Hydrodynamic Cavitation in Shockwave-Power-Reactor-Assisted Biodiesel Production in Continuous from Soybean and Waste Cooking Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Traditional Process
2.3. Cavitation Process
2.4. Oil and Biodiesel Characterization
2.5. Experimental Design
2.5.1. Factorial Design in the Traditional Process
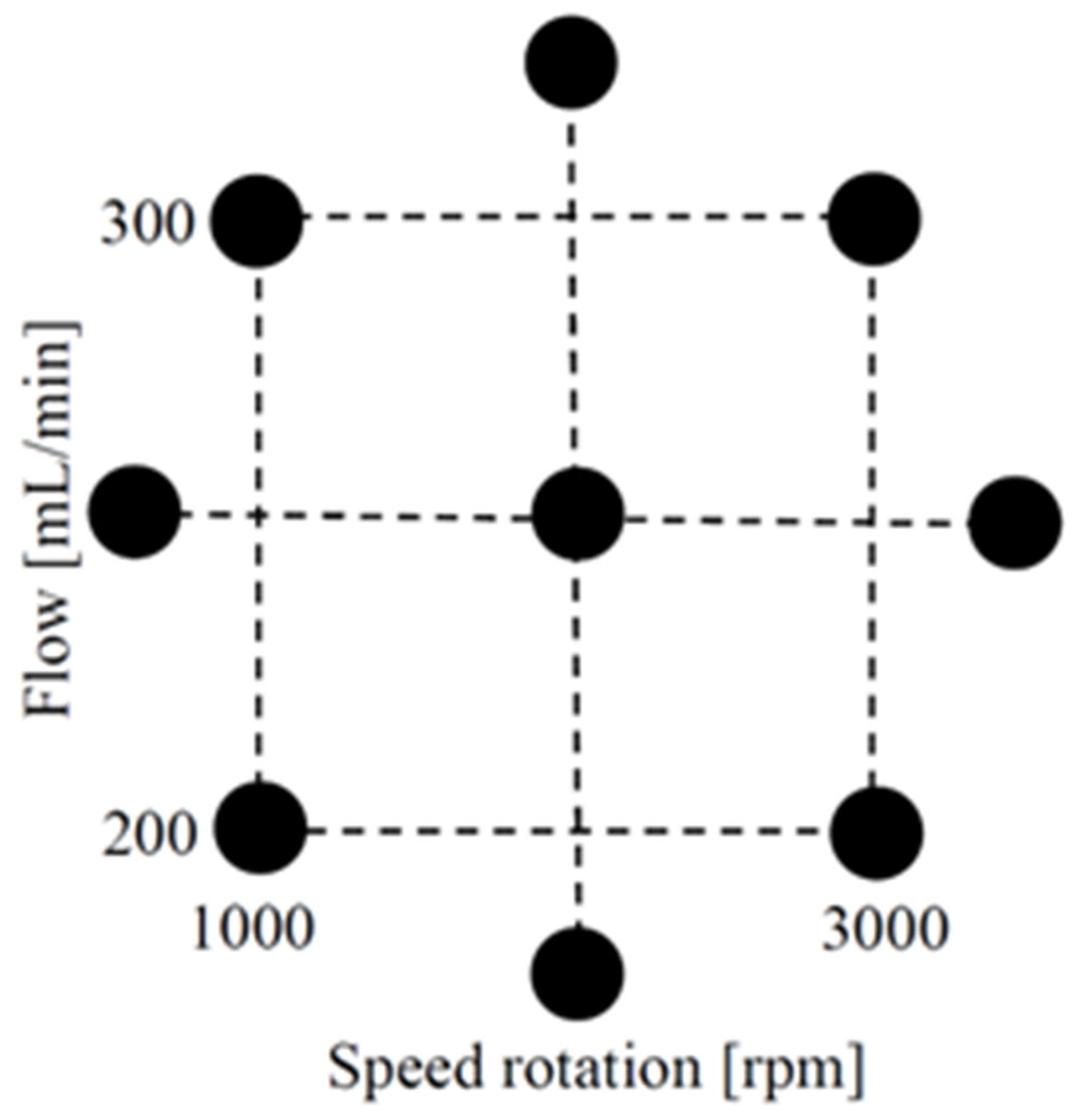
2.5.2. Central Composite Design in the Cavitation Process
3. Results and Discussions
3.1. Oil Characterization
3.2. Results of Factorial Design in the Traditional Process
3.3. Results of Central Composite Design in the Cavitation Process with Transient and Steady States
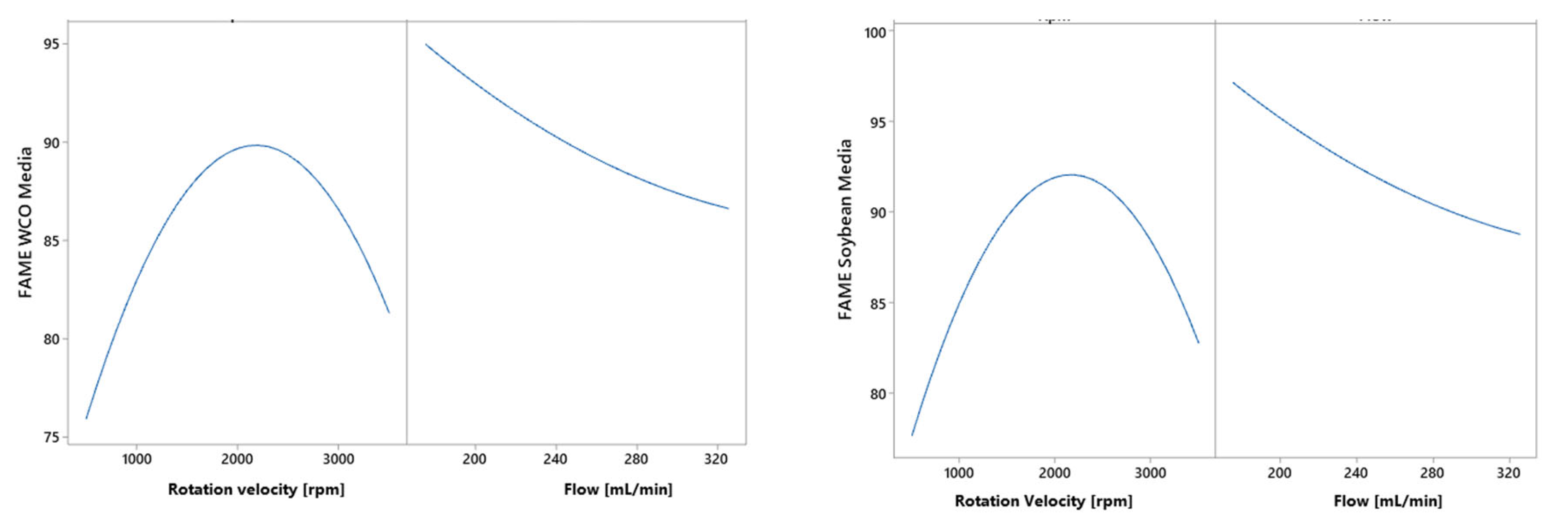
3.4. Results of Central Composite Design in the Cavitation Process with Flow and Rotation Speed Factors
3.5. Comparison of Biodiesel Obtained
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asmelash, E.; Prakash, G.; Gorini, R.; Gielen, D. Role of IRENA for global transition to 100% renewable energy. In Accelerating the Transition to a 100% Renewable Energy Era; Springer: Cham, Switzerland, 2020; pp. 51–71. [Google Scholar]
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Jaszczur, M. The renewable energy role in the global energy transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Aljaafari, A.; Fattah, I.M.R.; Jahirul, M.I.; Gu, Y.; Mahlia, T.M.I.; Islam, M.A.; Islam, M.S. Biodiesel emissions: A state-of-the-art review on health and environmental impacts. Energies 2023, 16, 2086. [Google Scholar] [CrossRef]
- de la Rosa-Urbalejo, D.; Reyes-Ramírez, B.; Riesco-Ávila, J.M.; Vera-Rozo, J.R.; Martínez-Martínez, S.; de la Garza, O.A. An on-road experimental emissions evaluation of a diesel engine fueled with pyrolysis oil from waste plastics, pure and blended with biodiesel—A Mexican case. Int. J. Engine Res. 2024, 26, 1234–1245. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Makertihartha, I.G.; Indarto, A.; Prakoso, T.; Soerawidjaja, T.H. A review of biodiesel cold flow properties and its improvement methods. Energies 2024, 17, 4543. [Google Scholar] [CrossRef]
