Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling
Abstract
1. Introduction
2. Literature Review
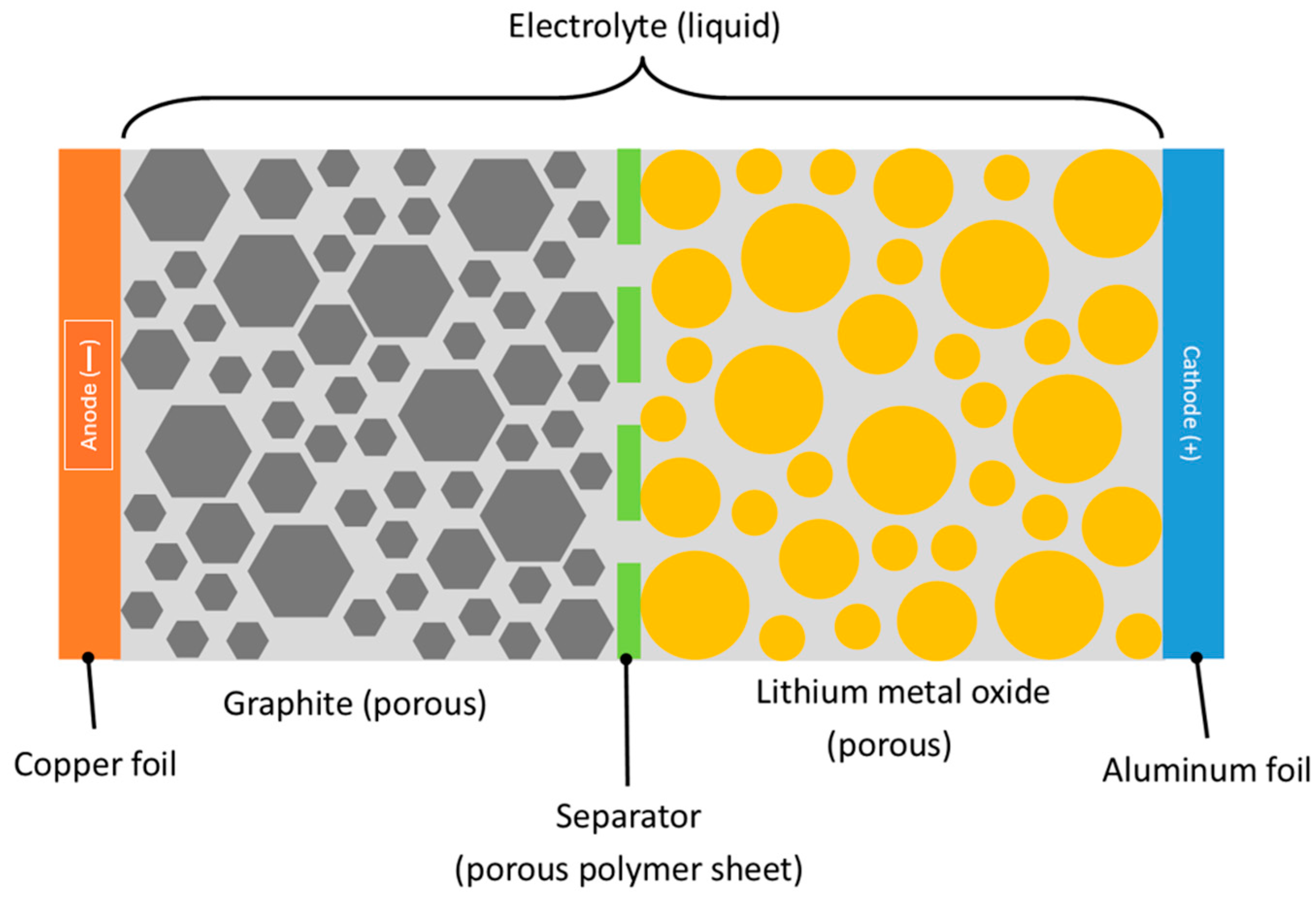
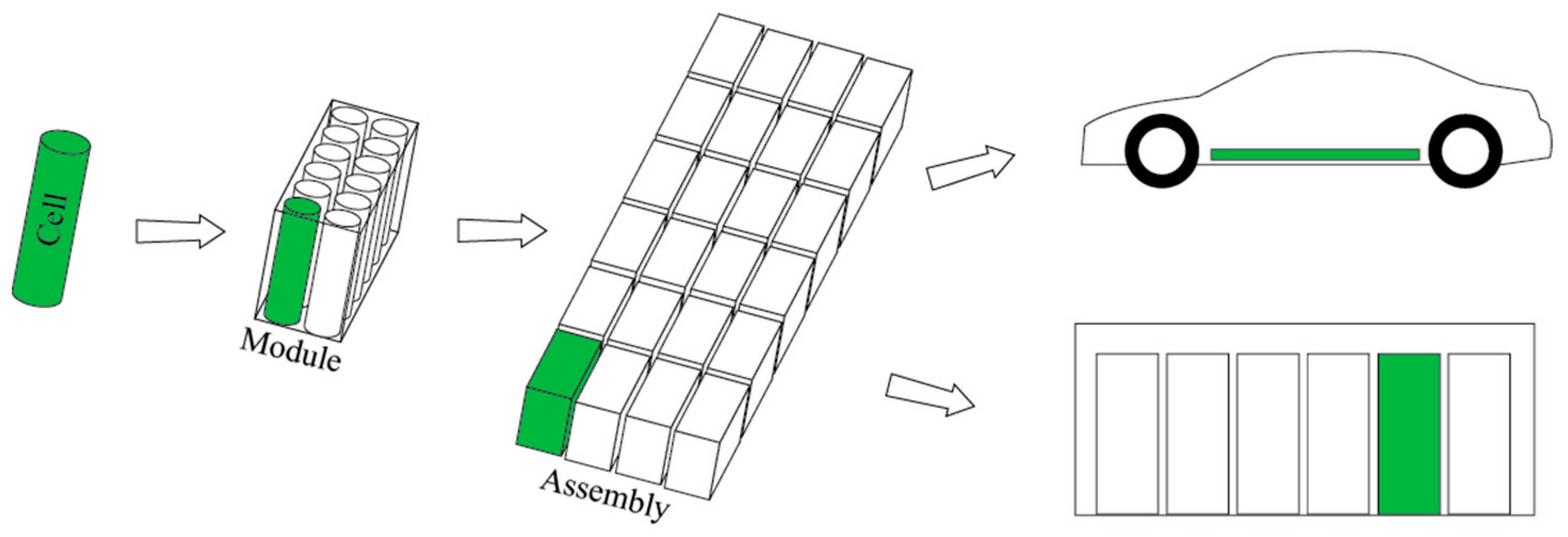
2.1. Mechanisms of Lithium-Ion Battery Fires
2.2. Advances Towards Safer LIB Technologies
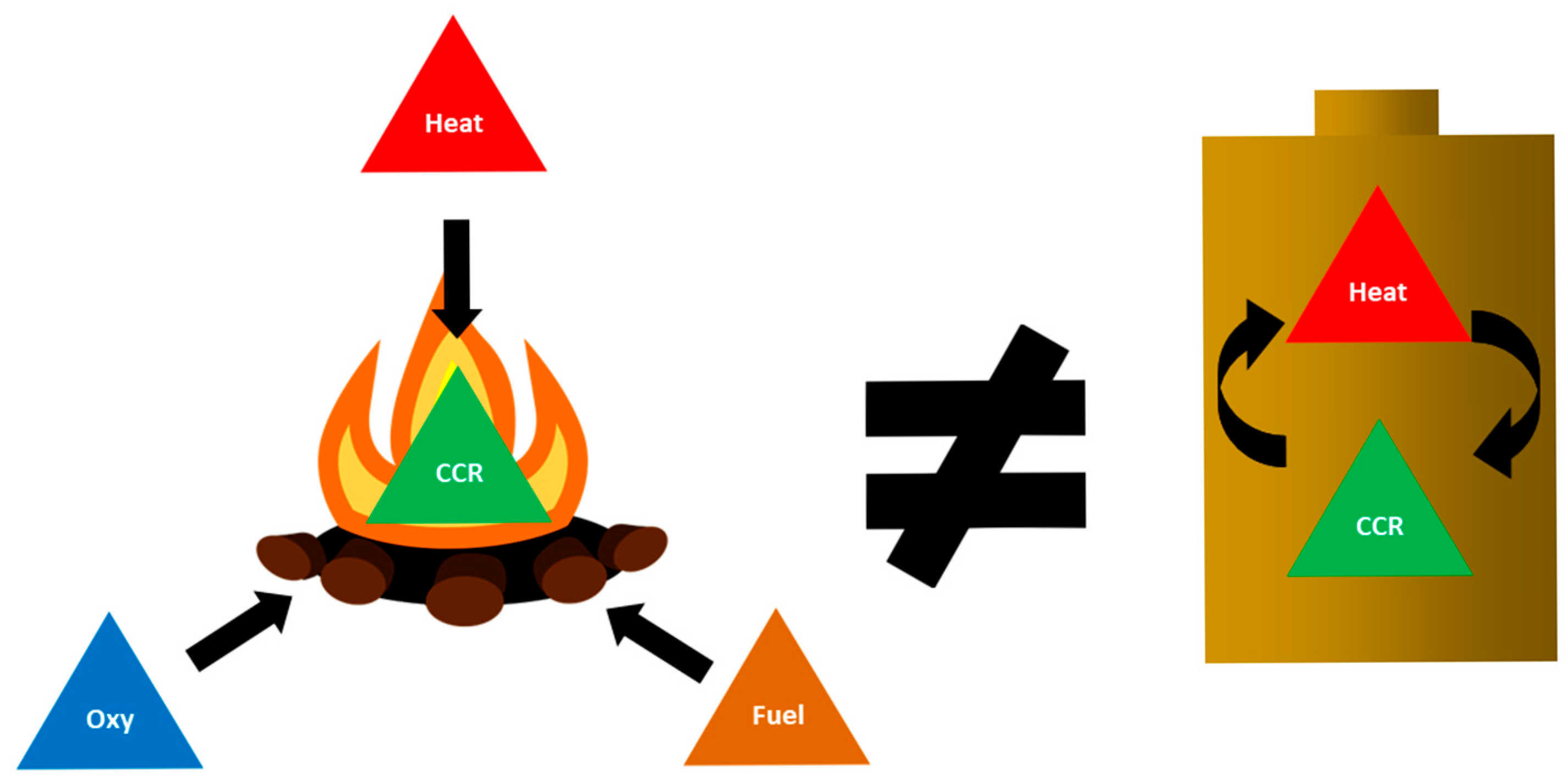
2.3. Overview of Fire Classifications and Typical Fire Suppression
2.4. Oxidation and Its Role in Combustion and Electrochemistry
