Electrostatic Dual-Layer Solvent-Free Cathodes for High-Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
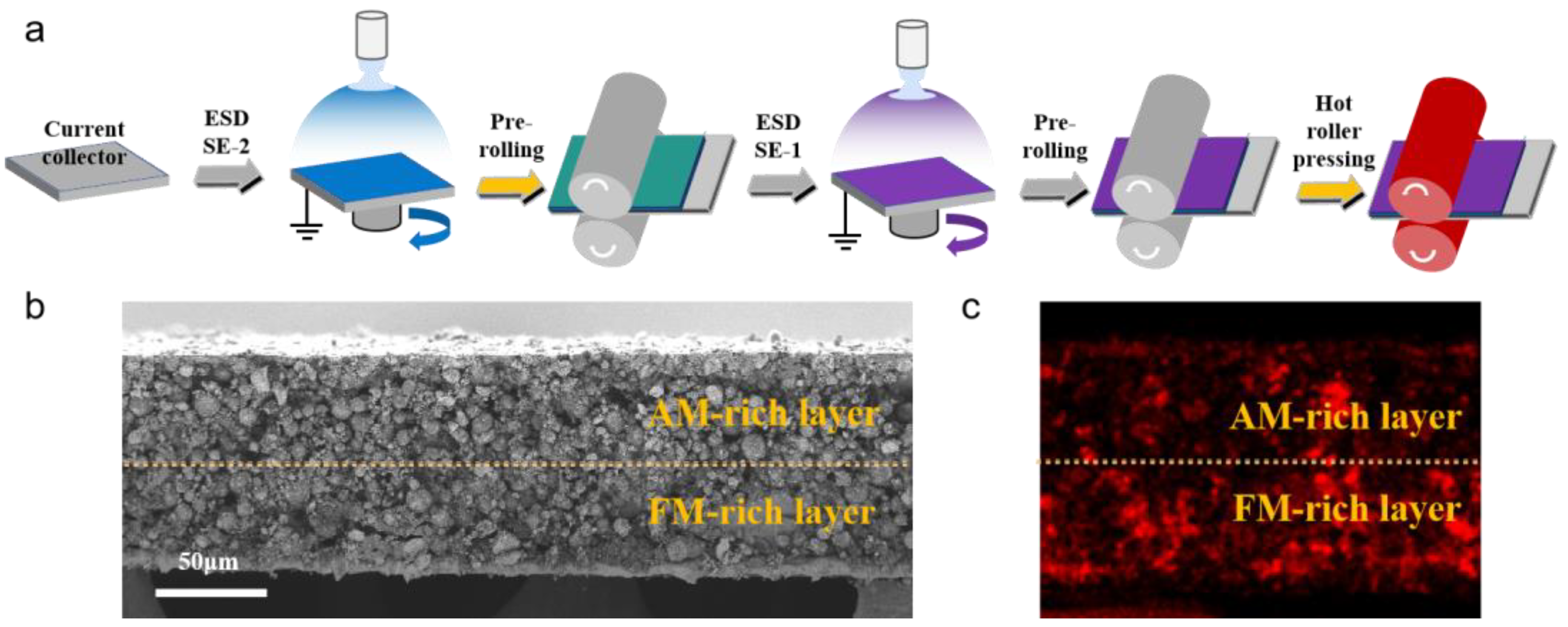
2.1. Material Preparation
2.2. Electrode Fabrication
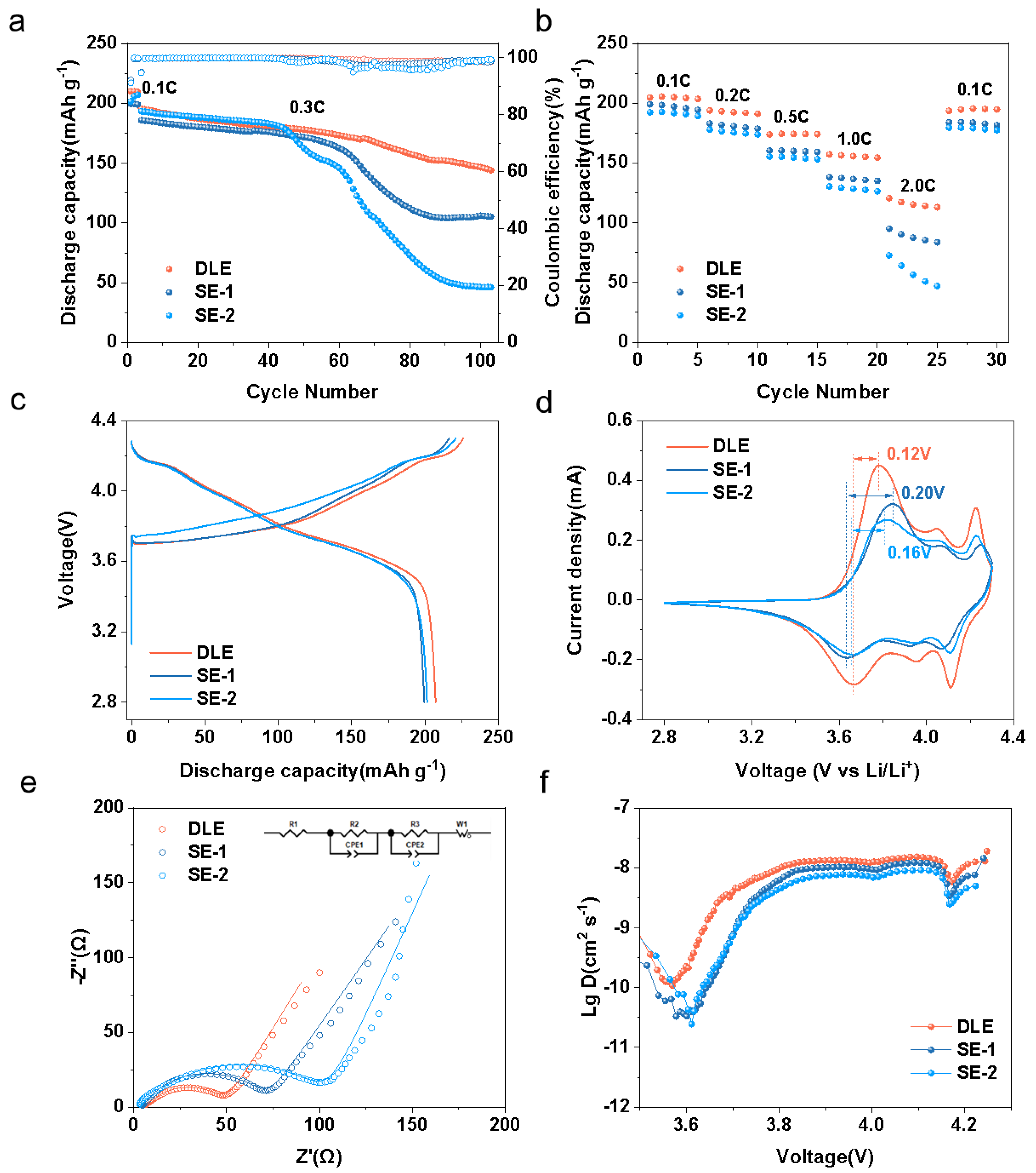
2.3. Electrochemical Characterization
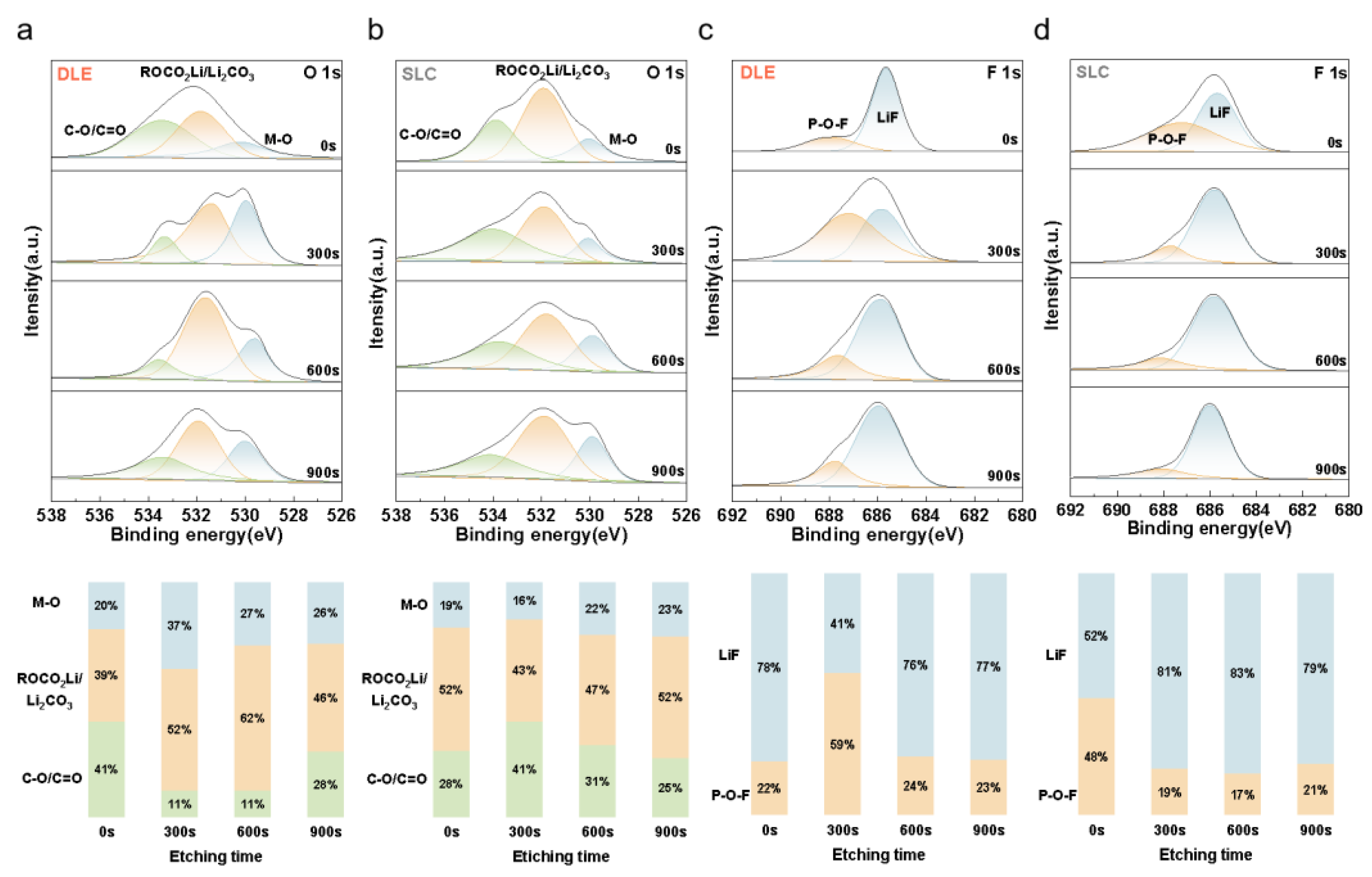
2.4. Experimental Instrument
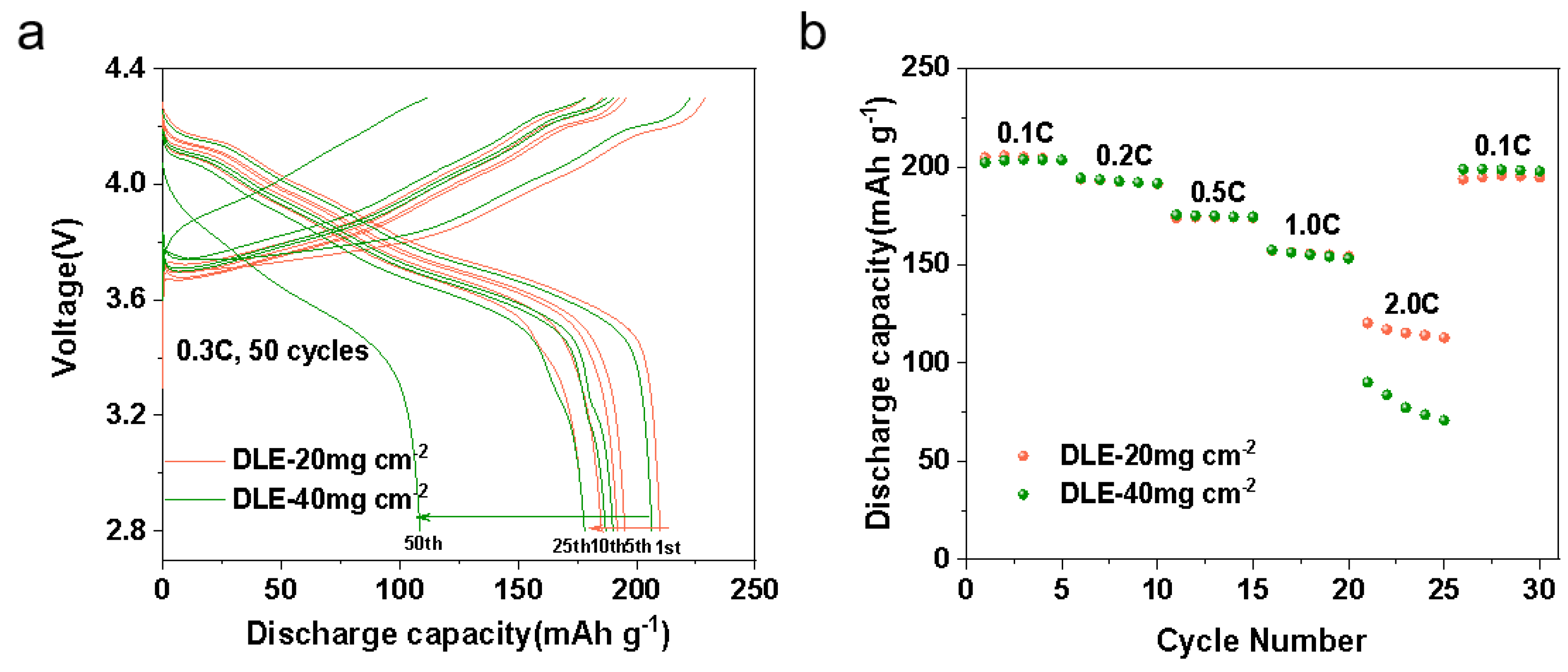
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Cao, C.C.; Zhao, L.Q.; Tadé, M.O.; Shao, Z.P. Optimization of two-dimensional solid-state electrolyte–anode interface by integrating zinc into composite anode with dual-conductive phases. Green Carbon 2024, 2, 94–100. [Google Scholar] [CrossRef]
- Liu, C.C.; Jia, S.e.; Yang, T.Z.; Liu, J.B.; Zhou, X.R.; Wang, Z.F.; Dong, H.C.; Shi, Z.J.; Zhang, Y.G.; Chen, Z.W. Scalable and ultrathin dual entangled network polymer electrolytes for safe solid-state sodium batteries. Angew. Chem. 2025, e202505938. [Google Scholar] [CrossRef]
- Yang, H.J.; Naveed, A.; Li, Q.Y.; Guo, C.; Chen, J.H.; Lei, J.Y.; Yang, J.; Nuli, Y.; Wang, J.L. Lithium sulfur batteries with compatible electrolyte both for stable cathode and dendrite-free anode. Energy Storage Mater. 2018, 15, 299–307. [Google Scholar] [CrossRef]
- Liu, I.P.; Chen, Y.Y.; Cho, Y.S.; Wang, L.W.; Chien, C.Y.; Lee, Y.L. Double-layered printable electrolytes for highly efficient dye-sensitized solar cells. J. Power Sources 2021, 482, 228962. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Xu, Z.Q.; Li, H.; Xiong, H.Q.; Liu, X.J.; Qin, C.L.; Wang, Z.F. Silicon/biomass carbon composite as a low-cost anode for lithium-ion batteries. Energies 2025, 18, 972. [Google Scholar] [CrossRef]
