Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge
Abstract
1. Introduction
2. Materials and Methods
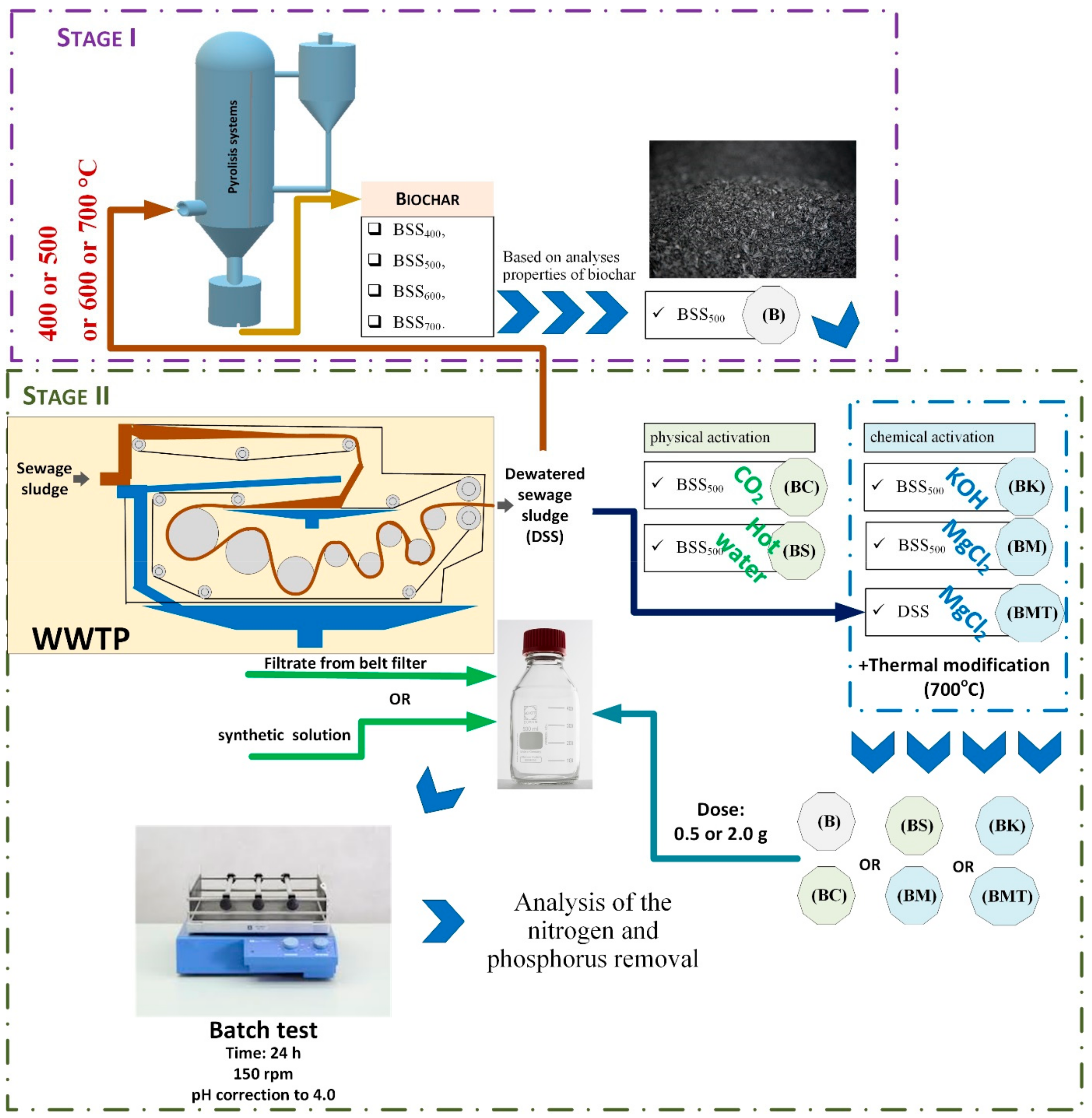
2.1. Experimental Procedure
2.2. Raw Materials for Biochar Production
2.3. Preparation of Biochar
2.4. Modification of Biochar
2.5. Batch Adsorption Experiments
- c0—the initial concentration of the ammonia nitrogen or phosphorus in solution, mg/L;
- ceq—the equilibrium concentration of the ammonia nitrogen and phosphorus in solution, mg/L;
- V—the volume of the solution, L;
- m—the weight of biochar, g.
