Recent Developments, Challenges, and Environmental Benefits of Using Hermetia illucens for Bioenergy Production Within a Circular Economy Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. BSFL Rearing Experiments
2.2.1. Diets and Experimental Design
2.2.2. Statistical Analysis
2.3. Integrated Refinery Process Design
3. Results and Discussion
3.1. Literature Review
- -
- Limited scalability: Most studies remain at the lab scale, with few insights on pilot- or industrial-scale feasibility. Research on scale-up strategies—also accounting for regulatory constraints—is urgently needed.
- -
- Geographical concentration: Research is geographically skewed, with a strong focus on China and Malaysia, while other regions remain under-represented. Expanding studies to these areas could yield new perspectives, especially in relation to local waste streams and legal frameworks.
- -
- Economic viability: There is a lack of data on the cost effectiveness of larval biodiesel compared to conventional sources. More techno-economic analyses are required to assess its market competitiveness.
- -
- Environmental assessments: Future work should include more comprehensive life-cycle assessments and compare BSF-based systems with conventional biofuel pathways across multiple impact categories and under realistic production scenarios.
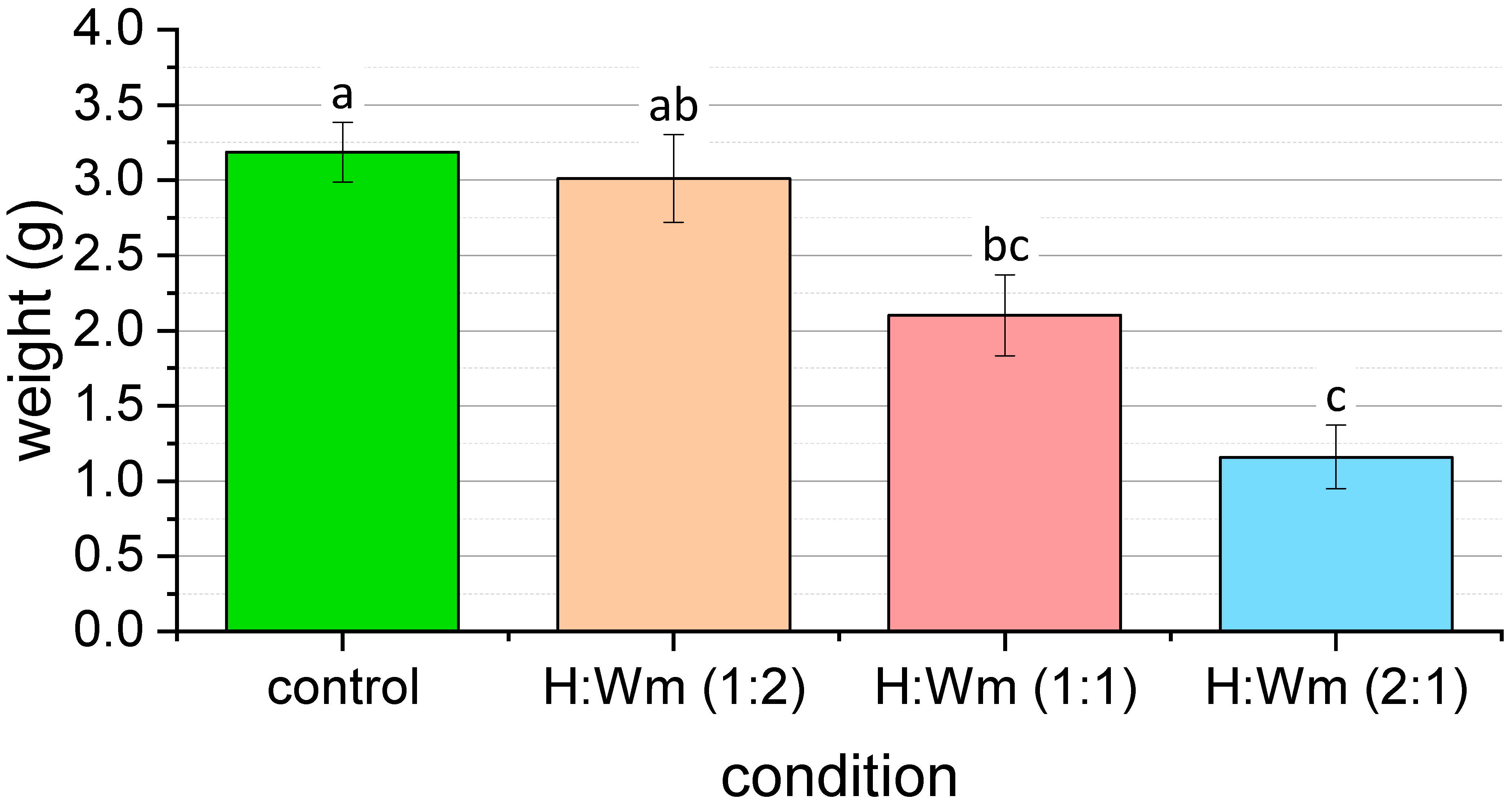
3.2. BSFL Rearing Experiments
3.3. Integrated Process Design
- Three main process units: these are bioconversion with H. illucens, anaerobic digestion (AD), and hydrothermal carbonisation (HTC);
- Thermally self-sustainable process: the system is designed to be thermally self-sufficient through the partial use of the produced hydrochar as a fuel in boiler B-01; this boiler provides heat to both the HTC process and the anaerobic digestion unit;
- Thermal integration strategies for HTC: to reduce energy consumption, water is employed as a heat transfer medium for HTC feed preheating (via heat exchanger E-01) and for cooling the HTC product (via E-02);
- Heat recovery to support anaerobic digestion: waste heat recovered from the HTC process using exchanger E-03 is utilised to partially meet the thermal energy demand of the anaerobic digestion reactor R-02.

