Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis
Abstract
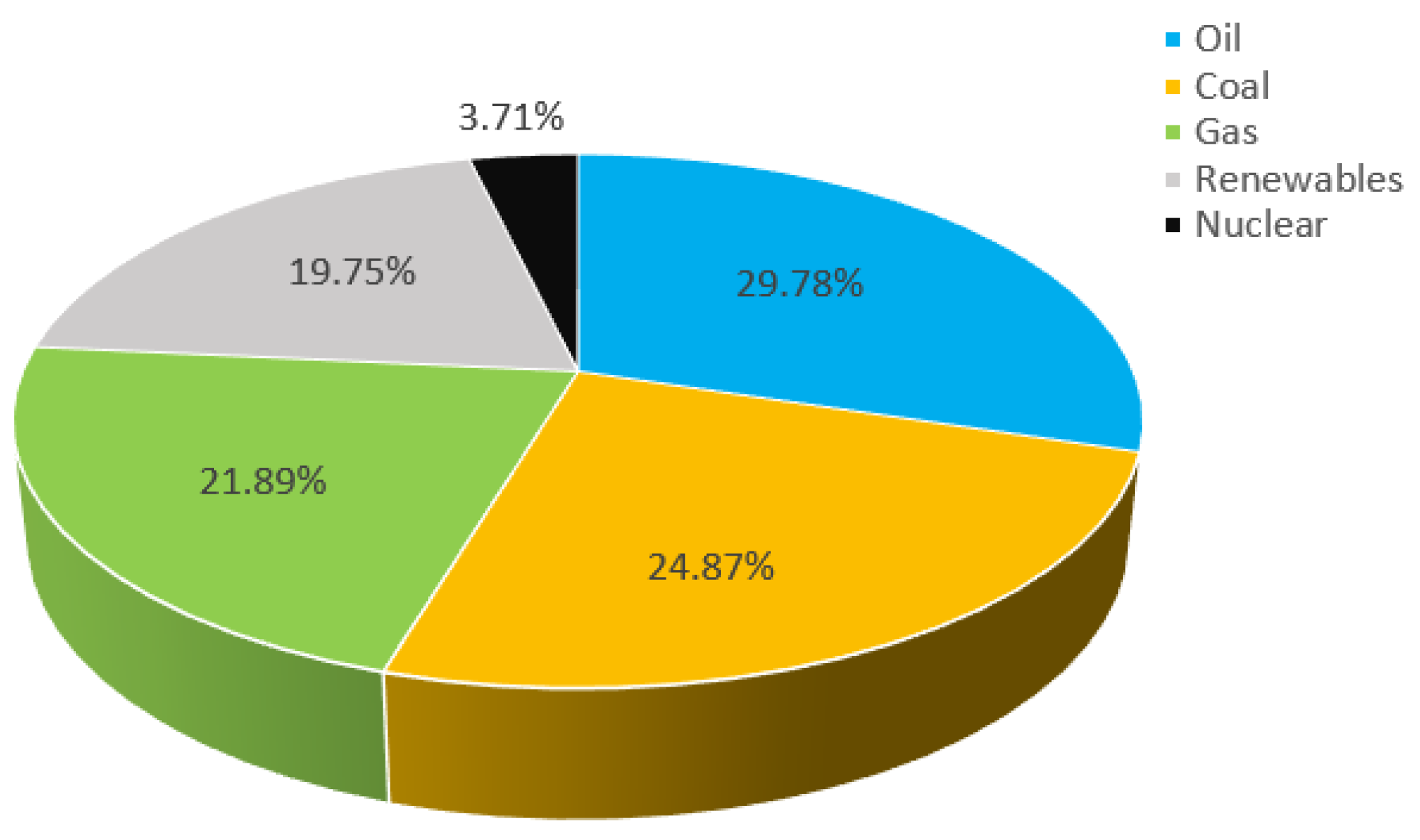
1. Introduction
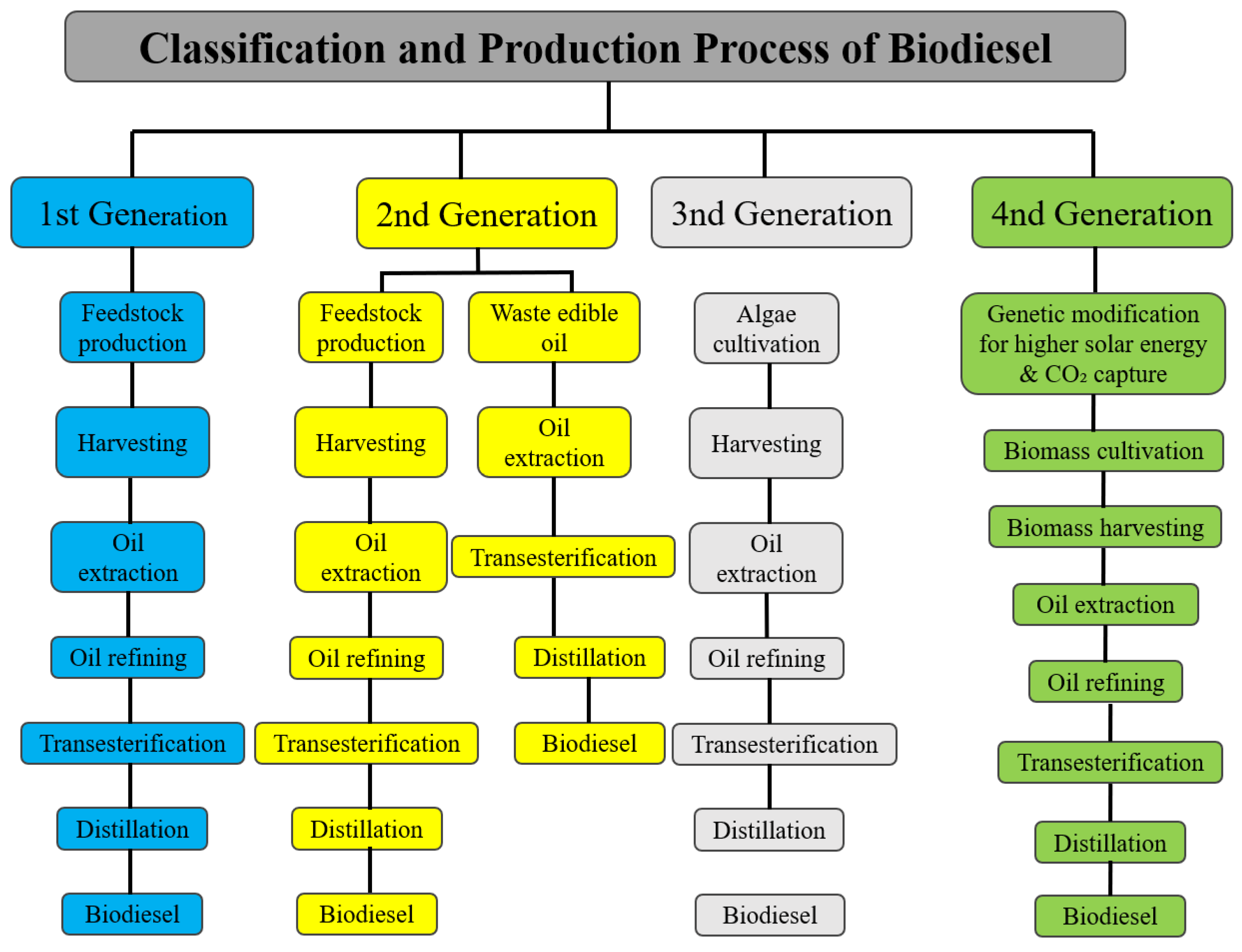
2. Evolution of Biodiesel Feedstocks
2.1. First-Generation Feedstock
2.2. Second-Generation Feedstock
2.3. Third-Generation Feedstock
2.4. Fourth-Generation Feedstock
3. Production Method of Biodiesel
3.1. Transesterification Reaction
3.2. Direct Esterification Reaction
3.3. Supercritical Alcohol Process
3.4. Enzymatic Transesterification
4. Factors Affecting the Production and Quality of Biodiesel
4.1. Molar Ratio
4.2. Time of Reaction
4.3. Temperature of Reaction
5. Catalysts
5.1. Homogeneous Catalyst
5.2. Heterogeneous Catalyst
6. Life Cycle Assessment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Pranta, M.H.; Cho, H.M. A comprehensive review of the evolution of biodiesel production technologies. Energy Convers. Manag. 2025, 328, 119623. [Google Scholar] [CrossRef]
- Cako, E.; Soltani, R.D.C.; Sun, X.; Boczkaj, G. Desulfurization of raw naphtha cuts using hybrid systems based on acoustic cavitation and advanced oxidation processes (AOPs). Chem. Eng. J. 2022, 439, 135354. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.-H.; Tian, B.; Ashokkumar, V.; Lim, S.; Seljak, T.; Józsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Hosseinzadeh-Bandbafha, H.; Dehhaghi, M.; Orooji, Y.; Mahian, O.; Shahbeik, H.; Kiehbadroudinezhad, M.; Kalam, M.A.; Karimi-Maleh, H.; Jouzani, G.S.; et al. Nanotechnology applications in biodiesel processing and production: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 192, 114219. [Google Scholar] [CrossRef]
- Muhammad, K.M.; Adeyemi, M.M.; Jacob, J.; Koko, A.R.; Dauda, K.; Tamasi, A.A.; Yahuza, I. Biodiesel production in Africa from non-edible sources: Sources, production, properties and policies. Sustain. Chem. Environ. 2025, 9, 100201. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, S.M.Z.; Ahmed, U.; Hossain, M.M. Cleaner biodiesel production from waste oils (cooking/vegetable/frying): Advances in catalytic strategies. Fuel 2025, 393, 134901. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Advances in photocatalytic biodiesel production: Preparation methods, modifications and mechanisms. Fuel 2024, 362, 130749. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Arslan, E.; Atelge, M.R.; Kahraman, N.; Ünalan, S. A study on the effects of nanoparticle addition to a diesel engine operating in dual fuel mode. Fuel 2022, 326, 124847. [Google Scholar] [CrossRef]
- Akram, F.; Aslam, H.; Suhail, M.; Fatima, T.; Haq, I.U. Divulging the future of sustainable energy: Innovations and challenges in algal biodiesel production for green energy. Sustain. Energy Technol. Assess. 2025, 75, 104266. [Google Scholar] [CrossRef]
- Dhamodaran, G.; Elumalai, A. Effect of ternary nanocomposite in margosa biodiesel microemulsion blends on performance, emission, and combustion characteristics of a diesel engine. Energy 2025, 326, 136362. [Google Scholar] [CrossRef]
- Tec-Caamal, E.N.; Kamarudin, S.K.; Pugazhendhi, A. Synergistic effects of CeO2 nanoparticles and HHO gas on biodiesel blends in CI engine performance and emissions. Int. J. Hydrogen Energy 2025. [Google Scholar] [CrossRef]
- Deep, A.; Sandhu, S.S.; Chander, S. Experimental Investigations on Castor Biodiesel as an Alternative Fuel for Single Cylinder Compression Ignition Engine. Environ. Prog. Sustain. Energy 2017, 36, 1139–1150. [Google Scholar] [CrossRef]
- Kondaiah, A.; Rao, Y.S.; Satishkumar; Kamitkar, N.D.; Ibrahim, S.J.A.; Chandradass, J.; Kannan, T.T.M. Influence of blends of castor seed biodiesel and diesel on engine characteristics. Mater. Today Proc. 2021, 45 Pt 7, 7043–7049. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Veličković, A.V.; Rajković, D.D.; Avramović, J.M.; Jeromela, A.M.M.; Krstić, M.S.; Veljković, V.B. Advancements in biodiesel production from castor oil: A comprehensive review. Energy Convers. Manag. 2025, 330, 119622. [Google Scholar] [CrossRef]
