Abstract
Lithium–sulfur batteries suffer from a reduced cycle life and diminished coulombic efficiency, which is attributed to the polysulfide shuttle effect. We herein present a process for the fabrication of lithium–sulfur battery cathode material via the recrystallization of dissolved sulfur inside self-assembled carbon nanospheres synthesized through the carbonization of d-glucose. Trapping sulfur in the carbonaceous matrix lessens the rapid dissolution of polysulfides and minimizes the loss of active sulfur, thus extending the cycling stability of these batteries. The carbon–sulfur composite material was characterized via X-ray diffraction (XRD), field emission scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). Electrochemical analysis of the material and its functionality as an electrode for lithium–sulfur battery systems was evaluated in a coin cell format using impedance spectroscopy and a life cycle study. The as-prepared cathode has shown remarkable electrochemical performance with a specific capacity of 781 mA/g at 0.1 C after 500 charge/discharge cycles and 83.4% capacity retention.
Keywords:
lithium–sulfur; Li-S; carbon; nanospheres; nanotechnology; cathode synthesis; carbonization; d-glucose; hydrothermal 1. Introduction
Lithium–sulfur batteries are thought to herald the next energy storage revolution due to their elevated theoretical energy density of 2600 Wh/kg, a significant increase over current technologies. This amelioration of the performance stems from the ability of this system to utilize lithium metal anodes by donating 2 Li+ ions to bond with each sulfur atom available at the cathode. This added electron transfer expands the energy of the system beyond what traditional lithium-ion batteries can offer and allows the replacement of expensive cobalt with abundant, cheap sulfur. Despite the remarkable feats of the lithium–sulfur system (Li-S), its commercialization has been plagued by a short cycle life, attributed to the polysulfide shuttle reaction, which reduces active sulfur products into resistive insoluble species such as Li2S and LiS. Briefly, during the redox reaction for lithium–sulfur batteries, lithium sulfide crystals formed through binding with lithium ions during previous discharge cycles are converted back into elemental sulfur through a long string of intermediate steps. The reaction is responsible for the production of subspecies of polysulfides which are highly soluble in organic electrolytes typical of Li-S batteries. Several methods have been proposed to curb the transfer of resistive sulfide species to the anode or their dissolution into the electrolyte: engineering of the carbon surface with functional groups to chemically anchor the polysulfides to the carbon pores [1]; employing catalytic transition metal nitrides, sulfide, or oxides to increase the efficiency of the chain reaction [2,3]; or physical entrapment of sulfur into conductive matrices [4,5] to limit the deposition of resistive lithium sulfides at the anode. Previous efforts have shown that capacity degradation of the lithium–sulfur system stems from the higher discharge plateau [6], since that is where nonrecoverable products of the redox reaction involving long chain polysulfide are formed; therefore, efforts to create carbon–sulfur nanocomposites and the confinement of sulfur would cull the full reduction in polysulfide chain products, as wrapped sulfur sites would act as sites with limited lithium reactivity [7,8,9]. Additionally, encapsulation of recrystallized sulfur aims to circumvent drawbacks in the conventional methods of carbon–sulfur integration, namely, the limited effectiveness of the ball milling technique and the energy cost associated with the melt method [7].
Carbon stands out among sulfur hosts for its abundance and its ability to form covalent bonds with other elements, including itself. This permitted researchers to develop many allotropes for use in various fields from energy storage to medicine delivery. Various methods have been employed for the synthesis of carbonaceous nanomaterials such as Chemical Vapor Deposition (CVD) [8] and polymer pyrolysis. Walle et al. [9] used the melt method to confine sulfur in double hollow nanospheres (S-DHCS) integrated into carbon nanotubes (CNTs). The diameter of these S-DHCS/CNTs ranged from 20 to 200 nm and demonstrated decent electrochemical performance in a lithium–sulfur system of 730 mAh/g after 48 cycles at 0.2 C. The authors attributed the superior functioning to the successful confinement of sulfur into the carbonaceous vessel, which minimized both the polysulfide dissolution reaction and the volumetric growth of sulfur during cycling [9]. Other efforts concentrated on hosting sulfur in carbonaceous structures formed through the carbonization of polyacrylonitrile (PAN) to obtain SPAN electrodes with excellent electrochemical performance. These efforts customarily involve processes necessitating high-energy ball milling accompanied with high-temperature annealing or the utilization of high-cost, complicated carbonaceous frameworks such as carbon nanoribbons [10,11].
