Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials
Abstract
1. Introduction
2. Methods
2.1. Experimental Setup
2.2. Experimental Design
2.3. Materials
3. Results and Discussion
3.1. Sensitive Heat: Thermal Behavior of Volcanic Stone and Biochar
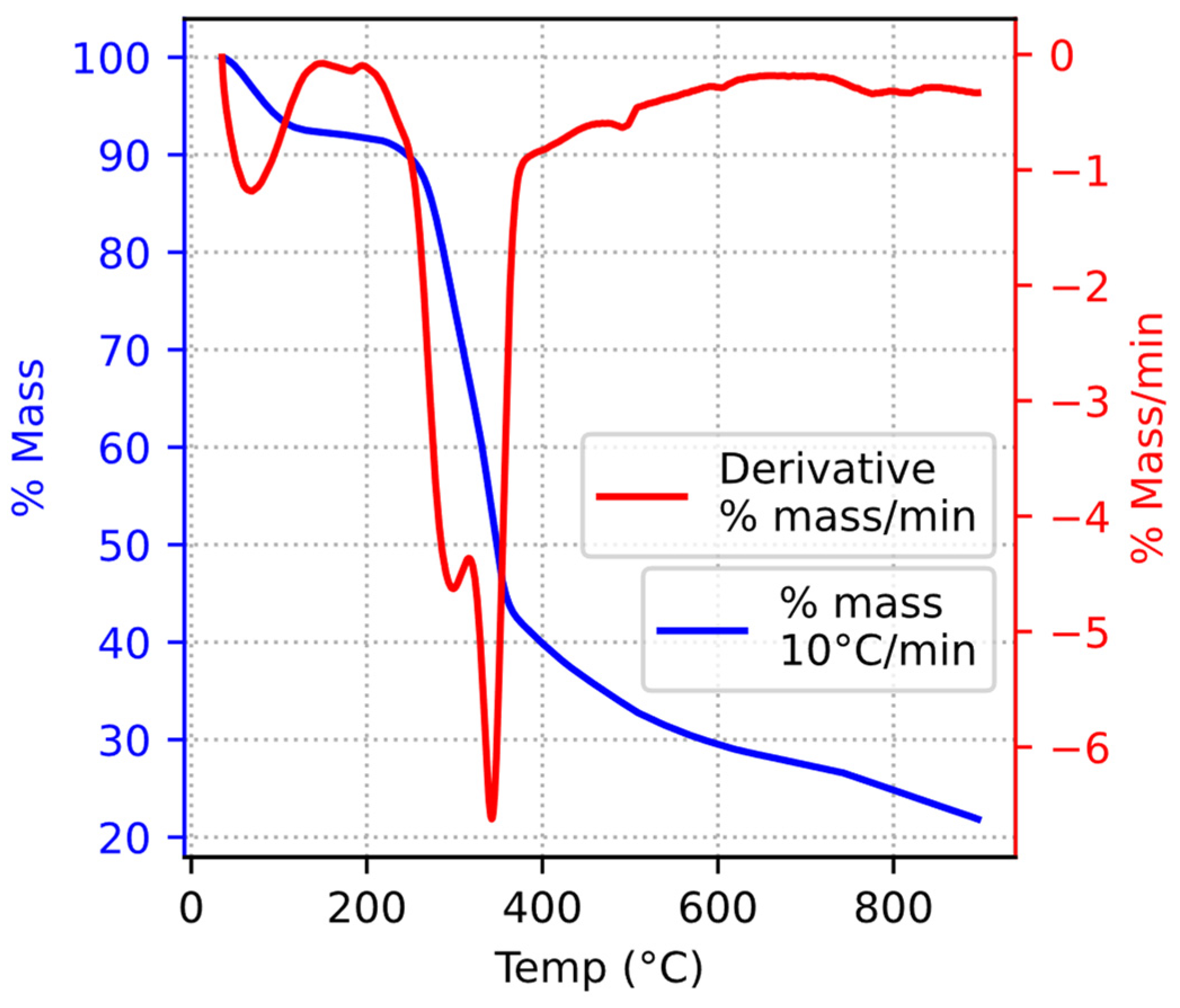
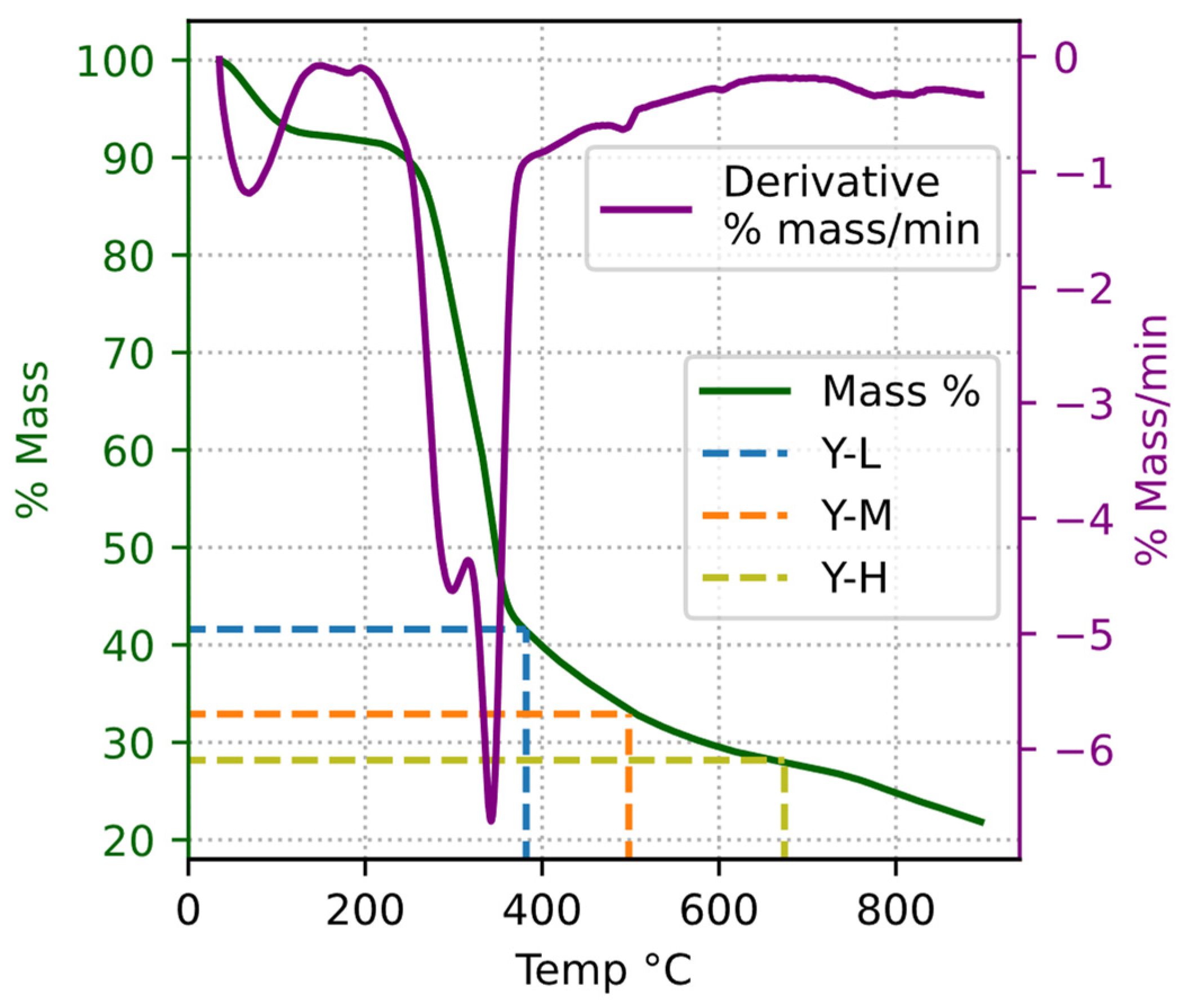
3.2. Latent Heat and Interpretation of Pyrolysis Stages