- Gopi, R.; Thangarasu, V.; Vinayakaselvi M, A.; Ramanathan, A. A critical review of recent advancements in continuous flow reactors and prominent integrated microreactors for biodiesel production. Renew. Sustain. Energy Rev. 2022, 154, 111869. [Google Scholar]
- Datta, I.; Ghosh, A.; Acharjee, A.; Rakshit, A.; Saha, B. Overview on biodiesel market. Vietnam J. Chem. 2021, 59, 271–284. [Google Scholar] [CrossRef]
- Akande, O.; Okolie, J.A.; Kimera, R.; Ogbaga, C.C. A Comprehensive Review on Deep Learning Applications in Advancing Biodiesel Feedstock Selection and Production Processes. Green Energy Intell. Transp. 2025, 100260. [Google Scholar] [CrossRef]
- Adamu, H.; Bello, U.; Yuguda, A.U.; Tafida, U.I.; Jalam, A.M.; Sabo, A.; Qamar, M. Production processes, techno-economic and policy challenges of bioenergy production from fruit and vegetable wastes. Renew. Sustain. Energy Rev. 2023, 186, 113686. [Google Scholar] [CrossRef]
- Jayabal, R. Advancements in catalysts, process intensification, and feedstock utilization for sustainable biodiesel production. Results Eng. 2024, 24, 103668. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Ali, S.S.; Ansari, K.B.; Athar, M.; Mesfer, M.K.A.; Shah, M.; Danish, M.; Kumar, R.; Shakeelur Raheman, A.R. Process intensification in biodiesel production using unconventional reactors. Fuel 2025, 380, 133263. [Google Scholar] [CrossRef]
- Powar, R.S.; Yadav, A.S.; Ramakrishna, C.S.; Patel, S.; Mohan, M.; Sakharwade, S.G.; Choubey, M.; Ansu, A.K.; Sharma, A. Algae: A potential feedstock for third generation biofuel. Mater. Today Proc. 2022, 63, A27–A33. [Google Scholar] [CrossRef]
- Aryasomayajula Venkata Satya Lakshmi, S.B.; Subramania Pillai, N.; Khadhar Mohamed, M.S.B.; Narayanan, A. Biodiesel production from rubber seed oil using calcined eggshells impregnated with Al2O3 as heterogeneous catalyst: A comparative study of RSM and ANN optimization. Braz. J. Chem. Eng. 2020, 37, 351–368. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Gendy, E.A.; Barasa, M.; Oyekunle, D.O.; Oni, B.; Tiong, S.K. Review on utilization of rubber seed oil for biodiesel production: Oil extraction, biodiesel conversion, merits, and challenges. Clean. Eng. Technol. 2024, 21, 100773. [Google Scholar] [CrossRef]
- Anil, N.; Piyush; Rao, K.; Sarkar, A.; Kubavat, J.; Vadivel, S.; Manwar, N.R.; Paul, B. Advancements in sustainable biodiesel production: A comprehensive review of bio-waste derived catalysts. Energy Convers. Manag. 2024, 318, 118884. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Yan, Q.; Wu, X.; Zhang, H. Superparamagnetic nanospheres with efficient bifunctional acidic sites enable sustainable production of biodiesel from budget non-edible oils. Energy Convers. Manag. 2023, 297, 117758. [Google Scholar] [CrossRef]
- Bekhradinassab, E.; Haghighi, M.; Shabani, M. A review on acidic metal oxide-based materials towards heterogeneous catalytic biodiesel production via esterification process. Fuel 2025, 379, 132986. [Google Scholar] [CrossRef]
- Yue, M.; Rokhum, S.L.; Ma, X.; Wang, T.; Li, H.; Zhao, Z.; Wang, Y.; Li, H. Recent advances of biodiesel production enhanced by external field via heterogeneous catalytic transesterification system. Chem. Eng. Process.-Process Intensif. 2024, 205, 109997. [Google Scholar] [CrossRef]
- Ao, Q.; Jiang, L.; Tang, J. Urea-crosslinked 3D graphene oxide/MXene-SO3/cyclodextrin films for efficient and sustainable biodiesel production. Carbohydr. Polym. 2025, 357, 123457. [Google Scholar] [CrossRef] [PubMed]
- Damian, C.S.; Yuvarajan, D.; Raja, T.; Choubey, G.; Munuswamy, D.B. Biodiesel production from shrimp shell lipids: Evaluating ZnO nanoparticles as a catalyst. Results Eng. 2024, 24, 103453. [Google Scholar] [CrossRef]