Hydrothermal polycondensation of glucose has been demonstrated as an efficient yet simple technique for the formation of carbon nanospheres of variable sizes [12,13]. The technique allows greater control of the sphere properties such as size and porosity. Sun et al. [14] demonstrated the possibility of encapsulating nanoparticles into the core of colloidal carbon nanospheres prepared hydrothermally. In their studies, they found that the diameter of the prepared spheres depended on the formation time almost linearly and identified 9 h as a cut off limit after which the diameter of the spheres would be negatively impacted [14]. Karna et al. found that increasing the formation temperature of the hydrothermally prepared carbon nanospheres beyond a critical optimal point negatively impacts the diameter of the prepared spheres [15].
Based on these findings, we developed a novel, facile synthesis and integration process for confining sulfur in hydrothermally grown carbon nanospheres using sulfur recrystallization. The extensive literature search on the topic of sulfur encapsulation into carbonaceous structures highlighted the benefits offered by using carbon nanospheres in the Li-S battery realm and the versatility of the facile recrystallization of sulfur. The drawbacks of the reported development techniques of these materials include their complicated formation methods and high consumption of time, materials and energy. Our approach presents a novel preparation process. The material development involves little reliance on toxic chemicals, is energy-efficient and takes advantage of basic properties (carbonization of glucose and recrystallization of sulfur), making this process easily scalable. Similar published efforts have typically relied on building scaffolds for the formation of spheres or fabricated spheres separately and inserted molten sulfur into their porous structure, which then necessitated elaborate engineering of surface pores. Our work provides competitive results with a simple, direct, one-pot synthesis technique. It was found that the confinement of sulfur into rigid carbonaceous spheres resulted in a specific capacity of 781 mA/g at 0.1 C after 500 charge/discharge cycles, with an 83.4% capacity retention.
2. Materials and Methods
D-glucose (MW: 180.16 g/mol), xylenes (Reagent grade, MW: 106.17 g/mol), sulfur powder (99.98% purity, MW: 32.07 g/mol) and polyvinylidene fluoride (PVDF, MW: 534,000 g/mol) were purchased from Sigma Aldrich (St. Louis, MO, USA); 1-methyl-2-pyrrolidone (NMP, MW: 99.13 g/mol) and Super P were obtained from Millipore Sigma (Burlington, MA, USA) and Timcal USA Corp. (Westlake, OH, USA), respectively. These chemicals were used without further modification.
The 0.5 M aqueous glucose solution was prepared by stirring d-glucose in deionized water until a clear solution was obtained. Concurrently, sulfur was stirred in an appropriate volume of xylenes at 115 °C to create a clear yellow solution. The glucose solution temperature was increased to 115 °C and mixed with the sulfur solution such that the glucose to sulfur mass ratio was 40:60. The mixture was stirred for 1 h until phase separation was no longer noticeable.
The mixture was subsequently placed in a Teflon-lined autoclave and kept at 180 °C in a box furnace for 9 h. The reacted samples were then centrifuged and washed repeatedly in DI water to remove unreacted sulfur and traces of xylene. Clean samples were dried for 24 h in a vacuum oven at 45 °C and labeled as CNS-S.
A slurry was fabricated by mixing CNS-S with Super P carbon and PVDF in an 8:1:1 ratio. The slurry was sonicated for 1 h, degassed for 30 min to remove all trapped gaseous bubbles, then stirred for 48 h. Using a doctor blade, the active material was spread on aluminum foil and dried at 70 °C for 12 h under vacuum to remove all trace of NMP. Then, 1 cm2 disks were cut to be used as cathodes for lithium|carbon nanospheres–sulfur (CNS-S) coin cells. Sulfur loading per electrode was 2.44 mg cm−2. Next, 20 µL of a basic electrolyte for Li-S batteries, prepared by mixing an appropriate amount of 1 M LiTFSI, 2 wt% LiNO3 in 1,2-dimethoxyethane (DME) and 1,3-dioxolane (DOL) (v/v = 1:1), was used to wet a polypropylene membrane (Celgard 240. Charlotte, NC, USA) used as a separator. Cell assembly post-synthesis was undertaken in an argon-filled glovebox (Gen1 NEXUS, Vacuum Atmospheres, Hawthorne, CA, USA) operating at <0.1 O2 PPM, <0.2 H2O PPM).