3.3. Analysis of Gas Outlet Temperatures and Tar Condensation
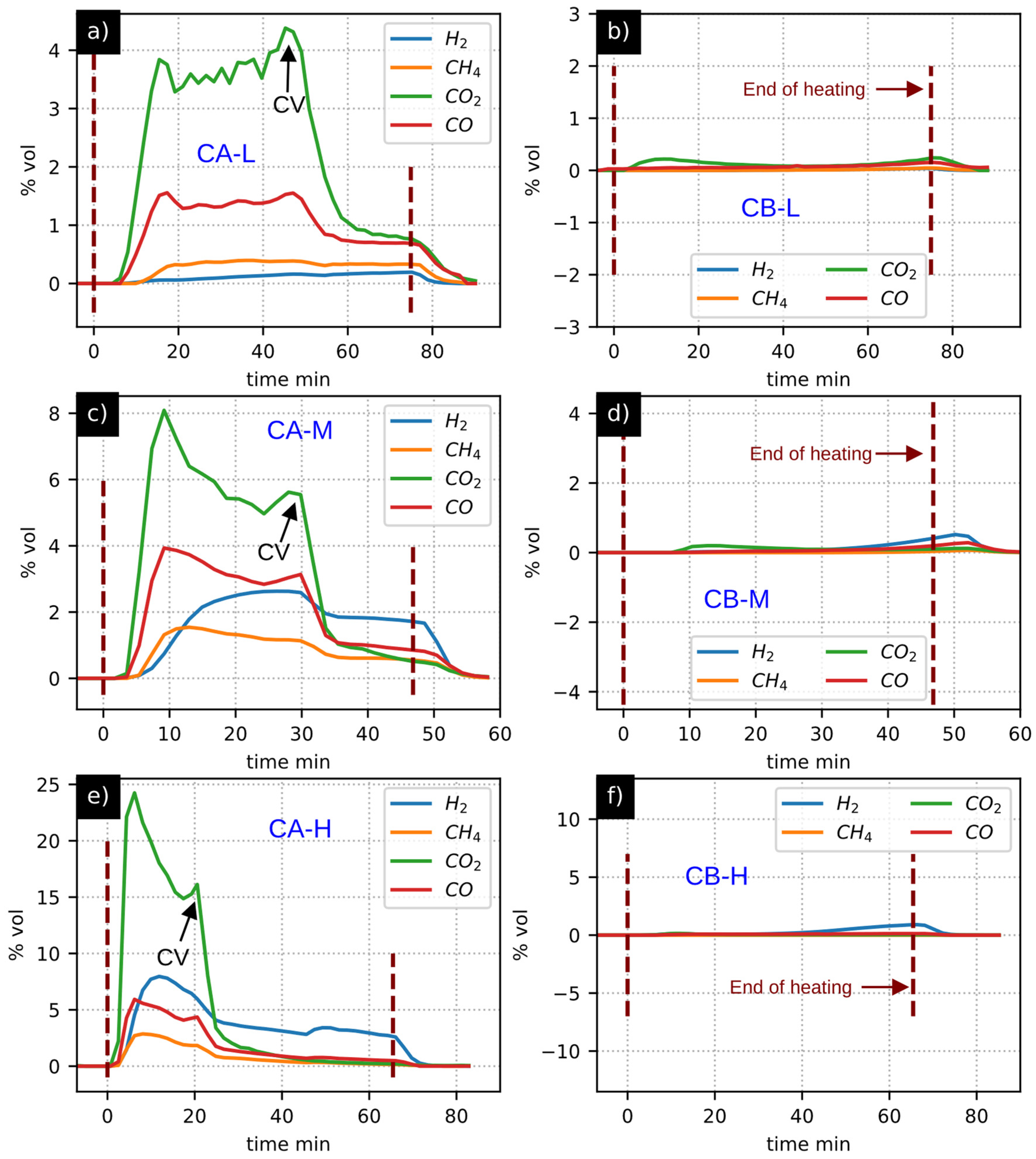
3.4. Gas Composition Analysis
3.5. Mass Balance
4. Conclusions
- (a)
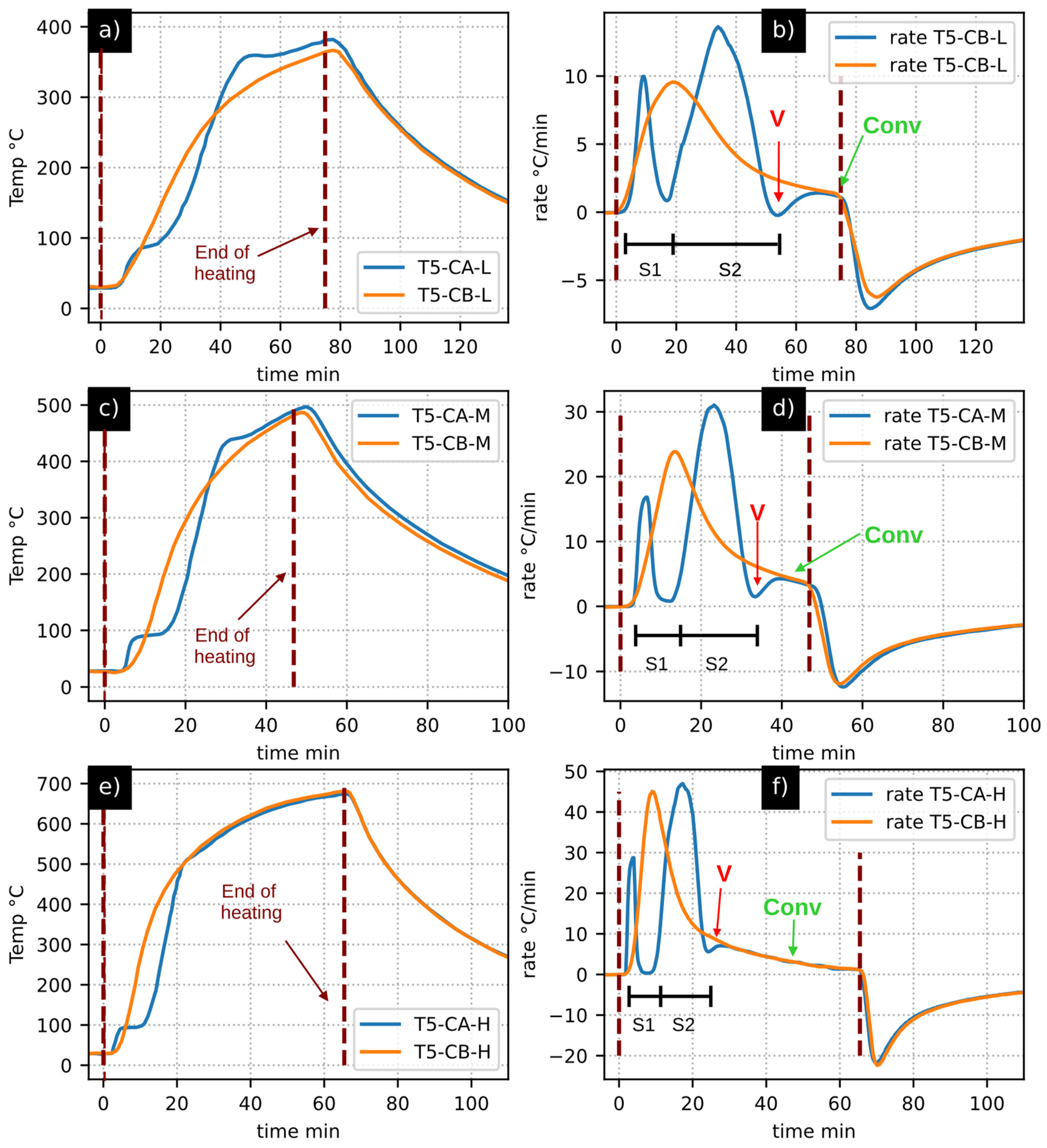
- Initially, a comparative investigation between the heating profiles of biochar and volcanic stone revealed a notable similarity in their thermal behaviors during the heating process.
