Stimulating Methane Production from Poultry Manure Digest with Sewage Sludge and Organic Waste by Thermal Pretreatment and Adding Iron or Sodium Hydroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
- The co-digestion mixture consisted of poultry manure and a mixture of primary and excess sludge. Sewage sludge (Ss) was collected from a municipal wastewater treatment plant located in the Silesian Voivodeship, while poultry manure (PM) was sourced from a laying poultry farm in the Silesia region of Poland. The manure was homogenized before the preparation of the co-digestion mixture, and the share of PM in the co-digestion mixture was 40% based on volatile solids (VSs);
- Inoculum—digested sludge collected from heat exchangers at a municipal wastewater treatment plant located in the Silesian Voivodeship (I). The WWTP produces annually approx. 3200 Mg dry mass of sewage sludge and treats about 90,000 m3/d of wastewater. In the mentioned WWTP, a mixture of waste-activated sludge and primary sludge was digested under mesophilic conditions, and the HRT of AD was equal to 30 d;
- Selected organic waste listed in Table 1.
2.2. Experimental Procedure
- Selecting, based on the results of the biomethane potential (BMP) tests, the third component of the co-digestion mixture (the methane potential of the waste itself was assessed, and then, for the selected waste, their joint anaerobic co-digestion with sewage sludge and poultry manure was carried out); based on our previously conducted research, it was assumed that the third component would constitute 30% of the co-digestion mixture based on the VS [27,28,29];