- -
- The composition of stream n. 1 (feedstock) is taken from Eisert’s report [76];
- -
- The specific electricity consumptions for the biogas upgrading unit are taken from Lombardi and Francini [77];
- -
- The specific heat capacity of the solid fraction entering the HTC reactor is based on data reported by Arlabosse et al. [78];
- -
- The high heating value (HHV) of the hydrochar, on a dry basis, is assumed to be 12 MJ/kg; this value aligns with the findings of Cao [79], who consider digestates from AD as feedstock for the HTC process;
- -
- The heat of reaction for the HTC process is considered negligible;
- -
- The equivalent electrical energy consumption of heat rejection is estimated at 2% of the removed thermal duty.
| Main Assumptions—Compositions of Relevant Streams | |||
|---|---|---|---|
| Stream n.1: feedstock | |||
| TS | 39% | %w | |
| VS | 37% | %w | |
| Ash | 2% | %w | |
| Water | 61% | %w | |
| Stream n.3: AD inlet | |||
| Water | 86% | %w | |
| TS | 14% | %w | |
| Stream n.4: Biogas | |||
| CO2 | 36% | %vol | |
| CH4 | 54% | %vol | |
| Water | 10% | %vol | |
| Stream n.8: water from separation unit | |||
| Water | 100% | %w | |
| Stream n.9: HTC input | |||
| Water | 75% | %w | |
| TS | 25% | %w | |
| Stream n.13: liquid from F-01 | |||
| Water | 94% | %w | |
| TS | 6% | %w | |
| Stream n.14: char from F-01 | |||
| Water | 40% | %w | |
| TS | 60% | %w | |
| Main Assumptions—Performance Indicators and Process Parameters | |||
|---|---|---|---|
| Process unit | Assumption | Value | Unit |
| R-02 (AD—anaerobic digestor) | Digestate mass yield | 94% | %w - kg(7)/kg(3) |
| Qlosses (%Qadiabatic) | 92% | %adiabatic duty | |
| cp_AD inlet | 3.847 | kJ/kg/K | |
| AD impeller | 13.760 | kJe/kg(1) | |
| Biogas upgrading | 94.319 | kJe/kg(1) | |
| HTC (E-01 + R-03 + E-02) | T9 | 55 | °C |
| T10 | 110 | °C | |
| T11 | 210 | °C | |
| T_E-02_out | 139 | °C | |
| cp_solid fraction stream n.9 | 1.763 | kJ/kg/K | |
| cp_sludges_HTC | 3.580 | kJ/kg/K | |
| Thermal integration: water E03-R-02 | T_water to E-03 | 60 | °C |
| T_water from E-03 | 75 | °C | |
| Boiler | η_boiler | 80% | |
| HHV | 12 | MJ/kg_dry fuel | |
| LHV | 6.32 | MJ/kg(15) | |
| cp_Flue gas | 1.300 | kJ/kg/K | |
| cp_Ash | 0.840 | kJ/kg/K | |
| cp_Air | 1.025 | kJ/kg/K | |
| Air-to-fuel ratio | 7 | w/w | |
| Specific electric consumption | 288 | kJ/kg(15) | |
| ρ_Oil | 700 | kg/m3 | |
| cp_Oil | 2.51 | kJ/kg/K | |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AD | Anaerobic digestion |
| HTC | Hydrothermal carbonization |
| IEA | International Energy Agency |
| SDS | Sustainable Development Scenario |
| VS | Volatile solids |
| TS | Total solids |
| BSF | Black Soldier Fly (Hermetia illucens) |
| BSF | Black Soldier Fly larvae (Hermetia illucens) |
| RH | Relative humidity |
| IPIFF | International Platform of Insects for Food and Feed |
| ABP | Animal By-Products |
| WD | Water depletion |
| LU | Land use |
| EGU | Energy use |
| EBA | European Biogas Association |
| EU | European Union |
| FHB | Fusarium Head Blight |
| REPowerEU | European energy plan aiming to reduce dependence on fossil fuels |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| FW | Food waste |
| BMP | Biochemical methane potential |
| R&D | Research and development |
| B-01 | Boiler unit (used in the integrated system) |
| E-01, E-02, E-03 | Heat exchangers (used in HTC and AD thermal integration) |
| R-02 | Anaerobic digestion reactor |
References
- Demir, E.; Alp, E. A framework for assessing the circular economy potential in the water and agriculture sectors in Türkiye through the water-energy-food-ecosystem nexus. Sustain. Prod. Consum. 2025, 54, 335–347. [Google Scholar] [CrossRef]
- Chaudhary, A.; Rathour, R.K.; Solanki, P.; Kakkar, P.M.; Pathania, S.; Walia, A.; Baadhe, R.R.; Bhatia, R.K. Recent technological advancements in biomass conversion to biofuels and bioenergy for circular economy roadmap. Renew. Energy 2025, 244, 122714. [Google Scholar] [CrossRef]
- Hu, X.; Elshkaki, A.; Shen, L. The implications of circular economy strategies on the future energy transition technologies and their impacts: Solar PV as a case study. Energy 2024, 313, 133972. [Google Scholar] [CrossRef]
- Voccia, D.; Abdel Sater, S.; Demichelis, F.; Froldi, F.; Savorani, F.; Tommasi, T.; Wachongkum, S.; Lamastra, L. Unlocking the power of Italy’s bioeconomy: A comparative analysis of immediate vs. deferred impact on energy generation through straw valorisation. J. Environ. Manag. 2025, 380, 125056. [Google Scholar] [CrossRef] [PubMed]
- Lemke, N.B.; Dickerson, A.J.; Tomberlin, J.K. No neonates without adults. BioEssays 2023, 45, 2200162. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Doelle, K.; Smith, R. External morphology of Hermetia illucens Stratiomyidae: Diptera (L.1758) based on electron microscopy. Annu. Res. Rev. Biol. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Demetriou, J.; Kalaentzis, K.; Kazilas, C.; Kunz, G.; Muller, B.; Mostovski, M.B.; Koutsoukos, E.C. An “alien” species on the loose: New records and updated distribution of the black soldier fly Hermetia illucens in the Western Palearctic. Bull. Insectol. 2022, 75, 125–130. [Google Scholar]
- James, M.T. The genus Hermetia in the United States (Diptera: Stratiomyidae). Bull. Brooklyn Entomol. Soc. 1935, 30, 165–170. [Google Scholar]
- Leclercq, M. Á propos de Hermetia illucens (Linnaeus, 1758) (“soldier fly”) (Diptera Stratiomyidae: Hermetiinae). Bull. Ann. Soc. R. Belge Déntomol. 1997, 133, 275–282. [Google Scholar]
- Kim, W.; Bae, S.; Park, H.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y.-H. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2010, 21, 185–187. [Google Scholar]
