A Prototype Reactor Promoting the Hg(0) Capture in the Simulated Flue Gas from Small-Scale Boilers by Using Copper Oxide- and Copper Sulfide-Coated Teflon Pipes
Abstract
1. Introduction
- -
- The MPR size can be adjusted to current needs;
- -
- Its construction is adapted, space-friendly;
- -
- It has easy installation, recycle, and reuse capacities;
- -
- It is easily connected to small-scale boilers or installed to attach to household chimneys;
- -
- It can also be installed at the inlet or outlet of a ventilator in compact locations;
- -
- It has long-lasting, accessible maintenance;
- -
- It has a relatively low cost, etc.
2. Methodology and Materials
2.1. Mercury Capture Reactors
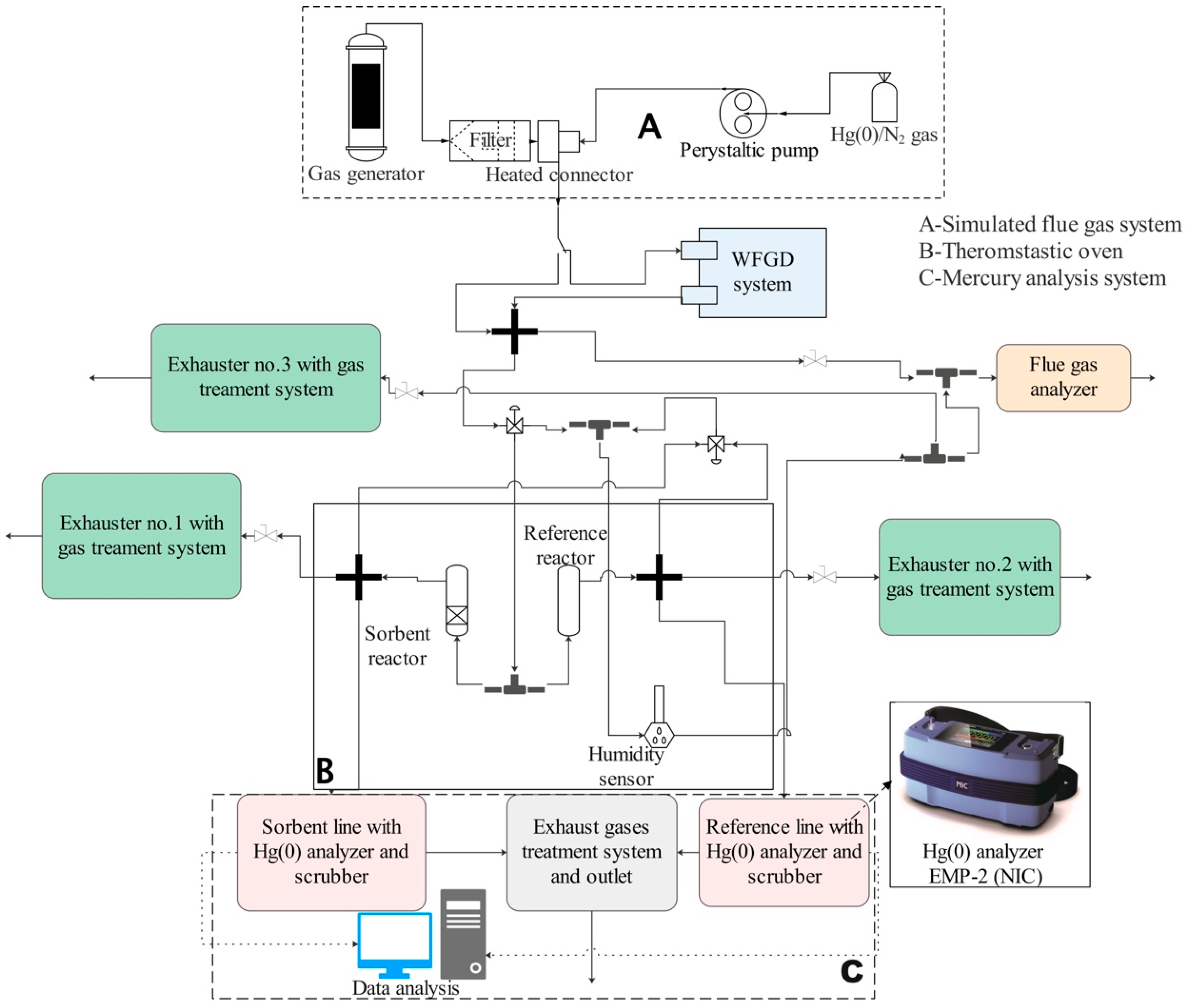
2.2. A Mercury Capture Efficiency Analysis System
- -
- The flue gas and elemental mercury supplying system (Part A);
- -
- The laboratory-scale thermostatic system for testing materials (Part B);
- -
- The elemental mercury analysis system (Part C).
- A.
- Preparation and cleaning of the experimental system and reactors at a temperature of approximately 150 °C for about 1.5 h. This step removes most of the mercury residue inside the pipelines and reactors, and ensures that the readings from the mercury analyzers are close to or below 0.4 µg/m3 and stable;
- B.
- Loading of Hg(0) into the system. The introduction of Hg(0) into the system takes approximately 20–30 min, during which the mercury concentration is adjusted and stabilized;
- C.