- Determination of the most favorable inoculum-to-substrate ratio (I/S) for the co-digestion mixture. This research determined the impact of I/S ratios of 0.5, 1.0, 1.5, and 2 on the methane yield factor;
- Selection of the most favorable parameters for the pretreatment of the feedstock based on changes in volatile fatty acids (VFAs), ammonium nitrogen, TS, VS, and non-purgeable organic carbon (NPOC), as well as the content of extracellular polymers substances (EPS) in individual fractions broken down by their concentration of carbohydrates, ammonium nitrogen, protein, and NPOC. The three-component co-digestion mixture and sewage sludge were treated with the following pretreatment methods (Figure 1): (a) the thermochemical method—thermal method using various doses of NaOH [g/L], temperature 121 °C, conditioning time 30 min (NaOH); (b) thermochemical method—thermal method using various doses of metallic iron, [g/L], temperature 121 °C, conditioning time 30 min (Fe); and (c) the thermal method—temperature 121 °C, conditioning time 30 min (A);
- Determining the impact of the pretreatment methods of the feedstock (three-component co-digestion mixture) selected in stage 3 on the effectiveness of the anaerobic co-digestion process measured by the methane yield, the VS removal, and the kinetic parameter.
2.3. Physicochemical and Physical Analyses
- A—weight of dried residue + dish, g
- B—weight of dish, g
- C—weight of wet sample + dish, g
- D—weight of residue + dish after ignition, g