- Mirshafiee, F.; Rezaei, M. Catalytic efficiency and reusability of K2O/M-aluminate (M = Mg, Zn, Cu) nanocatalyst in the esterification of sunflower oil with methanol for biodiesel production. J. Mol. Liq. 2025, 426, 127333. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: A review of membrane-integrated green approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del-Baño, J.M.; Chica, A.; Quesada-Medina, J. Waste animal fats as feedstock for biodiesel production using non-catalytic supercritical alcohol transesterification: A perspective by the PRISMA methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Saksono, N.; Muharam, Y.; Setiawan, M.A. Effect of mixing rate and molar ratio of methanol-oil to biodiesel synthesis from palm oil with plasma electrolysis method. AIP Conf. Proc. 2021, 2376, 020001. [Google Scholar]
- Fatimah, I.; Nugraha, J.; Sagadevan, S.; Kamari, A.; Oh, W.-C. Process intensification of biodiesel production by optimization using box-behnken design: A review. Chem. Eng. Process.-Process Intensif. 2025, 208, 110110. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, A.K. A Mathematical Study on the Effect of Molar Ratios of the Reactants for Biodiesel Production in Sc-Co2 Medium. Int. J. Math. Comput. Res. 2023, 11, 3729–3733. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Singh, M.V.P.; Fransila, B.; Kumar, R.P.; Devi, G.K. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Oza, S.; Kodgire, P.; Kachhwaha, S.S.; Lam, M.K.; Yusup, S.; Chai, Y.H.; Rokhum, S.L. A review on sustainable and scalable biodiesel production using ultra-sonication technology. Renew. Energy 2024, 226, 120399. [Google Scholar] [CrossRef]
- Girish, C.R. Review of various technologies used for biodiesel production. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 1379–1392. [Google Scholar]
- Thakkar, K.; Shah, K.; Kodgire, P.; Kachhwaha, S.S. In-situ reactive extraction of castor seeds for biodiesel production using the coordinated ultrasound—Microwave irradiation: Process optimization and kinetic modeling. Ultrason. Sonochem. 2019, 50, 6–14. [Google Scholar] [CrossRef]
- Moghadam, A.J.; Beni, A.A. Comparison of biodiesel production from Dunaliella salina teodor and Chlorella vulgaris microalgae using supercritical fluid technique. S. Afr. J. Chem. Eng. 2022, 41, 150–160. [Google Scholar] [CrossRef]
- Kasirajan, R. Biodiesel production by two step process from an energy source of Chrysophyllum albidum oil using homogeneous catalyst. S. Afr. J. Chem. Eng. 2021, 37, 161–166. [Google Scholar] [CrossRef]
- Prabakaran, P.; Karthikeyan, S. Algae biofuel: A futuristic, sustainable, renewable and green fuel for I.C. engines. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abedini, E.; hosein Darabi, A. Biodiesel synthesis from oleic acid by nano-catalyst (ZrO2/Al2O3) under high voltage conditions. RSC Adv. 2015, 5, 55027–55032. [Google Scholar] [CrossRef]
- Yahya, M.; Dutta, A.; Bouri, E.; Wadström, C.; Uddin, G.S. Ependence structure between the international crude oil market and the European markets of biodiesel and rapeseed oil. Renew. Energy 2022, 197, 594–605. [Google Scholar] [CrossRef]
- Körbitz, W. Biodiesel production in Europe and North America, an encouraging prospect. Renew. Energy 1999, 16, 1078–1083. [Google Scholar] [CrossRef]
- Gasol, C.M.; Salvia, J.; Serra, J.; Antón, A.; Sevigne, E.; Rieradevall, J.; Gabarrell, X. A life cycle assessment of biodiesel production from winter rape grown in Southern Europe. Biomass Bioenergy 2012, 40, 71–81. [Google Scholar] [CrossRef]
- Chang, T.-H.; Su, H.-M. The substitutive effect of biofuels on fossil fuels in the lower and higher crude oil price periods. Energy 2010, 35, 2807–2813. [Google Scholar] [CrossRef]
