Abstract
Global wine production reached about 226 million hectolitres in 2024, with Europe as the largest producer. The winemaking industry generates substantial amounts of by-products, presenting both economic and environmental challenges, as approximately 30% of processed grapes are discarded as waste. This study evaluates various polyphenol extraction techniques from wine residues, utilising data from the literature. Techniques assessed include subcritical water extraction, ultrasound-assisted extraction, conventional solvent extraction, and microwave-assisted extraction, each preceded by a suitable pretreatment. Results show that the extraction method, temperature, solvent, and feedstock type have a strong influence on environmental impacts. Microwave extraction from exhausted grape marc had the highest impact due to its low yields and high energy use during freeze drying. In contrast, subcritical water extraction from red wine residues was the most sustainable, benefiting from its high efficiency, use of water as a solvent, and the rich polyphenol content of red grape residues. When included, drying was the primary contributor to greenhouse gas emissions. Climate change and energy demand were key impact categories, with a renewable energy scenario potentially reducing impacts by up to 90%. Results demonstrate that no single extraction method is universally best; choices must balance efficiency and energy use. This work supports optimising sustainable polyphenol recovery within circular economy and climate goals.
1. Introduction
According to the International Organisation for Vine and Wine, world wine production has reached ~226 million hL in 2024, with Europe being the largest producer (~138 million hL) [1]. The winemaking industry generates substantial quantities of by-products, presenting significant economic and environmental challenges. It is estimated that about 30% of processed grapes are converted into by-products and waste [2]. During the grape pressing stage alone, over 20% of the grape’s weight becomes pomace—a residue primarily made up of grape skins and seeds, and in some cases stalks [3,4,5,6] that represent the primary by-product of winemaking. Typically, grape stalks (GSs) are removed before processing to prevent excessive astringency in the wine [3,7,8], resulting in pomace that generally consists of only skins, pulp, and seeds [3]. When grape pomace is further processed to extract alcohol and tartaric acid, it yields an additional residue known as spent grape pomace or exhausted grape marc (EGM) [9]. Another significant by-product is wine lees, the sediment that settles at the bottom of fermentation or storage vessels, or after authorised wine treatments [10]. Wine lees can account for up to 6% of the total wine volume [3]. This by-product mainly consists of fermentation-related yeasts and bacteria, tartaric acid salts, precipitated tannins, proteins, inorganic substances, and free phenolic compounds [11,12]. Globally, it is estimated that processing 100 tonnes of must, or wine, can generate approximately 38.9 tonnes of waste [3], implying that over 8 million tonnes of winemaking waste may have been produced in 2024.
In response, several applications for wine residues have been investigated, including their use as soil treatments and animal feed, as well as feedstock for producing bioethanol, biogas, and biochar [9]. Additionally, the recovery of various bioactive compounds—including phenolics, organic acids, lipids, proteins, and dietary fibre—has been explored as a promising biorefinery approach to transform these residues into valuable bioproducts for diverse applications [3,8]. Among these, phenolic compounds can serve as the basis for the development of functional ingredients with roles in the prevention of human disease, and, due to their antioxidant and antimicrobial properties, in replacing oenological additives [13,14]. Approximately 60–65% of phenolic compounds present in grapes remain in the “pomace” after juice or wine production; most of them, more than 50%, are found in grape seeds [15] that represent the main dry residue, followed by skin, while the rest is residual pulp and stems [16]. Vine shoots, grape pomace, and wine lees are recognised as some of the most promising biomasses for recovering phenolic compounds [17,18,19]. Therefore, integrating extraction methods, especially green technologies, into biorefinery processes offers a pathway toward more sustainable production models [17,18,19]. Nonetheless, beyond evaluating phenolic yield and composition, life cycle assessment is a crucial tool to guide the selection of the most sustainable extraction approach for phenolic compounds [20] and to drive the conversion from a linear to a circular approach.
In a circular economic approach, waste valorisation primarily aims to convert waste materials into valuable products, thereby reducing waste and promoting sustainable resource use. The energy requirements for waste valorisation vary depending on the techniques and processes employed. Some of the commonly researched techniques for waste valorisation include chemical, biochemical, thermochemical, and mechanical processes. Among the processes that require thorough investigation are also pretreatments, as certain stages—such as drying—can have a significant impact in terms of energy consumption. Innovative drying technologies play a critical role in advancing energy-efficient processing within the framework of a circular economy. Given that drying operations account for approximately 20–30% of the total energy consumption in agricultural and food production, there is a pressing need to develop more efficient systems that enhance sustainability and reduce operational costs [21]. Moreover, beyond technological advancements alone, the integration of these systems with renewable energy sources—such as solar-powered drying units—can significantly reduce fossil energy demand and further align waste valorisation processes with circular economy principles [22].
Winemaking by-products are abundant and have attracted growing interest as valuable resources within the framework of a circular economy. However, potential valorisation solutions must also be carefully evaluated from an environmental impact perspective to ensure their true sustainability. Given this context, the primary objective of this study is to promote the sustainable management and valorisation of these residues using the following ways:
- Investigating the current state of research on the extraction of antioxidant molecules from winemaking by-products, focusing on both traditional and innovative techniques.
- Selecting studies that provide comprehensive data and detailed process descriptions to enable a robust, also if preliminary, environmental assessment.
- Conducting a preliminary life cycle assessment (LCA) aimed at identifying the critical environmental impact categories associated with these valorisation processes.
- Pinpointing the most energy-intensive and environmentally sensitive stages of the extraction process that require targeted improvements, especially when scaling up to industrial applications.
- Emphasizing the importance of integrating environmental considerations—particularly energy consumption—early in the selection of extraction methods to foster circular economy principles within the wine industry, thereby contributing to waste reduction and resource efficiency throughout the supply chain.