- (b)
- Subsequently, through a comparative analysis of the thermal behaviors between biochar and biomass, different stages of walnut shell pyrolysis were discerned. As a corollary, the most notable findings included the unambiguous identification of moisture evaporation and the termination point of tar production.
- (a)
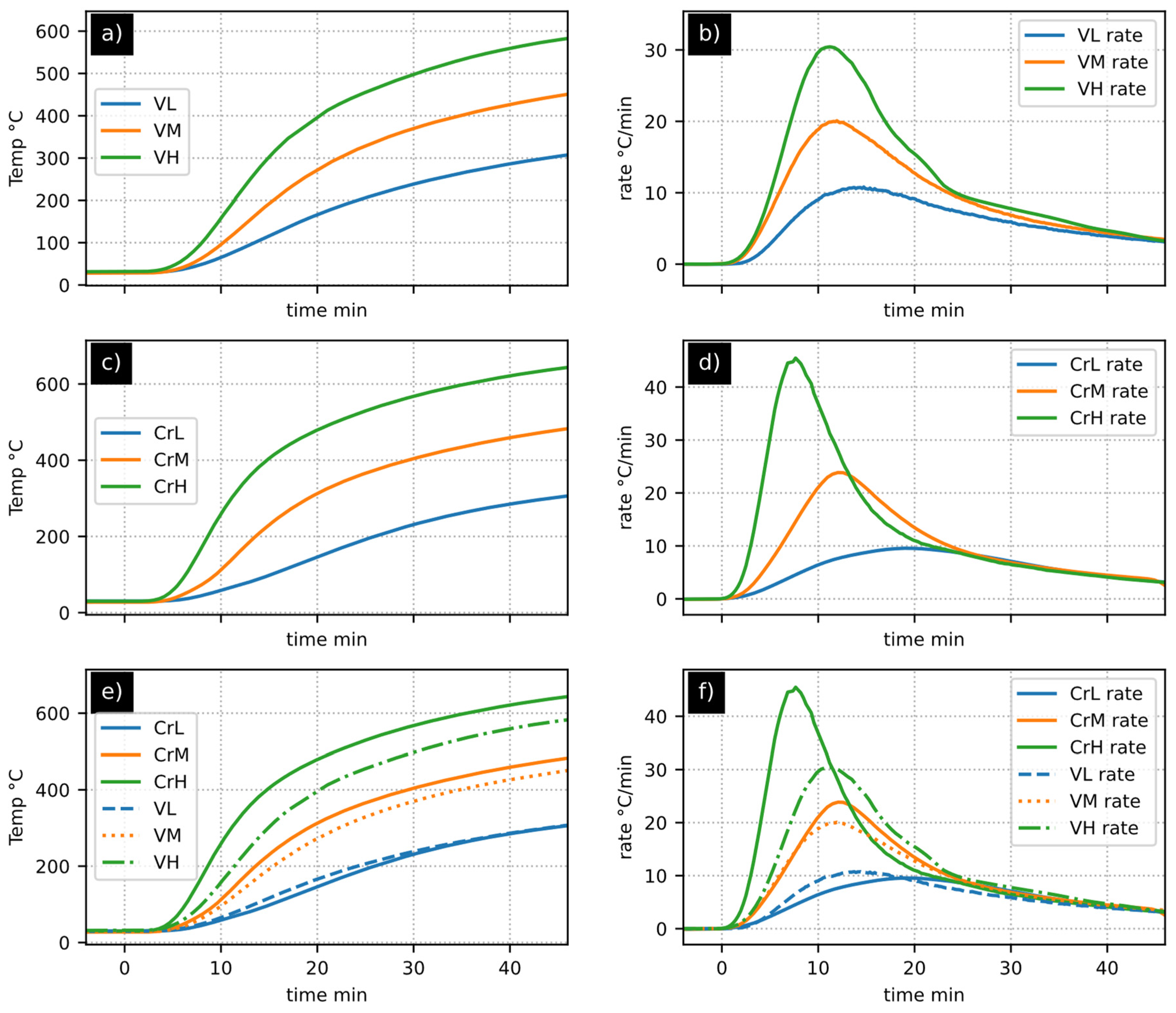
- In the specific context of solar reactors, generating consistent heating ramps proves highly intricate and impractical, particularly when dealing with materials undergoing phase changes or chemical reactions. Consequently, this variable goes from a controlled parameter to a subject of investigation. Accordingly, the analysis