1. Introduction
The energy crisis and rising energy prices are forcing humanity to look for alternatives to mineral resources, among which renewable energy sources occupy an important place. This is encouraged by national legislation in force in the countries and a unified EU policy. Over the past two decades, the production and use of biofuels has increased significantly throughout the European Union, and their use is also spreading to other countries of the world. Biofuel for diesel engines is produced from vegetable oil by applying transesterification it with methanol. Catalysts, usually homogeneous alkaline or acidic catalysts, are used to increase the rate of this process. As acidic homogeneous catalysts most commonly used are organic and inorganic acids, such phosphoric, hydrochloric and sulfonic acids. Potassium or sodium hydroxide or potassium or sodium methylate is used as an alkaline catalyst. Mechanism of action of alkali catalysts is based on the phenomenon of protonation. When applying homogeneous alkaline catalysis and using alkalis, the alcohol is first protonated, and when it reacts with the alkali, a hydrogen proton is separated from the alcohol molecule, forming an alkoxide ion, which reacts with a triglyceride molecule to form an intermediate compound. This intermediate is very unstable. There, a rearrangement occurs to obtain a stable form. This gives a new fatty acid alkyl ester. The transesterification produces a nucleophile as a by-product. Using acid catalysts, the carbonyl is first protonated. Which is then attacked by an alcohol to form a tetrahedral intermediate, which is unstable. Several protonation and deprotonation steps occur to obtain a new fatty acid alkyl ester.
However, the use of homogeneous catalysts, in addition to the advantages, also has disadvantages: it is difficult to purify the product, regenerate the catalysts and reuse them. In addition, these catalysts are obtained from mineral resources by applying the chemical processes. To solve these problems, it is proposed to use heterogeneous catalysts that have high catalytic activity in the transesterification process, can be obtained from natural resources, are easily separated from the transesterification product, regenerated and reused [
1]. They are tolerant to high free fatty acid feedstock containing high moisture content. These catalysts are classified as acidic and alkaline. Transesterification reactions in this case occur on the catalyst surface, on which the reacting substances are adsorbed, and the compounds formed during the reaction are desorbed from the surface. The catalytic mechanism of acidic heterogeneous catalysts is explained by the Bronsted-Lewis acid theory. In the case of heterogeneous acid catalysis, the process occurs on the active centers of the Brønsted acid catalyst surface. The carbonyl groups of triglycerides are protonated on them. The alcohol attacks the carbon proton, forming a reactive center and creating an intermediate compound. During the intermediate stages, bond breakage occurs and the final product is formed—alkyl esters of fatty acids. When a Lewis acid catalyst is used, the oxygen atoms of the carbonyl group in triglycerides interact with the Lewis acid and form electrophilic complexes, which react with alcohols and form nucleophilic bonds. Finally, fatty acid alkyl esters are formed, and the Lewis acid is separated from the carbonyl oxygen. The mechanism of action of a heterogeneous base catalyst is similar to that of a homogeneous base catalyst. First, after methanol is adsorbed on the surface of the solid base, ion exchange occurs, forming a catalytically active compound that is strongly basic and very catalytically active. It attacks the carbonyl carbon atom of the triglyceride and forms a tetrahedral intermediate, which is unstable and is converted to fatty acid alkyl esters.
Among heterogeneous catalysts, much attention is paid to biocatalysts—lipases, which are obtained from natural sources, work effectively when using oils of higher acidity or in the presence of water in the reaction mixture, and are easily separated from the reaction product—biodiesel [
2]. However, lipases are expensive compared to chemical catalysts, making their industrial use difficult.
Another groups of heterogeneous catalysts are mixed metal oxides, alkali-doped metal oxides and hydrotalcites [
3]. CaO and MgO are most commonly used in biodiesel synthesis. They are characterized by low cost and high catalytic efficiency. They can be used more than once. These compounds can be obtained by chemical synthesis, but from an environmental point of view it is advisable to use naturally occurring materials or various wastes containing calcium and magnesium compounds, after appropriate preparation for biodiesel synthesis. The main disadvantage of CaO when using biodiesel in synthesis is its sensitivity to free fatty acids. During the transesterification process, calcium leaching occurs, which reacts with free fatty acids to form soaps. Due to the reduction of calcium content in the catalyst, its activity decreases, thus ways are sought to stabilize calcium by modifying the catalyst with oxides of other metals [
4].
