Review of Hydrogen Storage in Solid-State Materials
Abstract
1. Introduction
2. MOF Hydrogen Storage
2.1. Concept of MOF Hydrogen Storage
2.2. MOF Hydrogen Storage Density
| MOFs | Temperature (K) | Pressure (MPa) | Hydrogen Storage Density (wt%) | References |
|---|---|---|---|---|
| MOF-5 | 77 | 0.1 | 2.49 | [44] |
| 1Li-MOF-5 | 77 | 0.1 | 3.09 | [44] |
| 2Na-MOF-5 | 77 | 0.1 | 4.18 | [44] |
| 1K-MOF-5 | 77 | 0.1 | 4.23 | [44] |
| Cu3(BTC)2 | 77 | 0.1 | 2.41 | [45] |
| LiCu3(BTC)2 | 77 | 0.1 | 3.50 | [45] |
| MIL-101(Cr) | 77 | 0.1 | 2.37 | [45] |
| LiMIL-101(Cr) | 77 | 0.1 | 3.39 | [45] |
| IRMOF-9 | 298 | 10 | ~0.35 | [46] |
| MOF-C30 | 300 | 10 | ~1.0 | [43] |
| Li-MOF-C30 | 300 | 10 | ~5.0 | [43] |
2.3. Thermodynamics Conditions of MOF Hydrogen Storage
2.4. Stability of MOF Hydrogen Storage Cycle
2.5. Commerciality of MOF Hydrogen Storage
3. Solid Alloy Hydrogen Storage
3.1. Concept of Hydrogen Storage in Solid Alloys
3.2. Hydrogen Storage Density of Alloy
3.3. Temperature and Pressure Conditions of Hydrogen Storage in Alloys
3.4. Cyclic Decay of Hydrogen Storage in Alloys
3.5. Economy of Hydrogen Storage in Alloys
4. Hydrate Hydrogen Storage
4.1. Concept of Hydrogen Hydrate
4.2. Hydrogen Storage Density of Hydrate
4.3. Temperature and Pressure Conditions for Hydrogen Storage of Hydrate
4.4. Economy of Hydrogen Storage in Hydrate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, C.; Ma, F.; Pan, S.; Lin, M.; Zhang, G.; Xiong, B.; Wang, Y.; Liang, Y.; Yang, Z. Earth energy evolution, human development and carbon neutral strategy. Pet. Explor. Dev. 2022, 49, 468–488. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent progress using solid-state materials for hydrogen storage: A short review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Ait Ousaleh, H.; Mehmood, S.; Baba, Y.F.; Bürger, I.; Linder, M.; Faik, A. An analytical review of recent advancements on solid-state hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 1182–1193. [Google Scholar]
- Hui, Y.; Wang, M.; Guo, S.; Akhtar, S.; Bhattacharya, S.; Dai, B.; Yu, J. Comprehensive review of development and applications of hydrogen energy technologies in China for carbon neutrality: Technology advances and challenges. Energy Convers. Manag. 2024, 315, 118776. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Bajaber, M.A.; Javed, M.S.; Junk, P.C.; Nanjundan, A.K.; Qian, J.; Dubal, D.P. Design and Advanced Manufacturing of NU-1000 Metal–Organic Frameworks with Future Perspectives for Environmental and Renewable Energy Applications. Small 2024, 20, 2306353. [Google Scholar] [CrossRef] [PubMed]
- Addai, F.P.; Wu, J.; Lin, F.; Ma, X.; Han, J.; Liu, Y.; Zhou, Y.; Wang, Y. Alloyed Trimetallic Nanocomposite as an Efficient and Recyclable Solid Matrix for Ideonella sakaiensis Lipase Immobilization. Langmuir 2024, 40, 8921–8938. [Google Scholar] [CrossRef]
- Pleshivtseva, Y.; Derevyanov, M.; Pimenov, A.; Rapoport, A. Comparative analysis of global trends in low carbon hydrogen production towards the decarbonization pathway. Int. J. Hydrogen Energy 2023, 48, 32191–32240. [Google Scholar] [CrossRef]
- Chai, H.; Chen, J.; Yu, Y.; Zhao, C. Grand canonical monte carlo simulations of Cu&Li-based metal organic framework for desirable hydrogen storage. Int. J. Hydrogen Energy 2024, 61, 424–431. [Google Scholar] [CrossRef]
