Simulation of Underground Coal-Gasification Process Using Aspen Plus
Abstract
1. Introduction
2. Principles of Underground Coal Gasification
2.1. Oxidation Zone
2.2. Reduction Zone
2.3. Methanation Reaction Zone
2.4. Dry Distillation and Drying Zone
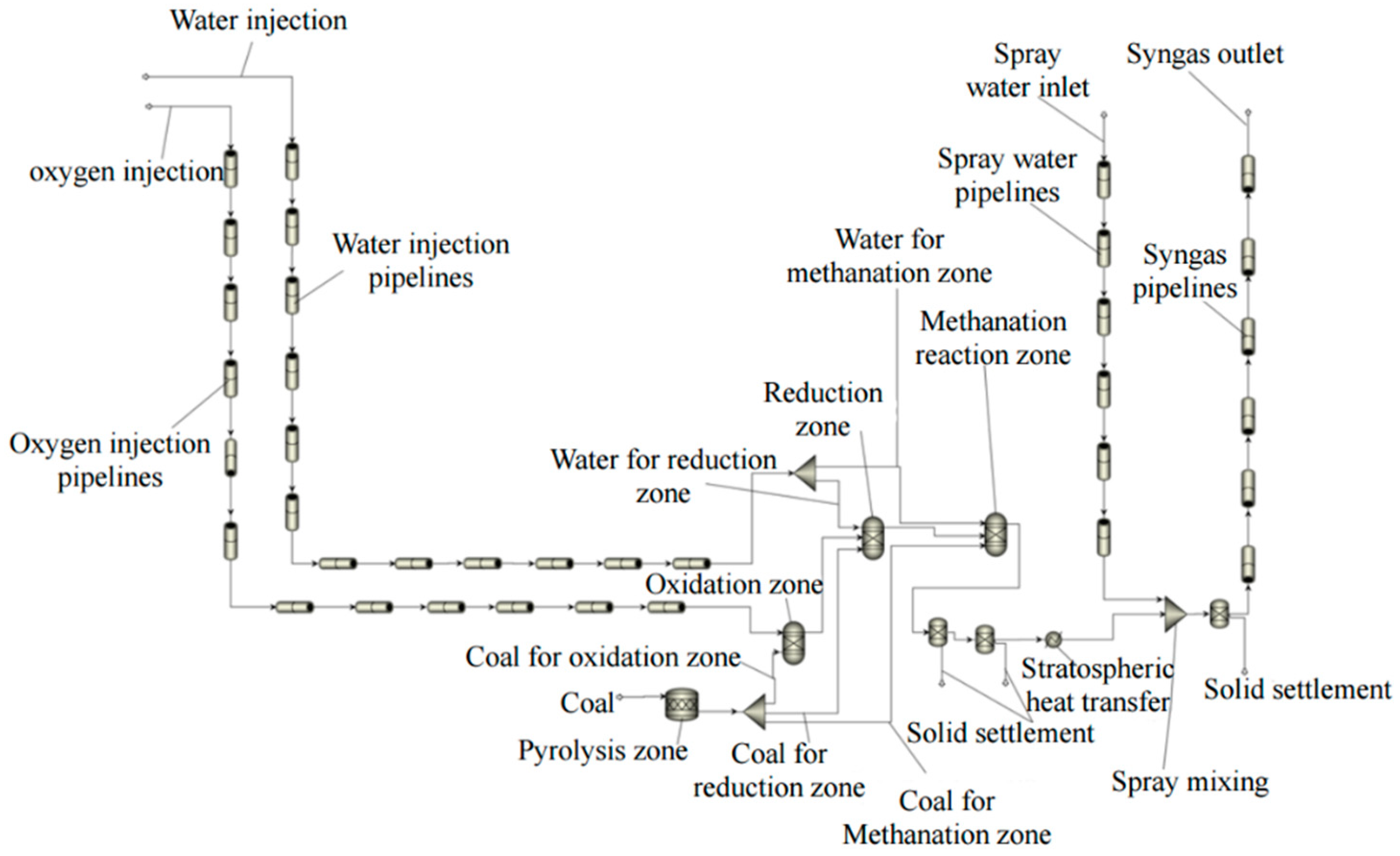
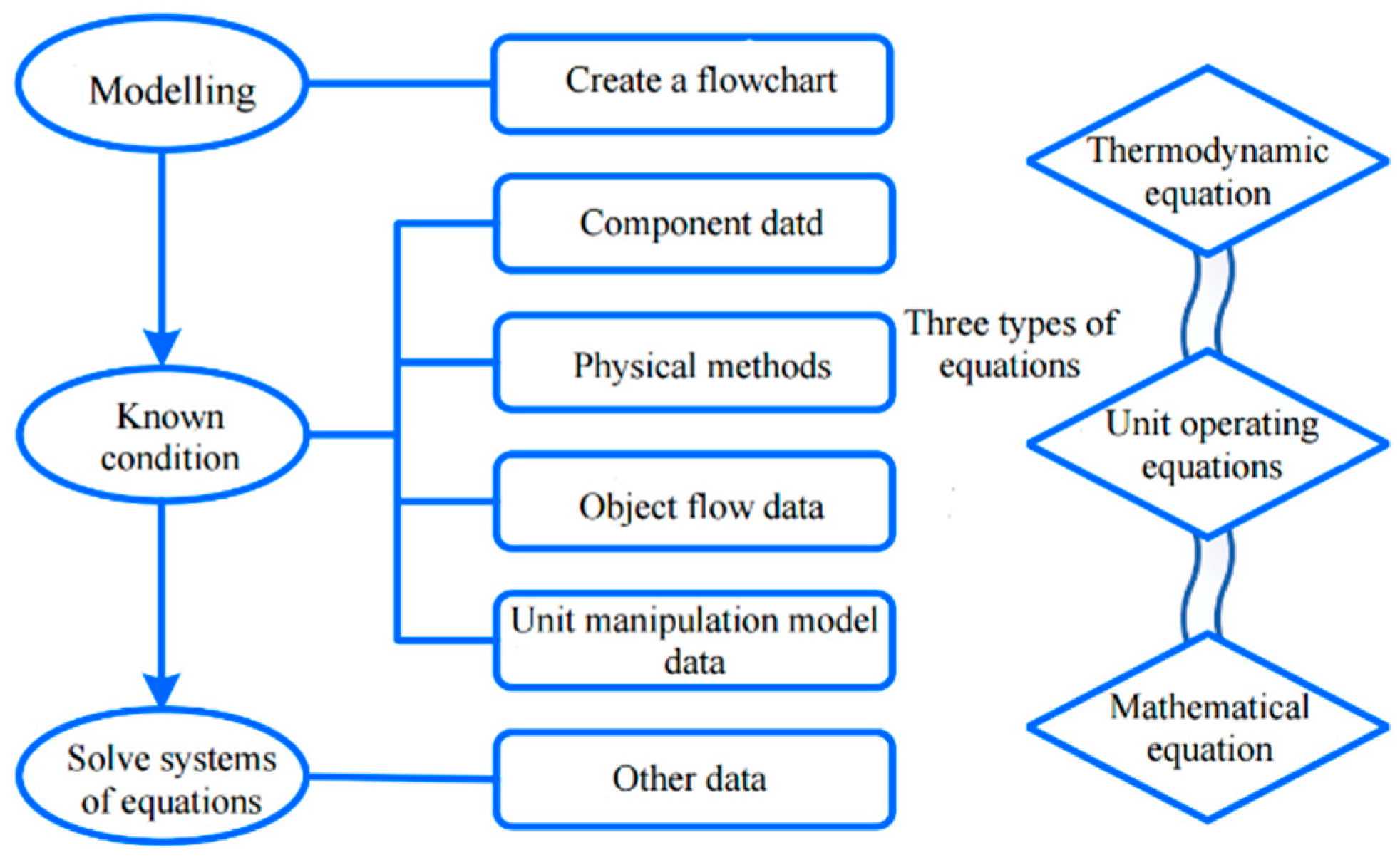
3. Model Building
3.1. Process Simulation Models