- Activation of the fuel loader for 40–60 min to generate the simulated flue gas, which is mixed with Hg(0); An optional procedure, (C-1), may be performed, in which the laboratory-scale WFGD system is run for 20–30 min to remove SO2 from the simulated flue gas. The purpose of this test is to measure the impact of SO2 on sorbents;
- D.
- The fuel loader is turned off and only a Hg(0)/N2 mixture is loaded into the system for 20–30 min;
- E.
- The final observation of the system response and sorbent activity after Hg(0) is switched off and the temperature is lowered for 30–60 min.
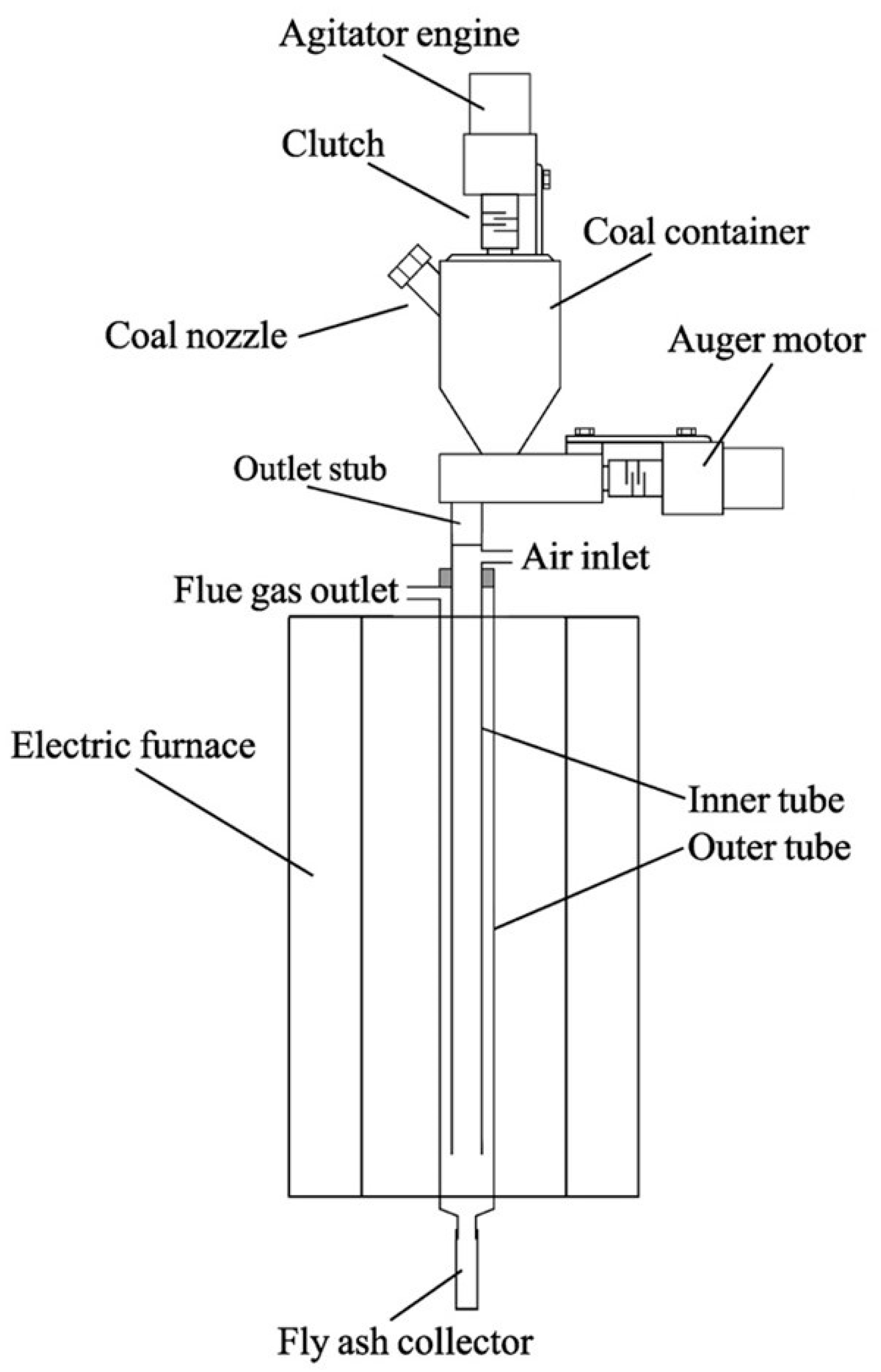
2.3. A Mobile Laboratory Flue Gas Generator and Gas Analysis
2.4. Synthesis of Sorbents
2.4.1. Granulate Form Sorbent Synthesis for VGR Experimental Tests
- (1)
- In total, 15.7 g of CuSO4·5H2O was dissolved in 150 mL of DW, and 11 g of KOH was dissolved in 100 mL of DW;
- (2)
- The CuSO4 solution was stirred at 250–300 rpm and heated to around 60 °C. Then, the KOH solution was added, drop by drop, and stirred for another 2 h until the reaction was completed;
- (3)
- The product was filtered, rinsed with DW, and dried at room temperature;
- (4)
- After the drying process, the synthesized sorbent was ground into small granules (1–3 mm), heated in an oven at 110 °C for 90 min to remove moisture, and stored in a desiccator.