- Dai, B.; Shen, X.; Chen, T.; Li, J.; Xu, Q. Porous layered ZnV2O4@C synthesized based on a bimetallic MOF as a stable cathode material for aqueous zinc ion batteries. Dalton Trans. 2024, 53, 8335–8346. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Zhang, B.; Kimura, H.; Xiao, L.R.; Liu, R.H.; Ni, C.; Hou, C.X.; Sun, X.Q.; Zhang, Y.P.; Yang, X.Y.; et al. Vermiform Ni@CNT derived from one-pot calcination of Ni-MOF precursor for improving hydrogen storage of MgH2. Trans. Nonferrous Met. Soc. China 2024, 34, 2629–2644. [Google Scholar] [CrossRef]
- Granja-DelRío, A.; Cabria, I. Grand Canonical Monte Carlo simulations of the hydrogen and methane storage capacities of JLU-MOF120 and JLU-MOF121. Int. J. Hydrogen Energy 2024, 61, 57–72. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, S.; Liu, J.; Lin, M.; Ye, R.; Chen, X. Ultrafast Hole Preservation with Undercoordinated Tungsten for Efficient Solar-to-Chemical Conversion. ACS Energy Lett. 2024, 9, 3252–3260. [Google Scholar] [CrossRef]
- Hoang, T.K.; Antonelli, D.M. Exploiting the Kubas interaction in the design of hydrogen storage materials. Adv. Mater. 2009, 21, 1787–1800. [Google Scholar] [CrossRef]
- Berijani, K.; Vakili-Nezhaad, G.R. The performance of MOFs and rich structure types of stable Zr-MOFs in storing clean energy: Hydrogen. J. Energy Storage 2025, 116, 115928. [Google Scholar] [CrossRef]
- Dincă, M.; Long, J.R. Hydrogen storage in microporous metal–organic frameworks with exposed metal sites. Angew. Chem. Int. 2008, 47, 6766–6779. [Google Scholar] [CrossRef]
- Jing, Y.; Gong, F.; Wang, S.; Wang, W.; Yang, P.; Fu, E.; Xiao, R. Activating the synergistic effect in Ni-Co bimetallic MOF for enhanced plasma-assisted ammonia synthesis. Fuel 2024, 368, 131686. [Google Scholar] [CrossRef]
- Al-Roomi, Y.; George, R.; Elgibaly, A.; Elkamel, A. Engineering. Use of a novel surfactant for improving the transportability/transportation of heavy/viscous crude oils. J. Pet. Sci. Eng. 2004, 42, 235–243. [Google Scholar] [CrossRef]
- Berry, G.D.; Pasternak, A.D.; Rambach, G.D.; Smith, J.R.; Schock, R.N. Hydrogen as a future transportation fuel. Energy 1996, 21, 289–303. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Irani, R. Hydrogen storage: High-pressure gas containment. MRS Bull. 2002, 27, 680–682. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Dornheim, M.; Baetcke, L.; Akiba, E.; Ares, J.-R.; Autrey, T.; Barale, J.; Baricco, M.; Brooks, K.; Chalkiadakis, N.; Charbonnier, V.; et al. Research and development of hydrogen carrier based solutions for hydrogen compression and storage. Prog. Energy 2022, 4, 042005. [Google Scholar] [CrossRef]
- Bouwman, P. Applications. In Fundamentals of Electrochemical Hydrogen Compression; Bessarabov, D., Wang, H., Li, H., Zhao, N., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 269–299. [Google Scholar]
- Badwal, S.P.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Chung, C.; Ihm, J.; Lee, H. Recent progress on Kubas-type hydrogen-storage nanomaterials: From theories to experiments. J. Korean Phys. Soc. 2015, 66, 1649–1655. [Google Scholar] [CrossRef]
- Mahamiya, V.; Dewangan, J.; Chakraborty, B. Interplay between van der Waals, Kubas, and chemisorption process when hydrogen molecules are adsorbed on pristine and Sc-functionalized BeN4. Int. J. Hydrogen Energy 2024, 50, 1302–1316. [Google Scholar] [CrossRef]
- Salehabadi, A.; Ahmad, M.I.; Ismail, N.; Morad, N.; Enhessari, M. Solid-state hydrogen storage materials. In Energy, Society and the Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 41–67. [Google Scholar] [CrossRef]