3.2. Component Definition
3.3. Definition of Physical Methods
3.4. Parameters and Boundary Conditions
3.5. Model Validation
4. Analysis and Discussion of Results
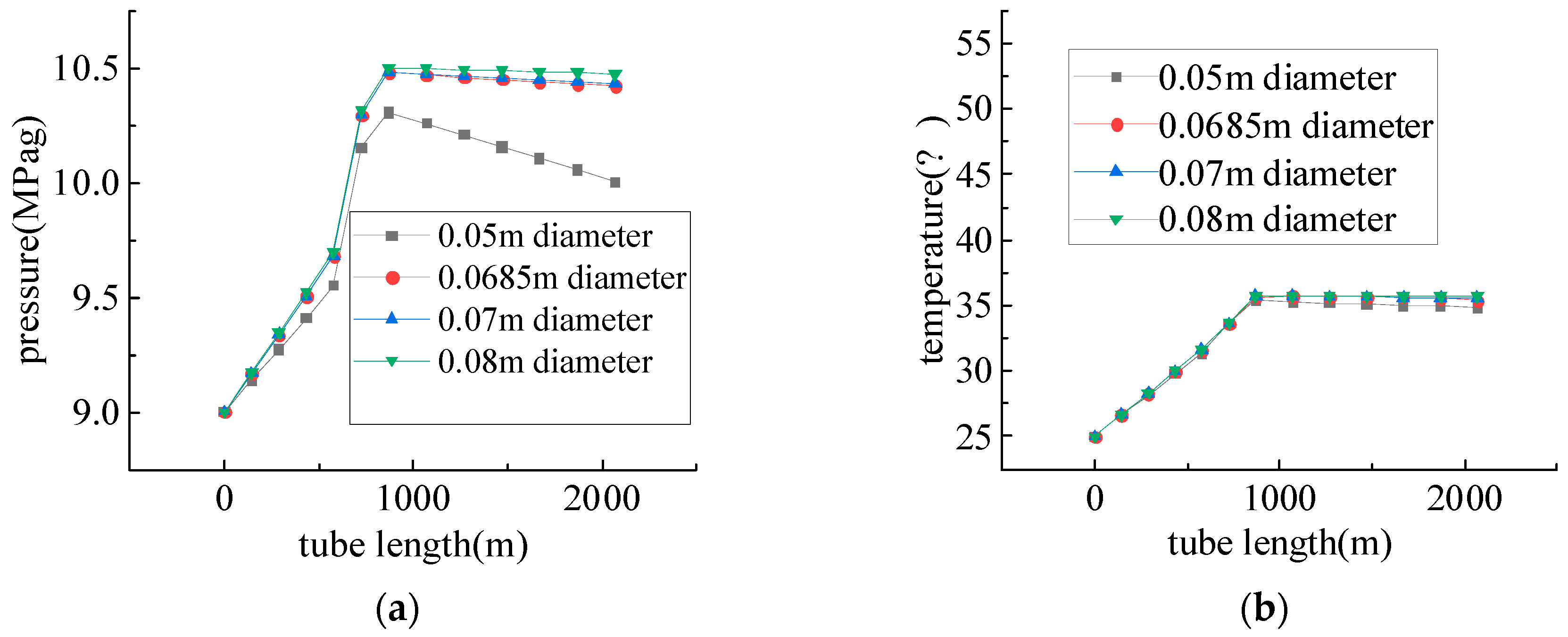
4.1. Pipeline Conveyance Performance Study
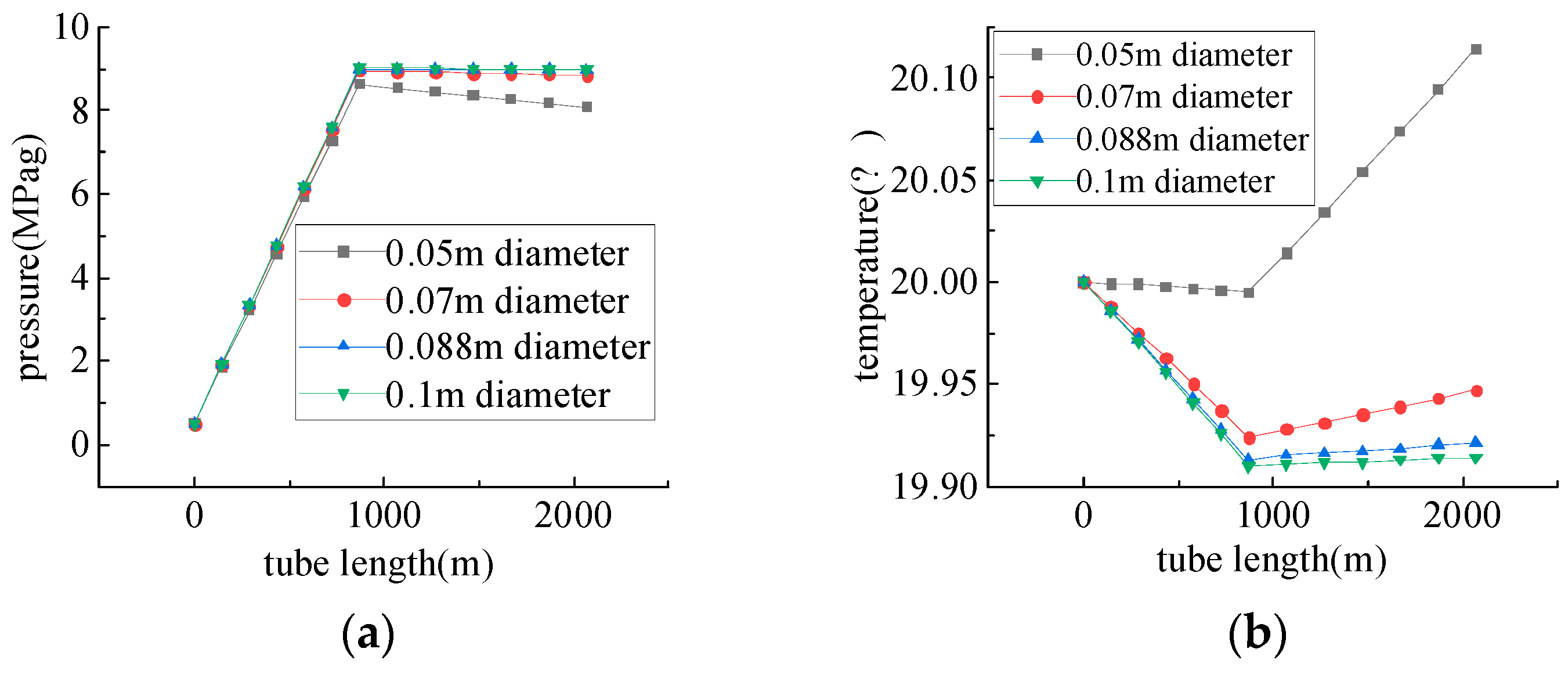
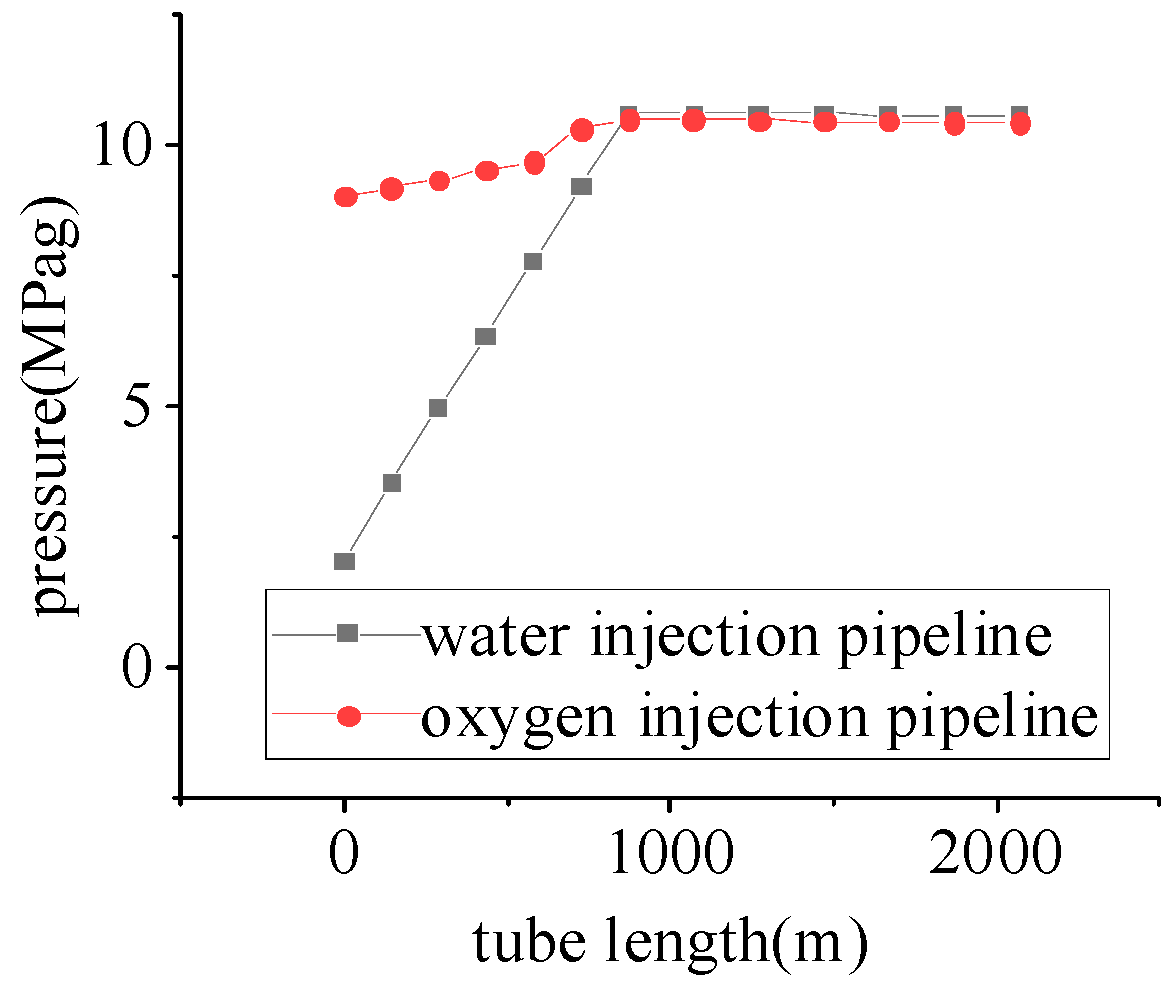
Study of Oxygen Injection Pipelines Conveyance Performance