of heating rates simplifies the interpretation of observed phenomena.
- (b)
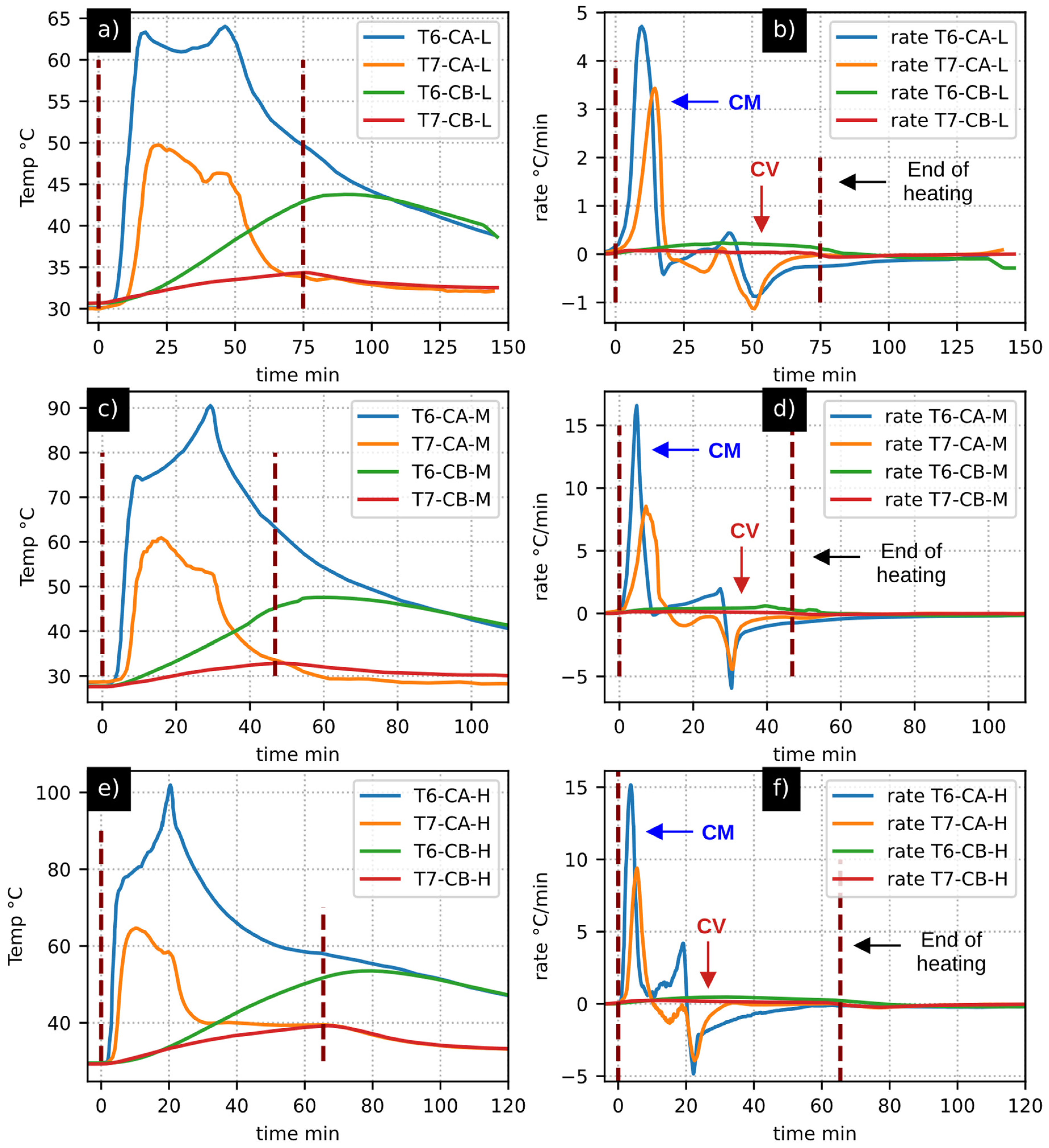
- While the observation and analysis of temperature curves provide insights into the process, heating rates amplify fluctuations in direct measurements. This amplification facilitates the identification of the quasi-inert thermal behavior of biochar.