- David, E. An overview of advanced materials for hydrogen storage. J. Mater. Process. Technol. 2005, 162–63, 169–177. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Williams, K.A.; Eklund, P.C. Hydrogen Adsorption in Carbon Materials. MRS Bull. 1999, 24, 45–50. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Towards a Hydrogen Economy. Electr. J. 2005, 18, 62–76. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, W.; Xu, Z. Overview of Key Technologies and Applications of Hydrogen Energy Storage in Integrated Energy Systems. In Proceedings of the 2020 12th IEEE PES Asia-Pacific Power and Energy Engineering Conference (APPEEC), Nanjing, China, 20–23 September 2020; pp. 1–5. [Google Scholar]
- Alnafisah, M.S.; Alharbi, K.N.; Almuqati, N.S.; Almalahi, K.M.; Almusawa, M.H.; Almotairy, D.D.; Alotaibi, G.; Alromaeh, A.I. Effect of solvent selection on Zn-MOFs synthesized for hydrogen storage applications. Int. J. Hydrogen Energy 2025, 120, 393–402. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.L.; Mardel, J.I.; Hill, M.R. Metal-Organic Frameworks (MOFs) as Hydrogen Storage Materials at Near-Ambient Temperature. Chem. A Eur. J. 2024, 30, e202400717. [Google Scholar] [CrossRef] [PubMed]
- Yifu, C. Metal-organic frameworks: Current trends, challenges, and future prospects. Met. Org. Framew. 2024, 3–23. [Google Scholar]
- Goel, N. Porous coordination polymers: A brief introduction. In Porous Coordination Polymers; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–9. [Google Scholar]
- Li, X.; Zhang, G.; Li, N.; Xu, Q.; Li, H.; Lu, J.; Chen, D. Solar-driven production of highly concentrated hydrogen peroxide by Zn3In2S6/PCN-222 heterostructure. Nano Energy 2024, 126, 109671. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zhu, F.; Liu, J.; Wan, X.; Liu, R.; Liu, X.; Shang, J.X.; Yu, R.; Feng, Q.; et al. Mg-MOF-74 Derived Defective Framework for Hydrogen Storage at Above-Ambient Temperature Assisted by Pt Catalyst. Adv. Sci. 2024, 11, e2401868. [Google Scholar] [CrossRef]
- Makhafola, M.D.; Balogun, S.A.; Modibane, K.D. A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction. Energies 2024, 17, 1646. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Han, S.S.; Goddard, W.A. Lithium-Doped Metal-Organic Frameworks for Reversible H2 Storage at Ambient Temperature. J. Am. Chem. Soc. 2007, 129, 8422–8423. [Google Scholar] [CrossRef]
- Chu, C.-L.; Chen, J.-R.; Lee, T.-Y. Enhancement of hydrogen adsorption by alkali-metal cation doping of metal-organic framework-5. Int. J. Hydrogen Energy 2012, 37, 6721–6726. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Yang, W.; Cao, D. Lithium doping on metal-organic frameworks for enhancing H2 Storage. Int. J. Hydrogen Energy 2012, 37, 946–950. [Google Scholar] [CrossRef]
- Meng, Z.; Lu, R.; Rao, D.; Kan, E.; Xiao, C.; Deng, K. Catenated metal-organic frameworks: Promising hydrogen purification materials and high hydrogen storage medium with further lithium doping. Int. J. Hydrogen Energy 2013, 38, 9811–9818. [Google Scholar] [CrossRef]
- Sengupta, D.; Melix, P.; Bose, S.; Duncan, J.; Wang, X.; Mian, M.R.; Kirlikovali, K.O.; Joodaki, F.; Islamoglu, T.; Yildirim, T.; et al. Air-stable Cu (I) metal–organic framework for hydrogen storage. J. Am. Chem. Soc. 2023, 145, 20492–20502. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.A.; Obeso, J.L.; Vaidyula, R.R.; López-Cervantes, V.B.; Peralta, R.A.; Marín Rosas, P.; de los Reyes, J.A.; Mullins, C.B.; Ibarra, I.A. From pollution to energy storage: Leveraging hydrogen sulfide with SU-101 cathodes in lithium-sulfur batteries. J. Mater. Chem. A 2024, 12, 32735–32744. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Im, K.; Tu, T.N.; Park, J.; Kim, J. ZIF67-derived ultrafine Co9S8 nanoparticles embedded in nitrogen-doped hollow carbon nanocages for enhanced performances of trifunctional ORR/OER/HER and overall water splitting. Carbon Lett. 2024, 34, 1915–1925. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A comprehensive review of the prospects for future hydrogen storage in materials-application and outstanding issues. Int. J. Energy Res. 2022, 46, 16150–16177. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, S.; Wu, C.; Park, S.-S.; Ye, J.; Wu, Y. Advancing integrated high–energy metal–gas batteries. J. Power Sources 2024, 612, 234797. [Google Scholar] [CrossRef]
- Prabu, S.; Vinu, M.; Mariappan, A.; Dharman, R.K.; Oh, T.H.; Chiang, K.Y. Synthesis of Cr(OH)3/ZrO2@Co-based metal-organic framework from waste poly (ethylene terephthalate) for hydrogen production via formic acid dehydrogenation at low temperature. Ceram. Int. 2024, 50, 24293–24301. [Google Scholar] [CrossRef]
- Said, M.I.; Dardir, F.M.; Gabr, R.M.; Ahmed, E.A.; Soliman, M.F.; Abukhadra, M.R. CuMOF@Faujasite nanocomposite as a novel catalyst for hydrogen production. Appl. Organomet. Chem. 2024, 38, e7455. [Google Scholar] [CrossRef]
- Seyyedattar, M.; Zendehboudi, S.; Ghamartale, A.; Afshar, M. Advancing hydrogen storage predictions in metal-organic frameworks: A comparative study of LightGBM and random forest models with data enhancement. Int. J. Hydrogen Energy 2024, 69, 158–172. [Google Scholar] [CrossRef]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.K.; Hua, T.Q. Sorbent material property requirements for on-board hydrogen storage for automotive fuel cell systems. Int. J. Hydrogen Energy 2015, 40, 6373–6390. [Google Scholar] [CrossRef][Green Version]
- Sinha, N.; Pakhira, S. Hydrogen: A Future Chemical Fuel. In Photoelectrochemical Hydrogen Generation: Theory, Materials Advances, and Challenges; Kumar, P., Devi, P., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 1–30. [Google Scholar]
- Singh, M.K.; Krishnan, S.; Singh, K.; Rai, D.K. CNT Interwoven Cu-MOF: A Synergistic Electrochemical Approach for Solid-State Supercapacitor and Hydrogen Evolution Reaction. Energy Fuels 2024, 38, 12098–12110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, Z.; Feng, X.; Hou, H.; Zhang, Y. Recent Advances of Metal–Organic Framework for Heavy Metal Ions Adsorption. Langmuir 2024, 40, 17868–17888. [Google Scholar] [CrossRef]
- Han, L. Engineering Smart Thermal Properties in Metal-Organic-Frameworks. Ph.D. Thesis, University of California, Riverside, CA, USA, 2018. [Google Scholar]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.-S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N. Strategy of Purifier Selection and Integration in Hydrogen Networks. Chem. Eng. Res. Des. 2004, 82, 1315–1330. [Google Scholar] [CrossRef]
- Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Wang, X.; Breunig, H.M.; Peng, P. Broad range material-to-system screening of metal–organic frameworks for hydrogen storage using machine learning. Appl. Energy 2025, 383, 125346. [Google Scholar] [CrossRef]
- Minuto, F.D.; Rozzi, E.; Borchiellini, R.; Lanzini, A. Modeling hydrogen storage at room temperature: Adsorbent materials for boosting pressure reduction in compressed H2 tanks. J. Energy Storage 2024, 90, 111758. [Google Scholar] [CrossRef]
