Produced Water Use for Hydrogen Production: Feasibility Assessment in Wyoming, USA
Abstract
1. Introduction
- Oil and gas activities in Wyoming, including production volumes and major basins;
- Chemical analysis of produced water and its suitability for electrolysis;
- Feasibility of water treatment to meet electrolysis standards;
- Renewable-energy potential for supporting hydrogen production in Wyoming.

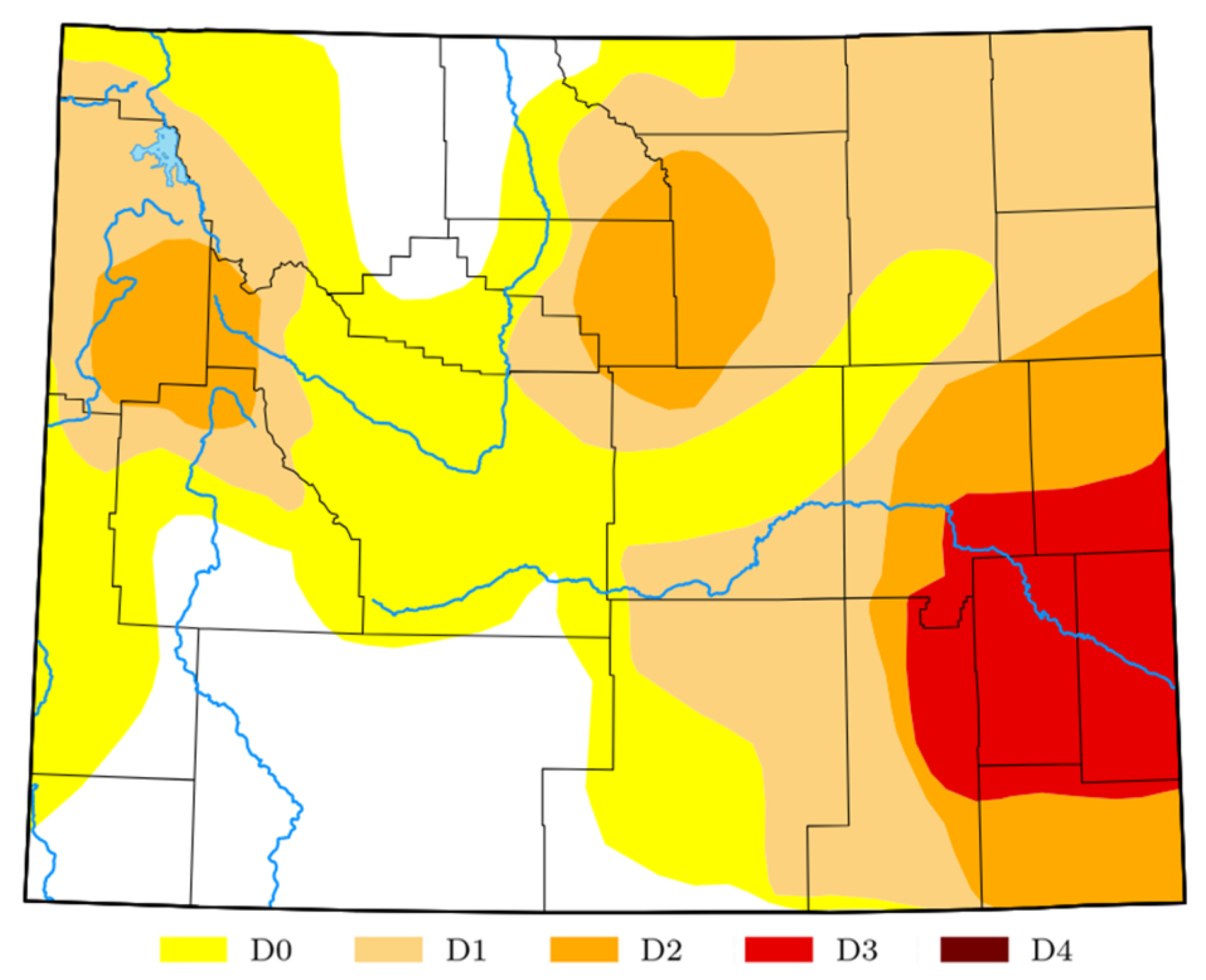
2. Area of Study
2.1. Powder River Basin