2.4. Statistical Analyses
3. Results and Discussion
3.1. Selection of the Third Component of the Co-Digestion Mixture
3.2. Determination of the Inoculum-to-Substrate Ratio (ISR, I/S)
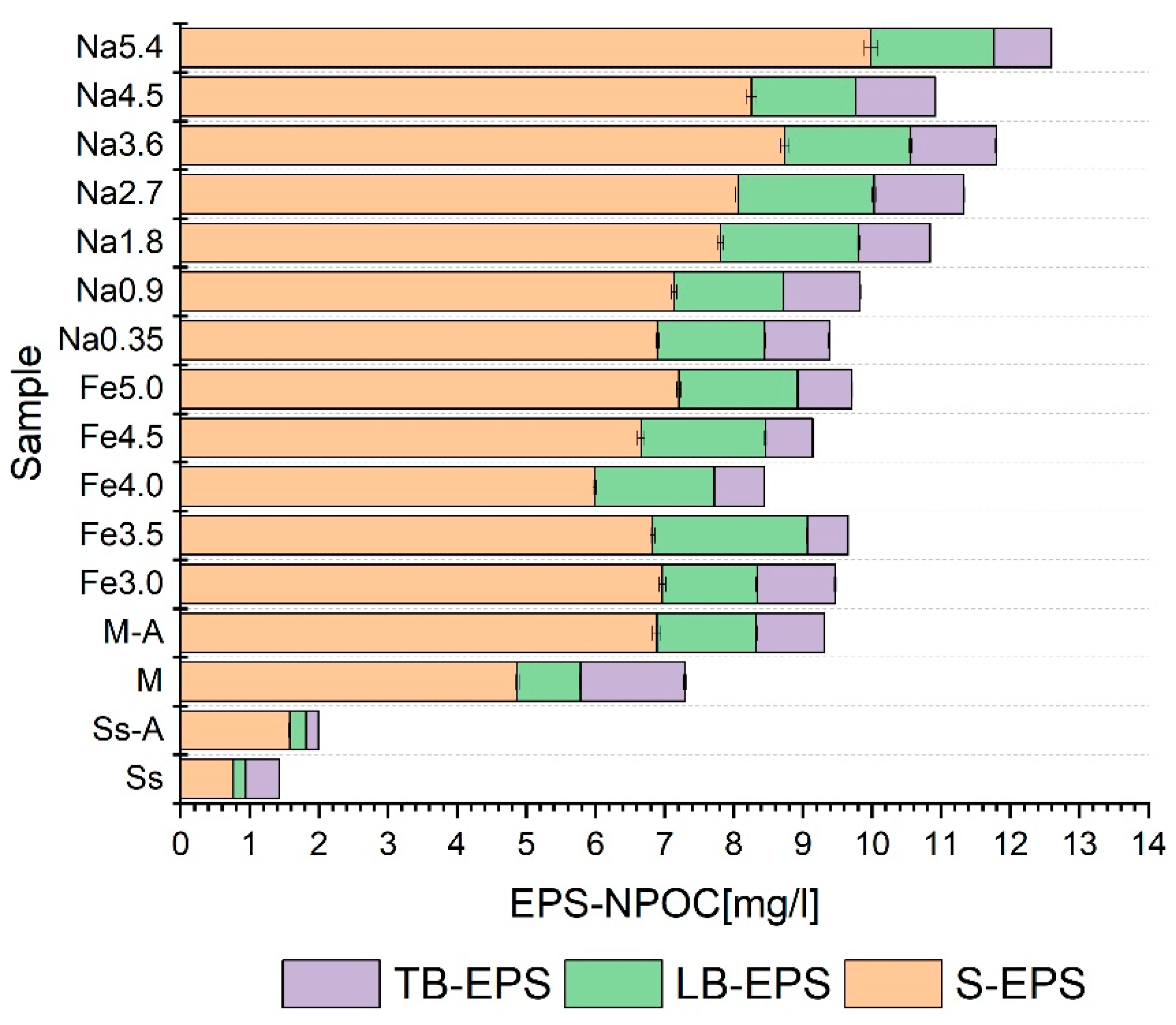
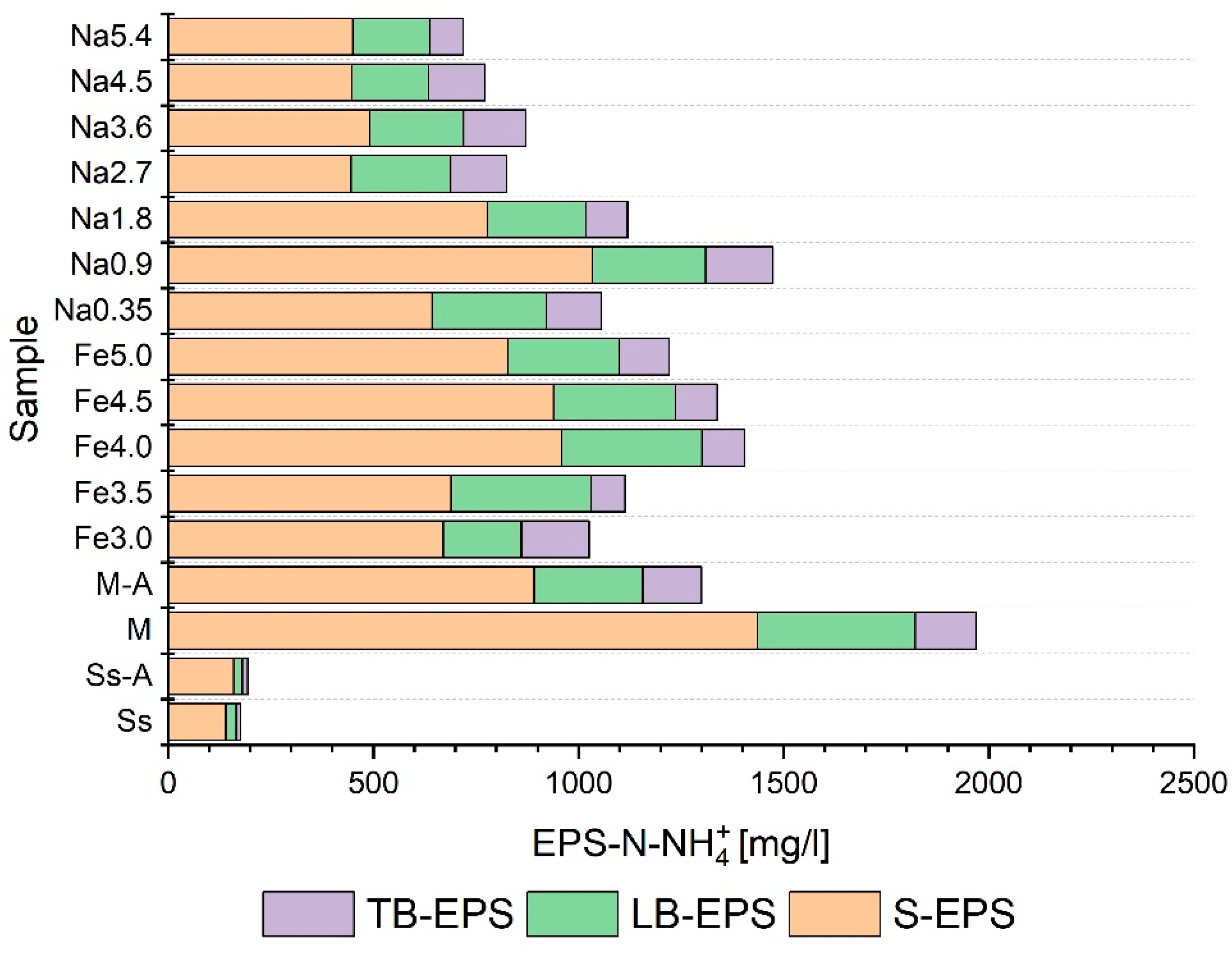
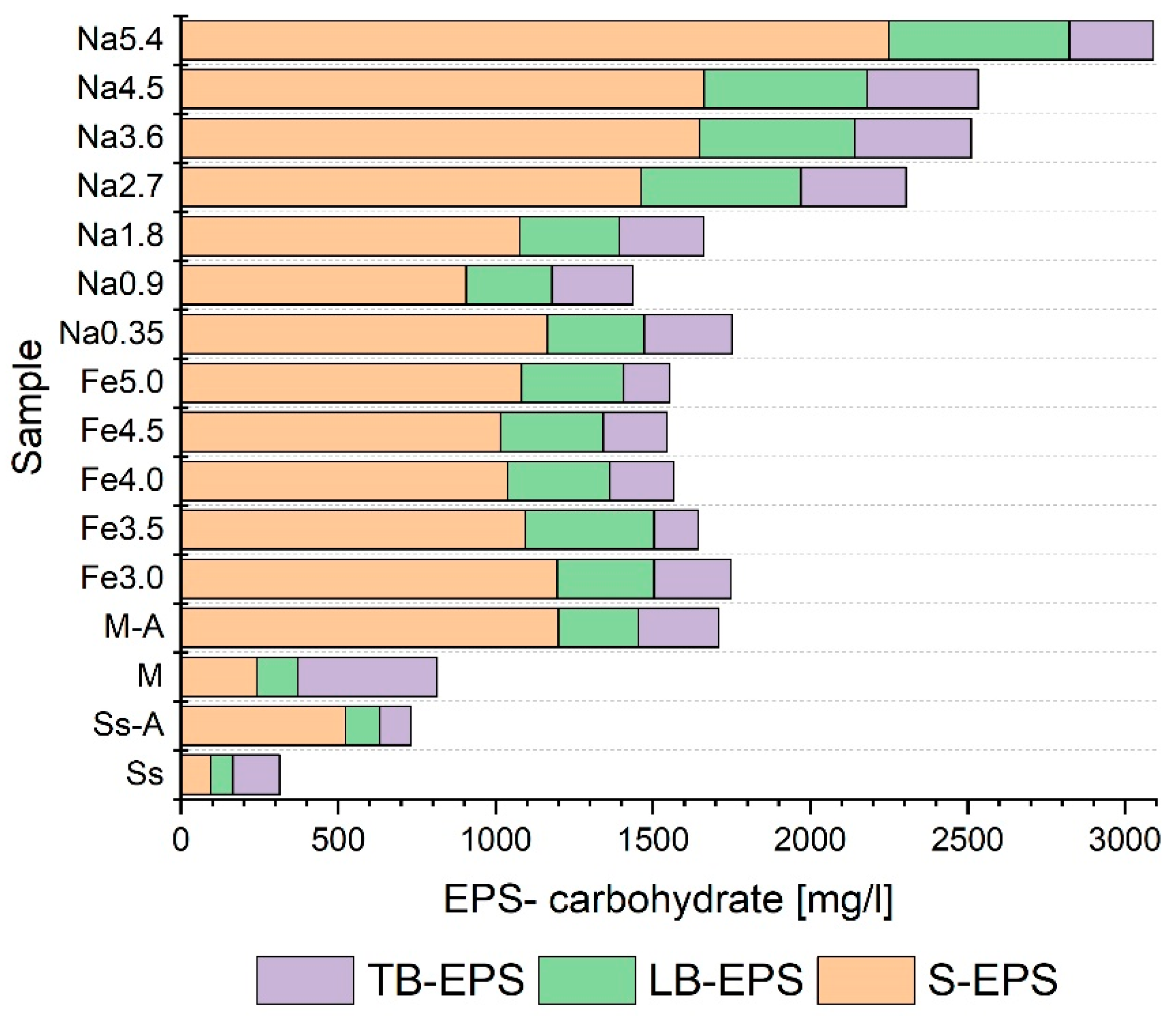
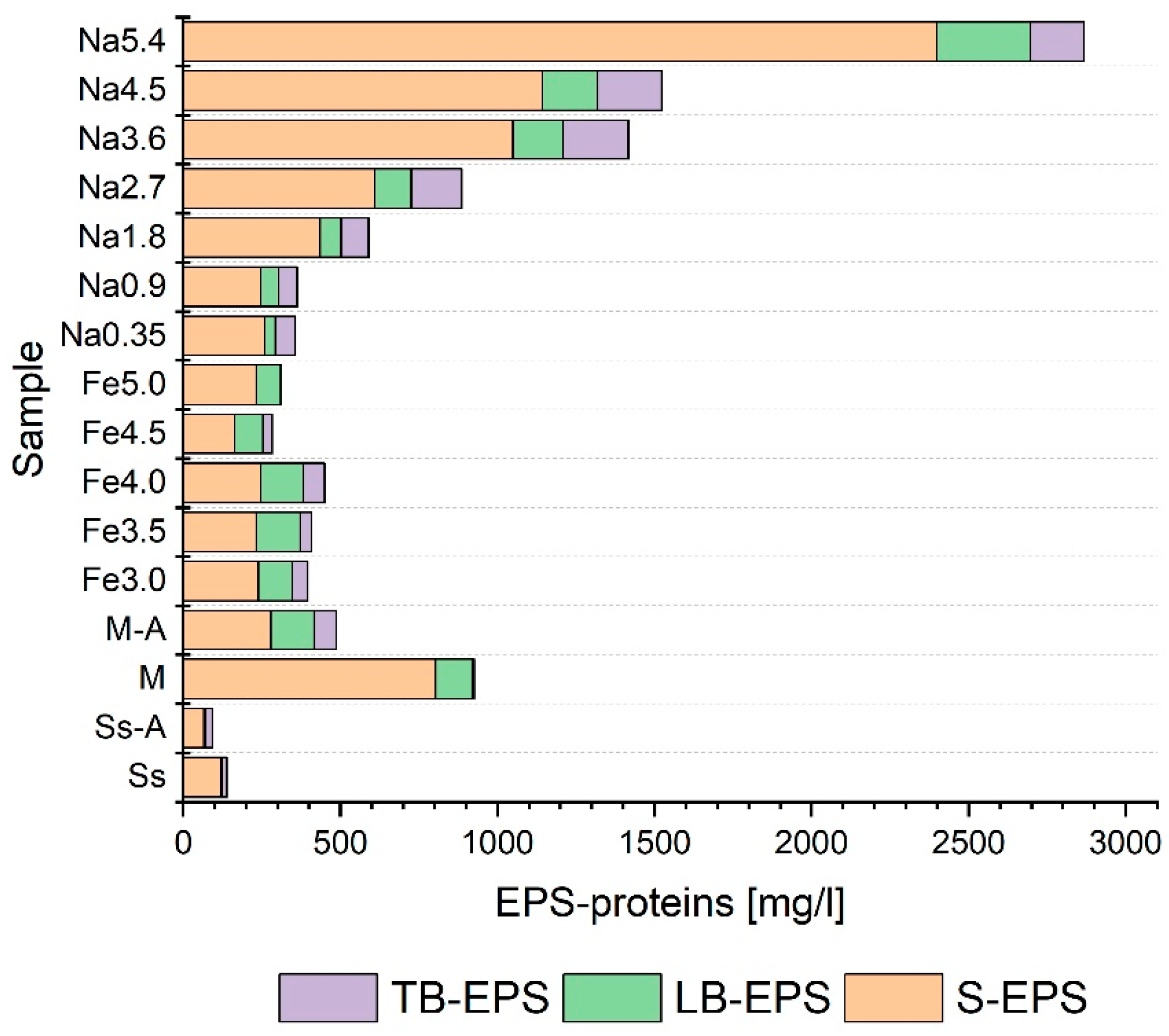
3.3. Selection of Pretreatment Conditions
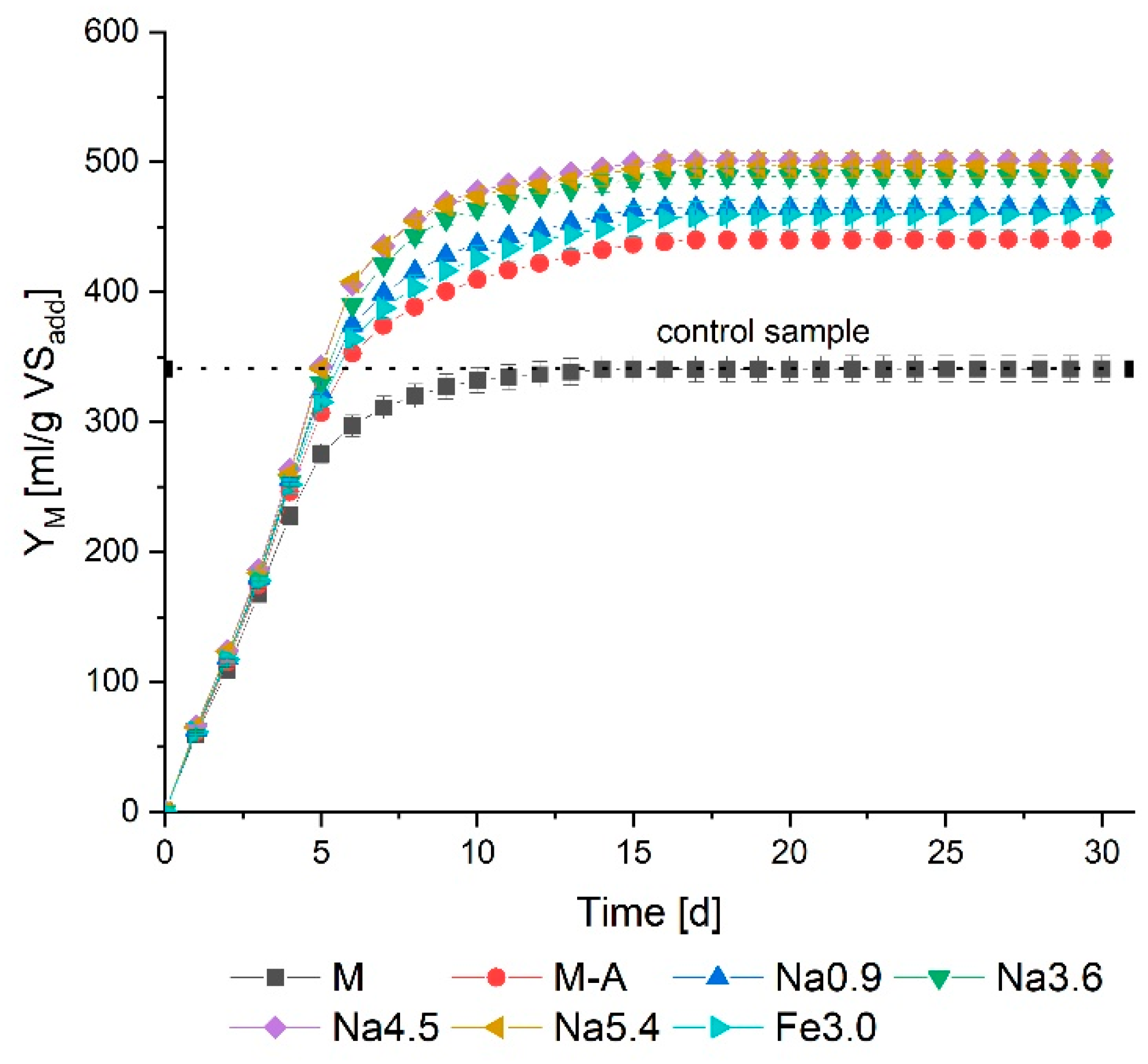
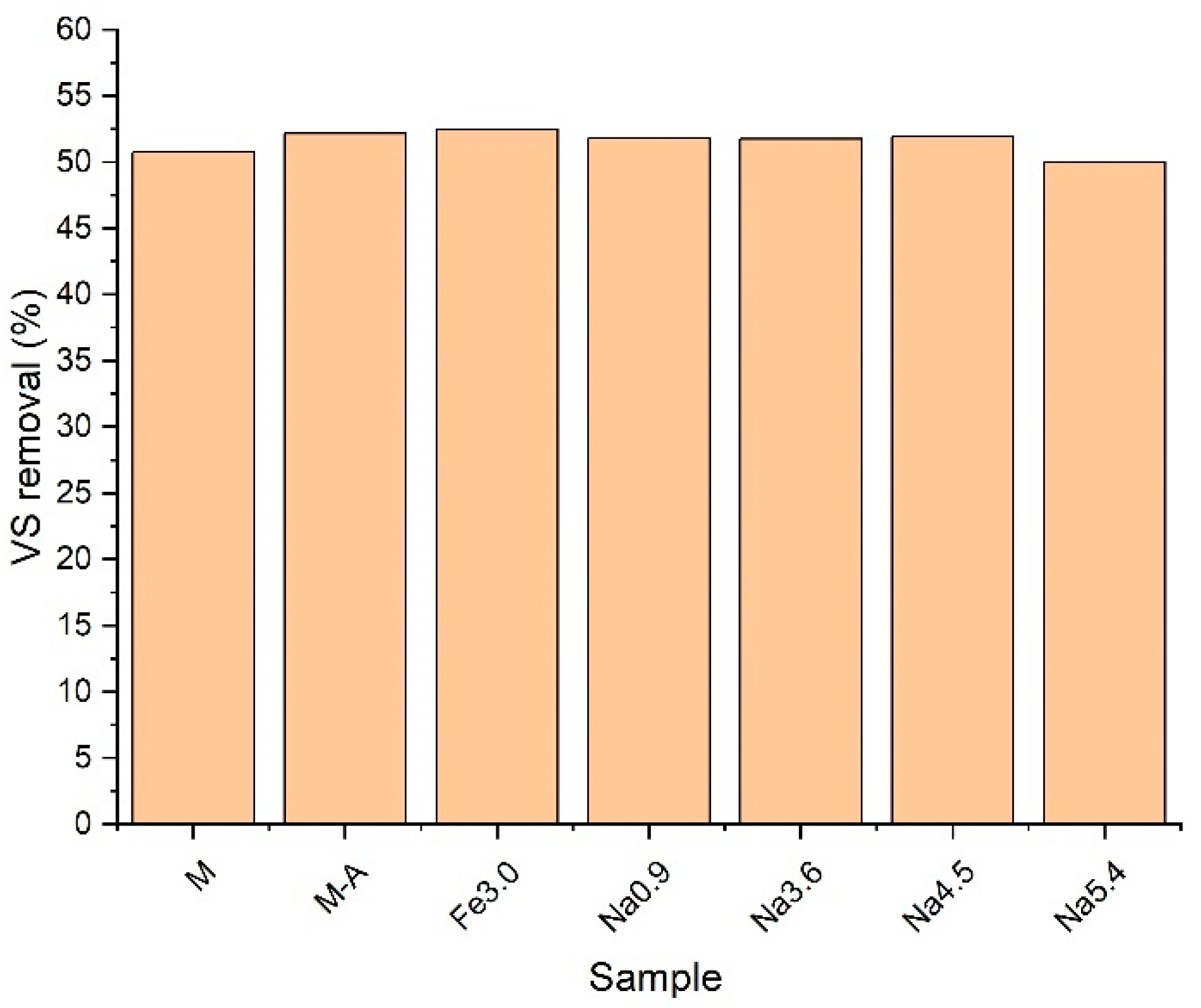
3.4. Methane Production and VS Removal
4. Conclusions
- Waste with a high fat content has the highest potential for methane production;
- The addition of a third co-substrate to the mixture of sewage sludge and poultry manure resulted in a substantial increase in methane production;
- The inoculum-to-substrate ratio (I/S) has a statistically significant impact on the methane yield;
- The highest value of methane yield was obtained for an I/S equal to 2;
- Pretreatment of the three-component co-digestion mixture frequently led to a reduction in volatile fatty acids (VFAs) within the digestion broth;
- All the pretreatment methods used resulted in an increase in the degree of liquefaction of organic matter measured by changes in the NPOC;
- With an increase in the dose of sodium hydroxide, an increase in the NPOC parameter was observed;
- Iron did not result in statistically significant changes in the NPOC levels;
- The pretreatment generally did not impact the total solid (TS) and volatile solid (VS) content of the three-component co-digestion mixture;