- Qi, W.; Lai, Y.Q.; Liu, F.Y.; Jiang, L.X.; Ming, J.; Wang, X.L. Sb2S3 nanorods/porous-carbon composite from natural stibnite ore as high-performance anode for lithium-ion batteries. Trans. Nonferrous Met. Soc. China 2021, 31, 2051–2061. [Google Scholar] [CrossRef]
- Houache, M.S.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the current and future outlook of battery chemistries for electric vehicles—Mini review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Liu, L.J.; Wang, T.; Sun, L.; Song, T.L.; Yan, H.; Li, C.L.; Mu, D.B.; Zheng, J.C.; Dai, Y. Stable Cycling of All-Solid-State Lithium Metal Batteries Enabled by Salt Engineering of PEO-Based Polymer Electrolytes. Energy Environ. Mater. 2024, 7, e12580. [Google Scholar] [CrossRef]
- Liu, Z.D.; Wang, C.Y.; Zhang, J.C.; Luo, J.W.; Zeng, C.H.; Liu, W.D.; Liu, R.; Chen, Y.N. Co-free/Co-poor high-Ni cathode for high energy, stable and low-cost lithium-ion batteries. Rare Met. 2023, 42, 2214–2225. [Google Scholar] [CrossRef]
- Mi, C.; Wang, Z.G.; Yang, S.B.; Liu, X.J.; Wang, Y.C.; Wang, Z.F. Porous Al11Ce3 intermetallics as effective sulphur host networks for stable lithium–sulphur batteries. J. Mater. Chem. C 2025, 13, 9014–9026. [Google Scholar] [CrossRef]
- Yang, X.; Tat, T.; Libanori, A.; Cheng, J.; Xuan, X.X.; Liu, N.; Yang, X.; Zhou, J.H.; Nashalian, A.; Chen, J. Single-atom catalysts with bimetallic centers for high-performance electrochemical CO2 reduction. Mater. Today 2021, 45, 54–61. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; He, X.M. Toward practical solid-state polymer lithium batteries by in situ polymerization process: A review. Adv. Energy Mater. 2023, 13, 2300798. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li–S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Fei, F.; Wen, Z.G.; De Clercq, D. Spatio-temporal estimation of landfill gas energy potential: A case study in China. Renew. Sustain. Energy Rev. 2019, 103, 217–226. [Google Scholar] [CrossRef]
- Awasthi, S.; Moharana, S.; Kumar, V.; Wang, N.; Chmanehpour, E.; Sharma, A.D.; Tiwari, S.K.; Kumar, V.; Mishra, Y.K. Progress in doping and crystal deformation for polyanions cathode based lithium-ion batteries. Nano Mater. Sci. 2024, 6, 504–535. [Google Scholar] [CrossRef]
- Sun, X.; Qin, C.L.; Zhao, B.Y.; Jia, S.F.; Wang, Z.F.; Yang, T.Z.; Liu, X.C.; Pan, L.N.; Zheng, L.L.; Luo, D. A cation and anion dual-doping strategy in novel Li-rich Mn-based cathode materials for high-performance Li metal batteries. Energy Storage Mater. 2024, 70, 103559. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yan, Y.J.; Zhang, Y.G.; Chen, Y.X.; Peng, X.Y.; Wang, X.; Zhao, W.M.; Qin, C.L.; Liu, Q.; Liu, X.J. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium–sulfur batteries. Carbon Energy 2023, 5, e306. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kim, J.H.; Koo, H.; Oh, J.; Pang, J.H.; Kang, K.D.; Chae, S.S.; Lim, J.; Nam, K.W.; Lee, S.Y. Material challenges facing scalable dry-processable battery electrodes. ACS Energy Lett. 2024, 9, 5688–5703. [Google Scholar] [CrossRef]