Scanning electron microscopy (SEM, JSM 7600 FE. JEOL Technology Center, New England, USA ) was used to study the morphology of the prepared carbon–sulfur electrode. Transmission Electron Microscopy (TEM) and Elemental Mapping (EDS) of the CNS-S material was conducted using a TALOS F200XS/TEM (Thermo Fisher Scientific, Waltham, MA, USA) device. An X-ray powder diffractometer (XRD, Bruker D2 Phaser, Bruker Corp., Allentown, PA, USA) was used to characterize the crystalline structure of the formed material through a 2Ɵ scan between 10 and 80 degrees. Thermogravimetric analysis (TGA, TA instruments Q5000, TA instruments, NEW Castle, DE, USA) was used to analyze the composition of the prepared samples between the temperatures of 0 and 800 °C. Electrochemical Impedance Spectroscopy (EIS) and cyclic voltammetry (CV) measurements were conducted on a PARSTAT 2200 (Ametek, Rochester, NY, USA, and charge/discharge tests were performed using a MACCOR 4200 (Maccor, Tulsa, OK, USA) system at 25 °C.
3. Results
The formed carbon spheres lack crystallinity, which is evident from the broadened peaks in the XRD spectra (CNS) in Figure 1A. The hydrothermal carbonization of biomass has been reported to yield varying carbonaceous structures at temperatures higher than 170 °C [16]. This polycondensation reaction of glucose proceeds through the development of intermediate furan species and culminates in the creation of long, carbon-rich, polymeric chains that curl into a nucleus under the influence of pressure of around 1 MPa. The standard (002) graphite peak (Figure 1A) at 26.6° has given way to a broad “hump” centered at 24°, typical of amorphous carbon that lacks periodic arrays with long-range orders. Previous efforts in sulfur hosting have shown the ease with which amorphous carbon can form stronger bonds with polysulfides because of the elevated adsorption energies it possesses over traditional crystalline carbons such as graphite [17].

Figure 1.
(A) X-ray diffraction of carbon nanospheres, carbon nanospheres–sulfur composite and elemental sulfur. (B) Thermogravimetric analysis of carbon nanospheres-sulfur composite sample.
Crystalline peaks appear in the spectra of the sample with integrated sulfur: standard sulfur peaks can be seen in the CNS-S sample (Figure 1A), where a strong characteristic peak at 23° (222), part of the orthorhombic phase of sulfur corresponding to the Fddd space group, is visible. Other standard sulfur peaks such as ones at 28.7° (026) and 37.4° are also present, indicating the successful recrystallization of sulfur in the CNS sample. Unlike XRD peaks for fully confined sulfur as demonstrated in Takahashi et al.’s work [18], Figure 1A shows the characteristic sulfur peaks in the CNS-S, which indicate the presence of free and carbon-bonded sulfur on the surface, similar to what was observed in the related literature [19,20].
Based on previous endeavors [7,18,21], it is likely that entrapment of sulfur in carbon or the creation of C-S composites alters the TGA curve of the material in such a way that the thermal decomposition of sulfur is slower and pushed to higher temperatures. In Figure 1B, complete weight loss of the sample occurs past 600 °C (zone 3), which is in agreement with references [18,22] and where the eventual burn off of carbon occurs, similar to biomass amorphous carbon TGA behavior reported elsewhere [22]. Typically, sublimed sulfur is entirely evaporated by 300 °C [22], and a sharp drop occurs between 200 and 300 °C (zone 1) to account for unbonded sulfur on the surface of the sample. The disappearance of the bound sulfur is slower (zone 2) and requires higher temperatures to overcome carbon–sulfur bonding [23]. This is likely due to the unraveling of the S8 chain into a covalently bonded chain as reported elsewhere [14,20]. The differential weight curve (Figure 1B) shows thermal evaporation events occurring in two regions. Encapsulation of sulfur likely resulted in the absence of traditional differential peaks around 240 °C, which are expected for standalone elemental sulfur and water vapor.