- Hamdy, S.; Morosuk, T.; Tsatsaronis, G. Exergetic and economic assessment of integrated cryogenic energy storage systems. Cryogenics 2019, 99, 39–50. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Grobivć Novaković, J.; Novaković, N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Lo Russo, S. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.; Knight, P.; Vincent, A.J. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 1999, 293, 877–888. [Google Scholar] [CrossRef]
- Panwar, K.; Srivastava, S. Theoretical model on the electronic properties of multi-element AB5-type metal hydride. Int. J. Hydrogen Energy 2021, 46, 10819–10829. [Google Scholar] [CrossRef]
- Xie, D. Effect of Surface Coating on Electrochemical Properties of Rare Earth-Based AB5-type Hydrogen Storage Alloys. Int. J. Electrochem. Sci. 2016, 11, 9153–9163. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xia, B.; Huang, T.; Chen, J.; Wang, Z.; Xu, N. Hydrogen storage in Ti–V-based body-centered-cubic phase alloys. J. Mater. Res. 2003, 18, 2533–2536. [Google Scholar] [CrossRef]
- Yang, B.; He, J.; Zhang, G.; Guo, J. Vanadium Series Products and Functional Materials. Vanadium Extr. Manuf. Appl. 2021, 395–413. [Google Scholar]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Zou, Y.; Chu, H.; Li, B.; Zhang, K. Enhanced hydrogen storage/sensing of metal hydrides by nanomodification. Mater. Today Nano 2020, 9, 100071. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Zhang, J.; Hou, Q.; Zou, J. Progress in improving hydrogen storage properties of Mg-based materials. Mater. Today Adv. 2023, 19, 100387. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Kang, W.; Cai, X.; Zhou, L. Hydrogen storage properties of MgTiVZrNb high-entropy alloy and its catalytic effect upon hydrogen storage in Mg. Int. J. Hydrogen Energy 2024, 50, 1113–1128. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Ding, X.; Chen, R.; Chen, X.; Cao, W.; Su, Y.; Ding, H.; Guo, J. Formation of Mg2Ni/Cu phase and de-/hydrogenation behavior of Mg91Ni9-xCux alloy at moderate temperatures. Renew. Energy 2020, 166, 81–90. [Google Scholar] [CrossRef]
- Yin, F.; Chen, Z.; Si, T.; Liu, D.; Li, Y.; Li, H.-W.; Zhang, Q. Structural-regulation of Laves phase high-entropy alloys to catalytically enhance hydrogen desorption from MgH2. J. Alloys Compd. 2024, 997, 174822. [Google Scholar] [CrossRef]
- Van Vleet, M.J.; Weng, T.; Li, X.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal–Organic Framework Nucleation and Growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Dansirima, P.; Pangon, A.; Utke, O.; Utke, R. Dehydrogenation kinetics of MgH2-based hydrogen storage tank at different operating temperatures and mass flow rates. Int. J. Hydrogen Energy 2022, 47, 7351–7361. [Google Scholar] [CrossRef]
- Wan, H.; Yang, X.; Zhou, S.; Ran, L.; Lu, Y.; Chen, Y.; Wang, J.; Pan, F. Enhancing hydrogen storage properties of MgH2 using FeCoNiCrMn high entropy alloy catalysts. J. Mater. Sci. Technol. 2023, 149, 88–98. [Google Scholar] [CrossRef]
- Yadav, Y.K.; Shaz, M.A.; Yadav, T.P. Al–Cu–Fe–Ni–Ti high entropy alloy nanoparticles as new catalyst for hydrogen sorption in MgH2. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Yu, R.; Yang, C.; Liu, R.; Xu, Z.; Hu, C.; Xia, L.; Jin, Y.; Liu, X.; Shui, J. Tungsten clusters derived from phosphotungstic acid to enhance hydrogen storage efficiency in MgH2. Int. J. Hydrogen Energy 2024, 55, 778–785. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, Z. Overview of hydrogen storage and transportation technology in China. Unconv. Resour. 2023, 3, 291–296. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, L.; Yan, W.; Li, Z.; Fan, M.; Du, G.; Zhao, W. Controlled preparation of nitrogen-doped hierarchical carbon cryogels derived from Phenolic-Based resin and their CO2 adsorption properties. Energy 2022, 246, 123367. [Google Scholar] [CrossRef]
