Nickel Selenide Electrodes with Tuned Deposition Cycles for High-Efficiency Asymmetric Supercapacitors
Abstract
1. Introduction
2. Methods and Materials
2.1. Material
2.2. Synthesis of Nickel Selenide
2.3. Material Characterization
2.4. Electrochemical Studies
3. Results and Discussion
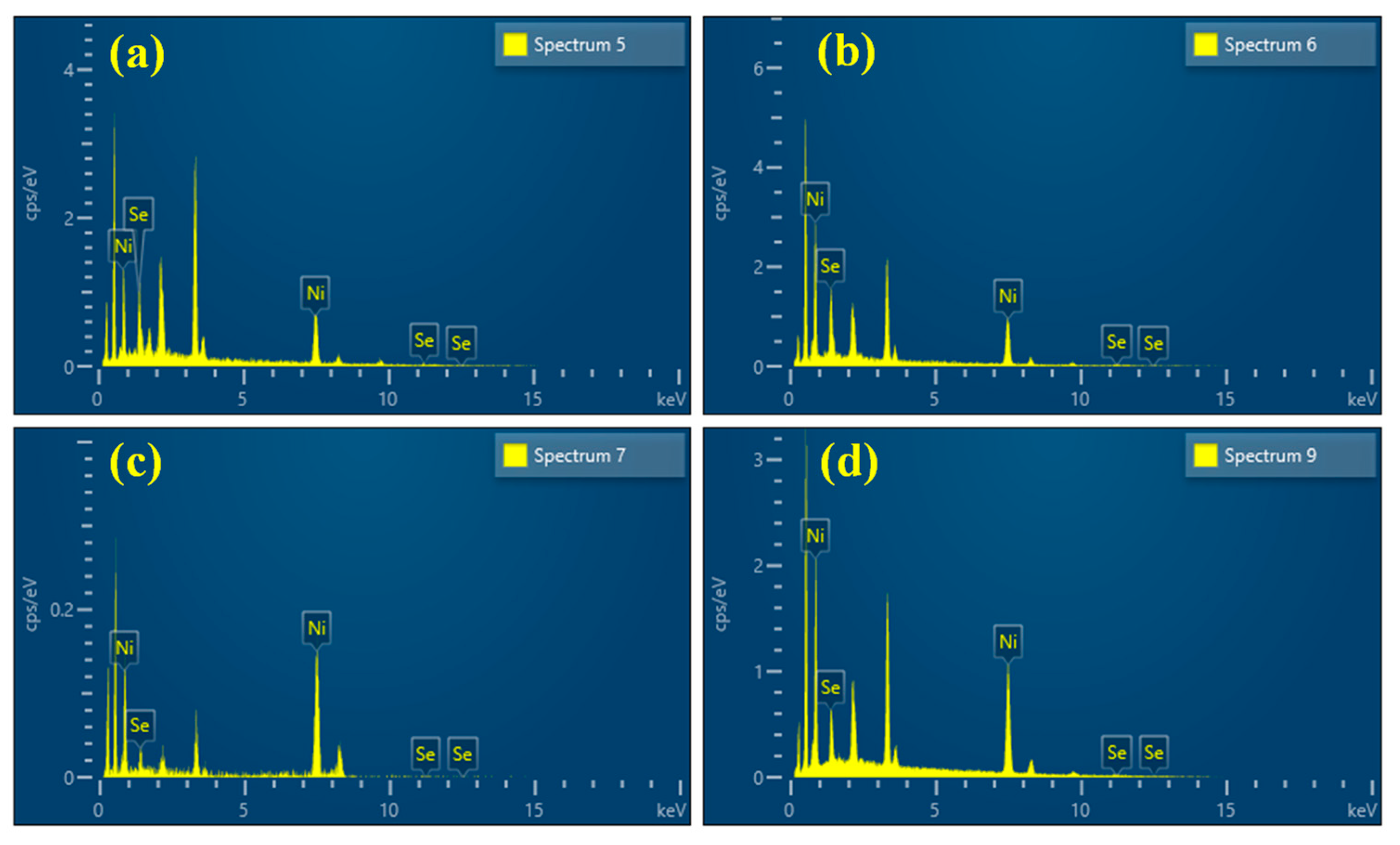
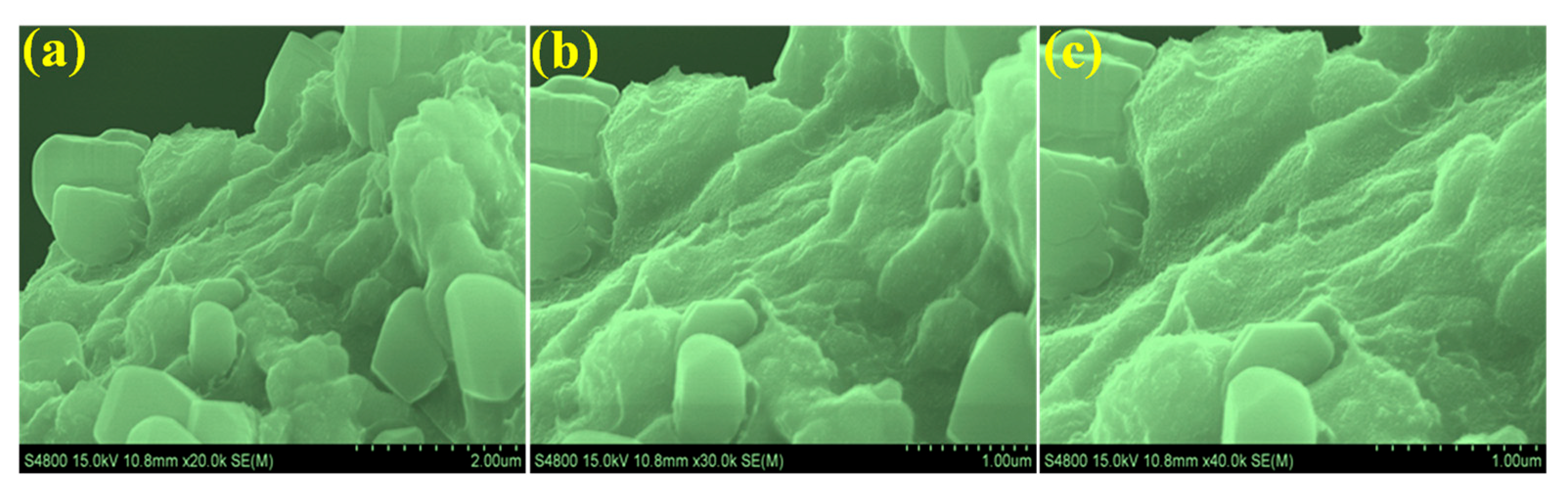
3.1. The Characterizations of Morphology, Composition, and Structure
3.2. Electrochemical Study
4. Asymmetric Supercapacitor (ASC) Device
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patil, A.M.; Jadhav, A.A.; Chodankar, N.R.; Avatare, A.T.; Hong, J.; Dhas, S.D.; Patil, U.M.; Jun, S.C. Recent progress of MXene synthesis, properties, microelectrode fabrication techniques for microsupercapacitors and microbatteries energy storage devices and integration: A comprehensive review. Coord. Chem. Rev. 2024, 517, 216020. [Google Scholar] [CrossRef]
- Yadav, M.S.; Kour, S.; Sharma, A.L. A critical review on various bimetallic chalcogenides (Sulfides, Selenides, and Tellurides) as Efficient Electrode Materials for advanced supercapacitors. Mater. Today Commun. 2024, 41, 110520. [Google Scholar] [CrossRef]
- Patil, A.M.; Moon, S.; Seo, Y.; Roy, S.B.; Jadhav, A.A.; Dubal, D.P.; Kang, K.; Jun, S.C. Reconfiguring the Electronic Structure of Heteroatom Doped Carbon Supported Bimetallic Oxide@Metal Sulfide Core–Shell Heterostructure via In Situ Nb Incorporation toward Extrinsic Pseudocapacitor. Small 2023, 19, 2205491. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Satale, V.V.; Amate, R.U.; Morankar, P.J.; Yewale, M.A.; Shin, J.C. A critical review of recent advancements in high-temperature supercapacitors: Thermal kinetics, interfacial dynamics, employed strategies, and prospective trajectories. Energy Storage Mater. 2024, 66, 103217. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kumar, V.; Kadam, R.A.; Kharade, R.B.; Teli, A.M.; Beknalkar, S.A.; Dhas, S.D.; Nakate, U.T.; Shin, D.K. Wrapped nanochain microstructures of Ni3V2O8 nanoparticles for supercapacitor applications using the hydrothermal method. J. Energy Storage 2023, 73, 109005. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Liu, Z.; Song, S.; Liu, S.; Liu, F.; Jin, X.; Ma, X.; Zhang, Y.; Zhang, K.; et al. Small and low-crystallinity NiO nanoparticles embedded in hollow carbon materials for high performance asymmetric supercapacitors. J. Energy Storage 2025, 113, 115738. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Huang, C.; Wang, Y.; Liu, C. One-step carbonisation synthesis of thorn-like NiO self-assembled on resin carbon to improve the stability of supercapacitor. J. Power Sources 2025, 637, 236595. [Google Scholar] [CrossRef]