4.2. Study of Gasification Water Injection Pipelines Conveyance Performance
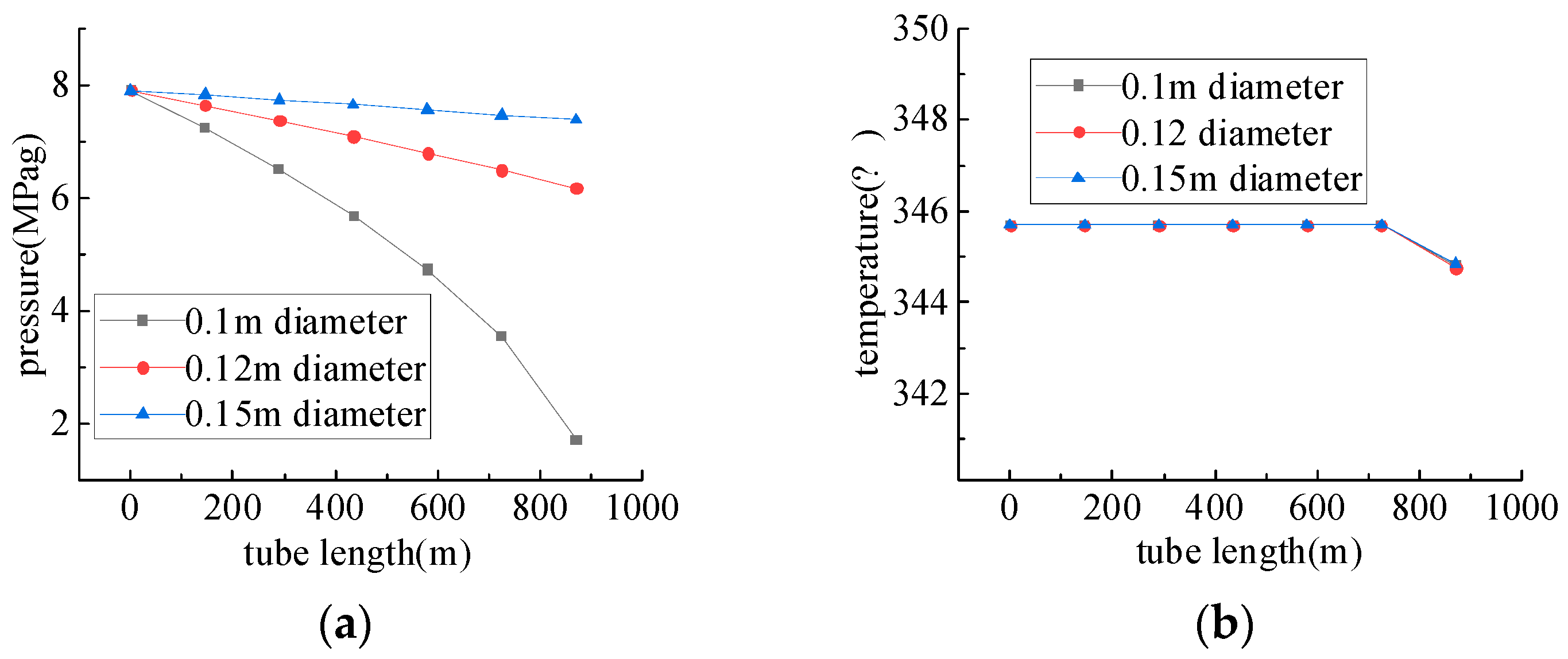
4.3. Study of Spray-Water Pipelines Conveyance Performance
4.4. Study of Syngas Pipeline Conveyance Performance
4.5. Study of Gasification Products under Different Conditions
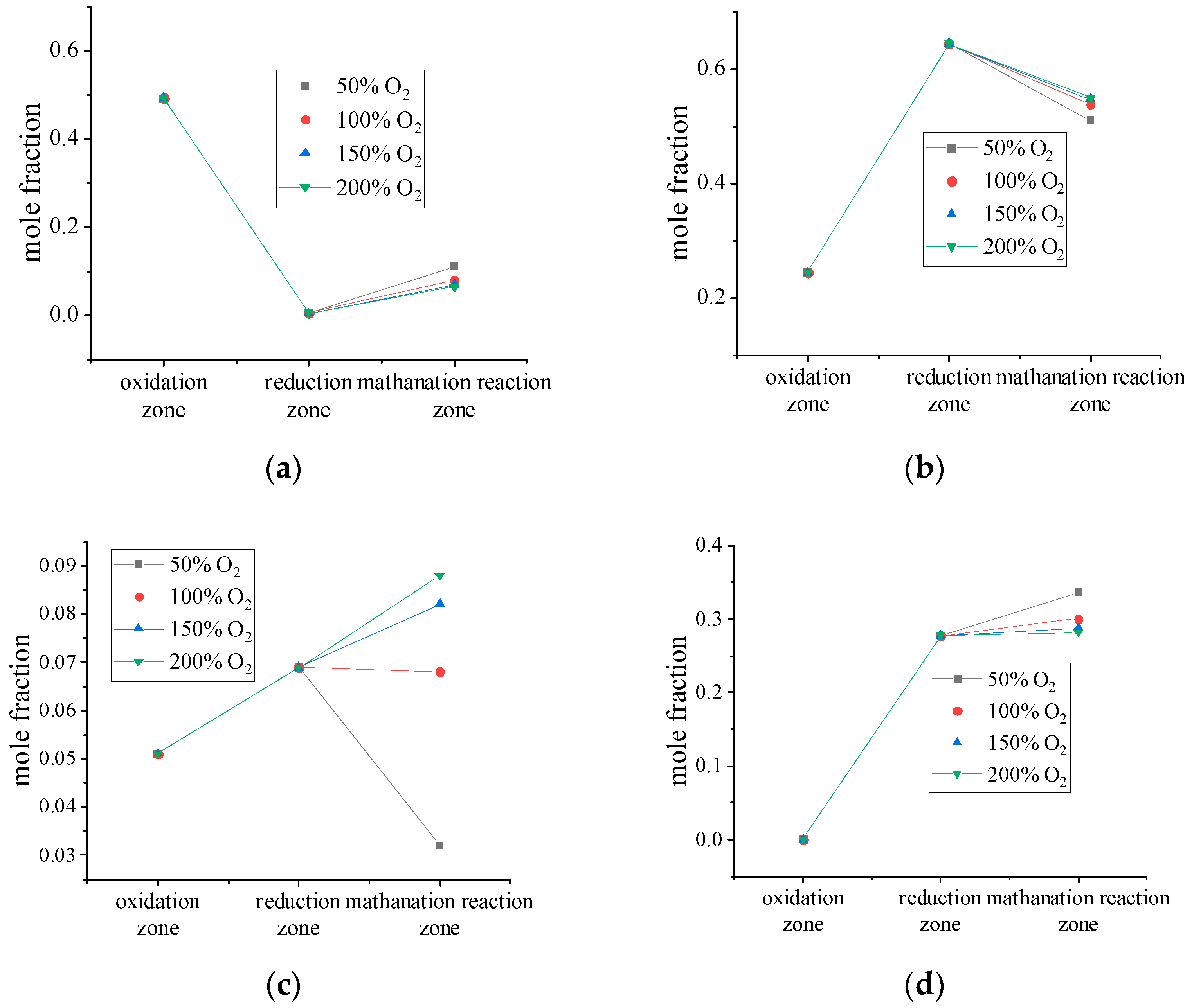
Effect of Oxygen/Water Ratio on Syngas Composition