- Ghorbani, B.; Zendehboudi, S.; Saady, N.M.C.; Dusseault, M.B. Hydrogen storage in North America: Status, prospects, and challenges. J. Environ. Chem. Eng. 2023, 11, 109957. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. An overview of TiFe alloys for hydrogen storage: Structure, processes, properties, and applications. J. Energy Storage 2023, 68, 107772. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Ma, H.; Wang, H.; Hua, L.; Fu, S. Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review. Metals 2021, 11, 1303. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Mao, W.L.; Mao, H.-K. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. USA 2004, 101, 708–710. [Google Scholar] [CrossRef]
- Cai, X.; Worley, J.; Phan, A.; Salvalaglio, M.; Koh, C.; Striolo, A. Understanding the effect of moderate concentration SDS on CO2 hydrates growth in the presence of THF. J. Colloid Interface Sci. 2024, 658, 1–11. [Google Scholar] [CrossRef]
- Fiedler, F.; Vinš, V.; Jäger, A.; Span, R. Modification of the van der Waals and Platteeuw model for gas hydrates considering multiple cage occupancy. J. Chem. Phys. 2024, 160. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lee, W.; Kim, M.K.; Lee, J.W.; Ahn, Y.H. Hydrogen separation from hydrogen-compressed natural gas blends through successive hydrate formations. Chem. Eng. J. 2024, 483, 149409. [Google Scholar] [CrossRef]
- Hu, W.; Tian, X.; Chen, C.; Cheng, C.; Zhu, S.; Zhang, J.; Qi, T.; Jin, T.; Wu, X. Molecular dynamic simulation of H2-CH4 binary hydrate growth induced by methane hydrate. Fuel 2024, 360, 130554. [Google Scholar] [CrossRef]
- Suzuki, K.; Wada, R.; Konno, Y.; Hiekata, K.; Nanjo, T.; Nagakubo, S. Impact of epistemic uncertainty on tradeoff in model-based decision support for methane hydrate development system design. Appl. Energy 2024, 356, 122408. [Google Scholar] [CrossRef]
- Wang, P.; Long, H.; Teng, Y.; Li, Y.; Li, Y.; Zhu, J.; Xie, H.; Han, S.; Zhao, Y.; Zhu, J. Investigation of hydrogen-propane hydrate formation mechanism and optimal pressure range via hydrate-based hydrogen storage. Fuel 2024, 361, 130791. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; He, S.; Zheng, J.N.; Jiang, L.; Song, Y. Enhanced CO2 hydrate formation using hydrogen-rich stones, L-Methionine and SDS: Insights from kinetic and morphological studies. Energy 2024, 291, 130280. [Google Scholar] [CrossRef]
- Khan, M.N. Hydrogen as future sustainable energy resource: An insight into technological advancements in hydrate-based hydrogen storage. Int. J. Hydrogen Energy 2025, 97, 1386–1398. [Google Scholar] [CrossRef]
- Kim, M.K.; Ahn, Y.H. Gas Hydrates for Hydrogen Storage: A Comprehensive Review and Future Prospects. Korean J. Chem. Eng. 2024, 41, 73–94. [Google Scholar] [CrossRef]
- Krishna, S.; Mahant, B.; Robustillo, M.D.; Sreenivasan, H.; Pandey, J.S. The Role of Gas Hydrates in Storing Natural Gas-Hydrogen Blends for Coupling Power-to-X and Decarbonization. Energy Fuels 2024, 38, 23192–23229. [Google Scholar] [CrossRef]
- Kummamuru, N.B.; Ciocarlan, R.G.; Houlleberghs, M.; Martens, J.; Breynaert, E.; Verbruggen, S.W.; Cool, P.; Perreault, P. Surface modification of mesostructured cellular foam to enhance hydrogen storage in binary THF/H2 clathrate hydrate. Sustain. Energy Fuels 2024, 8, 2824–2838. [Google Scholar] [CrossRef]