It should be noted that this approach explicitly excludes experimental validation and the integration of broader primary datasets, as it constitutes a preliminary eco-design study primarily based on secondary data sources. Eco-design methodologies rely on existing literature data, databases, and published process information to perform early-stage environmental assessments and guide decision making before extensive experimental work is undertaken. Therefore, the present study serves as an initial screening tool within a simplified eco-design framework, laying the groundwork for more detailed experimental validation and primary data collection in subsequent research phases.
2. Materials and Methods
2.1. Literature Review and Paper Selection
An initial literature search was conducted to identify relevant works. The research utilised the following keywords on the SCOPUS and Google Scholar websites: polyphenol, extraction, grape residue, grape waste, antioxidant, and phenolic compounds. The search was limited to the years 2019–2024. This search yielded a total of 53 articles, of which 31 were initially deemed potentially relevant to the objectives of this study following a preliminary screening process. Articles that did not directly pertain to the study’s objectives or were reviews were excluded. A further selection process was conducted to identify articles that provided detailed discussions on innovative and/or non-conventional extraction techniques, specifically those related to grape residues. This screening process reduced the number of relevant documents to 14, as reported in Table 1. Of these 14 articles, 11 were excluded due to the absence of critical information and data necessary for the study, or because of the absence of an innovative extraction technique. Specifically, these articles lacked details on the analytical methods, instruments used, raw materials, solvents employed in the experiments, and extraction yields, which are essential for constructing a comprehensive framework for this research [23,24,25]. Additionally, some of the papers lack innovation, because they consider conventional solid–liquid extraction (SLE) [23,25,26,27,28,29,30] or extraction techniques too complex for proper assessment [17,31]. The remaining 3 papers were selected based on their focus on extraction techniques, including subcritical water extraction (SWE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and conventional solid–liquid extraction (SLE). The first 4 rows of Table 1 detail the pretreatments and extraction methods used in the selected papers, including freeze drying—MAE from grape marc and grape stalk using ethanol (EtOH) [32]; vacuum drying—SWE from white and red grape stalks [2]; oven drying—UAE from white grape skin and seed using EtOH or 2-methyltetrahydrofuran (2MTHF) [16]; and oven drying—SLE from white grape skin and seed using EtOH or 2MTHF [16].

Table 1.
Phenolic extraction technique found in the literature. Abbreviations: subcritical water extraction (SWE), microwave-assisted extraction (MAE), solid-liquid extraction (SLE), ultrasound-assisted extraction (UAE), hot pressurised liquid extraction (HPLE), and deep eutectic solvent (DES).
2.2. LCA
The life cycle assessment (LCA) encompasses the environmental impacts associated with input materials, such as solvents and electricity, as well as the resultant outputs, including total phenolic content (TPC) extract and emissions. The assessments were conducted by the ISO 14040 and ISO 14044 standards [33,34]. SimaPro 10.2 Analyst software (PRé Consultants B.V., Amersfoort, The Netherlands) was employed to develop the life cycle inventory (LCI) models and to generate results and analyses using the IPCC 2021 GWP100 V1.03 and EF 3.1 methodologies [35]. The data collected includes information sourced from the literature, databases, technical manuals, other studies, and calculations. The functional unit (FU) for the LCAs of the selected extraction processes is defined as one gram (1 g) of TPC in the extract expressed as grams of gallic acid equivalent (g GAE). The system boundaries include the pretreatment phase requiring drying and milling, as well as the extraction and centrifugation processes (where applicable), culminating in the production of the extract, characterised by its TPC. The disposal of solid residues generated is excluded (Figure 1). Ecoinvent 3.11 (Ecoinvent, Zurich, Switzerland) served as the dataset for background data, with all background data utilising the allocation at point of substitution (APOS) approach [36].
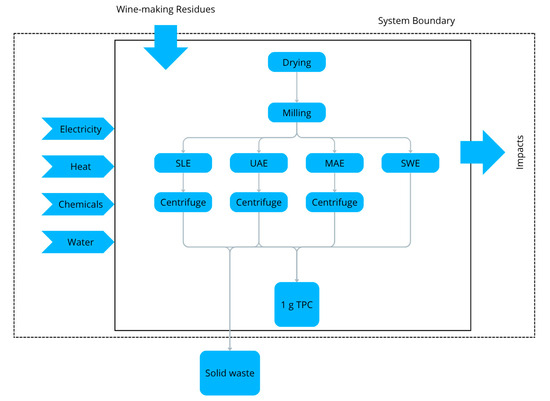
Figure 1.
System boundary of life cycle assessment. SLE = solid–liquid extraction; UAE = ultrasound-assisted extraction; MAE = microwave-assisted extraction; SWE = subcritical water extraction; TPC = total phenolic content.
2.3. Raw Material
Table 1 reports on the residues considered in the selected studies: GM, EGM, and GSs. Salgado-Ramos et al. [32] considered GSs, GM, and EGM and reported a moisture content of 66.82%, 76.35%, and 7.75%, respectively, following the 2017 harvesting season, and the grapes were provided by ALVINESA NATURAL INGREDIENTS S.A. (Daimiel, Ciudad Real, Spain), and the type of grapes used were not specified.
Canadas et al. [16] considered white grape waste from the winemaking process, consisting mainly of skins and seeds. Due to the lack of moisture information in the paper, a moisture content of 65.37%, as reported by Castillo et al. [37], has been assumed.
In their work, de Freitas et al. [2] reported the use of red and white GSs obtained from winemaking (2021) of the white grape variety cv. Malvasia and the red grape variety Bobal, both from the Utiel-Requena region (Finca Los Coloraos, Spain). No information about the moisture content was reported; therefore, a moisture content of 71.70% and 62.50% for white and red GS, respectively, was assumed, as reported by Blackford et al. [38].