2.2. Wind River Basin
2.3. Greater Green River Basin
2.4. Bighorn Basin
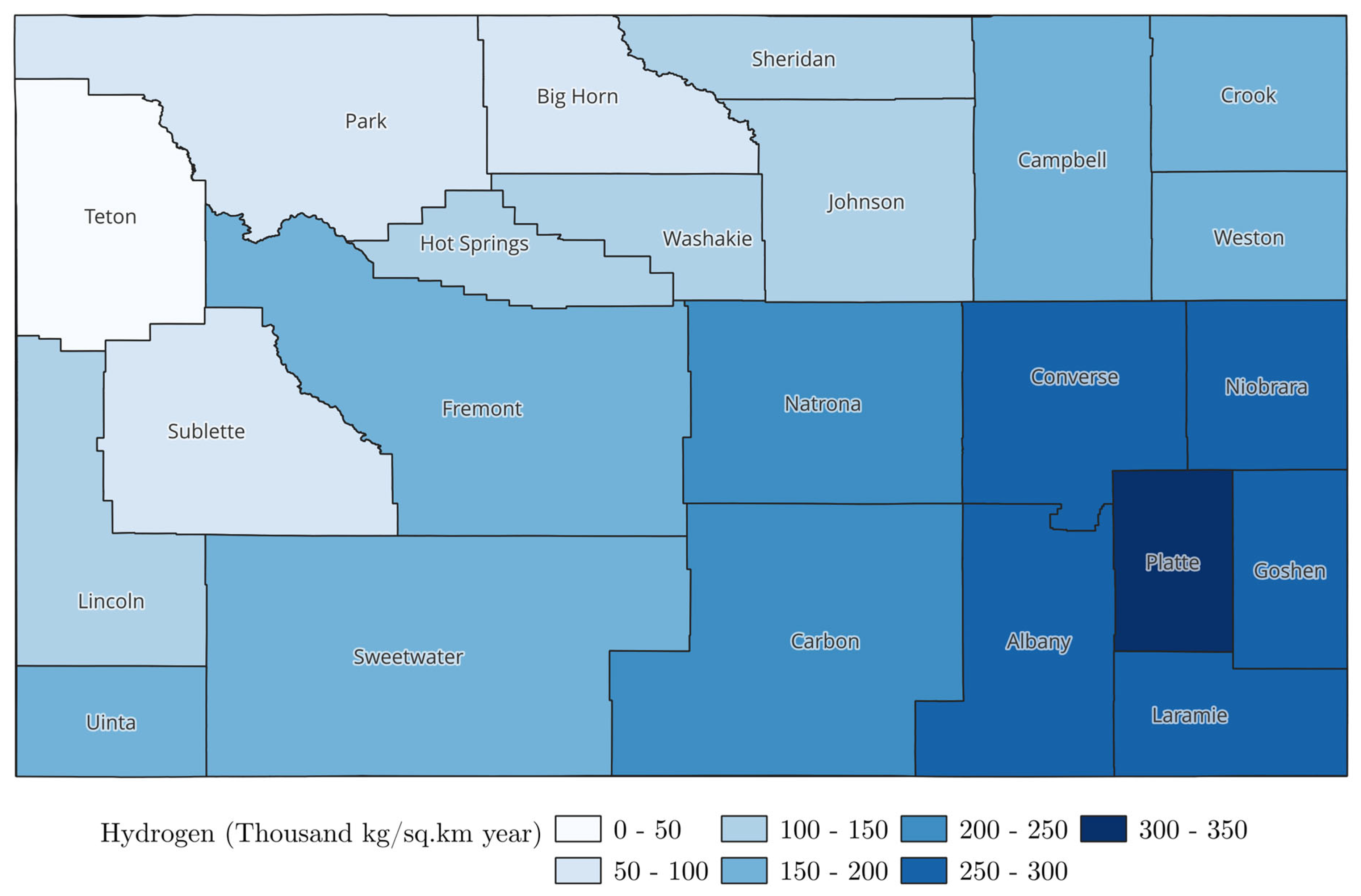
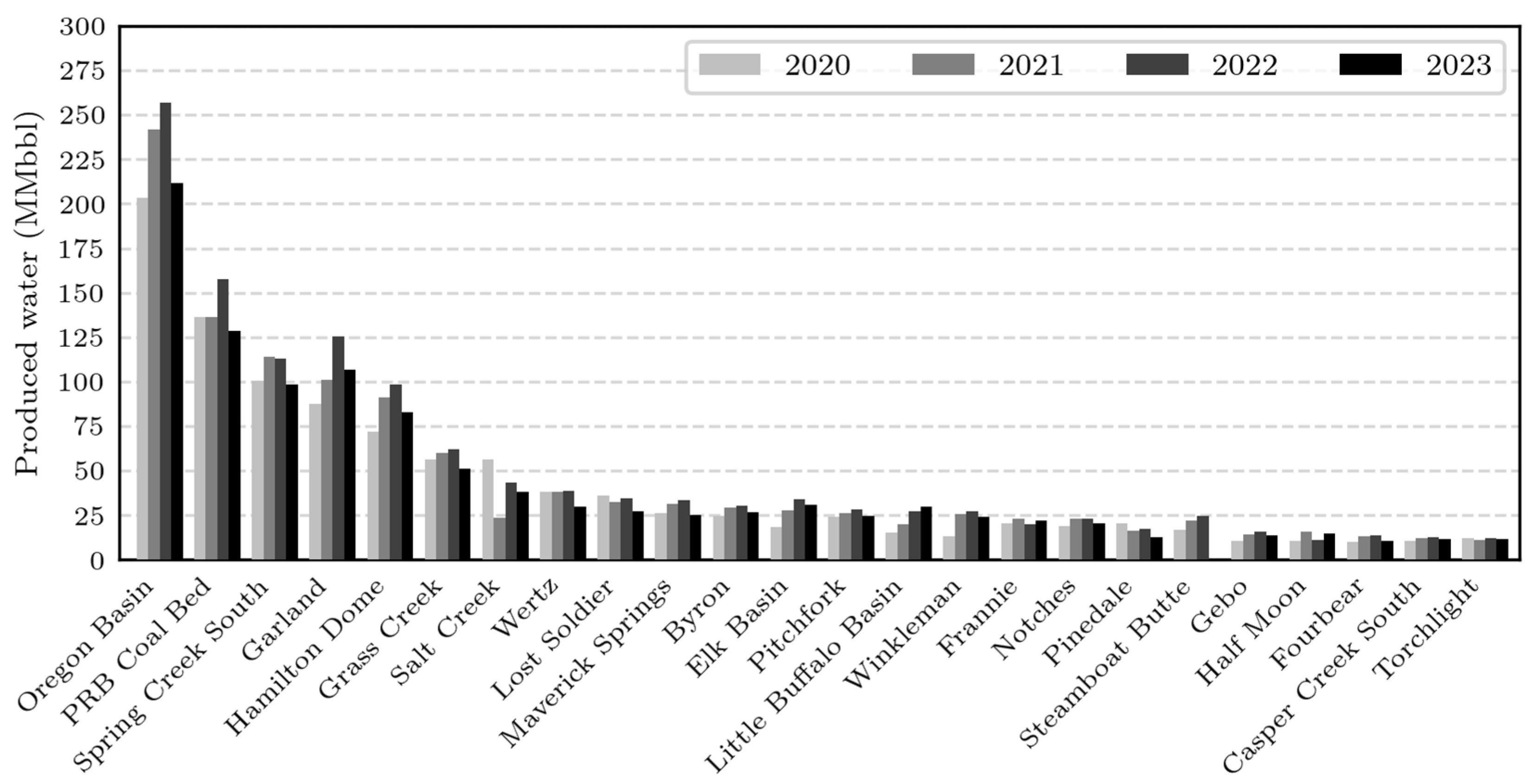
3. Geospatial Analysis of Produced Water
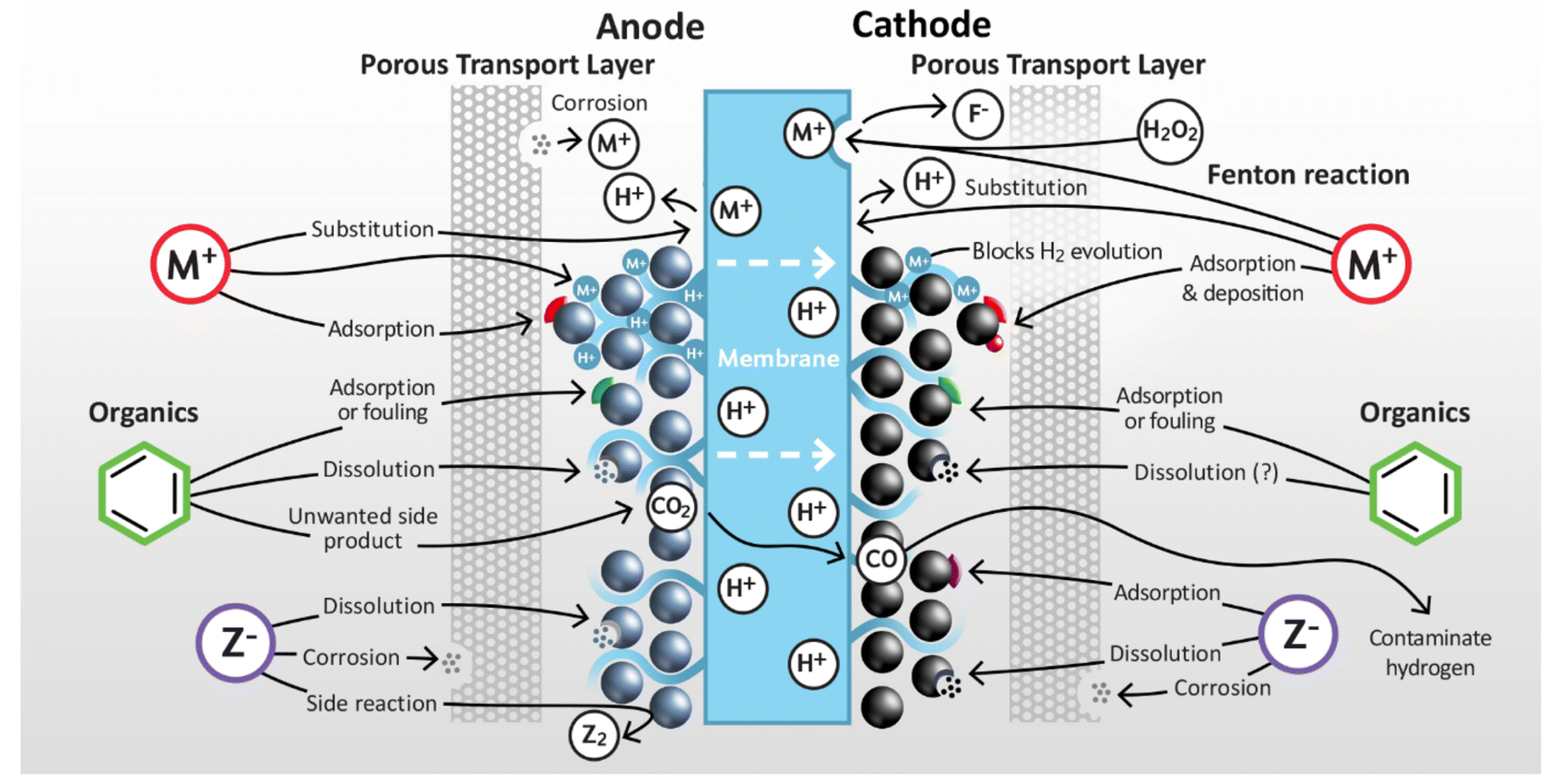
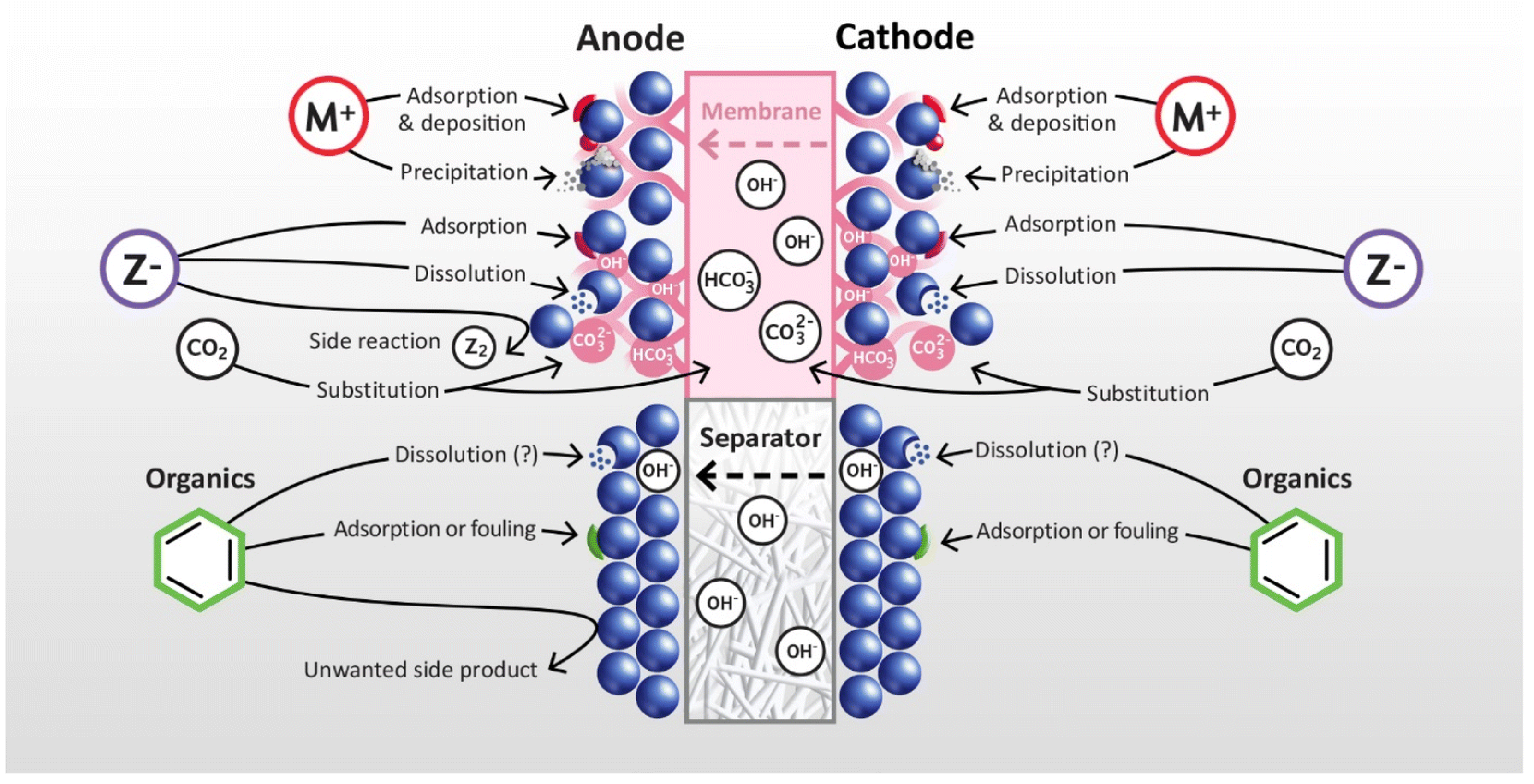
4. Electrolysis and Produced Water as a Feedstock
4.1. The Electrolysis Process
4.1.1. Proton Exchange Membrane Water Electrolyzers (PEMWE)
4.1.2. Alkaline Water Electrolyzers (AWEs)