- Tao, R.M.; Steinhoff, B.; Sawicki, C.H.; Sharma, J.; Sardo, K.; Bishtawi, A.; Gibbs, T.; Li, J.L. Unraveling the impact of the degree of dry mixing on dry-processed lithium-ion battery electrodes. J. Power Sources 2023, 580, 233379. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.K.; Lee, S.Y.; Park, J.H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef] [PubMed]
- He, R.J.; Zhong, W.; Cai, C.Y.; Li, S.P.; Cheng, S.J.; Xie, J. Flour-infused dry processed electrode enhancing lithium-ion battery performance. Adv. Energy Mater. 2024, 14, 2402109. [Google Scholar] [CrossRef]
- Horst, M.; Beverborg, F.; Bahlmann, L.; Schreiber, S.; Gerk, J.; Michalowski, P.; Kwade, A. Effect of active material morphology on PTFE-fibrillation, powder characteristics and electrode properties in dry electrode coating processes. Powder Technol. 2025, 451, 120451. [Google Scholar] [CrossRef]
- Ma, Y.J.; Guo, H.J.; Yang, T.; Wang, Z.F. Structural design of dry-processed lithium-rich Mn-based materials with high loading for enhanced energy density. Batteries 2025, 11, 146. [Google Scholar] [CrossRef]
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.D.; Pastel, G.; Xie, H.; Connell, J.W.; Lin, Y.; Hu, L.B. Scalable dry processing of binder-free lithium-ion battery electrodes enabled by holey graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.T.; Zheng, Z.F.; Wang, F.; Tang, M.; Wang, J.J.; Wang, J.; Pan, H.; Wang, Y. Scalable dry printing manufacturing to enable long-life and high energy lithium-ion batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.F.; Shou, W.; Wang, Y.; Pan, H. Solvent-free