- Lupi, D.; Savoldelli, S.; Leonardi, M.G.; Jucker, C. Feeding in the adult of Hermetia illucens (Diptera Stratiomyidae): Reality or fiction? J. Entomol. Acarol. Res. 2019, 51, 8046. [Google Scholar] [CrossRef]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Rehman, R.U.; Rehman, A.U.; Zhang, J.; Zheng, L.; Nienaber, T.; Heinz, V.; Aganovic, K. Black soldier fly, Hermetia illucens as a potential innovative and environmentally friendly tool for organic waste management: A mini-review. Waste Manag. Res. J. Sustain. Circ. Econ. 2023, 41, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Interspecific competition between the House Fly, Musca domestica L. (Diptera: Muscidae) and Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae) when reared on poultry manure. Insects 2019, 10, 440. [Google Scholar] [CrossRef]
- Reguzzi, M.; Cominelli, F.; Bardone, M.; Aldini, R.N.; Chiesa, O.; Panini, M.; Casu, G.; Mazzoni, E. Unwelcome guests at farms breeding the black soldier fly, Hermetia illucens (L.) (Diptera Stratiomyidae). J. Insects Food Feed 2021, 7, 1177–1181. [Google Scholar] [CrossRef]
- Joosten, L.; Lecocq, A.; Jensen, A.B.; Haenen, O.; Schmitt, E.; Eilenberg, J. Review of insect pathogen risks for the black soldier fly (Hermetia illucens) and guidelines for reliable production. Entomol. Exp. Appl. 2020, 168, 432–447. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; van Huis, A. Black soldier fly from pest to ‘crown jewel’ of the insects as feed industry: An historical perspective. J. Insects Food Feed 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.-Y.; Park, K.Y.; Chung, H. Use of Black Soldier Fly Larvae for Food Waste Treatment and Energy Production in Asian Countries: A Review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae Mediated Valorization of Industrial, Agriculture and Food Wastes: Biorefinery Concept through Bioconversion, Processes, Procedures, and Products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- Caruso, D.; Devic, E.; Subamia, I.W.; Talamond, P.; Baras, E. (Eds.) Technical Handbook of Domestication and Production of Diptera Black Soldier Fly (BSF) Hermetia illucens, Stratiomyidae; PT Penerbit IPB Press Kampus IPB Taman Kencana Bogor: Bogor, Indonesia, 2014. [Google Scholar]
- Kee, P.E.; Cheng, Y.-S.; Chang, J.-S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.-W.; Ng, H.S.; Khoo, K.S. Insect biorefinery: A circular economy concept for biowaste conversion to value-added products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect farming for feed and food production from a circular business model perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using Black Soldier Fly larvae: A review. J. Environ. Manag. 2019, 251, 109569. [Google Scholar] [CrossRef]
- Firmansyah, M.; Abduh, M.Y. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 2019, 5, e02005. [Google Scholar] [CrossRef] [PubMed]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, X.; Tu, F.; Wang, C.; Yang, F. Preparation, antioxidant activity evaluation, and identification of antioxidant peptide from black soldier fly (Hermetia illucens L.) larvae. J. Food Biochem. 2020, 44, e13186. [Google Scholar] [CrossRef]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive compounds from Hermetia illucens larvae as natural ingredients for cosmetic application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.-H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef]
- IPIFF. Guide on Good Hygiene Practices for European Union (EU) Producers of Insects as Food and Feed—February 2024; IPIFF: Brussels, Belgium, 2024. [Google Scholar]
- Nguyen, T.H.; Wang, X.; Utomo, D.; Gage, E.; Xu, B. Circular bioeconomy and sustainable food systems: What are the possible mechanisms? Clean. Circ. Bioecon. 2025, 11, 100145. [Google Scholar] [CrossRef]
- Perone, C.; Romaniello, R.; Leone, A.; Berardi, A.; Tamborrino, A. Towards energy efficient scheduling in the olive oil extraction industry: Comparative assessment of energy consumption in two management models. Energy Convers. Manag. X 2022, 16, 100287. [Google Scholar] [CrossRef]
- Islam, K.M.N.; Kenway, S.J.; Renouf, M.A.; Lam, K.L.; Wiedmann, T. A review of the water-related energy consumption of the food system in nexus studies. J. Clean. Prod. 2021, 279, 123414. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Wang, Y.; Zheng, L.; Zhang, Y.; Yu, Z.; Chen, H.; Zhang, J. Efficient bioconversion of organic wastes to value-added chemicals by soaking, black soldier fly (Hermetia illucens L.) and anaerobic fermentation. J. Environ. Manag. 2018, 227, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Win, S.S.; Ebner, J.H.; Brownell, S.A.; Pagano, S.S.; Cruz-Diloné, P.; Trabold, T.A. Anaerobic digestion of black solider fly larvae (BSFL) biomass as part of an integrated biorefinery. Renew. Energy 2018, 127, 705–712. [Google Scholar] [CrossRef]
- Kamarulzaman, M.K.; Hafiz, M.; Abdullah, A.; Chen, A.F.; Awad, O.I. Combustion, performances and emissions characteristics of black soldier fly larvae oil and diesel blends in compression ignition engine. Renew. Energy 2019, 142, 569–580. [Google Scholar] [CrossRef]
- Yusaf, T.; Kamarulzaman, M.K.; Adam, A.; Hisham, S.; Ramasamy, D.; Kadirgama, K.; Samykano, M.; Subramaniam, S. Physical-chemical properties modification of Hermetia illucens larvae oil and diesel fuel for the internal combustion engines application. Energies 2022, 15, 8073. [Google Scholar] [CrossRef]
- Elsayed, M.; Wang, J.; Wang, H.; Zhou, Z.; Osman, A.I.; Almutairi, A.W.; Faisal, S.; Abomohra, A. Conversion of protein-rich waste into biodiesel by Hermetia illucens: Enhanced energy recovery and reduced greenhouse gas emissions. Sustain. Energy Technol. Assess. 2024, 66, 103825. [Google Scholar] [CrossRef]
