Hydrothermal Co-Liquefaction of Food and Plastic Waste for Biocrude Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
2.2. Hydrothermal Liquefaction Experiments
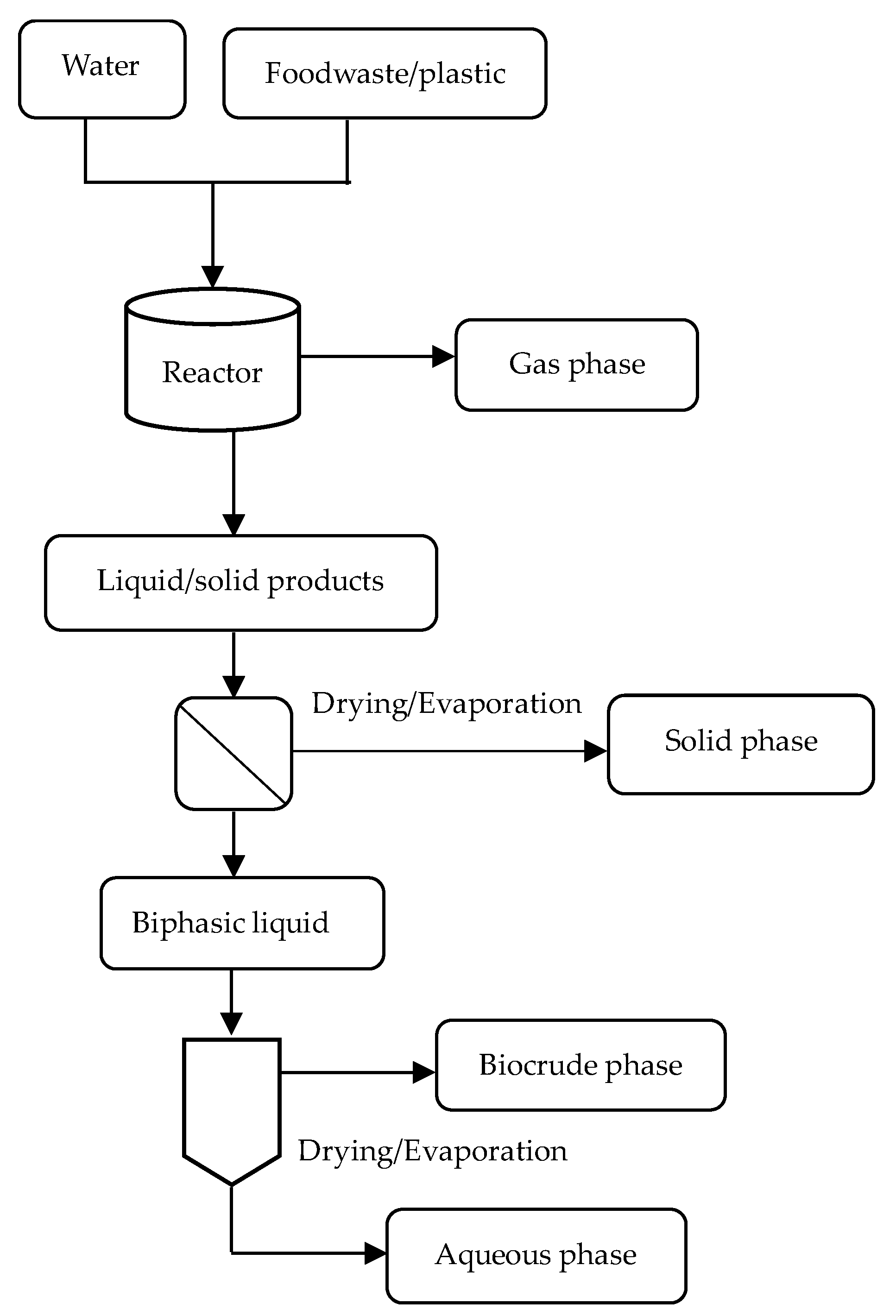
2.3. Product Separation and Characterization
2.4. Design of Experiments
3. Results and Discussion
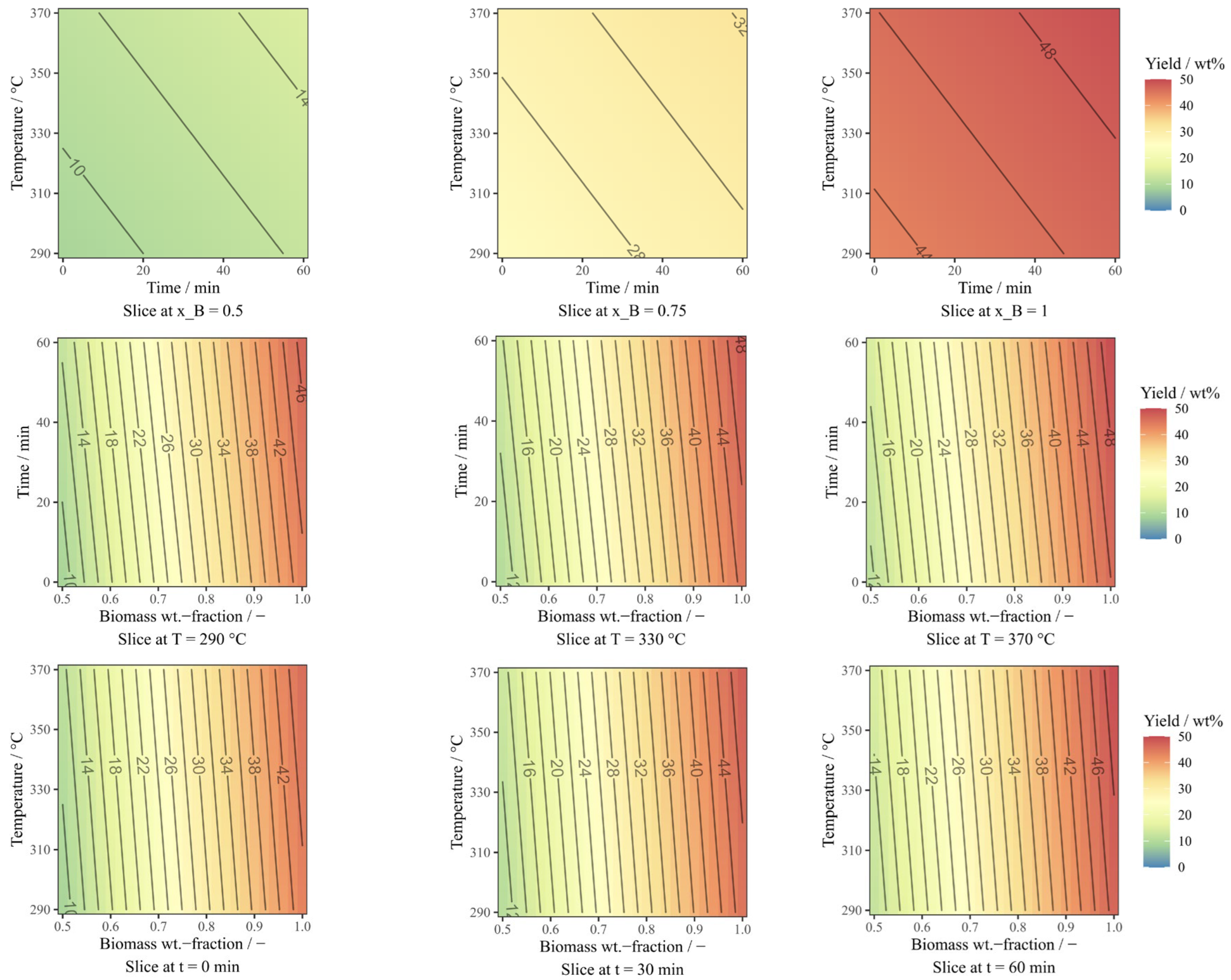
3.1. Statistical Evaluation & Response Surface Methodology
| Nr. - | Temp. °C | Time min | Biomass Frac wt.-frac | Gas a wt% | Aqu. b wt% | Biocrude wt% | Solids c wt% | Diff d wt% | HHVBiooil MJ/kg | ER e % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Experiments | T2 | 330 | 30 | 0.5 | 3.3 | 19.2 | 8.3 | 57.9 | 11.3 | 34.9 f | 8.0 |
| T7 | 370 | 30 | 0.5 | 7.5 | 15.8 | 13.8 | 51.4 | 11.5 | 34.9 | 15.1 | |
| T8 | 330 | 30 | 1 | 6.6 | 19.7 | 38.2 | 11.0 | 24.5 | 35.7 | 60.3 | |
| T9 | 330 | 30 | 0 | 5.5 | 6.5 | 0 | 101.3 | −13.4 | 22.9 f | 0 | |
| T10 | 370 | 30 | 0 | 1.6 | 4.5 | 6.1 | 87.9 | −0.1 | 41.9 f | 6.2 | |
| T11 | 330 | 30 | 0.9 | 5.1 | 20.7 | 36.7 | 20.5 | 17.1 | 35.5 | 53.1 | |
