Magnetron-Sputtered and Rapid-Thermally Annealed NiO:Cu Thin Films on 3D Porous Substrates for Supercapacitor Electrodes
Abstract
1. Introduction
2. Materials and Methods
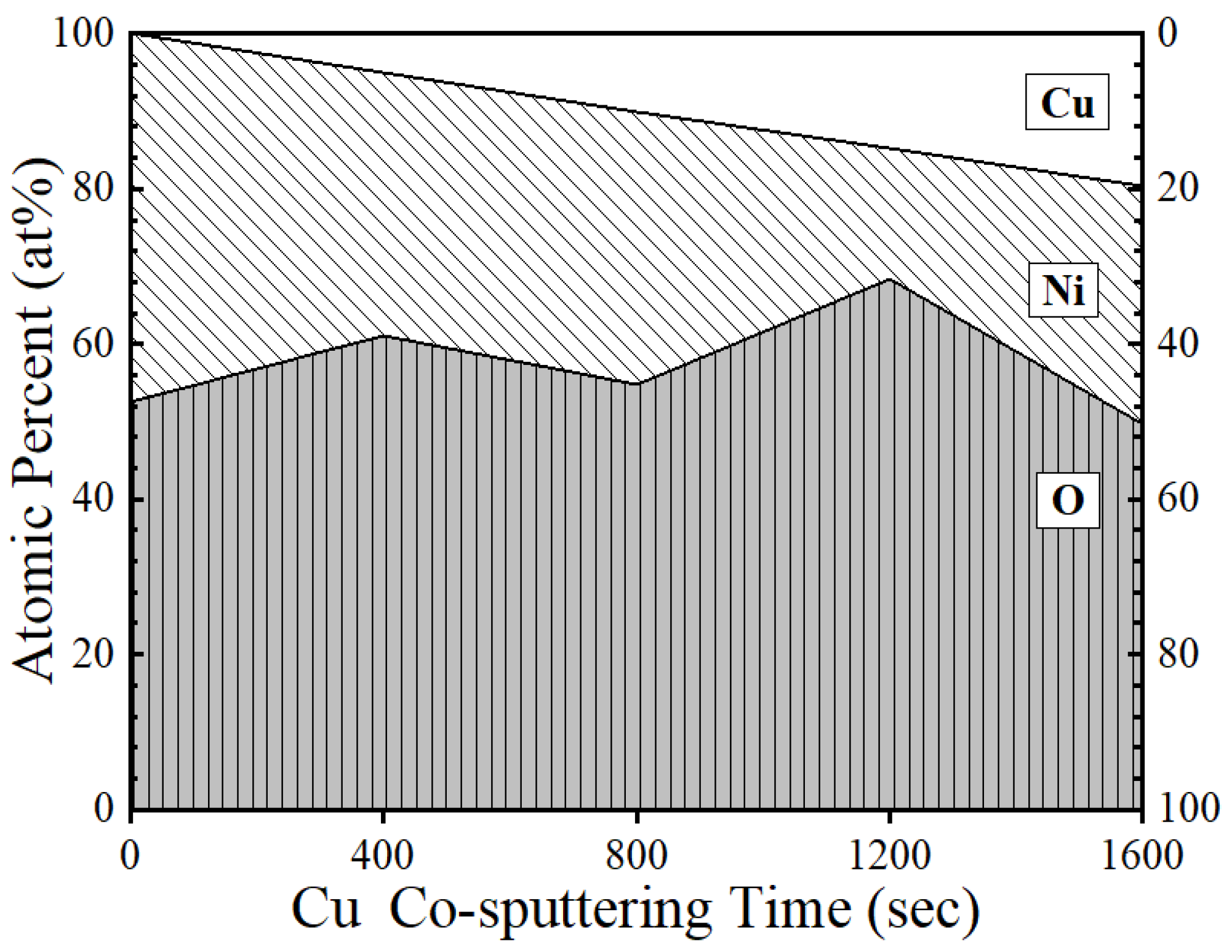
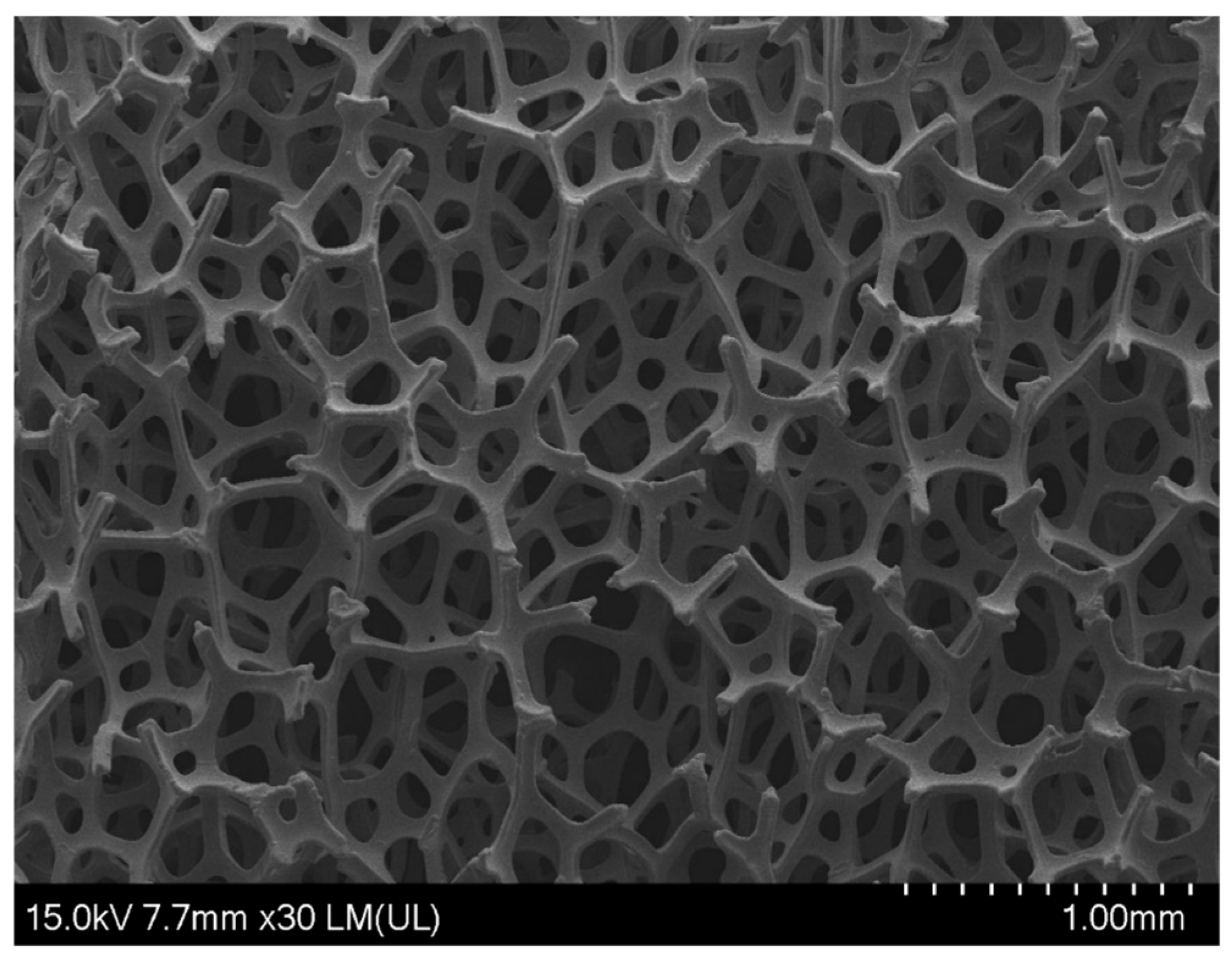
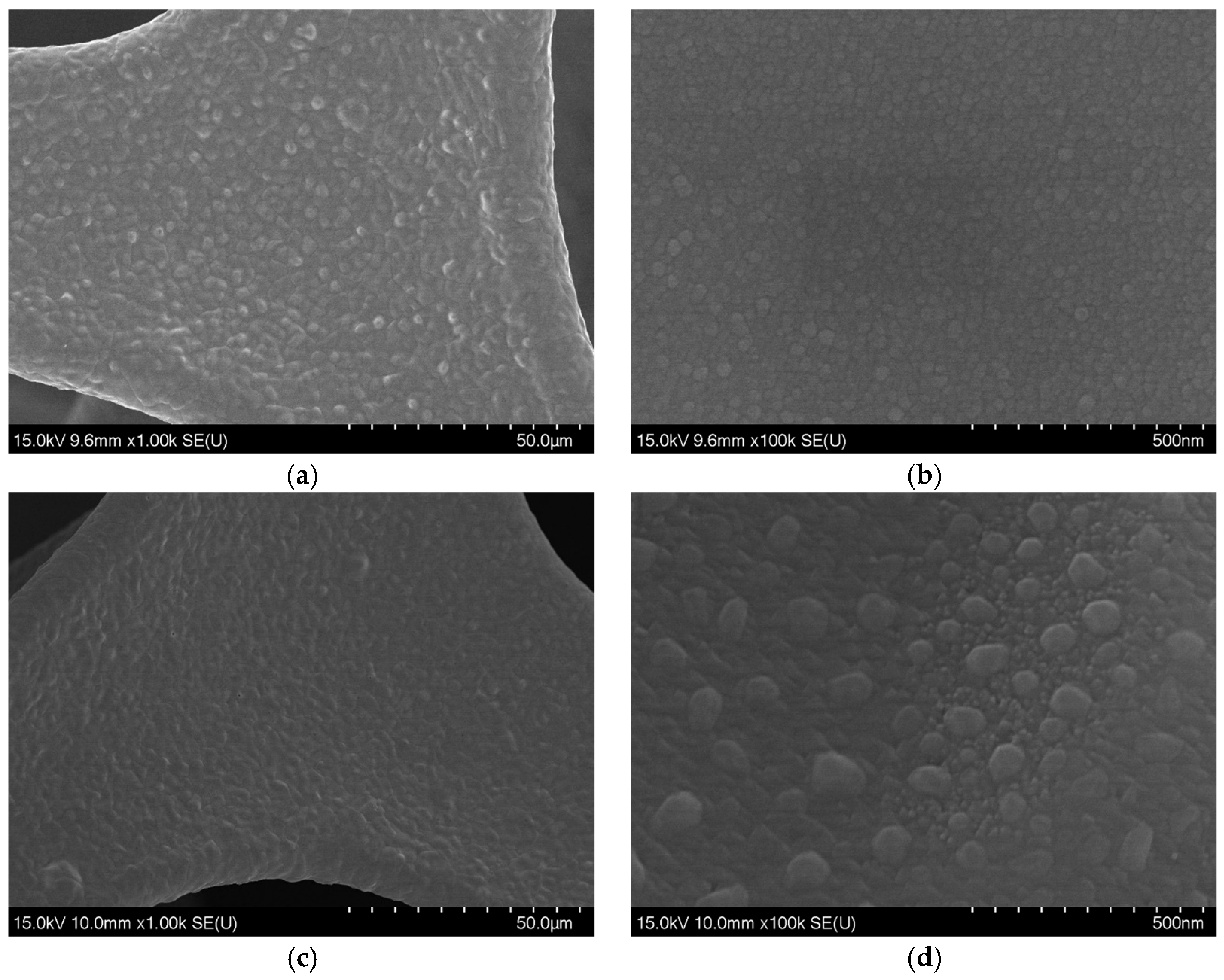
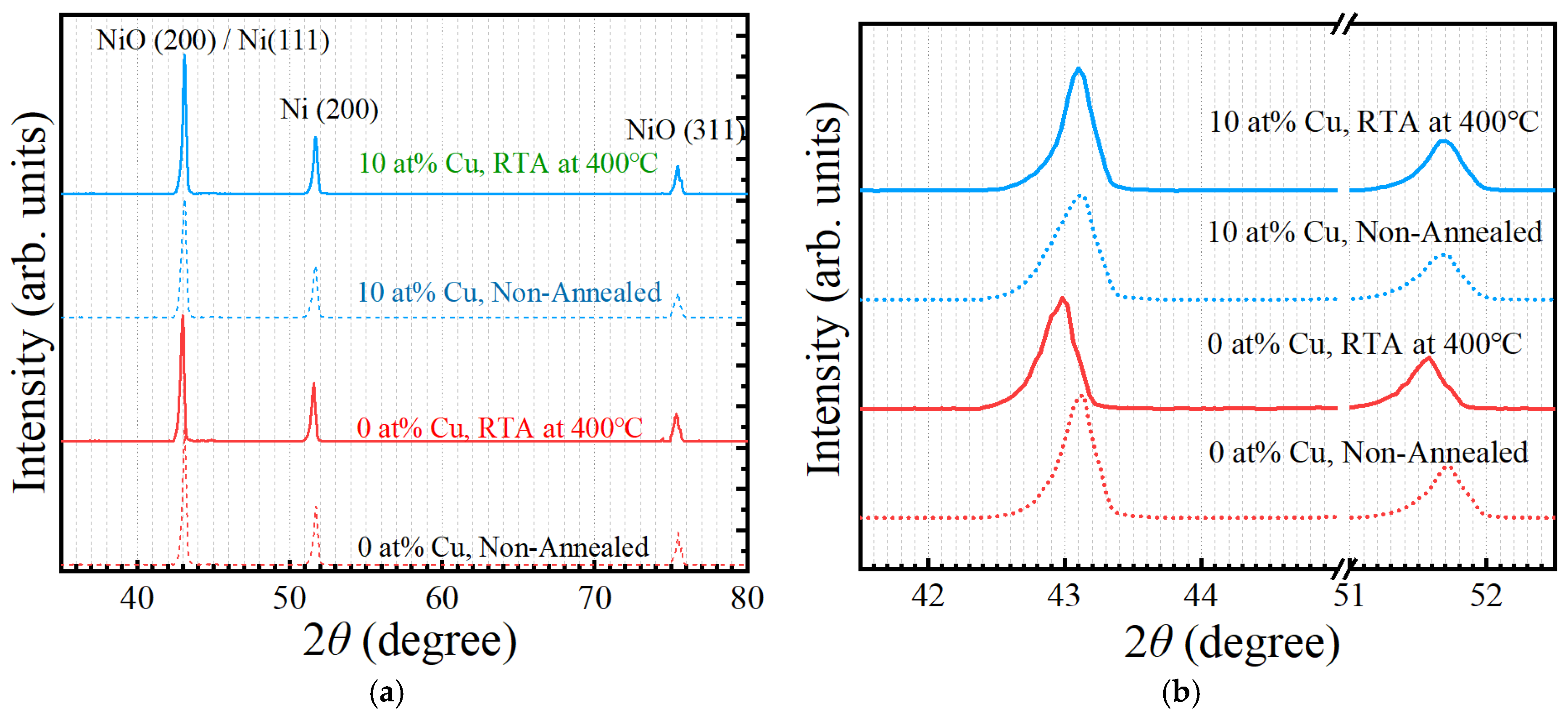
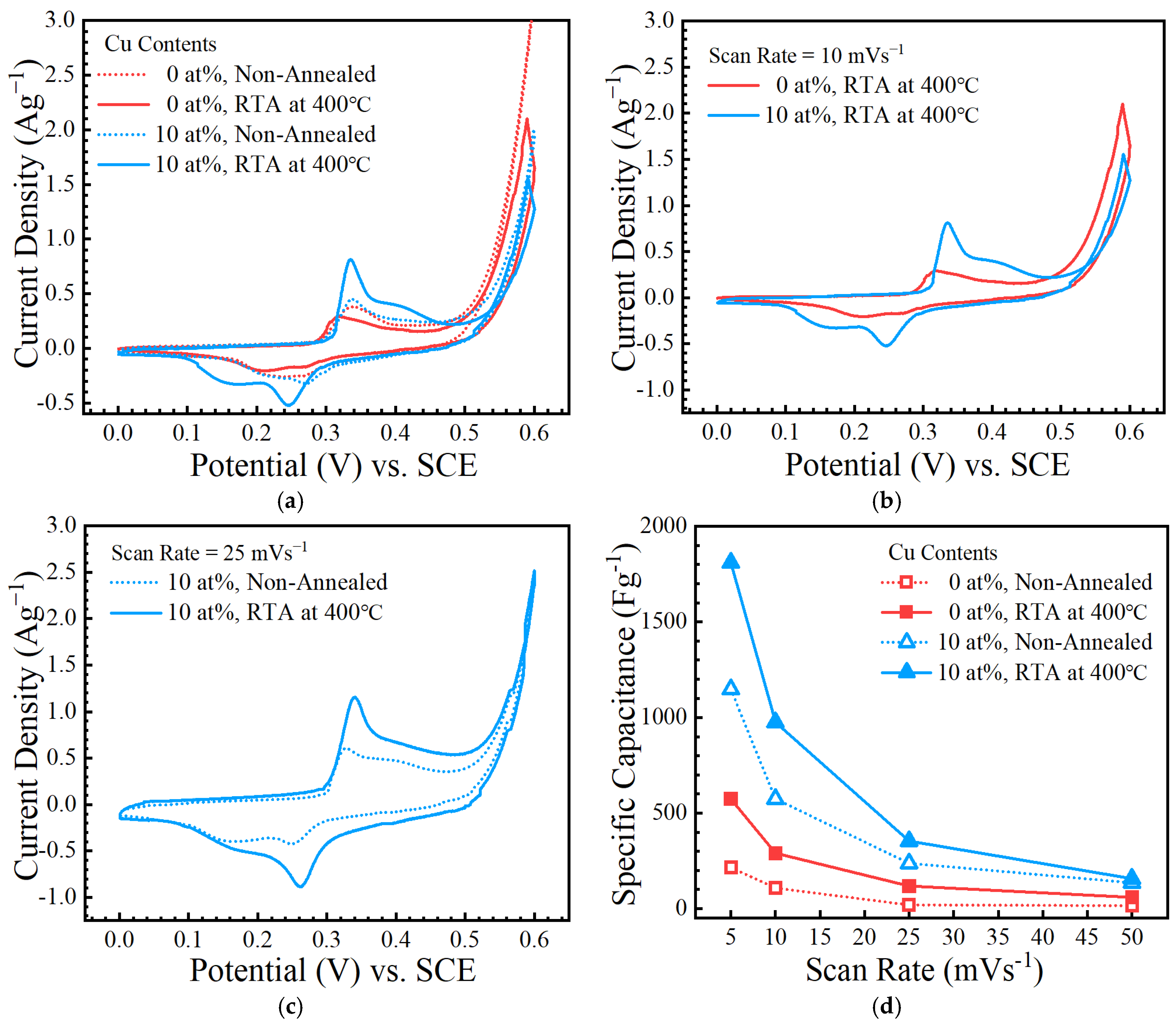
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Lin, J.; Wu, N.; Xie, S.; Meng, C.; Zheng, Y.; Wang, X.; Zhao, Y. Review and outlook on the international renewable energy development. Energy Built Environ. 2022, 3, 139–157. [Google Scholar] [CrossRef]
- Xia, C.; Alshareef, H.N. Self-templating scheme for the synthesis of nanostructured transition-metal chalcogenide electrodes for capacitive energy storage. Chem. Mat. 2015, 27, 4661–4668. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Lu, Y.; Wei, S.; Wujcik, E.K.; Guo, Z. Multifunctional carbon nanostructures for advanced energy storage applications. Nanomaterials 2015, 5, 755–777. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Hu, C.; Li, X.; Zhu, Y. Enhancing battery performance under motor overload drive with a battery–supercapacitor hybrid energy storage system. J. Power Sources 2025, 642, 236680. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mat. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Hu, H.; Yang, J.; Qian, B.; Ling, Z.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon nanofiber/graphene composite electrode for supercapacitors. J. Power Sources 2013, 243, 350–353. [Google Scholar] [CrossRef]