2.4. Pretreatments
Salgado-Ramos et al. [32] employed a lyophilisation process, performed using a “Telstar LyoQuest-85PLUS FREEZE DRYER” (Telstar Technologies, Terasa, Spain). The paper lacks information about the lyophilisation process, so theoretical energy consumption was assumed. Briefly, the energy required for cooling to 0 °C (Q1) a certain mass of water in the sample (m) was estimated considering a starting temperature of 20 °C and a heat capacity of liquid water (Cp,w) of 4.18 kJ/kg°C with Equation (1):
The energy required for freezing (Q2) the same mass of water is calculated considering a latent heat of fusion (ΔHfus) of 334 kJ/kg, estimated with Equation (2):
Once the ice is formed, the energy required to reduce the temperature (Q3) from 0 °C to −85 °C was estimated considering a heat capacity of ice (Cp,i) of 2.11 kJ/kg°C with Equation (3):
Increasing the temperature from −85 °C to −18 °C allows the water to sublimate, so the energy for heating the ice (Q4 and Q5) has been estimated considering Equations (4) and (5), using a sublimation enthalpy (ΔHsub) of 2838 kJ/Kg:
Assuming a remaining water content of 10%, the energy required to increase the temperature from −18 °C to 0 °C (Q6 and Q7) was estimated by Equations (6) and (7) using a melting enthalpy (ΔHmel) of 334 kJ/kg:
In the last process, the energy required to increase the temperature from 0 °C to 50 °C and the relative evaporation process of the remaining water (Q8 and Q9) are assessed, using an evaporation enthalpy (ΔHvap) of 2383 kJ/kg, with Equations (8) and (9):
Assuming a drying efficiency (Eff) of 0.012 as reported by Baeghbali et al. [39], it is possible to estimate the energy of the lyophilisation process with Equation (10):
All other energy consumption values in this study were derived from the power ratings specified by the instrument manufacturers, a conservative methodology adopted to model a worst-case scenario.
For vacuum generation, we considered the power consumption reported in the instrument’s manual and an operational time of 24 h, as reported by Baeghbali et al. [39]. A maximum load of 4.5 kg was considered, assuming three plates that were 22 cm in diameter and 1 cm in height, with a density of the raw material assumed to be 1 g/cm3. Subsequently, the dried residue was ground at 20,000 rpm for 1 min using Fritsch Pulverisette 15 (Idar-Oberstein, Germany). For this process, a power consumption of 1.1 kW was considered, as reported in the instrument’s operational manual.
In the UAE study performed by Canadas et al. [16], pretreatment consists of the conventional drying of the residues for 24 h at 40 °C, followed by grinding the dried residue into a powder (<2 mm). Due to the lack of information about the oven used and the grinding process, it was assumed that a Thermo Scientific drying oven (OMS60) with an estimated maximum load of 2 kg of raw material was employed (calculated considering two trays of 13.9 × 14.5 × 5 cm and a raw material density of 1 g/m3). Due to a lack of information about the milling process, it has been assumed to be equal to the one adopted by Salgado-Ramos et al. [32].
In the SWE conducted by de Freitas et al. [2], GSs were vacuum dried (60 ± 2 °C, 0.6 mbar for 16 h) in a vacuum oven, assuming the same energy consumption of 1.7 kW as a Thermo Fisher VT6060 M and an estimated maximum load of 7 kg (calculated considering two trays measuring 41.5 × 34.5 × 2.5 cm, assuming a density of 1 g/cm3). Subsequently, they were milled (2 cycles of 60 s) using a mill (Model SM300, stainless steel, Retsch GmbH, Germany), which has a reported power consumption of 3 kW according to the operational manual, and then sieved to achieve a particle size below 600 μm.
2.5. Extractions
Salgado-Ramos et al. [32] applied MAE using a CEM Discover monomodal microwave reactor (CEM Discover SP, Matthews, NC, USA). The extraction was performed at 100 °C for 120 min, assuming an operating power of 0.3 kW, as reported in the reactor user manual. A maximum load of 5 g of dried, blade-milled solid and 10 mL of solvent were used. After extraction, the crude was vacuum filtered and thoroughly washed with EtOH/H2O solution. A centrifuge from Thermo Fisher (Rockford, IL USA), with a power consumption of 1.95 kW, was used for separating solid waste from the extract, which was capable of processing up to 40 vials (50 mL).
Canadas et al. [16] applied both UAE and SLE. A 95 W and a 515 W operating power, along with operational times of 1 and 6 h for UAE and SLE, respectively, were considered, as reported by the authors. A maximum load of 9 g was considered for both extraction methods. A centrifuge by Thermo Fisher, with an operating power of 1.95 kW, was considered for solid waste separation from the extract, which was capable of processing a maximum of 96 vials (15 mL) in 10 min.
SWE, performed by de Freitas et al. [2], was carried out using a Pressure Reactor (Model 1-T-A-P-CE, 5 L capacity, Amar Equipment PVT. LTD, Mumbai, India) assuming an operational power of 3.5 kW as reported in the manual and an estimated maximum load of 2 kg (calculated considering an occupied volume of 2 L in the reactor chamber and a raw material density of 1 g/m3).
2.6. LCI
The life cycle inventory was developed using the quantities outlined in Table 2, Table 3, Table 4 and Table 5 for each extraction scenario. The data used have been classified to clearly indicate both their source and type, as detailed in Table 2, Table 3, Table 4 and Table 5.

Table 2.
Specific inventory data (input and output) relating to the grape marc (GM), grape stalk (GS) and exhausted grape marc (EGM) with the freeze-drying microwave-assisted extraction (MAE) combination.

Table 3.
Specific inventory data (input and output) relating to the ethanol (EtOH) and 2-methyltetrahydrofuran (2MTHF) oven-drying ultrasound-assisted extraction (UAE) combination.

Table 4.
Specific inventory data (input and output) relating to the ethanol (EtOH) and 2-methyltetrahydrofuran (2MTHF) oven-drying solid–liquid extraction (SLE) combination.

Table 5.