CaO and MgO can be obtained from carbonate rocks found in nature. One of the more promising such rocks is dolomite, which consists of calcium and magnesium carbonate that decomposes into calcium and magnesium oxides when heated to high temperatures. It is characterized by low cost, less toxicity, and is environmentally friendly [
5].
A lot of research has already been done in this area, with varying results. The aim of this article is to review the methods of preparation of dolomite and its efficiency in using them as heterogeneous catalysts in biodiesel synthesis.
2. Preparation of Dolomite for Use in the Transesterification Process
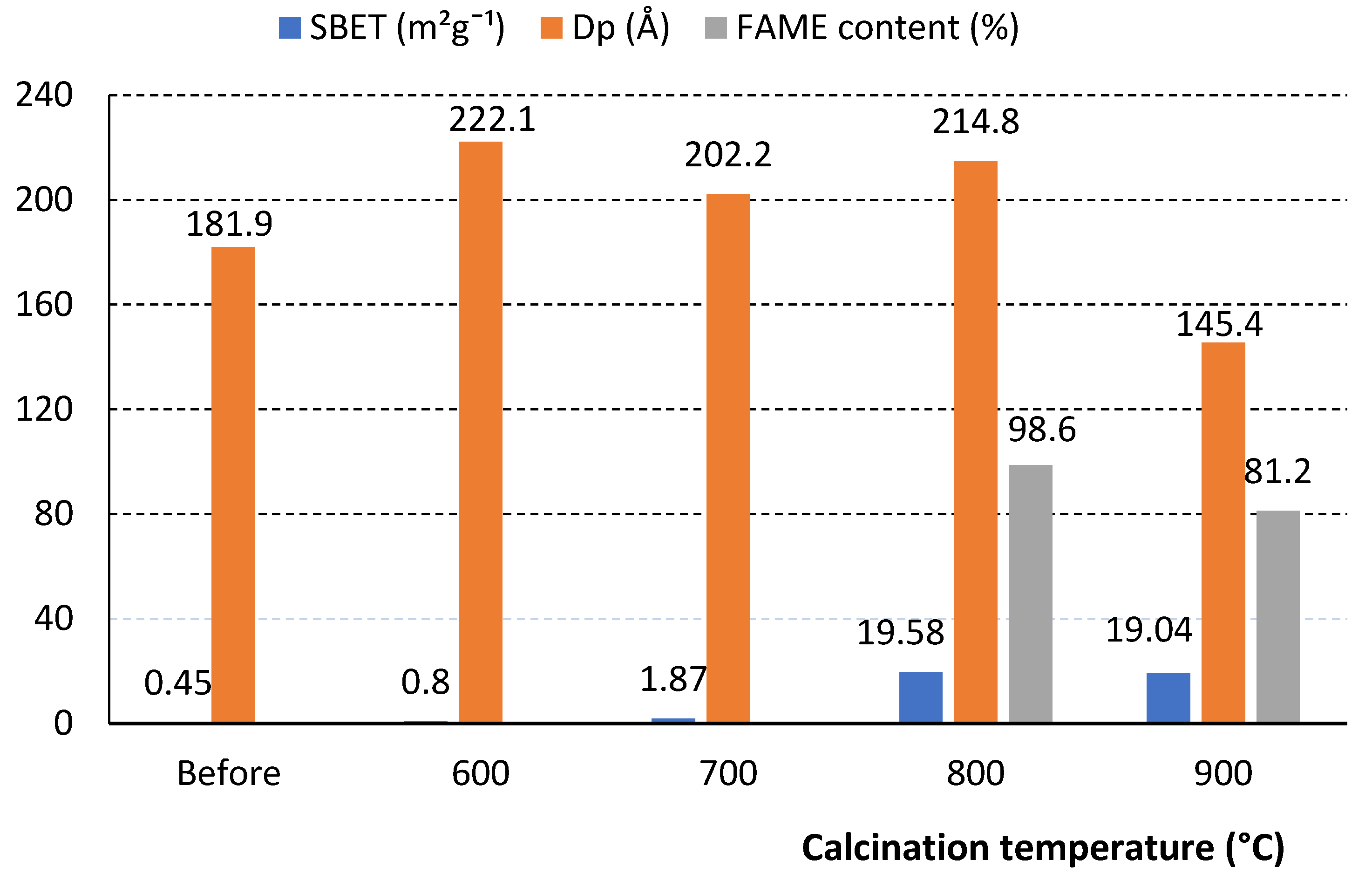
Among the natural rocks containing Ca and Mg compounds and most widely studied as potential raw materials for heterogeneous catalysts for biodiesel synthesis, such rocks as dolomite, limestone, calcite, silicate mineral albite, zeolite should be mentioned. Analyzing the efficiency of natural rocks in the biodiesel synthesis process, it was found that the highest yield of fatty acid methyl esters (FAME) is obtained using dolomite (98.6%) and calcite (46.8%). The Ca and Mg compounds in these rocks are in the form of carbonates. The transesterification reaction is catalyzed by oxides, therefore, before using these rocks for biodiesel synthesis, they must be additionally prepared—crushed and calcined at high temperatures. During calcination, the specific surface area of the catalyst, the volume and size of pores, and the number of active centers are increased, therefore, the catalytic activity improves, which depends on the calcination temperature and duration. Meanwhile, using calcined apatite and dicalcium phosphate, a very low yield of FAME was obtained.
The authors used calcite and dolomite, crushed them to <10 μm, and in order to determine the optimal decomposition temperature of calcium and magnesium carbonate, calcined them in a muffle furnace at 600–900 °C for 2 h [
5]. The optimal calcination temperature for dolomite was found to be 800 °C. It is indicated that dolomite is decarbonized at a lower temperature than calcite, the optimal decarbonization temperature of which is about 32 °C higher than that of dolomite. It was observed that dolomite calcined at 500–600 °C did not exhibit catalytic properties. When using it, no fatty acid methyl esters were found in the reaction product. Only when calcined at a temperature above 700 °C an increase in surface roughness observed in dolomite, which affected the catalytic activity. This is explained by the fact that although MgCO