2.6. Physicochemical and Physical Analyses
2.7. Statistical Analyses
3. Results and Discussion
3.1. Biochar Production Efficiency
3.2. Characteristics of Selected Biochar Properties
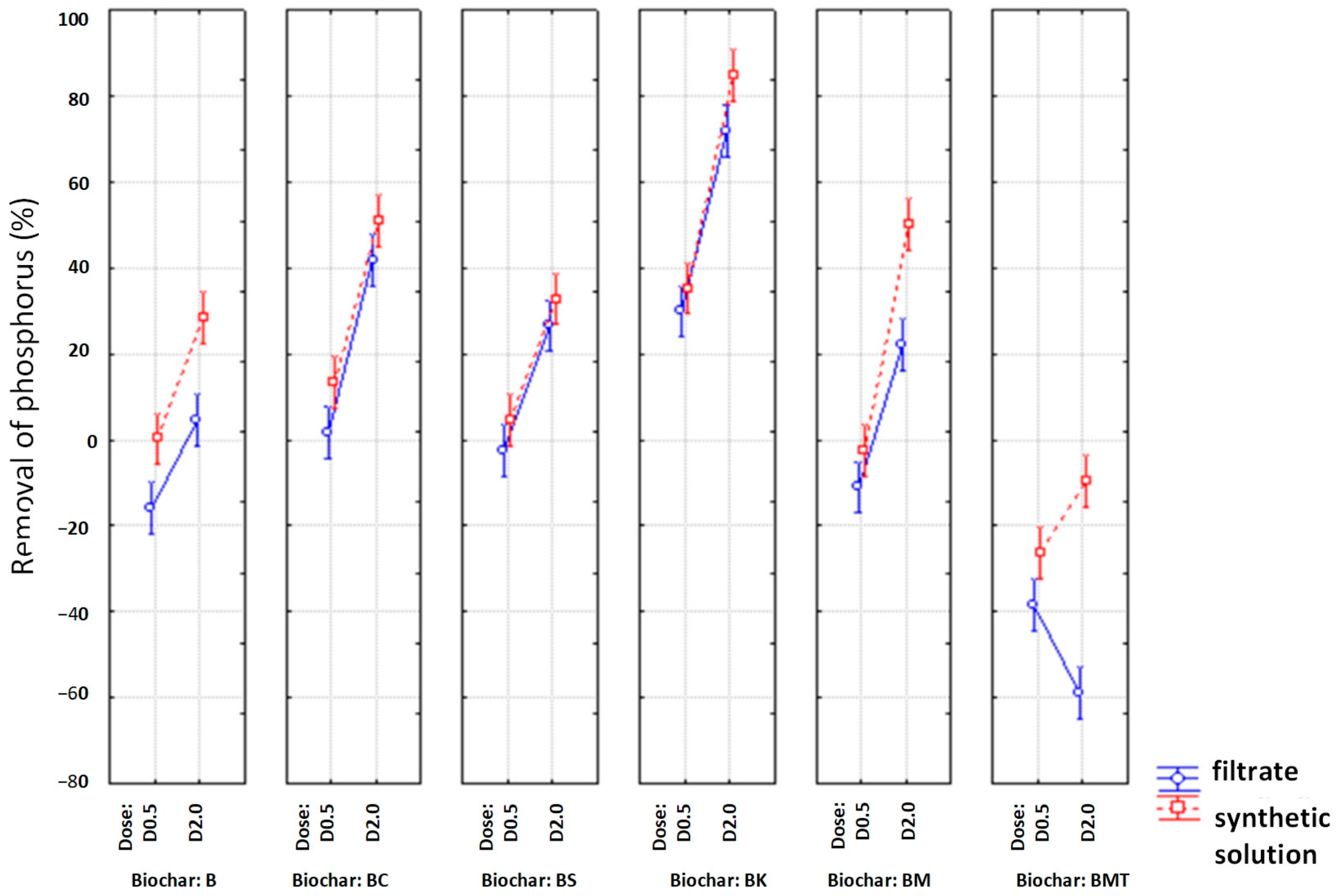
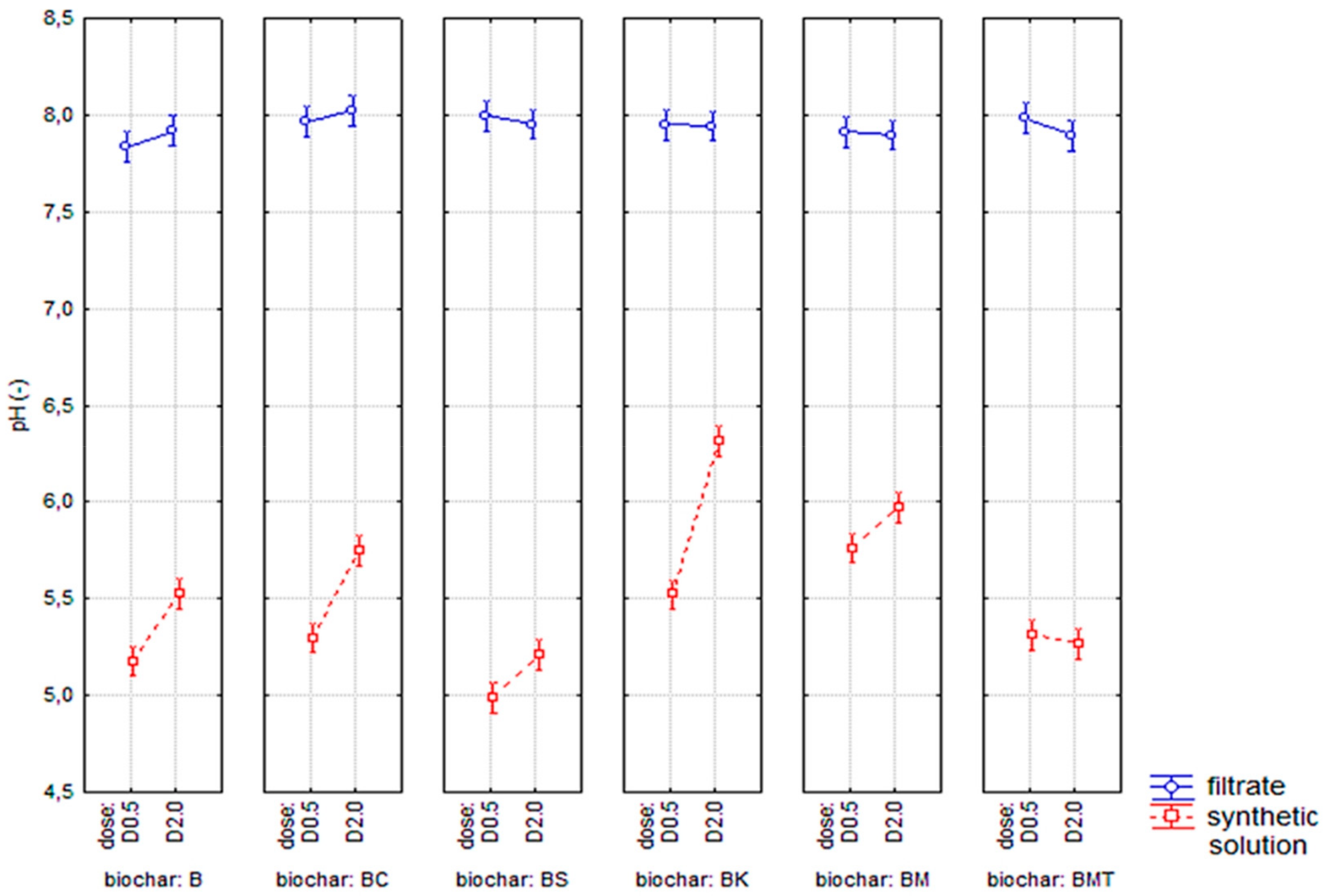
3.3. Biochar as an Adsorbent for Ammonia Nitrogen/Phosphorus Removal
4. Conclusions
- (1)
- The increase in temperature caused a decrease in the yield of biochar. There was a 21% reduction in the parameter when comparing the extreme tested in research temperatures from 62.41% to 49.33% for temperatures of 400 and 700 °C, respectively. A similar trend was observed for carbon (36% reduction, from 42.4% to 27.0%), hydrogen (66% reduction, from 3.22% to 1.4%), and nitrogen content and H/C (64% reduction, from 0.73% to 0.26%) in biochar.
- (2)
- An increase in the pyrolysis temperature of the digestate resulted in an increase in the biochar pH, ash content and BET surface area. The parameters mentioned above increased, respectively, from 7.13 to 11, 48.13 to 71.43 and from 11.09 to 45.53 m2/g.
- (3)
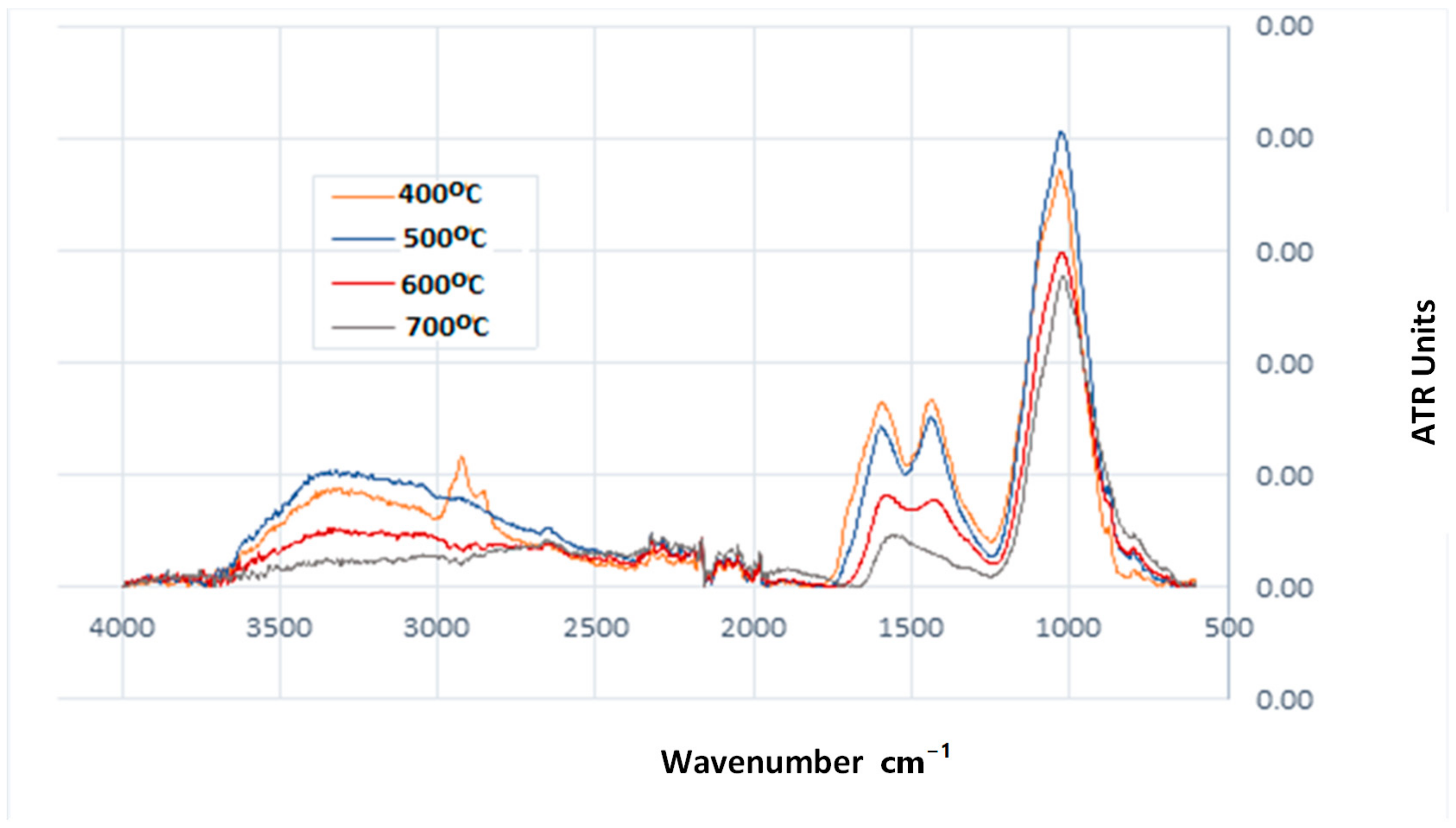
- The pyrolysis temperature influenced the change in the type and quantity of surface functional groups. A decreasing number of acidic functional groups and increasing aromaticity of biochar were observed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, S.T.; Yin, J.; Draper, K.; Trabold, T.A. Closing Nutrient Cycles with Biochar—From Filtration to Fertilizer. J. Clean. Prod. 2018, 197, 1597–1606. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative Effects of Bamboo Sawdust Addition on Pyrolysis of Sewage Sludge: Biochar Properties and Environmental Risk from Metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar Increases Nitrogen Retention and Lowers Greenhouse Gas Emissions When Added to Composting Poultry Litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, Y.; Wang, H.; Liu, Y. Ammonium Adsorption from Aqueous Solutions by Strawberry Leaf Powder: Equilibrium, Kinetics and Effects of Coexisting Ions. Desalination 2010, 263, 70–75. [Google Scholar] [CrossRef]