- Lan, X.; Chen, J.; Li, D.; Zheng, J.; Linga, P. Gas storage via clathrate hydrates: Advances, challenges, and prospects. Gas Sci. Eng. 2024, 129, 205388. [Google Scholar] [CrossRef]
- Kumar, R.; Klug, D.D.; Ratcliffe, C.I.; Tulk, C.A.; Ripmeester, J.A. Low-pressure synthesis and characterization of hydrogen-filled ice Ic. ChemInform 2013, 125, 1571. [Google Scholar]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Papaioannou, A.T.; Stubos, A.K. Evaluation of the Hydrogen-Storage Capacity of Pure H2 and Binary H2-THF Hydrates with Monte Carlo Simulations. J. Phys. Chem. C 2008, 112, 10294–10302. [Google Scholar] [CrossRef]
- Strobel, T.A.; Koh, C.A.; Sloan, E.D. Water Cavities of sH Clathrate Hydrate Stabilized by Molecular Hydrogen. J. Phys. Chem. B 2008, 112, 1885–1887. [Google Scholar] [CrossRef]
- Trueba, A.T.; Radović, I.R.; Zevenbergen, J.F.; Kroon, M.C.; Peters, C.J. Kinetics measurements and in situ Raman spectroscopy of formation of hydrogen–tetrabutylammonium bromide semi-hydrates. Int. J. Hydrogen Energy 2012, 37, 5790–5797. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.; Lee, J.; Ahn, Y.H.; Lee, J.W. Perspectives on facilitating natural gas and hydrogen storage in clathrate hydrates under a static system. Green Chem. 2024, 26, 7552–7578. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Lang, X.; Li, G.; Lu, E.; Tian, W.; Fan, S. Hydrate-Based Hydrogen Storage and Transportation System: Energy, Exergy, Economic Analysis. In Lecture Notes in Civil Engineering; Springer: Singapore, 2024; pp. 405–421. [Google Scholar]
- Mahant, B.; Kushwaha, O.S.; Kumar, R. Thermodynamic phase equilibria study of Hythane (methane + hydrogen) gas hydrates for enhanced energy storage applications. Fluid Phase Equilibria 2024, 582, 114089. [Google Scholar] [CrossRef]
- Mahant, B.; Swethika, C.S.; Kushwaha, O.S.; Kumar, R. Storage and Transportation of Hythane: Thermodynamics, Kinetics, and Stability Studies Using the Gas Hydrates Process. Energy Fuels 2024, 38, 17586–17607. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Mahmoudi, A.; Ghasemi, H.J.I. The potential of hydrogen hydrate as a future hydrogen storage medium. iScience 2021, 24, 101907. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.A. Gas hydrate technological applications: From energy recovery to carbon capture and storage. Gas Sci. Eng. 2024, 131, 205455. [Google Scholar] [CrossRef]
- Nguyen, N.N. Prospect and Challenges of Hydrate-Based Hydrogen Storage in the Low-Carbon Future. Energy Fuels 2023, 37, 9771–9789. [Google Scholar] [CrossRef]
- Dohrn, R.; Peper, S.; Fonseca, J.M.S. High-pressure fluid-phase equilibria: Experimental methods and systems investigated (2000–2004). Fluid Phase Equilibria 2010, 288, 1–54. [Google Scholar] [CrossRef]
- Hashimoto, S.; Murayama, S.; Sugahara, T.; Sato, H.; Ohgaki, K. Thermodynamic and Raman spectroscopic studies on H2+ tetrahydrofuran+ water and H2+ tetra-n-butyl ammonium bromide+ water mixtures containing gas hydrates. Chem. Eng. Sci. 2006, 61, 7884–7888. [Google Scholar] [CrossRef]
- Du, J.-W.; Liang, D.-Q.; Dai, X.-X.; Li, D.-L.; Li, X.-J. Hydrate phase equilibrium for the (hydrogen+ tert-butylamine+ water) system. J. Chem. Thermodyn. 2011, 43, 617–621. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Yang, J.; Zhu, J.; Zhao, Y.; Teng, Y. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate. Chem. Eng. J. 2021, 426, 131279. [Google Scholar] [CrossRef]