- Rivas, A.G.; Vázquez, V.Á.; Flores, M.M.A.; Cerrillo-Rojas, G.V.; Correa-Aguado, H.C. Sustainable castor bean biodiesel through Ricinus communis L. lipase extract catalysis. Appl. Biochem. Biotechnol. 2023, 195, 1297–1318. [Google Scholar] [CrossRef]
- Akpan, I.O.; Edeh, I.; Uyigue, L. A review on biodiesel production. Petro Chem. Indus. Int. 2023, 6, 131–141. [Google Scholar]
- Vera-Rozo, J.R.; Riesco-Ávila, J.M.; Elizalde-Blanca, F.; Cano-Andrade, S. Optimization of the real conversion efficiency of waste cooking oil to FAME. Therm. Sci. 2022, 26, 653–665. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Calabrò, V. Importance of the properties, collection, and storage of waste cooking oils to produce high-quality biodiesel. Biomass Bioenergy 2024, 189, 107363. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.A.; Ahmad, A. State of the art of catalysts for biodiesel production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Gopi, R.; Thangarasu, V.; Ramanathan, A.; Ramanathan, A. A critical review of continuous flow and integrated microreactors for biodiesel. Renew. Sustain. Energy Rev. 2022, 154, 111869. [Google Scholar]
- Qadeer, M.U.; Ayoub, M.; Komiyama, M.; Daulatzai, M.U.K.; Mukhtar, A.; Saqib, S.; Bokhari, A. Biodiesel synthesis technologies: Review and economic analysis. J. Clean. Prod. 2021, 309, 127388. [Google Scholar] [CrossRef]
- Patle, D.S.; Pandey, A.; Srivastava, S.; Sawarkar, A.N.; Kumar, S. Ultrasound-intensified biodiesel production from algal biomass. Environ. Chem. Lett. 2021, 19, 209–229. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Rokhum, S.L.; Halder, G. Current progress and future outlooks of microwave-irradiated biodiesel production: A holistic review. Chem. Eng. J. 2024, 482, 149033. [Google Scholar]
- Vera-Rozo, J.R.; Riesco-Ávila, J.M.; Poveda-Pachon, M.Y.; Zaleta-Aguilar, A. Biodiesel production by hydrodynamic cavitation through an orifice plate. Chem. Eng. Trans. 2022, 92, 565–570. [Google Scholar]
