Thermophysical Enhancement of Graphene Oxide-Enhanced Quaternary Nitrate for Concentrated Solar Power Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Synthesis of GO Sheets
2.2.2. QN Synthesis
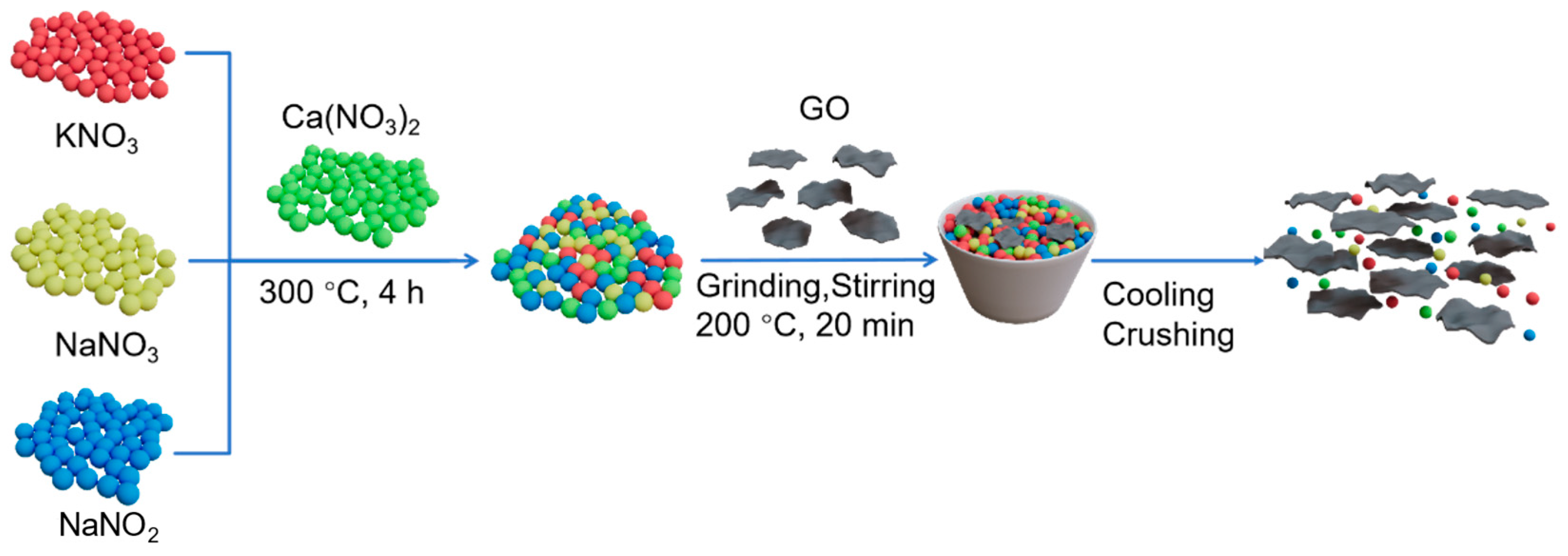
2.2.3. Synthesis of GO/QN CPCM
2.3. Material Characterization
2.4. Material Preparation
3. Results and Discussion
3.1. Characteristics of the Composite Material
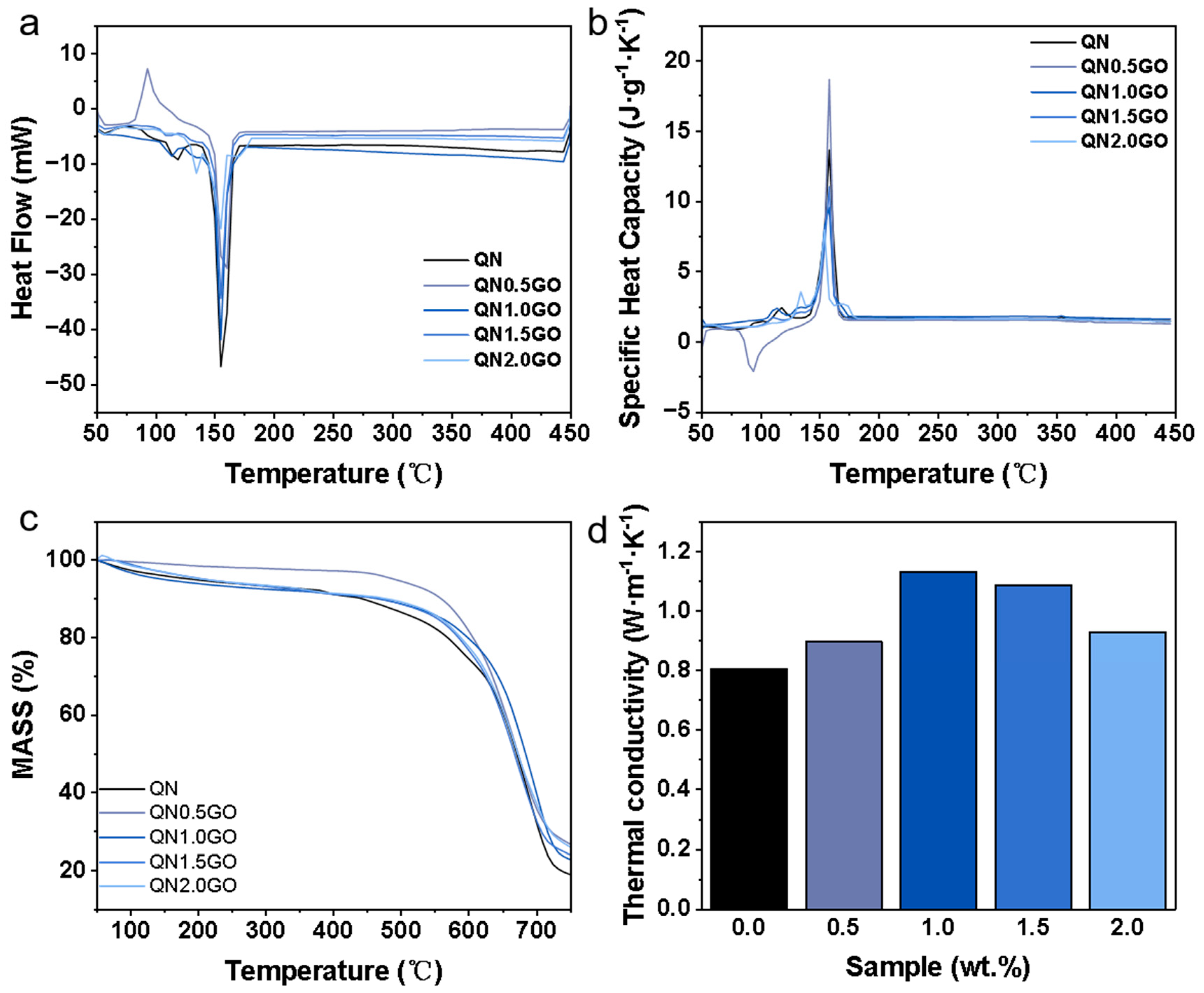
3.2. Thermophysical Properties of the QN/GO Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| BET | Brunauer–Emmett–Teller |
| CPCM | Composite-phase change material |
| CSP | Concentrated solar power |
| DSC | Differential scanning calorimetry |
| EDX | Energy-dispersive X-ray spectroscopy |
| EG | Expanded graphite |
| GO | Graphene oxide |
| GP | Graphite paper |
| PCM | Phase change material |
| QA | Quaternary nitrate |
| SEM | Scanning electron microscopy |
| SHM | Specific heat capacity |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| XPS | X-ray photoelectron spectroscopy |
References
- Zhang, M.H.; Qi, J.L.; Liu, Y.Q.; Lan, S.; Luo, Z.X.; Pan, H.; Lin, Y.H. High energy storage capability of perovskite relaxor ferroelectrics via hierarchical optimization. Rare Met. 2022, 41, 730–744. [Google Scholar] [CrossRef]
- Qin, Y.C.; Wang, F.Q.; Wang, X.M.; Wang, M.W.; Zhang, W.L.; An, W.K.; Wang, X.P.; Ren, Y.L.; Zheng, X.; Lv, D.C.; et al. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 2021, 40, 2354–2368. [Google Scholar] [CrossRef]
- Ke, G.L.; Jia, B.; He, H.C.; Zhou, Y.; Zhou, M. State-of-the-art advancements of transition metal oxides as photoelectrode materials for solar water splitting. Rare Met. 2022, 41, 2370–2386. [Google Scholar] [CrossRef]
- Han, D.; Lougou, B.G.; Shuai, Y.; Wang, W.; Jiang, B.; Shagdar, E. Study of thermophysical properties of chloride salts doped with CuO nanoparticles for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 234, 111432. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Togun, H.; Abed, A.M.; Mohammed, H.I.; Mahdi, J.M.; Ibrahem, R.K.; Yaïci, W.; Talebizadehsardari, P.; Keshmiri, A. Comprehensive analysis of melting enhancement by circular Y-shaped fins in a vertical shell-and-tube heat storage system. Eng. Appl. Comput. Fluid Mech. 2023, 17, 2227682. [Google Scholar] [CrossRef]
- Han, D.M.; Shuai, Y.; Lougou, B.G.; Geng, B.X.; He, X.B.; Yan, T.T.; Song, J.M. Corrosion evaluation and resistance study of alloys in chloride salts for concentrating solar power plants. Rare Met. 2024, 43, 1222–1233. [Google Scholar] [CrossRef]