- (1)
- In total, 15.7 g of CuSO4·5H2O was dissolved in 150 mL of DW, and 16 g of Na2S·9H2O was dissolved in 100 mL of DW;
- (2)
- The CuSO4 solution was stirred at 250–300 rpm and heated it up to around 60 °C. Then, it was titrated with Na2S solution drop by drop and stirred for 2 h until the reaction was completed;
- (3)
- The product was filtered and rinsed with DW;
- (4)
- The solid residue was dried at room temperature;
- (5)
- The product was ground quickly to break larger particles into small granules (1–3 mm), heated it in an oven at 110 °C for 90 min to remove moisture, and stored in a desiccator.
2.4.2. Synthesis of Thin Layer Coatings Inside Pipes
- (1)
- 15.7 g of CuSO4·5H2O was dissolved in 150 mL of DW, and 16.0 g of Na2S·9H2O was dissolved in 100 mL of DW;
- (2)
- Using two syringes, the CuSO4 and Na2S solutions were transferred into the selected pipes at room temperature;
- (3)
- CuSO4 and Na2S solutions were mixed inside each pipe several times;
- (4)
- The prepared pipes were dried at room temperature and then rinsed gently with DW;
- (5)
- The prepared pipes were placed horizontally in the oven at over 110 °C to remove moisture and stored in a desiccator.
- (1)
- The PTFE pipes were immersed in a CuSO4 solution in flat glassware (e.g., Petri dish);
- (2)
- A total of 3.0 g of FeS was placed in an Erlenmeyer flask;
- (3)
- The Erlenmeyer flask was poured with 200 mL of 10% solution of H2SO4 to produce H2S gas. The gas was transferred to a Tedlar bag using a peristaltic pump;
- (4)