3 decomposition to MgO occurs below 700 °C, such a temperature is insufficient for the decomposition of CaCO
3 to CaO, which is more active in the transesterification reaction than MgO.
Ngamcharussrivichai et al. found that using dolomite calcined at 800 °C, a 98.6% yield of (FAME) could be obtained, while dolomite calcined at 900 °C gave only 81.2% yield of FAME under the same transesterification conditions [
5]. Similar conclusions were made by most scientists who studied the influence of rock calcination temperature on catalytic activity. They found that the optimal limestone calcination temperature is 825–900 °C [
6]. Gaide et al. studied the influence of dolomite preparation (grinding, calcination) on catalytic efficiency and found that the highest amount of biodiesel is obtained using a 0.315–0.1 mm dolomite fraction and calcining at 800 °C for 2 h [
6]. Ergan et al. used industrially produced dolomite with a particle size of 200 mesh and heated it at 840 °C for 3 h [
7]. Korkut et al. used dolomite calcined at 840 °C for 3 h [
8]. Niu et al. studied the influence of calcination temperature on catalytic capacity of dolomite and found that calcination at 800 °C allows obtaining catalytic efficiency higher than that of calcium methoxide, and basic strange is similar to that obtained by other scientists who studied the dolomite calcination process at 750–900 °C [
9]. Wang et al. proposed a cheap method of preparing calcite and dolomite by calcining it with stearic acid. They obtained catalysts with good pore structure and high basic strength [
10]. A slightly higher calcination temperature was used by Wilson et al. [
11]. Before calcination, dolomite was ground and sieved to a particle size of 149–250 μm, then calcined at 900 °C for 3 h. Some authors indicate that MgO present in dolomite reduces its catalytic activity, therefore they propose a hydrothermal limestone treatment method to separate CaO from dolomite and calcinate it at 800 °C for 2 h to obtain 98% pure CaO and use it in biodiesel synthesis [
12].