| T12 | 370 | 30 | 1 | 8.1 | 18.4 | 49.9 | 8.8 | 14.9 | 32.3 | 71.2 | |
| T13 | 370 | 30 | 0.9 | 10.6 | 16 | 38.3 | 19.3 | 15.9 | 35.7 | 55.8 | |
| Cube design | 1 | 370 | 60 | 0.5 | 12.2 | 20.5 | 16.0 | 48.4 | 2.8 | 37.3 | 18.7 |
| 2 | 290 | 60 | 0.5 | 6.8 | 15.7 | 13.8 | 52.9 | 10.8 | 35.2 | 15.2 | |
| 3 | 370 | 0 | 0.5 | 1.0 | 16.4 | 15.2 | 55.6 | 11.9 | 36.1 | 17.1 | |
| 4 | 330 | 30 | 0.75 | 4.8 | 18.6 | 31.5 | 31.9 | 13.2 | 36.0 | 41.5 | |
| 5 | 290 | 0 | 0.5 | 7.1 | 22.2 | 5.6 | 56.7 | 8.4 | 35.8 | 6.2 | |
| 6 | 290 | 60 | 1 | 7.7 | 24.7 | 48.6 | 11.3 | 7.8 | 36.5 | 78.4 | |
| 7 | 290 | 0 | 1 | 7.4 | 25.7 | 50.1 | 17.7 | −0.9 | 36.3 | 80.3 | |
| 8 | 370 | 0 | 1 | 3.5 | 19.5 | 42.2 | 7.8 | 27.1 | 36.6 | 68.2 | |
| 9 | 370 | 60 | 1 | 8.1 | 24.2 | 51.9 | 7.4 | 8.4 | 37.6 | 86.2 | |
| 10 | 330 | 30 | 0.75 | 4.9 | 23.2 | 30.7 | 33.2 | 8.0 | 36.8 | 41.3 | |
| Face centers | 11 | 290 | 30 | 0.75 | 1.0 | 24.3 | 25.5 | 36.7 | 12.6 | 35.6 | 33.2 |
| 12 | 330 | 60 | 0.75 | 5.2 | 20.3 | 28.9 | 32.5 | 13.1 | 36.8 | 38.9 | |
| 13 | 370 | 30 | 0.75 | 2.2 | 17.1 | 31.3 | 30.1 | 19.2 | 37.2 | 42.7 | |