- H2S gas was directed into the PTFE pipes, where it reacted with CuSO4 solution;
- (5)
- The coated pipes were removed from the Petri dish and placed them in an oven heated to 110 °C to remove moisture;
- (6)
- All the pipes were rinsed with DW, dried again at 110 °C, and stored in a desiccator.
3. Results
3.1. The Contact Area and Contact Time between the Simulated Gas and the CuO or CuS Sorbents in VGRs and MPRs
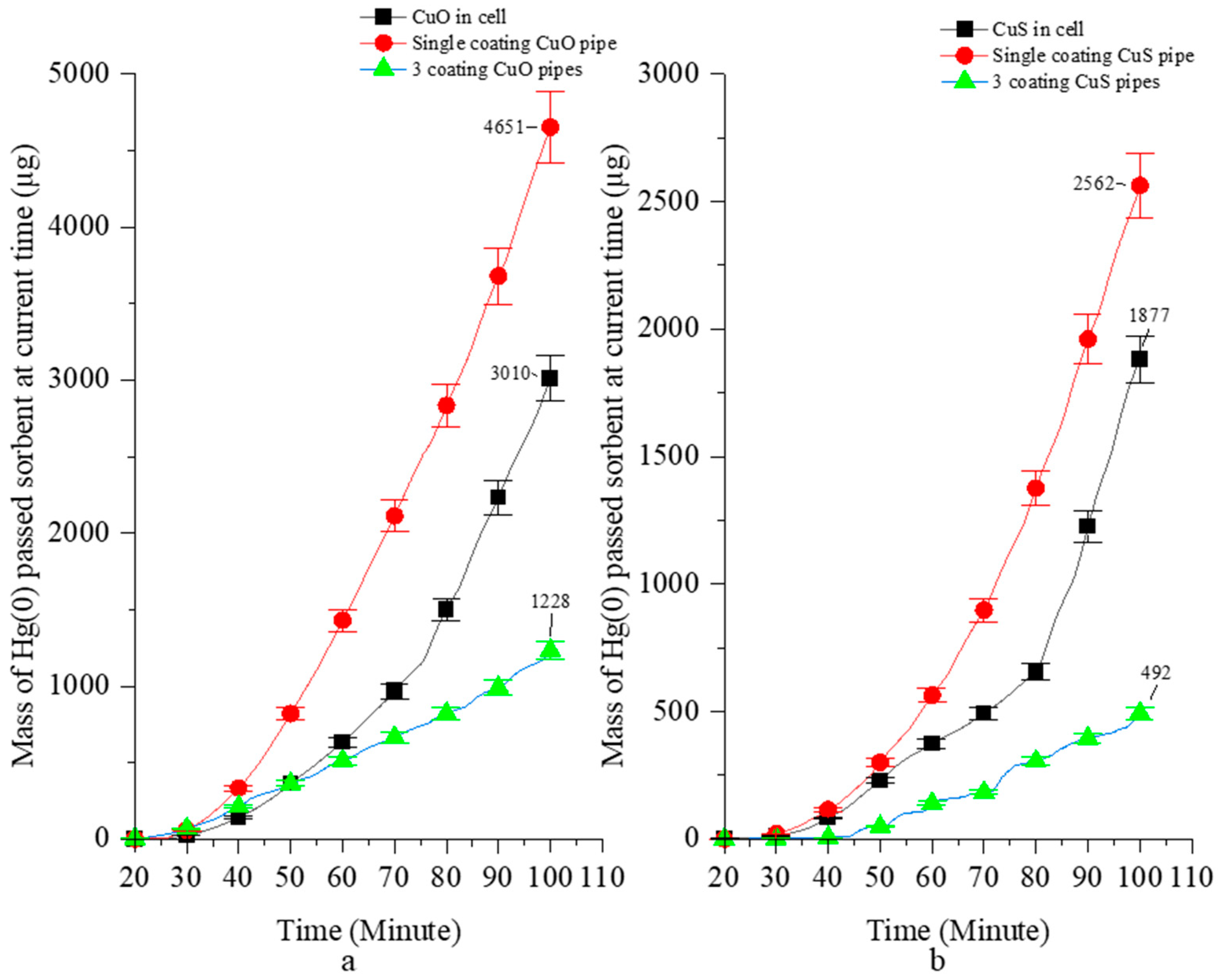
3.2. Laboratory Tests of the VGR and the MPR in the Simulated Flue Gas
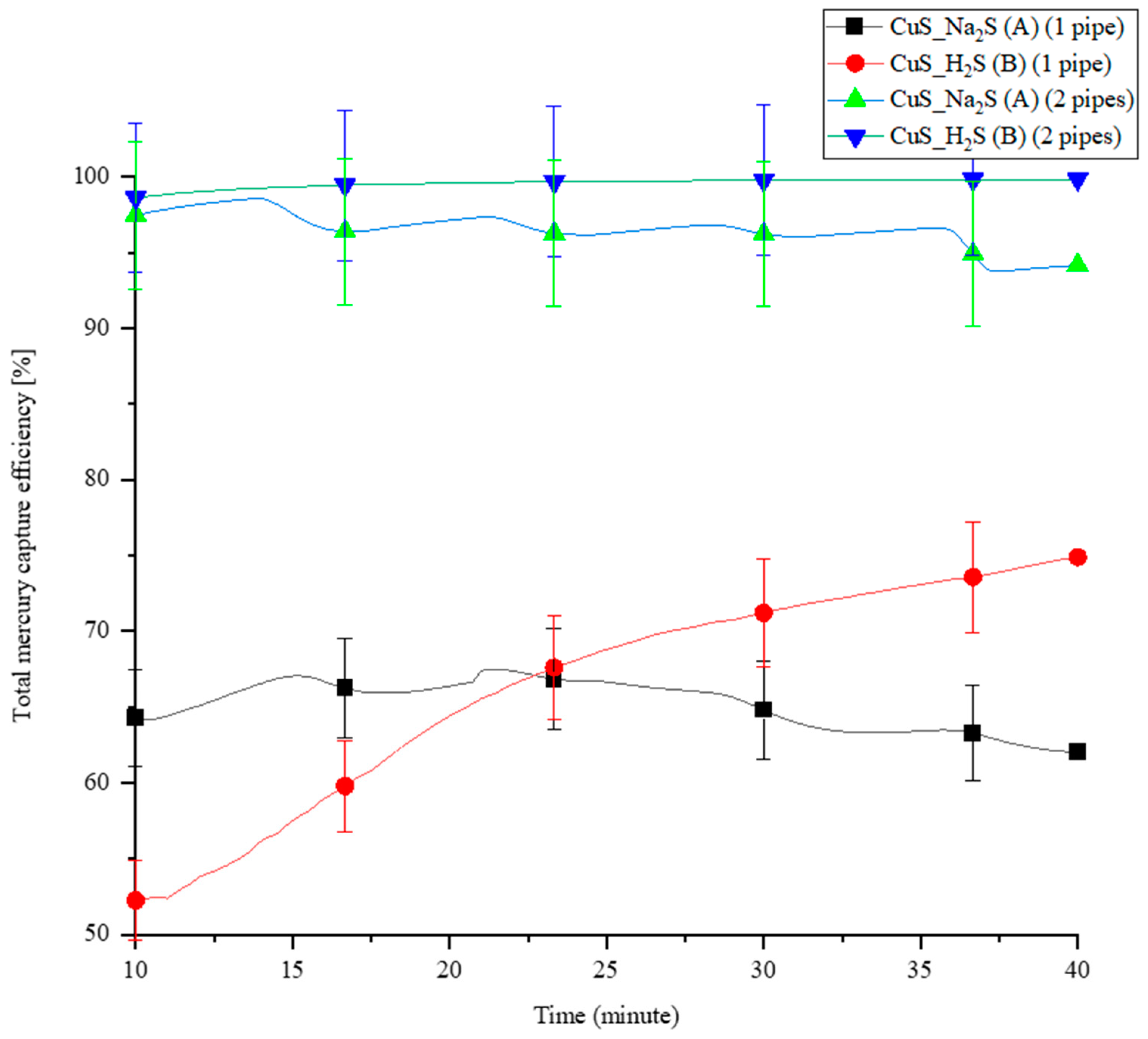
3.3. Comparison of the Coating Methodologies A and B by Using MPR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP. BP Statistical Review of World Energy 2022, 71st ed.; British Petroleum Co.: London, UK, 2022; pp. 1–60. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (accessed on 30 January 2024).
- BP Energy Outlook. Statistical Review of World Energy globally consistent data on world energy markets and authoritative publications in the field of energy. BP Energy Outlook 2021, 70, 8–20. [Google Scholar]
- Ryfa, A.; Żmuda, R.; Mandrela, S.; Białecki, R.; Adamczyk, W.; Nowak, M.; Lelek, Ł.; Bandoła, D.; Pichura, M.; Płonka, J.; et al. Experimental and numerical investigation of mercury removal from flue gas by sorbent polymer composite. Fuel 2023, 333, 126470. [Google Scholar] [CrossRef]
- Knotts, J.; Guenioui, K. A Complete Mercury Control System. Available online: https://www.gore.com/sites/default/files/2017-07/IEEE-article-July-2017.pdf (accessed on 17 November 2022).