- Singh, A.K.; Mandal, K. Engineering of high performance supercapacitor electrode based on Fe-Ni/Fe2O3-NiO core/shell hybrid nanostructures. J. Appl. Phys. 2015, 117, 105101. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, H.C.; Lu, S.Y. Manganese oxide/graphene aerogel composites as an outstanding supercapacitor electrode material. Chem. Eur. J. 2014, 20, 517–523. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, G.; Xiao, S.; Zhang, C.; Ma, Y. Effect of microstructure on electrochemical performance of electrode materials for microsupercapacitor. Mater. Lett. 2023, 346, 134481. [Google Scholar] [CrossRef]
- Wang, H.; Yi, H.; Chen, X.; Wang, X. Asymmetric supercapacitors based on nano-architectured nickel oxide/graphene foam and hierarchical porous nitrogen-doped carbon nanotubes with ultrahigh-rate performance. J. Mater. Chem. A 2014, 2, 3223–3230. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; Lou, X.W.D. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Yuan, S.; Ge, M.; Ren, M.; Sun, C.; Zhou, Z. Nanosheet-based NiO microspheres: Controlled solvothermal synthesis and lithium storage performances. J. Phys. Chem. C 2010, 114, 251–255. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Hierarchically ordered porous nickel oxide array film with enhanced electrochemical properties for lithium ion batteries. Electrochem. Commun. 2010, 12, 890–893. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, H.N.; Lim, Y.S.; Chee, W.K.; Huang, N.M. Fabrication of flexible polypyrrole/graphene oxide/manganese oxide supercapacitor. Int. J. Energy Res. 2015, 39, 344–355. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Qu, B.; Hu, L.; Mei, L.; Lei, D.; Li, Q.; Chen, L.; Li, Q.; Wang, T. Synthesis of mesoporous NiO nanospheres as anode materials for lithium ion batteries. Electrochim. Acta 2012, 80, 140–147. [Google Scholar] [CrossRef]
- Oh, S.; Park, Y.S.; Ko, P.J.; Kim, N.H. Effects of rapid thermal treatment on characteristics of magnetron-sputtered NiO thin films for supercapacitor applications. J. Nanosci. Nanotechnol. 2018, 18, 6213–6219. [Google Scholar] [CrossRef]
- Dar, F.I.; Moonoosawmy, K.R.; Es-Souni, M. Morphology and property control of NiO nanostructures for supercapacitor applications. Nanoscale Res. Lett. 2013, 8, 363. [Google Scholar] [CrossRef]
- Offiah, S.U.; Nwodo, M.O.; Nwanya, A.C.; Ezugwu, S.C.; Agbo, S.N.; Ugwuoke, P.U.; Osuji, R.U.; Malik, M.; Ezema, F.I. Effects of post-thermal treatments on morphological and optical properties of NiO/Ni(OH)2 thin films synthesized by solution growth. Optik 2014, 125, 2905–2908. [Google Scholar] [CrossRef]
- Hotovy, I.; Huran, J.; Spiess, L.; Hascik, S.; Rehacek, V. Preparation of nickel oxide thin films for gas sensors applications. Sens. Actuator B Chem. 1999, 57, 147–152. [Google Scholar] [CrossRef]
- Hotovy, I.; Huran, J.; Siciliano, P.; Capone, S.; Spiess, L.; Rehacek, V. The influences of preparation parameters on NiO thin film properties for gas-sensing application. Sens. Actuator B Chem. 2001, 78, 126–132. [Google Scholar] [CrossRef]
- Chen, H.L.; Lu, Y.M.; Hwang, W.S. Characterization of sputtered NiO thin films. Surf. Coat. Technol. 2005, 198, 138–142. [Google Scholar] [CrossRef]