- Fredsgaard, M.; Hulkko, L.S.S.; Chaturvedi, T.; Thomsen, M.H. Process simulation and techno-economic assessment of Salicornia sp. based jet fuel refinery through Hermetia illucens sugars-to-lipids conversion and HEFA route. Biomass Bioenergy 2021, 150, 106142. [Google Scholar] [CrossRef]
- Koyunoğlu, C. Biofuel production utilizing black soldier fly (Hermetia illucens): A sustainable approach for organic waste management. Int. J. Thermofluids 2024, 23, 100754. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Zhang, X.; Deng, B.; Xu, C.; Zhang, C.; Yuan, Q. Experimental study on black soldier fly (Hermetia illucens L.) larvae hydrothermal liquefaction in methanol-water Co-solvent: Bio-oil yields and properties. Renew. Energy 2023, 218, 119345. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; Hussein, M.Y.; Ibrahim, H.M.; Hanafy, M.H.; Salah, S.M.; El-Bassiony, G.M.; Abdelfattah, E.A. Mixed microalgae-food waste cake for feeding of Hermetia illucens larvae in characterizing the produced biodiesel. Biomass Bioenergy 2022, 165, 106586. [Google Scholar] [CrossRef]
- Jung, S.; Jung, J.-M.; Tsang, Y.F.; Bhatnagar, A.; Chen, W.-H.; Lin, K.-Y.A.; Kwon, E.E. Biodiesel production from black soldier fly larvae derived from food waste by non-catalytic transesterification. Energy 2022, 238, 121700. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M. Characteristic of Hermetia illucens Fatty Acid and that of the Fatty Acid Methyl Ester Synthesize Based on Upcycling of Perishable Waste. Waste Biomass Valorization 2020, 11, 5607–5614. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Ke, J.; Chen, J.; Holtzapple, M.T.; Tomberlin, J.K.; Chen, H.; Zhang, J.; Li, Q. Production of biodiesel from CO2 and organic wastes by fermentation and black soldier fly. Renew. Energy 2020, 149, 1174–1181. [Google Scholar] [CrossRef]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.-H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De novo transcriptome sequencing and analysis revealed the molecular basis of rapid fat accumulation by black soldier fly (Hermetia illucens, L.) for development of insectival biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Li, R.; Lin, T.; Fan, X.; Dai, X.; Huang, J.; Zhang, Y.; Guo, R.; Fu, S. Effects of salinity in food waste on the growth of black soldier fly larvae and global warming potential analysis. Chem. Eng. J. 2024, 480, 148221. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ohkawa, M.; Zhao, J.; Yano, K. Effect of dietary meat content on weight gain, mortality, and pre-pupal rate in Black Soldier Fly (Hermetia illucens) larvae. Insects 2022, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Camenzuli, L.; van Dam, R.; de Rijk, T.; Andriessen, R.; van Schelt, J.; van der Fels-Klerx, H.J.I. Tolerance and excretion of the mycotoxins aflatoxin B1, zearalenone, deoxynivalenol, and ochratoxin A by Alphitobius diaperinus and Hermetia illucens from contaminated substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef]
- Leni, G.; Cirlini, M.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Sforza, S.; Dall’asta, C. Impact of naturally contaminated substrates on Alphitobius diaperinus and Hermetia illucens: Uptake and excretion of mycotoxins. Toxins 2019, 11, 476. [Google Scholar] [CrossRef]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1410–1420. [Google Scholar] [CrossRef]
- European Biogas Association. Statistical Report 2024—Tracking Biogas and Biomethane Deployment Across Europe 2024. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2024/12/EBA_stats_report_complete_241204_preview.pdf (accessed on 30 April 2025).
- European Commission. Energy—Biomethane 2025. Available online: https://energy.ec.europa.eu/topics/renewable-energy/bioenergy/biomethane_en (accessed on 30 April 2025).
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Mikusińska, J.; Kuźnia, M.; Czerwińska, K.; Wilk, M. Hydrothermal Carbonization of Digestate Produced in the Biogas Production Process. Energies 2023, 16, 5458. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates—Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef]
- Wirth, B.; Mumme, J. Anaerobic digestion of waste water from hydrothermal carbonization of corn silage. Appl. Bioenergy 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Medina-Martos, E.; Istrate, I.-R.; Villamil, J.A.; Gálvez-Martos, J.-L.; Dufour, J.; Mohedano, Á.F. Techno-economic and life cycle assessment of an integrated hydrothermal carbonization system for sewage sludge. J. Clean. Prod. 2020, 277, 122930. [Google Scholar] [CrossRef]
- Kassim, F.O.; Thomas, C.L.P.; Afolabi, O.O.D. Integrated conversion technologies for sustainable agri-food waste valorization: A critical review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Kythreotou, N.; Florides, G.; Tassou, S.A. A review of simple to scientific models for anaerobic digestion. Renew. Energy 2014, 71, 701–714. [Google Scholar] [CrossRef]
- Conversano, A.; Porcu, A.; Mureddu, M.; Pettinau, A.; Gatti, M. Bench-scale experimental tests and data analysis on CO2 capture with potassium prolinate solutions for combined cycle decarbonization. Int. J. Greenh. Gas Control 2020, 93, 102881. [Google Scholar] [CrossRef]
- Lombardelli, G.; Scaccabarozzi, R.; Conversano, A.; Gatti, M. Bio-methanol with negative CO2 emissions from residual forestry biomass gasification: Modelling and techno-economic assessment of different process configurations. Biomass Bioenergy 2024, 188, 107315. [Google Scholar] [CrossRef]
- Eisert, T. Process Efficiencies in Black Soldier Fly Larvae Composting—Evaluation of Process Parameters of Food Industry Waste Treatment Using Hermetia illucens 2021. pp. 1–36. Available online: https://www.slu.se/globalassets/ew/org/inst/thv/dokument/publikationer-till-projektsidor/5tonfid/process-efficiencies-in-black-soldier-fly-larvae-composting1.pdf (accessed on 30 April 2025).