| 14 | 330 | 30 | 0.75 | 5.1 | 24.3 | 28.3 | 33.8 | 8.6 | 36.6 | 38.0 | |
| 15 | 330 | 0 | 0.75 | 5.8 | 25.2 | 28.9 | 34.0 | 6.0 | 34.5 | 36.5 | |
| 16 * | 330 | 30 | 0.5 | 3.3 | 19.2 | 8.3 | 57.9 | 11.3 | 34.9 f | 8.0 | |
| 17 * | 330 | 30 | 1 | 6.6 | 19.7 | 38.2 | 11.0 | 24.5 | 35.7 | 60.3 |
3.1.1. Bio-oil Yield Model
3.1.2. Energy Yield and Energy Recovery Model
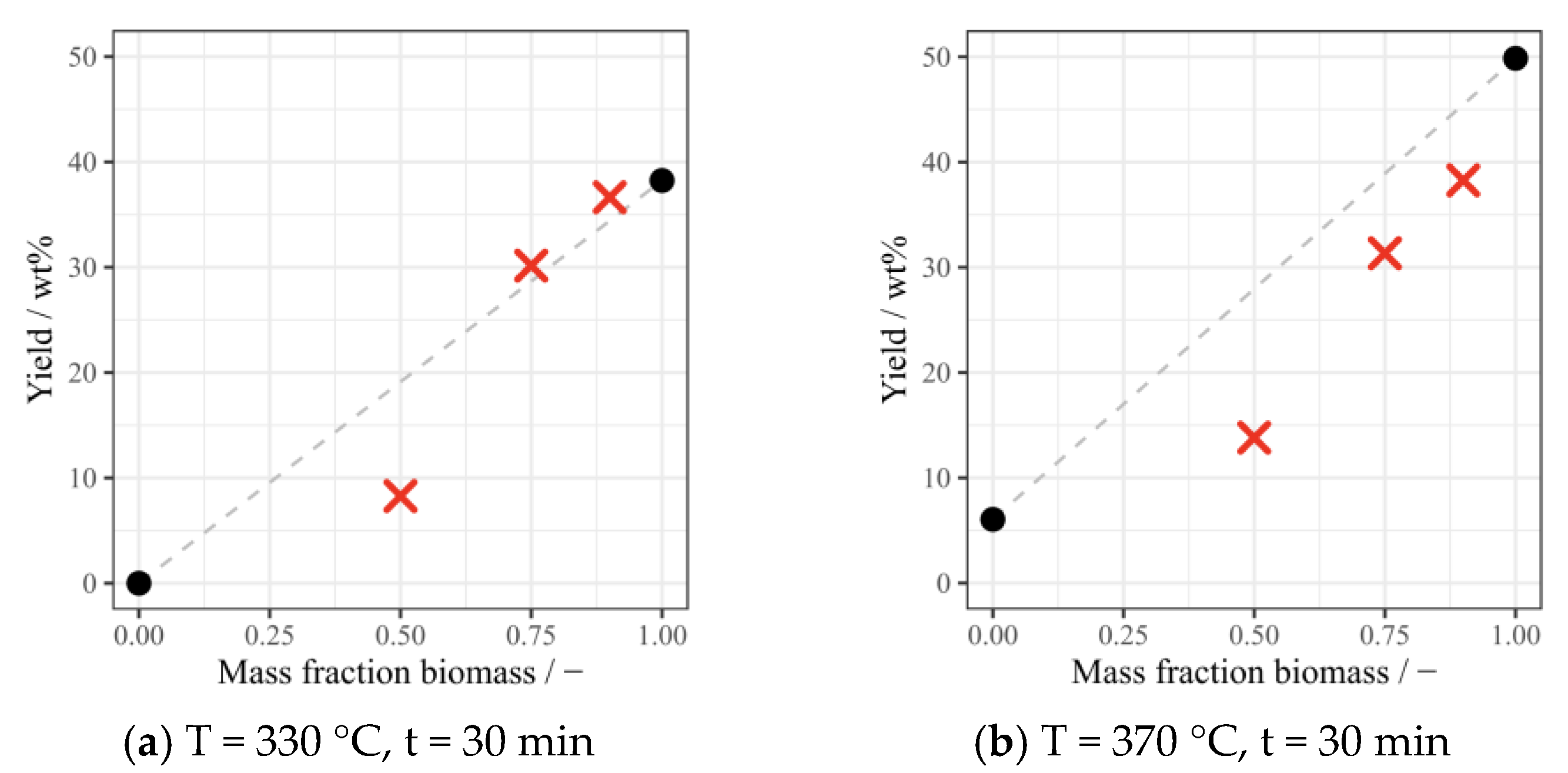
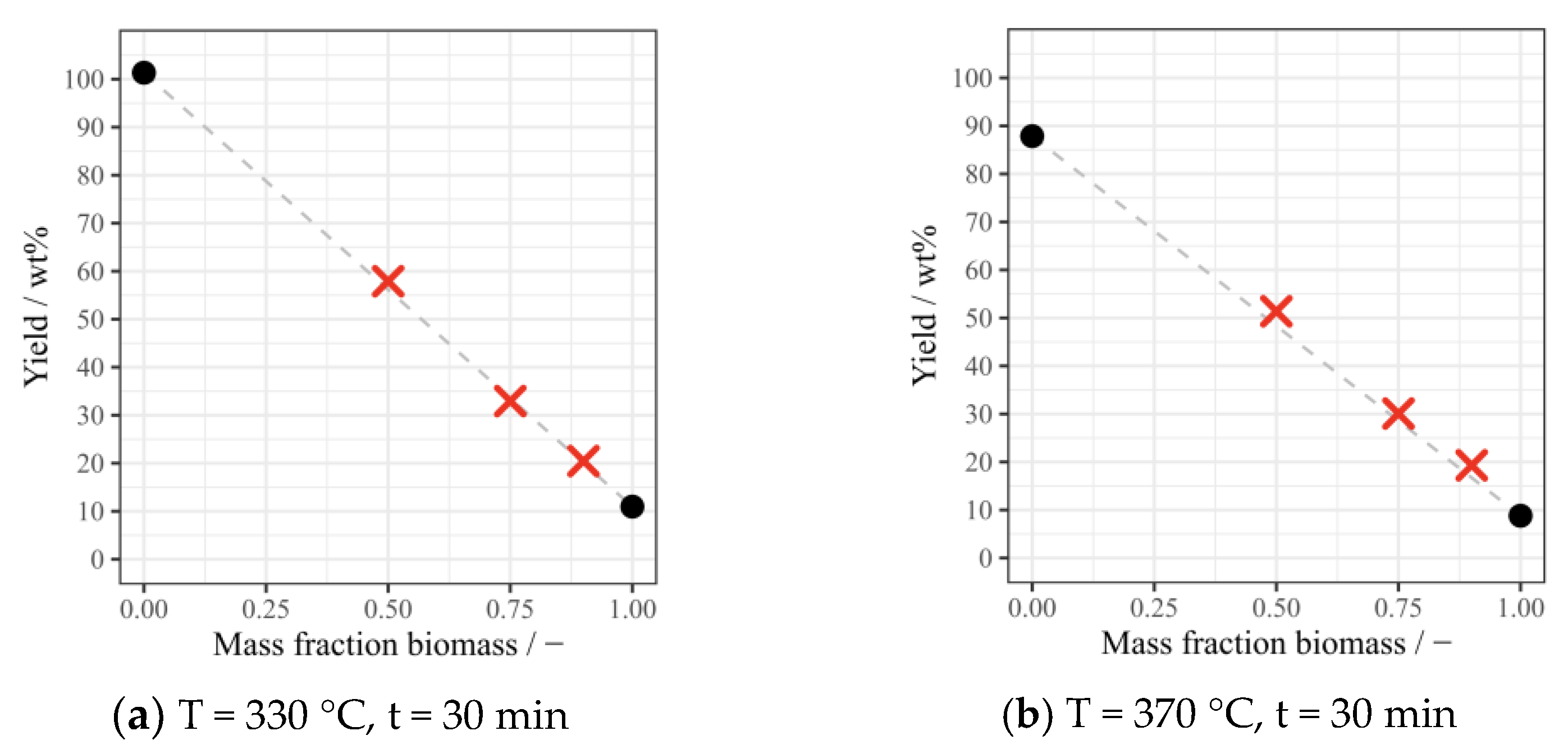
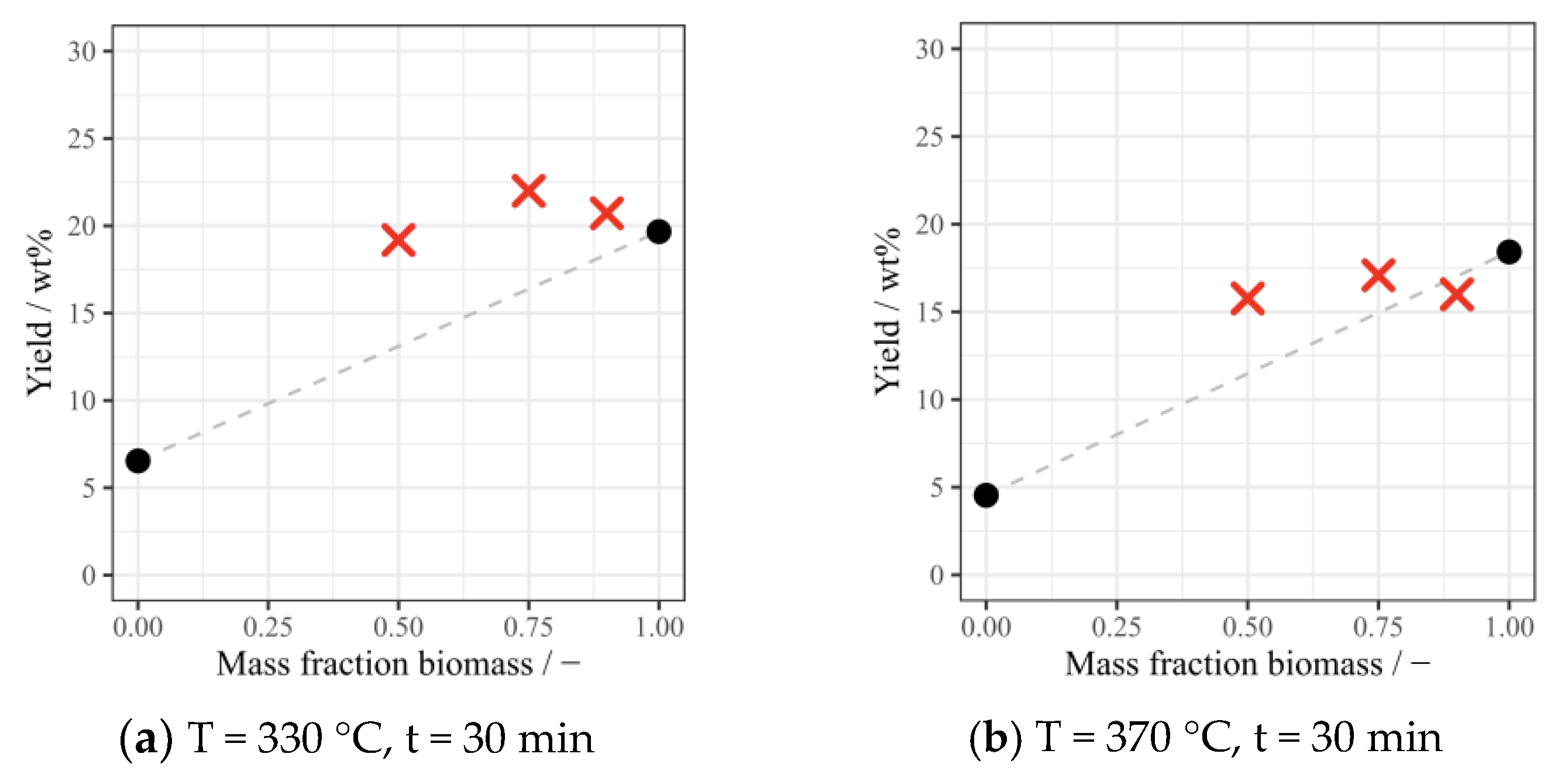
3.2. Yields of Bio-Oil and Solid Residue under Different Conditions
3.3. Chemical Characterization of Selected Results
3.3.1. Biocrude Phase
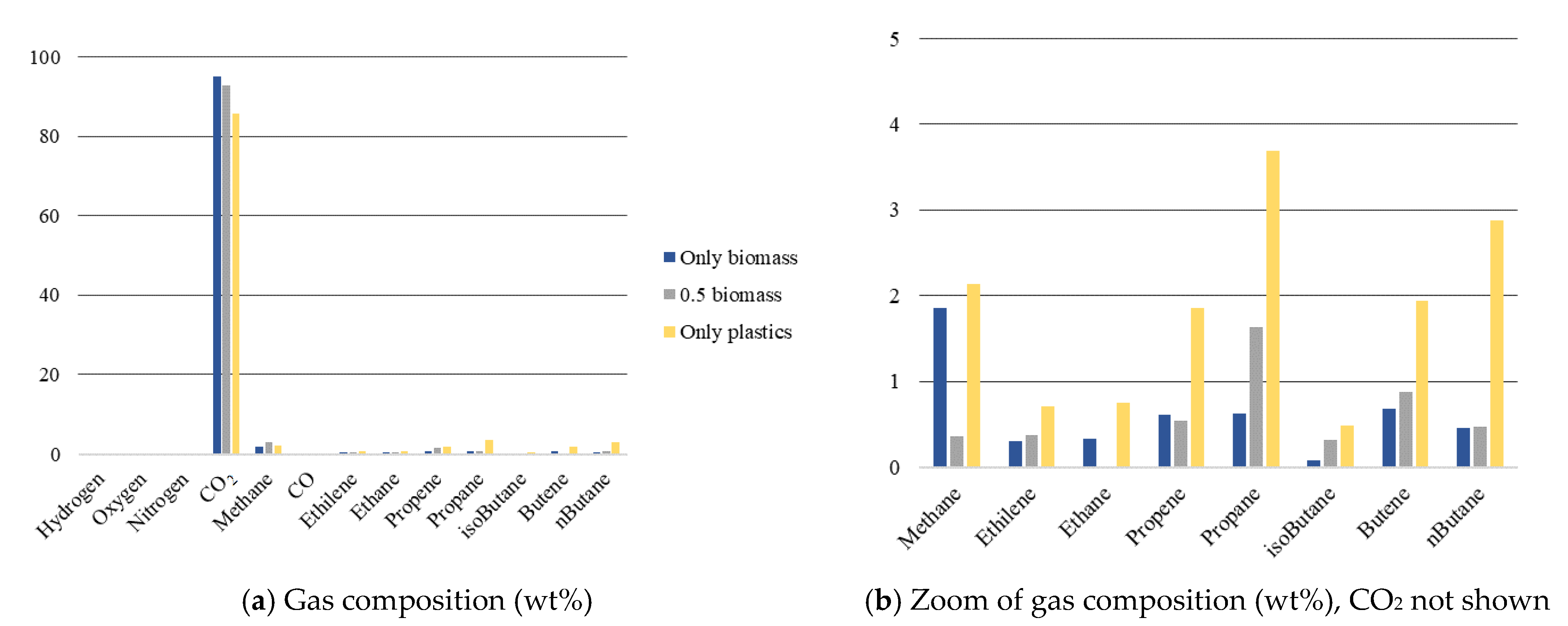
3.3.2. Gas Phase
3.3.3. Aqueous Phase
3.3.4. Solid Phase
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNFCCC. Paris Agreement; United Nations Framework Convention on Climate Change: Paris, France, 2015. [Google Scholar]
- WMO. State of the Global Climate 2023; World Meteorological Organization: Geneva, Switzerland, 2024; p. 53. [Google Scholar]
