Performance of Corn Cob Combustion in a Low-Temperature Fluidized Bed
Abstract
1. Introduction
2. Materials and Methods
2.1. Fuel Selection, Preparation, and Analysis
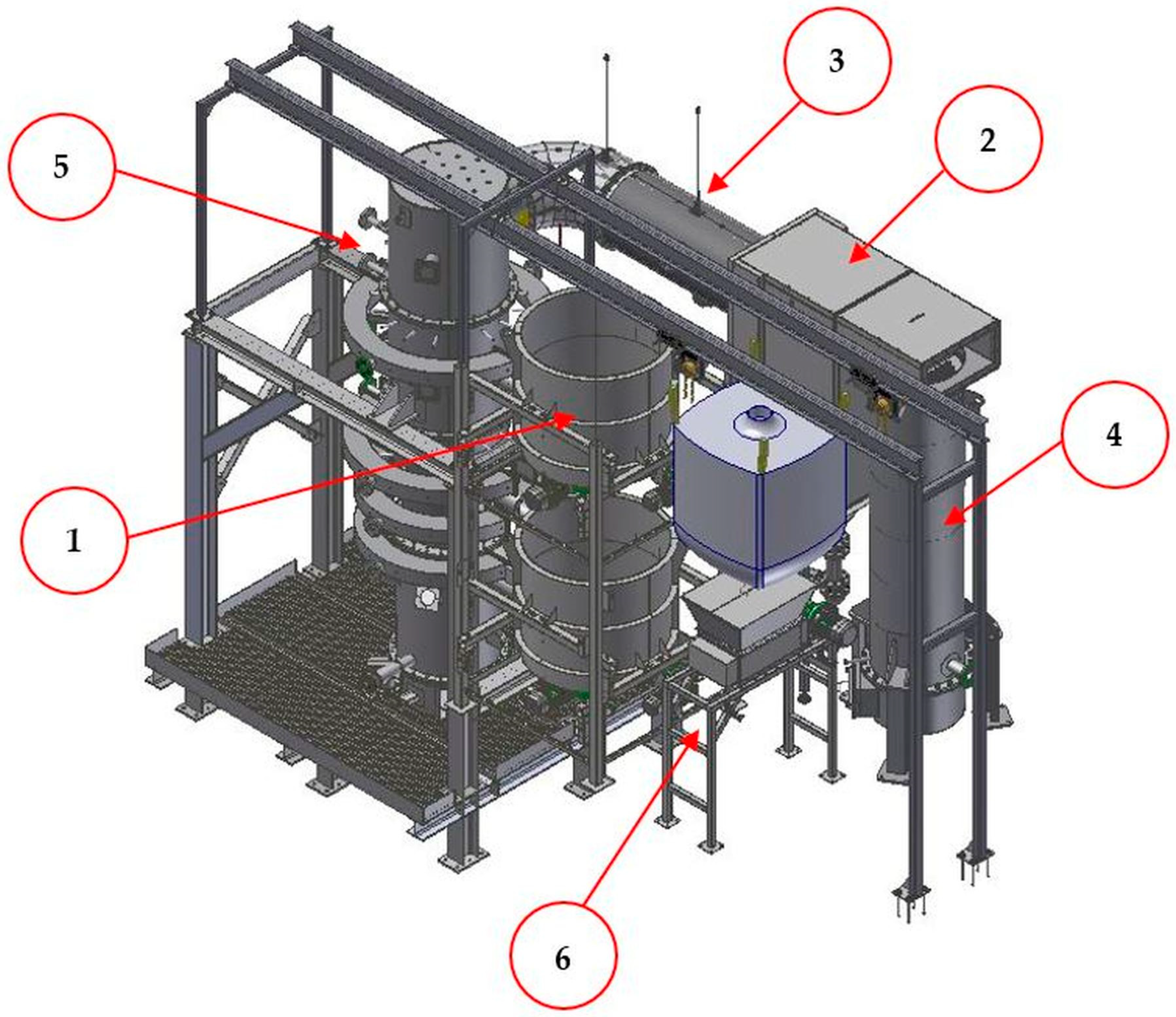
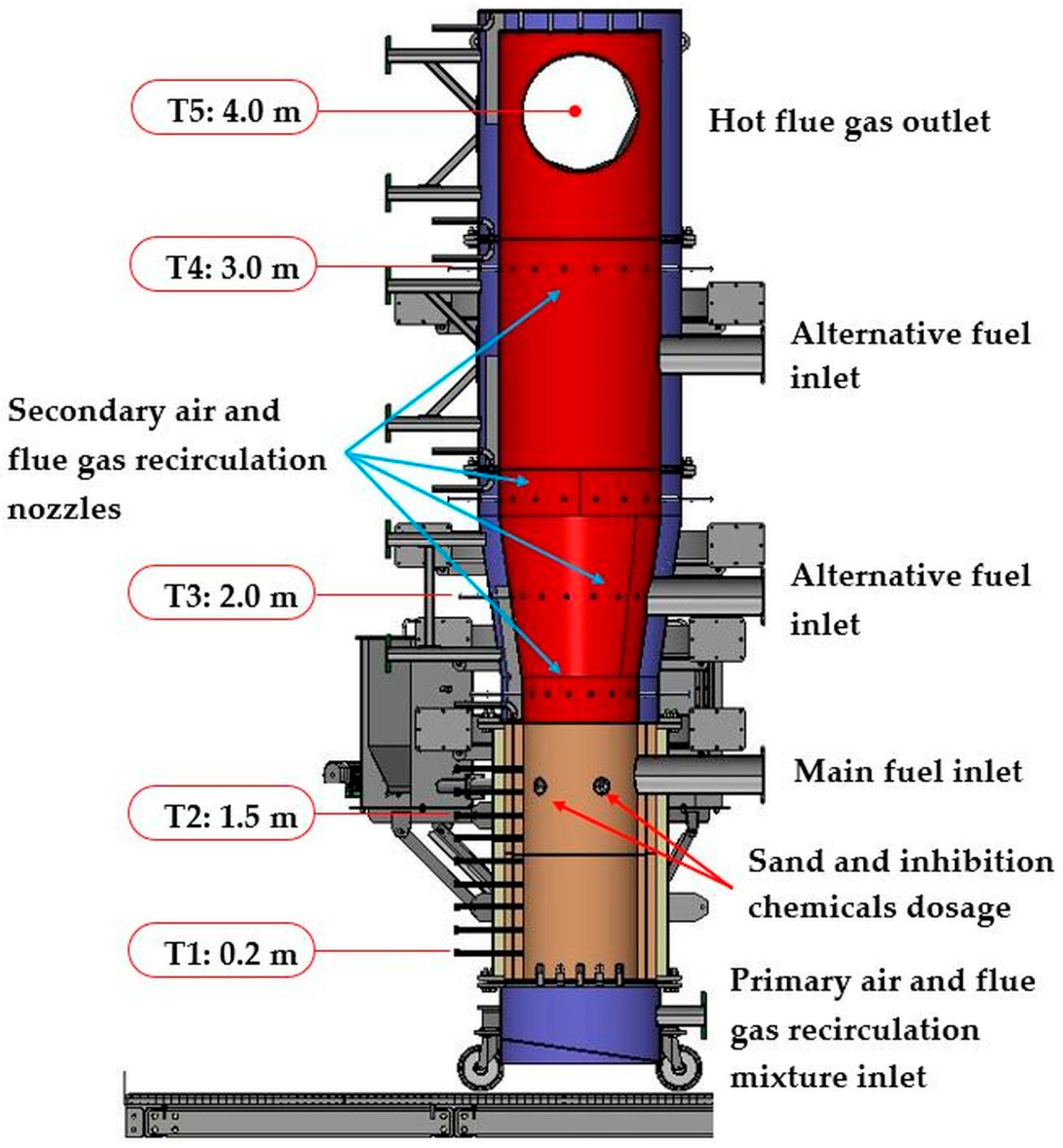
2.2. Experimental Setup and Procedure
2.3. Particulate Matter Sampling and Its Chemical Analysis
3. Results and Discussion
3.1. Chemical Composition of Raw Material
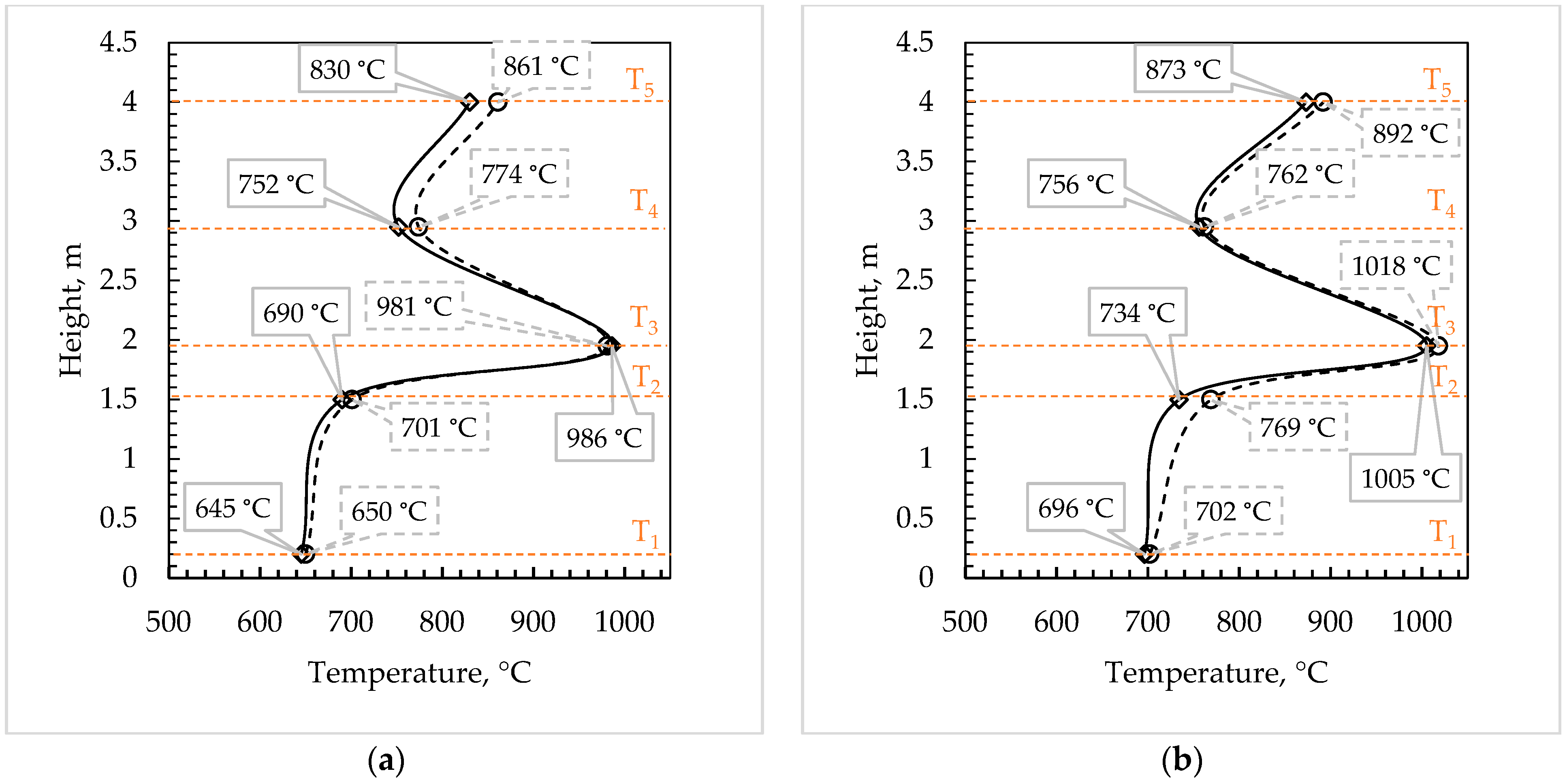
3.2. Combustion Tests of FBC and Optimal Parameters
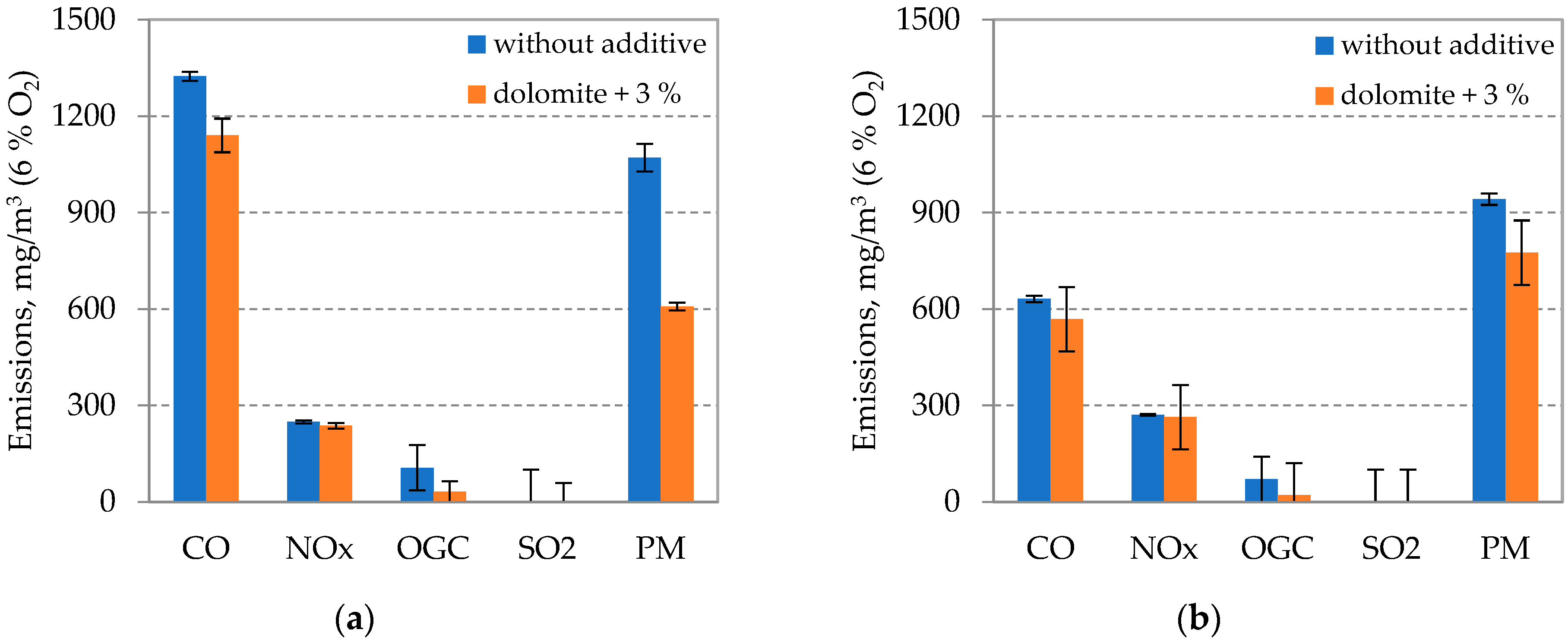
3.3. Pollutant Emissions during Combustion at Different Fluidized Bed Temperatures
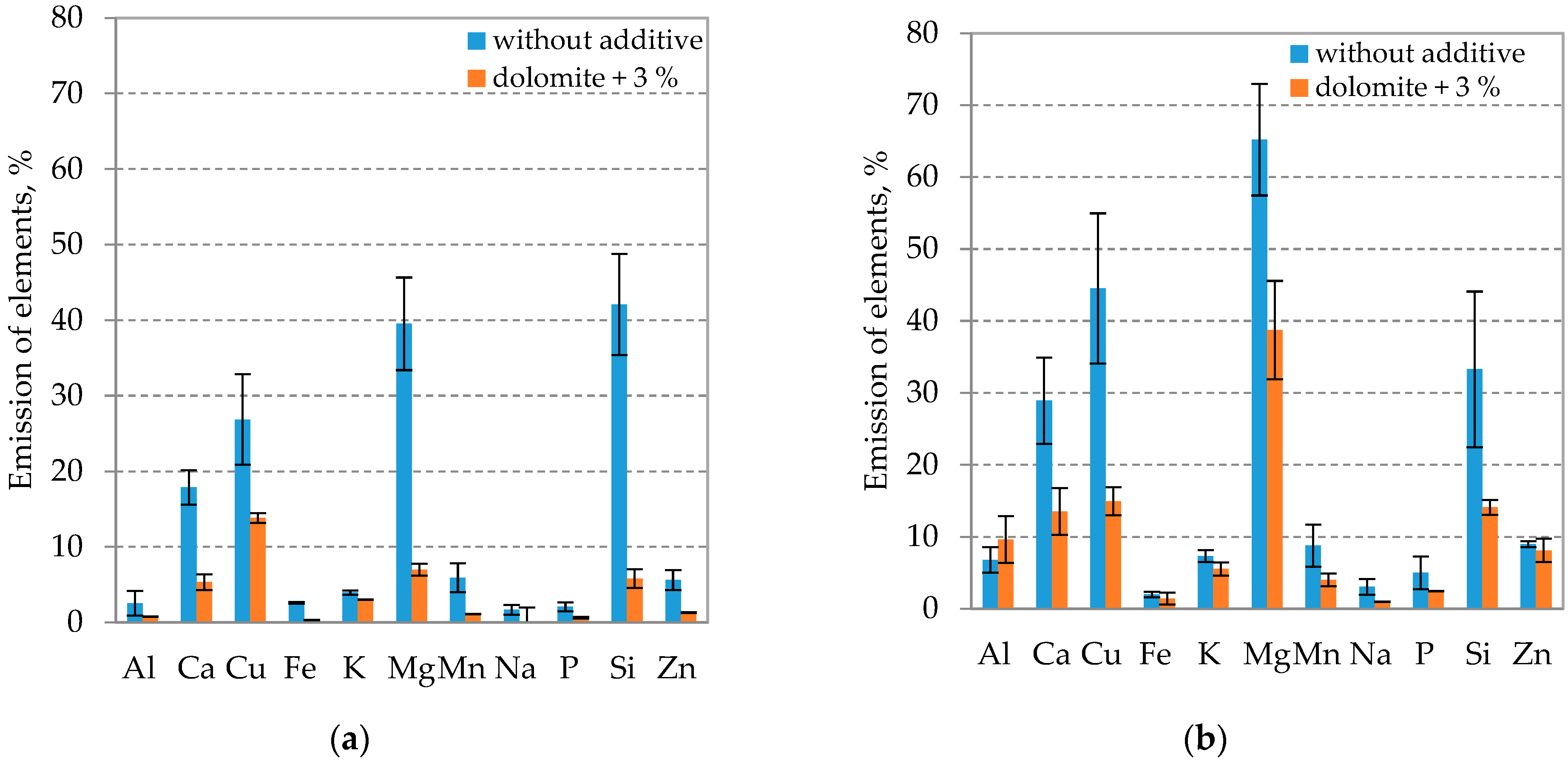
3.4. Release of Chemical Elements from Fuel during Combustion at Different Fluidized Bed Temperatures Using an Inhibitor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FBC | fluidized bed combustor |
| RES | renewable energy sources |
| HHV | higher heating value, MJ/kg |
| LHV | lower heating value, MJ/kg |
| SST | shrinkage starting temperature, °C |
| DT | deformation temperature, °C |
| HT | hemisphere temperature, °C |
| FT | flow temperature, °C |
| PA | primary air, m3/h |
| SA | secondary air, m3/h |
| FGR | flue gas recirculation, m3/h |
| OGC | organic gaseous compounds, mg/m3 |
| PM | particulate matter, mg/m3 |
References