- (c)
- The installed thermocouples exhibited variations, resulting in temperature curves of different natures. However, during pronounced fluctuations in the experiments, the analysis of heating rates revealed coincidences of nearly simultaneous shapes. Therefore, this tool not only served for result analysis but was also crucial for real-time monitoring throughout the experimental process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CA | Case A: walnut shell pyrolysis |
| CB | Case B: biochar heating |
| CM | Coincidence moisture drying |
| Conv | Convergence between CA and CB |
| CSP | Concentrated Solar Power |
| CV | Coincidence with valley |
| GC | Gas chromatography |
| S1 | Segment 1 |
| S2 | Segment 2 |
| Tg | Glass transition temperature of lignin |
| TGA | Thermogravimetric analysis |
| V | Valley: tar formation |
References
- Castillo, I.O.; Sangerman-Jarquín, D.M.; Vázquez, M.G.C.; Arellano, J.D.J.E.; Moreno, J.H.N. La producción y comercialización de nuez pecanera en México. Rev. Mex. Cienc. Agríc. 2019, 10, 1797–1808. [Google Scholar]
- Arancibia-Bulnes, C.; Peón-Anaya, R.; Riveros-Rosas, D.; Quiñones, J.; Cabanillas, R.; Estrada, C. Beam Solar Irradiation Assessment for Sonora, Mexico. Energy Procedia 2014, 49, 2290–2296. [Google Scholar] [CrossRef]
- Zeng, K.; Gauthier, D.; Soria, J.; Mazza, G.; Flamant, G. Solar pyrolysis of carbonaceous feedstocks: A review. Sol. Energy 2017, 156, 73–92. [Google Scholar] [CrossRef]
- Encinas-Vázquez, A.; Quezada-Renteria, J.A.; Cervantes, F.J.; Pérez-Rábago, A.C.; Molina-Freaner, F.E.; Pat-Espadas, A.M.; Estrada, C.A. Unraveling the mechanisms of lead adsorption and ageing process on high-temperature biochar. J. Chem. Technol. Biotechnol. 2021, 96, 775–784. [Google Scholar] [CrossRef]
- Poli, F.L.M.; Islas-Samperio, J.M.; Bustamante, C.A.G.; Rivero, J.C.S.; Grande-Acosta, G.K.; Gallardo-Álvarez, R.M.; Lagunes, R.M.; Pineda, F.N.; Escobedo, C.A. Sustainability Assessment of Solid Biofuels from Agro-Industrial Residues Case of Sugarcane Bagasse in a Mexican Sugar Mill. Sustainability 2022, 14, 1711. [Google Scholar] [CrossRef]
- Mazurek, K.; Drużyński, S.; Kiełkowska, U.; Wróbel-Kaszanek, A.; Igliński, B.; Cichosz, M. The Application of Pyrolysis Biochar Obtained from Waste Rapeseed Cake to Remove Copper from Industrial Wastewater: An Overview. Energies 2024, 17, 498. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. A method for screening the relative long-term stability of biochar. GCB Bioenergy 2013, 5, 215–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Li, Y.; Bao, H.; Xing, J.; Zhu, Y.; Nan, J.; Xu, G. Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review. Energies 2022, 16, 410. [Google Scholar] [CrossRef]
- Nayak, N.; Mehrotra, R.; Mehrotra, S. Carbon biosequestration strategies: A review. Carbon Capture Sci. Technol. 2022, 4, 100065. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; Murugavelh, S.; Leu, S. A critical review on biomass pyrolysis: Reaction mechanisms, process modeling and potential challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Reed, T.B.; Das, A. Handbook of Biomass Downdraft Gasifier Engine Systems; Solar Energy Research Institute: Golden, CO, USA, 1988. [Google Scholar]
- Patra, T.K.; Sheth, P.N. Biomass gasification models for downdraft gasifier: A state-of-the-art review. Renew. Sustain. Energy Rev. 2015, 50, 583–593. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; del Olmo, O.A.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Maciejewska, M.; Rogulska, M. Thermal Behavior of Polymeric and Other Advanced Materials. Materials 2023, 16, 2875. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Cárdenas, N.; Pérez-Enciso, R.; Pérez-Rábago, C.; Calleja-Valdez, R.; Maytorena-Soria, V.; García-Gutiérrez, R.; Cabanillas-Lopez, R. Thermal experimental study of a volumetric receiver-reactor using a Mini-Solar furnace. Appl. Therm. Eng. 2023, 234, 121276. [Google Scholar] [CrossRef]