- The pretreatment methods had a significant statistical impact on the distribution of the analyzed indicators in individual EPS fractions;
- The samples treated using thermochemical methods exhibited lower total ammonium nitrogen contents in extracellular polymers than the control sample;
- The use of pretreatment on the co-digestion mixture usually resulted in an increase in the degree of liquefaction of organic matter (measured by changes in NPOC, protein, and carbohydrates) in all the fractions of extracellular polymers;
- Pretreatment intensifies methane production (for all the tested methods, the methane yield was higher than for the control sample);
- The most substantial methane yield was observed following thermochemical pretreatment with 4.5 g NaOH/L (501 mL/g VS);
- An increase in the sodium hydroxide concentration prolonged the adaptation time of microorganisms (lag phase extension);
- The pretreatment methods used slightly improved VS removal. This indicator compared to the unconditioned co-digestion mixture increased from 50.8 to 52.5%; however, the one-way analysis of variance showed that this change was not statistically significant. It is worth emphasizing that, compared to the AD of sewage sludge itself, for which VS removal is usually around 30–40%, the values obtained in the tests were satisfactory.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.; Subrahmanyam, B.; Akhil, P.; Prashanth, B. Biogas: A Renewable Energy for Future. Int. J. Mech. Eng. Res. Dev. 2017, 6. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3516504 (accessed on 3 May 2024).