- Lombardi, L.; Francini, G. Techno-economic and environmental assessment of the main biogas upgrading technologies. Renew. Energy 2020, 156, 440–458. [Google Scholar] [CrossRef]
- Arlabosse, P.; Chavez, S.; Prevot, C. Drying of municipal sewage sludge: From a laboratory scale batch indirect dryer to the paddle dryer. Braz. J. Chem. Eng. 2005, 22, 227–232. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Lombardelli, G.; Consonni, S.; Conversano, A.; Mureddu, M.; Pettinau, A.; Gatti, M. Process Design and Techno-Economic Assessment of biogenic CO2 Hydrogenation-to-Methanol with innovative catalyst. J. Phys. Conf. Ser. 2022, 2385, 012038. [Google Scholar] [CrossRef]
- Conversano, A.; Gatti, M.; Scaccabarozzi, R.; Martelli, E.; Ali, I.; Moure, G.; Consonni, S. Techno-Economic Assessment of Novel vs. Standard 5m Piperazine CCS Absorption Processes for Conventional and High-efficiency NGCC Power Plants. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–26 October 2018. [Google Scholar]
- Cotrina-Teatino, M.A.; Marquina-Araujo, J.J. Circular economy in the mining industry: A bibliometric and systematic literature review. Resour. Policy 2025, 102, 105513. [Google Scholar] [CrossRef]
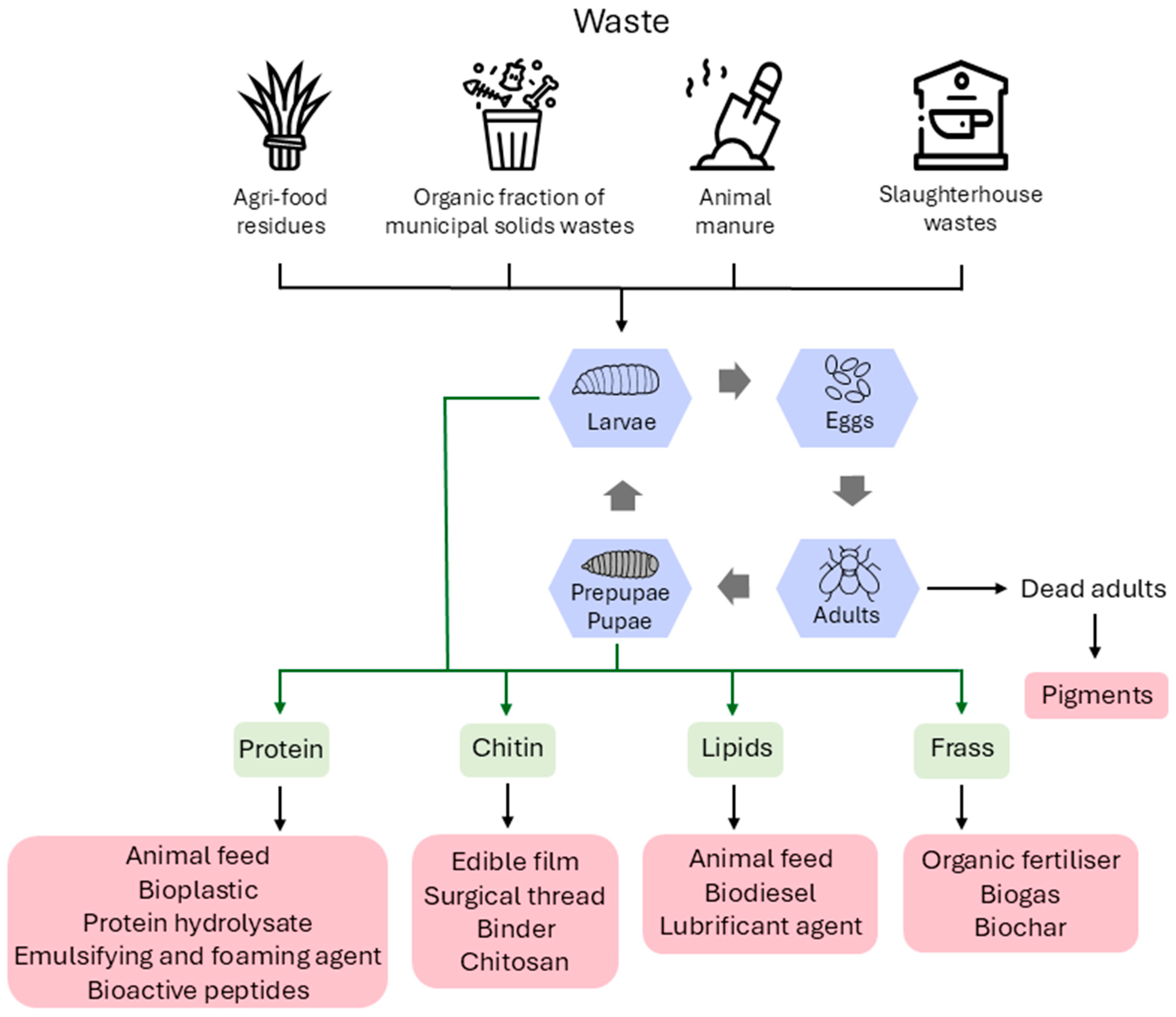
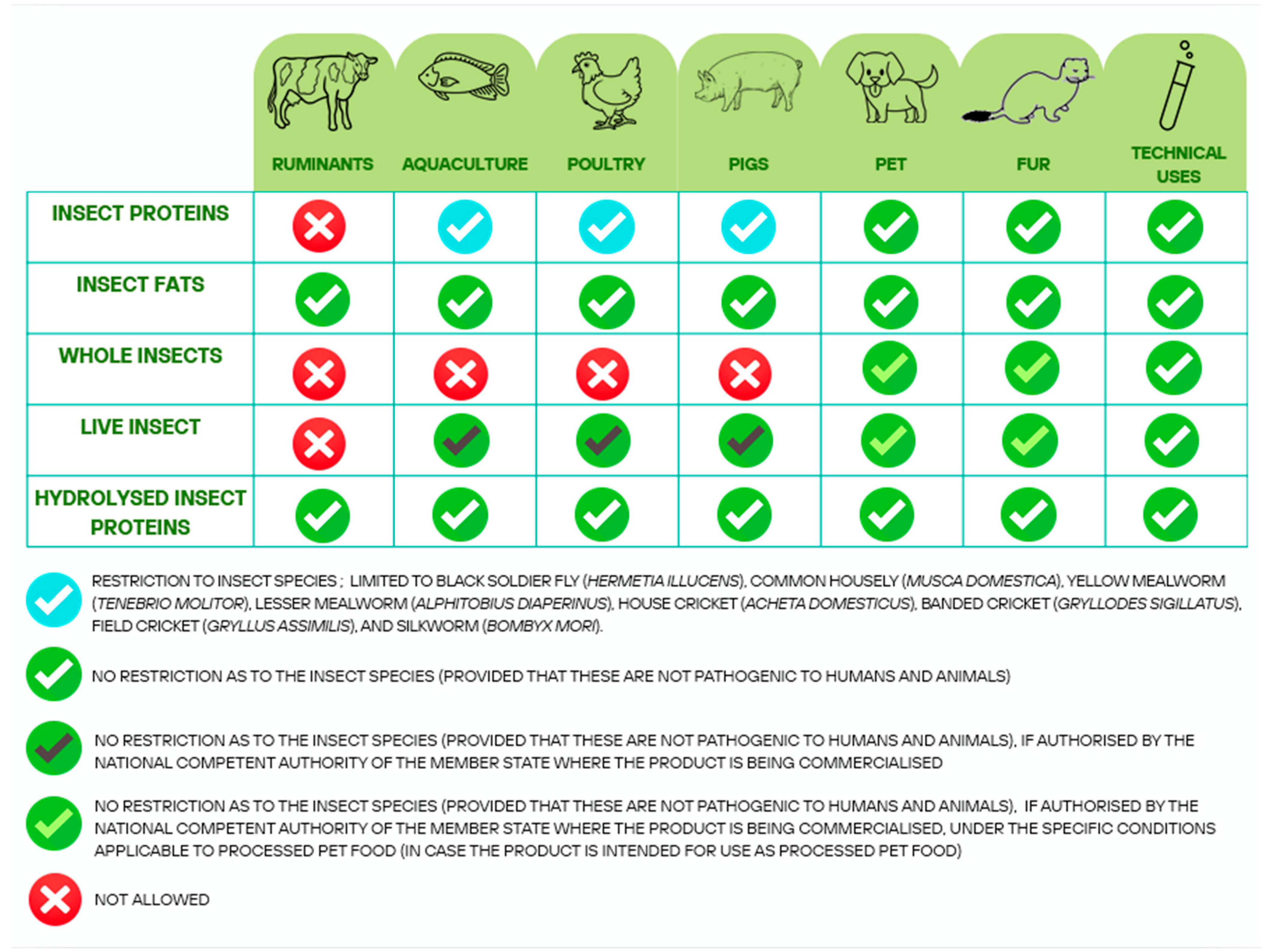
| Authors | Year | Geographical Position | Methods | Substrate for Larval Growth | Main Products | Economic Aspects | Environmental Aspects |
|---|---|---|---|---|---|---|---|
| [47] | 2024 | No info | Theoretical | Residual biomass (rice straw + restaurant waste; dairy manure; restaurant waste) | biodiesel | x | CC, WD, EGU, and LU |
| [45] | 2024 | No info | Experimental (lab scale) | Protein-rich waste (bovine powder of MBM) | biodiesel | CC | |
| [48] | 2023 | China | Experimental (lab scale) | Kitchen waste | biodiesel | ||
| [44] | 2022 | No info | Experimental (lab scale) | No info | biodiesel blends | ||
| [49] | 2022 | Egypt | Experimental (lab scale) | Organic waste + microalgae + antioxidants | biodiesel | ||
| [50] | 2022 | No info | Experimental (lab scale) | Food waste | biodiesel from non-catalytic transesterification | ||
| [46] | 2021 | Denmark | Experimental (lab scale) | Fermented Salicornia sp. | biodiesel | x | CC |