- Ozdemir, S.; Ozer, H.; Ozdemir, S.; Dede, O.H. Sustainable biodiesel production from oil crops: The impact of bio-nutrient recycling on yield and farmer technology acceptance. Ind. Crops Prod. 2025, 225, 120541. [Google Scholar] [CrossRef]
- Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M.B.; Akhtar, M.N.; Iqbal, H.M.N. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials—A review. Fuel 2022, 328, 125254. [Google Scholar] [CrossRef]
- Surriya, O.; Saleem, S.S.; Waqar, K.; Gul Kazi, A.; Öztürk, M. Bio-fuels: A blessing in disguise. In Phytoremediation for Green Energy; Springer: Dordrecht, The Netherlands, 2015; pp. 11–54. [Google Scholar]
- Malik, M.A.I.; Zeeshan, S.; Khubaib, M.; Ikram, A.; Hussain, F.; Yassin, H.; Qazi, A. A review of major trends, opportunities, and technical challenges in biodiesel production from waste sources. Energy Convers. Manag. X 2024, 23, 100675. [Google Scholar]
- Ruatpuia, J.V.L.; Halder, G.; Vanlalchhandama, M.; Lalsangpuii, F.; Boddula, R.; Al-Qahtani, N.; Niju, S.; Mathimani, T.; Rokhum, S.L. Jatropha curcas oil a potential feedstock for biodiesel production: A critical review. Fuel 2024, 370, 131829. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, X.-F.; Fang, Z.; Shah, M.; Wang, Y.-T.; Jiang, W.; Yao, M. Catalytic production of Jatropha biodiesel and hydrogen with magnetic carbonaceous acid and base synthesized from Jatropha hulls. Energy Convers. Manag. 2021, 142, 107–116. [Google Scholar] [CrossRef]
- Das, A.; Jati, A.P.; Selvaraj, M.; Kataki, R.; Baskar, G.; Halder, G.; Rokhum, S.L. Psidium guajava (guava) leaves derived functional activated carbon as a heterogeneous catalyst for conversion of Jatropha curcas oil to biodiesel. J. Anal. Appl. Pyrolysis 2024, 181, 106636. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Z.; Liu, K.; Zhou, Y.; Ba, J.; Wei, G.; Yang, Q.; Liu, Y. Green and economical transesterification of castor oil for biodiesel production via a novel bentonite based Li2SiO3-LiAlO2 composite: Process technology & production feasibility. Process Saf. Environ. Prot. 2024, 192, 1037–1050. [Google Scholar]
- Phewphong, S.; Roschat, W.; Ratchatan, T.; Suriyafai, W.; Khotsuno, N.; Janlakorn, C.; Leelatam, T.; Namwongsa, K.; Moonsin, P.; Yoosuk, B.; et al. Physicochemical exploration of castor seed oil for high-quality biodiesel production and its sustainable application in agricultural diesel engines. Chem. Eng. Res. Des. 2024, 205, 207–220. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, J.; Tan, P.; Lou, D. Life cycle carbon footprint of biodiesel production from waste cooking oil based on survey data in Shanghai. China Energy 2025, 320, 135318. [Google Scholar] [CrossRef]
- Qadeer, M.U.; Ayoub, M.; Nazir, M.H.; Javed, M.A.; Zulqarnain; Zahid, I.; Ameen, M.; Sher, F.; Ibrahim, A. Interesterification of waste cooking oil via microwave irradiation for glycerol-free biodiesel production. Biomass Bioenergy 2025, 197, 107739. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429. [Google Scholar] [CrossRef]
- Kouhgardi, E.; Zendehboudi, S.; Mohammadzadeh, O.; Lohi, A.; Chatzis, I. Current status and future prospects of biofuel production from brown algae in North America: Progress and challenges. Renew. Sustain. Energy Rev. 2023, 172, 113012. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S.; Pandian, E. A review on biodiesel production by algal biomass: Outlook on lifecycle assessment and techno-economic analysis. Fuel 2022, 324 Pt C, 124774. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Huang, Z.; Liu, T.; Li, H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef]
- Kishor, R.; Raj, A.; Bharagava, R.N. Synergistic role of bacterial consortium (RKS-AMP) for treatment of recalcitrant coloring pollutants of textile industry wastewater. J. Water Process Eng. 2022, 47, 102700. [Google Scholar] [CrossRef]