- Depren, S.K.; Kartal, M.T.; Çelikdemir, N.Ç.; Depren, Ö. Energy Consumption and Environmental Degradation Nexus: A Systematic Review and Meta-Analysis of Fossil Fuel and Renewable Energy Consumption. Ecol. Inform. 2022, 70, 101747. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Jasińska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure—A Critical Review. Energies 2023, 16, 3885. [Google Scholar] [CrossRef]
- Maj, I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies 2022, 15, 8981. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A Comprehensive Review of Green Policy, Anaerobic Digestion of Animal Manure and Chicken Litter Feedstock Potential–Global and Irish Perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D.W. Bioenergy Production from Chicken Manure: A Review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- de Oliveira Paranhos, A.G.; Adarme, O.F.H.; Barreto, G.F.; de Queiroz Silva, S.; de Aquino, S.F. Methane Production by Co-Digestion of Poultry Manure and Lignocellulosic Biomass: Kinetic and Energy Assessment. Bioresour. Technol. 2020, 300, 122588. [Google Scholar]
- Rahman, M.A.; Shahazi, R.; Nova, S.N.B.; Uddin, M.R.; Hossain, M.S.; Yousuf, A. Biogas Production from Anaerobic Co-Digestion Using Kitchen Waste and Poultry Manure as Substrate—Part 1: Substrate Ratio and Effect of Temperature. Biomass Convers. Biorefin. 2023, 13, 6635–6645. [Google Scholar] [CrossRef]
- Rahman, M.A.; Møller, H.B.; Saha, C.K.; Alam, M.M. The Effect of Temperature on the Anaerobic Co-Digestion of Poultry Droppings and Sugar Mill Press Mud. Biofuels 2022, 13, 139–147. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Improvement of the Anaerobic Digestion of Sewage Sludge by Co-Digestion with Wine Vinasse and Poultry Manure: Effect of Different Hydraulic Retention Times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Bista, U.; Rayamajhi, B.; Dhungana, B.; Lohani, S.P. Biogas Production by Co-Digestion of Food Waste with Sewage Sludge and Poultry Litter: A Way Towards Sustainable Waste-to-Energy Conversion. J. Renew. Energy Environ. 2023, 10, 39–44. [Google Scholar]
- Burka, M.S.; Basamykina, A.N.; Kharlamova, M.D. Technological Features of Biogas Production While Anaerobic Co-Digestion of Faecal Sludge, Sewage Sludge and Livestock. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 666, p. 42052. [Google Scholar]
- Hassan, M.; Aboelenin, S.M.; Soliman, M.M.; Attia, H.F.; Metwally, K.A.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T.; Khalaphallah, R. Biogas Manufacture from Co-Digestion of Untreated Primary Sludge with Raw Chicken Manure under Anaerobic Mesophilic Environmental Conditions. Saudi J. Biol. Sci. 2022, 29, 2969–2977. [Google Scholar]
- Okewale, A.O.; Adesina, O.A. Evaluation of Biogas Production from Co-Digestion of Pig Dung, Water Hyacinth and Poultry Droppings. Waste Dispos. Sustain. Energy 2019, 1, 271–277. [Google Scholar] [CrossRef]
- Song, L.; Li, D.; Cao, X.; Tang, Y.; Liu, R.; Niu, Q.; Li, Y.-Y. Optimizing Biomethane Production of Mesophilic Chicken Manure and Sheep Manure Digestion: Mono-Digestion and Co-Digestion Kinetic Investigation, Autofluorescence Analysis and Microbial Community Assessment. J. Environ. Manag. 2019, 237, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic Co-Digestion: Current Status and Perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B. Impacts of Anaerobic Co-Digestion on Different Influencing Parameters: A Critical Review. Sustainability 2022, 14, 9387. [Google Scholar] [CrossRef]
- Kadam, R.; Jo, S.; Lee, J.; Khanthong, K.; Jang, H.; Park, J. A Review on the Anaerobic Co-Digestion of Livestock Manures in the Context of Sustainable Waste Management. Energies 2024, 17, 546. [Google Scholar] [CrossRef]
- Jasińska, A.; Grosser, A.; Meers, E.; Robles, A. Energy Recovery from Poultry Manure in the Process of Semi-Continuous Anaerobic Co-Digestion with Sewage Sludge. [CrossRef]
- Obileke, K.; Nwokolo, N.; Makaka, G.; Mukumba, P.; Onyeaka, H. Anaerobic Digestion: Technology for Biogas Production as a Source of Renewable Energy—A Review. Energy Environ. 2021, 32, 191–225. [Google Scholar] [CrossRef]
- Lisowyj, M.; Wright, M.M. A Review of Biogas and an Assessment of Its Economic Impact and Future Role as a Renewable Energy Source. Rev. Chem. Eng. 2020, 36, 401–421. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199. [Google Scholar] [CrossRef]
- Grosser, A. Determination of Methane Potential of Mixtures Composed of Sewage Sludge, Organic Fraction of Municipal Waste and Grease Trap Sludge Using Biochemical Methane Potential Assays. A Comparison of BMP Tests and Semi-Continuous Trial Results. Energy 2018, 143, 488–499. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E. Sewage Sludge and Fat Rich Materials Co-Digestion-Performance and Energy Potential. J. Clean. Prod. 2018, 198, 1076–1089. [Google Scholar] [CrossRef]
- Grosser, A. The Influence of Decreased Hydraulic Retention Time on the Performance and Stability of Co-Digestion of Sewage Sludge with Grease Trap Sludge and Organic Fraction of Municipal Waste. J. Environ. Manage. 2017, 203, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Schillak, R. Oznaczanie Azotu Mineralnego w Glebie. Rocz. Glebozn. 1958, 7, 185–189. [Google Scholar]
- Yang, Z.; Kang, X.; Chen, B.; Qiu, G.; Wei, J.; Li, F.; Wei, C. Effects of Alkali, Autoclaving, and Fe+ Autoclaving Pretreatment on Anaerobic Digestion Performance of Coking Sludge from the Perspective of Sludge Extracts and Methane Production. Environ. Sci. Pollut. Res. 2020, 28, 13151–13161. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Nielsen, S.S. Total Carbohydrate by Phenol-Sulfuric Acid Method. Food Anal. Lab. Man. 2017, 137–141. [Google Scholar]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic Co-Digestion of Commercial Food Waste and Dairy Manure: Characterizing Biochemical Parameters and Synergistic Effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.Q.; Borja, V.M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Pearse, L.F.; Hettiaratchi, J.P.; Kumar, S. Towards Developing a Representative Biochemical Methane Potential (BMP) Assay for Landfilled Municipal Solid Waste–A Review. Bioresour. Technol. 2018, 254, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Influence of Inoculum to Substrate Ratio and Substrates Mixing Ratio on Biogas Production from the Anaerobic Co-Digestion of Phragmites Australis and Food Waste. Bioenergy Res. 2023, 17, 1277–1287. [Google Scholar] [CrossRef]
- Agrawal, A.; Chaudhari, P.K.; Ghosh, P. Effect of Inoculums Type and Optimization of Inoculum to Substrate Ratio on the Kinetics of Biogas Production of Fruit and Vegetable Waste. Environ. Eng. Res. 2024, 29, 220518. [Google Scholar] [CrossRef]
- Chamchoi, N.; Garcia, H.; Angelidaki, I. Methane Potential of Household Waste; Batch Assays Determination. Appl. Environ. Res. 2010, 33, 13–26. [Google Scholar]
- Elsayed, M.; Andres, Y.; Blel, W.; Hassan, R.; Ahmed, A. Effect of Inoculum VS, Organic Loads and I/S on the Biochemical Methane Potential of Sludge, Buckwheat Husk and Straw. Desalin. Water Treat. 2019, 157, 69–78. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; de la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical Methane Potential (BMP) of Solid Organic Substrates: Evaluation of Anaerobic Biodegradability Using Data from an International Interlaboratory Study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; OJ L 312, 22.11.2008, p. 0003–0030. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 3 May 2024).