Specific inventory data (input and output) relating to the red stalk extraction at 160 °C (R160) and at 180 °C (R180) and the white stalk extraction at 160 °C (W160) and 180 °C (W180) with the vacuum-drying subcritical water extraction (SWE) combination.
2.7. Sensitivity Analysis
To assess uncertainty and the robustness of the results related to the most promising extraction technique, a Monte Carlo simulation was conducted within SimaPro 10.2. The simulation consisted of 10,000 iterations, during which key input parameters were assigned probabilistic distributions using the IPCC 2021 GTP100 V1.03 method with a 95% confidence interval. These distributions were derived from default uncertainty values provided by the Ecoinvent 3.11 database. Outputs from the Monte Carlo analysis were used to generate confidence intervals. Other potentials sources of uncertainty in the input data were not taken into consideration (like yields or energy consumptions) due to the lack of information from the selected literature.
In addition to statistical uncertainty addressed through Monte Carlo simulation, LCA are also subject to uncertainty stemming from modelling and methodological choices. These choices are inherently discrete and cannot be meaningfully described using continuous probability distributions. As highlighted in the ISO 14044 [34] standard, this type of choice-related uncertainty is more appropriately explored through scenario analysis, whereby distinct, plausible modelling assumptions are assessed independently. Therefore, to understand to what extent results could be improved in the future, an alternative Italian energy scenario has been created that excludes contributions from imported coal and natural gas. The renewable energy mix for Italy was obtained by spreading the eliminated contribution to all renewable energy used in Italy, except for hydroelectric energy. The hydroelectric contribution to the mix was maintained due to the assumption that it would be more challenging to increase the number of hydro plants compared to wind, solar, and geothermal energy sources.
3. Results and Discussion
Characterised results of extraction techniques are shown in Table 6, while Figure 2 shows single-score results. Results demonstrate that the extraction factors, including extraction technique, temperature, residues, and solvent, have a significant impact on the related environmental impacts. Among the techniques, MAE is the most impactful, with the MAE_EGM extraction technique having the highest impact per gram of TPC across all 16 impact categories investigated. Significant improvements are observed using the same methods and shifting to other residues (GM and GSs). The best results are achieved with the SWE technique, with SWE_180 showing the lowest impact in all 16 analysed impact categories. SLE and UAE exhibit similar performance, with SLE having a more significant impact than UAE, and MTHF in both cases being more impactful than ethanol. Overall, the single-score results obtained using the EF 3.1 methodology represent an aggregated index that synthesises environmental impacts across multiple categories, each weighted according to its relative importance. Analysis of the contributions from individual impact categories reveals that specific categories play a dominant role in determining the overall score. Specifically, categories such as climate change (CC); ozone depletion; resource use, fossils; and resource use, minerals and metals contribute the most, reflecting both the magnitude of their measured impacts and their assigned weighting factors within the EF 3.1 framework. Their contribution to the overall single-score results is greater than 80% in all scenarios investigated, with climate change accounting for more than 50%. This distribution of contributions highlights the most critical environmental aspects within the assessed scenarios, thereby guiding the selection of the most appropriate impact categories for further investigation. For this reason, aligning with the new goal of achieving net-zero carbon emissions by 2050, as outlined in the European Green Deal, the focus of this paper was on climate change (expressed in kg CO2eq/FU).

Table 6.
Impact categories for all polyphenol extraction method scenarios related to 1 g produced. CC = climate change; OD = ozone depletion; IR = ionising radiation; POF = photochemical ozone formation; PM = particulate matter; HTNC = human toxicity, non-cancer; HTC = human toxicity, cancer; AC = acidification; EF = eutrophication, freshwater; EM = eutrophication, marine; ET = eutrophication, terrestrial; ETF = ecotoxicity, freshwater; LU = land use; WU = water use; RUF = resource use, fossils; RUM = resource use, minerals and metals.
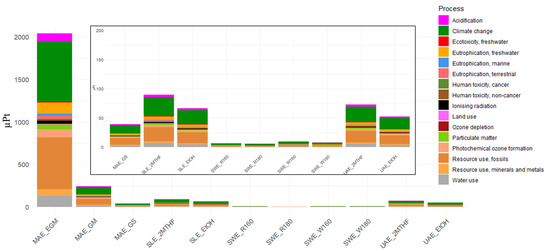
Figure 2.
Single-score results of all the extractions considered. MAE: microwave-assisted extraction; EGM: exhausted grape marc; GM: grape mark; GS: grape stalk; SLE: solid–liquid extraction; EtOH: ethanol; 2MTHF: 2-methyltetrahydrofuran; SWE: subcritical water extraction; R160: red grape residue extraction at 160 °C; R180: red grape residue extraction at 180 °C; W160: white grape residue extraction at 160 °C; W180: white grape residue extraction at 180 °C; UAE: ultrasound-assisted extraction.