- Mia, S.; Uddin, M.E.; Kader, M.A.; Ahsan, A.; Mannan, M.A.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and Co-Composting of Municipal Organic Waste in Bangladesh: A Quantitative Estimate of Recyclable Nutrients, Greenhouse Gas Emissions, and Economic Benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of Biochar as an Additive in Organic Waste Composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U. Modeling and Evaluation of Chromium Remediation from Water Using Low Cost Bio-Char, a Green Adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The Role of Biochar and Biochar-Compost in Improving Soil Quality and Crop Performance: A Review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Zama, E.F.; Zhu, Y.G.; Reid, B.J.; Sun, G.X. The Role of Biochar Properties in Influencing the Sorption and Desorption of Pb(II), Cd(II) and As(III) in Aqueous Solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Manera, C.; Silvestre, W.P.; Pauletti, G.F.; Altafini, C.R.; Godinho, M. Use of Biochar Produced from Elephant Grass by Pyrolysis in a Screw Reactor as a Soil Amendment. Waste Biomass Valorization 2019, 10, 3089–3100. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 Activated Biochar from Solid Wastes of a Beer Industry and Its Application for Methylene Blue Adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for Composting Improvement and Contaminants Reduction. A Review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover- and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of Biochar on Nitrogen Transformation and Heavy Metals in Sludge Composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, G. Thermogravimetric, Thermochemical, and Infrared Spectral Characterization of Feedstocks and Biochar Derived at Different Pyrolysis Temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, M. Quality Variations of Poultry Litter Biochar Generated at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Włodarczyk, R.; Chajczyk, O. Effects of Pyrolysis Parameters on the Yield and Properties of Biochar from Pelletized Sunflower Husk. E3S Web Conf. 2018, 44, 00197. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Dinelli, F.D.; Kenar, J.A.; Jackson, M.A.; Thomas, A.J.; Peterson, S.C. Physical and Chemical Properties of Pyrolyzed Biosolids for Utilization in Sand-Based Turfgrass Rootzones. Waste Manag. 2018, 76, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wystalska, K.; Malińska, K.; Barczak, M. Poultry Manure Derived Biochars—The Impact of Pyrolysis Temperature on Selected Properties and Potentials for Further Modifications. J. Sustain. Dev. Energy Water Environ. Syst. 2021, 9, 1080337. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of Pyrolysis Temperature, Heating Rate, and Residence Time on Rapeseed Stem Derived Biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of Feedstock and Pyrolysis Temperature on Properties of Biochar Governing End Use Efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Bai, X.; Li, Z.; Zhang, Y.; Ni, J.; Wang, X.; Zhou, X. Recovery of Ammonium in Urine by Biochar Derived from Faecal Sludge and Its Application as Soil Conditioner. Waste Biomass Valorization 2018, 9, 1619–1628. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of Nitrogen and Phosphorus Adsorption by Mg-Loaded Biochar from Different Feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.J.; Liu, Y. Influence of Pyrolysis Temperature on Production of Digested Sludge Biochar and Its Application for Ammonium Removal from Municipal Wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of Sludge-Based Adsorbents: Preparation, Characterization, Utilization and Its Feasibility Assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Utilization of Sludge Based Adsorbents for the Removal of Various Pollutants: A Review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Yu, K. Effect of Pyrolysis Temperature on Characteristics of Biochars Derived from Different Feedstocks: A Case Study on Ammonium Adsorption Capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of Biochar for the Removal of Nitrogen and Phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Gajewska, M.; Obarska-Pempkowiak, H. Effect of Recycling of Reject Waters from Dewatering the Sludges on the Operation Efficiency of a Wastewater Treatment Plant. Przem. Chem. 2008, 87, 448–451. [Google Scholar]
- Tayibi, S.; Monlau, F.; Bargaz, A.; Jimenez, R.; Barakat, A. Synergy of Anaerobic Digestion and Pyrolysis Processes for Sustainable Waste Management: A Critical Review and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 152, 111603. [Google Scholar] [CrossRef]
- Sobik-Szołtysek, J.; Bień, J.; Grosser, A. Assessment of the Sorption Properties of Materials Proposed for the Construction of Insulation Barriers. Environ. Prot. Eng. 2016, 42, 169–189. [Google Scholar] [CrossRef]
- PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN 16169:2012; Sewage Sludge. Treated Bio-Waste and Soil. Determination of Nitrogen by the Kjeldahl Method. Polish Committee for Standardization: Warsaw, Poland, 2012.