- Seok, J.; Lee, Y.Y.; Hyun, T. Bioresource Technology A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and Potential of the Anaerobic Digestion of Waste-Activated Sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Y.; Qi, Q.; Zhang, P.; Zhang, Y.; Tong, Y.W.; He, Y. The Bio-Chemical Cycle of Iron and the Function Induced by ZVI Addition in Anaerobic Digestion: A Review. Water Res. 2020, 186, 116405. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Lee, C. A Review of the Effects of Iron Compounds on Methanogenesis in Anaerobic Environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Yang, Q.; Wu, Y.; Li, Y.; Fu, Q.; Yang, F.; Liu, Y.; Ni, B.J.; et al. Thermal-Alkaline Pretreatment of Polyacrylamide Flocculated Waste Activated Sludge: Process Optimization and Effects on Anaerobic Digestion and Polyacrylamide Degradation. Bioresour. Technol. 2019, 281, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mehari, B.B.; Chang, S.; Hong, Y.; Chen, H. Temperature-Phased Biological Hydrolysis and Thermal Hydrolysis Pretreatment for Anaerobic Digestion Performance Enhancement. Water 2018, 10, 1812. [Google Scholar] [CrossRef]
- Rahman, A.; Bjarne, H.; Kumer, C.; Alam, M.; Wahid, R.; Feng, L. Anaerobic Co-Digestion of Poultry Droppings and Briquetted Wheat Straw at Mesophilic and Thermophilic Conditions: In Fl Uence of Alkali Pretreatment. Renew. Energy 2018, 128, 241–249. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A Review of the Role of Extracellular Polymeric Substances (EPS) in Wastewater Treatment Systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef] [PubMed]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, Structure, and Function of Extracellular Polymeric Substances (EPS) of Microbial Biofilm in Biological Wastewater Treatment Systems: A Review. Desalin. Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting Extracellular Polymeric Substances (EPS) Controlling Strategies for Performance Enhancement of Biological Wastewater Treatments: An Overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef]
- Park, J.; Cayetano, R.D.A.; Kim, G.B.; Jo, Y.; Kwon, Y.; Lei, Z.; Kim, S.H. Sludge Disintegration and Anaerobic Digestion Enhancement by Alkaline-Thermal Pretreatment: Economic Evaluation and Microbial Population Analysis. Bioresour. Technol. 2022, 346, 126594. [Google Scholar] [CrossRef]
- Awe, O.W.; Lu, J.; Wu, S.; Zhao, Y.; Nzihou, A.; Lyczko, N.; Minh, D.P. Effect of Oil Content on Biogas Production, Process Performance and Stability of Food Waste Anaerobic Digestion. Waste Biomass Valorization 2018, 9, 2295–2306. [Google Scholar] [CrossRef]
- Diamantis, V.; Eftaxias, A.; Stamatelatou, K.; Noutsopoulos, C.; Vlachokostas, C.; Aivasidis, A. Bioenergy in the Era of Circular Economy: Anaerobic Digestion Technological Solutions to Produce Biogas from Lipid-Rich Wastes. Renew. Energy 2021, 168, 438–447. [Google Scholar] [CrossRef]
- Salama, E.S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.H. Recent Trends in Anaerobic Co-Digestion: Fat, Oil, and Grease (FOG) for Enhanced Biomethanation. Prog. Energy Combust. Sci. 2019, 70, 22–42. [Google Scholar] [CrossRef]
- Konkol, I.; Świerczek, L.; Cenian, A. Chicken Manure Pretreatment for Enhancing Biogas and Methane Production. Energies 2023, 16, 5442. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z. Bioresource Technology E Ff Ect of Pre-Treatment on Sequential Anaerobic Co-Digestion of Chicken Litter with Agricultural and Food Wastes under Semi-Solid Conditions and Comparison with Wet Anaerobic Digestion. Bioresour. Technol. 2019, 281, 286–295. [Google Scholar] [CrossRef]
- Costa, J.C.; Barbosa, S.G.; Alves, M.M.; Sousa, D.Z. Thermochemical Pre- and Biological Co-Treatments to Improve Hydrolysis and Methane Production from Poultry Litter. Bioresour. Technol. 2012, 111, 141–147. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of Substrate Pretreatment on Biogas Production through Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Ahamed, J.U.; Raiyan, M.F.; Hossain, M.S.; Rahman, M.M.; Salam, B. Production of Biogas from Anaerobic Digestion of Poultry Droppings and Domestic Waste Using Catalytic Effect of Silica Gel. Int. J. Automot. Mech. Eng. 2016, 13, 3503–3517. [Google Scholar] [CrossRef]
- Schumacher, B.; Wedwitschka, H.; Weinrich, S.; Mühlenberg, J.; Gallegos, D.; Oehmichen, K.; Liebetrau, J. The Influence of Pressure Swing Conditioning Pre-Treatment of Chicken Manure on Nitrogen Content and Methane Yield. Renew. Energy 2019, 143, 1554–1565. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.J. Enhancing Hyper-Thermophilic Hydrolysis Pre-Treatment of Chicken Manure for Biogas Production by in-Situ Gas Phase Ammonia Stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef]
- Raju, C.S.; Sutaryo, S.; Ward, A.J.; Møller, H.B. Effects of High-Temperature Isochoric Pre-Treatment on the Methane Yields of Cattle, Pig and Chicken Manure. Environ. Technol. 2013, 34, 239–244. [Google Scholar] [CrossRef]
- Nava-Valente, N.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Effect of Acid Pretreatment on the Anaerobic Codigestion of Sewage Sludge, Chicken Litter, and Sugarcane Waste (SCW) for Biogas Production. Bioenergy Res. 2023, 16, 1889–1901. [Google Scholar] [CrossRef]
- Alkhrissat, T.; Kassab, G.; Abdel-Jaber, M. Impact of Iron Oxide Nanoparticles on Anaerobic Co-Digestion of Cow Manure and Sewage Sludge. Energies 2023, 16, 5844. [Google Scholar] [CrossRef]
- Mansour, M.N.; Lendormi, T.; Louka, N.; Maroun, R.G.; Hobaika, Z.; Lanoisellé, J.L. Anaerobic Digestion of Poultry Droppings in Semi-Continuous Mode and Effect of Their Co-Digestion with Physico-Chemical Sludge on Methane Yield. Sustainability 2023, 15, 5997. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Enhancing the Production of Biogas through Anaerobic Co-Digestion of Agricultural Waste and Chemical Pre-Treatments. Chemosphere 2020, 255, 126805. [Google Scholar] [CrossRef]