Figure 3 presents the GHG emissions associated with the extraction scenarios investigated and the process contribution to the CC impact category. CC showed values ranging from 0.08 kg CO2eq/FU for SWE at 180 °C from red grape GSs to 25.4 kg CO2 eq/FU for MAE from EGM. The drying phase, regardless of the technique applied, consistently represents the most impactful process (>85%), except in scenarios involving microwaves, in which the extraction process accounts for 56% to 82% of the GHG emissions, and drying becomes the second-largest contributor to CC, except in MAE_EGM, where solvent precedes it. SWE techniques perform better due to the vacuum drying of grape stalks, which requires less energy compared to other drying technologies. For instance, in SWE_R180, 0.175 kWh/FU is required for drying, compared to 0.419 kWh/FU for the freeze drying of MAE_GS. The MAE_EGM is the most impactful extraction process. This can be explained by considering that, among all the drying methods considered, freeze drying requires the most energy. Furthermore, the yield of polyphenols extracted from EGM is lower compared to GM and GS substrates. Among all extraction methods, SWE is the most promising with a CC between 7.8 × 10−2 and 1.3 × 10−1 kg CO2eq/FU for R180 and W180, respectively, and an average energy consumption of approximately 2.00 × 10−2 kWh/FU. This finding can be explained by considering the maximum load of dried residues of the instrument involved. In SWE, a larger extraction chamber has been considered, allowing for the processing of kilograms of materials, compared to the grams processed in UAE and MAE techniques. In the literature, a similar study conducted by Croxatto Vega et al. [20] reported emissions of approximately 0.15 kg CO2eq/g GAE for acetone extraction at 50 °C for 2 h, and 0.70 kg CO2eq/g GAE for pressurised ethanol extraction at 80 °C and 100 bar. By comparison, the MAE_GM method evaluated in the present study shows higher emissions (3.04 kg CO2eq/FU), mainly due to the high energy consumption associated with the freeze-drying process and the limited capacity of the microwave extraction reactor, which can process only 5 g of GM per batch, requiring multiple runs to obtain 1 g of extract. When considering SLE from skin and seeds, impacts vary from 0.874 and 1.16 kg CO2eq/FU for EtOH and 2MTHF, respectively. This result reflects the differences due to the use of different solvents and is more in line with Croxatto Vega et al., for which the main difference is the extraction time (2 h vs. 6 h in the current study). The results of SLE are slightly higher than those of UAE. Differences are primarily due to the higher energy consumption associated with the orbital shaker used in SLE compared to the ultrasonic bath, as well as the slightly higher yield of UAE compared to SLE with the same solvent. The differences in the use of two different solvents are attributable to the higher extraction yield achieved with EtOH compared to MTHF, as well as the higher environmental impact of MTHF.
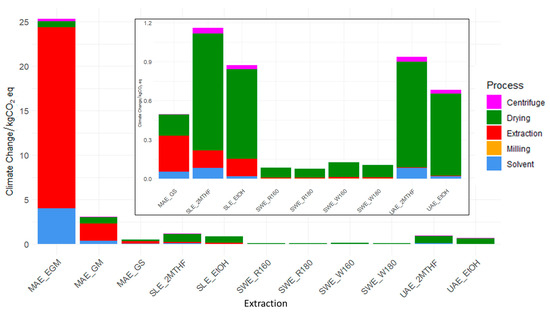
Figure 3.
Climate change process contribution of all the extractions considered. MAE: microwave-assisted extraction; EGM: exhausted grape marc; GM: grape mark; GS: grape stalk; SLE: solid–liquid extraction; EtOH: ethanol; 2MTHF: 2-methyltetrahydrofuran; SWE: subcritical water extraction; R160: red grape residue extraction at 160 °C; R180: red grape residue extraction at 180 °C; W160: white grape residue extraction at 160 °C; W180: white grape residue extraction at 180 °C; UAE: ultrasound-assisted extraction.
Finally, among the residues considered, GSs from red grapes are the most promising in terms of environmental impact, due to its higher extraction yields.
In the study by Fraterrigo Garofalo et al. [40], compared to MAE, UAE exhibited the highest environmental impact for two main reasons: the use of a higher temperature (60 °C) and a higher apple pomace-to-water ratio (1:30). In the present study, MAE is more environmentally favoured compared to UAE only when starting from GSs. The difference is primarily due to the different starting materials, instruments used for extraction, and the resulting yields. Comparing similar starting materials and various techniques, MAE_GM and UAE_EtOH, the lower impacts associated with the second one can be attributed to both higher yields of extraction (0.8% vs. 1.0%) and lower energy consumption (6.90 kWh/FU vs. 2.06 kWh/FU).
In the present study, various extraction scenarios were tested using different solvents across MAE, UAE, and SLE methods, with ethanol (EtOH) and 2-methyltetrahydrofuran (MTHF) as the primary solvents. While the drying phase tends to mask the impact of solvents on the overall footprint, isolating the extraction and solvent contributions reveals significant differences: in SLE and UAE, solvents account up to 95% of the impact. In contrast, the solvent contribution is notably lower in MAE (16%), and negligible in SWE, where only water is used as the extraction medium.
When comparing different starting materials processed with the same extraction technology (e.g., SWE_R180 vs. SWE_W180; SWE_R160 vs. SWE_W160), grape marc from red wine production consistently yields better results. This can be attributed to the significantly higher polyphenol content in red grape marc compared to white grape marc, which reflects both the natural differences in grape composition and the specific winemaking practices applied to red and white varieties. Red grape marc consistently exhibits higher polyphenol contents compared to white grape marc. Research indicates that the total phenolic content in white grape marc ranges widely, with reported values of 1634 to 3727 mg gallic acid equivalents (GAE) per litre for extracts obtained through various extraction methods [37]. Conversely, red grape marc yields significantly more polyphenols, with estimates suggesting total polyphenol content in dry matter ranging from 4.8% to 5.4%, highlighting its superior polyphenolic richness compared to white grape marc [41]. In this specific case, although reaching higher temperatures requires greater energy input, this expenditure is offset by the improved extraction yield, which allows for a higher recovery of polyphenols, as also reported by Barjoveanu et al. [42]. In their study, Barjouveany et al. stated that a reduction of 11–12% in all categories occurs when considering an increase in the spruce bark UAE temperature from 25 °C to 50 °C. In the present study, an increase from 160°C to 180°C results in an overall reduction of 10% and 17% in SWE for red and white GSs, respectively.