- IEA. World Energy Outlook 2023; International Energy Agency: Paris, France, 2023. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F.; lonkova, K.; Morton, J.; Poveda, R.A.; Sarraf, M.; Malkawi, F.; Harinath, A.S.; et al. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development Series; World Bank Group: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- Agência Portuguesa do Ambiente. Relatório Anual Resíduos Urbanos 2021; Agência Portuguesa do Ambiente: Amadora, Portugal, 2022. [Google Scholar]
- Woidasky, J. Plastics Recycling. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: London, UK, 2020; pp. 1–29. ISBN 978-3-527-30673-2. [Google Scholar]
- Kruse, A.; Dahmen, N. Water—A Magic Solvent for Biomass Conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Swetha, A.; ShriVigneshwar, S.; Gopinath, K.P.; Sivaramakrishnan, R.; Shanmuganathan, R.; Arun, J. Review on Hydrothermal Liquefaction Aqueous Phase as a Valuable Resource for Biofuels, Bio-Hydrogen and Valuable Bio-Chemicals Recovery. Chemosphere 2021, 283, 131248. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and Characterization of Hydrochars and Their Application in Soil Improvement and Environmental Remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Marin, N.; Beregovtsova, N.G.; Baryshnikov, S.V.; Kuznetsov, B.N.; Cebolla, V.L.; Weber, J.V. Co-Pyrolysis of Wood Biomass and Synthetic Polymer Mixtures. Part I: Influence of Experimental Conditions on the Evolution of Solids, Liquids and Gases. J. Anal. Appl. Pyrolysis 2002, 64, 15–28. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, H.; Li, H.; Zeng, G.; Tong, J.; Wang, L. Quantitative and Qualitative Analysis of Products Formed during Co-Liquefaction of Biomass and Synthetic Polymer Mixtures in Sub- and Supercritical Water. Fuel Process. Technol. 2009, 90, 428–434. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Savage, P.E. Oil from Plastic via Hydrothermal Liquefaction: Production and Characterization. Appl. Energy 2020, 278, 115673. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Savage, P.E. Synergistic Interactions during Hydrothermal Liquefaction of Plastics and Biomolecules. Chem. Eng. J. 2021, 417, 129268. [Google Scholar] [CrossRef]
- Dahlbo, H.; Poliakova, V.; Mylläri, V.; Sahimaa, O.; Anderson, R. Recycling Potential of Post-Consumer Plastic Packaging Waste in Finland. Waste Manag. 2018, 71, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Martín-Lara, M.; Godoy, V.; Quesada, L.; Martínez, D.; Peula, F.; Soto, J. Characterization of Plastic Materials Present in Municipal Solid Waste: Preliminary Study for Their Mechanical Recycling. Detritus 2018, 4, 104–112. [Google Scholar] [CrossRef]
- ISO 18134-3:2015; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 3: Moisture in General Analysis Sample. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 18122:2015; Solid Biofuels: Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 18123:2023; Solid Biofuels: Determination of Volatile Matter. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 16948:2015; Solid Biofuels: Determination of Total Content of Carbon, Hydrogen and Nitrogen. International Organization for Standardization: Geneva, Switzerland, 2015.