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Llorach-Massana, P.; Lopez-Capel, E.; Peña, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical Feasibility and Carbon Footprint of Biochar Co-Production with Tomato Plant Residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Dunnigan, L.; Morton, B.J.; Ashman, P.J.; Zhang, X.; Kwong, C.W. Emission Characteristics of a Pyrolysis-Combustion System for the Co-Production of Biochar and Bioenergy from Agricultural Wastes. Waste Manag. 2018, 77, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-Pyrolysis of Sewage Sludge and Manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Version, D.; Wildt, D. Biomass Pyrolysis for Chemicals de Wildt, Paulus; University of Groningen: Groningen, The Netherlands, 2011; ISBN 9789036749947. [Google Scholar]
- Brown, R.; Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; Volume 8, pp. 127–146. [Google Scholar]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and Phytoavailability of Lead and Antimony in a Small Arms Range Soil Amended with Mussel Shell, Cow Bone and Biochar: EXAFS Spectroscopy and Chemical Extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic Temperatures Impact Lead Sorption Mechanisms by Bagasse Biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of Pyrolysis Temperature on Characteristics and Heavy Metal Adsorptive Performance of Biochar Derived from Municipal Sewage Sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Schmidt, H.P.; Bucheli, T.; Kammann, C.; Glaser, B.; Abiven, S.; Leifeld, J. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012. [Google Scholar]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Production and Characterization of a Value Added Biochar Mix Using Seaweed, Rice Husk and Pine Sawdust: A Parametric Study. J. Clean. Prod. 2018, 200, 641–656. [Google Scholar] [CrossRef]
- Zolfi Bavariani, M.; Ronaghi, A.; Ghasemi, R. Influence of Pyrolysis Temperatures on FTIR Analysis, Nutrient Bioavailability, and Agricultural Use of Poultry Manure Biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Qi, F.; Yan, Y.; Lamb, D.; Naidu, R.; Bolan, N.S.; Liu, Y.; Ok, Y.S.; Donne, S.W.; Semple, K.T. Thermal Stability of Biochar and Its Effects on Cadmium Sorption Capacity. Bioresour. Technol. 2017, 246, 48–56. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Smernik, R.; Das, O.; Farid, M.; Gao, W. A Feasibility Study of Agricultural and Sewage Biomass as Biochar, Bioenergy and Biocomposite Feedstock: Production, Characterization and Potential Applications. Sci. Total Environ. 2015, 512–513, 495–505. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Zhang, H.; Shao, L.; Chen, D.; He, P. Multiscale Visualization of the Structural and Characteristic Changes of Sewage Sludge Biochar Oriented towards Potential Agronomic and Environmental Implication. Sci. Rep. 2015, 5, 9406. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Martoglio-Smith, P.A.; Hajaligol, M.R. Characterization of Char from the Pyrolysis of Tobacco. J. Agric. Food Chem. 2002, 50, 771–783. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tsai, W.T.; Chen, J.W.; Lin, Y.Q.; Chang, Y.M. Characterization of Biochar Prepared from Biogas Digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, F.I.; Iamail, K.N.; Musa, M.; Jaapar, J.; Alwi, H.; Hamid, K.K.K. Characterization of Bio Char Derived from Tapioca Skin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 12016. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, K.K. A Short Note on Investigation and Remediation of Contaminated Groundwater and Soil in Korea. J. Eng. Geol. 2004, 14, 123–130. [Google Scholar]