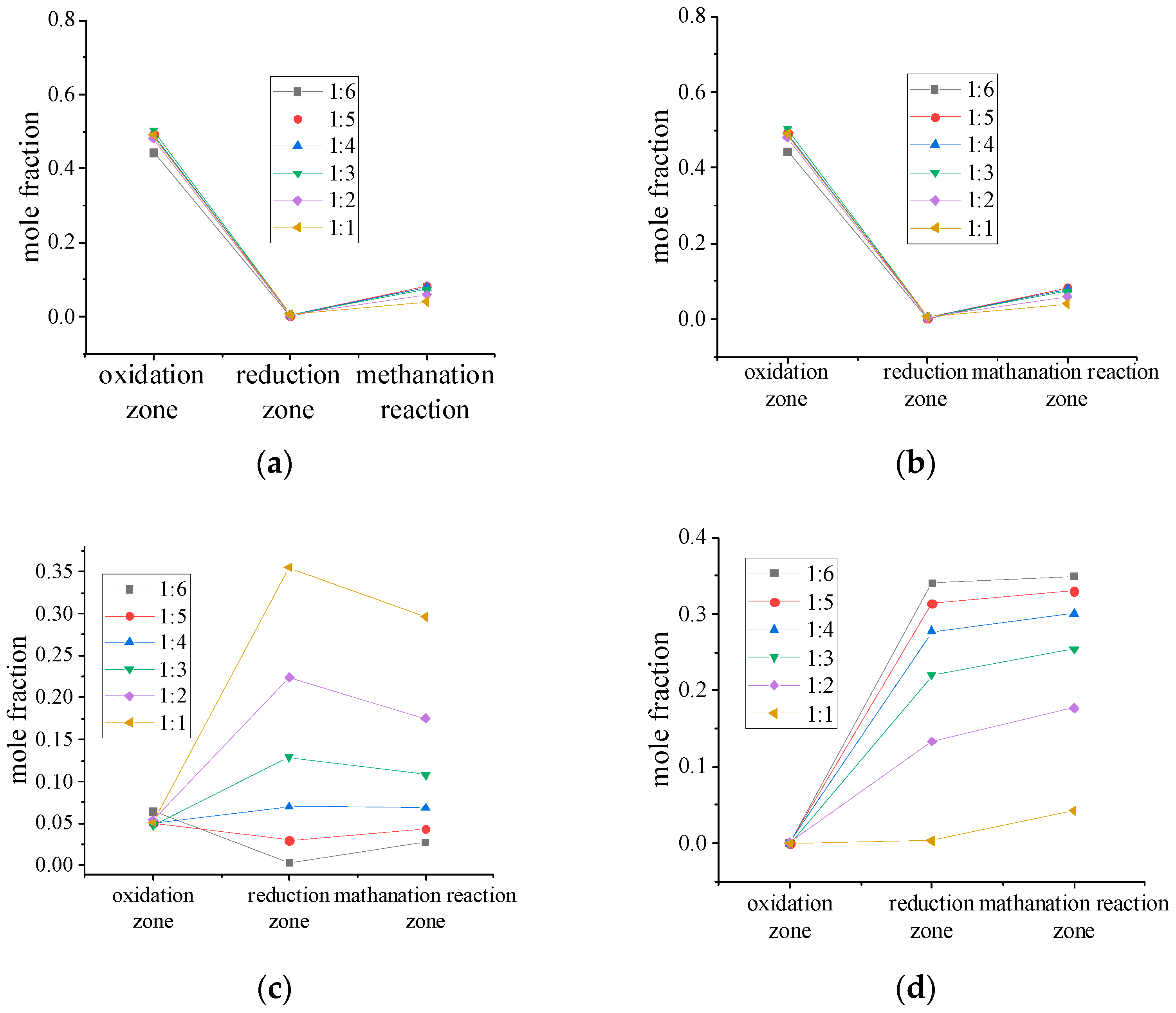
4.6. Analysis of Gas Components in Different Reaction Zones
4.7. Effect of Processing Capacity on Syngas Composition
4.8. Effect of Spray Water on Syngas Composition
5. Conclude
- (1)
- Due to the influence of gravitational potential energy, the flow of medium in the injection pipeline is a pressurizing process. In horizontal wells, the pressure drop decreases with the increase in wellbore diameter, which is similar to the result obtained by Zhang et al. [34]: the hydraulic molar resistance decreases with the increase in wellbore diameter, and when injecting the gasifier, it is necessary to determine a reasonable injection pressure according to the formation pressure and the change in pressure during the injection process.