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef]
- Janiszewska, D.; Ossowska, L. The Role of Agricultural Biomass as a Renewable Energy Source in European Union Countries. Energies 2022, 15, 6756. [Google Scholar] [CrossRef]
- Krishna Koundinya, K.; Dobhal, P.; Ahmad, T.; Mondal, S.; Kumar Sharma, A.; Kumar Singh, V. A technical review on thermochemical pathways for production of energy from corncob residue. Renew. Energy Focus 2023, 44, 174–185. [Google Scholar] [CrossRef]
- Choi, J.Y.; Nam, J.; Yun, B.Y.; Kim, Y.U.; Kim, S. Utilization of corn cob, an essential agricultuaral residue difficult to disposal: Composite board manufactured improved thermal performance using microencapsulated PCM. Ind. Crops Prod. 2022, 183, 114931. [Google Scholar] [CrossRef]
- Gani, A.; Reza, M.; Desvita, H. Proximate and ultimate analysis of corncob biomass waste as raw material for biocoke fuel production. Case Stud. Chem. Environ. Eng. 2023, 8, 100525. [Google Scholar] [CrossRef]
- Honorato-Salazar, J.A.; Sadhukhan, J. Annual biomass variation of agriculture crops and forestry residues, and seasonality of crop residues for energy production in Mexico. Food Bioprod. Process. 2020, 119, 1–19. [Google Scholar] [CrossRef]