- Li, J.; Cao, L.; Yuan, Y.; Wang, R.; Wen, Y.; Man, J. Comparative Study for Microcystin-LR Sorption onto Biochars Produced from Various Plant- and Animal-Wastes at Different Pyrolysis Temperatures: Influencing Mechanisms of Biochar Properties. Bioresour. Technol. 2018, 247, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H.; Xu, D.; Han, L.; Niu, D.; Tian, B.; Zhang, J.; Zhang, L.; Wu, W. Removal of Ammonium from Aqueous Solutions Using Zeolite Synthesized from Fly Ash by a Fusion Method. Desalination 2011, 271, 111–121. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of Highly Ordered Cubic NaA Zeolite from Halloysite Mineral for Adsorption of Ammonium Ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lin, F.; Dou, X.; Zheng, M.; Tan, W.; Wang, C. Recovery of Ammonium and Phosphate from Urine as Value-Added Fertilizer Using Wood Waste Biochar Loaded with Magnesium Oxides. J. Clean. Prod. 2018, 187, 205–214. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an Adsorbent for Inorganic Nitrogen and Phosphorus Removal from Water: A Review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Melia, P.M.; Busquets, R.; Hooda, P.S.; Cundy, A.B.; Sohi, S.P. Driving Forces and Barriers in the Removal of Phosphorus from Water Using Crop Residue, Wood and Sewage Sludge Derived Biochars. Sci. Total Environ. 2019, 675, 623–631. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, F.; Liu, Z.; Ai, L. Removal of Ammonia Nitrogen and Phosphorus by Biochar Prepared from Sludge Residue after Rusty Scrap Iron and Reduced Iron Powder Enhanced Fermentation. J. Environ. Manag. 2021, 282, 111970. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of Slow Pyrolyzed Wood and Rice Husks Biochar for Adsorption of Ammonium Nitrogen from Piggery Manure Anaerobic Digestate Slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and Application of Chars Produced from Pinewood Pyrolysis and Hydrothermal Treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of Magnesium Modified Corn Biochar for Phosphorus Removal and Recovery from Swine Wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of Biochar for Sorption of Ammonium and Phosphate from Dairy Effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar Produced from Oak Sawdust by Lanthanum (La)-Involved Pyrolysis for Adsorption of Ammonium (NH4+), Nitrate (NO3−), and Phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Mahmood, I.B. Recovery of NH4+ by Corn Cob Produced Biochars and Its Potential Application as Soil Conditioner. Front. Environ. Sci. Eng. 2014, 8, 825–834. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Evaluation of Phosphorus Adsorption Capacity of Sesame Straw Biochar on Aqueous Solution: Influence of Activation Methods and Pyrolysis Temperatures. Environ. Geochem. Health 2015, 37, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Da Zhang, S.; Li, T.Q.; Zhao, F.L.; He, Z.L.; Zhao, H.P.; Yang, X.E.; Wang, H.L.; Zhao, J.; Rafiq, M.T. Sorption of Ammonium and Phosphate from Aqueous Solution by Biochar Derived from Phytoremediation Plants. J. Zhejiang Univ. Sci. B 2013, 14, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar Derived from Anaerobically Digested Sugar Beet Tailings: Characterization and Phosphate Removal Potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, N.; Li, L.; An, J.-K.; Zhao, L.; Ren, N.-Q. Granulation and Ferric Oxides Loading Enable Biochar Derived from Cotton Stalk to Remove Phosphate from Water. Bioresour. Technol. 2015, 178, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate from Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- El Sharkawi, H.M.; Tojo, S.; Chosa, T.; Malhat, F.M.; Youssef, A.M. Biochar-Ammonium Phosphate as an Uncoated-Slow Release Fertilizer in Sandy Soil. Biomass Bioenergy 2018, 117, 154–160. [Google Scholar] [CrossRef]


| pHH2O | M% | Ash% | C% | NK% | |
|---|---|---|---|---|---|
| SS | 7.02 ± 0.22 | 6.25 ± 0.59 | 55.18 ± 0.31 | 33.11 ± 1.01 | 4.47 ± 0.52 |
| Parameter | The Filtrate from the Belt Filter | Synthetic Solution |
|---|---|---|
| pH, - | 7.75 | 4.73 |
| N-NH4+, mg/L | 864.27 ± 6.47 | 890.40 ± 14.82 |
| P, mg/L | 20.09 ± 0.87 | 18.95 ± 0.5 |
| NPOC, mg/L | 189.20 ± 1.72 | - |
| BSS400 | BSS500 | BSS600 | BSS700 | |
|---|---|---|---|---|
| Yield, % | 62.41 ± 0.56 | 56.12 ± 1.05 | 52.79 ± 0.20 | 49.33 ± 1.75 |
| pHH2O | 7.13 ± 0.07 | 7.53 ± 0.39 | 10.76 ± 0.06 | 11.03 ± 0.15 |
| moisture, % | 2.21 ± 0.04 | 4.08 ± 0.04 | 3.59 ± 0.06 | 3.13 ± 0.02 |
| Ash, % | 48.13 ± 12.69 | 63.10 ± 0.47 | 67.16 ± 0.08 | 71.43 ± 0.22 |
| C, % | 42.40 ± 11.55 | 26.98 ± 0.25 | 27.32 ± 0.02 | 27.07 ± 0.15 |
| H,% | 2.59 ± 0.07 | 1.44 ± 0.00 | 0.93 ± 0.00 | 0.59 ± 0.00 |
| N, % | 3.22 ± 0.04 | 2.60 ± 0.01 | 2.29 ± 0.02 | 1.40 ± 0.00 |
| S, % | - | - | - | - |
| TOC, % | 27.9 ±7.3 | 23.5 ± 6.1 | 27.0 ± 7.2 | 26.6 ± 6.9 |
| H/C, % | 0.73 | 0.64 | 0.41 | 0.26 |
| BET, m2/g | 11.09 ± 0.16 | 40.97 ± 0.14 | 43.33 ± 0.24 | 45.53 ± 0.31 |
| Type of Biochar | Dose, g | qm, mg/g for NH4+ | qm, mg/g for PO43− | ||
|---|---|---|---|---|---|
| Filtrate | Synthetic Solution | Filtrate | Synthetic Solution | ||
| B | 0.5 | 5.97 | 6.72 | - | 0.01 |
| 2 | 1.68 | 3.45 | 0.05 | 0.27 | |
| BC | 0.5 | 7.47 | 8.21 | 0.07 | 0.5 |
| 2 | 1.12 | 3.08 | 0.42 | 0.48 | |
| BS | 0.5 | 6.72 | 7.47 | - | 0.17 |
| 2 | 2.52 | 2.52 | 0.27 | 0.31 | |
| BK | 0.5 | 15.31 | 14.19 | 1.2 | 1.33 |
| 2 | 6.44 | 7.65 | 0.72 | 0.8 | |
| BM | 0.5 | 14.56 | 8.21 | - | - |
| 2 | 2.89 | 2.33 | 0.22 | 0.47 | |
| BMT | 0.5 | 11.57 | 6.72 | - | - |
| 2 | 2.89 | 1.59 | - | - | |
| Factors | F | p |
|---|---|---|
| solution | 0.052 | 0.821128 |
| dose | 41.060 | 0.000000 |
| biochar | 68.035 | 0.000000 |
| solution × dose | 13.056 | 0.000722 |
| solution × biochar | 10.228 | 0.000001 |
| dose × biochar | 17.783 | 0.000000 |
| solution × dose × biochar | 1.890 | 0.113642 |
| Factors | F | p |
|---|---|---|
| solution | 170.5697 | 0.000000 |
| dose | 602.2890 | 0.000000 |