2.5. Industry Perspectives
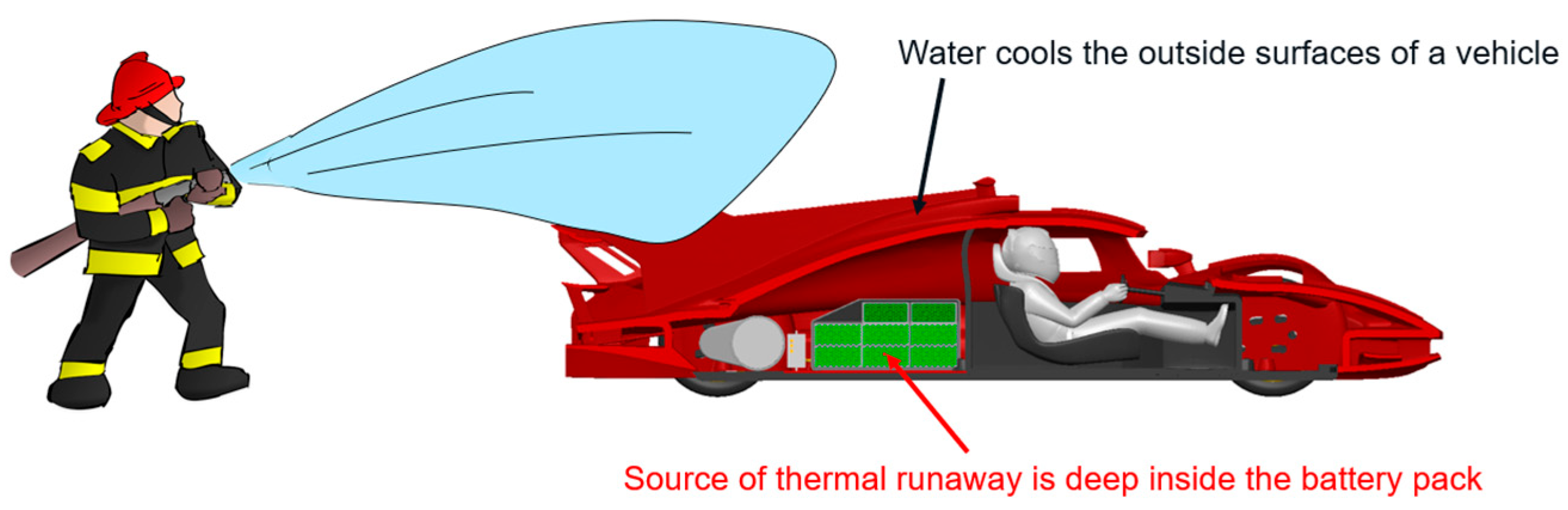
2.6. Firefighting Effectiveness
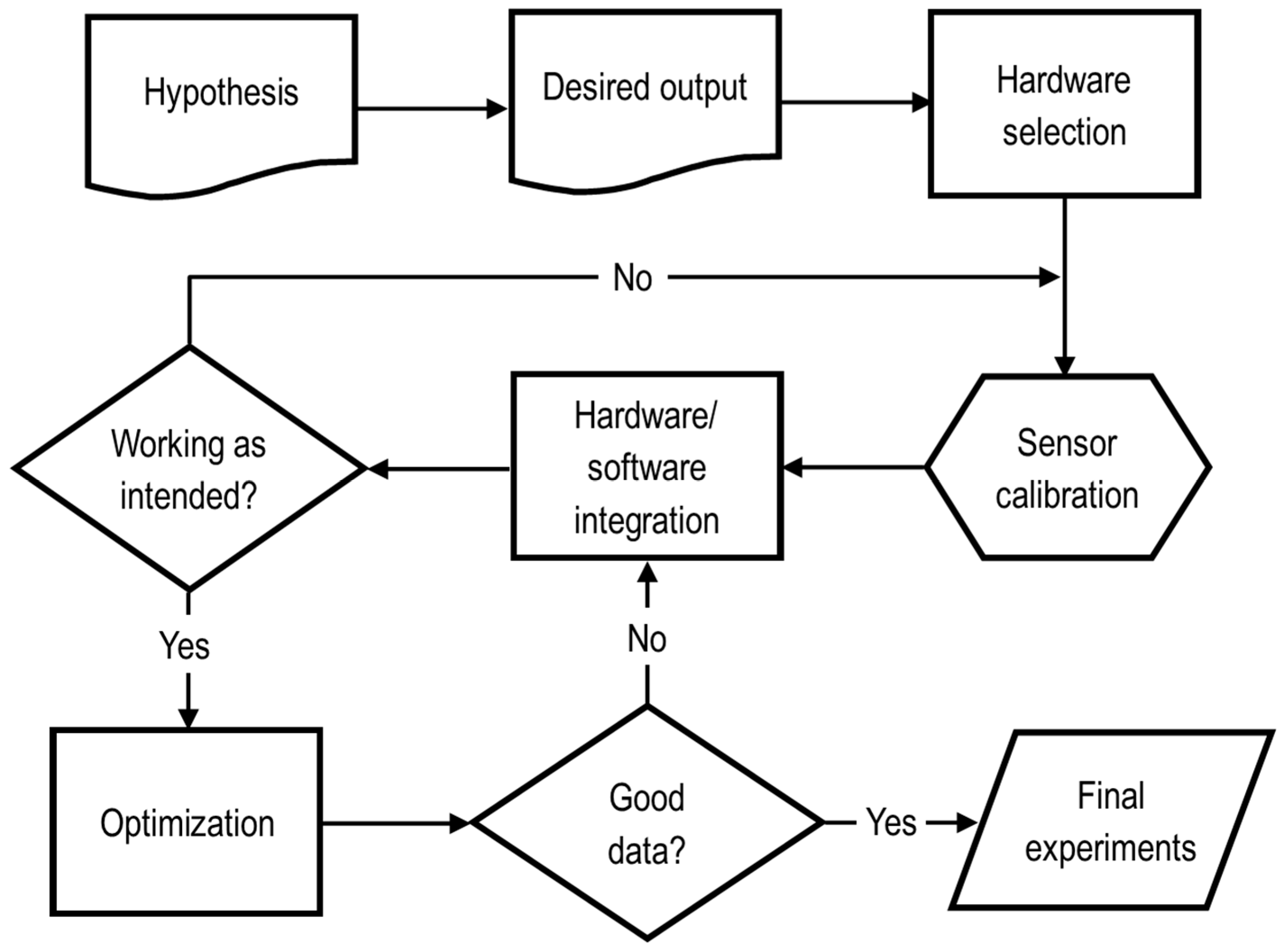
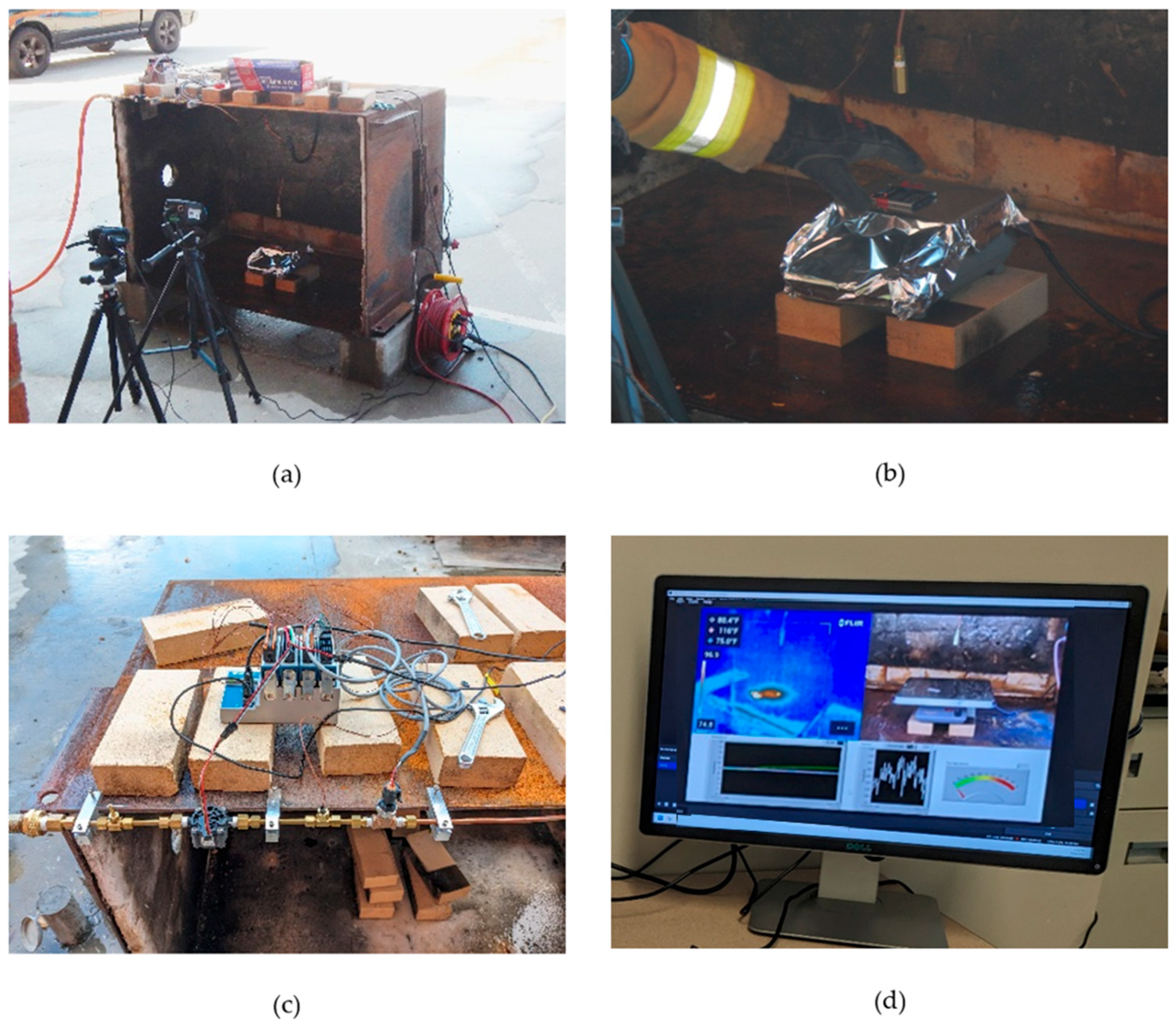
3. Experimental Methodology
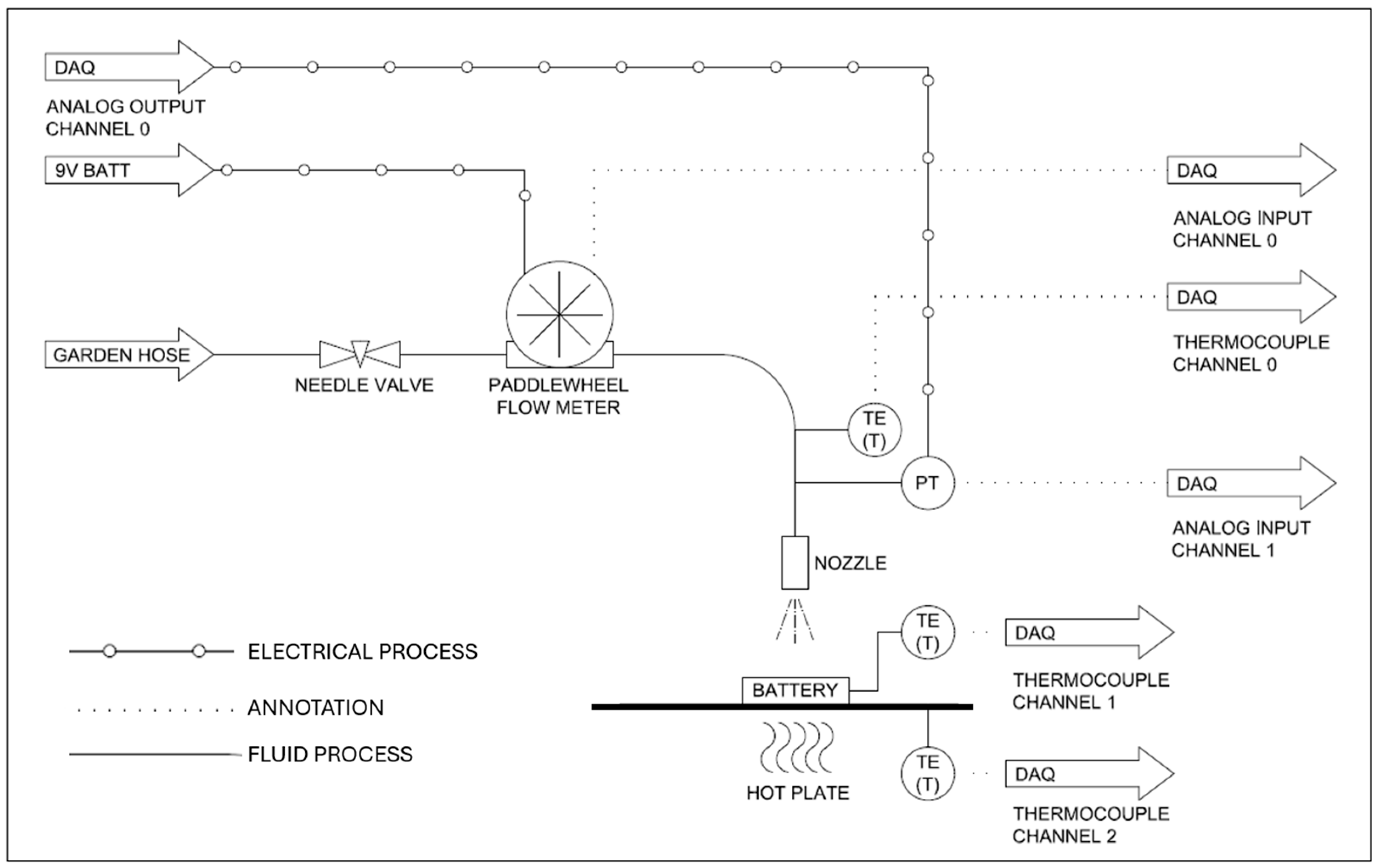
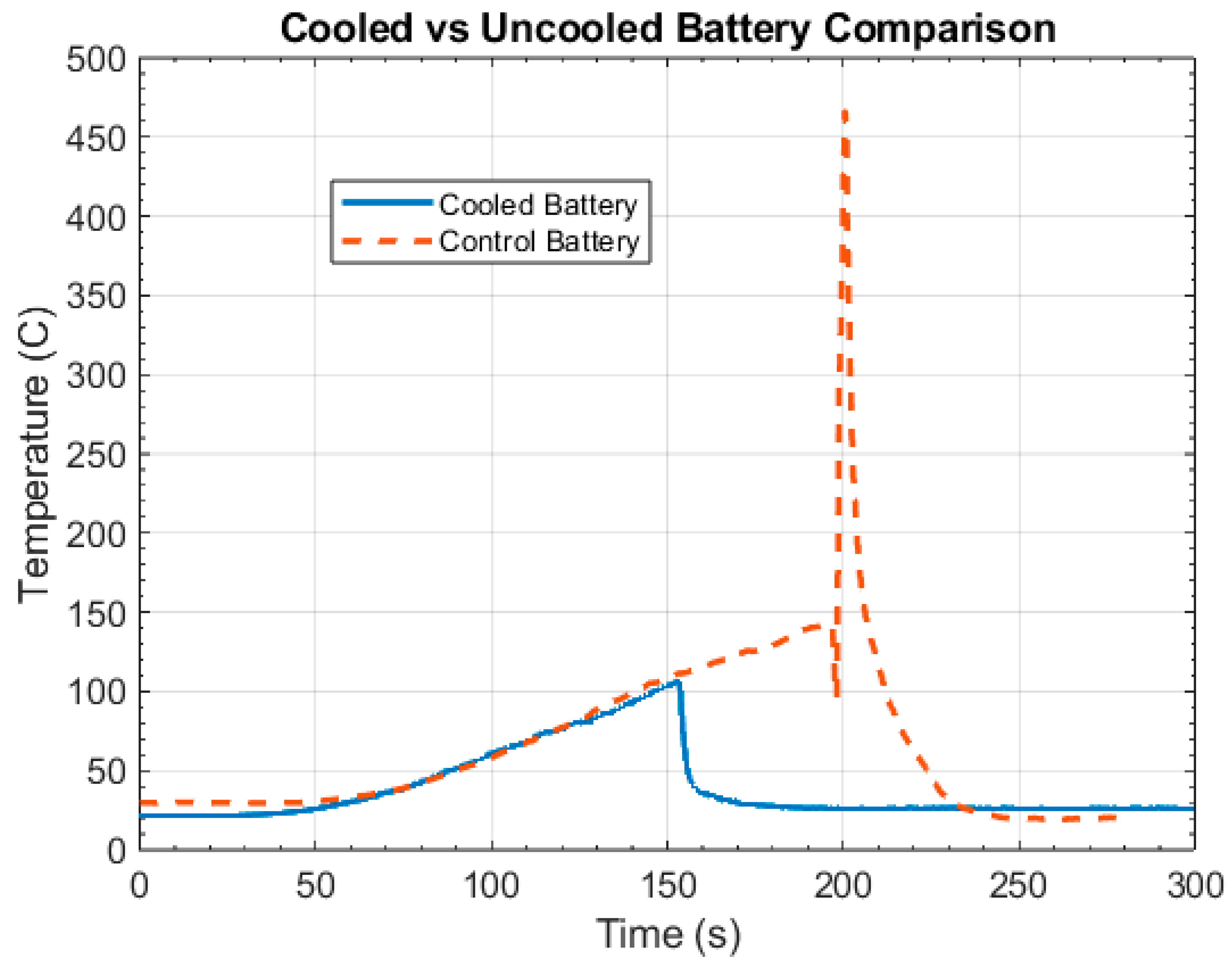
4. Results and Discussion
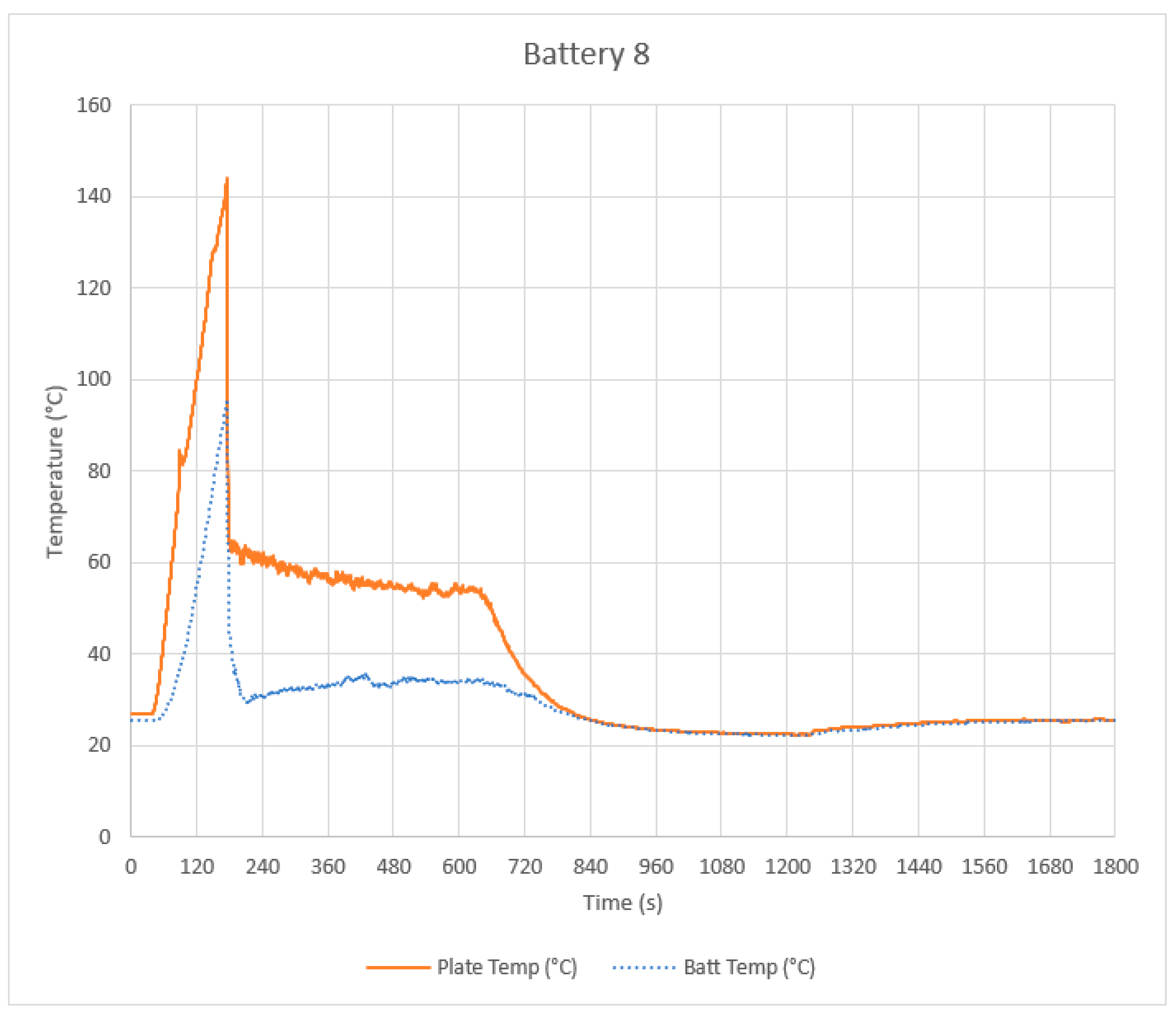
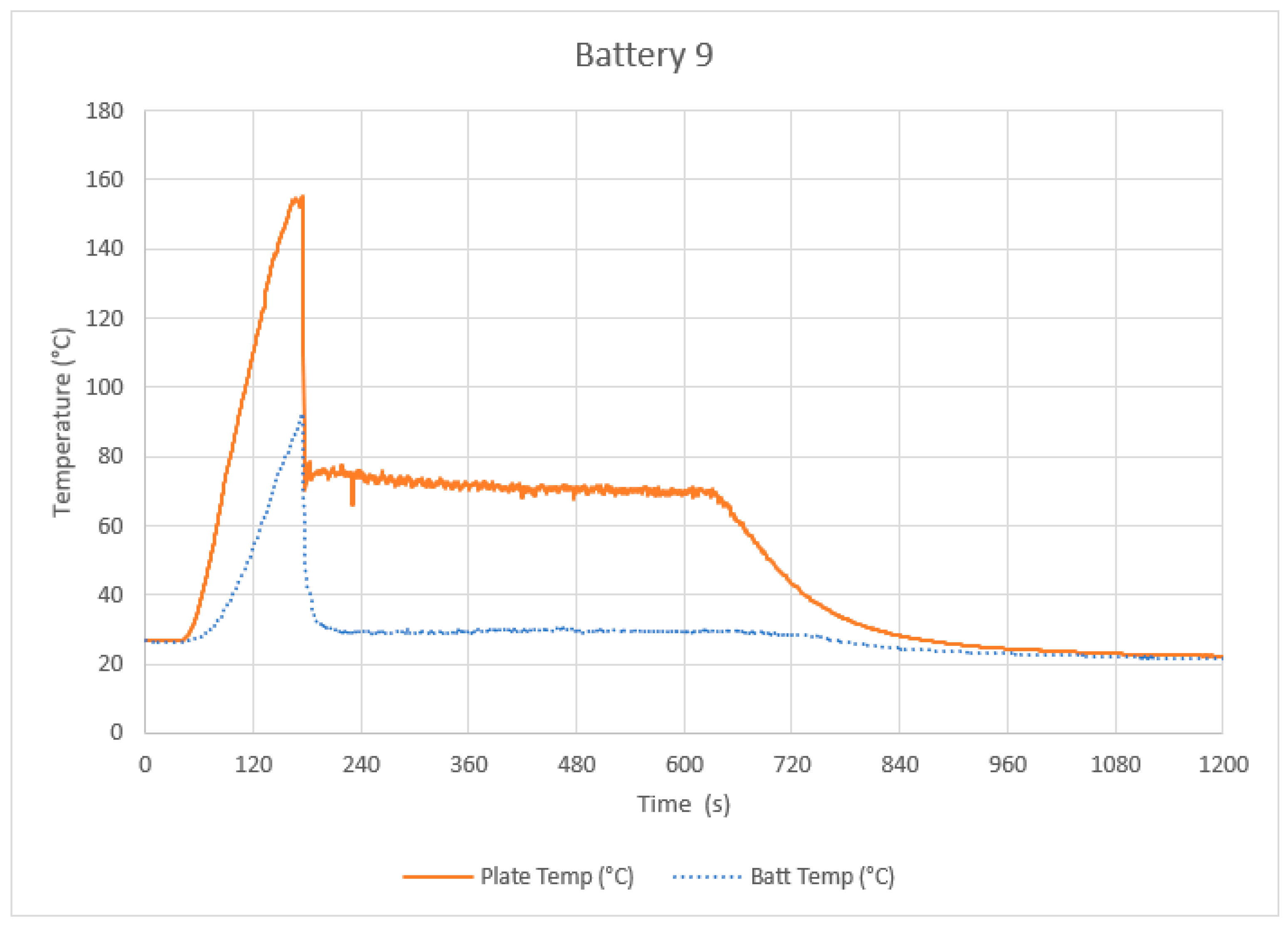