- Trobajo, J.R.; Antuña-Nieto, C.; Rodríguez, E.; García, R.; López-Antón, M.A.; Martínez-Tarazona, M.R. Carbon-based sorbents impregnated with iron oxides for removing mercury in energy generation processes. Energy 2018, 159, 648–655. [Google Scholar] [CrossRef]
- Zhou, Q.; Duan, Y.F.; Zhao, S.L.; Zhu, C.; She, M.; Zhang, J.; Wang, S.Q. Modeling and experimental studies of in-duct mercury capture by activated carbon injection in an entrained flow reactor. Fuel Process. Technol. 2015, 140, 304–311. [Google Scholar] [CrossRef]
- Qi, H.; Xu, W.; Wang, J.; Tong, L.; Zhu, T. Hg0removal from flue gas over different zeolites modified by FeCl3. J. Environ. Sci. 2015, 28, 110–117. [Google Scholar] [CrossRef]
- Wdowin, M.; Macherzyński, M.; Panek, R.; Górecki, J.; Franus, W. Investigation of the sorption of mercury vapour from exhaust gas by an Ag-X zeolite. Clay Miner. 2015, 50, 31–40. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Liu, F. Heterogeneous reaction kinetics of mercury oxidation by HCl over Fe2O3 surface. Fuel Process. Technol. 2017, 159, 266–271. [Google Scholar] [CrossRef]
- Li, H.; Zhu, W.; Yang, J.; Zhang, M.; Zhao, J.; Qu, W. Sulfur abundant S/FeS2 for efficient removal of mercury from coal-fired power plants. Fuel 2018, 232, 476–484. [Google Scholar] [CrossRef]
- UNEP. Global mercury assessment 2018. In Global Mercury Assessment 2018: Sources, Emissions, Releases and Environmental Transport, November; UNEP: Geneva, Switzerland, 2018; pp. 1–60. [Google Scholar] [CrossRef]
- James, B.S.; Shetty, R.S.; Kamath, A.; Shetty, A. Household cooking fuel use and its health effects among rural women in southern India-A cross-sectional study. PLoS ONE 2020, 15, e0231757. [Google Scholar] [CrossRef]
- Ravindra, K.; Kaur-Sidhu, M.; Mor, S.; John, S. Trend in household energy consumption pattern in India: A case study on the influence of socio-cultural factors for the choice of clean fuel use. J. Clean. Prod. 2019, 213, 1024–1034. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Z.; Xu, Y.; Wang, C.; Gu, Y.; Xu, H.; Streets, D.G. Black carbon emissions from biomass and coal in rural China. Atmos. Environ. 2017, 176, 158–170. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T.; Marchewicz, A.; Krupa, A.; Czech, T. Particulate matter emission control from small residential boilers after biomass combustion. A review. Renew. Sustain. Energy Rev. 2021, 137, 110446. [Google Scholar] [CrossRef]
- Shen, H.; Luo, Z.; Xiong, R.; Liu, X.; Zhang, L.; Li, Y.; Du, W.; Chen, Y.; Cheng, H.; Shen, G.; et al. A critical review of pollutant emission factors from fuel combustion in home stoves. Environ. Int. 2021, 157, 106841. [Google Scholar] [CrossRef] [PubMed]
- Dziok, T.; Kołodziejska, E.K.; Kołodziejska, E.L. Mercury content in woody biomass and its removal in the torrefaction process. Biomass Bioenergy 2020, 143, 105832. [Google Scholar] [CrossRef]
- De Simone, F.; Artaxo, P.; Bencardino, M.; Cinnirella, S.; Carbone, F.; D’amore, F.; Dommergue, A.; Feng XBin Gencarelli, C.N.; Hedgecock, I.M.; Landis, M.S.; et al. Particulate-phase mercury emissions from biomass burning and impact on resulting deposition: A modelling assessment EPA Public Access. Atmos. Chem. Phys. 2017, 17, 1881–1899. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Assad, N.A.; Barnes, P.J.; Churg, A.; Gordon, S.B.; Harrod, K.S.; Irshad, H.; Kurmi, O.P.; Martin, W.J.; Meek, P.; et al. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur. Respir. J. 2018, 51, 1700698. [Google Scholar] [CrossRef]