- Gao, Q.; Lu, Y.; Yu, Q.; Wu, Y.; Zhang, C.; Zhi, R. High-temperature corrosion behavior of austenitic stainless steel in quaternary nitrate molten salt nanofluids for concentrated solar power. Sol. Energy Mater. Sol. Cells 2022, 245, 111851. [Google Scholar] [CrossRef]
- Sutter, F.; Oskay, C.; Galetz, M.C.; Diamantino, T.; Pedrosa, F.; Figueira, I.; Glumm, S.; Bonk, A.; Agüero, A.; Rodríguez, S.; et al. Dynamic corrosion testing of metals in solar salt for concentrated solar power. Sol. Energy Mater. Sol. Cells 2021, 232, 111331. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Mahdi, J.M.; Dulaimi, A.; Chatroudi, I.S.; Tiji, M.E.; Ibrahem, R.K.; Yvaz, A.; Talebizadehsardari, P. On the application of novel arc-shaped fins in a shell-and-tube type of latent heat storage for energy charge enhancement. J. Energy Storage 2023, 73, 108697. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cabeza, L.F. Anodic protection assessment using alumina-forming alloys in chloride molten salt for CSP plants. Coatings 2020, 10, 138. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; Anagnostopoulos, A.; Carbó-Argibay, E.; Bondarchuk, O.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Navarro, M.E.; Ding, Y.; et al. Nanoparticles as a high-temperature anticorrosion additive to molten nitrate salts for concentrated solar power. Sol. Energy Mater. Sol. Cells 2019, 203, 110171. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Ullah, Z.; Alturki, M.; Mirza, C.R.; Eldin, S.M. Effect of Joule heating and MHD on periodical analysis of current density and amplitude of heat transfer of electrically conducting fluid along thermally magnetized cylinder. Ain Shams Eng. J. 2024, 15, 102374. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Sheremet, M.; Hussin, A.M.; Mehryan, S.; Ghalambaz, M. The effect of hot wall configuration on melting flow of nano-enhanced phase change material inside a tilted square capsule. J. Energy Storage 2023, 69, 107921. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.; Palacios, A.; Navarro, M.H.; Fereres, S.; Ding, Y. Effect of SiO2 nanoparticle addition on the wetting and rheological properties of solar salt. Sol. Energy Mater. Sol. Cells 2020, 210, 110483. [Google Scholar] [CrossRef]
- Han, Z.; Ram, M.K.; Kamal, R.; Alamro, T.; Goswami, D.Y.; Jotshi, C. Characterization of molten salt doped with different size nanoparticles of Al2O3. Int. J. Energy Res. 2019, 43, 3732–3745. [Google Scholar] [CrossRef]