- Wu, K.; Xu, W.; Lu, J.; Wang, C.; Liao, J.; He, X. Saponification with Calcium Enhanced Methane Yield in Anaerobic Digestion of Fat, Oil, and Grease: The Essential Role of Calcium. Renew. Energy 2022, 195, 1103–1112. [Google Scholar] [CrossRef]
- Aromolaran, A.; Sartaj, M.; Abdallah, M. Supplemental Sewage Scum and Organic Municipal Solid Waste Addition to the Anaerobic Digestion of Thickened Waste Activated Sludge: Biomethane Potential and Microbiome Analysis. Fermentation 2023, 9, 237. [Google Scholar] [CrossRef]
- Grosser, A.; Grobelak, A.; Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemière, S.; Guyoneaud, R.; Attard, E.; Celary, P. Effects of Silver Nanoparticles on Performance of Anaerobic Digestion of Sewage Sludge and Associated Microbial Communities. Renew. Energy 2021, 171, 1014–1025. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Demir, S.; Oda, V. Anaerobic Digestion of Cattle Manure, Corn Silage and Sugar Beet Pulp Mixtures after Thermal Pretreatment and Kinetic Modeling Study. Fuel 2020, 263, 116651. [Google Scholar] [CrossRef]
- Yu, Q.; Cui, S.; Sun, C.; Liu, R.; Sarker, M.; Guo, Z.; Lai, R. Synergistic Effects of Anaerobic Co-Digestion of Pretreated Corn Stover with Chicken Manure and Its Kinetics. Appl. Biochem. Biotechnol. 2021, 193, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Jin, R.; Liu, G.; Dong, B.; Zhou, J.; Xing, D. Improving Waste Activated Sludge Dewaterability with Sodium Periodate Pre-oxidation on Extracellular Polymeric Substances. Water Environ. Res. 2021, 93, 1680–1689. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Collection Site |
|---|---|
| By-product of the refining of vegetable fats (waste soap—WS) | Refining technological line |
| Fats contained in wastewater from the production of dairy products—flotation-thickened sludge (F) | Grease trap sludge |
| Grease trap sludge from meat-processing plant (GTS) | Grease trap sludge |
| Fruit waste (cherry processing period) (FW) | Waste storage yard |
| Substrate | TS (%) | VS (% TS) | VS (%) | VS/TS | pH (−) | |||
|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | − | Average | SD | |
| F | 11.38 | 0.12 | 77.22 | 0.11 | 8.79 | 0.77 | 6.18 | 0.01 |
| GTS | 14.62 | 0.02 | 89.05 | 0.56 | 13.02 | 0.89 | 5.44 | 0.01 |
| WS | 17.01 | 0.10 | 91.85 | 0.06 | 15.63 | 0.92 | 6.02 | 0.01 |
| FW | 12.87 | 0.07 | 87.88 | 0.29 | 11.31 | 0.88 | 4.21 | 0.01 |
| PM | 27.44 | 0.24 | 73.58 | 0.81 | 20.19 | 0.74 | 5.41 | 0.01 |
| Ss | 3.21–3.43 | 77.77–80.67 | 2.59–2.66 | 0.77–0.81 | 5.69–5.81 | |||
| I | 2.46–2.47 | 62.85–63.32 | 1.55–1.56 | 0.62–0.63 | 8.47–8.51 | |||
| Model | Equal |
|---|---|
| Modified Gompertz equation (MG) | |
| Logistic function model (LF) | |
| Transference function (TF) |
| Feedstock | YM (mL/g VSadd) | SD |
|---|---|---|
| By-product of the refining of vegetable fats (waste soap—WS) | 630 | 50 |
| Fats contained in wastewater from the production of dairy products—flotation-thickened sludge (F) | 750 | 70 |
| Grease trap sludge from meat-processing plant (GTS) | 740 | 60 |
| Fruit waste (cherry processing period) (FW) | 250 | 30 |
| Poultry manure (PM) | 270 | 20 |
| Sewage sludge (Ss) | 307 | 40 |
| Ss + PM | 320 | 30 |
| Feedstock | YM (mL/g VS) |
|---|---|
| Sewage sludge | 143–460 |
| Fruit and vegetable waste | 420 |
| Fruit waste | 180–732 |
| Fats from flotation | 600–700 |
| Grease trap sludge | 700 (1000) |
| Waste from grease trap sludge | 845–928 |
| Poultry manure | 150–410 |
| Feedstock | Cumulative Methane Production (mL) | SD |
|---|---|---|
| Ss + PM + F | 1061.8 | 7.933 |
| Ss + PM + GTS | 1297.4 | 31.8 |
| Ss + PM + WS | 1110.27 | 28.5 |
| Ss + PM + FW | 696.7 | 4.8 |
| I/S | Cumulative Methane Production (mL) | SD | YM (l/kg VS) | SD |
|---|---|---|---|---|
| 0.5 | 2137.57 | 9.15 | 372.05 | 1.34 |
| 1.0 | 1356.93 | 19.98 | 370.51 | 4.57 |
| 1.5 | 1028.57 | 14.15 | 380.43 | 4.41 |
| 2.0 | 826.77 | 6.35 | 385.50 | 2.50 |
| I/S | YM (l/kg VS) | a | b | c |
|---|---|---|---|---|
| I/S_1.0 | 372.05 | **** | ||
| I/S_0.5 | 370.51 | **** | **** | |
| I/S_1.5 | 380.43 | **** | **** | |
| I/S_2.0 | 385.50 | **** |
| Indicator | Sample | ||||||
|---|---|---|---|---|---|---|---|
| M-A | Na-0.9 | Na-3.6 | Na-4.5 | Na-5.4 | Fe-3.0 | ||
| VFAs | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | |
| N-NH4+ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | |
| NPOC | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| N-NH4+ | SB-EPS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| LB-EPS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| TB-EPS | ↓ | ↑ | - | ↓ | ↓ | ↑ | |
| Sum | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| NPOC | SB-EPS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| LB-EPS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| TB-EPS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Sum | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| carbohydrate | SB-EPS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| LB-EPS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| TB-EPS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Sum | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Protein | SB-EPS | ↓ | ↓ | ↑ | ↑ | ↑ | ↓ |
| LB-EPS | ↑ | ↓ | ↑ | ↑ | ↑ | ↓ | |
| TB-EPS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Sum | ↓ | ↓ | ↑ | ↑ | ↑ | ↓ | |
| Feedstock | Pretreatment | AD Conditions | Remarks | Reference |
|---|---|---|---|---|
| Chicken manure | Extraction (by shaking) of chicken manure with water in the amount of 5 g fresh weight of manure per 100 mL of deionized water. | Batch assay, 2 L reactor, 38 ± 2 °C | Without pretreatment YB: 362.5 mL/g VS With pretreatment YB: 420.6–527.8 mL/g VS Enhancement: +45% | [59] |