- Usha, K.S.; Sivakumar, R.; Sanjeeviraja, C.; Sathe, V.; Ganesan, V.; Wang, T.Y. Improved electrochromic performance of a radio frequency magnetron sputtered NiO thin film with high optical switching speed. RSC Adv. 2016, 6, 79668–79680. [Google Scholar] [CrossRef]
- Usha, K.S.; Sivakumar, R.; Sanjeeviraja, C. Optical constants and dispersion energy parameters of NiO thin films prepared by radio frequency magnetron sputtering technique. J. Appl. Phys. 2013, 114, 123501. [Google Scholar] [CrossRef]
- Karabiberoğlu, Ş.; Dursun, Z. Synthesis and characterization of polyindole-NiO-graphene composite for supercapacitor electrode. Fuller. Nanotub. Carbon Nanostruct. 2025, 1–16. [Google Scholar] [CrossRef]
- Bachankar, S.; Kumbhar, A.; Mullani, S.; Malavekar, D.; Park, J.; Kim, C.; Ji, T. Enhanced Supercapacitive Charge Storage in a Nickel Oxide-Graphene Oxide Composite: Synergistic Effect. Korean J. Mater. Res. 2024, 34, 609–619. [Google Scholar] [CrossRef]
- Shah, A.; Senapati, S.; Murthy, H.A.; Singh, L.R.; Mahato, M. Supercapacitor performance of NiO, NiO-MWCNT, and NiO–Fe-MWCNT composites. ACS Omega 2023, 8, 33380–33391. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Jiménez-Marín, E.; Villalpando, I.; Trejo-Valdez, M.; Cervantes-Sodi, F.; Vargas-García, J.R.; Torres-Torres, C. Coexistence of positive and negative photoconductivity in nickel oxide decorated multiwall carbon nanotubes. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2017, 220, 22–29. [Google Scholar] [CrossRef]