- Li, Q.; Wei, W.; Li, Y.; Li, C.; Ge, R.; Du, Y.; Zhang, X.; Wu, Y. Development and investigation of form-stable quaternary nitrate salt based composite phase change material with extremely low melting temperature and large temperature range for low-mid thermal energy storage. Energy Rep. 2022, 8, 1528–1537. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Zaki, A.; Grosu, Y.; González-Fernández, L.; Anagnostopoulos, A.; Navarro, M.E.; Ding, Y.; Igartua, J.M.; Faik, A. Effect of silica nanoparticle size on the stability and thermophysical properties of molten salts based nanofluids for thermal energy storage applications at concentrated solar power plants. J. Energy Storage 2022, 51, 104276. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, J.; Wang, H.; Zhao, Y. Improving thermal energy storage and transfer performance in solar energy storage: Nanocomposite synthesized by dispersing nano boron nitride in solar salt. Sol. Energy Mater. Sol. Cells 2021, 232, 111378. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, H.; Pervaiz, S.; Wen, D. Molten salt/metal foam/graphene nanoparticle phase change composites for thermal energy storage. ACS Appl. Nano Mater. 2020, 3, 5240–5251. [Google Scholar] [CrossRef]
- Madathil, P.K.; Balagi, N.; Saha, P.; Bharali, J.; Rao, P.V.C.; Choudary, N.V.; Ramesh, K. Preparation and characterization of molten salt based nanothermic fluids with enhanced thermal properties for solar thermal applications. Appl. Therm. Eng. 2016, 109, 901–905. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.H.; Wang, S.; Ma, K.Q.; Zhu, Q.Z. Preparation and thermal properties of a novel ternary molten salt/expanded graphite thermal storage material. J. Energy Storage 2023, 74, 109273. [Google Scholar] [CrossRef]
- Liu, J.; Xie, M.; Ling, Z.; Fang, X.; Zhang, Z. Novel MgCl2-KCl/expanded graphite/graphite paper composite phase change blocks with high thermal conductivity and large latent heat. Sol. Energy 2018, 159, 226–233. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Wu, H.; Sun, S.; Chen, L.; Wu, K.; Lin, X.; Qin, Y. In-situ construction of dense thermal conduction networks endow the polymeric composites with advanced thermal management capability and superior dielectric properties. Chem. Eng. J. 2022, 449, 137753. [Google Scholar] [CrossRef]