- Gupta, J.; Ahmed, A.S.; Pushpendra; Azam, A. Comparative study of NiO based core-shell nanocomposites to high performance supercapacitor electrode materials. Phys. E Low-Dimens. Syst. Nanostruct. 2025, 165, 116121. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Cui, L.; Zhang, M.; Huo, X.; Guo, M. Ammonia controlled synthesis of NiO nanosheet arrays for high-performance electrochromic-supercapacitors. J. Alloys Compd. 2024, 1008, 176696. [Google Scholar] [CrossRef]
- Yaldiz, H.; Kocal, A.C.; Gultepe, O.; Gür, E.; Atay, F. Effect of Hydrothermal Reaction Time on Supercapacitor Properties of Nanostructured NiS Electrodes. Electrochim. Acta 2025, 526, 146197. [Google Scholar] [CrossRef]
- Tan, Y.; Long, Y.; Liu, Z.; Li, L.; Jin, H.; Wang, M. 3D layer shape electrode of NiS in-situ growth on shaddock peel derived carbon for high-performance supercapacitors. J. Electroanal. Chem. 2025, 980, 118995. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, X.; Wang, Y.; Luo, C.; Wang, G.; Xu, C.; Chen, H. Simple synthesis of high-performance α-NiS particles as battery-type cathode material for advanced hybrid supercapacitor application. J. Energy Storage 2025, 116, 116091. [Google Scholar] [CrossRef]
- Roy, K.; Marilingaiah, N.R.; Kumar, A.; Palya Narayanaswamy, M.; Yelamaggad, C.V.; Doddakunche Shivaramu, P.; Vidyashankar, S.; Singh, S.K.; Rangappa, D. Phase-controlled construction of copper-leaf-like 2D NiS for enhanced supercapacitor performance: A supercritical fluid approach. Mater. Today Commun. 2025, 42, 111202. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Liu, H.; Umar, A.; Wu, X. High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide. Chin. Chem. Lett. 2019, 30, 1105–1110. [Google Scholar] [CrossRef]
- Liu, G.; Guo, T.; Kang, J.; Wang, Z.; Guo, L. Robust self-standing multiscale engineered Ni(OH)2 film for flexible high-capacity supercapacitor. J. Power Sources 2025, 641, 236892. [Google Scholar] [CrossRef]
- Patil, S.A.; Jagdale, P.B.; Sfeir, A.; Pathak, M.; Royer, S.; Samal, A.K.; Rout, C.S.; Saxena, M. Flexible micro-supercapacitors with high-energy-density Ni(OH)2 nanosheet: A scalable approach for enhanced volumetric performance. J. Energy Storage 2025, 121, 116526. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, Q.; Gao, L.; Wang, L.; Liu, M.; Xu, S.; Zhang, G. Improving energy density of battery-type electrode material by growing β-Ni(OH)2 in situ on biochar for hybrid supercapacitors. J. Energy Storage 2024, 100, 113530. [Google Scholar] [CrossRef]