- Donskoy, I. Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion. Energies 2023, 16, 4589. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash deposition in biomass combustion or co-firing for power/heat generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Alam, T.; Hoadley, A.; Dai, B.; Zhang, L. Impact of potassium on bio-ash slagging and resultant slag flowing characteristics under mild reducing environment. Fuel Process. Technol. 2023, 243, 107672. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Leckner, B.; Szentannai, P.; Winter, F. Scale-up of fluidized-bed combustion—A review. Fuel 2011, 90, 2951–2964. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, S.-J.; Baek, G.-U.; Moon, J.H.; Jo, S.H.; Park, S.J.; Kim, J.-Y.; Yoon, S.-J.; Ra, H.W.; Yoon, S.-M.; et al. Operational optimization of air staging and flue gas recirculation for NOx reduction in biomass circulating fluidized bed combustion. J. Clean. Prod. 2023, 387, 135878. [Google Scholar] [CrossRef]
- Peng, T.-H.; Lin, C.-L.; Wey, M.-Y. Development of a low-temperature two-stage fluidized bed incinerator for controlling heavy-metal emission in flue gases. Appl. Therm. Eng. 2014, 62, 706–713. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Hauggaard-Nielsen, H.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Henriksen, U.B.; Müller-Stöver, D.S. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 2: Evaluation of ash materials as phosphorus fertilizer. Waste Manag. 2017, 66, 145–154. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Frandsen, F.J.; Henriksen, U.B. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 1: Process performance and gas product characterization. Waste Manag. 2017, 66, 123–133. [Google Scholar] [CrossRef]
- Karel, T.; Fürsatz, K.; Priscak, J.; Kuba, M.; Skoglund, N.; Hofbauer, H. Influence of fuel characteristics of alternative residual biomass and ash chemistry on fluidized bed combustion and gasification. Energy 2020, 219, 119650. [Google Scholar]
- Silvennoinen, J.; Hedman, M. Co-firing of agricultural fuels in a full-scale fluidized bed boiler. Fuel Process. Technol. 2013, 105, 11–19. [Google Scholar] [CrossRef]
- Saastamoinen, H.; Leino, T. Fuel Staging and Air Staging To Reduce Nitrogen Emission in the CFB Combustion of Bark and Coal. Energy Fuels 2019, 33, 5732–5739. [Google Scholar] [CrossRef]
- Chyang, C.-S.; Qian, F.-P.; Lin, Y.-C.; Yang, S.-H. NO and N2O Emission Characteristics from a Pilot Scale Vortexing Fluidized Bed Combustor Firing Different Fuels. Energy Fuels 2008, 22, 1004–1011. [Google Scholar] [CrossRef]
