Porous Prussian Blue Analogs Decorated Electrospun Carbon Nanofibers as Efficient Electrocatalyst for Overall Water Splitting
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
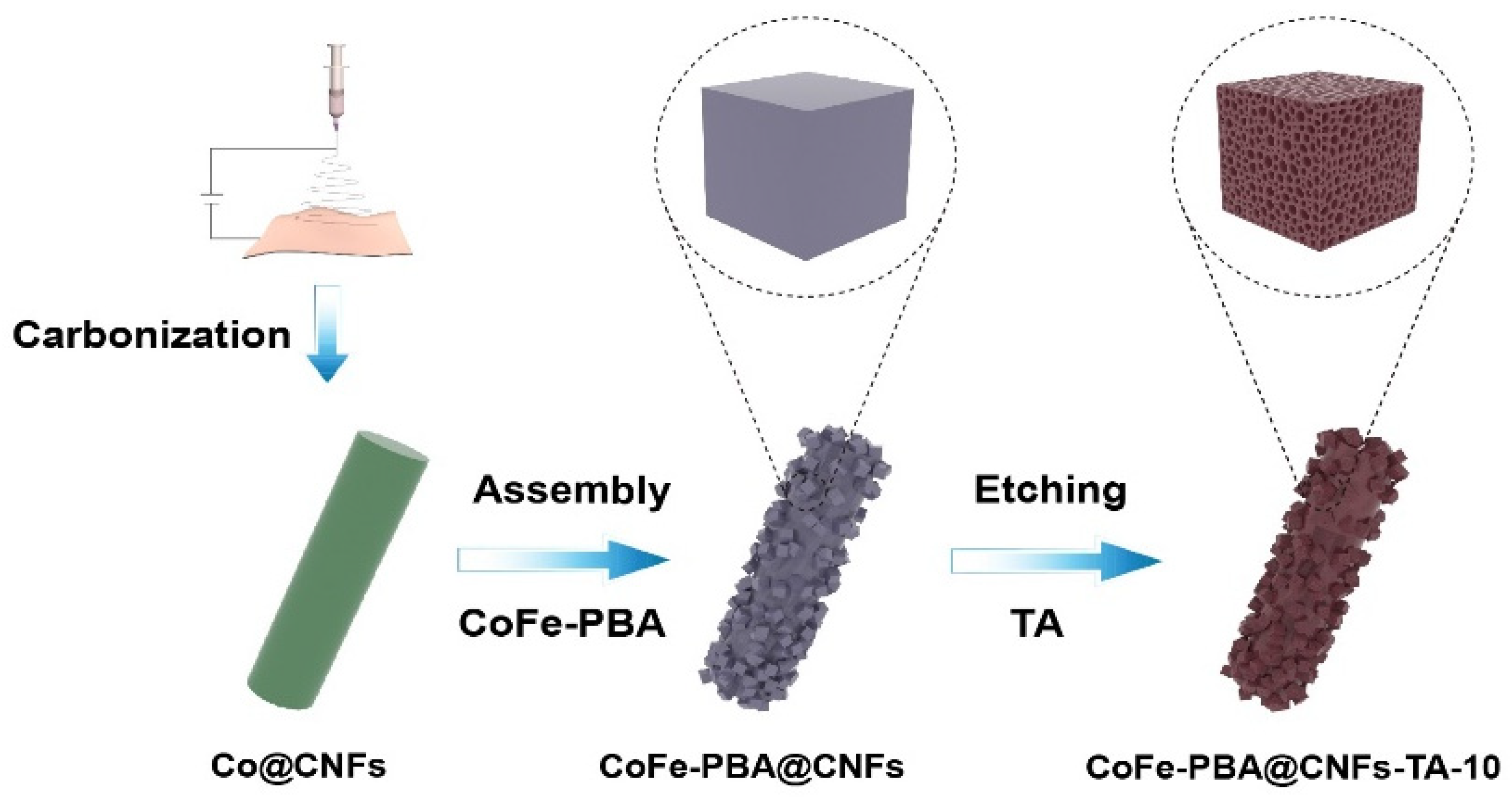
2.2. Preparation of Catalysts