| [51] | 2020 | Malaysia | Experimental (lab scale) | Perishable waste (fruits waste and food waste) | biodiesel | ||
| [52] | 2020 | China | Experimental (lab scale) | VFA from pig manure and rice straw | biodiesel | ||
| [43] | 2019 | Malaysia | Experimental (lab scale) | No info (commercial BSFL oil) | biodiesel | ||
| [53] | 2019 | No info | Experimental (lab scale) | Commercial chicken feed used to rear BSFs | biodiesel | ||
| [38] | 2018 | China | Experimental (lab scale) | Residues of corncob soaking with restaurant wastewater | larval grease and biogas | ||
| [42] | 2018 | USA | Experimental (lab scale) | Food waste | biomethane or biomethane + biodiesel | ||
| [41] | 2017 | China | Experimental (lab scale) | Corn stover + waste carrots | biodiesel + protein feed + bio-fertilisers | ||
| [40] | 2016 | USA | Experimental (lab scale) | Food wastes | biodiesel + animal feed | ||
| [39] | 2015 | China | Experimental (lab scale) | Biogas digestate from corncob and pig manure | biodiesel + biogas | ||
| [54] | 2012 | China | Experimental (lab scale) | Restaurant wastes | biodiesel | ||
| [55] | 2012 | China | Experimental (lab scale) | Restaurant wastes + rice straw | biodiesel | ||
| [56] | 2011 | China | Experimental (lab scale) | Organic waste (cattle manure, pig manure, and chicken manure) | biodiesel |
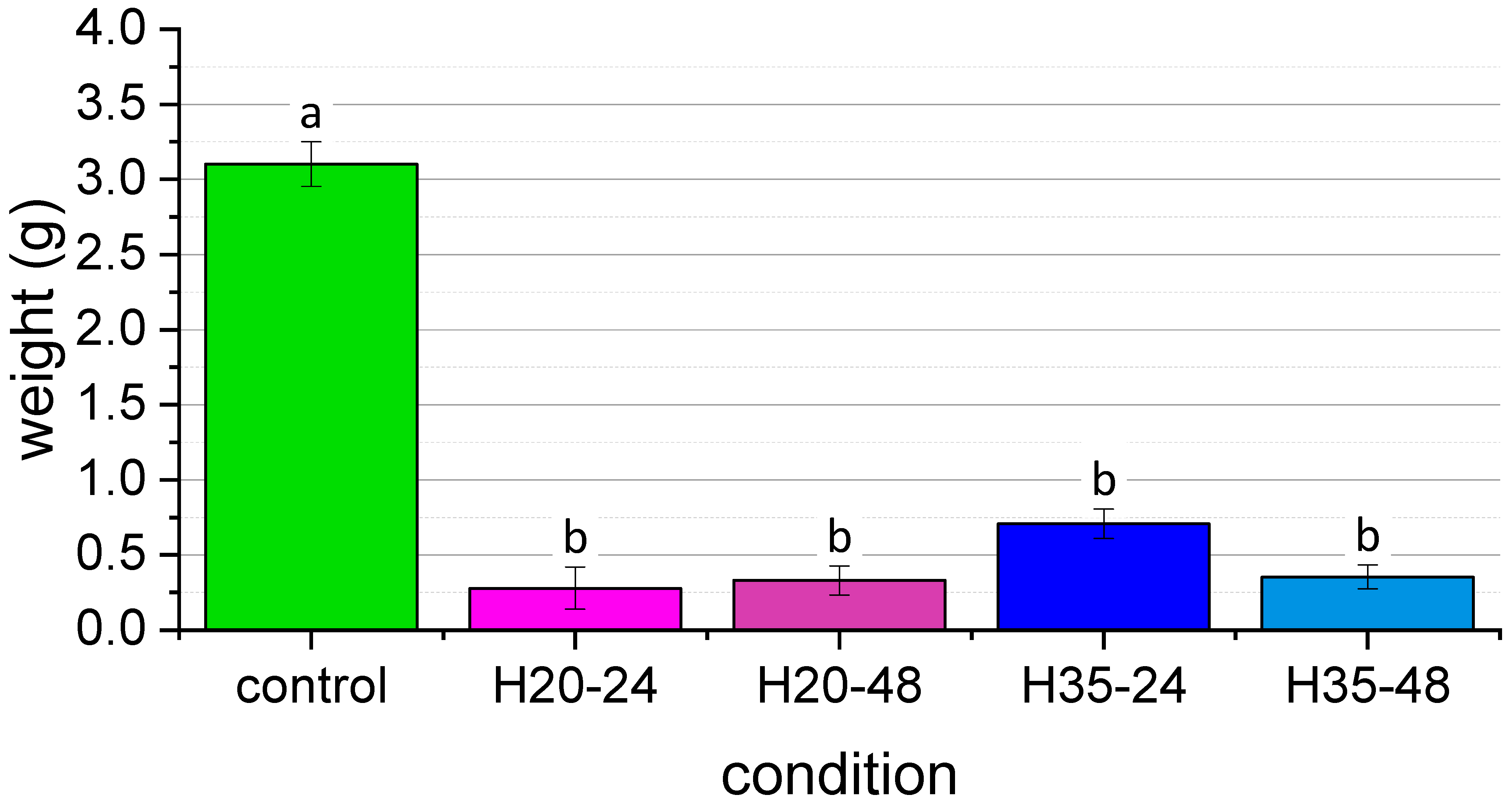
| Condition | Initial Weight of Larvae (g) | Final Number of Live Larvae | Final Weight of Live Larvae (g) | Final Weight of Substrate (g) |
|---|---|---|---|---|
| C1 | 0.0968 | 20 | 2.9229 | 2.3166 |
| C2 | 0.078 | 20 | 3.4237 | 2.5037 |
| C3 | 0.0732 | 20 | 2.7803 | 2.4666 |
| C4 | 0.0752 | 20 | 3.2834 | 2.0058 |
| H20-24-1 | 0.0826 | 4 | 0.1372 | 9.6217 |
| H20-24-2 | 0.104 | 4 | 0.0856 | 9.9723 |
| H20-24-3 | 0.0732 | 8 | 0.2015 | 9.8835 |
| H20-24-4 | 0.054 | 15 | 0.6921 | 11.2466 |
| H20-48-1 | 0.0852 | 4 | 0.1028 | 10.0014 |
| H20-48-2 | 0.0635 | 10 | 0.3313 | 12.0008 |
| H20-48-3 | 0.062 | 16 | 0.5708 | 11.3126 |
| H20-48-4 | 0.0787 | 11 | 0.3167 | 10.7964 |
| H35-24-1 | 0.0736 | 19 | 0.9801 | 11.3415 |
| H35-24-2 | 0.0697 | 14 | 0.7229 | 9.5901 |