- Shi, C.; Yan, L.; Wu, Z.; Lu, M.; Li, Z. Ultrathin Ni(OH)2 nanosheets: Microemulsion assisted hydrothermal synthesis and application in advanced hybrid supercapacitors. Surf. Interfaces 2024, 55, 105472. [Google Scholar] [CrossRef]
- Jagtap, C.; Kadam, V.; Kamble, B.; Lokhande, P.E.; Pakdel, A.; Kumar, D.; Udayabhaskar, R.; Vedpathak, A.; Chaure, N.B.; Pathan, H.M. Synergistic growth of cobalt hydroxide on reduced graphene oxide/nickel foam for supercapacitor application. J. Energy Storage 2024, 83, 110666. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Gaikwad, M.A.; Patil, K.D.; Jang, S.; Park, S.W.; Kim, J.H. Nanoarchitectonics of self-grown copper selenide on copper for solid-state asymmetric supercapacitor. J. Energy Storage 2023, 68, 107675. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Construction of complex copper-cobalt selenide hollow structures as an attractive battery-type electrode material for hybrid supercapacitors. Chem. Eng. J. 2020, 402, 126241. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; Saleki, F.; Mohammadi, A.; Hafizi, A.; Rahimpour, M.R. Construction of hierarchical nanoporous bimetallic copper-cobalt selenide hollow spheres for hybrid supercapacitor. J. Electroanal. Chem. 2020, 871, 114295. [Google Scholar] [CrossRef]
- Xie, X.; Xu, Y.; Yuan, F.; Wu, Q.; Liu, P.; Liu, J.; Wang, D.; Lv, T.; Zhang, Q. One-step microwave synthesis of in situ grown NiSe microdendrite arrays on Ni foam for high-performance supercapacitors. J. Power Sources 2024, 620, 235256. [Google Scholar] [CrossRef]
- Shankar, E.G.; Das, A.K.; Yu, J.S. Binder-free pinecone-like NiSe/MnSe nanostructure arrays via electrochemically controlled one-step synthesis towards high-performance semi-solid-state supercapacitor. J. Energy Storage 2023, 68, 107746. [Google Scholar] [CrossRef]
- Wan, L.; Ye, G.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Synthesis of nickel selenide/manganese selenide@cobalt sulfide heterostructure with superior stability for supercapacitors. Appl. Surf. Sci. 2024, 670, 160638. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, Y.; Sarwar, S.; Luo, J.; Zhang, X. Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors. J. Power Sources 2020, 452, 227793. [Google Scholar] [CrossRef]
- Shahidani, H.S.; Seifi, M.; Bagher Askari, M. Design of NiSe2@MoSe2 nanocomposite anchored on multi-walled carbon nanotubes as advanced supercapacitor applications. Inorg. Chem. Commun. 2024, 170, 113218. [Google Scholar] [CrossRef]
- Khan, A.U.; Liu, Y.; Tahir, K.; Almarhoon, Z.M.; Chen, Q.; Zaki, M.E.A.; Badi, N.; Al-Saeedi, S.I.; Mao, B. A facile synthesis of novel binder-free NiSe-SnSe electrode: With enhanced electrochemical performance and superior life span. J. Energy Storage 2024, 91, 112068. [Google Scholar] [CrossRef]