- Hariana; Prismantoko, A.; Prabowo; Hilmawan, E.; Darmawan, A.; Aziz, M. Effectiveness of different additives on slagging and fouling tendencies of blended coal. J. Energy Inst. 2023, 107, 101192. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, J.; Zheng, L.; Zhang, R.; Dong, B.; Liang, G.; Zhai, Z. Adhesion strength of straw biomass ash: Effect of dolomite additive. Energy 2023, 262, 125320. [Google Scholar] [CrossRef]
- Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. [Google Scholar] [CrossRef]
- Benny, M.; Suraj, P.; Arun, P.; Muraleedharan, C. Agglomeration behavior of lignocellulosic biomasses in fluidized bed gasification: A comprehensive review. J. Therm. Anal. Calorim. 2023, 148, 9289–9308. [Google Scholar] [CrossRef]
- Hervy, M.; Olcese, R.; Bettahar, M.M.; Mallet, M.; Renard, A.; Maldonado, L.; Remy, D.; Mauviel, G.; Dufour, A. Evolution of dolomite composition and reactivity during biomass gasification. Appl. Catal. A Gen. 2019, 572, 97–106. [Google Scholar] [CrossRef]
- Çelikler, C.; Varol, M. Investigation of control methods for agglomeration and slagging during combustion of olive cake in a bubbling fluidized bed combustor. J. Clean. Prod. 2021, 320, 128841. [Google Scholar] [CrossRef]
- TNO Biobased and Circular Technologies Phyllis2, Database for (Treated) Biomass, Algae, Feedstocks for Biogas Production and Biochar. Available online: https://phyllis.nl/ (accessed on 4 April 2024).