| H35-24-3 | 0.0674 | 16 | 0.544 | 10.5957 |
| H35-24-4 | 0.0902 | 16 | 0.5881 | 11.3494 |
| H35-48-1 | 0.072 | 4 | 0.1366 | 9.9077 |
| H35-48-2 | 0.0803 | 11 | 0.4869 | 12.2903 |
| H35-48-3 | 0.0618 | 13 | 0.4579 | 10.581 |
| H35-48-4 | 0.0887 | 8 | 0.3295 | 12.0488 |
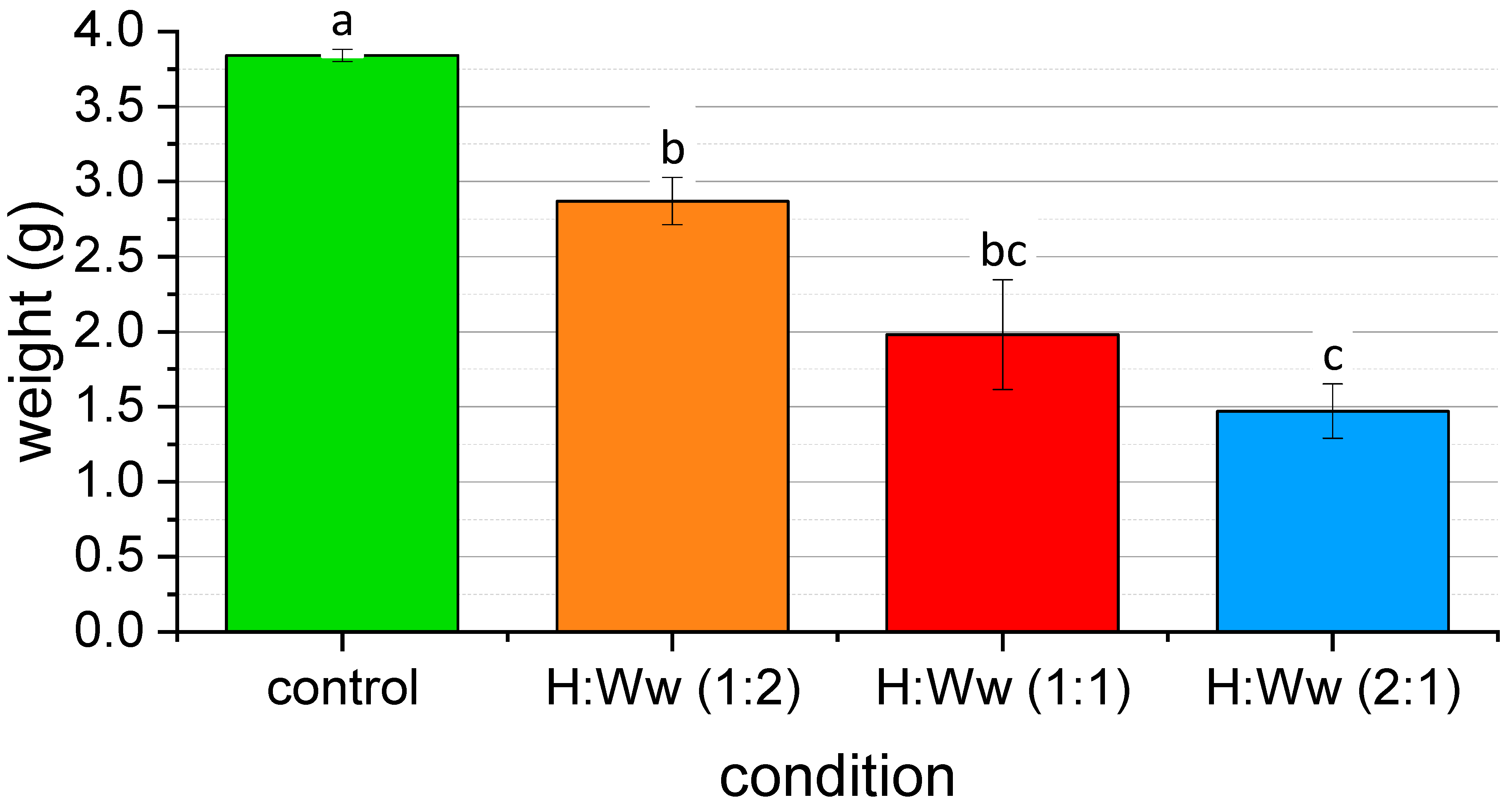
| Condition | Initial Weight of Larvae (g) | Final Number of Live Larvae | Final Weight of Live Larvae (g) | Final Weight of Substrate (g) |
|---|---|---|---|---|
| C1 | 0.0326 | 20 | 3.7909 | 2.8482 |
| C2 | 0.0312 | 20 | 3.9045 | 3.2135 |
| C3 | 0.0269 | 20 | 3.9149 | 5.1445 |
| C4 | 0.0299 | 20 | 3.7564 | 3.2185 |
| H:Ww - 1:2-1 | 0.0339 | 20 | 2.7292 | 9.784 |
| H:Ww - 1:2-2 | 0.0271 | 20 | 3.0678 | 10.2723 |
| H:Ww - 1:2-3 | 0.03 | 20 | 2.4968 | 10.0944 |
| H:Ww - 1:2-4 | 0.0289 | 17 | 3.1838 | 11.3387 |
| H:Ww - 1:1-1 | 0.022 | 20 | 2.056 | 12.265 |
| H:Ww - 1:1-2 | 0.0331 | 18 | 1.995 | 10.2389 |
| H:Ww - 1:1-3 | 0.0268 | 20 | 2.8283 | 10.0753 |
| H:Ww - 1:1-4 | 0.0337 | 18 | 1.0433 | 10.9851 |
| H:Ww - 2:1-1 | 0.0311 | 20 | 1.6396 | 12.2697 |
| H:Ww - 2:1-2 | 0.0297 | 20 | 1.1315 | 11.3488 |
| H:Ww - 2:1-3 | 0.0313 | 17 | 1.2129 | 11.0729 |
| H:Ww - 2:1-4 | 0.0281 | 19 | 1.8984 | 10.9928 |
| Condition | Initial Weight of Larvae (g) | Final Number of Live Larvae | Final Weight of Live Larvae (g) | Final Weight of Substrate (g) |
|---|---|---|---|---|
| C1 | 0.038 | 20 | 3.435 | 4.1136 |
| C2 | 0.0396 | 20 | 2.6019 | 2.5495 |
| C3 | 0.0395 | 20 | 3.4191 | 3.0033 |
| C4 | 0.037 | 20 | 3.2873 | 2.4235 |
| H:Wm - 1:2-1 | 0.04 | 19 | 3.7746 | 8.7996 |
| H:Wm - 1:2-2 | 0.0412 | 20 | 2.9228 | 7.8382 |
| H:Wm - 1:2-3 | 0.037 | 19 | 2.9918 | 9.6619 |
| H:Wm - 1:2-4 | 0.0474 | 19 | 2.3569 | 8.3898 |
| H:Wm - 1:1-1 | 0.0541 | 19 | 1.3304 | 9.6389 |
| H:Wm - 1:1-2 | 0.045 | 20 | 2.5733 | 8.7385 |
| H:Wm - 1:1-3 | 0.0436 | 17 | 2.3186 | 9.34 |
| H:Wm - 1:1-4 | 0.0468 | 18 | 2.188 | 8.5014 |
| H:Wm - 2:1-1 | 0.0407 | 18 | 1.7308 | 10.7884 |
| H:Wm - 2:1-2 | 0.0422 | 16 | 0.7182 | 10.6487 |
| H:Wm - 2:1-3 | 0.0454 | 18 | 1.0626 | 11.089 |
| H:Wm - 2:1-4 | 0.0337 | 13 | 1.1283 | 9.0354 |
| Mass Balance | |||
|---|---|---|---|
| Stream # | Description | Value | Unit |