- SO 18125:2017; Solid Biofuels: Determination of Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 18134-1:2022; Solid Biofuels, Determination of Moisture Content, Part 1: Reference Method. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 18122:2022; Solid Biofuels: Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2022.
- DIN EN 15414-3:2011; Solid Recovered Fuels—Determination of Moisture Content Using the oven Dry Method—Part 3: Moisture in General Analysis Sample. Deutsches Institut für Normung e.V.: Berlin, Germany, 2011.
- ISO 1171: 2010; Solid Mineral Fuels: Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 2010.
- DIN EN 15402:2011; Solid Recovered Fuels—Determination of the Content of Volatile Matter. Deutsches Institut für Normung e.V.: Berlin, Germany, 2011.
- DIN EN15407:2011; Solid Recovered Fuels—Methods for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content. Deutsches Institut für Normung e.V.: Berlin, Germany, 2011.
- DIN EN 15408:2011; Solid Recovered Fuels—Methods for the Determination of Sulphur (S), Chlorine (Cl), Fluorine (F) and Bromine (Br) Content. Deutsches Institut für Normung e.V.: Berlin, Germany, 2011.
- DIN EN 15400:2011; Solid biofuels Determination of Calorific Value. Deutsches Institut für Normung e.V.: Berlin, Germany, 2011.
- ASTM D 240; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2019.
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R, Using Rsm. J. Stat. Soft. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 3rd ed.; Worth Publishers: New York, NY, USA, 2000; ISBN 978-1-57259-153-0. [Google Scholar]
- Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, Y.; Zhan, L.; Xie, B.; Gao, B.; Wang, J. Hydrothermal Treatment of E-Waste Plastics for Tertiary Recycling: Product Slate and Decomposition Mechanisms. ACS Sustain. Chem. Eng. 2019, 7, 1464–1473. [Google Scholar] [CrossRef]
- Su, L.; Wu, X.; Liu, X.; Chen, L.; Chen, K.; Hong, S. Effect of Increasing Course of Temperature and Pressure on Polypropylene Degradation in Supercritical Water. Chin. J. Chem. Eng. 2007, 15, 738–741. [Google Scholar] [CrossRef]
- Huang, J.B.; Zeng, G.S.; Li, X.S.; Cheng, X.C.; Tong, H. Theoretical Studies on Bond Dissociation Enthalpies for Model Compounds of Typical Plastic Polymers. IOP Conf. Ser. Earth Environ. Sci. 2018, 167, 012029. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A Review on Process Conditions for Optimum Bio-Oil Yield in Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]

| Feedstocks | MC | Ash a | VM a | FC b | C c | H c | N c | O c | S | Cl | HHV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | wt% | wt% | wt% | wt% | wt% | wt% | wt% | wt% | wt% | MJ/kg | |
| Food Waste | 7.4 | 11.5 | 74.4 | 6.7 | 54.4 | 8.7 | 5.5 | 16.84 | 0.36 | 1.8 | 24.11 |
| Paper | 8.4 | 1.6 | 77.8 | 12.2 | 46.5 | 5.0 | 0.4 | 45.98 | 0.12 | 0.2 | 18.24 |
| LDPE | 0.2 | 0.6 | 99.1 | 0.1 | 85.2 | 13.6 | 0.3 | 0 | 0.3 | n/a | 45.68 |
| PP | 0.1 | 1.6 | 98.4 | 0 | 78.8 | 12.2 | 0.4 | 7 | 0.1 | n/a | 44.32 |
| PS | 0.3 | 0 | 99.5 | 0.2 | 86.0 | 7.5 | 1.6 | 4.8 | 0.1 | n/a | 38.95 |
| PET * | 0.0 | 0.2 | 86.0 | 13.8 | 61.76 | 5.62 | 0.01 | 32.61 | n/a | n/a | 23.16 |
| Method: Food waste | ISO 18134-3: 2015 [17] | ISO 18122: 2015 [18] | ISO 18123: 2023 [19] | By difference | ISO 16948 [20] | By difference | Burning + ion chromatography | ISO 18125 [21] | |||