- ISO 18134-1:2016; LST EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640308-84af-17ba-8184-c3c1fbec00ea (accessed on 4 April 2024).
- ISO 18122:2016; Standartizacijos LST LT EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a64031d-8211-1029-8184-762685b30677 (accessed on 4 April 2024).
- ISO 16994:2016; Standartizacijos LST EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640324-6747-1da0-8168-815006b5174d (accessed on 4 April 2024).
- ISO 16948:2015; Standartizacijos LST EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640324-6747-1da0-8168-8adfadca1c24 (accessed on 4 April 2024).
- ISO 16967:2015; Standartizacijos LST EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640324-6747-1da0-8168-8adfadc81c1d (accessed on 4 April 2024).
- ISO 18125:2017; Standartizacijos LST EN. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640324-6747-1da0-8168-7c8308020f6d (accessed on 4 April 2024).
- ISO 21404:2020; Standartizacijos. ISO Tarptautinė Standartizacijos Organizacija: Geneva, Switzerland. Available online: https://eshop.lsd.lt/public#!/product/info/0a640325-6f72-19c7-816f-f758e2d2014c, (accessed on 4 April 2024).
- BIODAT Database. Available online: https://biodat.eu/pages/Search.aspx (accessed on 9 April 2024).
- Chandrasekaran, S.R.; Hopke, P.K.; Rector, L.; Allen, G.; Lin, L. Chemical Composition of Wood Chips and Wood Pellets. Energy Fuels 2013, 26, 4932–4937. [Google Scholar] [CrossRef]
- Zhai, M.; Li, X.; Yang, D.; Ma, Z.; Dong, P. Ash fusion characteristics of biomass pellets during combustion. J. Clean. Prod. 2022, 336, 130361. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Álvarez, X.; Valero, E.; Ortiz, L.; de la Torre-Rodríguez, N.; Acuña-Alonso, C. Influence of ashes in the use of forest biomass as source of energy. Fuel 2021, 283, 119256. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Čajová Kantová, N.; Holubčík, M.; Trnka, J.; Čaja, A. Analysis of Ash Melting Temperatures of Agricultural Pellets Detected during Different Conditions. Fire 2023, 6, 88. [Google Scholar] [CrossRef]
- Radačovská, L.; Holubčík, M.; Nosek, R.; Jandačka, J. Influence of Bark Content on Ash Melting Temperature. Procedia Eng. 2017, 192, 759–764. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Zhang, M.; Cui, L.; Dong, Y. Ash problems and prevention measures in power plants burning high alkali fuel: Brief review and future perspectives. Sci. Total Environ. 2023, 901, 165985. [Google Scholar] [CrossRef] [PubMed]
- Al-Qayim, K.; Nimmo, W.; Hughe, K.J.; Pourkashanian, M. Effect of oxy-fuel combustion on ash deposition of pulverized wood pellets. Biofuel Res. J. 2019, 6, 927–936. [Google Scholar] [CrossRef]
- Miranda, M.T.; García-Mateos, R.; Arranz, J.I.; Sepúlveda, F.J.; Romero, P.; Botet-Jiménez, A. Selective use of corn crop residues: Energy viability. Appl. Sci. 2021, 11, 3284. [Google Scholar] [CrossRef]
- Yao, X.; Hu, Y.; Ge, J.; Ma, X.; Mao, J.; Sun, L.; Xu, K.; Xu, K. A comprehensive study on influence of operating parameters on agglomeration of ashes during biomass gasification in a laboratory-scale gasification system. Fuel 2020, 276, 118083. [Google Scholar] [CrossRef]
- Quoc Viet, D.; Van Vinh, N.; Luong, P.H.; Dinh, V.; Tho, S. Thermogravimetric Study on Rice, Corn and Sugar Cane Crop Residue. J. Sustain. Energy Environ. 2015, 6, 87–91. [Google Scholar]