- (2)
- The liquid-water injection process generates a high water-column pressure, and a lower injection pressure should be selected, ensuring that the pressure in the inner pipeline of the injection well is higher than that in the outer pipeline. The initial injection pressure of the liquid is lower than that of the gas, which should be injected into the inner pipeline of the injection well and the outer pipeline, and should be injected with the gas and into the liquid.
- (3)
- Changes in the oxygen/water ratio have a greater effect on the molar fractions of H2 and CH4 and a smaller effect on CO2 and CO. Under high-pressure conditions, increasing the oxygen/water ratio is beneficial to H2 generation because H2 mainly comes from the decomposition reaction of water vapor [35]. Lowering the oxygen/water ratio results in a lower gasification temperature, which is favorable for CH4 generation, so the syngas composition can be controlled by changing the oxygen/water ratio.
- (4)
- CO2 is the highest in the oxidation zone and the lowest in the reduction zone, and the methanation zone increases with the transformation reaction. CO is the lowest in the oxidation zone and the highest in the reduction zone. This indicates that the combustion reaction of C mainly occurs in the early stage of gasification, and the CO2 produced has not yet participated in the reduction reaction in large quantities, leading to an increase in the concentration of CO2. As the gasification process proceeds, the heat generated by the combustion reduces the CO2 to CO, resulting in a decrease in the content of CO2. As the gasification temperature decreases, some CO is converted to H2 and CO2 and the CO content decreases. A high content of CO2 means a higher temperature in the zone, while a high content of CO means a lower temperature.
- (5)
- The syngas temperature decreases with the increase in the injection volume of spray water, and the syngas temperature has a great influence on the stress of the production wellbore, so the spray-cooling of the syngas can effectively reduce the internal stress of the wellbore [36] and ensure that the wellbore will not be destabilized. The spray-cooling process does not affect the original components of the gas but will increase the water vapor content of the gas, which indirectly leads to changes in the fraction of wet gas components.
- (6)
- During gasification reactions with O2/H2O as gasification agents under high pressure, the proportion of H2O in the gasification agent should be increased if it is oriented towards the generation of CH4 and decreased if it is oriented towards the generation of H2.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Yang, Z.; Yi, T.; Qin, Y.; Yang, Y.; Jin, J. Research Status of Underground Coal Gasification Abroad. Coal Geol. China 2023, 4, 1–10. [Google Scholar]
- British Petroleum. BP Statistical Yearbook of World Energy; British Petroleum: London, UK, 2023. [Google Scholar]
- Hamanaka, A.; Ishii, Y.; Itakura, K.I.; Sasaoka, T.; Shimada, H.; Widodo, N.P.; Deguchi, G. Monitoring the gasification area and its behavior in underground coal gasification by acoustic emission technique instead of temperature measurement. Sci. Rep. 2023, 13, 9757. [Google Scholar] [CrossRef]
- Mandapati, R.N.A.; Ghodke, P.B. Modeling of gasification process of Indian coal in perspective of underground coal gasification (UCG). Environ. Dev. Sustain. 2020, 22, 6171–6186. [Google Scholar] [CrossRef]
- Sadovenko, I.A.; Inkin, A.V. Method for Stimulating Underground Coal Gasification. J. Min. Sci. 2018, 3, 514–521. [Google Scholar] [CrossRef]
- Yang, L. Characteristics of “Three Zones” in Underground Coal gasification and Its Study influence Variables. J. Nanjing Univ. Sci. Technol. 2001, 25, 533–537. [Google Scholar]
- Wiatowski, M.; Kapusta, K.; Strugała-Wilczek, A.; Stańczyk, K.; Castro-Muñiz, A.; Suárez-García, F.; Paredes, J.I. Large-Scale Experimental Simulations of In Situ Coal Gasification in Terms of Process Efficiency and Physicochemical Properties of Process By-Products. Energies 2023, 16, 4455. [Google Scholar] [CrossRef]
- Liu, J.; Wu, M.; Wang, S.; Qin, Y.; Zhu, S.; Sun, Q.; Yang, L.; Sun, P. Progress in Research on Coal Underground Gasification Impacting Factors. Coal Geol. China 2020, 32, 155–158. [Google Scholar]
- Chen, H.; Qin, Y.; Chen, Y.; Dong, Z.; Xue, J.; Chen, S.; Zhao, Y. Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent. Energies 2023, 16, 6993. [Google Scholar] [CrossRef]
- Zong, K.; Chai, X.; Zhang, H.; Yang, X.; Zhai, C. Analysis and Optimization of Hydrogen Extraction Process from Underground Coal Gasification. Chem. Prod. Technol. 2022, 28, 20–25+9. [Google Scholar]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K.; Zagorščak, R.; Thomas, H.R. Large-scale Experimental Investigations to Evaluate the Feasibility of Producing Methane-Rich Gas (SNG) through Underground Coal Gasification Process. Effect of Coal Rank and Gasification Pressure. Energies 2020, 13, 1334. [Google Scholar] [CrossRef]
- Krzysztof, S.; Krzysztof, Z.; Anna, Z. Determination of the Extent of the Rock Destruction Zones around a Gasification Channel on the Basis of Strength Tests of Sandstone and Claystone Samples Heated at High Temperatures up to 1200 °C and Exposed to Water. Energies 2021, 14, 6464. [Google Scholar] [CrossRef]
- Laciak, M.; Durdán, M.; Kačur, J.; Flegner, P. The Underground Coal Gasification Process in Laboratory Conditions: An Experimental Study. Energies 2023, 16, 3266. [Google Scholar] [CrossRef]
- Wang, J.; Meng, Z.; Shi, F.; Guan, J. Study on roof subsidence law and related parameters of gasifier under thermodynamic coupling action. J. China Coal Soc. 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=b4E8SuETvlK5M1N-T65--G-WxLUeSjFGdLy8T4EwowFDK6u9MGDDmUk3QfX1xHEBCRyd4Th0e0Hnk7GK0Y2_6beOlnabhaPvpE6BO4h2iu1dQNCLwfSy_6AN6ykWRm9ihSwaVSO52bA=&uniplatform=NZKPT&language=CHS (accessed on 15 March 2024).