Many authors indicate that the decomposition of CaCO
3 takes place at atmospheric pressure at a temperature of 825–920 °C over 2 h, forming CaO basic sites. XRD analysis results showed that during calcination at a temperature of 800 °C, almost all diffraction peaks associated with calcium carbonates disappear, but intense cubic CaO peaks are found. TGA analysis showed that the maximum weight loss of dolomite was observed at a temperature of 754 °C. Considering that it is necessary to break the CO
2 film forming on the surface of dolomite, the calcination temperature at which calcium carbonate completely decomposes must be higher than the temperature obtained during TG analysis. However, it was observed that increasing the duration from 2 to 6 h does not have a significant effect. Results of SEM analysis confirmed that the optimal dolomite calcination temperature is 800–900 °C. At 700 °C, the calcined dolomite showed no noticeable roughness of the dolomite surface. It has been observed that the highest surface area and average pore diameter were obtained by calcining dolomite at 800 °C, while calcining at lower temperatures showed only slight changes in surface area according to BET analysis. At this temperature, calcined dolomite used in the transesterification reaction yielded the highest amount of FAME (
Figure 1).
Calcining at temperatures higher than 900 °C resulted in a significant decrease in surface area, pore size, and pore volume. Although the MgCO
3 present in dolomite degrades at lower temperatures, MgO has a low basic strength, leading to the conclusion that CaO is the main compound effective in transesterification process, which requires a higher calcination temperature than MgO [
5].
One of the main properties that depends on the preparation of the dolomite catalyst and shows the catalytic activity is its basic strength. The basic strength shown in
Table 1 was obtained by different authors who studied the properties of calcined dolomite. The basic strength data confirm that calcination at lower temperatures is not effective. In order to convert all carbonates into oxides, a temperature higher than 700 °C is necessary, and the results of ester yields show that the optimal calcination temperature is 800–850 °C [
13].
Although the research results show that calcined dolomite has prospects for use as a catalyst for biodiesel synthesis, its activity is lower than that of other homogeneous catalysts used in the synthesis. Therefore, ways are being sought to increase the catalytic activity of dolomite and at the same time reduce the transesterification process duration and the amount of catalyst used. Recently, researchers have been interested in the possibilities of increasing its catalytic activity through additional processes and additives. Most research has been conducted on modifying dolomite. The main goal of modification is to increase catalytic efficiency by enhancing the factors that determine it, such as pore volume, surface area, crystallinity, and basic strength. Yoosuk et al. proposed the hydration–dehydration process of calcined dolomite and demonstrated that it improved the basic strength and catalytic properties. In this process, dolomite is calcined to produce CaO, then hydrated to CaOH, which is then transformed back to CaO. This changes the chemical composition and textural properties of the catalyst [
14]. A similar process was proposed by Widiarti et al., who investigated the method of increasing the catalytic activity of limestone using the calcination–hydration–dehydration method and found that its basic strength is greater than 15.0 [
12]. Even higher catalyst activity was obtained by dolomite impregnating it with NiO. Çakırca et al. proposed modifying dolomite by impregnating it with calcium acetate and obtained a yield of more than 90% of microalgal oil methyl esters [
15]. Ground and sieved dolomite to a particle size of 50 μm and calcined at 900 °C for 4 h was also used to prepare heterogeneous Na-CaO/MgO dolomite catalysts by doping with sodium nitrate and subsequent calcination. The catalyst prepared in this way was characterized by high catalytic activity, the obtained yield of canola oil methyl esters was higher than 95% within 7 h [
16]. Zhao et al. propose the use of a catalyst obtained by the impregnation method. Dried dolomite powder is mixed with a lanthanum nitrate solution and calcined at 800 °C. Using this catalyst under optimal conditions, a biodiesel yield of 98.6% was obtained [
17].