| Method: Paper | ISO 18134-1: 2022 [22] | ISO 18122: 2022 [23] | |||||||||
| Method: Plastic | DIN EN 15414-3:2011 [24] | ISO 1171: 2010 [25] | DIN EN 15402:2011 [26] | DIN EN 15407:2011 [27] | DIN EN 15408:2011 [28] | DIN EN 15400:2011 [29] | |||||
| Factor | Unit | Levels |
|---|---|---|
| Temperature | °C | [290, 330, 370] |
| Time | min | [0 a, 30, 60] |
| Biomass weight fraction | wt.% | [0.5, 0.75, 1] |
| S.No. | RT | Compounds | Rel. Area |
|---|---|---|---|
| 1 | 10 | Linoleic acid | 20.3 |
| 2 | 10.1 | Oleic acid | 20.3 |
| 3 | 9.2 | Hexadecanoic/palmitic acid | 14.6 |
| 4 | 10.2 | Octadecanoic/stearic acid | 6.8 |
| 5 | 9.1 | Palmitoleic acid | 3.3 |
| 6 | 10.3 | Hexadecanamide | 3.2 |
| 7 | 10.4 | Heptadecanamide | 3.0 |
| 8 | 11.4 | 9-Octadecenamide | 3.0 |
| 9 | 11.2 | cis-13-Eicosenoic acid | 2.3 |
| 10 | 10.7 | Octadecanamide | 1.8 |
| Total area detected | |||
| S.No. | RT | Compounds | Rel. Area |
|---|---|---|---|
| 1 | 7.9 | Diphenylpropane | 5.2 |
| 2 | 9.3 | C20 | 3.3 |
| 3 | 8.5 | C18 | 3.3 |
| 4 | 8.9 | C19 | 3.2 |
| 5 | 9.4 | 2-Phenylnaphtalene | 3.0 |
| 6 | 10.0 | Dihydro-cyclo-pentaphenanthrene | 2.8 |
| 7 | 8.0 | C17 | 2.8 |
| 8 | 8.3 | Cyclopropane | 2.7 |
| 9 | 9.8 | C21 | 2.5 |
| 10 | 8.6 | 1,3-Diphenyl-1-butene | 2.5 |
| Total area detected | |||
| Feedstocks | C (%) | H (%) | N (%) | Ash + O (%) b | S | HHV (MJ/kg) a |
|---|---|---|---|---|---|---|
| Only biomass (T8) | 72.86 | 10.10 | 3.0 | 13.74 | ˂0.3 | 35.71 |
| Only plastics (T10) | 85.05 | 11.54 | ˂0.3 | 2.71 | ˂0.4 | 41.9 |
| Compound | Only Biomass (T8), g/L | Only Plastics (T10), g/L |
|---|---|---|
| Cellobiose | - | - |
| Glucose | 0.449 | 0.133 |
| Xylose | 0.346 | - |
| Arabinose | 0.756 | - |
| Succinic acid | 0.555 | - |
| Lactic acid | - | - |
| Glycerol | 7.552 | - |
| Formic acid | 0.477 | 7.185 |
| Acetic acid | 3.661 | 0.07 |
| Propanoic acid | 0.286 | - |
| Isobutyric acid | - | - |
| Butyric acid | - | - |
| Feedstocks | C (%) | H (%) | N (%) | S (%) | O + Ash (%) | HHV (MJ/kg) a | Yield (wt%) |
|---|---|---|---|---|---|---|---|
| T2-unconverted | 83.31 | 12.53 | 0.3 | 0.4 | 3.46 | 43.7 | 51.2 |
| T2-converted | 49.55 | 2.98 | 1.92 | 0.6 | 44.95 | 18.5 | 6.7 |
| T8-converted | 40.13 | 4.07 | 1.88 | 0.4 | 53.52 | 16 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feuerbach, S.; Toor, S.S.; Costa, P.A.; Paradela, F.; Marques, P.A.A.S.; Castello, D. Hydrothermal Co-Liquefaction of Food and Plastic Waste for Biocrude Production. Energies 2024, 17, 2098. https://doi.org/10.3390/en17092098
Feuerbach S, Toor SS, Costa PA, Paradela F, Marques PAAS, Castello D. Hydrothermal Co-Liquefaction of Food and Plastic Waste for Biocrude Production. Energies. 2024; 17(9):2098. https://doi.org/10.3390/en17092098
Chicago/Turabian StyleFeuerbach, Silvan, Saqib Sohail Toor, Paula A. Costa, Filipe Paradela, Paula A.A.S. Marques, and Daniele Castello. 2024. "Hydrothermal Co-Liquefaction of Food and Plastic Waste for Biocrude Production" Energies 17, no. 9: 2098. https://doi.org/10.3390/en17092098
APA StyleFeuerbach, S., Toor, S. S., Costa, P. A., Paradela, F., Marques, P. A. A. S., & Castello, D. (2024). Hydrothermal Co-Liquefaction of Food and Plastic Waste for Biocrude Production. Energies, 17(9), 2098. https://doi.org/10.3390/en17092098