- Mehra, K.S.; Abrar, I.; Bhatia, R.K.; Goel, V. A comprehensive review of algae consortium for wastewater bioremediation and biodiesel production. Energy Convers. Manag. 2025, 325, 119428. [Google Scholar] [CrossRef]
- Shaitor, N.; Yakimovich, B.; Gorpinchenko, A. Electromechanical wave systems for mineral extraction. Acta Montan. Slovaca 2022, 27, 2. [Google Scholar]
- McBride, R.C.; Lopez, S.; Meenach, C.; Burnett, M.; Lee, P.A.; Nohilly, F.; Behnke, C. Contamination management in low cost open algae ponds for biofuels production. Ind. Biotechnol. 2014, 10, 221–227. [Google Scholar] [CrossRef]
- Razminienė, K.; Vinogradova-Zinkevič, I.; Tvaronavičienė, M. Clusters in transition to circular economy: Evaluation of relation. Acta Montan. Slovaca. 2021, 26, 455–465. [Google Scholar]
- Amer, L.; Adhikari, B.; Pellegrino, J. Technoeconomic analysis of five microalgae-to-biofuels processes of varying complexity. Bioresour. Technol. 2011, 102, 9350–9359. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wei, C.; Yan, Q.; Shan, X.; Wu, M.; Zhao, X.; Song, Y. Optimization of a novel lipid extraction process from microalgae. Sci. Rep. 2021, 11, 20221. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Khoiroh, I.; Vo, D.V.N.; Senthil Kumar, P.; Show, P.L. Techniques of lipid extraction from microalgae for biofuel production: A review. Environ. Chem. Lett. 2021, 19, 231–251. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45, 24–31. [Google Scholar] [CrossRef]
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014, 12, 381–390. [Google Scholar] [CrossRef]
- Abdelrahman, A.A.; El-Khair, M.A.A. Advanced Biodiesel Production: Feedstocks, Technologies, Catalysts, Challenges, and Environmental Impacts. J. Environ. Chem. Eng. 2025, 13, 114966. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Hossain, M.N.; Siddik Bhuyan, M.S.U.; Md Ashraful Alam, A.H.; Seo, Y.C. Optimization of biodiesel production from waste cooking oil using S–TiO2/SBA-15 heterogeneous acid catalyst. Catalysts 2019, 9, 67. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Hu, Y.; Rao, K.T.V.; Xu, C.; Yang, S. Advances in production of bio-based ester fuels with heterogeneous bifunctional catalysts. Renew. Sustain. Energy Rev. 2019, 114, 109296. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Kitrys, S. Kinetics of free fatty acids esterification with methanol in the production of biodiesel fuel. Eur. J. Lipid Sci. Technol. 2004, 106, 831–836. [Google Scholar] [CrossRef]
- Thaiyasuit, P.; Pianthong, K.; Worapun, I. Acid esterification-alkaline transesterification process for methyl ester production from crude rubber seed oil. J. Oleo Sci. 2012, 61, 81–88. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. State of the art of biodiesel production under supercritical conditions. Prog. Energy Combust. Sci. 2017, 63, 173–203. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Sunol, A.K.; Philippidis, G. Status and prospects of supercritical alcohol transesterification for biodiesel production. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e252. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. A review on supercritical fluids (SCF) technology in sustainable biodiesel production: Potential and challenges. Renew. Sustain. Energy Rev. 2011, 15, 2452–2456. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Cheng, J.; Mao, Y.; Guo, H.; Qian, L.; Shao, Y.; Yang, W.; Park, J.-Y. Synergistic and efficient catalysis over Brønsted acidic ionic liquid [BSO3HMIm][HSO4]–modified metal–organic framework (IRMOF-3) for microalgal biodiesel production. Fuel 2022, 322, 124217. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Binnal, P.; Amruth, A.; Basawaraj, M.P.; Chethan, T.S.; Murthy, K.R.S.; Rajashekhara, S. Microwave-assisted esterification and transesterification of dairy scum oil for biodiesel production: Kinetics and optimisation studies. Indian Chem. Eng. 2021, 63, 374–386. [Google Scholar] [CrossRef]