- Zhang, M.; Ni, D. Evaluating the gas storage capacity of 1,3-dioxolane–hydrogen binary hydrates via molecular simulations. J. Mol. Liq. 2024, 393, 123542. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Veluswamy, H.P.; Kumar, A.; Linga, P. Stability analysis of methane hydrates for gas storage application. Chem. Eng. J. 2021, 415, 123542. [Google Scholar] [CrossRef]
- Borah, U.B.; Pandey, G.; Sarma, S.; Molokitina, N.; Chauhan, G. Affordable and Clean Energy: Natural Gas Hydrates and Hydrogen Storage. In Clean and Renewable Energy Production; Wiley-Scrivener: Hoboken, NJ, USA, 2024; pp. 87–121. [Google Scholar]
- Kong, Y.; Yu, H.; Liu, M.; Zhang, G.; Wang, F. Ultra-rapid formation of mixed H2/DIOX/THF hydrate under low driving force: Important insight for hydrate-based hydrogen storage. Appl. Energy 2024, 362, 123029. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Q.; Liu, Y.; Cheng, L.; Taylor Isimjan, T.; Tian, J.; Yang, X. Electronic metal-support interactions for defect-induced Ru/Co-Sm2O3 mesosphere to achieve efficient NaBH4 hydrolysis activity. J. Catal. 2024, 433, 115491. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Kumar, R.; Linga, P. Solidified Hydrogen Storage (Solid-HyStore) via Clathrate Hydrates. Chem. Eng. J. 2022, 431, 133702. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Linga, P. Kinetic Study of Mixed Hydrogen/1,3-Dioxolane Hydrates in the Presence of Amino Acids. Energy Proc. 2021, 18, 169. [Google Scholar]









| Mode of Transportation | Cost |
|---|---|
| state hydrogen trailers | 1.5–3 USD/kg |
| liquid hydrogen tankers | 2–4 USD/kg |
| pipeline transportation | 0.5–1.5 USD/kg |
| pipelines | 0.05–0.15 USD per liter |
| oil tankers | 0.02–0.05 USD per liter |
| rail/road tankers | 0.1–0.3 USD per liter |
| gas transportation | 0.001–0.03 USD/m3 |
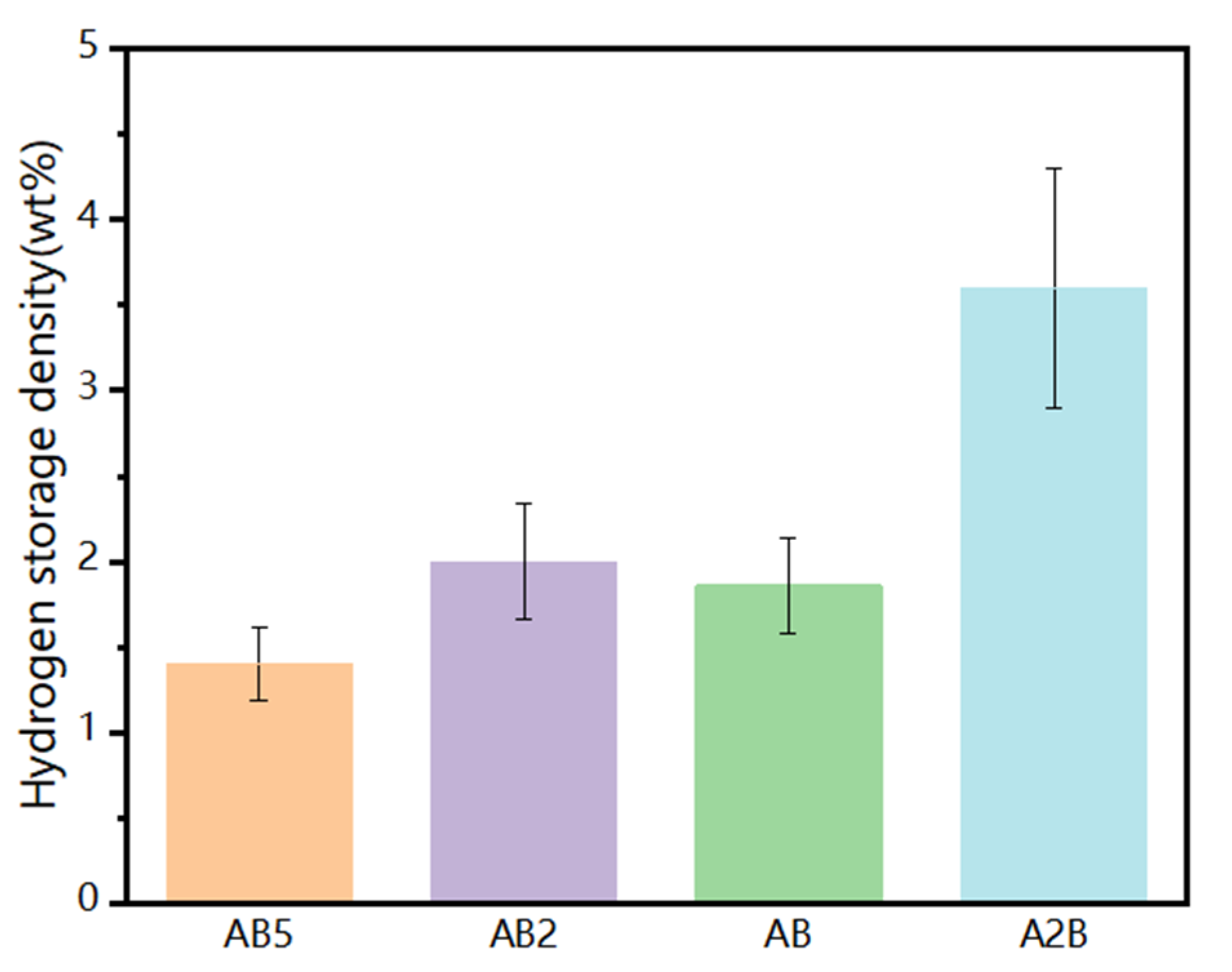
| Hydrogen Storage Alloy | Represents Alloy | Temperature (K) | Quality Hydrogen Storage Capacity (wt%) |
|---|---|---|---|
| AB5 | LaNi5 | 298 | 1.4 |
| AB2 | TiMn2 | 298 | 2.0 |
| AB | TiFe | 298 | 1.86 |
| A2B | Mg2Ni | 523 | 3.6 |
| Country Case | Hydrogen Storage Materials | Cost of Hydrogen Storage |
|---|---|---|
| China (Xiamen Tungsten Industry) | Magnesium-based materials | 4 USD/kg (within a transport radius of 100 km) 20 USD/kg (within a transport radius of 500 km) |
| United States (2020 DOE Report) | NaAlH4 | 43 USD/kg |
| Japan (Japan Metals & Chemicals) | LaNi5 | 15–25 USD/kg |
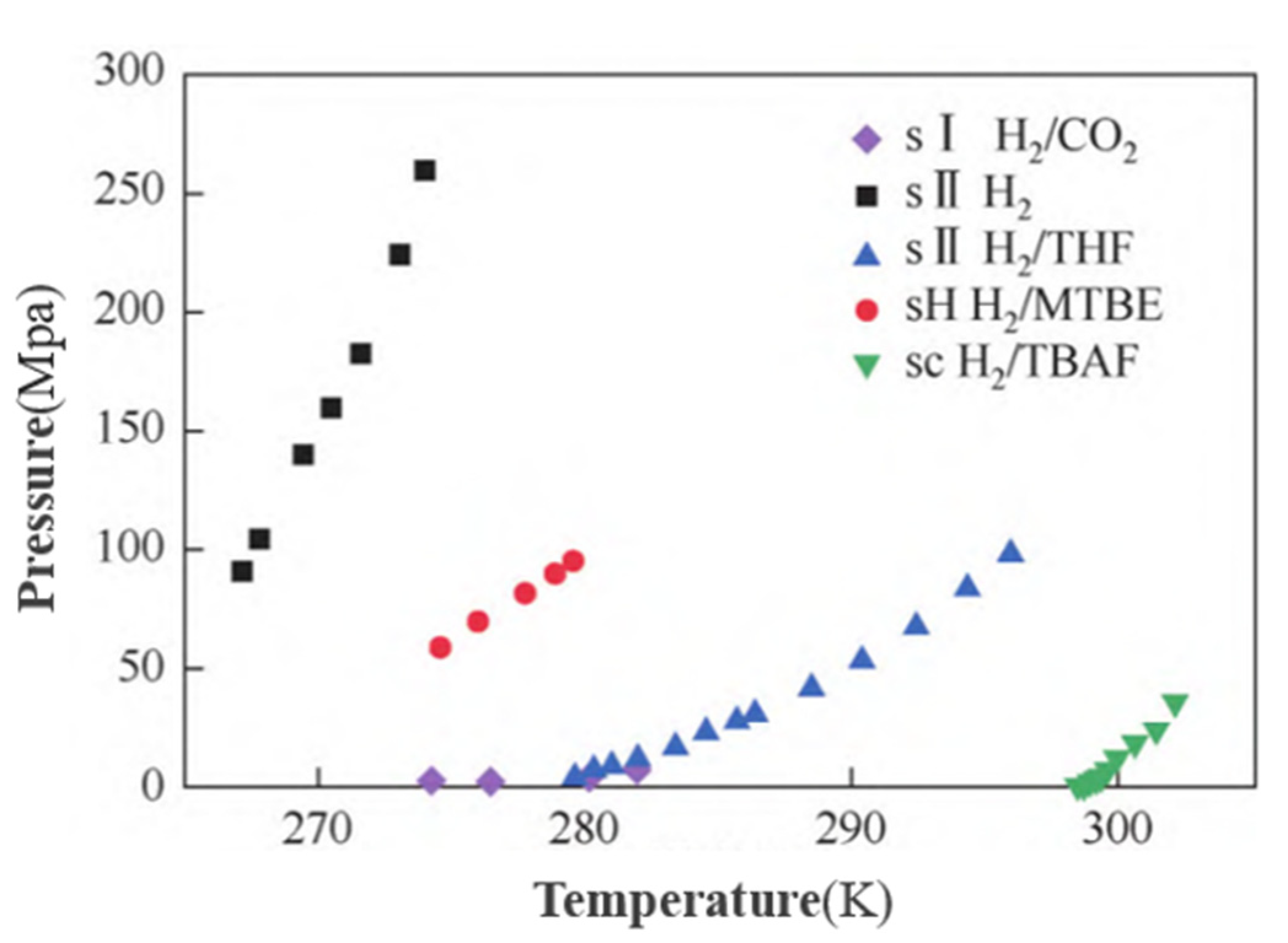
| Crystal Structure | System | Temperature (K) | Pressure (MPa) | Hydrogen Storage Density (wt%) |
|---|---|---|---|---|
| sI | H2/CO2 | 270 | 200 | 0.37 |
| sII | Pure water | 273 | 200~300 | 5.3 |
| sH | H2/MTBE | 270 | 100 | 1.4 |
| sc | H2/TBAB | 281.15 | 16 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Liang, D.; Kang, Z.; Fan, J.; Fan, S.; Zhou, X. Review of Hydrogen Storage in Solid-State Materials. Energies 2025, 18, 2930. https://doi.org/10.3390/en18112930
Chen G, Liang D, Kang Z, Fan J, Fan S, Zhou X. Review of Hydrogen Storage in Solid-State Materials. Energies. 2025; 18(11):2930. https://doi.org/10.3390/en18112930
Chicago/Turabian StyleChen, Gelin, Deqing Liang, Zhanxiao Kang, Jintu Fan, Shuanshi Fan, and Xuebing Zhou. 2025. "Review of Hydrogen Storage in Solid-State Materials" Energies 18, no. 11: 2930. https://doi.org/10.3390/en18112930
APA StyleChen, G., Liang, D., Kang, Z., Fan, J., Fan, S., & Zhou, X. (2025). Review of Hydrogen Storage in Solid-State Materials. Energies, 18(11), 2930. https://doi.org/10.3390/en18112930