The SEM image in Figure 2a shows a relatively uniform field of nanospheres that range in size from 1 to 10 µm. Figure 2b is a zoomed-in view of a sphere cluster, taken using the lower detector in the SEM (L mode) to reduce the charging effect, which highlights the sphere size distribution. Figure 2c,d are TEM images that present standalone nanospheres demonstrating the versatility and the success of the technique of d-glucose carbonization in producing uniform, nanosized particles. These images show colloidal, independent spheres without the exclusions of hexagonally shared borders. Multitudinous studies of colloidal carbon spheres [12,13,14] have linked their formation and diameter size to the unraveling of the glucose during a polymerization process into an arboreal oligosaccharide form which culminates in the formation of a carbon core through dehydration [24]. EDS of the sample (Figure 2e,f) shows sulfur particles scattered on the sample surface, with the highest concentration present on the sphere itself. This further demonstrates that the reactions of the cell take place primarily at the spheres themselves without discounting the contribution from free sulfur.

Figure 2.
(a) SEM images of CNS-S sample showing the relative homogeneity of the formed spheres. (b) Focused image on a cluster of spheres with shared edges. (c) TEM Images of a standalone nanosphere. (d) TEM image of identical nanospheres (e) EDS of sulfur. (f) EDS of carbon.
Figure 3 shows the electrochemical performance of CNS-S as a cathode for lithium–sulfur batteries. Figure 3A shows the specific discharge capacity of the cell during its life cycle test. The capacity for the initial cycles increases as the SEI layer matures and the lithium redistributes within the layer into a stable aggregation. This maturation of the SEI layer is reflected in the fluctuation in the coulombic efficiency during the earlier cycling phases. Typically, lithium–sulfur batteries suffer from low coulombic efficiency due to the polysulfide shuttle [25], and that effect is also likely taking place with the unbound surface sulfur that is likely dissolving into the electrolyte during redox. The coulombic efficiency of the sample is less than 100, however stable. This reduction in efficiency may be attributed to the difficulty of sulfur extraction through the confines of the carbonaceous structure in the sense that there is limited polysulfide loss during cycling. The sample demonstrates an excellent capacity retention between cycles 112 and cycle 500 (83.4%), which outperforms some of the reported works investigating other entrapment techniques: Fan et al. achieved 54.4% capacity retention after 400 cycles through the entrapment of sulfur into carbon nanotubes [4]. Kim et al. confined sulfur in carbon micropores and demonstrated 36% retention of capacity after 250 cycles [26]. Walle et al. trapped sulfur in double hollow spheres to achieve 57% retention at a 0.2 C rate and 48 cycles [9]. Some of these findings are summarized in Table 1:
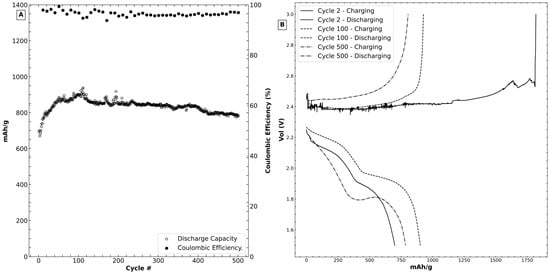

Figure 3.
(A) The cycle life of a CNS-S cell at C/10. (B) Charge and discharge curves corresponding to cycles 2, 100 and 500. (C) Rate capability of the CNS-S cell. (D) Cyclic voltammogram of the CNS-S cell.

Table 1.
A concise summary of previous efforts on cathode designs for Li-S electrodes.