- Bhujel, K.; Thangavel, R.; Pal, K.K.; Sardar, P.; Nayak, D.; Singh, N.S.; Rai, S. Cu-doped NiO thin film’s structural, optical, and electrical properties and its negative absorption behaviour in the Infra-Red region. Physica B 2024, 688, 416129. [Google Scholar] [CrossRef]
- Rihia, G.; Ghougali, M.; Beggas, A.; Mimouni, M.; Mahboub, M.S. Effects of Cu doping on the structural, optical and electrical characterizations of spray-deposited Ni-O thin films. Dig. J. Nanomater. Biostruct. 2025, 20, 139–147. [Google Scholar] [CrossRef]
- Issatayev, N.; Abdumutaliyeva, D.; Tashenov, Y.; Yeskozha, D.; Seipiyev, A.; Bakenov, Z.; Nurpeissova, A. Three-dimensional carbon coated and high mass-loaded NiO@Ni foam anode with high specific capacity for lithium ion batteries. RSC Adv. 2024, 14, 40069–40076. [Google Scholar] [CrossRef] [PubMed]
- Pech, S.; Rou, Y.J.; Kim, S.; Lee, K.Y.; Kim, N.H. Cu(In,Ga)Se2: Te thin films for stoichiometric compensation by using co-sputtering and rapid thermal annealing. Appl. Sci. 2023, 13, 4284. [Google Scholar] [CrossRef]
- Yang, J.L.; Lai, Y.S.; Chen, J.S. Effect of heat treatment on the properties of non-stoichiometric p-type nickel oxide films deposited by reactive sputtering. Thin Solid Films 2005, 488, 242–246. [Google Scholar] [CrossRef]
- Jang, W.L.; Lu, Y.M.; Hwang, W.S.; Hsiung, T.L.; Wang, H.P. Point defects in sputtered NiO films. Appl. Phys. Lett. 2009, 94, 062103. [Google Scholar] [CrossRef]
- Aftab, M.; Butt, M.Z.; Ali, D.; Bashir, F.; Khan, T.M. Optical and electrical properties of NiO and Cu-doped NiO thin films synthesized by spray pyrolysis. Opt. Mater. 2021, 119, 111369. [Google Scholar] [CrossRef]