- Dziok, T.; Penkała, K. The possibility of reducing emissions from households by using coal briquettes. Polityka Energetyczna 2020, 23, 55–70. [Google Scholar] [CrossRef]
- Ruan, T.; Rim, D. Indoor air pollution in office buildings in mega-cities: Effects of filtration efficiency and outdoor air ventilation rates. Sustain. Cities Soc. 2019, 49, 101609. [Google Scholar] [CrossRef]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A.; Kumar, K.; Singh, B.; Mina, U.; Singh, B.B.; Jain, V.K. Statistical modeling of O3, NOx, CO, PM2.5, VOCs and noise levels in commercial complex and associated health risk assessment in an academic institution. Sci. Total Environ. 2016, 572, 586–594. [Google Scholar] [CrossRef]
- Macherzyński, M. Redukcja Emisji Rtęci do Środowiska—Wybrane Problemy w Świetle Badań Laboratoryjnych i przemysłowych; Wydawnictwa AGH: Kraków, Poland, 2018. [Google Scholar]
- Wdowin, M.; MacHerzyński, M.; Panek, R.; Wałȩka, M.; Górecki, J. Analysis of selected mineral and waste sorbents for the capture of elemental mercury from exhaust gases. Mineralogia 2020, 51, 17–35. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, A.; Zhou, Z.; Wang, C.; Deng, L.; Liu, L.; Xia, H.; Xu, M. Elemental Mercury Removal from Flue Gas over Silver-Loaded CuS-Wrapped Fe3O4 Sorbent. Energy Fuels 2021, 35, 13975–13983. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Qu, W.; Zhang, M.; Feng, Y.; Zhao, J.; Yang, J.; Shih, K. Role of Sulfur Trioxide (SO3) in Gas-Phase Elemental Mercury Immobilization by Mineral Sulfide [Research-article]. Environ. Sci. Technol. 2019, 53, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mei, J.; Sun, P.; Zhao, H.; Guo, Y.; Yang, S. Mechanism of Elemental Mercury Oxidation over Copper-Based Oxide Catalysts: Kinetics and Transient Reaction Studies. Ind. Eng. Chem. Res. 2020, 59, 61–70. [Google Scholar] [CrossRef]
- Sharma, M.; Poddar, M.; Gupta, Y.; Nigam, S.; Avasthi, D.K.; Adelung, R.; Abolhassani, R.; Fiutowski, J.; Joshi, M.; Mishra, Y.K. Solar light assisted degradation of dyes and adsorption of heavy metal ions from water by CuO–ZnO tetrapodal hybrid nanocomposite. Mater. Today Chem. 2020, 17, 100336. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, L.; Wang, T.; Bai, Y.; Guan, Y. Fabrication of highly efficient Bi2WO6/CuS composite for visible-light photocatalytic removal of organic pollutants and Cr(VI) from wastewater. Front. Environ. Sci. Eng. 2021, 15, 52. [Google Scholar] [CrossRef]
- Isac, L.; Cazan, C.; Andronic, L.; Enesca, A. CuS-Based Nanostructures as Catalysts for Organic Pollutants Photodegradation. Catalysts 2022, 12, 1135. [Google Scholar] [CrossRef]
- Liu, W.; Xu, H.; Liao, Y.; Quan, Z.; Li, S.; Zhao, S.; Qu, Z.; Yan, N. Recyclable CuS sorbent with large mercury adsorption capacity in the presence of SO2 from non-ferrous metal smelting flue gas. Fuel 2019, 235, 847–854. [Google Scholar] [CrossRef]
- Jia, L.; Fan, B.G.; Yao, Y.X.; Han, F.; Huo, R.P.; Zhao, C.W.; Jin, Y. Study on the Elemental Mercury Adsorption Characteristics and Mechanism of Iron-Based Modified Biochar Materials. Energy Fuels 2018, 32, 12554–12566. [Google Scholar] [CrossRef]
- Yang, W.; Adewuyi, Y.G.; Hussain, A.; Liu, Y. Recent developments on gas–solid heterogeneous oxidation removal of elemental mercury from flue gas. Environ. Chem. Lett. 2019, 17, 19–47. [Google Scholar] [CrossRef]
- Gorecki, J.; Macherzynski, M.; Chmielowiec, J.; Borovec, K.; Wałeka, M.; Deng, Y.; Sarbinowski, J.; Pasciak, G. The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases. Energies 2022, 15, 8891. [Google Scholar] [CrossRef]