During discharge, solid sulfur (S8) is reduced into a lithium polysulfide after dissolution in the electrolyte, which is reflected in a stepwise voltage discharge profile analogous to what is shown in Figure 3B (cycle 2). A plateau is visible in the discharge voltage vs. Li reference (2.4 to 2.2 V) associated with reducing elemental sulfur to Li2S4. A secondary plateau (around 2.1 V vs. lithium) is strongly associated with the formation of Li2S2. The tail end of this discharge region, where the plateau begins to noticeably slope downwards, brings about the formation of the insoluble Li2S species [28]. This behavior is reflected in the literature and can be due to the activation of the cathode [26], which in turn leads to the better distribution of the sulfur species and a slower formation of a stable SEI layer. It has been demonstrated in previous efforts that capacity degradation of the lithium–sulfur system occurs mostly in the higher plateau region [6], which is present in the discharge voltage plot in Figure 3B (cycles 2 and 100) but clearly absent from cycle 500. This in turn might explain the discharge capacity retention that the CNS-S cell demonstrates after the 112th cycle [13,16,27].
The disappearance of the high plateau post-cycle 100 may be the result of the depletion of surface sulfur, and hence the only reactive sulfur remaining in the system is trapped within the confines of the nanospheres, where it may act as a polysulfide reserve, which parallels findings by other groups [23]. The discharge plot of cycle 500 exhibits a complete absence of the upper plateau, which in turn is reflected by lower capacity compared to earlier cycles. At the lower plateau, the lithiation process likely encounters a mature SEI with areas of differing reaction kinetics leading to a slight rise in the plateau at 1.8 V. On the charge side, we notice that the noisy and extended plateau seen in cycle 2 in Figure 2B is absent as the SEI matures. The root cause of this initial charge behavior is arduous to deconvolute because of the profuse possible factors that contribute to it. Initial lithium–sulfur formation reactions are marred by volumetric expansion and morphological cathodic changes, which are translated into problems in mass transport within the cell. Additionally, embryonic cycles on confined sulfur may exhibit slower reaction kinetics because of sulfur dissolution or the deposition of a passivating layer on the electrolyte/electrode interface [29]. Subsequent cycles show symmetrical reversibility with their discharge counterparts. To gain insights into the power-handling ability of the developed material, a rate capability study of the cell was conducted (Figure 3C). Much like cycling at 0.1 C, the initial capacity of the cell rises initially before stabilizing even at the high rate of 0.5 C. The SEI layer likely forms in a drastically faster fashion than the 0.1 C cell; however, considering the recovery and the capacity retention when the current load returns to 0.5 C, this rapid SEI formation likely does not cause any lasting physical damage to the electrode. The capacity drops as a response to increased current loading, which highlights the difficulty of rapid sulfur extraction from the innards of the carbonaceous matrix. The electrode capacity recovers its original value as the current loading returns to C/2, possibly indicating minimal physical damage to the SEI, or likely no rapid decline of the electrochemical redox reaction takes place due to the high-power demand. Between each group of discharge rate cycles, the decrease in lower capacity is marked by a midpoint value for capacity, indicating that not all sulfur is trapped inside carbon, as some of the surface sulfur contributes to the capacity even at higher rates. This excellent capacity retention at the end of the 500th cycle (83.4%) of the material is likely due to the sequestration of sulfur into the carbonaceous matrix, which might slow the loss and aggregation of the active material through the polysulfide redox mechanism. Cyclic voltammetry of the cell (Figure 3D) was undertaken by sweeping a 0.1 mV/s potential and monitoring the redox reactions at the interface of the electrodes with the electrolyte through the collection of the current response. The reduction peak between 1.9 and 2.0 V is reduced in magnitude as the cell cycles. Another noticeable feature present in the post-cycling sweep is the superposition of the reduction curves, indicating the occurrence of changes in the level of reduced species between cycles. We notice that the oxidation peak for the initial cycle between 2.6 V and 3 V exhibits noise, which is likely due to the SEI formation. These turbulences are absent from subsequent cycles.