- Wen, Z.; Wang, F.; Zhang, L.; Cai, Z.; Liu, S. Application of ultrasonic cavitation in biodiesel production: A review. Renew. Energy 2021, 169, 1–12. [Google Scholar]
- Patil, P.D.; Gude, V.G.; Deng, S. Hydrodynamic cavitation as a novel intensification approach for biodiesel production. Energy Convers. Manag. 2022, 251, 114940. [Google Scholar]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Giannakis, N.; Carmona-Cabello, M.; Makri, A.; Leiva-Candia, D.; Filippi, K.; Argeiti, C.; Pateraki, C.; Dorado, M.P.; Koutinas, A.; Stylianou, E. Spent coffee grounds and orange peel residues based biorefinery for microbial oil and biodiesel conversion estimation. Renew. Energy 2023, 209, 382–392. [Google Scholar] [CrossRef]
- Al-Hasan, M.; Al-Mazidi, M.; Khan, Z. Influence of hydrodynamic cavitation on biodiesel yield from WCO. J. Clean. Prod. 2021, 316, 128214. [Google Scholar]
- Zhang, H.; Liu, Y.; Wu, X.; Lu, X. Advances in cavitation technology for renewable energy production. Renew. Sustain. Energy Rev. 2023, 154, 111876. [Google Scholar]
- Patel, A.; Joshi, R.; Upadhyay, S. Cavitation-based reactors for biodiesel production: Design and analysis. Chem. Eng. Process. 2021, 168, 108564. [Google Scholar]
- Wang, Y.; Liu, H.; Zhang, J. Application of SPR for biodiesel production: A review. Renew. Energy 2020, 150, 578–585. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Kim, H. Enhancing biodiesel yield using Shockwave Power Reactor technology. J. Clean. Prod. 2022, 310, 127430. [Google Scholar]
- Jiang, T.; Zhao, L.; Ma, X. Optimizing biodiesel production using SPR: Low-temp kinetics and energy savings. Energy Convers. Manag. 2021, 228, 113646. [Google Scholar]
- Vera-Rozo, J.R.; Gonzalez-Aguilar, A.M.; Riesco-Ávila, J.M. Geometric characterization of a Shockwave Power Reactor for biodiesel. Chem. Eng. Process. 2023, 190, 109418. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, W.; Liu, P. Sustainable biodiesel production using SPR: Towards scalability. Fuel Process. Technol. 2023, 234, 107369. [Google Scholar]