The energy production mix affects the result. Gargiulo et al. showcased how LCA could be applied to assess different electricity mixes in Italy up to 2030. Their comprehensive study underscored the varied impacts across multiple environmental categories, demonstrating that the evaluated energy scenarios greatly influence the LCA outcomes, providing insights into potential future trends and trade-offs associated with renewable energy deployment [43]. The current and future scenarios will also be influenced by the geopolitical changes we are already witnessing. The ongoing conflict between Russia and Ukraine has exhibited profound implications for the energy landscape within Italy and across Europe. Italian energy production from imported natural gas [44] underwent a significant shift from 2021 to 2022 [44], with natural gas imports from Russia decreasing from 40% to 20% and further reducing to 5% in 2023 [45]. This situation highlights the need to invest in expanding renewable energy infrastructure, thereby providing more opportunities to adopt sustainable policies. Carà et al. [46] predict that, over the next 30 years, a reduction in fossil energy sources will be accompanied by an expansion of renewable energy infrastructure. Considering that energy consumption is the primary contributor to the overall environmental impact, the choice of energy mix plays a crucial role in determining the results. A sensitivity analysis was therefore conducted on the most promising scenario by varying the energy mix. The results presented in Table 6 and Figure 2 and Figure 3 are based on the Italian energy mix, which in 2023 relied on fossil fuels for nearly 80% of its supply [44]. Replacing all fossil-based and imported energy with renewable sources leads to a reduction of approximately 90% in environmental impact, as illustrated in Figure 4 for the SWE scenarios. Under the current Italian energy mix, uncertainty values range from 0.06 to 0.132 kg CO2eq/FU (mean: 0.09 kg CO2eq/FU), while with a fully renewable mix, the range narrows to 0.009–0.024 kg CO2eq/FU (mean: 0.01 kg CO2eq/FU).
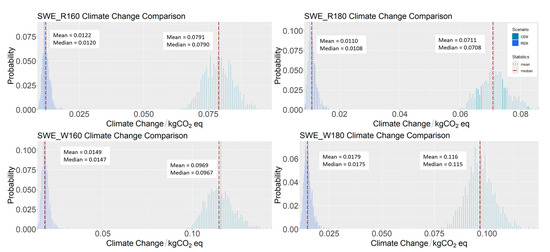
Figure 4.
GWP sensitivity analysis comparison of climate change contribution of polyphenol SWE from grape stalks with two different energy mixes: current Italian energy mix (CEM) and 100% renewable Italian energy mix (REM).
Although preliminary and limited, the data from this study highlight the key parameters that may influence the outcomes. One of the most significant limitations of the present work, as well as of all LCA assessments conducted on the laboratory scale in general, is the difficulty in translating laboratory results to real-world applications. Various factors contribute to discrepancies in results when moving from controlled laboratory environments to complex real-world systems. These issues often include methodological limitations, data gaps, and variations in process efficiencies. The choice of the proper technique is influenced by the starting material and the instrument’s efficiency, considering not only the yields but also the maximum load and, consequently, the number of runs required to process the starting materials. Besides this general consideration, among all the processes considered, the pretreatment, in this case the drying process, is a critical step due to the high energy required to remove water from a starting material characterised as having high water content. As the demand for energy-efficient processes rises, the integration of advanced drying methods can significantly reduce energy consumption in production cycles. Efficient drying technologies, such as solar and hybrid systems, can lower greenhouse gas emissions compared to conventional methods [47]. This shift towards energy-efficient drying processes supports the circular economy by reducing reliance on nonrenewable resources, thereby promoting environmental sustainability. On the other hand, a greener drying process could compromise the final product itself in terms of polyphenol content and antioxidant activity, making the drying process an essential component when developing an efficient and sustainable polyphenol extraction method [48,49,50].
Finally, a limitation of this study is the exclusion of post-extraction solid residues from the system boundaries. Although their management may have relevant environmental implications, detailed information on the composition, quantity, and destination of these residues was not available from the selected literature papers. Including them without such data would have introduced significant uncertainty into the assessment. Nonetheless, the existing literature suggests that these by-products have potential for sustainable valorisation. For example, pyrolysis can convert exhausted grape marc into biochar, contributing to carbon sequestration and soil enhancement, while pelletisation enables bioenergy production from agricultural residues [51]. In the MAE_GM scenario, energy recovery from solid waste could offset between 0.03% and 1.4% of the process’s energy demand [52]. Although the direct contribution is minimal, the integration of such strategies could offer broader environmental benefits and should be considered in future studies when more detailed data become available.
4. Conclusions
This study aimed to characterise different polyphenol extraction processes from various residual biomasses related to wine production, as sourced from the literature, within the framework of a preliminary eco-design approach. The goal was to assess and compare the environmental performance of these processes to inform more sustainable extraction strategies at early design stages. The results demonstrate that the extraction technique, temperature, solvent, and type of feedstock have a significant influence on the environmental impact of polyphenol recovery. Among the evaluated methods, MAE_EGM showed the most significant impact due to low yields and high energy use for freeze drying. At the same time, SWE_R180 achieves the lowest impacts across all 16 categories, thanks to its higher efficiency and the use of water as a solvent, as well as the use of red grape residues with a higher polyphenol content. The drying phase emerged as the primary contributor to GHG emissions, except in MAE scenarios where extraction dominates. Climate change and energy demand were the most influential impact categories, with results strongly affected by the energy mix used. A complete renewable energy scenario could reduce impacts by up to 90%.
While no method can be universally recommended, the choice of technique must consider both extraction efficiency and energy demand.
This study offers valuable insights into optimising polyphenol extraction within the context of current geopolitical challenges, the European Green Deal’s climate targets, and the urgent need to transition towards circular, resource-efficient production systems. Its findings highlight critical parameters for enhancing environmental sustainability in bio-based industries.