- Zheng, L.; Jin, J.; Zhang, R.; Liu, Z.; Zhang, L. Understanding the effect of dolomite additive on corrosion characteristics of straw biomass ash through experiment study and molecular dynamics calculations. Energy 2023, 271, 126950. [Google Scholar] [CrossRef]
- Chi, H.; Pans, M.A.; Sun, C.; Liu, H. Effectiveness of bed additives in abating agglomeration during biomass air/oxy combustion in a fluidised bed combustor. Renew. Energy 2022, 185, 945–958. [Google Scholar] [CrossRef]
- Duan, F.; Chyang, C.-S.; Zhang, L.; Yin, S.-F. Bed agglomeration characteristics of rice straw combustion in a vortexing fluidized-bed combustor. Bioresour. Technol. 2015, 183, 195–202. [Google Scholar] [CrossRef]
- Li, L.; Mao, J.; Tang, W.; Sun, G.; Gu, Q.; Lu, X.; Shao, K.; Chen, Y.; Duan, L. Experimental study on coal combustion by using the ilmenite ore as active bed material in a 0.3 MWth circulating fluidized bed. Fuel 2023, 342, 127007. [Google Scholar] [CrossRef]
- Schmid, D.; Karlström, O.; Yrjas, P. Release of NH3, HCN and NO during devolatilization and combustion of washed and torrefied biomass. Fuel 2020, 280, 118583. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Z.; Wang, S.; Zhang, Y.; Da, Y.; Dong, H.; Wen, J.; Du, Q.; Gao, J. Effects of Temperature and Chemical Speciation of Mineral Elements on PM10 Formation during Zhundong Coal Combustion. Energies 2022, 16, 310. [Google Scholar] [CrossRef]

| Parameter | Smashed Corn Cob | Non-Specified Wood Pellets [27,35] |
|---|---|---|
| Ultimate analysis | ||
| Carbon, % (d.b) | 48.6 ± 0.31 | 48.90–50.80 |
| Hydrogen, % (d.b) | 5.56 ± 0.11 | 6.17–7.51 |
| Nitrogen, % (d.b) | 0.3 ± 0.04 | 0.03–1.19 |
| Sulphur, % (d.b) | 0.3 ± 0.01 | 0.01–0.1 |
| Oxygen (by difference), % (d.b) | 44.29 | 41.67–44.94 |
| Proximate analysis | ||
| Volatile matter, % (d.b) | 81.62 ± 2.11 | 77.26–83.10 |
| Fixed carbon (calculated), % (d.b) | 16.3 | 16.90–20.39 |
| Ash, % (d.b) | 2.07 ± 0.07 | 0.38–4.60 |
| Moisture (water), % | 11.66 ± 0.11 | 5.20–14.65 |
| HHV, MJ/kg (d.b) | 18.86 ± 0.52 | 20.83–19.47 |
| LHV, MJ/kg (d.b) | 17.71 ± 0.57 | 19.37– 17.22 |
| Specific ash melting temperatures (prepared at 550 °C) | ||
| SST, °C | 794 ± 10 | 1000–1300 |
| DT, °C | 802 ± 6 | 1100–1200 |
| HT, °C | 810 ± 4 | 1160–1350 |
| FT, °C | 819 ± 4 | 1160 → 1350 |
| Element | Concentration of Corn Cob, mg/kg | Non-Specified Wood Pellets [35,36] | Concentration of Dolomite, mg/kg |
|---|---|---|---|
| Al | 408.88 ± 17.79 | 4.9–1360 | 7.12 ± 0.73 |
| Ca | 2781.00 ± 249.46 | 303–16,000 | 25651.98 ± 502.78 |
| Cr | <0.90 | 0.083–27 | <0.90 |
| Cu | 3.86 ± 0.01 | 0.36–46 | 0.41 ± 0.0002 |
| Fe | 316.76 ± 10.39 | 9.5–1460 | 2212.55 ± 100.67 |
| K | 6035.85 ± 300.59 | 167–9833 | 5502.78 ± 12.66 |
| Mg | 291.48 ± 17.02 | 58–1620 | 4669.38 ± 153.62 |
| Mn | 7.81 ± 1.03 | 22–702 | 317.71 ± 9.50 |
| Na | 322.32 ± 12.70 | 8.4–973 | 386.34 ± 11.01 |
| P | 347.91 ± 24.35 | 22.1–1012 | 110.13 ± 4.09 |
| Si | 5749.60 ± 320.25 | 115–2694 | 4.00 ± 0.29 |
| Ti | 27.16 ± 1.70 | 2.65–3.42 | 7267.67 ± 210.04 |
| Zn | 7.63 ± 0.50 | 1.20–90 | 108.71 ± 5.21 |
| Pb | 10.36 ± 0.89 | 0.04–11 | 11.60 ± 0.09 |
| Parameter | Data |
|---|---|
| Thermal power | 500 kW |
| FBC height | 4.5 m |
| Bed height | 0.3 m |
| Internal diameter of fluidization area | 0.75 m |
| Internal diameter of combustion chamber | 0.85 m |
| Fluidizing agent | Quartz sand, 0.8–1.0 mm |
| Fluidizing air distribution plate | 19 bubble caps with 5 holes each |