- Salamatinia, B.; Abdullah, A.Z.; Bhatia, S. Quality evaluation of biodiesel produced through ultrasound-assisted heterogeneous catalytic system. Fuel Process. Technol. 2012, 97, 1–8. [Google Scholar] [CrossRef]
- Naseef, H.H.; Tulaimat, R.H. Transesterification and esterification for biodiesel production: A comprehensive review of catalysts and palm oil feedstocks. Energy Convers. Manag. X 2025, 26, 100931. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Quesada-Medina, J. Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis. Appl. Energy 2020, 264, 114753. [Google Scholar] [CrossRef]
- dos Passos, R.M.; Ferreira, R.S.; Morgano, M.A.; de Souza, P.T.; Meirelles, A.J.; Batista, E.A.; Maximo, G.J.; Ferreira, M.C.; Sampaio, K.A. Ethyl biodiesel production from crude soybean oil using enzymatic degumming-transesterification associated process. Ind. Crops Prod. 2024, 222 Pt 4, 119930. [Google Scholar] [CrossRef]
- Ghedini, E.; Taghavi, S.; Menegazzo, F.; Signoretto, M. A review on the efficient catalysts for algae transesterification to biodiesel. Sustainability 2021, 13, 10479. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Xu, Y.; Zhou, Y.; Li, S.; Zheng, M. Broadly adapted and efficient enzymatic transesterification production of medium and long-chain triglycerides via coconut oil and long-chain triacylglycerols. Food Chem. 2025, 469, 142499. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- da Costa, J.M.; de Andrade Lima, L.R.P. Transesterification of cotton oil with ethanol for biodiesel using a KF/bentonite solid catalyst. Fuel 2021, 293, 120446. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Yang, X.-X. Biodiesel production from high acid value oils with a highly active and stable bifunctional magnetic acid. Appl. Energy 2017, 204, 702–714. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Synthesis of biodiesel from tall oil fatty acids by homogeneous and heterogeneous catalysis. Sustain. Chem. 2021, 2, 206–221. [Google Scholar] [CrossRef]
- Abdel-Hamid, S.M.S.; Tharwat, K.M.; Gad, A.M.; Mahmoud, A.T.; Emam, M.A.; Khaled, A.M.; Elshishiny, M.B.; El-Sayed, A.A.Y.; Aly, S.T. Process Optimization for Biodiesel Production and Its Effect on the Performance of Diesel Engines. Chem. Eng. Technol. 2022, 46, 694–701. [Google Scholar] [CrossRef]
- Celante, D.; Schenkel, J.V.D.; de Castilhos, F. Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide. Fuel 2018, 212, 101–107. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rokhum, L.; Gupta, R.; Chatterjee, S. Zinc oxide supported silver nanoparticles as a heterogeneous catalyst for production of biodiesel from palm oil. Environ. Prog. Sustain. Energy 2020, 39, e13369. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Lourenço Mares, E.K.; Luz, P.T.S.D.; Zamian, J.R.; Castro, H.F.D.; Conceição, L.R.V.D. Biodiesel synthesis from waste cooking oil using heterogeneous acid catalyst: Statistical optimization using linear regression model. J. Renew. Sustain. Energy 2021, 13, 043101. [Google Scholar] [CrossRef]
- Al Hatrooshi, A.S.; Eze, V.C.; Harvey, A.P. Production of biodiesel from waste shark liver oil for biofuel applications. Renew. Energy 2020, 145, 99–105. [Google Scholar] [CrossRef]
- Khiratkar, A.G.; Balinge, K.R.; Patle, D.S.; Krishnamurthy, M.; Cheralathan, K.K.; Bhagat, P.R. Transesterification of castor oil using benzimidazolium based Brønsted acid ionic liquid catalyst. Fuel 2018, 231, 458–467. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.-H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Okolie, J.A.; Escobar, J.I.; Umenweke, G.; Khanday, W.; Okoye, P.U. Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel 2022, 307, 121821. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Dybiński, O.; Milewski, J.; Szabłowski, Ł.; Szczęśniak, A.; Martinchyk, A. Methanol, ethanol, propanol, butanol and glycerol as hydrogen carriers for direct utilization in molten carbonate fuel cells. Int. J. Hydrogen Energy 2023, 48, 37637–37653. [Google Scholar] [CrossRef]