- Arreola, C. Reactor Solar de Receptor Volumétrico Poroso para la Producción de Combustibles Solares. Ph.D. Thesis, UNAM, Temixco, Mexico, 2023. [Google Scholar]
- Rolt, S.; Clark, P.; Schmoll, J.; Shaw, B.J. Xenon arc lamp spectral radiance modelling for satellite instrument calibration. In Proceedings of the Space Telescopes and Instrumentation 2016: Optical, Infrared, and Millimeter Wave, Edinburgh, UK, 26 June–1 July 2016. [Google Scholar]
- Sznajder, M.; Renger, T.; Witzke, A.; Geppert, U.; Thornagel, R. Design and performance of a vacuum-UV simulator for material testing under space conditions. Adv. Space Res. 2013, 52, 1993–2005. [Google Scholar] [CrossRef][Green Version]
- Dibowski, G.; Esser, K. Hazards Caused by UV Rays of Xenon Light Based High Performance Solar Simulators. Saf. Health Work. 2017, 8, 237–245. [Google Scholar] [CrossRef]
- Gooty, A.T.; Li, D.; Berruti, F.; Briens, C. Kraft-lignin pyrolysis and fractional condensation of its bio-oil vapors. J. Anal. Appl. Pyrolysis 2014, 106, 33–40. [Google Scholar] [CrossRef]
- Miyashiro, A. Nature of alkalic volcanic rock series. Contrib. Mineral. Petrol. 1978, 66, 91–104. [Google Scholar] [CrossRef]
- Kushiro, I.; Kuno, H. Origin of Primary Basalt Magmas and Classification of Basaltic Rocks. J. Pet. 1963, 4, 75–89. [Google Scholar] [CrossRef]
- Barros, M.D.R.M. El tezontle, la piedra de México. MoleQla Rev. Cienc. Univ. Pablo Olavide 2019, 33, 3. [Google Scholar]
- Nahhas, T.; Py, X.; Sadiki, N. Experimental investigation of basalt rocks as storage material for high-temperature concentrated solar power plants. Renew. Sustain. Energy Rev. 2019, 110, 226–235. [Google Scholar] [CrossRef]
- Popescu, F.; Mahu, R.; Ion, I.V.; Rusu, E. A Mathematical Model of Biomass Combustion Physical and Chemical Processes. Energies 2020, 13, 6232. [Google Scholar] [CrossRef]
- Boumaaza, M.; Belaadi, A.; Bourchak, M.; Juhany, K.A.; Jawaid, M.; Marvila, M.T.; de Azevedo, A.R. Optimization of flexural properties and thermal conductivity of Washingtonia plant biomass waste biochar reinforced bio-mortar. J. Mater. Res. Technol. 2023, 23, 3515–3536. [Google Scholar] [CrossRef]
- Raj, R.A.; Anand, M.D.; Ramabalan, S. Mathematical Modelling and Volume Prediction of Metal Melted by Electron Beam Welding in Copper and Stainless Steel 304 Dissimilar Metal Joints. Int. J. Recent Technol. Eng. 2019, 8, 0782S319. [Google Scholar]
- Grønli, M.; Antal, M.J.; Várhegyi, G. A Round-Robin Study of Cellulose Pyrolysis Kinetics by Thermogravimetry. Ind. Eng. Chem. Res. 1999, 38, 2238–2244. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Chaos, M.; Chen, R.; Lu, S. Estimation of beech pyrolysis kinetic parameters by Shuffled Complex Evolution. Bioresour. Technol. 2016, 200, 658–665. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Iswanto, A.H.; Nawawi, D.S.; Lee, S.H.; Fatriasari, W. Improvement of Thermal Behavior of Rattan by Lignosulphonate Impregnation Treatment. Forests 2022, 13, 1773. [Google Scholar] [CrossRef]
- Shang, Z.; Li, H. Unraveling pyrolysis mechanisms of lignin dimer model compounds: Neural network-based molecular dynamics simulation investigations. Fuel 2024, 357, 129909. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Jiaqiang, E.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Shen, J.; Igathinathane, C.; Yu, M.; Pothula, A.K. Biomass pyrolysis and combustion integral and differential reaction heats with temperatures using thermogravimetric analysis/differential scanning calorimetry. Bioresour. Technol. 2015, 185, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Galgano, A.; Di Blasi, C. Multi-scale analysis of the exothermic behavior of agricultural biomass pyrolysis. J. Anal. Appl. Pyrolysis 2023, 173, 106040. [Google Scholar] [CrossRef]