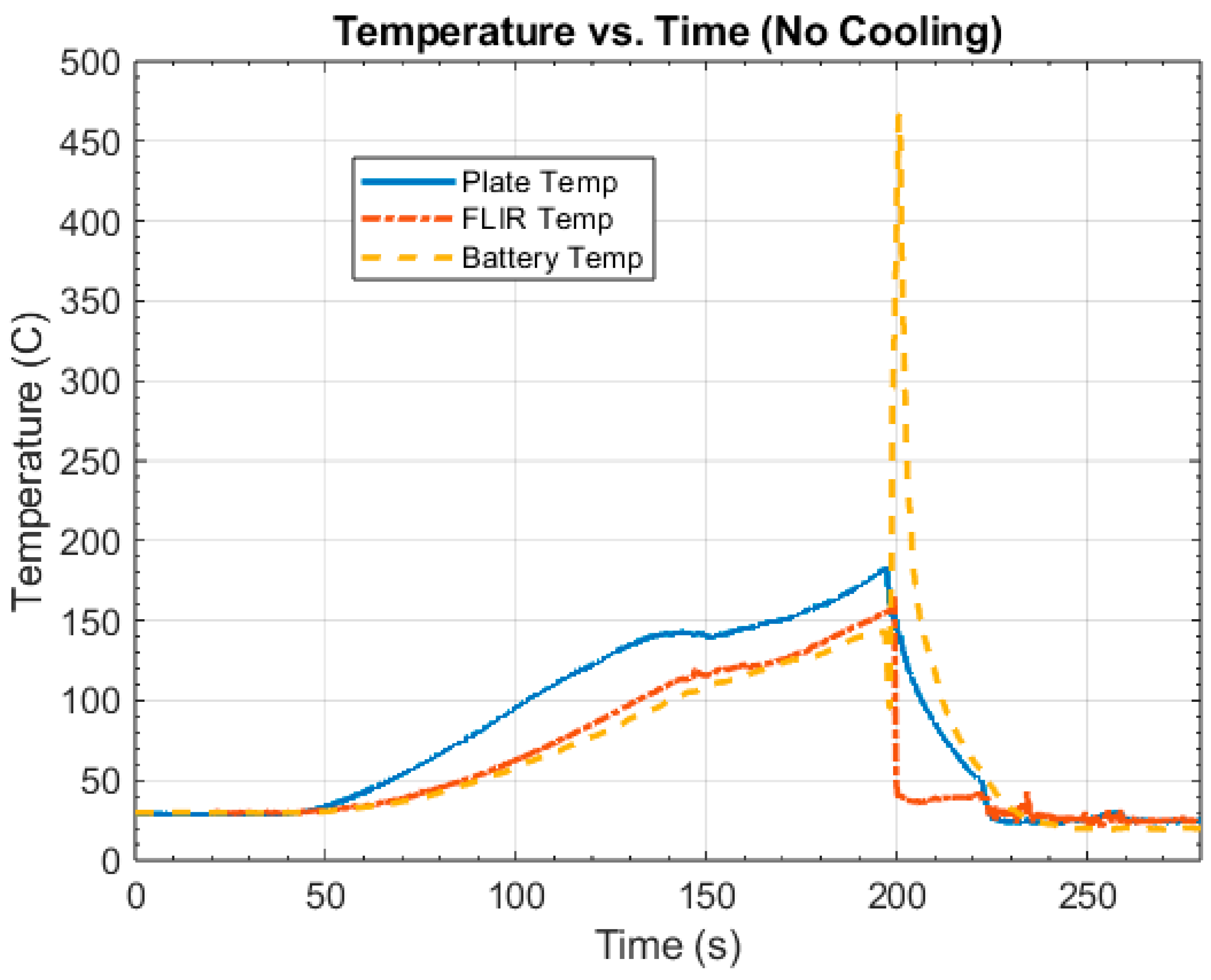
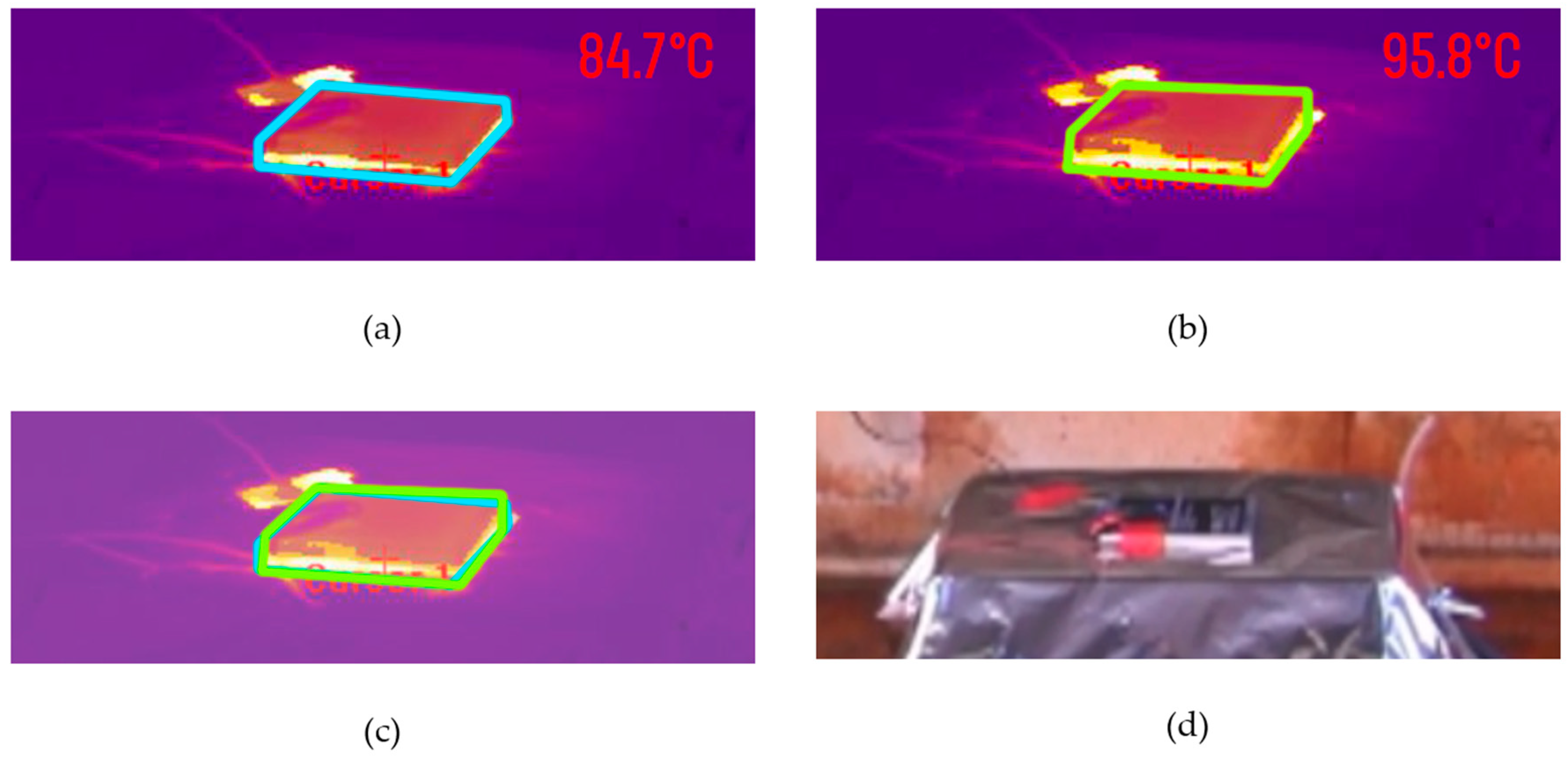
4.1. Control Tests
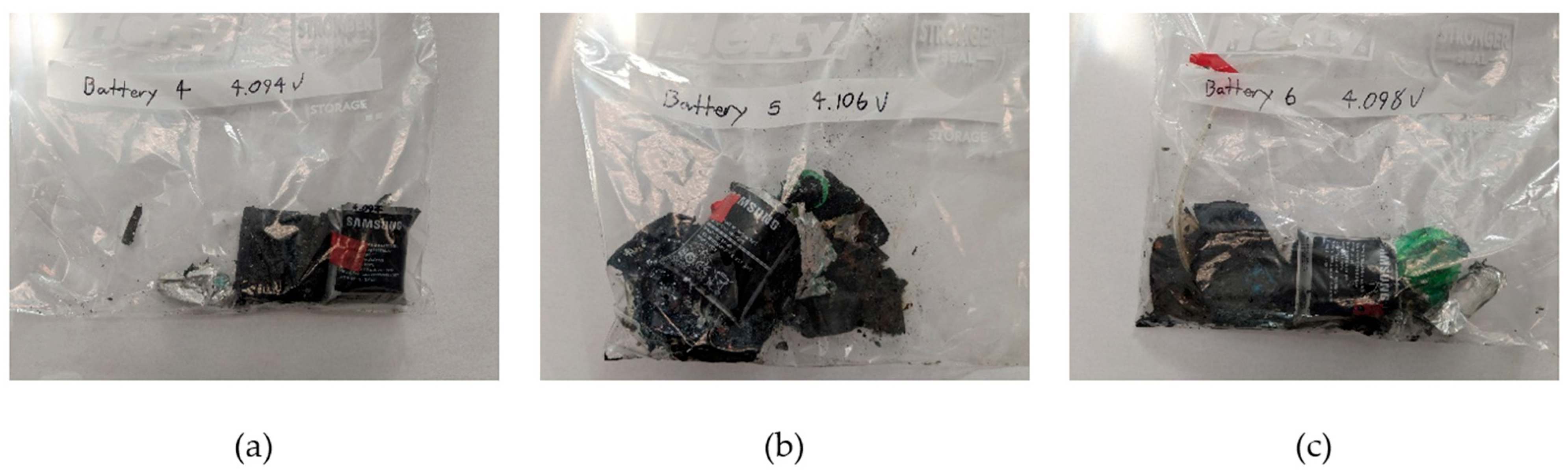
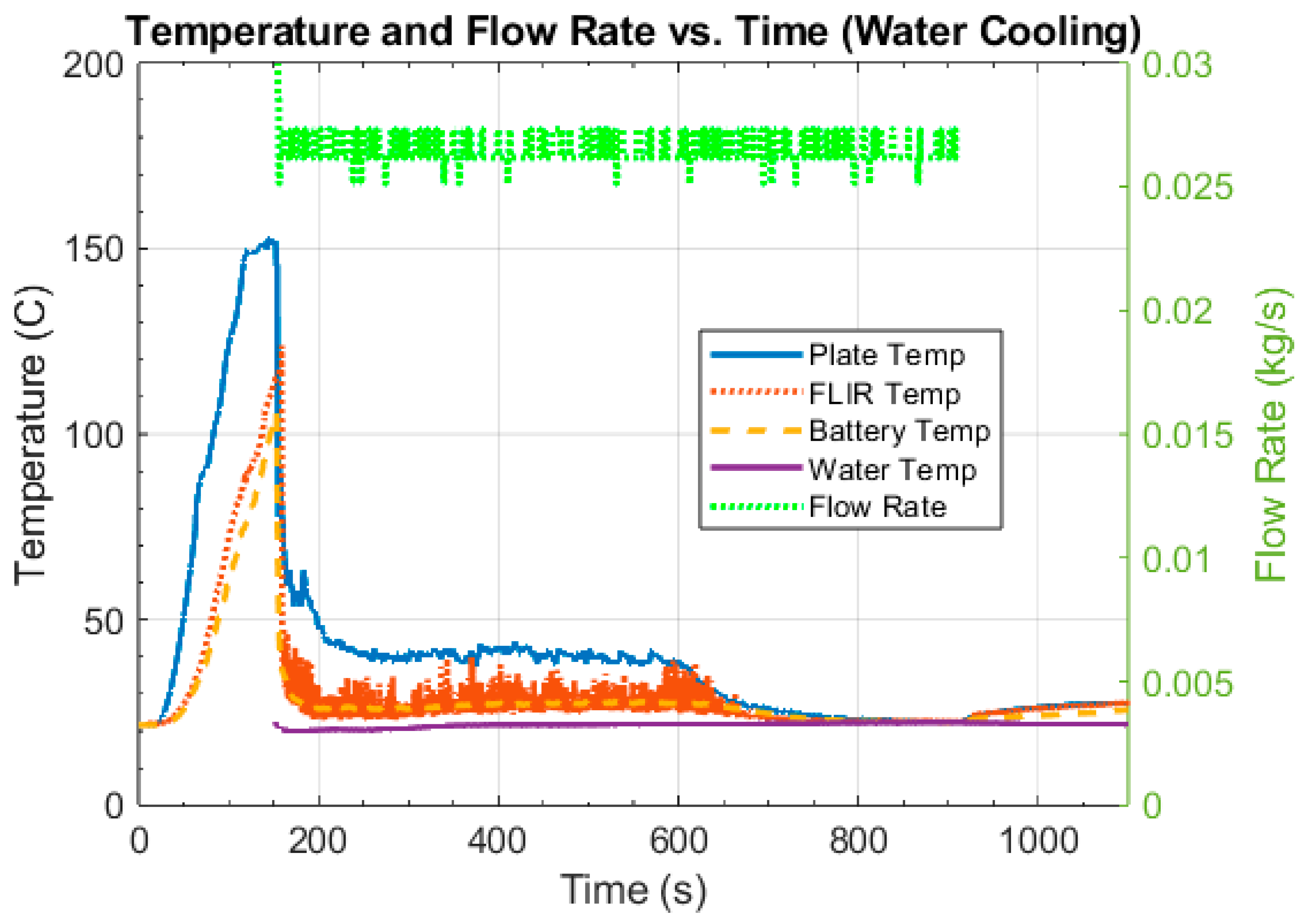
4.2. Intervention Tests
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIB | Lithium Ion Battery |
| SOC | State of Charge |
| SOH | State of Health |
| SOP | State of Power |
| SOE | State of Energy |
| SOL | State of Life |