- Sobczak, J.; Vallejo, J.P.; Traciak, J.; Hamze, S.; Fal, J.; Estellé, P.; Lugo, L.; Żyła, G. Thermophysical profile of ethylene glycol based nanofluids containing two types of carbon black nanoparticles with different specific surface areas. J. Mol. Liq. 2021, 326, 115255. [Google Scholar] [CrossRef]
- Abeykoon, A.M.K.L.; De Silva, R.C.L.; Kottegoda, I.R.M.; Gofer, Y.; Shmerling, B. Spectroscopic analysis of mass-scale prepared GO and rGO from vein graphite through compositional improvement. Sri Lankan J. Phys. 2024, 25, 13–34. [Google Scholar] [CrossRef]
- Bheel, N.; Mohammed, B.S. Modelling and optimization of long-term modulus of elasticity and Poisson’s ratio of graphene oxide based engineered cementitious composites by using response surface methodology. Diam. Relat. Mater. 2024, 143, 110949. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Wei, J.; Zhang, T. Research progress on properties of cement-based composites incorporating graphene oxide. Rev. Adv. Mater. Sci. 2023, 62, 20220329. [Google Scholar] [CrossRef]
- Hamdy, E.; Saad, L.; Abulfotuh, F.; Soliman, M.; Ebrahim, S. Enhancement of Molten Nitrate Thermal Properties by Reduced Graphene Oxide and Graphene Quantum Dots. ACS Omega 2020, 5, 21345–21354. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Khalid, M.; Walvekar, R.; Vaka, M.; Mubarak, N.M.; Chamkha, A.; Khalid, M. Investigating the effect of graphene on eutectic salt properties for thermal energy storage. Mater. Res. Bull. 2019, 119, 110568. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, H.; Liu, H.; Hou, H.; Guo, Y.; Chang, W. Thermophysical Enhancement of Graphene Oxide-Enhanced Quaternary Nitrate for Concentrated Solar Power Applications. Energies 2025, 18, 2607. https://doi.org/10.3390/en18102607
Wang Y, Zhang H, Liu H, Hou H, Guo Y, Chang W. Thermophysical Enhancement of Graphene Oxide-Enhanced Quaternary Nitrate for Concentrated Solar Power Applications. Energies. 2025; 18(10):2607. https://doi.org/10.3390/en18102607
Chicago/Turabian StyleWang, Yingchun, Haonan Zhang, Hantao Liu, Hong Hou, Yonghong Guo, and Wenrui Chang. 2025. "Thermophysical Enhancement of Graphene Oxide-Enhanced Quaternary Nitrate for Concentrated Solar Power Applications" Energies 18, no. 10: 2607. https://doi.org/10.3390/en18102607
APA StyleWang, Y., Zhang, H., Liu, H., Hou, H., Guo, Y., & Chang, W. (2025). Thermophysical Enhancement of Graphene Oxide-Enhanced Quaternary Nitrate for Concentrated Solar Power Applications. Energies, 18(10), 2607. https://doi.org/10.3390/en18102607