- Oza, S.; Kodgire, P.; Kachhwaha, S.S. Shockwave power reactor (SPR)-assisted esterification of blended non-edible oils: Optimization and kinetics studies. Therm. Sci. Eng. Prog. 2025, 59, 103337. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Ali, S.S.; Ansari, K.B.; Athar, M.; Al Mesfer, M.K.; Shah, M.; Danish, M.; Kumar, R.; Raheman, S. Process intensification in biodiesel production using unconventional reactors. Fuel 2025, 380, 133263. [Google Scholar] [CrossRef]
- Miyuranga, K.A.V.; Arachchige, U.S.P.R.; Marso, T.M.M.; Samarakoon, G. Biodiesel production through the transesterification of waste cooking oil over typical heterogeneous base or acid catalysts. Catalysts 2023, 13, 546. [Google Scholar] [CrossRef]
- EN 14214:2012; Liquid Petroleum Products–Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications–Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- Vera-Rozo, J.R.; Sáez-Bastante, J.; Carmona-Cabello, M.; Riesco-Ávila, J.M.; Avellaneda, F.; Pinzi, S.; Dorado, M.P. Cetane index prediction based on biodiesel distillation curve. Fuel 2022, 321, 124063. [Google Scholar] [CrossRef]
- ASTM D1298; Standard Test Method for Density, Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2020.
- EN 14103:2020; Fat and Oil Derivatives – Fatty Acid Methyl Esters (FAME) – Determination of Ester and Linolenic Acid Methyl ester Contents. European Committee for Standardization (CEN): Brussels, Belgium, 2020.
- ASTM D86; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D240; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2019.
- Antony, J. Design of Experiments for Engineers and Scientists, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA. Available online: https://wiki.anton-paar.com/jp-jp/density-and-density-measurement/astm-d4052/ (accessed on 18 May 2025).
- NMX-F-101-SCFI-2012; Alimentos–Aceites Y Grasas Vegetales O Animales–Determinación De Ácidos Grasos Libres-Método De Prueba (CANCELA A LA NMX-F-101-SCFI-2006). Secretaría de Economía: Mexico City, Mexico. Available online: http://www.economia-nmx.gob.mx/normas/nmx/2010/nmx-f-101-scfi-2012.pdf (accessed on 18 May 2025).