- Shah, M.S.U.; Zuo, X.; Shah, M.Z.U.; Hou, H.; Ahmad, S.A.; Haq, T.U.; Aftab, J.; Sajjad, M.; Shah, A. Supercapacitive performance of a novel binary nanocomposite of metal chalcogenides for advanced hybrid supercapacitor. J. Energy Storage 2023, 65, 107268. [Google Scholar] [CrossRef]
- Xie, Y.; Nuñez, C.G.; Wang, H.; Gao, X.; Zhang, H.; Jiang, F.; Jia, K.; Li, Q.; Bai, H.; Yao, F.; et al. Hollow transition metal chalcogenides derived from vanadium-based metal organic framework for hybrid supercapacitors with excellent energy-density and stability. J. Colloid Interface Sci. 2025, 680, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, S.M.; Metwally, W.; Mohamed, S.G.; Abdel-Karim, R.; El-Raghy, S.M.; Ghayad, I.M. Electrodeposition of nickel selenides thin films for high-performance hybrid supercapacitor. J. Energy Storage 2024, 83, 110744. [Google Scholar] [CrossRef]
- Zhu, J.; Ni, Y. Phase-controlled synthesis and the phase-dependent HER and OER performances of nickel selenide nanosheets prepared by an electrochemical deposition route. CrystEngComm 2018, 20, 3344–3352. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Y.; Zhang, X.; Zhang, Q.; Wei, Z.; Wang, D. One-step synthesis of 2D vertically-aligned hybrid CuSe@NiSe nanosheets for high performance flexible supercapacitors. J. Alloys Compd. 2022, 892, 162159. [Google Scholar] [CrossRef]
- Bin Farukh, S.F.; Javed, Y.; Qadir, M.B.; Jamil, Y.; Sarfraz, R.A. Optimization of zinc doping in nickel selenide nanorods for efficient hybrid energy storage devices. Inorg. Chem. Commun. 2025, 174, 114108. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Xu, Y.; Cai, W.; Zhu, M.; Wang, H. Facile synthesis of core-shell NiSe@α-Ni(OH)2 as battery-type electrode for high-performance hybrid supercapacitor. J. Alloys Compd. 2021, 876, 160164. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Xu, Q.; Shi, Y.; Tian, Z.; Wang, R.; Zhang, G.; Chen, J.; Wang, Z.; Zheng, W. Controllable synthesis of NiSe/MoSe2/MoO2 3D hierarchical hollow microspheres with enhanced performance for asymmetric supercapacitors. Chem. Eng. J. 2020, 387, 124121. [Google Scholar] [CrossRef]
- Tao, K.; Gong, Y.; Lin, J. Epitaxial grown self-supporting NiSe/Ni3S2/Ni12P5 vertical nanofiber arrays on Ni foam for high performance supercapacitor: Matched exposed facets and re-distribution of electron density. Nano Energy 2019, 55, 65–81. [Google Scholar] [CrossRef]
- Aftabuzzaman, M.; Kim, H.K. Solid-state synthesis of nickel selenide for high-performance supercapacitors. Mater. Chem. Phys. 2025, 329, 130052. [Google Scholar] [CrossRef]