- Liu, Y.; Jiang, Y.; Deng, S.; Xiong, Z.; Wang, M. A Review of Coal Gasification Process Simulation Based on Aspen Plus Software. Henan Chem. Ind. 2017, 49, 5–8. [Google Scholar]
- Cheng, S.; Cao, Z.; Shi, J.; Jiang, Q.; Zhou, H. Steady-state simulation of fixed bed foe coal gasification using ASPEN PLUS. J. China Coal Soc. 2012; 37, (Suppl. S1), 167–172+6. [Google Scholar]
- Hao, P.; Wang, C.; Liu, M.; Cao, W.; Li, X.; Yuan, S.; Wu, S.; Qi, W. Analysis of Recovery Rate for Underground Coal Gasification Based on Strength and Permeability. J. China Coal Soc. 2023, 48, 3562–3572. [Google Scholar]
- Wu, M.; Qin, Y.; Li, G.; Shen, J.; Song, X.; Zhu, S.; Han, L. Research progress on influencing factors and evaluation methods of underground coal gasification. Coal Sci. Technol. 2022, 50, 259–269. [Google Scholar]
- Wu, C.; Jiang, X. Research progress of temperature field and heat transfer characteristics in process of underground coal gasification. Coal Sci. Technol. 2022, 50, 275–285. [Google Scholar]
- Xin, L.; Wu, J.; Wang, B.; Niu, M.; Li, J.; Xu, W.; Wang, X.; Shang, Z.; Li, H.; Ma, Y. Pyrolysis yield of typical organics in underground coal gasification. Coal Eng. 2023, 55, 170–174. [Google Scholar]
- Zhou, H.; Huang, G.; Chen, Y.; Wang, S.; Wang, Y.; Wang, W. Research on phase change model and characteristics of crude coal gas in underground coal gasification. Coal Chem. Ind. 2023, 51, 19–23. [Google Scholar]
- Xu, Y.; Wu, Y.; Wei, S. Simulation of the Performance Based on ASPEN PLUS for Dry Feed Entrained Flow Coal Gasification of Entrained-Bed Gasifier. J. Xi’an Jiaotong Univ. 2003, 7, 692–694+706. [Google Scholar]
- Fu, X.; Li, K.; Xin, L.; Feng, M.; Wu, J.; Li, J.; Wang, Z. Analysis of the Physical and Chemical Properties of Fat Coal at Different Pyrolysis Temperatures in UCG Environment. Solid Fuel Chem. 2022, 56, 384–409. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Zhou, S.; Zhang, C.; Ren, X.; Yang, C. Analysis of Spray Cooling of High-temperature Gas Produce by Underground Coal Gasification Based on DMP Model and Realizable k-ε Turbulence Model. Mech. Electr. Eng. Technol. 2023, 52, 98–101. [Google Scholar]
- Shi, L.; Liang, J.; Wang, Y.; Wang, L.; Zhao, X.; Li, Y.; Zhang, Y. Simulation of lignite underground gasification using Aspen Plus. Coal Eng. 2017, 49, 5–8. [Google Scholar]
- Zhou, Y.; He, X.; Lin, K.; Qin, S.; Zhang, C.; Liu, Z. Formation Pressure Estimation Method Based on Dyna -mic Effective Stress Coefficient. Xinjiang Pet. Geol. 2023, 44, 245–251. [Google Scholar]
- Sun, L.; Wang, J.; Qi, P. Simulation Analysis with Aspen Plus for a Chemical System Process. Anhui Chem. Ind. 2015, 41, 48–50+52. [Google Scholar]
- Lin, L. Aspen Plus Software Application for Coal Gasification Simulation. Shanghai Chem. Ind. 2006, 5, 10–13. [Google Scholar]
- Pan, Z.; Wang, J.; Liu, R.; Hua, Z.; Zhang, C.; Han, L.; Zhang, Y.; Xu, X.; Ren, X. Structural Design of Multi-channel Coiled Tubing for Underground Coal Gasification. Pet. Tubul. Goods Instrum. 2023, 9, 44–47. [Google Scholar]
- Liu, Y.; Huang, S.; Yuan, G.; Tang, Y. Study on Temperature Field of High Temperature Wellbore of Underground Coal Gasification. Coal Convers. 2022, 45, 58–64. [Google Scholar]