manufacturing of electrodes for lithium-ion batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Oh, H.; Kim, G.S.; Bang, J.; Kim, S.; Jeong, K.M. Dry-processed thick electrode design with a porous conductive agent enabling 20 mAh cm−2 for high-energy-density lithium-ion batteries. Energy Environ. Sci. 2025, 18, 645–658. [Google Scholar] [CrossRef]
- Park, D.; Kim, S.; Ha, S.; Hwa, Y.; Kim, J.; Park, J.; Son, Y. Pitch-based quasi-dry thick electrode fabrication process of NCM cathode for lithium-ion batteries. J. Power Sources 2024, 614, 235037. [Google Scholar] [CrossRef]
- Yonaga, A.; Kawauchi, S.; Mori, Y.; Xuanchen, L.; Ishikawa, S.; Nunoshita, K.; Inoue, G.; Matsunaga, T. Effects of dry powder mixing on electrochemical performance of lithium-ion battery electrode using solvent-free dry forming process. J. Power Sources 2023, 581, 233466. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, D.H.; Liang, C.F.; Han, Y.; Li, Z.; Huang, Y.H. Design of double layer cathode electrode for improving the safety and stability of lithium-ion batteries. Chem. Eng. J. 2024, 495, 153344. [Google Scholar] [CrossRef]
- Chen, W.B.; Wang, K.; Li, Y.L.; Chen, J.; Wang, H.B.; Li, L.W.; Li, H.; Ren, X.Z.; Ouyang, X.P.; Liu, J.H. Minimize the electrode concentration polarization for high-power lithium batteries. Adv. Funct. Mater. 2024, 34, 2410926. [Google Scholar] [CrossRef]
- Kang, J.; Lim, J.; Lee, H.; Park, S.; Bak, C.; Shin, Y.; An, H.; Lee, M.; Lee, M.; Lee, S. Sequential effect of dual-layered hybrid graphite anodes on electrode utilization during fast-charging Li-ion batteries. Adv. Sci. 2024, 11, 2403071. [Google Scholar] [CrossRef]