- Deyab, M.A.; Keera, S.T. Cyclic voltammetric studies of carbon steel corrosion in chloride-formation water solution and effect of some inorganic salts. Egypt. J. Pet. 2012, 21, 31–36. [Google Scholar] [CrossRef]
- Wu, M.S.; Lin, C.J.; Ho, C.L. Multilayered architecture of graphene nanosheets and MnO2 nanowires as an electrode material for high-performance supercapacitors. Electrochim. Acta 2012, 81, 44–48. [Google Scholar] [CrossRef]
- Liang, K.; Tang, X.; Hu, W. High-performance three-dimensional nanoporous NiO film as a supercapacitor electrode. J. Mater. Chem. 2012, 22, 11062–11067. [Google Scholar] [CrossRef]
- Sarma, B.; Jurovitzki, A.L.; Smith, Y.R.; Ray, R.S.; Misra, M. Influence of annealing temperature on the morphology and the supercapacitance behavior of iron oxide nanotube (Fe-NT). J. Power Sources 2014, 272, 766–775. [Google Scholar] [CrossRef]
- Fouda, A.N.; Abu-Assy, M.K.; Yousf, N. Structural and capacitive characterizations of high temperature nitrogen annealed graphene oxide. IOSR J. Appl. Phys. 2014, 6, 33–37. [Google Scholar] [CrossRef]
- Firat, Y.E.; Peksoz, A. Efficiency enhancement of electrochromic performance in NiO thin film via Cu doping for energy-saving potential. Electrochim. Acta 2019, 295, 645–654. [Google Scholar] [CrossRef]
- Masood, A.; Afzal, N.; Ahmed, A.A.; Qahtan, T.F.; Rafique, M.; Ahmad, R.; Imran, M. Structural, surface and optical investigations of Cu+ implanted NiO film prepared by reactive sputtering. Ceram. Int. 2023, 49, 4435–4448. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Shi, L.; Xu, J.; Zhang, L.; Huang, L.; Li, H.; Zhang, J. Porous Ni–Mn oxide nanosheets in situ formed on nickel foam as 3D hierarchical monolith de-NO x catalysts. Nanoscale 2014, 6, 7346–7353. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Qian, Z.; Zhang, X.; Wang, J.; Li, Z.; Yan, H.; Gao, Z.; Zhao, F.; Liu, L. Two steps in situ structure fabrication of Ni–Al layered double hydroxide on Ni foam and its electrochemical performance for supercapacitors. J. Power Sources 2014, 246, 747–753. [Google Scholar] [CrossRef]
- Kang, M.; Gewirth, A.A. Voltammetric and force spectroscopic examination of oxide formation on Cu (111) in basic solution. J. Phys. Chem. B 2002, 106, 12211–12220. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Cyclic voltammetric behavior of copper powder immobilized on paraffin impregnated graphite electrode in dilute alkali solution. Int. J. Electrochem. Sci. 2008, 3, 1277–1287. [Google Scholar] [CrossRef]
- He, J.B.; Lu, D.Y.; Jin, G.P. Potential dependence of cuprous/cupric duplex film growth on copper electrode in alkaline media. Appl. Surf. Sci. 2006, 253, 689–697. [Google Scholar] [CrossRef]
- Burke, L.D.; Ahern, M.J.G.; Ryan, T.G. An investigation of the anodic behavior of copper and its anodically produced oxides in aqueous solutions of high pH. J. Electrochem. Soc. 1990, 137, 553. [Google Scholar] [CrossRef]
- Brisard, G.M.; Rudnicki, J.D.; McLarnon, F.; Cairns, E.J. Application of probe beam deflection to study the electrooxidation of copper in alkaline media. Electrochim. Acta 1995, 40, 859–865. [Google Scholar] [CrossRef]