4.1.3. Anion Exchange Membrane Water Electrolyzers (AEMWE)
4.2. Water Analysis Results
4.3. Hydrogen Production Volumes: An Estimation
5. Economic Feasibility
5.1. Water-Treatment Costs
5.2. Transportation Expenses
5.3. Impact on Levelized Cost of Hydrogen (LCOH)
5.4. Comparative Analysis with Conventional Disposal Methods
5.5. Comparison with Conventional Hydrogen Production
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masson-Delmotte; Allan, R.P.; Arias, P.A.; Berger, S.; Canadell, J.G.; Cassou, C.; Chen, D.; Cherchi, A.; Connor, S.L.; Coppola, E.; et al. Summary for Policymakers. Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM.pdf (accessed on 30 March 2025).
- Department of Energy. U.S. National Clean Hydrogen Strategy and Roadmap at a Glance. Tech. Rep. October 2019. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/clean-hydrogen-strategy-roadmap-at-a-glance.pdf?Status=Master (accessed on 30 March 2025).
- Can the Inflation Reduction Act Unlock a Green Hydrogen Economy?—International Council on Clean Transportation. Available online: https://theicct.org/ira-unlock-green-hydrogen-jan23/ (accessed on 14 May 2025).
- Murdoch, H.; Munster, J.; Satyapal, S.; Rustagi, N. Pathways to Commercial Liftoff: Clean Hydrogen. 2023. Available online: https://trid.trb.org/View/2494936 (accessed on 14 May 2025).
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2023—Analysis—IEA. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 30 March 2025).