- EN 14203:2011; Blinds and Shutters-Capability for Use of Gears with Crank Handle-Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium. Available online: https://standards.iteh.ai/catalog/standards/cen/c700dfda-b681-4cdf-900f-475f017a76da/en-14203-2004?srsltid=AfmBOopyTwjA97sDqHZigGvRLBKuRS_Bvx29a92PGRTqPI9uRkx0qDzy (accessed on 18 May 2025).
- EN 14112:2021; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Oxidation Stability (Accelerated Oxidation Test). SIST Slovenian Institute for Standardization: Ljubljana, Slovenia. Available online: https://cdn.standards.iteh.ai/samples/66922/49757a201d91465c837f02ac2541337d/SIST-EN-14112-2021.pdf (accessed on 18 May 2025).







| Properties | Standard | Soybean | WCO |
|---|---|---|---|
| Density at 15 °C (kg/m3) | ASTM D4052 [40] | 920.30 ± 0.1 | 925.1 ± 0.1 |
| Kinematic Viscosity at 40 °C (mm2/s) | ASTM D445 | 51.32 ± 0.01 | 70.46 ± 0.01 |
| Free FA, wt (%) | NMX-F-101 [41] | 0.13 ± 0.03 | 0.72 ± 0.04 |
| Molecular Weight, g/mol | EN 14203:2011 [42] | 928.39 ± 0.05 | 928.83 ± 0.05 |
| Fatty Acid | Formula | %wt ± 0.01 | %wt ± 0.01 |
| Palmitic (C16:0) | C16H28O2 | 10.74 | 11.71 |
| Palmitoleic (C16:1) | C16H30O2 | 0.10 | - |
| Stearic (C18:0) | C18H36O2 | 4.05 | 4.24 |
| Oleic (C18:1) | C18H34O2 | 24.05 | 29.96 |
| Linoleic (C18:2) | C18H32O2 | 53.36 | 48.36 |
| Linolenic (C18:3) | C18H30O2 | 7.48 | 5.72 |
| Arachidonic (C20:0) | C20H32O2 | 0.17 | 0.01 |
| Others (-) | - | 0.05 | 0.01 |
| Properties | Standard | Catalyst Percentage | Soybean | WCO | ||
|---|---|---|---|---|---|---|
| Conversion [%wt] | FAME [%wt] | Conversion [%wt] | FAME [%wt] | |||
| NaOH | 6.0:1 | 0.60 | 95.87 ± 1.11 | 96.47 ± 1.67 | 92.13 ± 1.16 | 84.76 ± 0.86 |
| 1.50 | 70.96 ± 0.74 | 90.01 ± 0.85 | 91.81 ± 1.72 | 80.83 ± 0.59 | ||
| 7.5:1 | 1.05 | 89.51 ± 1.30 | 94.51 ± 1.09 | 75.17 ± 1.52 | 80.27 ± 0.72 | |
| 9.0:1 | 0.60 | 95.06 ± 2.84 | 98.58 ± 1.85 | 88.39 ± 1.22 | 95.30 ± 0.26 | |
| 1.50 | 63.80 ± 0.08 | 96.82 ± 0.04 | 91.91 ± 1.17 | 88.10 ± 0.34 | ||
| KOH | 6.0:1 | 0.60 | 97.51 ± 0.72 | 90.50 ± 0.54 | 93.98 ± 1.20 | 87.50 ± 0.70 |
| 1.50 | 87.06 ± 2.47 | 86.59 ± 1.41 | 74.90 ± 1.38 | 88.50 ± 0.48 | ||
| 7.5:1 | 1.05 | 92.77 ± 0.41 | 89.20 ± 0.17 | 86.57 ± 1.50 | 79.90 ± 0.87 | |
| 9.0:1 | 0.60 | 97.08 ± 1.01 | 93.65 ± 0.87 | 92.55 ± 1.14 | 79.70 ± 0.52 | |
| 1.50 | 84.07 ± 0.48 | 96.94 ± 0.10 | 93.13 ± 1.67 | 94.10 ± 0.98 | ||
| Experiment | Rotation Velocity [rpm] | Flow [mL/min] | FAME to Soybean Oil [%wt] | FAME to WCO [%wt] |