Calcined at 700 °C for 2 h and ground to a uniform particle size, dolomite rock was used for impregnation with Fe
3O
4 to obtain a magnetite catalyst [
18]. This catalyst was tested in the synthesis of palm oil methyl esters and a biodiesel yield of 96.6% was obtained. Dolomite was also used for impregnation with Ce(NO
3)
3·9H
2O and calcined at 800 °C for 4 h to obtain a highly efficient catalyst [
9]. Ngamcharussrivichai et al. studied the possibilities of modifying calcined dolomite with various metals using the precipitation method. It was found that the catalyst in which the dolomite was modified with calcium had the highest activity. When using it, the biodiesel yield reached 99.9%. Additional treatment is intended not only to increase the catalytic activity but also to solve the problems of CaO leaching [
19]. Woo et al. studied a bead-type dolomite catalyst. It was prepared from dolomite calcined at 800 °C and 20 wt% pseudoboehmite sol as an inorganic binder and found that more than 80% jatropha oil conversion was obtained within 2 h [
20]. Widayat et al. attempted to increase the catalytic activity of dolomite by synthesizing a catalyst obtained from dolomite and iron sand by calcining the product at 1000 °C. Using such a catalyst for the transesterification of waste cooking oil, they obtained a 95.64% ester yield [
18].
3. Efficiency of Dolomite in the Triglyceride Transesterification Process
Dolomite is a calcium magnesium carbonate (CaMg(CO
3)
2), which can be converted to CaO and MgO. For this reason, dolomite is a promising catalyst for the transesterification of triglycerides. A considerable number of studies have been conducted using various types of oil and methanol as acyl receptor and dolomite as a heterogeneous catalyst, either calcined alone or in combination with other elements or compounds.
Table 2 presents the results obtained. The yield of the transesterification process depends on three main independent variables and their interaction. The amount of catalyst is important in heterogeneous catalysis. When the transesterification reaction occurs on the active sites of catalyst its surface must be sufficient to adsorb the reactants and desorb the reaction products. The molar ratio of methanol to oil is the second variable that affects the rate of the process and the yield of FAME. The transesterification process is an equilibrium process. In order to shift the equilibrium towards the product side, the concentration of one of the reactants in the reaction medium should be increased. During transesterification, the excess of alcohol is used. In the conventional homogeneous catalysis process, the molar ratio of methanol to oil is 6:1. Using a higher amount of alcohol is uneconomical due to the need to evaporate and regenerate an excess of methanol. In heterogeneous catalysis, the duration of the process is also important, which is longer than in the case of homogeneous catalysis. This is related to the slower transfer of reactants to the catalyst surface and the desorption of the reaction product from it. All these variables interact with each other: by increasing the duration, the same yield of FAME can be obtained with a smaller amount of catalyst and methanol, by increasing the amount of catalyst, the same yield can be obtained using a lower duration and a smaller amount of methanol. However, the interaction between the independent variables and the effect on the yield of the product are not the same.
The data presented in
Table 2 show that the transesterification was performed at a temperature close to the boiling point of methanol. This temperature is also commonly used for studies of conventional biodiesel synthesis by applying homogeneous catalysts. The amount of catalyst is a very important parameter affecting the yield of the transesterification process. It depends on the activity of the catalyst, the calcination conditions and modification processes. As can be seen from the results obtained, the optimal amount of catalyst determined by different scientists is very different. High FAME yields were obtained using from 2% to 15.6% calcined dolomite. Using 3% dolomite, Ilgen et al. obtained less than 96% FAME yield and the yield did not meet the requirements of the standard EN 14214 (Liquid petroleum products. Fatty acid methyl esters (FAME) for use in diesel engines and heating applications. Requirements and test methods) which defines the quality of biodiesel [
13]. This was also related to the small excess of methanol in the reaction medium, which did not compensate for the lack of catalyst and the short reaction time. However, Correia et al. obtained a biodiesel yield of 96.52 using only 2% calcined dolomite over an average time, but this was compensated by a higher methanol excess in the reaction medium (molar ratio 9:1) [
21]. In summary, it can be concluded that in order to obtain a high biodiesel yield under average process conditions, it is appropriate to use 4% or more dolomite calcined at 800–850 °C. On the other hand, Wilson et al. used a high amount of dolomite, which reached 15.6%, but the FAME yield was only a few percent higher than that obtained by other researchers [
11]. This could be explained by the fact that the dolomite was calcined at 900 °C and its activity was probably lower than that calcined at 800–850 °C, as indicated by other authors [
5].