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative effect of reaction time on biodiesel production from low free fatty acid beef tallow: A definition of product yield. SN Appl. Sci. 2019, 1, 140. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME. Fuel Process. Technol. 2015, 130, 127–135. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Haigh, K.F.; Saha, B. Esterification of free fatty acids in used cooking oil using ion-exchange resins as catalysts: An efficient pretreatment method for biodiesel feedstock. Ind. Eng. Chem. Res. 2012, 51, 14653–14664. [Google Scholar] [CrossRef]
- Latchubugata, C.S.; Kondapaneni, R.V.; Patluri, K.K.; Virendra, U.; Vedantam, S. Kinetics and optimization studies using Response Surface Methodology in biodiesel production using heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 135, 129–139. [Google Scholar] [CrossRef]
- Ansari, M.; Jamali, H.; Ghanbari, R.; Ehrampoush, M.H.; Zamani, P.; Hatami, B. Heterogeneous solid acid catalysts for sustainable biodiesel production from wastewater-derived sludge: A systematic and critical review. Chem. Eng. J. Adv. 2025, 22, 100718. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Enzymatic conversion of waste edible oil to biodiesel fuel in a fixed-bed bioreactor. J. Am. Oil Chem. Soc. 2001, 78, 703–707. [Google Scholar] [CrossRef]
- Mohandass, R.; Ashok, K.; Selvaraju, A.; Rajagopan, S. Homogeneous catalysts used in biodiesel production: A review. Int. J. Eng. Res. 2016, 5, 264–268. [Google Scholar] [CrossRef]
- Abelniece, Z.; Laipniece, L.; Kampars, V. Biodiesel production by interesterification of rapeseed oil with methyl formate in presence of potassium alkoxides. Biomass Convers. Biorefinery 2022, 12, 2881–2889. [Google Scholar] [CrossRef]
- Vicente, G.; Martı, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Rajali, N.A.; Radzi, S.M.; Rehan, M.M.; Amin, N.A.M. Optimization of the biodiesel production via transesterification reaction of palm oil using response surface methodology (RSM): A review. Malays. J. Sci. Health Technol. 2022, 8, 58–67. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.A.; Ahmad, A. State of the art of catalysts for biodiesel production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Al-Humairi, S.T.; Lee, J.G.; Harvey, A.P. Direct and rapid production of biodiesel from algae foamate using a homogeneous base catalyst as part of an intensified process. Energy Convers. Manag. X 2022, 16, 100284. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of biodiesel from waste cooking oil using a homogeneous catalyst: Study of semi-industrial pilot of microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Esposito, R.; Lega, M.; Turco, R.; Ruffo, F.; Di Serio, M. A novel and robust homogeneous supported catalyst for biodiesel production. Fuel 2016, 171, 1–4. [Google Scholar] [CrossRef]
- Su, C.-H. Recoverable and reusable hydrochloric acid used as a homogeneous catalyst for biodiesel production. Appl. Energy 2013, 104, 503–509. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Juan, J.C.; Yun Hin, T.Y.; Ong, H.C. Environment-friendly heterogeneous alkaline-based mixed metal oxide catalysts for biodiesel production. Energies 2016, 9, 611. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Shaheen, A.; Sultana, S.; Lu, H.; Ahmad, M.; Asma, M.; Mahmood, T. Assessing the potential of different nano-composite (MgO, Al2O3-CaO and TiO2) for efficient conversion of Silybum eburneum seed oil to liquid biodiesel. J. Mol. Liq. 2018, 249, 511–521. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Farhadi, K.; Mansourpanah, Y.; Nikbakht, A.M.; Molaei, R.; Forough, M. Application of CaO-based/Au nanoparticles as heterogeneous nanocatalysts in biodiesel production. Fuel 2016, 164, 119–127. [Google Scholar] [CrossRef]