Author Contributions
Conceptualization, L.L., D.V. and G.M.; methodology, L.L., D.V. and G.M.; software, L.L., G.M. and D.V.; validation, D.V., G.L. and L.L.; formal analysis, L.L., D.V., G.L. and G.M.; investigation, L.L., D.V. and G.M.; resources, L.L., D.V. and G.M.; data curation, L.L. and D.V.; writing—original draft preparation, D.V. and G.M.; writing—review and editing, L.L., D.V. and G.L.; visualisation, L.L. and D.V.; supervision, L.L.; project administration, L.L.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”, grant number PE0000003. CUP B83C22005120006.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GM | Grape marc |
| GS | Grape stalk |
| EGM | Exhausted grape marc |
| SWE | Subcritical water extraction |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| SLE | Solid–liquid extraction |
| EtOH | Ethanol |
| 2MTHF | 2-Methyltetrahydrofuran |
| LCA | Life cycle assessment |
| TPC | Total phenolic content |
| LCI | Life cycle inventory |
| FU | Functional unit |
| APOS | Allocation at point of substitution |
| GAE | Gallic acid equivalent |
| Cp,w | Heat capacity of liquid water |
| Cp,i | Heat capacity of ice |
| ΔHfus | Latent heat of fusion for ice |
| ΔHsub | Sublimation heat for ice |
| ΔHmel | Melting enthalpy for ice |
| ΔHvap | Evaporation enthalpy for water |
| Eff | Drying efficiency |
| CC | Climate change |
| CE | Circular economy |
| CEM | Current Italian energy mix |
| REM | Renewable Italian energy mix |
References
- International Organisation of Vine. Wine State of the World Vine and Wine Sector in 2024; International Organisation of Vine: Dijon, France, 2025. [Google Scholar]
- de Freitas, P.A.V.; Meyer, S.; Hernández-García, E.; Rebaque, D.; Vilaplana, F.; Chiralt, A. Antioxidant and Antimicrobial Extracts from Grape Stalks Obtained with Subcritical Water. Potential Use in Active Food Packaging Development. Food Chem. 2024, 451, 139526. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero-García, J.M.; López-Linares, J.C.; Romero, I.; Castro, E. Residues from Grapevine and Wine Production as Feedstock for a Biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of Grape Byproducts as Functional Ingredients in Baked Goods and Pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef]
- Baptista, S.L.; Romaní, A.; Cunha, J.T.; Domingues, L. Multi-Feedstock Biorefinery Concept: Valorization of Winery Wastes by Engineered Yeast. J. Environ. Manag. 2023, 326, 116623. [Google Scholar] [CrossRef]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated Biorefinery Approach to Valorize Winery Waste: A Review from Waste to Energy Perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Amico, V.; Tringali, C. Volatile Components of Grape Pomaces from Different Cultivars of Sicilian Vitis vinifera L. Bioresour. Technol. 2008, 99, 260–268. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Graça, A.; Corbet-Milward, J.; Schultz, H.R.; Ozer, C.; de La Fuente, M. Managing By-Products of Vitivinicultural Origin; OIV–International Organization of Vine and Wine: Paris, France, 2018. [Google Scholar]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Marangon, M.; Lomolino, G.; Crapisi, A.; Curioni, A. Red and White Wine Lees as a Novel Source of Emulsifiers and Foaming Agents. LWT 2021, 152, 112273. [Google Scholar] [CrossRef]
- Bulos, R.B.d.A.; Paz, F.d.G.; Machado, C.G.; Tavares, P.P.L.G.; de Souza, C.O.; Umsza-Guez, M.A. Scientific and Technological Research on the Use of Wine Lees. Food Prod. Process. Nutr. 2023, 5, 25. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the Genus Vitis: Phenolic Compounds, Anticancer Properties and Clinical Relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Cañadas, R.; Díaz, I.; Sánchez-Monedero, A.; González, E.J.; González-Miquel, M. Green Extraction of Natural Antioxidants from White Grape Waste Using Bio-Renewable Solvents and Ultrasonic Process Intensification. Chem. Eng. Process.-Process Intensif. 2024, 196, 109644. [Google Scholar] [CrossRef]
- Pedras, B.M.; Regalin, G.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Fractionation of Red Wine Grape Pomace by Subcritical Water Extraction/Hydrolysis. J. Supercrit. Fluids 2020, 160, 104793. [Google Scholar] [CrossRef]
- Freitas, L.C.; dos Santos, R.W.S.; Reis, F.R.; Haminiuk, C.W.I.; Corazza, M.L.; Masson, M.L. Green Extraction Technologies: A Path to the Amazon Bioeconomy Development. Trends Food Sci. Technol. 2024, 147, 104462. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies-A Review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Croxatto Vega, G.; Sohn, J.; Voogt, J.; Birkved, M.; Olsen, S.I.; Nilsson, A.E. Insights from Combining Techno-Economic and Life Cycle Assessment—A Case Study of Polyphenol Extraction from Red Wine Pomace. Resour. Conserv. Recycl. 2021, 167, 105318. [Google Scholar] [CrossRef]
- Ye, L.; El-Mesery, H.S.; Ashfaq, M.M.; Shi, Y.; Zicheng, H.; Alshaer, W.G. Analysis of Energy and Specific Energy Requirements in Various Drying Process of Mint Leaves. Case Stud. Therm. Eng. 2021, 26, 101113. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A Comprehensive Review of Sustainable Bioremediation Techniques: Eco Friendly Solutions for Waste and Pollution Management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.; Bajić, S.S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic Compounds and Biopotential of Grape Pomace Extracts from Prokupac Red Grape Variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Daniela, T.-R.; del Socorro, L.-C.M.; Fortunata, S.-T.; Patricia, R.-M.; Felipe, G.-O.; Teresa, H.-B.M.; de la Paz, S.-C.M. Optimization of the Extraction of Bioactive Compounds from Cabernet Sauvignon Grape Pomace from Querétaro, Mexico, Using MSPD. Separations 2023, 11, 13. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Enhancement of Phenolic Compounds Extraction from Grape Pomace by High Voltage Atmospheric Cold Plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Conidi, C.; Cassano, A. A Membrane-Assisted Green Strategy for Purifying Bioactive Compounds from Extracted White Wine Lees. Sep. Purif. Technol. 2024, 336, 126183. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Zhang, Z.; Zhang, L.; Fei, Q.; Ma, Y. New Insights into Wine Waste Management: Zero Waste Discharge-Driven Full Energy/Resource Recovery Strategy. Results Eng. 2022, 15, 100606. [Google Scholar] [CrossRef]