The reaction time for transesterification using calcined dolomite ranged from 1.5 to 5 h. The shortest process was performed by Korkut and Bayramoglu and a high methyl ester yield of 97.4% was obtained [
8]. However, in this case, ultrasound was used, which resulted in the process being more efficient even at a transesterification temperature of 60 °C. Niu et al. also obtained a biodiesel yield of 96.07% in 2 h, but used a higher molar ratio of methanol to oil of 12:1 [
22]. Other researchers indicate an average optimal duration of 3–4 h, which is required to obtain a yield of esters greater than 95%. It is not suggested to increase the process further, since a significant increase of FAME yield is not observed. Some authors indicate that if the process duration is longer, hydrolysis of fatty acid esters may begin and the yield of esters decreases [
21]. However, this was not observed by Sendzikiene et al., who determined the optimal process duration of 5 h, during which they obtained a biodiesel yield greater than 97% [
23].
The molar ratio of methanol to oil studied by the researchers varied quite significantly—from 6:1 to 30:1. The highest molar ratio of 30:1 gave the highest ester yield: >98% yield was obtained by Wilson et al. [
11] and 98% yield was obtained by Ngamcharussrivichai et al. [
5]. Ilgen obtained a lower ester yield of 91.78% using a 6:1 molar ratio of methanol to oil [
13], although at the same molar ratio, a 98.81% biodiesel yield was obtained by Correia et al., but a higher amount of catalyst and process duration were applied [
21]. The double excess of methanol used by Sendzikiene et al. did not show a significant effect on the yield, the obtained methyl ester yield was even lower and reached 98.66% and 97.6%, respectively [
23].
As can be seen, all the studied factors and their interaction affect the biodiesel yield. At the lowest dolomite amount and molar ratio of methanol to oil, the ester yield obtained is less than 96%. Meanwhile, using a higher molar ratio of methanol to oil, this yield is higher. And in all other cases it corresponds to the value specified in the standard EN 14214.
Although a number of studies have been conducted on modifying dolomite to increase its catalytic activity in the transesterification reaction, no significant increase in biodiesel yield has been observed using such catalysts. From the data presented in
Table 2, it can be concluded that biodiesel yields greater than 95% are obtained only at a relatively high molar ratio of methanol to oil. Murguía-Ortiz et al. used 12:1 [
16], Nur et al., Niu et al. and Ngamcharussrivichai et al. used 15:1 and Zhao et al. used as much as 18:1 molar ratio of methanol to oil [
17]. When molar ratio of methanol to oil was 6:1, only 90% biodiesel yield was obtained [
15]. However, a large excess of methanol is not desirable due to dilution of the reaction medium and subsequent problems associated with methanol evaporation and regeneration.