- Shi, H.F.; Niu, S.Z.; Lv, W.; Zhou, G.M.; Zhang, C.; Sun, Z.H.; Li, F.; Kang, F.Y.; Yang, Q.H. Easy fabrication of flexible and multilayer nanocarbon-based cathodes with a high unreal sulfur loading by electrostatic spraying for lithium-sulfur batteries. Carbon 2018, 138, 18–25. [Google Scholar] [CrossRef]
- Song, K.F.; Zhang, C.; Hu, N.F.; Wu, X.K.; Zhang, L. High performance thick cathodes enabled by gradient porosity. Electrochim. Acta 2021, 377, 138105. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.C.; Sun, S.M.; Sun, X.L.; Cao, F.T.; Ohsaka, T.; Wu, J.F. Analysis of the relationship between vertical imparity distribution of conductive additive and electrochemical behaviors in lithium ion batteries. Electrochim. Acta 2018, 269, 422–428. [Google Scholar] [CrossRef]
- Chen, L.C.; Liu, D.; Liu, T.J.; Tiu, C.; Yang, C.R.; Chu, W.B.; Wan, C.C. Improvement of lithium-ion battery performance using a two-layered cathode by simultaneous slot-die coating. J. Energy Storage 2016, 5, 156–162. [Google Scholar] [CrossRef]
- Wulandari, T.; Fawcett, D.; Majumder, S.B.; Poinern, G.E. Lithium-based batteries, history, current status, challenges, and future perspectives. Battery Energy 2023, 2, 20230030. [Google Scholar] [CrossRef]
- Yang, X.P.; Cheng, F.; Ka, O.; Wen, L.; Gu, X.Y.; Hou, W.T.; Lu, W.; Dai, L.M. High-voltage lithium-ion capacitors enabled by a multifunctional phosphite electrolyte additive. Energy Storage Mater. 2022, 46, 431–442. [Google Scholar] [CrossRef]