| HP | Hewlett Packard |
| AT&T | American Telephone and Telegraph Company |
| LMP | Lithium Metal Polymer |
| NHTSA | National Highway Traffic Safety Administration |
| BESS | Battery Energy Storage System |
| AZ | Arizona |
| NV | Nevada |
| GWh | Gigawatt Hour |
| NFPA | National Fire Protection Association |
| TX | Texas |
| MWh | Megawatt Hour |
| NY | New York |
| UL | Underwriters Laboratory |
| EV | Electric Vehicle |
| LMO | Lithium Metal Oxide |
| NMC | Lithium Nickel Manganese Cobalt Oxide |
| LFP | Lithium Iron Phosphate |
| NCA | Lithium Nickel Cobalt Aluminum Oxide |
| ISC | Internal Short Circuit |
| V | Volt |
| CCR | Chemical Chair Reaction |
| US | United States |
| FDNY | Fire Department of New York |
| FSRI | Fire Safety Research Institute |
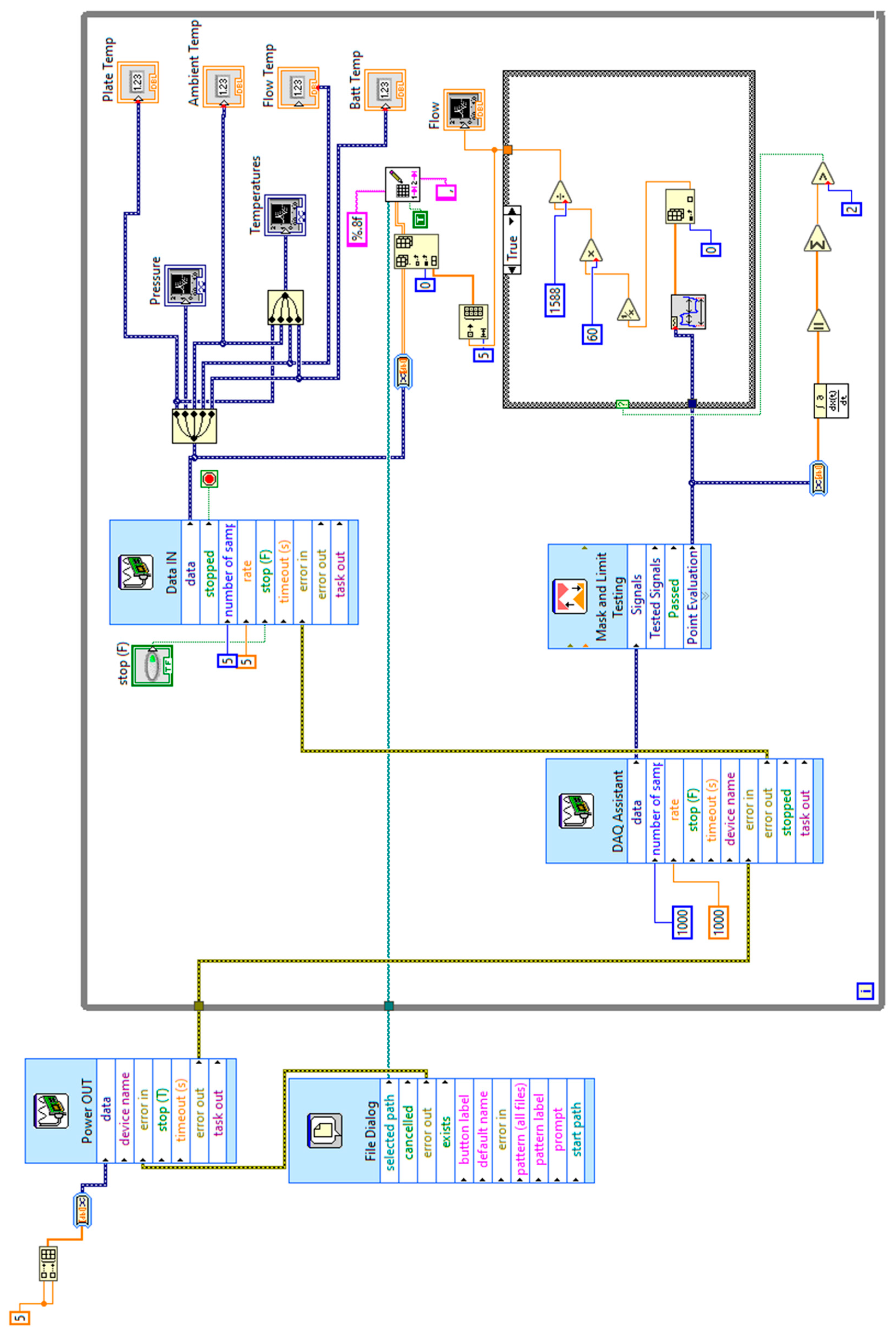