Compared to the efficiency of using unmodified calcined dolomite as a catalyst in the transesterification process, it was not observed that a lower amount of modified catalyst was required even when using a large excess of methanol. The catalyst amount ranged between 0.05 and 10%. The lowest amount of cerium-impregnated dolomite was reported by Niu et al., who obtained a 97.21% biodiesel yield in 2 h with a 15:1 methanol to oil molar ratio [
9]. The highest amount of modified with calcium dolomite, reaching 10%, was used by Ngamcharussrivichai et al., who obtained a 99.9% palm methyl ester yield in 3 h and using a relatively high 15:1 methanol to oil molar ratio [
19]. The SnO
2 doped activated dolomite and CaO/MgO/Fe
3O
4 nanomagnetic catalyst obtained from dolomite were characterized by high activity, requiring only 1% of catalyst to obtain 99.98% and 95.64% biodiesel yields using 15:1 and 9:1 molar methanol to oil ratios, respectively [
18,
24]. Murguía-Ortiz et al. used an average of 6% Na nitrate doped dolomite at a relatively high 12:1 methanol to oil molar ratio and obtained over 95% methyl ester yields, while the reaction time was relatively short—7 h [
16]. Meanwhile, when using hydrothermal and coprecipitated CaO from dolomite as a catalyst in biodiesel synthesis, the product yield was low and did not reach 93%. The incorporation of cerium, SnO, lanthanum, and sodium did not give the expected good results in increasing the catalytic efficiency of dolomite. When using modified dolomite, a high molar ratio of methanol to oil was required to obtain a higher yield of esters.
The results of the studies show that modification did not increase the catalytic activity of dolomite in all cases. These results are controversial, as most authors studying the properties of modified dolomite by applying XRD, SEM, and thermogravimetric analysis found that during hydrothermal treatment, pore volume nearly doubles and surface area triples, but pore shape remains unchanged. Additionally, most authors note that applying various modification methods with metals results in catalysts with stronger basic strength, smaller grain size, higher surface area, and narrower pore diameter than calcined dolomite. This should significantly increase the catalytic activity of modified dolomite in the transesterification reaction, although such a significant increase is not observed when comparing with efficiency of pure calcined dolomite [
15,
16,
17,
18].
There are very few research results on the use of other types of alcohols for transesterification using dolomite, therefore, it is difficult to compare and comment on the obtained results. Gaidė et al. studied the transesterification process with butanol. It was chosen for its environmental benefits because butanol, unlike methanol, can be produced biochemically by bacteria. The optimal determined process were determined: the highest yield of rapeseed butyl esters, which reached 94.55%, was obtained within 8 h by taking 5.24% of calcined 850 °C dolomite, using a 13.71:1 molar ratio of butanol to rapeseed oil at a temperature of 110 °C [
25].
4. Regeneration and Reuse of Dolomite
Heterogeneous catalysts are attractive because they are easily separated from the reaction medium and can be reused several times after regeneration. Methods of regeneration of dolomite as a catalyst and the possibilities of its subsequent use have been studied by a number of authors. Two main regeneration methods of dolomite have been studied: solvent washing and calcination or a combined application of the two methods. Methanol was most commonly used for washing. The properties of the regenerated material were investigated by applying a transesterification process and evaluating the biodiesel yield over several regeneration and transesterification cycles. Ngamcharussrivichai et al. used methanol for regeneration and found that the FAME content in the product was greater than 90% after 7 reuse cycles [
5]. Ilgen also used methanol for washing and found that dolomite could be reused 3 times: the resulting ester yield was greater than 90% [
13]. It was observed that the biodiesel yield significantly decreases when dolomite is used for the fourth time. Similar results were obtained by Korkur and Bayramoglu when studying the ultrasound-assisted transesterification process. They washed the spent dolomite with methanol under the influence of ultrasound and found that the yield of fatty acid methyl esters after 4 catalyst washing cycles decreased from 96.5% to 89.5% [
8]. The decrease in the catalytic activity of dolomite is explained by the solubility of CaO in methanol [
5]. Part of the CaO is dissolved in methanol both during the transesterification process and during the regeneration of dolomite by washing, which reduces the amount of active sites on the catalyst surface and, at the same time, the catalytic activity. This was confirmed by the results of the studies of Correia et al. [
21]. When studying the reusability of dolomite, it was observed that its activity decreases after each cycle and only during the first transesterification cycle a high yield of rapeseed or sunflower methyl esters is obtained, which reaches 96%. After the third and subsequent cycles, the yields of esters obtained are less than 85%. In addition, greater deactivation was observed when using canola oil than sunflower oil. Similar results were obtained by Šlinkšienė et al. when studying the possibilities of dolomite regeneration by applying two different processes—calcination at 850 °C and washing with methanol and hexane (1:10 by volume) and drying [
26]. It was found that dolomite regenerated by washing lost catalytic activity after the first regeneration cycle: the yield of methyl esters decreased from 98.66% to 78.85%, while the catalytic efficiency of dolomite regenerated by calcination only slightly decreased even after the 5th regeneration cycle: a yield of 97.23% methyl esters was obtained (
Table 3).