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Wilkinson, J.; Mays, T.; McManus, M. Review and meta-analysis of recent life cycle assessments of hydrogen production. Clean. Environ. Syst. 2023, 9, 100116. [Google Scholar] [CrossRef]
- Miller, J.E. Review of Water Resources and Desalination Technologies; Sandia National Lab: Albuquerque, NM, USA, 2003. [CrossRef]
- Zhang, L.; Hascakir, B. A review of issues, characteristics, and management for wastewater due to hydraulic fracturing in the U.S. J. Pet. Sci. Eng. 2021, 202, 108536. [Google Scholar] [CrossRef]
- Hamiche, A.M.; Stambouli, A.B.; Flazi, S. A review of the water-energy nexus. Renew. Sustain. Energy Rev. 2016, 65, 319–331. [Google Scholar] [CrossRef]
- Saada, H.; Fabre, B.; Loget, G.; Benoit, G. Is Direct Seawater Splitting Realistic with Conventional Electrolyzer Technologies? ACS Energy Lett. 2024, 9, 3351–3368. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nawaz, M.H.; Rout, P.R.; Lim, J.W.; Mainali, B.; Shahid, M.K. Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives. Water 2023, 15, 2980. [Google Scholar] [CrossRef]
- Zawadzki, P.; Kończak, B.; Smoliński, A. Municipal wastewater reclamation: Reclaimed water for hydrogen production by electrolysis—A case study. Measurement 2023, 216, 112928. [Google Scholar] [CrossRef]
- Chauhan, D.; Ahn, Y.-H. Alkaline electrolysis of wastewater and low-quality water. J. Clean. Prod. 2023, 397, 136613. [Google Scholar] [CrossRef]
- Cassol, G.S.; Shang, C.; An, A.K.; Khanzada, N.K.; Ciucci, F.; Manzotti, A.; Westerhoff, P.; Song, Y.; Ling, L. Ultra-fast green hydrogen production from municipal wastewater by an integrated forward osmosis-alkaline water electrolysis system. Nat. Commun. 2024, 15, 2617. [Google Scholar] [CrossRef]
- Huang, L.; Fang, C.; Pan, T.; Zhu, Q.; Geng, T.; Li, G.; Li, X.; Yu, J. Hydrogen Production via Electrolysis of Wastewater. Nanomaterials 2024, 14, 567. [Google Scholar] [CrossRef]
- Dong, X.; Pang, D.; Luo, G.; Zhu, X. Microbial Water Electrolysis Cells for Efficient Wastewater Treatment and H2 Production. ACS Sustain. Chem. Eng. 2024, 12, 4203–4212. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Zhang, F.; Zou, M.; Li, J.-C.; Zhang, L.; Hou, P.-X.; Shi, C.; Zhang, L.; Cheng, H.-M. Green and efficient electrolysis of seawater using carbon nanotube-based hybrid films. Nano Energy 2024, 123, 109356. [Google Scholar] [CrossRef]
- Konkel, L. Salting the Earth: The Environmental Impact of Oil and Gas Wastewater Spills. Environ. Health Perspect. 2016, 124, A230–A235. [Google Scholar] [CrossRef] [PubMed]
- National Drought Mitigation Center. Available online: https://drought.unl.edu/ (accessed on 30 March 2025).
- Slack, P.B. Paleotectonics and Hydrocarbon Accumulation, Powder River Basin, Wyoming. Am. Assoc. Pet. Geol. Bull. 1981, 65, 730–743. [Google Scholar] [CrossRef]
- Bauer, C.M. Wind River Basin. GSA Bull. 1934, 45, 665–696. [Google Scholar] [CrossRef]
- Roehler, H.W. Eocene Climates, Depositional Environments, and Geography, Greater Green River Basin, Wyoming, Utah, and Colorado; U.S. Geological Survey: Reston, VA, USA, 1993. Available online: https://books.google.it/books?hl=en&lr=&id=jA4-isGCWsYC&oi=fnd&pg=PA1&dq=greater+green+river+basin&ots=UGm7jO0pbZ&sig=MJHiQY5xnsSKggwA8Nx3tjbT78A&redir_esc=y#v=onepage&q=greater%20green%20river%20basin&f=false (accessed on 30 March 2025).
- Clyde, W.C.; Hamzi, W.; Finarelli, J.A.; Wing, S.L.; Schankler, D.; Chew, A. Basin-wide magnetostratigraphic framework for the Bighorn Basin, Wyoming. GSA Bull. 2007, 119, 848–859. [Google Scholar] [CrossRef]
- Zaidi, S.Z.; Shafeeq, A.; Sajjad, M.; Hassan, S.; Aslam, M.S.; Saeed, T.; Walsh, F.C. Benchmarking of scaling and fouling of reverse osmosis membranes in a power generation plant of paper and board mill: An industrial case of a paper and board mill study. Int. J. Environ. Sci. Technol. 2021, 18, 2511–2518. [Google Scholar] [CrossRef]
- Meng, M.; Chen, M.; Sanders, K.T. A Geospatial Feasibility Assessment of Utilizing Produced Water from Oil and Natural Gas Production in California for Beneficial Use. In Proceedings of the World Environmental and Water Resources Congress 2016, West Palm Beach, FL, USA, 22–26 May 2016; pp. 207–216. [Google Scholar] [CrossRef]
- Gardiner, J.; Thomas, R.B.; Phan, T.T.; Stuckman, M.; Wang, J.; Small, M.; Lopano, C.; Hakala, J.A. Utilization of produced water baseline as a groundwater monitoring tool at a CO2-EOR site in the Permian Basin, Texas, USA. Appl. Geochem. 2020, 121, 104688. [Google Scholar] [CrossRef]
- Na, J.; Yu, H.; Jia, S.; Chi, J.; Lv, K.; Li, T.; Zhao, Y.; Zhao, Y.; Zhang, H.; Shao, Z. Electrochemical reconstruction of non-noble metal-based heterostructure nanorod arrays electrodes for highly stable anion exchange membrane seawater electrolysis. J. Energy Chem. 2024, 91, 370–382. [Google Scholar] [CrossRef]
- Li, L.; Chen, G.; Shao, Z.; Huang, H. Progress on smart integrated systems of seawater purification and electrolysis. Energy Environ. Sci. 2023, 16, 4994–5002. Available online: https://pubs.rsc.org/en/content/articlehtml/2023/ee/d3ee02712k (accessed on 30 March 2025). [CrossRef]
- IRENA. Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; p. 105. Available online: www.irena.org/publications (accessed on 30 March 2025).
