S. Experimental Study on the Effects of pilot injection Strategy on Combustion and Emission Characteristics of Ammonia/Diesel Dual Fuel Engine under low load. Energy 2024, 303, 131913. [Google Scholar] [CrossRef]
- Huang, Y.; Lyu, L.; Liang, J.; Yang, H.; Zhu, N.; Sang, H.; Zhang, X. Performance and emission characteristics of marine ammonia/diesel dual-fuel engines at different diesel substitution rates. Fuel 2025, 379, 132967. [Google Scholar] [CrossRef]
- Bjørgen, K.O.P.; Emberson, D.R.; Terese Løvås, T. Combustion of liquid ammonia and diesel in a compression ignition engine operated in high-pressure dual fuel mode. Fuel 2024, 360, 130269. [Google Scholar] [CrossRef]
- Lewandowski, M.T.; Pasternak, M.; Haugsvær, M.; Terese Løvås, T. Simulations of ammonia spray evaporation, cooling, mixture formation and combustion in a direct injection compression ignition engine. Int. J. Hydrogen Energy 2024, 52, 916–935. [Google Scholar] [CrossRef]
- Drazdauskas, M.; Lebedevas, S. Optimization of Combustion Cycle Energy Efficiency and Exhaust Gas Emissions of Marine Dual-Fuel Engine by Intensifying Ammonia Injection. J. Mar. Sci. Eng. 2024, 12, 309. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, J.; Fu, L.; Li, Z.; Liu, F.; Wang, Z.; Liu, X.; Dong, Q. Effects of Pre-Injection Strategy on Combustion Characteristics of Ammonia/Diesel Dual-Fuel Compression Ignition Mode. Energies 2023, 16, 7687. [Google Scholar] [CrossRef]
- Ghadamkheira, K.; Safamanesha, F.; Moghimana, M.; Hatami, M. Numerical investigation of the effects of hydrogen/ammonia addition to n-heptane combustion and emissions for a dual injection compression-ignition engine. Heliyon 2025, 11, e42106. [Google Scholar] [CrossRef]
- Park, C.; Jang, I.; Kim, M.; Park, G.; Kim, Y. Effect of high compression ratio on thermal efficiency and unburned ammonia emissions of a dual-fuel high-pressure direct injection marine ammonia engine. Appl. Therm. Eng. 2025, 261, 125183. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Løvås, T.; Adamczyk, W. Effects of biodiesel injector configuration and its injection timing on performance, combustion and emissions characteristics of liquid ammonia dual direct injection engine. J. Energy Inst. 2024, 114, 101605. [Google Scholar] [CrossRef]
- Shin, J.; Park, S. Numerical analysis and optimization of combustion and emissions in an ammonia-diesel dual-fuel engine using an ammonia direct injection strategy. Energy 2024, 289, 130014. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Bereczky, Á.; Lukacs, K. Effects of injection timing of diesel fuel on performance and emission of dual fuel diesel engine powered by diesel/E85 fuels. Transport 2018, 33, 633–646. [Google Scholar] [CrossRef]
- El-Adawy, M.; Nemitallah, M.A.; Abdelhafez, A. Towards sustainable hydrogen and ammonia internal combustion engines: Challenges and opportunities. Fuel 2024, 364, 131090. [Google Scholar] [CrossRef]
- Tutak, W. Co-combustion of ammonia and hydrogen in spark ignition engines—State-of-the-art and challenges. Int. J. Hydrogen Energy 2024, 80, 188–205. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Yang, D.; Yin, Z.; Lu, K.; Tan, D. A comprehensive assessment over the environmental impact and combustion efficiency of using ammonia/ hydrogen/diesel blends in a diesel engine. Energy 2024, 303, 131955. [Google Scholar] [CrossRef]
- Paterson, G.; Tingas, E.A.; Hardalupas, Y.; Taylor, A.M.K.P. Engine performance and emissions from a fumigated hydrogen/ammonia compression ignition engine with a hydrogen peroxide pilot. Int. J. Hydrogen Energy 2024, 67, 334–350. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Yang, C.; Hu, D.; Duan, B.; Wang, Y. Study on dual injection strategy of diesel ignition ammonia/hydrogen mixture fuel engine. Fuel 2023, 348, 128526. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Hu, D.; Yang, C.; Duan, B.; Wang, Y. Study on the performance of premixed natural gas/ammonia engine with diesel ignition. Energy 2023, 271, 127056. [Google Scholar] [CrossRef]
- Bayramoğlu, K.; Bahlekeh, A.; Masera, K. Numerical investigation of the hydrogen, ammonia and methane fuel blends on the combustion emissions and performance. Int. J. Hydrogen Energy 2023, 48, 39586–39598. [Google Scholar] [CrossRef]
- Fakhari, A.H.; Gharehghani, A.; Salahi, M.M.; Amin Andwari, M. RCCI combustion of ammonia in dual fuel engine with early injection of diesel fuel. Fuel 2024, 365, 131182. [Google Scholar] [CrossRef]
- Elumalai, R.; Ravi, K. Strategy to reduce carbon emissions by adopting ammonia–Algal biodiesel in RCCI engine and optimize the fuel concoction using RSM methodology. Int. J. Hydrogen Energy 2022, 47, 39701–39718. [Google Scholar] [CrossRef]
- Elumalai, R.; Ravi, K.; Elumalai, P.V.; Chanduveetil Ahamed, S.; Saboor, S. Investigation on Ammonia–Biodiesel Fueled RCCI Combustion Engine Using a Split Injection Strategy. ACS Omega 2023, 8, 30990–31001. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Zhang, Y.; Wang, J.; Huang, Z. Effects of temperature and composition inhomogeneity on the ignition characteristics of NH3/H2 co-firing fuels under HCCI operating conditions. Appl. Energy Combust. Sci. 2023, 15, 100194. [Google Scholar] [CrossRef]
- Pfahl, U.J.; Ross, M.C.; Shepherd, J.E.; Pasamehmetoglu, K.O.; Unal, C. Flammability limits, ignition energy, and flame speeds in H2–CH4–NH3–N2O–O2–N2 mixtures. Combust. Flame 2000, 123, 140–158. [Google Scholar] [CrossRef]
- Wang, C.; Deng, J.; Ding, W.; Huang, Y.; Tang, Y.; Li, L. Thermodynamic analysis of employing argon as the diluent and adding hydrogen in an HCCI ammonia engine: Ignition characteristics and performances of combustion and NO emissions. Int. J. Hydrogen Energy 2024, 49, 293–300. [Google Scholar] [CrossRef]
- Pochet, M.; Truedsson, I.; Foucher, F.; Jeanmart, H.; Contino, F. Ammonia-Hydrogen Blends in Homogeneous-Charge Compression-Ignition Engine. SAE Tech. Pap. 2017. [Google Scholar] [CrossRef]
- Shafiq, O.; Tingas, E.-A. Computational investigation of ammonia-hydrogen peroxide blends in HCCI engine mode. Int. J. Engine Res. 2023, 24, 2279–2294. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Kociszewski, A.; Sosnowski, M. Numerical simulation of two-stage combustion in SI engine with prechamber. Appl. Math. Model. 2013, 37, 2961–2982. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Q.; Im, H.G. Enhancing ammonia engine efficiency through pre-chamber combustion and dual-fuel compression ignition techniques. J. Clean. Prod. 2024, 436, 140622. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Comparative assessments of two integrated systems with/without fuel cells utilizing liquefied ammonia as a fuel for vehicular applications. Int. J. Hydrogen Energy 2018, 43, 4597–4608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