Some authors indicate that the activity of dolomite remains similar for several cycles even without washing it, only after separation from the reaction medium and drying. Yoosuk et al. using hydration–dehydration processed dolomite reused the catalyst without any additional treatment, and found that the Ca content in the catalyst changed slightly, and after 4 reuses for palm olein transesterification, 91% methyl ester content was obtained [
14]. Similar results were obtained by Niu et al., who separated the spent catalyst by vacuum filtration and directly reused it without any additional treatment. Over 8 cycles, the activity of this catalyst decreased slightly, the obtained methyl ester yield was 92.11% [
22]. Zhao et al. using lanthanum impregnated dolomite obtained a high palm oil methyl ester yield of 95.9% over 5 cycles [
17]. Sendžikienė et al. also found that dolomite as a catalyst can be used without additional treatment for 2 cycles. When dolomite was used for the second time, the sunflower methyl ester yield was 85.1%, while if it was used for the third time, it was only 43.8% [
23].
6. Conclusions
This review summarizes the use of the natural mineral dolomite as a heterogeneous catalyst in the transesterification of triglycerides. Dolomite preparation is an important process that has a significant impact on catalytic activity. The main method of activating dolomite is calcination at high temperatures, during which calcium and magnesium carbonate are decomposed to calcium oxide, which is effective catalyst in the transesterification process. Studies have shown that the optimal calcination temperature is 800–850 °C. Using at higher temperatures calcined dolomite, the yield of fatty acid methyl esters is lower. The catalytic activity of calcined dolomite can be improved by modifying it with other elements. The biodiesel yield is influenced by the amount of dolomite, the molar ratio of methanol to oil and the duration of the process. The highest yields were obtained using calcined dolomite using an average of 4% catalyst over an average of 4 h, while the excess of methanol was very variable. Using modified dolomite with a large excess of methanol results in a higher yield of methyl esters than using calcined dolomite. Dolomite can be regenerated and reused. Some scientists suggest using only dried dolomite several times, while others have obtained good results by washing dolomite with methanol and calcining it. The physicochemical properties of biodiesel obtained by heterogeneous catalysis were studied, and they met the standards for biodiesel fuel.
Future Prospects
Summarizing the obtained research data, it can be stated that dolomite as a cheap mineral can be used for biodiesel synthesis. However, it is necessary to prepare it accordingly—calcinate and/or modify it with other elements, respectively. However, such preparation or modification requires additional energy and material costs, which increase the cost of the catalyst. Unfortunately, a detailed analysis of how much additional resources are required for dolomite preparation has not been performed, and there is no comparison of the economic benefits of using dolomite instead of homogeneous catalysts. Such studies would be useful in discussing the economic benefits of using heterogeneous catalysts. There is also no information about the costs of the dolomite regeneration process and how much this affects the cost of biodiesel production by using the catalyst repeatedly, because the currently declared benefits have not been confirmed by specific calculations. Therefore, it would be useful to continue further research in this direction. As can be seen from scientific publications, in recent years, more research has been conducted not on the dolomite preparation process, but on the possibilities of its modification. Various options for modification with additional compounds have been evaluated, but this is again related to additional costs, although no special catalytic effect has been observed when using modified dolomites, transesterification requires a fairly large amount of catalyst and excess methanol, the process duration does not differ significantly when using calcined or modified dolomite. In this case, too, an economic analysis is necessary, showing the benefits of modification and the cost-effectiveness of catalyst preparation. Recently, efforts have been made to use as many environmentally friendly materials as possible, including dolomite. However, its preparation requires additional operations, so it is necessary to analyze the environmental impact of dolomite use through a life cycle perspective, comparing the life cycle indicators of the biodiesel synthesis process using dolomite and other catalysts.