Appendix A. LabVIEW Data Acquisition Block Diagram
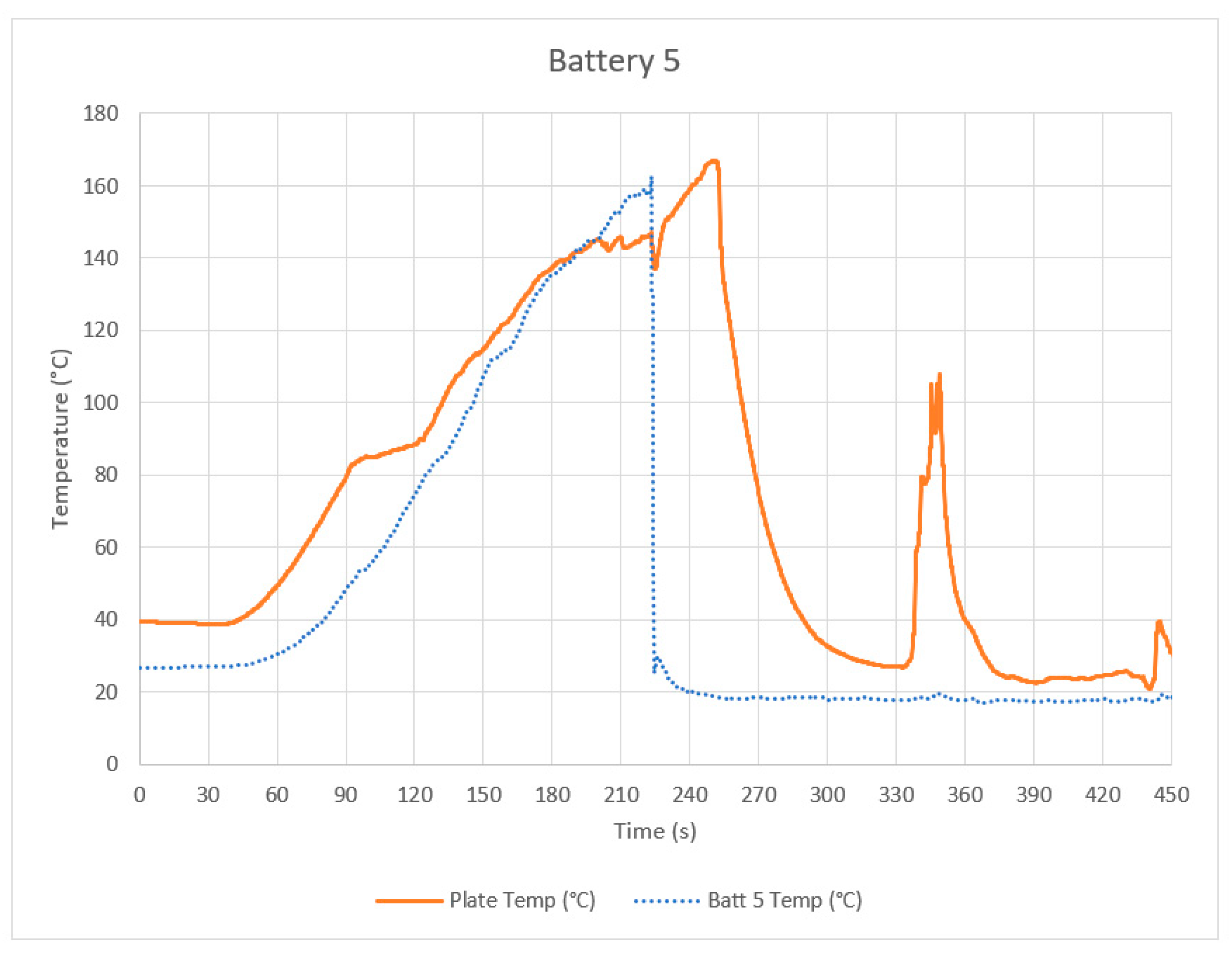
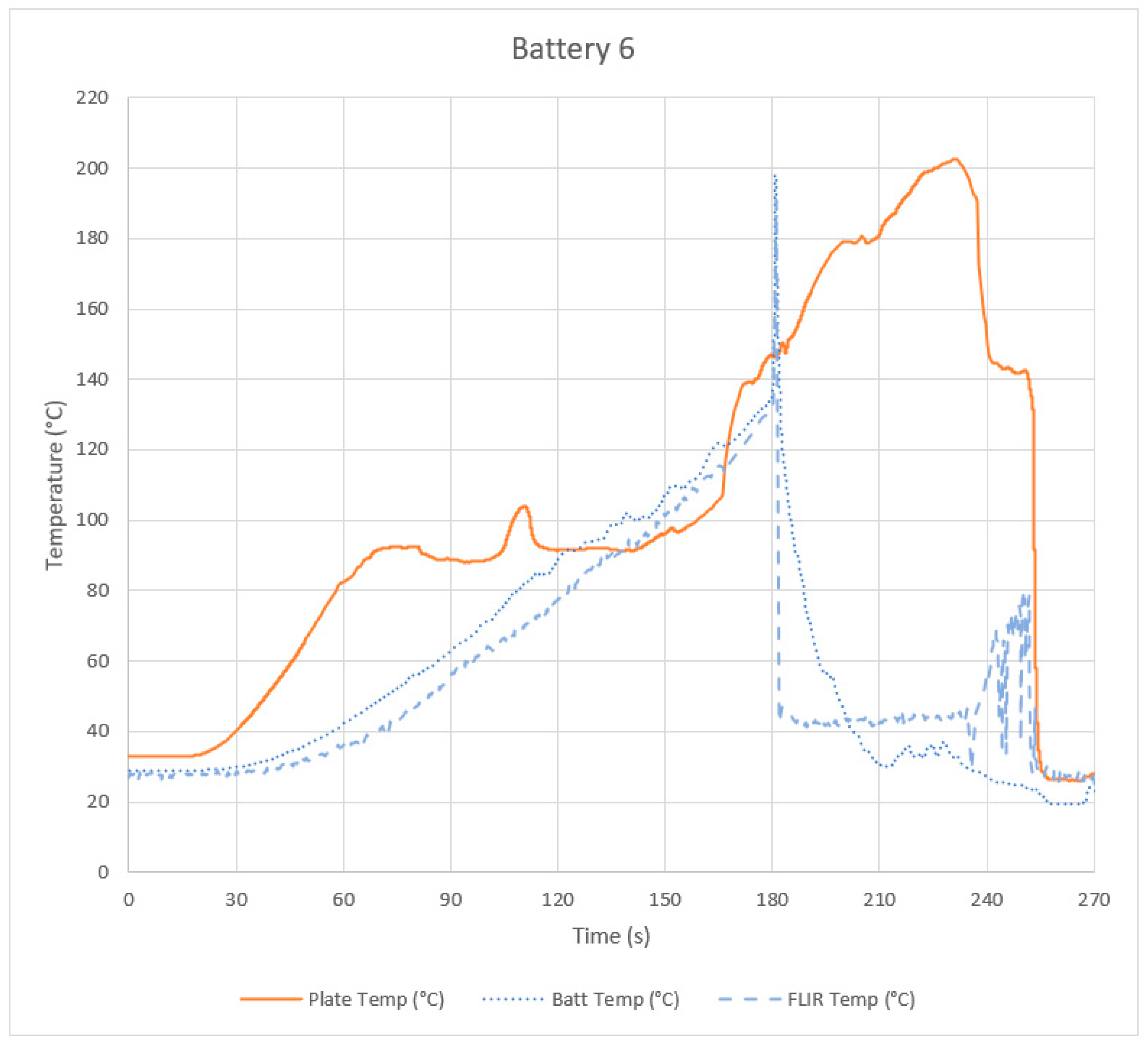
Appendix B. Data from Other Tests
References
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- CPSC. HP Recalls Notebook Computer Batteries Due to Fire Hazard. Available online: https://www.cpsc.gov/Recalls/2006/hp-recalls-notebook-computer-batteries-due-to-fire-hazard (accessed on 5 February 2024).