| biochar | 383.0134 | 0.000000 |
| solution × dose | 22.1578 | 0.000022 |
| solution × biochar | 9.2743 | 0.000003 |
| dose × biochar | 35.1475 | 0.000000 |
| solution × dose × biochar | 6.2098 | 0.000162 |
| Type of Feedstock | Pyrolysis Temperature °C | Treatment (Modification) Method | Biochar Dose g | Initial Concentration mg/L | Adsorption Capacity mg/g | Reference |
|---|---|---|---|---|---|---|
| NH4+ | ||||||
| Pine sawdust | 300 | pristine | 3 | 100 | 5.38 | [62] |
| Wheat straw | 550 | pristine | 3 | 100 | 2.08 | |
| Wood waste | 600 | MgO | 2 | 8203 | 47.5 | |
| Sugarcane harvest residue | 550 | MgO | 1.25 | 200 | 22 | |
| Oak sawdust | 300 | - | 0.1 | 25.7 | 3.12 | [72] |
| LaCl3 | 4.13 | |||||
| Mixed hardwood | 300 | - | 2 | 980 | 2.8 | [71] |
| - | 2 | 713 (dairy manure) | 5.3 | |||
| Corn cob | 400 | - | - | 100 | 1.09 | [73] |
| Corn cob | 600 | - | - | 100 | 0.69 | |
| PO42− | ||||||
| Wood waste | 600 | MgO | 2 | 318.5 | 116.40 (PO42) | [62] |
| Bamboo | 600 | Mg-Al layered double hydroxide | 2 | 50 | 13.11 (PO42−) | |
| Sugar beet tailings | 600 | MgCl2 solution immersed | 2 | 1600 | 135.00 (PO42−) | |
| Sesame straw | 600 | ZnCl2 | 0.1 | 20 (P) | 9.39 | [74] |
| MgO | 0.1 | 8.42 | ||||
| Thalia dealbata | 500 | CO2 at 500 °C | 0.2 | 30 | 2.9 | [75] |
| 500 | CO2 at 600 °C | 0.2 | 38.12 | |||
| Sugar beet tailings | 600 | MgCl2 | 0.1 | 20 | 66.7 | [76] |
| Pine wood | 600 | MgCl2 | 0.1 | 20 | 0.5 | |
| Mixed hardwood | 300 | - | 2 | 24 | 0.48 | [72] |
| 300 | - | 2 | 24 (dairy manure) | 0.24 | ||
| Cotton stalks | 350 | immobilization of ferric oxides on the biochar granule | 2 | 20 | 0.963 | [77] |
| FeCl3, then granulation | 2 | 0.399 | ||||
| chemical precipitation of ferric oxide on biochar | 2 | 0.319 | ||||
| Factors | F | p |
|---|---|---|
| solution | 24355.3 | 0 |
| dose | 109.9 | 0 |
| biochar | 71.4 | 0 |
| solution × dose | 113.7 | 0 |
| solution × biochar | 78.7 | 0 |
| dose × biochar | 17.5 | 0 |
| solution × dose × biochar | 11.1 | 0 |
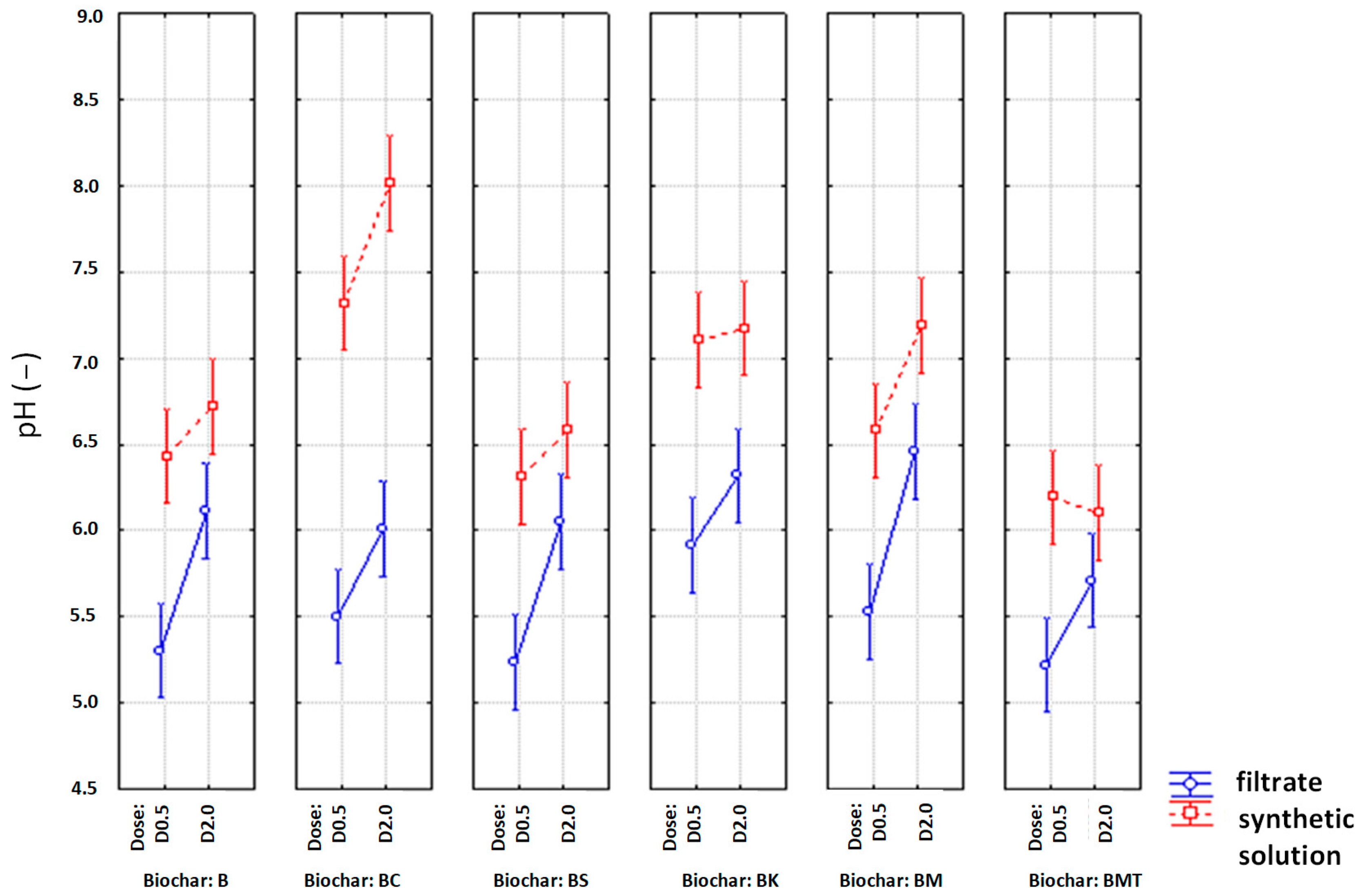
| Filtrate | Synthetic Solution | |||||||
|---|---|---|---|---|---|---|---|---|
| pH before | pH after | pH before | pH after | |||||
| Factors | F | p | F | p | F | p | F | p |
| type of biochar | 13 | 0.000004 | 3.17 | 0.024491 | 82.1 | 0.000000 | 379.0 | 0.000000 |
| Dose biochar | 0 | 0.781917 | 36.68 | 0.000003 | 124.4 | 0.000000 | 182.2 | 0.000000 |
| type of biochar × Dose biochar | 7 | 0.000400 | 0.67 | 0.652760 | 15.1 | 0.000001 | 29.2 | 0.000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wystalska, K.; Grosser, A. Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge. Energies 2024, 17, 1310. https://doi.org/10.3390/en17061310
Wystalska K, Grosser A. Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge. Energies. 2024; 17(6):1310. https://doi.org/10.3390/en17061310
Chicago/Turabian StyleWystalska, Katarzyna, and Anna Grosser. 2024. "Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge" Energies 17, no. 6: 1310. https://doi.org/10.3390/en17061310
APA StyleWystalska, K., & Grosser, A. (2024). Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge. Energies, 17(6), 1310. https://doi.org/10.3390/en17061310