- Jaiser, S.; Sanchez Salach, N.; Baunach, M.; Scharfer, P.; Schabel, W. Impact of drying conditions and wet film properties on adhesion and film solidification of lithium-ion battery anodes. Dry. Technol. 2017, 35, 1807–1817. [Google Scholar] [CrossRef]
- Zhong, W.H.; Tao, J.M.; Chen, Y.; White, R.G.; Zhang, L.; Li, J.X.; Huang, Z.G.; Lin, Y.B. Unraveling the evolution of Cathode–Solid electrolyte interface using operando X-ray Photoelectron spectroscopy. Adv. Powder Mater. 2024, 3, 100184. [Google Scholar] [CrossRef]
- Liu, W.B.; Li, D.Y.; Liu, Y.Q.; Luo, D.; Xu, R. A critical review of single-crystal LiNixMnyCo1-x-yO2 cathode materials. Renewables 2024, 2, 25–51. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zhang, C.; Ma, Y.; Liu, N.; Wang, Z. Electrostatic Dual-Layer Solvent-Free Cathodes for High-Performance Lithium-Ion Batteries. Energies 2025, 18, 3112. https://doi.org/10.3390/en18123112
Guo H, Zhang C, Ma Y, Liu N, Wang Z. Electrostatic Dual-Layer Solvent-Free Cathodes for High-Performance Lithium-Ion Batteries. Energies. 2025; 18(12):3112. https://doi.org/10.3390/en18123112
Chicago/Turabian StyleGuo, Haojin, Chengrui Zhang, Yujie Ma, Ning Liu, and Zhifeng Wang. 2025. "Electrostatic Dual-Layer Solvent-Free Cathodes for High-Performance Lithium-Ion Batteries" Energies 18, no. 12: 3112. https://doi.org/10.3390/en18123112
APA StyleGuo, H., Zhang, C., Ma, Y., Liu, N., & Wang, Z. (2025). Electrostatic Dual-Layer Solvent-Free Cathodes for High-Performance Lithium-Ion Batteries. Energies, 18(12), 3112. https://doi.org/10.3390/en18123112