- CPSC. Dell Announces Recall of Notebook Computer Batteries Due To Fire Hazard. Available online: https://www.cpsc.gov/Recalls/2006/dell-announces-recall-of-notebook-computer-batteries-due-to-fire-hazard (accessed on 5 February 2024).
- Green Car Congress. Avestor Shuts Down. Available online: https://www.greencarcongress.com/2006/11/avestor_shuts_d.html (accessed on 5 February 2024).
- Shepard, J. AT&T Begins Battery Replacement Program After Reports of Fires. Available online: https://eepower.com/news/att-begins-battery-replacement-program-after-reports-of-fires/# (accessed on 5 February 2024).
- Searcey, D. Batteries Hamper AT&T TV Effort. Available online: https://www.wsj.com/articles/SB120045143379793329 (accessed on 7 February 2024).
- Shahan, Z. Electric Car Evolution. Available online: https://cleantechnica.com/2015/04/26/electric-car-history/ (accessed on 7 February 2024).
- Qian, H.; Zhang, J.; Lai, J.S.; Yu, W. A high-efficiency grid-tie battery energy storage system. IEEE Trans. Power Electron. 2011, 26, 886–896. [Google Scholar] [CrossRef]
- NHTSA. Chevrolet Volt Battery Incident Overview Report; U.S. Department of Transportation: Washington, DC, USA, 2012. [Google Scholar]
- Ferguson, J. APS Fire Probed. Available online: https://azdailysun.com/news/local/aps-fire-probed/article_1de2e924-ab0a-5e71-9a3a-6942c2d1c9bb.html (accessed on 7 February 2024).
- Electric Power Research Institute. BESS Failure Event Database; Electric Power Research Institute: Palo Alto, CA, USA, 2024. [Google Scholar]
- Wilson, G. Timeline: Tesla’s Construction of Gigafactories. Available online: https://manufacturingdigital.com/digital-factory/timeline-teslas-construction-gigafactories (accessed on 7 February 2024).
- IEA. Commissioned EV and Energy Storage Lithium-Ion Battery Cell Production Capacity by Region, and Associated Annual Investment; IEA: Paris, France, 2022. [Google Scholar]
- Hollister, S. Here’s Why Samsung Note 7 Phones Are Catching Fire. Available online: https://www.cnet.com/tech/mobile/why-is-samsung-galaxy-note-7-exploding-overheating/ (accessed on 7 February 2024).
- Blum, A.F.; Long, R.T.J. Hazard Assessment of Lithium Ion Battery Energy Storage Systems; NFPA: Quincy, MA, USA, 2016; p. 130. [Google Scholar]
- Strobel, P. Hoverboard Recalls List: Are They Safe in 2021? Available online: https://eridehero.com/ (accessed on 7 February 2024).
- KHOU. Train Car Carrying Lithium Batteries Explodes Near Downtown Houston. Available online: https://www.khou.com/article/news/local/train-car-carrying-lithium-batteries-explodes-near-downtown-houston/285-433576556 (accessed on 7 February 2024).
- Branquinho, L. Richard Hammond’s Crash: Why Did His EV Catch Fire? Available online: https://www.news24.com/life/motoring/cardoctor/fuel_focus/richard-hammonds-crash-why-did-his-ev-catch-fire-20170614 (accessed on 7 February 2024).
- NFPA. NFPA 855: Standard for the Installation of Stationary Energy Storage Systems; NFPA: Quincy, MA, USA, 2023. [Google Scholar]
- Morones, A. BESS Part 4: Flammable Hazards of BESS Failures, Technical Report; Baker Engineering and Risk Consultants: San Antonio, TX, USA, 2021; p. 5. [Google Scholar]
- Charalambous, P. Amid a Rise in Fires and Deaths, New York City Enacts New e-Bike Rules. Available online: https://abcnews.go.com/US/amid-rise-fires-deaths-new-york-city-enacts-e-bike-law/story?id=97984159 (accessed on 7 February 2024).
- NTSB. Electric Truck-Tractor Roadway Departure and Postcrash Fire; NTSB: Washington, DC, USA, 2024. [Google Scholar]
- Rodriguez, O.R.; O’Malley, I. Smoke from fire at California Lithium Battery Plant Raises Concerns About Air Quality. Available online: https://apnews.com/article/battery-storage-plant-fire-california-moss-landing-7c561fed096f410ddecfb04722a8b1f8 (accessed on 27 March 2025).
- Bay City News Service. Battery Storage Facility; Residents Told to Close Windows. Available online: https://www.cbsnews.com/sanfrancisco/news/moss-landing-lithium-battery-storage-vistra-monterey-county/ (accessed on 27 March 2025).
- Durham, P. Lithium-Ion Battery Fires: The Missing Data. Available online: https://www.firerescue1.com/lithium-ion-battery-fires/articles/lithium-ion-battery-fires-the-missing-data-vuR8fCKUZgq55Vpm/ (accessed on 16 November 2024).
- Rubin, A. Lithium-Ion Batteries in E-Bikes and Other Devices Pose Fire Risks. Available online: https://www.nytimes.com/2022/11/14/us/lithium-ion-ebike-battery-fires.html (accessed on 16 November 2024).
- DNV GL. McMicken Battery Energy Storage System Event Technical Analysis and, Recommendations; Report No. 10209302-HOU-R-01; DNV GL: Phoenix, AZ, USA, 2020. [Google Scholar]
- Kim, T.; Makwana, D.; Adhikaree, A.; Vagdoda, J.S.; Lee, Y. Cloud-Based Battery Condition Monitoring and Fault Diagnosis Platform for Large-Scale Lithium-Ion Battery Energy Storage Systems. Energies 2018, 11, 125. [Google Scholar] [CrossRef]
- NTSB. Safety Risks to Emergency Responders from Lithium-Ion battery Fires in Electric Vehicles; Safety Report NTSB/SR-20/01; NTSB: Washington, DC, USA, 2020. [Google Scholar]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A Review on the Thermal Hazards of the Lithium-Ion Battery and the Corresponding Countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Rivian. R1T Emergencty Response. Available online: https://rivian.com/emergency-response (accessed on 20 May 2025).
- Tariq, M.; Maswood, A.I.; Gajanayake, C.J.; Gupta, A.K. Aircraft batteries: Current trend towards more electric aircraft. IET Electr. Syst. Transp. 2017, 7, 93–103. [Google Scholar] [CrossRef]
- Rao, H.; Huang, Z.; Zhang, H.; Xiao, S. Study of fire tests and fire safety measures on lithiumion battery used on ships. In Proceedings of the 2015 International Conference on Transportation Information and Safety (ICTIS), Wuhan, China, 25–28 June 2015; pp. 865–870. [Google Scholar]
- Geisige, J. How a Lithium Ion Battery Actually Works//Photorealistic//16 Month Project. 2022. Available online: https://www.linkedin.com/posts/ed-perez-130025242_how-a-lithium-ion-battery-actually-works-activity-7248548898238001153-CIRD (accessed on 5 February 2024).
- Orendorff, C.J. The role of separators in lithium-ion cell safety. Electrochem. Soc. Interface 2012, 21, 61. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Ziebert, C. Thermal and Mechanical Safety Assessment of Type 21700 Lithium-Ion Batteries with NMC, NCA and LFP Cathodes–Investigation of Cell Abuse by Means of Accelerating Rate Calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Ghiji, M.; Edmonds, S.; Moinuddin, K. A Review of Experimental and Numerical Studies of Lithium Ion Battery Fires. Appl. Sci. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Lamb, J.; Torres-Castro, L.; Hewson, J.C.; Shurtz, R.C.; Preger, Y. Investigating the Role of Energy Density in Thermal Runaway of Lithium-Ion Batteries with Accelerating Rate Calorimetry. J. Electrochem. Soc. 2021, 168, 60516. [Google Scholar] [CrossRef]
- Börger, A.; Mertens, J.; Wenzl, H. Thermal runaway and thermal runaway propagation in batteries: What do we talk about? J. Energy Storage 2019, 24, 100649. [Google Scholar] [CrossRef]
- Guo, R.; Ouyang, M.; Lu, L.; Feng, X. Mechanism of the entire overdischarge process and overdischarge-induced internal short circuit in lithium-ion batteries. Sci. Rep. 2016, 6, 30248. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Ramadass, P.; Zhang, J. Analysis of internal short-circuit in a lithium ion cell. J. Power Sources 2009, 194, 550–557. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Juan, L.; Sha, Y.; Chunhao, Y.; Zihan, H.; Wang, L.; Yangxing, L.; Jun, X. Safety issues caused by internal short circuits in lithium-ion batteries. J. Mater. Chem. A 2018, 6, 21475–21484. [Google Scholar] [CrossRef]
- Mikolajczak, C.; Kahn, M.; White, K.; Long, R.T. Lithium-Ion Batteries Hazard and Use Assessment; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lee, C.; Said, A.O.; Stoliarov, S.I. Impact of State of Charge and Cell Arrangement on Thermal Runaway Propagation in Lithium Ion Battery Cell Arrays. Transp. Res. Rec. 2019, 2673, 408–417. [Google Scholar] [CrossRef]
- Doose, S.; Hahn, A.; Fischer, S.; Müller, J.; Haselrieder, W.; Kwade, A. Comparison of the consequences of state of charge and state of health on the thermal runaway behavior of lithium ion batteries. J. Energy Storage 2023, 62, 106837. [Google Scholar] [CrossRef]
- Kumar, R.R.; Bharatiraja, C.; Udhayakumar, K.; Devakirubakaran, S.; Sekar, K.S.; Mihet-Popa, L. Advances in Batteries, Battery Modeling, Battery Management System, Battery Thermal Management, SOC, SOH, and Charge/Discharge Characteristics in EV Applications. IEEE Access 2023, 11, 105761–105809. [Google Scholar] [CrossRef]
- Lelie, M.; Braun, T.; Knips, M.; Nordmann, H.; Ringbeck, F.; Zappen, H.; Sauer, D.U. Battery Management System Hardware Concepts: An Overview. Appl. Sci. 2018, 8, 534. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; Ouyang, M. Safety of Lithium Battery Materials Chemistry. J. Mater. Chem. A 2023, 11, 25236–25246. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, X.; Zhang, H.; Yin, Y.; Yu, Z.; Shi, D.; Fang, Y.; Xu, R. Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries. Batteries 2024, 10, 62. [Google Scholar] [CrossRef]
- Luo, Y.; Sang, C.; Le, K.; Chen, H.; Li, H.; Ai, X. Self-actuating protection mechanisms for safer lithium-ion batteries. J. Energy Chem. 2024, 94, 181–198. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, T.; Han, Z.; Chen, H.; Chen, H.; Liu, Z.; Huang, H. Study on the effect of immersion thermal management for high-current rate fast charging of 21700 Li-ion batteries. J. Energy Storage 2024, 85, 111061. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Y.; Tang, H.; Zhang, S.; Yuan, W.; Zhu, L.; Tang, Y. A review on the liquid cooling thermal management system of lithium-ion batteries. Appl. Energy 2024, 375, 124173. [Google Scholar] [CrossRef]
- Biharta, M.A.S.; Santosa, S.P.; Widagdo, D. Design and optimization of lithium-ion battery protector with auxetic honeycomb for in-plane impact using machine learning method. Front. Energy Res. 2023, 11, 1114263. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat Mass Transf. 2022, 184, 122288. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, K.; Sun, J.; Wang, Q. A Review of Fire-Extinguishing Agents and Fire Suppression Strategies for Lithium-Ion Batteries Fire. Fire Technol. 2024, 60, 817–858. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Gao, H.; Wan, X.; Qiu, H.; Zhang, J.; Di, J. Inhibitory effect of water mist containing composite additives on thermally induced jet fire in lithium-ion batteries. J. Therm. Anal. Calorim. 2022, 147, 2171–2185. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Li, H.; Sun, J.; Wang, Q. Experimental study on a novel safety strategy of lithium-ion battery integrating fire suppression and rapid cooling. J. Energy Storage 2020, 28, 101185. [Google Scholar] [CrossRef]
- Deng, J.; Chen, B.; Lu, J.; Wu, C.; Zhou, T.; Chen, J. Experimental Study Regarding the Characteristics of Using Dry Ice Sprays to Inhibit the Thermal Runaway of Lithium-Ion Batteries. SSRN 2023. [Google Scholar] [CrossRef]
- Fire. Merriam-Webster Dictionary, 16th ed.; Merriam-Webster: Springfield, MA, USA, 1971; p. 854. [Google Scholar]
- NFPA. Fire Protection Handbook, 19th ed.; National Fire Protection Association: Quincy, MA, USA, 2003; Volume 1. [Google Scholar]
- Papathanasiou, S. Wood Fire & Chemical Reactions. Available online: https://seetheair.org/2018/08/03/wood-fire-chemical-reactions/ (accessed on 24 January 2024).