- Tabakaev, R.; Astafev, A.; Shanenkova, Y.; Dubinin, Y.; Yazykov, N.; Yakovlev, V. Thermal effects investigation during biomass slow pyrolysis in a fixed bed reactor. Biomass Bioenergy 2019, 126, 26–33. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Rath, J.; Wolfinger, M.; Steiner, G.; Krammer, G.; Barontini, F.; Cozzani, V. Heat of wood pyrolysis. Fuel 2003, 82, 81–91. [Google Scholar] [CrossRef]
- Fasina, O.; Littlefield, B. TG-FTIR analysis of pecan shells thermal decomposition. Fuel Process. Technol. 2012, 102, 61–66. [Google Scholar] [CrossRef]
- Medrano, J.A.L.; Martínez, D.B.; De la Rosa, J.R.; Pedraza, E.S.C.; Flores-Escamilla, G.A.; Ciuta, S. Particle pyrolysis modeling and thermal characterization of pecan nutshell. J. Therm. Anal. 2016, 126, 969–979. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabo, T.; Trif, L.; Felhősi, I.; Abbi, K.; Shaban, A. Biomass valorization of walnut shell into biochar as a resource for electrochemical simultaneous detection of heavy metal ions in water and soil samples: Preparation, characterization, and applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- Jia, X.; Che, Y.; Li, J.; Yan, B.; Cheng, Z.; Chen, G.; Zhao, J. From cellulose to tar: Analysis of tar formation pathway with distinguishing the primary and secondary reactions. Bioresour. Technol. 2023, 390, 129846. [Google Scholar] [CrossRef] [PubMed]

| Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||
|---|---|---|---|
| Moisture | 7.73 | C | 47.97 |
| Ash | 1.19 | H | 5.41 |
| Volatiles | 80.46 | O | 45.92 |
| Fixed carbon | 10.62 | N | 0.61 |
| Material | Mass (g) | Specific Heat Capacity (J g−1K−1) | Heat Capacity (J K−1) | Thermal Conductivity (W m−1K−1) |
|---|---|---|---|---|
| Volcanic stone | 71.5 | 0.89 [25] | 63.6 | 1.55 [25] |
| Biochar | 17.3 | 1.5 [26] | 25.9 | 0.4 [27] |
| Stainless steel | 1260 | 0.53 [28] | 667.8 | 16.2 [28] |
| Power (W) | Max Reaction Temp (°C) [T5] | Char (%) | Tar (%) | Gas (%) |
|---|---|---|---|---|
| 234 | 382 | 41.61 | 49.37 | 9.02 |
| 482 | 498 | 32.90 | 57.61 | 9.49 |
| 725 | 674 | 28.17 | 53.29 | 18.54 |
| Power (W) | Max Reaction Temp (°C) (T5) | C (%) | H (%) | O (%) | N (%) |
|---|---|---|---|---|---|
| - | Nutshell (raw material) | 47.97 | 5.41 | 45.92 | 0.61 |
| 234 | 382 | 73.70 | 3.18 | 22.43 | 0.69 |
| 482 | 498 | 77.88 | 3.15 | 18.02 | 0.95 |
| 725 | 674 | 82.84 | 2.26 | 14.22 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspiazu-Méndez, A.; Cisneros-Cárdenas, N.A.; Pérez-Rábago, C.; Pat-Espadas, A.M.; Manzini-Poli, F.; Estrada, C.A. Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials. Energies 2024, 17, 1435. https://doi.org/10.3390/en17061435
Aspiazu-Méndez A, Cisneros-Cárdenas NA, Pérez-Rábago C, Pat-Espadas AM, Manzini-Poli F, Estrada CA. Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials. Energies. 2024; 17(6):1435. https://doi.org/10.3390/en17061435
Chicago/Turabian StyleAspiazu-Méndez, Arturo, Nidia Aracely Cisneros-Cárdenas, Carlos Pérez-Rábago, Aurora M. Pat-Espadas, Fabio Manzini-Poli, and Claudio A. Estrada. 2024. "Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials" Energies 17, no. 6: 1435. https://doi.org/10.3390/en17061435
APA StyleAspiazu-Méndez, A., Cisneros-Cárdenas, N. A., Pérez-Rábago, C., Pat-Espadas, A. M., Manzini-Poli, F., & Estrada, C. A. (2024). Analysis of the Solar Pyrolysis of a Walnut Shell: Insights into the Thermal Behavior of Biomaterials. Energies, 17(6), 1435. https://doi.org/10.3390/en17061435