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, I.E.L.; Hinds, G.; Smith, G. Impact of impurities on water electrolysis: A review. Sustain. Energy Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- HyLYZERTM—Hydrogen Systems—PDF Catalogs|Technical Documentation|Brochure. Available online: https://pdf.directindustry.com/pdf/hydrogen-systems/hylyzer/14703-642453.html (accessed on 30 March 2025).
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Jeong, N.; Wiltse, M.E.; Boyd, A.; Blewett, T.; Park, S.; Broeckling, C.; Borch, T.; Tong, T. Efficacy of Nanofiltration and Reverse Osmosis for the Treatment of Oil-Field Produced Water Intended for Beneficial Reuse. ACS ES T Eng. 2023, 3, 1568–1581. [Google Scholar] [CrossRef]
- Wang, X.; Xia, J.; Ding, S.; Zhang, S.; Li, M.; Shang, Z.; Lu, J.; Ding, J. Removing organic matters from reverse osmosis concentrate using advanced oxidation-biological activated carbon process combined with Fe3+/humus-reducing bacteria. Ecotoxicol. Environ. Saf. 2020, 203, 110945. [Google Scholar] [CrossRef]
- Khan, A.A.; Minai, A.F.; Godi, R.K.; Sharma, V.S.; Malik, H.; Afthanorhan, A. Optimal Sizing, Techno-Economic Feasibility and Reliability Analysis of Hybrid Renewable Energy System: A Systematic Review of Energy Storage Systems’ Integration. IEEE Access 2025, 13, 59198–59226. [Google Scholar] [CrossRef]
- Potential for Hydrogen Production from Key Renewable Resources in the United States|Request PDF. Available online: https://www.researchgate.net/publication/237735832_Potential_for_Hydrogen_Production_from_Key_Renewable_Resources_in_the_United_States (accessed on 30 March 2025).
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 10070. [Google Scholar] [CrossRef]
- Sanchez-Rosario, R.; Hildenbrand, Z.L. Produced Water Treatment and Valorization: A Techno-Economical Review. Energies 2022, 15, 4619. [Google Scholar] [CrossRef]