EIS of the cell (Figure 4A) was used to study the shift in impedances of the cell upon cycling. Post-assembly (pre-cycling), a charge transfer impedance ending at unusually higher ohmic values than a traditional carbon–sulfur electrode is observed: A semi-circle is apparent as the response to the current excitation signal that starts near 10 ohms and diverges from the x axis towards the Walburg tail at the x axis at 340 ohms. The semi-circle indicates ionic transfer kinetics through the SEI, and this elevated value in the CNS-S sample highlights the difficulty of the ionic transfer. There is a slight amelioration of the charge transfer impedance post-cycling (100 ohm), which confirms the view that the stability of the SEI is improved upon cycling. The higher charge transfer impedance is not unexpected as carbon formed through carbonization of sugars is rarely graphitic in nature [15], and, as observed during charge/discharge cycling, the initial capacities grow as the interfaces stabilize. Post-cycling, there is a growth of a secondary diffusion semi-circle terminating near 250 ohms. This is an indicator of the change in the source of impedance in this system as the cell goes through charge/discharge cycles. The Warburg tail in the low-frequency region is at 45 degrees in both pre- and post-cycling plots, suggesting that the real and imaginary parts of the impedance are near equal in absolute value, which in turn is an indicator of the reversibility of the reaction occurring inside the cell. Typical impedance spectra of redox systems involving diffusion processes often display more than 1 semi-circle. While it is established that the semi-circle in the high-frequency region can be attributed to the charge transfer resistance of the sample, the secondary semi-circle, as shown in Figure 4B, occurring in the mid region before the Warburg tail, is commonly ascribed to diffusion impedance. As the shuttling of ions is restricted by both the entrapment of sulfur and the likely arduous lithium penetration into the confines of the carbon, frequency-dependent impedance of diffusion likely takes place as attested by other endeavors on the matter [30]. A third, barely discernible semi-circle in the low-frequency region is visible due to inconsistent diffusion behavior in the bulk of electrode.

Figure 4.
(A) EIS scan for CNS-S prior to cycling. (B) EIS scan for CNS-S post-500 cycles.
4. Conclusions
A novel facile synthesis technique for a carbon–sulfur system was developed to deliver stable capacity to lithium–sulfur batteries. Trapping recrystallized sulfur into the carbonaceous matrix of nanospheres successfully curtailed the loss of polysulfides to the shuttle effect and bestowed a reversible high capacity of the lithium–sulfur system. Thus, the CNS-S system delivered a stable, room-temperature-specific capacity of 781 mA/g at 0.1 C after 500 charge/discharge cycles and 83.4% capacity retention, which is noticeably superior to analogous previous efforts. The entrapment technique shows great potential as a means of producing long-life lithium–sulfur batteries.
Author Contributions
Conceptualization, W.F. and K.Y.S.N.; formal analysis, W.F. and K.Y.S.N.; funding acquisition, K.Y.S.N.; investigation, W.F. and Z.W.; methodology, W.F. and Z.W.; supervision, W.F. and K.Y.S.N.; validation, W.F. and Z.W.; writing—original draft, W.F.; writing—review and editing, K.Y.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fawaz, W.; Mosavati, N.; Abdelhamid, E.; Ng, K.Y.S. Synthesis of activated carbons derived from avocado shells as cathode materials for lithium–sulfur batteries. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Mosavati, N.; Salley, S.O.; Ng, K.S. Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J. Power Sources 2017, 340, 210–216. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, W.; Ng, K.Y.S. Facile Synthesis of CoS Nanoparticles Anchored on the Surface of Functionalized Multiwalled Carbon Nanotubes as Cathode Materials for Advanced Li–S Batteries. Ind. Eng. Chem. Res. 2022, 61, 9322–9330. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Li, J.; Yang, K.; Liang, Z.; Chen, Y.; Zhao, C.; Zhang, Z.; Mai, K. A general dissolution–recrystallization strategy to achieve sulfur-encapsulated carbon for an advanced lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 11664–11669. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Shen, Z.; Zhang, H.; Han, T.; Guan, Y.; Tian, Y.; Braun, P.V. A Lamellar Yolk–Shell Lithium-Sulfur Battery Cathode Displaying Ultralong Cycling Life, High Rate Performance, and Temperature Tolerance. Adv. Sci. 2022, 9, 2103517. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Fu, Y.; Cochell, T.; Manthiram, A. A strategic approach to recharging lithium-sulphur batteries for long cycle life. Nat. Commun. 2013, 4, 2985. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Cui, J.; Li, J.; Li, Q.; Liu, M.; Xi, Y. Mesoporous carbon prepared by etching halloysite nanotubes (HNTs) with pyrrole as a precursor for a sulfur carrier of superior lithium–sulfur batteries. RSC Adv. 2019, 9, 12331–12338. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Walle, M.D.; Liu, Y.-N. Confine sulfur in double-hollow carbon sphere integrated with carbon nanotubes for advanced lithium–sulfur batteries. Mater. Renew. Sustain. Energy 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–Sulfur Battery Cathodes Based on PAN–Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, S.; Sun, S.; Zhang, X. Fe3C Decorated N, Fe Co-Doped Hollow Carbon Microspheres as Efficient Air Electrode Catalyst for Zinc-Air Battery. ChemistrySelect 2022, 7, e202201503. [Google Scholar] [CrossRef]
- Yamuna, A.; Sundaresan, P.; Chen, S.-M. Citrate stabilized gold nanoparticles on graphenic carbon spheres for the selective detection of hydrazine. Microchem. J. 2019, 151, 104234. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Karna, P.; Ghimire, M.; Mishra, S.; Karna, S. Synthesis and Characterization of Carbon Nanospheres. OALib 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Jeon, T.; Lee, Y.C.; Hwang, J.-Y.; Choi, B.C.; Lee, S.; Jung, S.C. Strong lithium-polysulfide anchoring effect of amorphous carbon for lithium–sulfur batteries. Curr. Appl. Phys. 2020, 22, 94–103. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamagata, M.; Ishikawa, M. A sulfur–microporous carbon composite positive electrode for lithium/sulfur and silicon/sulfur rechargeble batteries. Prog. Nat. Sci. 2015, 25, 612–621. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gomez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef]
- Yang, X.; Yan, N.; Zhou, W.; Zhang, H.; Li, X.; Zhang, H. Sulfur embedded in one-dimensional French fries-like hierarchical porous carbon derived from a metal–organic framework for high performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 15314–15323. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, J.W.; Park, J.; Ahn, J.-P.; Kim, K.-W.; Ahn, J.-H.; Nam, T.-H.; Wang, G.; Ahn, H.-J. High capacity cathode materials for Li–S batteries. J. Mater. Chem. A 2012, 1, 1573–1578. [Google Scholar] [CrossRef]
- De Fatima Salgado, M.; Abioye, A.M.; Junoh, M.M.; Santos JA, P.; Ani, F.N. Preparation of activated carbon from babassu endocarpunder microwave radiation by physical activation. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012116. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, K.; Tian, J.; Tang, Q.; Shan, Z. Preparation of yolk-shell sulfur/carbon nanocomposite via an organic solvent route for lithium–sulfur batteries. J. Solid State Electrochem. 2014, 18, 2077–2085. [Google Scholar] [CrossRef]
- La Mer, V.K. Nucleation in Phase Transitions. Ind. Eng. Chem. 1952, 44, 1270–1277. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Yu, S.-H.; Sung, Y.-E. Enhancement of cycle performance of Li–S batteries by redistribution of sulfur. Chem. Commun. 2015, 52, 1198–1201. [Google Scholar] [CrossRef]
- Lai, C.; Wu, Z.; Gu, X.; Wang, C.; Xi, K.; Kumar, R.V.; Zhang, S. Reinforced Conductive Confinement of Sulfur for Robust and High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23885–23892. [Google Scholar] [CrossRef]
- Zhou, L. Sulfur Reduction Reaction in Lithium–Sulfur Batteries: Mechanisms, Catalysts, and Characterization. Adv. Energy Mater. 2022, 12, 2202094. [Google Scholar] [CrossRef]
- Li, B.; Kong, L.; Zhao, C.; Jin, Q.; Chen, X.; Peng, H.; Qin, J.; Chen, J.; Yuan, H.; Zhang, Q.; et al. Expediting redox kinetics of sulfur species by atomic-scale electrocatalysts in lithium–sulfur batteries. InfoMat 2019, 1, 533–541. [Google Scholar] [CrossRef]
- Loew, N.; Watanabe, H.; Shitanda, I.; Itagaki, M. Electrochemical impedance spectroscopy: Simultaneous detection of different diffusion behaviors as seen in finite element method simulations of mediator-type enzyme electrodes. Electrochimica Acta 2022, 421, 140467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).