- Wang, C.; Wang, J.; Jing, X.; Qiao, L.; He, A.; Liu, Y.; Tang, Y. Simulation Study on Erosion Law of Wellhead Equipment in Underground Coal Gasification Production Well. Surf. Technol. 2023, 5, 101–110, 130. [Google Scholar]
- Xin, L.; An, M.; Feng, M.; Li, K.; Cheng, W.; Liu, W.; Han, L. Study on pyrolysis characteristics of lump coal in the context of underground coal gasification. Energy 2021, 237, 121626. [Google Scholar] [CrossRef]
- Li, Q. Research on High Temperature Pyrolysis Characteristics of Pulverized Coal, Design and Experiment of Pilot-Scale Pyrolysis-Gasification System. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Zhang, Y.; Yang, P.; Liu, J.; Mao, L.; Yuan, J.; Fu, D. Pressure Control of Underground Coal Gasification Injection Well. Sci. Technol. Eng. 2023, 23, 15036–15043. [Google Scholar]
- Liang, J.; Ling, Y.; Cheng, S.; Yang, Y.; Zhao, Z.; Yuan, H.; Li, Z. Evolution law of the characteristic field of channel-type underground coal gasification process. J. China Coal Soc. 2022, 47, 2218–2227. [Google Scholar]
- Tang, Y.; Xie, N.; Xiong, H.; He, Y.; Huang, S. Stress field of high-temperature wellbore under spray cooling for underground coal gasification. Coal Geol. Explor. 2023, 51, 13–23. [Google Scholar]
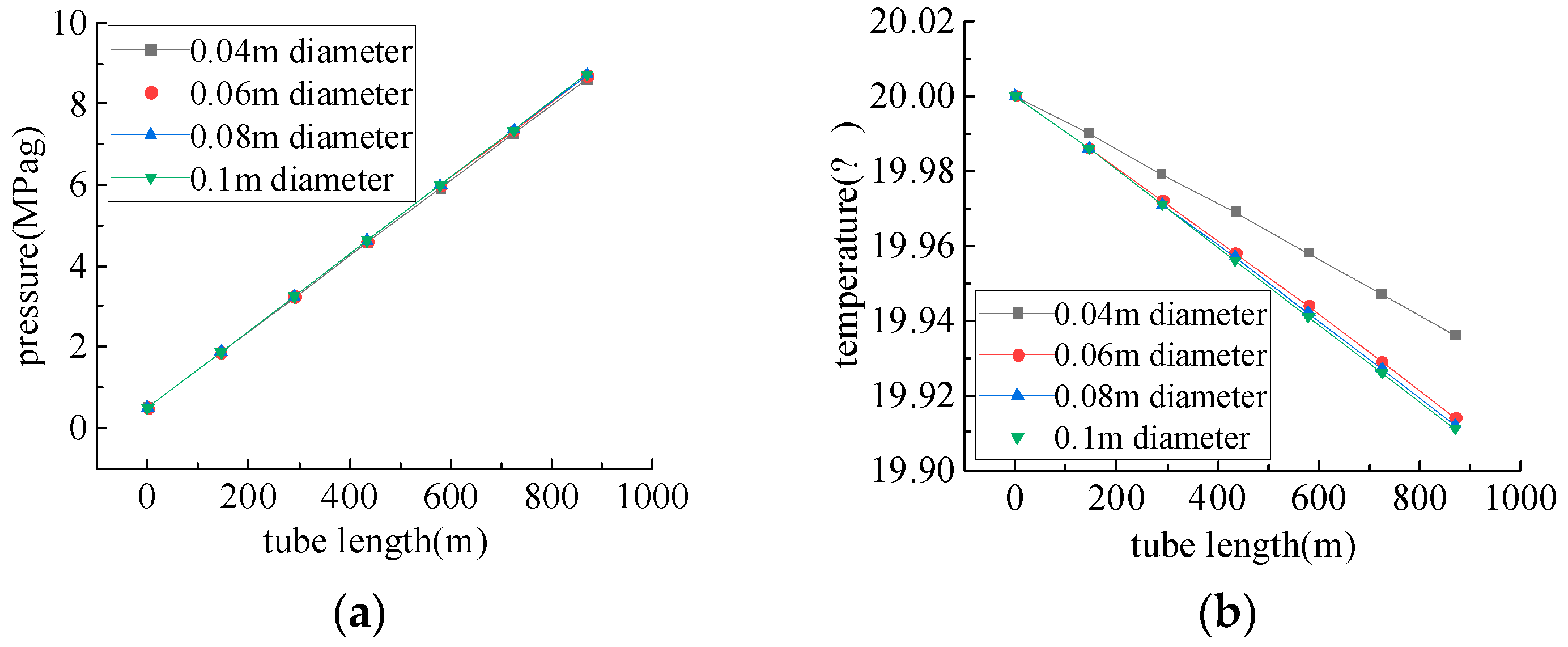


| Elemental Analysis | Industrial Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Cad | Had | Oad | Nad | Sad | Mad | Aad | FCad | Vad |
| 70.82 | 3.95 | 15.34 | 0.26 | 0.77 | 2.56 | 6.30 | 61.68 | 29.46 |
| Oxygen Injection Pipeline | Water Injection Pipeline | Spray-Water Pipeline | Syngas Pipeline | |
|---|---|---|---|---|
| Tube length (vertically)/m | 870 | 870 | 870 | 870 |
| Tube length (level)/m | 1200 | 1200 | / | / |
| Tube diameter (vertically)/m | 0.0685 | 0.088 | 0.08 | 0.1 |
| Tube diameter (level)/m | 0.0685 | 0.088 | / | / |
| Roughness/×10−5 m | 4.57 | 4.57 | 4.57 | 4.57 |
| Elemental Analysis | Industrial Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Cad | Had | Oad | Nad | Sad | Mad | Aad | FCad | Vad |
| 58.27 | 3.01 | 18.38 | 0.70 | 0.32 | 10.36 | 8.96 | 51.28 | 29.40 |
| Value Type | ψ/% | Syngas Temperature/°C | ||||
|---|---|---|---|---|---|---|
| N2 | H2 | CO | CO2 | CH4 | ||
| Experimental results | 0 | 30.20 | 59.31 | 11.24 | 0.29 | 1198 |
| Simulation results | 0.33 | 28.37 | 60.8 | 10.32 | 0.21 | 1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Jiao, W.; Wang, C.; Wu, S.; Jiang, Q. Simulation of Underground Coal-Gasification Process Using Aspen Plus. Energies 2024, 17, 1619. https://doi.org/10.3390/en17071619
Yuan S, Jiao W, Wang C, Wu S, Jiang Q. Simulation of Underground Coal-Gasification Process Using Aspen Plus. Energies. 2024; 17(7):1619. https://doi.org/10.3390/en17071619
Chicago/Turabian StyleYuan, Shuxia, Wanwan Jiao, Chuangye Wang, Song Wu, and Qibin Jiang. 2024. "Simulation of Underground Coal-Gasification Process Using Aspen Plus" Energies 17, no. 7: 1619. https://doi.org/10.3390/en17071619
APA StyleYuan, S., Jiao, W., Wang, C., Wu, S., & Jiang, Q. (2024). Simulation of Underground Coal-Gasification Process Using Aspen Plus. Energies, 17(7), 1619. https://doi.org/10.3390/en17071619