| Chicken litter | 5% of NaOH 90 min, 120 °C +3% of H2SO4 90 min, 120 °C | With pretreatment YM: 137 mL/g VS Enhancement: - | [60] | |
| Chicken litter and chicken feathers | Ca(OH)2 at 90 °C, 1.27 bar pressure | Batch assay, 50 mL bottles, 37 °C | With pretreatment YM: 101 mL/g VS Enhancement: - | [61] |
| Poultry manure | Clostridium cellulolyticum, Clostridium saccharolyticum, and Clostridium thermocellum as bioaccumulation strains | With pretreatment YM: 102 mL/g VS Enhancement: +15% | ||
| Food waste + poultry manure | 120 °C, 10 bar, 30 min | Batch assay, 2.0 L bottles, 30 d | Untreated: 8921 mL Enhancement: +4–11% | [62] |
| 2450 MHz, power 1460 W, wavelength of 12.24 cm, and cavity size of 470 | ||||
| Ultrasonication: 20 kHz, 130 W, 30 min | ||||
| Poultry droppings + kitchen waste | The addition of silica gel as a catalyst | 26–31 °C, 8% TS | With pretreatment YM: 10,545 mL/kg Enhancement: +31.12% compared to trail without silica gel | [63] |
| Chicken litter (CL) + wheat straw (WS) + food waste (FW) | NaOH (1–5% w/v), 30–90 min, 60–120 °C | Batch assay, 1 L, 37 °C | CL YM: 321.1 mL/g VSadded With pretreatment YM: 440–590 mL/g VSadded Enhancement: up to 83.7% | [60] |
| Sequential alkaline acid pretreatment 1%, 2%, and 3% (w/v) H2SO4 | With pretreatment YM: 470–625 mL/g VSadded Enhancement: up to 94.6% | |||
| Poultry manure | 150 °C/5 min and 4.8 ba | AMPTS device (Bio- Process control, Lund, Sweden), 39 ± 1 °C | With pretreatment YM: 288 mL/g VSadded Enhancement: up to 14.4% | [64] |
| Chicken manure | hyper-thermophilic (70 °C) pretreatment | continuously stirred tank reactors, 12 L, 100 rpm, 55 °C | With pretreatment YM: 518 mL/g VSadded Enhancement: up to 54.6% | [65] |
| Chicken manure (CM) | pretreated at 100 °C, 125 °C, 150 °C, 175 °C, 200 °C, and 225 °C | Batch assay, 35 ± 1 °C | CM: YM: 340 mL/g VSadded Enhancement: −7.86 | [66] |
| Co-digestion (AcD) a mixture of 60% sewage sludge, 20% chicken manure, and 20% sugarcane waste (SCW) | Acid pretreatment with different doses of acetic acid (2%, 3%, and 4% v/v); duration: 30–90 min | Batch assay, 450 mL bottles, 30 ± 4 °C, 150 rpm | Raw agroindustrial waste: YM: 426.9 mL/g VSrem Enhancement: up to 1392.9 mL/g VSrem | [67] |
| Chicken manure (CM) and sewage sludge (SS) | Iron oxide nanoparticles at dosages of 40, 80, 120, and 160 mg/L | Batch assay, 1 L, 35 °C | SS YM: 278.8 mL/g VS CM: SS (70:30 on VS base): 3389.7 mL/g VS AcD with iron: YM: 421.9–560.6 mL/g VS | [68] |
| Poultry manure (PM) and lignocellulosic materials | - | Batch assay, 120 mL bottles, 35 °C, 180 rpm | The best result was achieved for a food-to-inoculum ratio equal to 0.5 YM for PM: 13–302 mL/g VS YM for co-digestion PM with rice straw, corn cob, peanut shell, sawdust, coffee husks, or sugarcane bagasse lower than for AD of PM alone | [8] |
| Kitchen waste (KW) and poultry manure (wet weight basis) at ratios of 1:0, 1:1, 2:1, and 3:1 | - | Batch assay, 1 L bottles, 28 and 37 °C | KW YB: 312 mL/g VS (28 °C); 532 mL/g VS (37 °C) AcD: 360–396 mL/g VS (28 °C); 452–920 mL/g VS (37 °C) | [9] |
| Poultry droppings (PDs) with two different ratios of physicochemical sludge (20% and 40%, based on the VS) | Continuously stirred anaerobic digestor, 12 L, 37 °C, HRT 30 d | PD, YM: 270 mL/g VS Enhancement: up to 54% | [69] |
| Sample | P [mL CH4/g VS] | Rm [mL CH4/(g VS d)] | λ [d] | R2 |
|---|---|---|---|---|
| Modified Gompertz equation (MG) | ||||
| M | 340.3624 | 63.49502 | 0.235276 | 0.99933 |
| M-A | 438.4465 | 66.90778 | 0.265849 | 0.99895 |
| Na0.9 | 463.6653 | 72.92838 | 0.389854 | 0.99915 |
| Na3.6 | 488.7484 | 77.75526 | 0.548816 | 0.99912 |
| Na4.5 | 501.0150 | 81.16676 | 0.552048 | 0.99900 |
| Na5.4 | 496.8261 | 81.76055 | 0.589216 | 0.99862 |
| Fe3.0 | 457.6325 | 68.74786 | 0.290088 | 0.99895 |
| Logistic function model (LF) | ||||
| M | 338.9935 | 58.93189 | 0.177459 | 0.99879 |
| M-A | 435.8587 | 62.60483 | 0.213661 | 0.99705 |
| Na0.9 | 461.0132 | 68.71367 | 0.373178 | 0.99800 |
| Na3.6 | 485.9906 | 73.89803 | 0.575518 | 0.99902 |
| Na4.5 | 498.2668 | 77.18721 | 0.581775 | 0.99908 |
| Na5.4 | 494.0965 | 78.13411 | 0.636446 | 0.99896 |
| Fe3.0 | 454.8350 | 64.42861 | 0.242888 | 0.99693 |
| Transference function (TF) | ||||
| M | 342.8943 | 114.4020 | 0.609348 | 0.99474 |
| M-A | 443.7058 | 117.5256 | 0.673899 | 0.99564 |
| Na0.9 | 469.2031 | 126.1682 | 0.721710 | 0.99437 |
| Na3.6 | 494.8274 | 131.5803 | 0.786065 | 0.99226 |
| Na4.5 | 507.0182 | 137.4199 | 0.781930 | 0.99190 |
| Na5.4 | 502.7924 | 137.5215 | 0.792250 | 0.99115 |
| Fe3.0 | 463.4191 | 120.1680 | 0.691750 | 0.99576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, A.; Grosser, A.; Meers, E.; Piłyp, D. Stimulating Methane Production from Poultry Manure Digest with Sewage Sludge and Organic Waste by Thermal Pretreatment and Adding Iron or Sodium Hydroxide. Energies 2024, 17, 2679. https://doi.org/10.3390/en17112679
Jasińska A, Grosser A, Meers E, Piłyp D. Stimulating Methane Production from Poultry Manure Digest with Sewage Sludge and Organic Waste by Thermal Pretreatment and Adding Iron or Sodium Hydroxide. Energies. 2024; 17(11):2679. https://doi.org/10.3390/en17112679
Chicago/Turabian StyleJasińska, Anna, Anna Grosser, Erik Meers, and Dagmara Piłyp. 2024. "Stimulating Methane Production from Poultry Manure Digest with Sewage Sludge and Organic Waste by Thermal Pretreatment and Adding Iron or Sodium Hydroxide" Energies 17, no. 11: 2679. https://doi.org/10.3390/en17112679
APA StyleJasińska, A., Grosser, A., Meers, E., & Piłyp, D. (2024). Stimulating Methane Production from Poultry Manure Digest with Sewage Sludge and Organic Waste by Thermal Pretreatment and Adding Iron or Sodium Hydroxide. Energies, 17(11), 2679. https://doi.org/10.3390/en17112679