- CK-12 Foundation. Combustion Reactions. Available online: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/11%3A_Chemical_Reactions/11.06%3A_Combustion_Reactions (accessed on 25 January 2024).
- Grant, G.B.; Drysdale, D.D. The Suppression and Extinction of Class ’A’ Fires Using Water Sprays; Home Office Fire Research and Development Group: London, UK, 1997. [Google Scholar]
- NFPA. Fire Protection Handbook, 20th ed.; National Fire Protection Association: Quincy, MA, USA, 2008; Volume 2. [Google Scholar]
- Bombik, A. Thermal Runaway: Why it can’t be stopped. In Proceedings of the EVRSafe, Charlotte, NC, USA, 18 October 2023. [Google Scholar]
- CK-12 Foundation. Oxidation-Reduction Reactions. Available online: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/04%3A_Reactions_in_Aqueous_Solution/4.04%3A_Oxidation-Reduction_Reactions (accessed on 31 March 2024).
- NFPA. NFPA 10: Standard for Portable Fire Extinguishers; NFPA: Quincy, MA, USA, 2022. [Google Scholar]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Halton, B. It’s What We Do. Available online: https://www.fireengineering.com/leadership/its-what-we-do/ (accessed on 20 May 2025).
- Ambrose, P. FDNY Shares Fire, Hazmat Lessons on Lithium-Ion Batteries. Available online: https://www.hazmatnation.com/news/fdny-shares-fire-hazmat-lessons-on-lithium-ion-batteries/ (accessed on 20 May 2025).
- Tesla. Fire Responders Information. Available online: https://www.tesla.com/firstresponders (accessed on 10 February 2023).
- GM. First Responders Guides, Rescue Sheets, and Quick Reference Sheets. Available online: https://www.gmstc.com/index.php/first-responders/ (accessed on 10 February 2023).
- CTIF. Up to 150,000 Liters of Water Needed to Put Out a Fire in an Electric Car. Available online: https://ctif.org/news/150-000-liters-water-needed-put-out-fire-electric-car#:~:text=...Teslas%20may%20take%20up,a%20parking%20lot%20car%20fire (accessed on 20 May 2025).
- Coe, C.S. Experimental Study for Detection of Thermal Runaway, Explosion, and Fire in Li-Ion Batteries Initiated by Hot Plate Method. M.S.; The University of North Carolina at Charlotte: Charlotte, NC, USA, 2022. [Google Scholar]
- Feng, X.; He, X.; Ouyang, M.; Wang, L.; Lu, L.; Ren, D.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Y.; Liu, B. Experimental investigation into the use of emergency spray on suppression of battery thermal runaway. J. Energy Storage 2021, 38, 102546. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Lu, Y.; Liu, B. Investigation into the effects of emergency spray on thermal runaway propagation within lithium-ion batteries. J. Energy Storage 2023, 66, 107505. [Google Scholar] [CrossRef]


| Year | Event |
|---|---|
| 1817 | Lithium metal discovered [1] |
| 1901 | Swedish engineer Waldmar Jungner invents a rechargeable nickel-cadmium battery [2] |
| 1969 | W.F. Myers and J.W. Simmons patent the Li-SO2 battery [1] |
| 1975 | Sanyo CS-8176L solar-rechargeable calculator powered by a Li-MnO2 battery [1] |
| 1977 | Exxon exhibits rechargeable Li-TiS2 battery at Chicago Electric Vehicle Show [3] |
| 2006 | Dell and HP recall laptops due to fire risk caused by li-ion batteries [4,5] |
| 2008 | AT&T begins replacing 17,000 LMP batteries made by Avestor (who went bankrupt and ceased operations in 2006) after several fires [6,7,8] |
| 2008 | The Tesla Roadster is the first production electric vehicle to use lithium-ion battery technology [9] |
| 2010 | Lithium-ion batteries begin to be considered for grid-scale energy storage [10] |
| 2011 | NHTSA begins investigation into li-ion batteries in the Chevrolet Volt after numerous fires [11] |
| 2012 | First recorded Li-Ion BESS fire in Flagstaff, AZ [12,13] |
| 2014 | Tesla breaks ground on the first Gigafactory outside of Sparks, NV, USA [14] |
| 2016 | Global lithium-ion battery production tops 100 GWh [15] |
| 2016 | Samsung Note 7 cell phones recalled due to fires [16] |
| 2016 | NFPA publishes LIB hazard assessment [17] |
| 2017 | “Drone Nerds” brand recalls “hoverboards” because of fires [18] |
| 2017 | Rail car carrying Li-Ion batteries explodes outside of Houston, TX [19] |
| 2017 | Automotive journalist Richard Hammond crashes a Rimac Concept One electric hypercar. The vehicle caught fire on-scene and spontaneously reignited for several days afterward [20]. |
| 2017 | The “first draft committee” for the future NFPA 855 standard (Standard for the Installation of Stationary Energy Storage Systems) meets for the first time [21]. |
| 2019 | 2 MWh BESS explodes in Surprise, AZ, USA, seriously injuring five firefighters [22] |
| 2020 | NFPA 855 is published [21] |
| 2023 | New York City, NY enacts legislation prohibiting the use of li-ion powered e-mobility devices without a UL registration [23] |
| 2024 | Tesla tractor trailer crashes along Interstate Highway 80 in AZ. The crash resulted in the truck’s battery going into thermal runaway. Firefighters reportedly used 50,000 gallons of water to control the fire. The highway was closed for more than 12 h [24]. |
| 2025 | A BESS facility in Moss Landing, CA, experienced a large fire due to a thermal runaway prompting the evacuation of nearly 1500 people. A month later, smaller fires were still being found due to continued thermal runaway propagation [25,26]. |
| Manufacturer | Samsung |
| Model | EB-B220AC |
| Capacity | 2600 mAh/9.88 Wh |
| Voltage (Nominal/Maximum) | 3.8 V/4.35 V |
| Date of Manufacture | Sept. 2020 |
| Description | Manufacturer | Model |
|---|---|---|
| Hot plate | Corning, Corning, NY, USA | PC-400D |
| Paddlewheel flow meter | Advanced Thermal Solutions, Norwood, MA, USA | ATS-FM-34 |
| Pressure transducer (PT) | Honeywell, Charlotte, NC, USA | 480-MIPAN2XX500PSAXX-ND |
| Thermocouples (TE) | Nanmac, Milford, MA, USA | A4A-T-2-2-PK |
| Video camera | Canon, Tokyo, Japan | Aixia HF S200 |
| Thermal imaging camera | FLIR, Wilsonville, OR, USA | A600 |
| Data acquisition system (DAQ) | National Instruments, Austin, TX, USA | cDAQ-9174 (with modules 9213, 9205, 9264, and 9219) running through LabVIEW 21.0 |
| Live image feed management | Open Broadcaster Software | OBS Studio 30.0.0 |
| Water flow control needle valve | McMaster-Carr, Elmhurst, IL, USA | 455K13 |
| Water spray nozzle | McMaster-Carr | 32885K144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huhn, E.; Braxtan, N.; Chen, S.-E.; Bombik, A.; Zhao, T.; Ma, L.; Sherman, J.; Roghani, S. Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling. Energies 2025, 18, 2709. https://doi.org/10.3390/en18112709
Huhn E, Braxtan N, Chen S-E, Bombik A, Zhao T, Ma L, Sherman J, Roghani S. Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling. Energies. 2025; 18(11):2709. https://doi.org/10.3390/en18112709
Chicago/Turabian StyleHuhn, Eric, Nicole Braxtan, Shen-En Chen, Anthony Bombik, Tiefu Zhao, Lin Ma, John Sherman, and Soroush Roghani. 2025. "Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling" Energies 18, no. 11: 2709. https://doi.org/10.3390/en18112709
APA StyleHuhn, E., Braxtan, N., Chen, S.-E., Bombik, A., Zhao, T., Ma, L., Sherman, J., & Roghani, S. (2025). Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling. Energies, 18(11), 2709. https://doi.org/10.3390/en18112709