- Frieden, F.; Leker, J. Future costs of hydrogen: A quantitative review. Sustain. Energy Fuels 2024, 8, 1806–1822. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Grimmer, I.; Ranz, M.; Höglinger, M.; Kartusch, S.; Rauh, J.; Macherhammer, M.-G.; Grabner, B.; Trattner, A. A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: Insights for stack application, development, and research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. The role of hydrogen-based power systems in the energy transition of the residential sector. J. Chem. Technol. Biotechnol. 2022, 97, 561–574. [Google Scholar] [CrossRef]



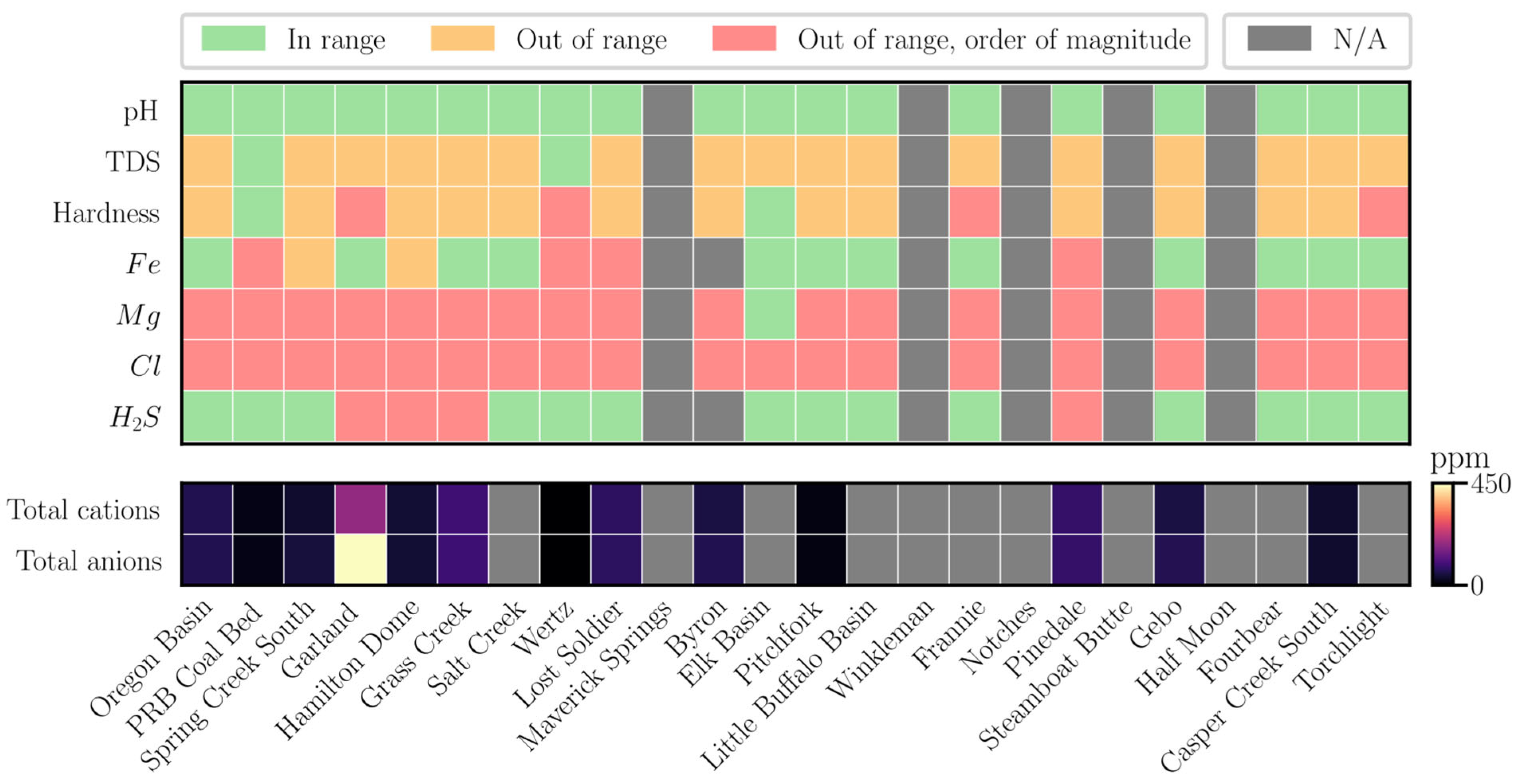
| Variable | Unit | Value |
|---|---|---|
| pH | Range | 3–11 |
| Maximum TDS | ppm | 2000 |
| Iron | ppm | 0.1 |
| Manganese | ppm | 0.1 |
| Chlorine | ppm | 0.1 |
| Hydrogen sulfide | ppm | 0.1 |
| Hardness | Grains (ppm) | 10 grains (170 ppm as CaCO3) |
| Maximum turbidity | NTU | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, C.; Latrach, A.; Rabiei, M.; Venugopal, K. Produced Water Use for Hydrogen Production: Feasibility Assessment in Wyoming, USA. Energies 2025, 18, 2756. https://doi.org/10.3390/en18112756
Abdelhamid C, Latrach A, Rabiei M, Venugopal K. Produced Water Use for Hydrogen Production: Feasibility Assessment in Wyoming, USA. Energies. 2025; 18(11):2756. https://doi.org/10.3390/en18112756
Chicago/Turabian StyleAbdelhamid, Cilia, Abdeldjalil Latrach, Minou Rabiei, and Kalyan Venugopal. 2025. "Produced Water Use for Hydrogen Production: Feasibility Assessment in Wyoming, USA" Energies 18, no. 11: 2756. https://doi.org/10.3390/en18112756
APA StyleAbdelhamid, C., Latrach, A., Rabiei, M., & Venugopal, K. (2025). Produced Water Use for Hydrogen Production: Feasibility Assessment in Wyoming, USA. Energies, 18(11), 2756. https://doi.org/10.3390/en18112756