- Ramkumar, R.; Dhakal, G.; Shim, J.J.; Kim, W.K. NiO/Ni nanowafer aerogel electrodes for high performance supercapacitors. Nanomaterials 2022, 12, 3813. [Google Scholar] [CrossRef]
- Patil, P.A.; Khalate, S.A.; Patil, U.M.; Kale, R.D.; Kulkarni, S.B. Cavity structured S-NiO with improved energy density for aqueous asymmetric hybrid supercapacitors by CDA mechanism. Mater. Adv. 2023, 4, 4607–4619. [Google Scholar] [CrossRef]
- Hanbo, W.; Dongyu, P.; Sheng, W.; Ziming, W.; Zhitian, F.; Yumei, T.; Kechang, L.; Haiyan, L. One-step potential-cycling method to fabricate NiO nanospheres for high performance supercapacitor application. J. Energy Storage 2024, 86, 111134. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Jun, Y.-K.; Kim, N.-H. Magnetron-Sputtered and Rapid-Thermally Annealed NiO:Cu Thin Films on 3D Porous Substrates for Supercapacitor Electrodes. Energies 2025, 18, 2704. https://doi.org/10.3390/en18112704
Oh S, Jun Y-K, Kim N-H. Magnetron-Sputtered and Rapid-Thermally Annealed NiO:Cu Thin Films on 3D Porous Substrates for Supercapacitor Electrodes. Energies. 2025; 18(11):2704. https://doi.org/10.3390/en18112704
Chicago/Turabian StyleOh, Seongha, Young-Kil Jun, and Nam-Hoon Kim. 2025. "Magnetron-Sputtered and Rapid-Thermally Annealed NiO:Cu Thin Films on 3D Porous Substrates for Supercapacitor Electrodes" Energies 18, no. 11: 2704. https://doi.org/10.3390/en18112704
APA StyleOh, S., Jun, Y.-K., & Kim, N.-H. (2025). Magnetron-Sputtered and Rapid-Thermally Annealed NiO:Cu Thin Films on 3D Porous Substrates for Supercapacitor Electrodes. Energies, 18(11), 2704. https://doi.org/10.3390/en18112704