- Macherzyński, M.; Deng, Y.; Górecki, J. Wykorzystanie technik spektroskopowych w laboratoryjnych testach kontroli oczyszczania gazów procesowych. In Nauka i Przemysł: Metody Spektroskopowe w Praktyce: Nowe Wyzwania i Możliwości: Praca Zbiorowa; Wydawnictwo UMCS: Lublin, Poland, 2021; pp. 390–394. ISBN 978-83-227-9504-0. [Google Scholar]
- Krzywanski, J.; Rajczyk, R.; Bednarek, M.; Wesolowska, M.; Nowak, W. Gas emissions from a large scale circulating fluidized bed boilers burning lignite and biomass. Fuel Process. Technol. 2013, 116, 27–34. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Nyashina, G.S.; Anand, R.; Strizhak, P.A. Composition of gas produced from the direct combustion and pyrolysis of biomass. Process Saf. Environ. Prot. 2021, 156, 43–56. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, H.; He, Y.; Yang, Y.; Sui, R.; Lu, Q. Combustion of biomass pyrolysis gas: Roles of radiation reabsorption and water content. Renew. Energy 2023, 205, 864–872. [Google Scholar] [CrossRef]
- Zhakupov, D.; Kulmukanova, L.; Sarbassov, Y.; Shah, D. Flue gas analysis for biomass and coal co-firing in fluidized bed: Process simulation and validation. Int. J. Coal Sci. Technol. 2022, 9, 59. [Google Scholar] [CrossRef]
- Li, G.; Shen, B.; Li, Y.; Zhao, B.; Wang, F.; He, C.; Wang, Y.; Zhang, M. Removal of element mercury by medicine residue derived biochars in presence of various gas compositions. J. Hazard. Mater. 2015, 298, 162–169. [Google Scholar] [CrossRef]
- He, Z.; Xie, Y.; Wang, Y.; Xu, J.; Hu, J. Removal of Mercury from Coal-Fired Flue Gas and Its Sulfur Tolerance Characteristics by Mn, Ce Modified γ-Al2O3Catalyst. J. Chem. 2020, 2020, 8702745. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Zhao, L.; Zhang, J.; Zeng, G.; Zhang, X.; Zhang, W.; Tao, S. Experimental study on Hg0 removal from flue gas over columnar MnOx-CeO2/activated coke. Appl. Surf. Sci. 2015, 333, 59–67. [Google Scholar] [CrossRef]
- Deng, Y.; Macherzyński, M. Mercury capture in process gases and its mechanisms in different industries: Theoretical and practical aspects, including the influence of sulfur compounds on mercury removal. Chem. Process Eng. New Front. 2023, 44, e5. [Google Scholar] [CrossRef]

| Rector’s Name | VGR | MPR |
|---|---|---|
| Total flow rate (Q) | 108 L/h (54 L/h for each gas stream-line and mercury analyzer) | |
| Flow condition | Laminar flow, perpendicular to the sorbent bed, static or semi-fluidized conditions | Laminar flow, parallel along the sorbent layer on a wall |
| Temperature of thermostatic oven during the main part of experiment | 125 °C | |
| Total number of sorbent cells or PTFE pipes in the reactor (N) | 3 | 1 and (5) |
| Number of used sorbent cells or coating pipes in the reactor (n) | 1 | 1 and (3) |
| The selected sorbent | CuO or CuS (granulate) | CuO or CuS (active layer) |
| Mass of sorbent | 200 mg | 50 mg in a 1 coated pipe 200 mg in 3 coated pipes |
| Radius of sorbent bed (r1) in the VGR | 13 mm | N/A |
| Inner radius of pipe (r2) in the MPR | N/A | 2 mm |
| Sorbent thickness (l1) in the VGR | 1 mm (depends on mass of sorbent) | N/A |
| Coating sorbent length (l2) in the MPR | N/A | 35 mm in CuO-coating pipes 50 mm in CuS-coating Pipes (depends on the coating processes) |
| Fuel | λ | O2 [%] | CO2 [%] | CO [mg/m3] | SO2 [mg/m3] | NOx [mg/m3] |
|---|---|---|---|---|---|---|
| Bituminous | 1.48 | 6.8 | 13.9 | 101 | 1212 | 885 |
| Bituminous | 1.75 | 9.0 | 11.7 | 113 | 1002 | 1315 |