|---|---|---|---|---|
| Factor points | ||||
| 1 | 1000 | 200 | 87.01 ± 0.04 | 84.46 ± 0.03 |
| 2 | 1000 | 300 | 85.08 ± 0.89 | 83.31 ± 0.87 |
| 3 | 3000 | 200 | 93.97 ± 0.04 | 92.16 ± 0.04 |
| 4 | 3000 | 300 | 91.89 ± 1.05 | 90.28 ± 0.20 |
| Axial points | ||||
| 5 | 586 | 250 | 79.18 ± 0.03 | 77.65 ± 0.03 |
| 6 | 3415 | 250 | 97.98 ± 0.05 | 95.85 ± 0.05 |
| 7 | 2000 | 150 | 97.00 ± 0.05 | 95.22 ± 0.50 |
| 8 | 2000 | 350 | 84.05 ± 0.03 | 81.59 ± 0.03 |
| Central point | ||||
| 9 | 2000 | 250 | 91.91 ± 0.04 | 89.68 ± 0.04 |
| Parameter | Standard | Soybean Biodiesel | WCO Biodiesel | ||
|---|---|---|---|---|---|
| Traditional Process | Cavitation Process | Traditional Process | Cavitation Process | ||
| FAME (%wt) | EN 14103 Min: 96.5 | 98.58 ± 0.01 | 97.98 ± 0.02 | 95.3 ± 0.01 | 95.85 ± 0.01 |
| Linolenic | EN 14103 Max: 12 | 7.48 ± 0.05 | 7.36 ± 0.08 | 5.72 ± 0.12 | 5.81 ± 0.03 |
| High Calorific Value, HCV (MJ/kg) | ASTM D240 [39] | 39.82 ± 0.01 | 38.84 ± 0.01 | 39.80 ± 0.01 | 39.81 ± 0.01 |
| Density at 15 °C (kg/m3) | ASTM D4052 Min 860 Max 900 | 0.884 ± 0.021 | 0.884 ± 0.050 | 0.886 ± 0.036 | 0.886 ± 0.007 |
| Kinematic Viscosity at 40 °C (mm2/s) | ASTM D445 Min 3.5 Max 5.0 | 5.45 ± 0.01 | 5.36 ± 0.01 | 5.81 ± 0.01 | 5.58 ± 0.01 |
| Cetane Number (CN) | EN 14112 Min.51 [43] | 49 | 50 | 51 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Rozo, J.R.; Caicedo-Peñaranda, E.A.; Riesco-Avila, J.M. Hydrodynamic Cavitation in Shockwave-Power-Reactor-Assisted Biodiesel Production in Continuous from Soybean and Waste Cooking Oil. Energies 2025, 18, 2761. https://doi.org/10.3390/en18112761
Vera-Rozo JR, Caicedo-Peñaranda EA, Riesco-Avila JM. Hydrodynamic Cavitation in Shockwave-Power-Reactor-Assisted Biodiesel Production in Continuous from Soybean and Waste Cooking Oil. Energies. 2025; 18(11):2761. https://doi.org/10.3390/en18112761
Chicago/Turabian StyleVera-Rozo, James R., Edison A. Caicedo-Peñaranda, and José M. Riesco-Avila. 2025. "Hydrodynamic Cavitation in Shockwave-Power-Reactor-Assisted Biodiesel Production in Continuous from Soybean and Waste Cooking Oil" Energies 18, no. 11: 2761. https://doi.org/10.3390/en18112761
APA StyleVera-Rozo, J. R., Caicedo-Peñaranda, E. A., & Riesco-Avila, J. M. (2025). Hydrodynamic Cavitation in Shockwave-Power-Reactor-Assisted Biodiesel Production in Continuous from Soybean and Waste Cooking Oil. Energies, 18(11), 2761. https://doi.org/10.3390/en18112761