- Jume, B.H.; Gabris, M.A.; Nodeh, H.R.; Rezania, S.; Cho, J. Biodiesel production from waste cooking oil using a novel heterogeneous catalyst based on graphene oxide doped metal oxide nanoparticles. Renew. Energy 2020, 162, 2182–2189. [Google Scholar] [CrossRef]
- Changmai, B.; Rano, R.; Vanlalveni, C.; Rokhum, S.L. A novel Citrus sinensis peel ash coated magnetic nanoparticles as an easily recoverable solid catalyst for biodiesel production. Fuel 2021, 286 Pt 2, 119447. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Pulidindi, I.N.; Pulidindi, I.N. Strontium Oxide Nanoparticles for Biodiesel Production: Fundamental Insights and Recent Progress. Energy Fuels 2021, 35, 187–200. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Basha, S.C.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 6–7. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]

| Type | Applicable Feedstocks | Reaction Conditions | Reaction Time | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|---|
| Transesterification | Low FFA oils (FFA < 2%) | 60–70 °C, atmospheric pressure | 30–60 min | Mature technology, high conversion efficiency, scalable | Soap formation with high FFA; pretreatment often required | [80,81,82] |
| Direct Esterification | High-FFA oils | 60–80 °C, atmospheric pressure | Several hours | Effectively reduces acid value; suitable for pretreatment | Slow reaction; equipment corrosion risk | [83,84,85] |
| Supercritical Alcohol Process | All types of oils, including high FFA | 240–350 °C, >80 bar (high temperature/ pressure) | Minutes to 1 h | Catalyst-free; no soap formation; fast reaction | High energy consumption; complex and costly equipment; safety risks | [86,87,88] |
| Enzymatic Transesterification | All types of oils, including waste oils | 30–60 °C, atmospheric pressure | 8–24 h | Mild, eco-friendly, fewer by-products, easy separation | High enzyme cost; sensitive to inhibition; limited reusability | [89,90] |
| Feedstock | Molar Ratio | Catalyst | Temperature (°C) | Reaction Time | Yield (%) | Refs. |
|---|---|---|---|---|---|---|
| Chicken fat | 15:1 | CaO/CuFe2O4 nanoparticles | 70 °C | 4 h | 94.52% | [101] |
| Cotton oil | 13:1 | Solid basic heterogeneous catalysts | 120 °C | 6 h | 95% | [102] |
| Jatropha oil | 12:1 | Functionalized magnetic solid acid catalysts | 90 °C | 4 h | 97.39% | [103] |
| Tall oil | 15:1 | H2SO4 | 55 °C | 1 h | 96.76% | [104] |
| Waste cooking oil | 9:1 | NaOH | 40 °C | 2 h | 98.22% | [105] |
| Soybean oil | 6:1 | CH3KO | 80 °C | 15 min | 91% | [106] |
| Palm oil | 10:1 | ZnO-silver nanoparticles | 80 °C | 1 h | 97% | [107] |
| Waste cooking oil | 90:1 | H3Mo12O40P | 190 °C | 4 h | 94.5% | [108] |
| Waste shark liver oil | 10:1 | H2SO4 | 60 °C | 6.5 h | 99% | [109] |
| Castor oil | 12:1 | transesterification of castor oil using benzimidazolium-based Brønsted acid ionic liquid | 70 °C | 24 h | 59% | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Cho, H.M. Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis. Energies 2025, 18, 2533. https://doi.org/10.3390/en18102533
Zheng F, Cho HM. Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis. Energies. 2025; 18(10):2533. https://doi.org/10.3390/en18102533
Chicago/Turabian StyleZheng, Fangyuan, and Haeng Muk Cho. 2025. "Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis" Energies 18, no. 10: 2533. https://doi.org/10.3390/en18102533
APA StyleZheng, F., & Cho, H. M. (2025). Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis. Energies, 18(10), 2533. https://doi.org/10.3390/en18102533