- Su, H.; Niu, C.; Zhang, R.; Huang, M.; Li, Z. Construction of a Ni2P/NiSe2/MoSe2 hybrid for advanced supercapacitors. J. Alloys Compd. 2025, 1010, 178074. [Google Scholar] [CrossRef]
- Manikandan, R.; Justin Raj, C.; Nagaraju, G.; Velayutham, R.; Moulton, S.E.; Puigdollers, J.; Chul Kim, B. Selenium enriched hybrid metal chalcogenides with enhanced redox kinetics for high-energy density supercapacitors. Chem. Eng. J. 2021, 414, 128924. [Google Scholar] [CrossRef]
- Patil, A.M.; Moon, S.; Jadhav, A.A.; Hong, J.; Kang, K.; Jun, S.C. Modifying Electronic Structure of Cation-Exchanged Bimetallic Sulfide/Metal Oxide Heterostructure through In Situ Inclusion of Silver (Ag) Nanoparticles for Extrinsic Pseudocapacitor. Adv. Funct. Mater. 2023, 33, 2305264. [Google Scholar] [CrossRef]
- Yewale, M.A.; Desarada, S.V.; Teli, A.M.; Chavan, K.B.; Morankar, P.J.; Shin, D.K.; Choi, S.T. Synthesis of the ZnCo2O4 Positive Electrode Using a Urea-Assisted Hydrothermal Approach for Supercapacitor Applications. Energy Fuels 2025, 39, 2281–2293. [Google Scholar] [CrossRef]
- Patil, A.M.; Wang, J.; Li, S.; Hao, X.; Du, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Bilateral growth of monoclinic WO3 and 2D Ti3C2Tx on 3D free-standing hollow graphene foam for all-solid-state supercapacitor. Chem. Eng. J. 2021, 421, 127883. [Google Scholar] [CrossRef]
- Qu, J.; Bai, Y.; Li, X.; Song, K.; Zhang, S.; Wang, X.; Wang, X.; Dai, S. Rational design of NiSe2@rGO nanocomposites for advanced hybrid supercapacitors. J. Mater. Res. Technol. 2021, 15, 6155–6161. [Google Scholar] [CrossRef]
- Shah, M.S.U.; Zuo, X.; Shah, A.; Al-Saeedi, S.I.; Shah, M.Z.U.; Alabbad, E.A.; Hou, H.; Ahmad, S.A.; Arif, M.; Sajjad, M.; et al. CoSe nanoparticles supported NiSe2 nanoflowers cathode with improved energy storage performance for advanced hybrid supercapacitors. J. Energy Storage 2023, 65, 107267. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, D.; Gao, S.; Yang, B.; Meng, X.; He, Y.; Wang, B.; Han, Z.; Wang, K. Porous corallite-like NiSe2/CNTs nanocomposite fabricated by a convenient one-step in-suit solid-phase synthesis method with high performance in both supercapacitor and sodium-ion battery. J. Alloys Compd. 2023, 958, 170364. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Satale, V.V.; Yewale, M.A.; Amate, R.U.; Morankar, P.J.; Wu, Y.H.; Kim, H.H.; Shin, J.C. Double-layered nano-composite of copper-manganese oxide/rGO-palladium for asymmetric supercapacitors. J. Alloys Compd. 2025, 1014, 178633. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Yewale, M.A.; Jeon, C.W. Nanospheres of TiO2/MoS2 composites synthesized via two-step chemical route for high-performance supercapacitor electrodes. Ceram. Int. 2025, 51, 15613–15626. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Amte, R.U.; Morankar, P.J.; Yewale, M.A.; Burungale, V.V.; Jeon, C.W.; Efstathiadis, H.; Shin, J.C. Investigating into the intricacies of charge storage kinetics in NbMn-oxide composite electrodes for asymmetric supercapacitor and HER applications. J. Alloys Compd. 2023, 965, 171305. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.E. Li+ ion insertion in TiO2 (anatase). 1. Chronoamperometry on CVD films and nanoporous films. J. Phys. Chem. B 1997, 101, 7710–7716. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2000; Volume 30, ISBN 0471043729. [Google Scholar]
- Jang, G.S.; Ameen, S.; Akhtar, M.S.; Kim, E.; Shin, H.S. Electrochemical Investigations of Hydrothermally Synthesized Porous Cobalt Oxide (Co3O4) Nanorods: Supercapacitor Application. ChemistrySelect 2017, 2, 8941–8949. [Google Scholar] [CrossRef]







| Sr. No. | Method | Electrode Material | Capacitance | Ref. |
|---|---|---|---|---|
| 1. | Electrodeposition | NiSe | 507 F/g, 8 A/g | Present work |
| 2. | Hydrothermal | NiSe2@rGO | 467 mAh/g, 1 A/g | [47] |
| 3. | Hydrothermal | NiSe2-CoSe | 374 F/g, 8 A/g | [48] |
| 4. | Hydrothermal | NiSe-SnSe | 217 F/g, 5 A/g | [31] |
| 5. | In-suit solid-phase synthesis (ISPS) | NiSe2/CNTs | 172.70 mAh/g, 1 A/g | [49] |
| 6. | Hydrothermal | NiSe2-MoSe2 | 121 F/g, 7 A/g | [30] |
| Parameter and Electrode code | 2CY | 3CY | 5CY | 5CY |
| b-value | 0.49 | 0.5 | 0.47 | 0.49 |
| Transfer coefficient (α) | 0.17 | 0.19 | 0.17 | 0.18 |
| Standard rate constant (k0) (×10−4) cm/S | 3.3 | 3.7 | 3.5 | 3.7 |
| Diffusion coefficient (D) (×10−6) cm2/S | 1.01 | 1.70 | 1.56 | 1.56 |
| Rs(Ω) | 1.86 | 1.59 | 1.59 | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yewale, M.A.; Shin, D.-k. Nickel Selenide Electrodes with Tuned Deposition Cycles for High-Efficiency Asymmetric Supercapacitors. Energies 2025, 18, 2606. https://doi.org/10.3390/en18102606
Yewale MA, Shin D-k. Nickel Selenide Electrodes with Tuned Deposition Cycles for High-Efficiency Asymmetric Supercapacitors. Energies. 2025; 18(10):2606. https://doi.org/10.3390/en18102606
Chicago/Turabian StyleYewale, Manesh Ashok, and Dong-kil Shin. 2025. "Nickel Selenide Electrodes with Tuned Deposition Cycles for High-Efficiency Asymmetric Supercapacitors" Energies 18, no. 10: 2606. https://doi.org/10.3390/en18102606
APA StyleYewale, M. A., & Shin, D.-k. (2025). Nickel Selenide Electrodes with Tuned Deposition Cycles for High-Efficiency Asymmetric Supercapacitors. Energies, 18(10), 2606. https://doi.org/10.3390/en18102606