| Lignite | 1.57 | 7.6 | 13.1 | 71 | 1157 | 311 |
| Lignite | 1.69 | 8.6 | 12.1 | 184 | 1102 | 159 |
| Reactor | Sorbent | Number of Beds or Coating Pipes Applied | Velocity of Gas Passes through Sorbent [m/s] | Contact Area [cm2] | Contact Time [s] |
|---|---|---|---|---|---|
| VGR | CuO | 1 | 0.028 | 5.31 | 0.035 |
| CuS | 1 | 5.31 | 0.035 | ||
| MPR | CuO | 1 (one-pipe module) | 1.19 | 4.39 | 0.03 |
| 3 (five-pipe module) | 0.239 | 13.19 | 0.44 | ||
| CuS | 1 (one-pipe module) | 1.19 | 6.28 | 0.04 | |
| 3 (five-pipe module) | 0.239 | 18.84 | 0.63 |
| λ | O2 [%] | CO2 [%] | CO [mg/m3] | SO2 [mg/m3] | H2S [mg/m3] | NOx [mg/m3] | WFGD |
|---|---|---|---|---|---|---|---|
| 1.69 | 8.6 | 12.1 | 184 | 1102 | 43 | 158.6 | Switched off |
| 1.65 | 8.1 | 12.6 | Below 100 | 25 | Below DL | 146.0 | Switched on |
| Sorbent | Reactor | Sorbent [mg] | B Stage [%] | C Stage [%] | C-1 Stage [%] | D Stage [%] | Mean [%] | Hg(0) Capture Capacity [µg/mg] |
|---|---|---|---|---|---|---|---|---|
| CuO (granulate) | VGR | 200 | 62.2 | 59.6 | 48 | 33.6 | 50.85 | 20.3 |
| CuO (coating) | MPR 1 (one-pipe module) | 50 | 13.3 | 24.8 | 45.4 | 13.9 | 24.35 | 23.0 |
| 3 (five-pipe module) | 200 | 49.6 | 77.3 | 78.2 | 55.8 | 65.23 | 15.7 | |
| CuS (granulate) | VGR | 200 | 74.2 | 85 | 60 | 47.6 | 66.70 | 30.4 |
| CuS (coating) | MPR 1 (one-pipe module) | 50 | 67.4 | 54.9 | N/A | 55.83 | 59.38 | 75.9 |
| 3 (five-pipe module) | 200 | 97.9 | 92 | N/A | 92.1 | 93.93 | 22.1 |
| Sorbent (Reactor) | 20–40 min [µg/g∙min] | 40–60 min [µg/g∙min] | 60–80 min [µg/g∙min] | 80–100 min [µg/g∙min] | Mean Hg(0) Pass through Rate [µg/g∙min] |
|---|---|---|---|---|---|
| CuO (VGR) | 35.5 | 123 | 216.3 | 377.8 | 188.1 |
| CuO (MPR) 1 (one-pipe module) | 332 | 1096 | 1402 | 1820 | 1162.5 |
| CuO (MPR) 3 (five-pipe module) | 51.8 | 75.3 | 77 | 103 | 76.8 |
| CuS (VGR) | 20 | 73.3 | 70.8 | 305.8 | 117.4 |
| CuS (MPR) 1 (one-pipe module) | 115 | 449 | 811 | 1187 | 640.5 |
| CuS (MPR) 3 (five-pipe module) | 1.5 | 33.5 | 41.3 | 47 | 30.8 |
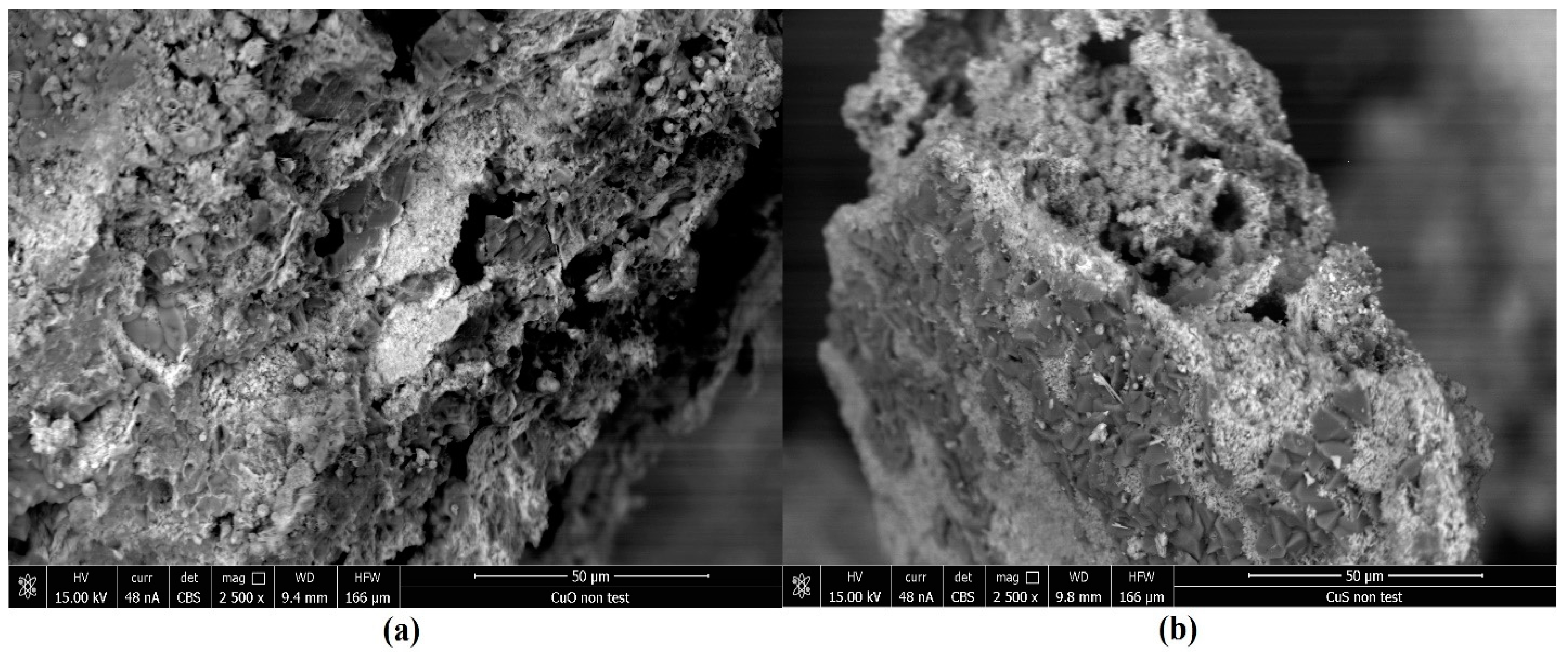
| Element | CuO | CuS |
|---|---|---|
| Atomic [%] | Atomic [%] | |
| O | 55.8 | 21.12 |
| Al | 0.47 | 1.78 |
| S | 0.51 | 37.83 |
| Cl | 0.28 | N/A |
| K | 19.19 | N/A |
| Cu | 23.68 | 37.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Górecki, J.; Szramowiat-Sala, K.; Macherzynski, M. A Prototype Reactor Promoting the Hg(0) Capture in the Simulated Flue Gas from Small-Scale Boilers by Using Copper Oxide- and Copper Sulfide-Coated Teflon Pipes. Energies 2024, 17, 1236. https://doi.org/10.3390/en17051236
Deng Y, Górecki J, Szramowiat-Sala K, Macherzynski M. A Prototype Reactor Promoting the Hg(0) Capture in the Simulated Flue Gas from Small-Scale Boilers by Using Copper Oxide- and Copper Sulfide-Coated Teflon Pipes. Energies. 2024; 17(5):1236. https://doi.org/10.3390/en17051236
Chicago/Turabian StyleDeng, Yinyou, Jerzy Górecki, Katarzyna Szramowiat-Sala, and Mariusz Macherzynski. 2024. "A Prototype Reactor Promoting the Hg(0) Capture in the Simulated Flue Gas from Small-Scale Boilers by Using Copper Oxide- and Copper Sulfide-Coated Teflon Pipes" Energies 17, no. 5: 1236. https://doi.org/10.3390/en17051236
APA StyleDeng, Y., Górecki, J., Szramowiat-Sala, K., & Macherzynski, M. (2024). A Prototype Reactor Promoting the Hg(0) Capture in the Simulated Flue Gas from Small-Scale Boilers by Using Copper Oxide- and Copper Sulfide-Coated Teflon Pipes. Energies, 17(5), 1236. https://doi.org/10.3390/en17051236