| 1 | Feedstock | 1.000 | kg/kg(1) |
| 3 | AD input | 3.056 | kg/kg(1) |
| 5 | Bio-CH4 | 0.060 | kg/kg(1) |
| 9 | HTC input | 1.028 | kg/kg(1) |
| 13 | HTC liquid to AD | 0.666 | kg/kg(1) |
| 14 | Hydrochar | 0.362 | kg/kg(1) |
| 15 | Hydrochar to B-01 | 0.105 | kg/kg(1) |
| Ash | Ashes from B-01 | 0.003 | kg/kg(1) |
| Purge | Excess water from separation unit | 0.455 | kg/kg(1) |
| Energy Balance | |||
| Unit | Description | Value | Unit |
| AD | Pumps (P-01, P-02, and P-03) | 0.90 | kJe/kg(1) |
| AD impeller | 13.76 | kJe/kg(1) | |
| Biogas upgrading | 94.32 | kJe/kg(1) | |
| Thermal duty (Qth) | 20.35 | kJth/kg(1) | |
| HTC | Pumps (P-04, P-05, P-06, and P-07) | 10.77 | kJe/kg(1) |
| Heat removal and integration, electric equivalent | 4.56 | kJe/kg(1) | |
| B-01 | Auxiliaries (pump and fan) | 35.29 | kJe/kg(1) |
| Boiler duty B-01 | 485.59 | kJth/kg(1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataglia, L.; Conversano, A.; Di Bona, D.; Sogni, D.; Voccia, D.; Mazzoni, E.; Lamastra, L. Recent Developments, Challenges, and Environmental Benefits of Using Hermetia illucens for Bioenergy Production Within a Circular Economy Approach. Energies 2025, 18, 2826. https://doi.org/10.3390/en18112826
Bataglia L, Conversano A, Di Bona D, Sogni D, Voccia D, Mazzoni E, Lamastra L. Recent Developments, Challenges, and Environmental Benefits of Using Hermetia illucens for Bioenergy Production Within a Circular Economy Approach. Energies. 2025; 18(11):2826. https://doi.org/10.3390/en18112826
Chicago/Turabian StyleBataglia, Luana, Antonio Conversano, Daniele Di Bona, Davide Sogni, Diego Voccia, Emanuele Mazzoni, and Lucrezia Lamastra. 2025. "Recent Developments, Challenges, and Environmental Benefits of Using Hermetia illucens for Bioenergy Production Within a Circular Economy Approach" Energies 18, no. 11: 2826. https://doi.org/10.3390/en18112826
APA StyleBataglia, L., Conversano, A., Di Bona, D., Sogni, D., Voccia, D., Mazzoni, E., & Lamastra, L. (2025). Recent Developments, Challenges, and Environmental Benefits of Using Hermetia illucens for Bioenergy Production Within a Circular Economy Approach. Energies, 18(11), 2826. https://doi.org/10.3390/en18112826