| Element | Part by Weight of Corn Cob Ash, % | Part by Weight of Wood Pellet Ash [35,46], % |
|---|---|---|
| Al2O3 | 0.33 | 1.66–283 |
| CaO | 1.20 | 25.18–56.28 |
| Fe2O3 | 0.37 | 2.11–3.67 |
| MgO | 2.32 | 4.58–8.72 |
| P2O5 | 3.07 | 0.22–4.68 |
| K2O | 56.03 | 2.68–17.18 |
| SiO2 | 31.02 | 4.75–30.31 |
| Na2O | 0.05 | 0.63–1.09 |
| TiO2 | 0.01 | <0.01–0.29 |
| ZnO | 0.26 | <0.05 |
| PbO | 0.0004 | <0.01 |
| Bed Temperature | 650 °C | 700 °C | ||||||
| Fuel amount, kg/h | ~132 | ~132 | ||||||
| Thermal power, kW | ~500 | ~500 | ||||||
| Supply of PA and FGR mixture, m3/h | 180 | 180 | ||||||
| T2, °C | 690 | 689 | 715 | 705 | 734 | 756 | 803 | 744 |
| T3, °C | 986 | 981 | 1014 | 989 | 1005 | 1008 | 1014 | 995 |
| T4, °C | 752 | 778 | 740 | 733 | 756 | 754 | 726 | 692 |
| T5, °C | 830 | 865 | 805 | 800 | 873 | 889 | 819 | 829 |
| Ratio of PA/FGR | 0.42 | 0.49 | 0.58 | 0.65 | 0.22 | 0.33 | 0.38 | 0.44 |
| Secondary air, m3/h | 656 | 911 | 954 | 717 | 603 | 642 | 667 | 658 |
| SA to zone I, % | 34 | 25 | 29 | 31 | 19 | 20 | 33 | 30 |
| SA to zone II, % | 42 | 32 | 27 | 39 | 26 | 28 | 20 | 28 |
| SA to zone III, % | 5 | 4 | 3 | 4 | 13 | 15 | 1 | 3 |
| SA to zone IV, % | 43 | 39 | 41 | 40 | 42 | 38 | 46 | 49 |
| O2 concentration in flue gas, % | 3.1 | 3.3 | 3.8 | 4.1 | 2.27 | 2.98 | 3.5 | 3.1 |
| O2 concentration in PA/FGR mixture, % | 10.4 | 10 | 10.4 | 10.4 | 12.6 | 11.9 | 12.6 | 10.2 |
| Air equivalence ratio | 1.17 | 1.19 | 1.22 | 1.24 | 1.12 | 1.17 | 1.2 | 1.17 |
| CO, mg/m3 | 1168 | 1354 | 1402 | 1468 | 693 | 1018 | 971 | 748 |
| NOx, mg/m3 | 379 | 222 | 285 | 289 | 426 | 323 | 333 | 343 |
| Bed Temperature | 650 °C | 700 °C | ||
|---|---|---|---|---|
| Condition | W/O Dolomite | With Dolomite | W/O Dolomite | With Dolomite |
| Fuel amount, kg/h | 132 | |||
| Thermal power, kW | ~500 | |||
| Dolomite amount, kg/h | 0 | ~3.9 | 0 | ~3.9 |
| Supply of PA and FGR mixture, m3/h | 180 | |||
| Ratio of FGR/PA | 0.51 | 0.42 | 0.22 | 0.24 |
| Secondary air, m3/h | 736 | 769 | 658 | 667 |
| SA to zone I, % | 25 | 29 | 19 | 20 |
| SA to zone II, % | 32 | 36 | 26 | 27 |
| SA to zone III, % | 4 | 4 | 13 | 14 |
| SA to zone IV, % | 40 | 37 | 42 | 38 |
| O2 concentration in flue gas, % | 3.4 | 4.1 | 2.27 | 2.32 |
| O2 concentration in PA/FGR mixture, % | 10.1 | 10.4 | 12.6 | 11.9 |
| Air equivalence ratio | 1.19 | 1.24 | 1.12 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulauskas, R.; Praspaliauskas, M.; Ambrazevičius, I.; Zakarauskas, K.; Lemanas, E.; Eimontas, J.; Striūgas, N. Performance of Corn Cob Combustion in a Low-Temperature Fluidized Bed. Energies 2024, 17, 2196. https://doi.org/10.3390/en17092196
Paulauskas R, Praspaliauskas M, Ambrazevičius I, Zakarauskas K, Lemanas E, Eimontas J, Striūgas N. Performance of Corn Cob Combustion in a Low-Temperature Fluidized Bed. Energies. 2024; 17(9):2196. https://doi.org/10.3390/en17092196
Chicago/Turabian StylePaulauskas, Rolandas, Marius Praspaliauskas, Ignas Ambrazevičius, Kęstutis Zakarauskas, Egidijus Lemanas, Justas Eimontas, and Nerijus Striūgas. 2024. "Performance of Corn Cob Combustion in a Low-Temperature Fluidized Bed" Energies 17, no. 9: 2196. https://doi.org/10.3390/en17092196
APA StylePaulauskas, R., Praspaliauskas, M., Ambrazevičius, I., Zakarauskas, K., Lemanas, E., Eimontas, J., & Striūgas, N. (2024). Performance of Corn Cob Combustion in a Low-Temperature Fluidized Bed. Energies, 17(9), 2196. https://doi.org/10.3390/en17092196