- Montagner, G.E.; Wingert, N.R.; Stein, C.d.S.; Moresco, R.N.; Fogaça, A.d.O.; Gomes, P. Optimization of the Extraction of Antioxidant Compounds from Grape Seed from Winemaking Waste. Sustain. Chem. Pharm. 2022, 30, 100856. [Google Scholar] [CrossRef]
- Ivanov, Y. Optimization of the Extraction Procedure of Polyphenols from Red Pinot Noir Grape Seeds. BIO Web Conf. 2024, 102, 02002. [Google Scholar] [CrossRef]
- Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Gajardo-Parra, N.; Pérez-Correa, J.R.; Canales, R.I.; Martínez-Cifuentes, M.; Contreras-Contreras, G.; Mariotti-Celis, M.S. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants 2023, 12, 1446. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Huertas-Alonso, A.J.; Martí-Quijal, F.J.; Barba, F.J.; Sánchez-Verdú, M.P.; Moreno, A.; Cabañas, B. Sustainable Management of Wine-Derived Leftovers: Enhanced Extraction of Antioxidants and Production of Levulinic Acid by Microwave-Assisted Processing. J. Environ. Chem. Eng. 2024, 12, 112411. [Google Scholar] [CrossRef]
- ISO14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Damiani, M.; Ferrara, N.; Ardente, F. Understanding Product Environmental Footprint and Organisation Environmental Footprint Methods; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of Bioactives Extraction from Grape Marc via a Medium Scale Ambient Temperature System and Stability Study. Front. Nutr. 2022, 9, 1008457. [Google Scholar] [CrossRef]
- Blackford, M.; Comby, M.; Zeng, L.; Dienes-Nagy, Á.; Bourdin, G.; Lorenzini, F.; Bach, B. A Review on Stems Composition and Their Impact on Wine Quality. Molecules 2021, 26, 1240. [Google Scholar] [CrossRef] [PubMed]
- Baeghbali, V.; Niakousari, M.; Farahnaky, A. Refractance Window Drying of Pomegranate Juice: Quality Retention and Energy Efficiency. LWT—Food Sci. Technol. 2016, 66, 34–40. [Google Scholar] [CrossRef]
- Fraterrigo Garofalo, S.; Demichelis, F.; Peletti, V.; Picco, L.; Tommasi, T.; Fino, D. Comparative Study of Polyphenol Extraction Using Physical Techniques and Water as a Solvent: A Sustainable Approach for the Valorization of Apple Pomace. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Matei, P.M.; Iacomi, B.M.; Nieves, M.B.T.; Alvarez, F.L.; Barbulescu, I.D.; Teodorescu, R.I.; Banita, D.C.; Grosu, A.C.; Nitu, M. The Composting Potential of the By-Product Marc Resulting from the White and Red Winemaking Process. BIO Web Conf. 2023, 68, 01041. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life Cycle Assessment of Polyphenols Extraction Processes from Waste Biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef]
- Gargiulo, A.; Carvalho, M.L.; Girardi, P. Life Cycle Assessment of Italian Electricity Scenarios to 2030. Energies 2020, 13, 3852. [Google Scholar] [CrossRef]
- Statista. Distribution of the Total Energy Supply in Italy in 2023, by Energy Source. Available online: https://www.statista.com/statistics/873552/energy-mix-in-italy/#:~:text=In (accessed on 25 July 2025).
- Statista. Gross Imports of Natural Gas in Italy in 2023, by Country of Origin. Available online: https://www.statista.com/statistics/787720/natural-gas-imports-by-country-of-origin-in-italy/#:~:text=In (accessed on 25 July 2025).
- Carà, C.; Marocco, P.; Novo, R.; Koivisto, M.; Santarelli, M.; Mattiazzo, G. Modeling the Long-Term Evolution of the Italian Power Sector: The Role of Renewable Resources and Energy Storage Facilities. Int. J. Hydrogen Energy 2024, 59, 1183–1195. [Google Scholar] [CrossRef]
- Uzoma, S.; Nwakuba, N.; Anyaoha, K. Performance of Hybrid Photovoltaic/Thermal Crop Dryer in Hot Humid Nigerian Region. Poljopr. Teh. 2019, 44, 56–75. [Google Scholar] [CrossRef]
- Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.C.; Irakli, M. Comparison of Drying Methods for the Retention of Phenolic Antioxidants in Post-Distillation Solid Residues of Aromatic Plants. LWT 2023, 189, 115463. [Google Scholar] [CrossRef]
- Soto, B.; Gatica, M.; Uribe, E.A.; Rozas, A.; Cabezas, R.; Pino, L.; Román, J. Evaluation of Antioxidant Activity and Polyphenol Preservation in Rosehip (Rosa spp.) during Storage and Convective Drying. LWT 2025, 228, 118089. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of Seven Different Drying Treatments in Respect to Total Flavonoid, Phenolic, Vitamin C Content, Chlorophyll, Antioxidant Activity and Color of Green Tea (Camellia sinensis or C. Assamica) Leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef]
- Frikha, K.; Limousy, L.; Arif, M.B.; Thevenin, N.; Ruidavets, L.; Zbair, M.; Bennici, S. Exhausted Grape Marc Derived Biochars: Effect of Pyrolysis Temperature on the Yield and Quality of Biochar for Soil Amendment. Sustainability 2021, 13, 11187. [Google Scholar] [CrossRef]
- Zhang, N.; Hoadley, A.; Patel, J.; Lim, S.; Li, C. Sustainable Options for the Utilization of Solid Residues from Wine Production. Waste Manag. 2017, 60, 173–183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).