2.3. Materials Characterization
2.4. Electrochemical Measurements
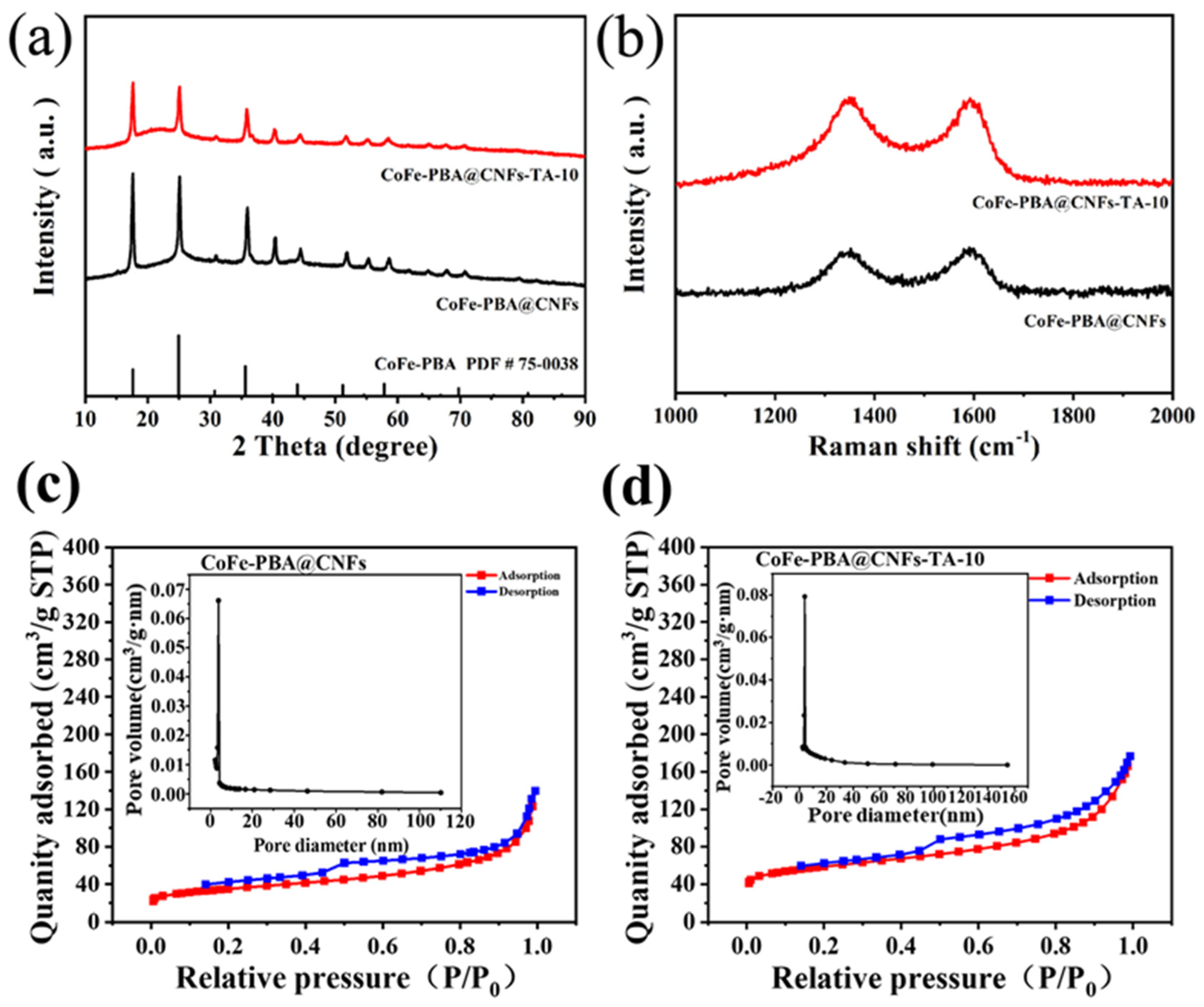
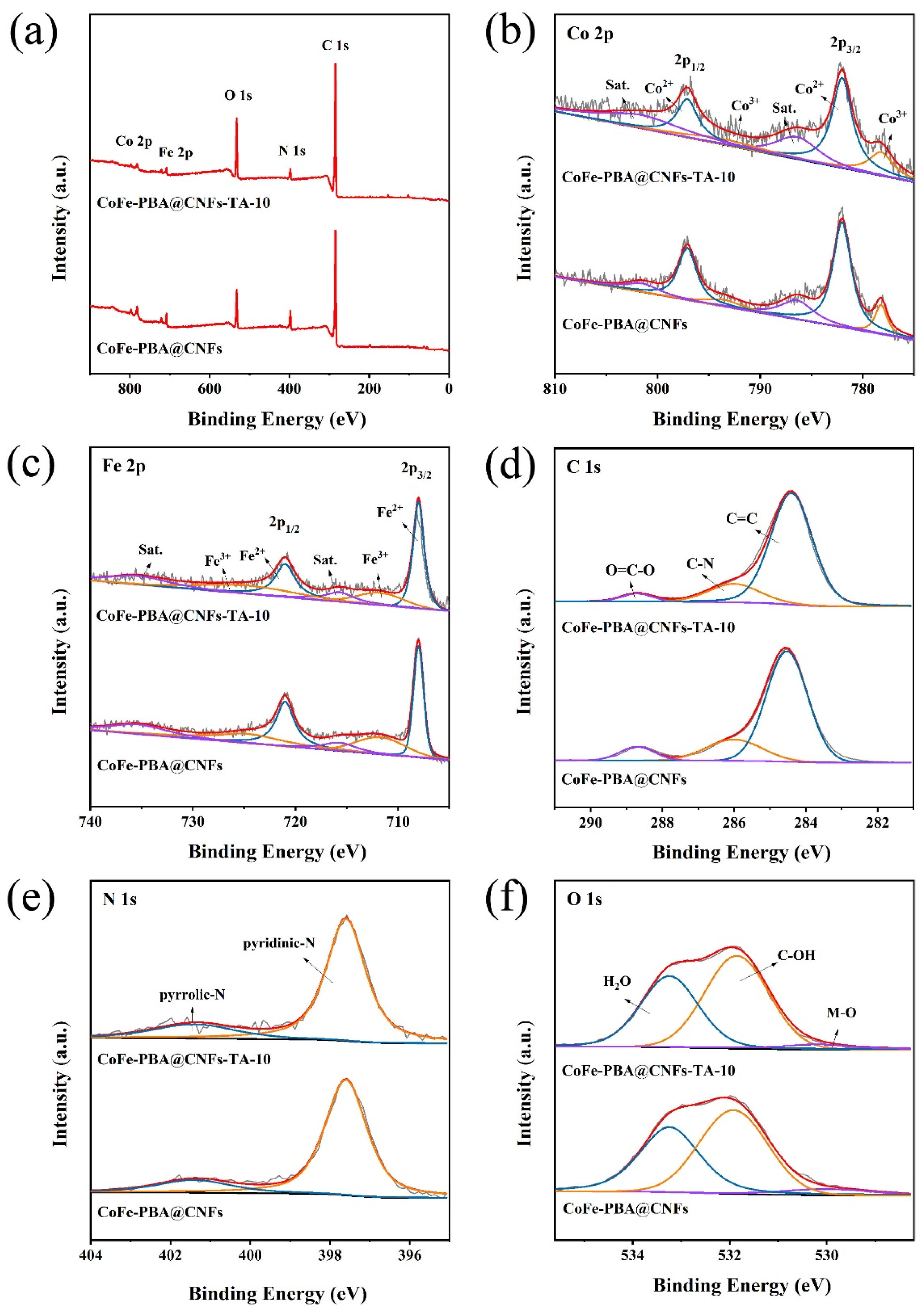
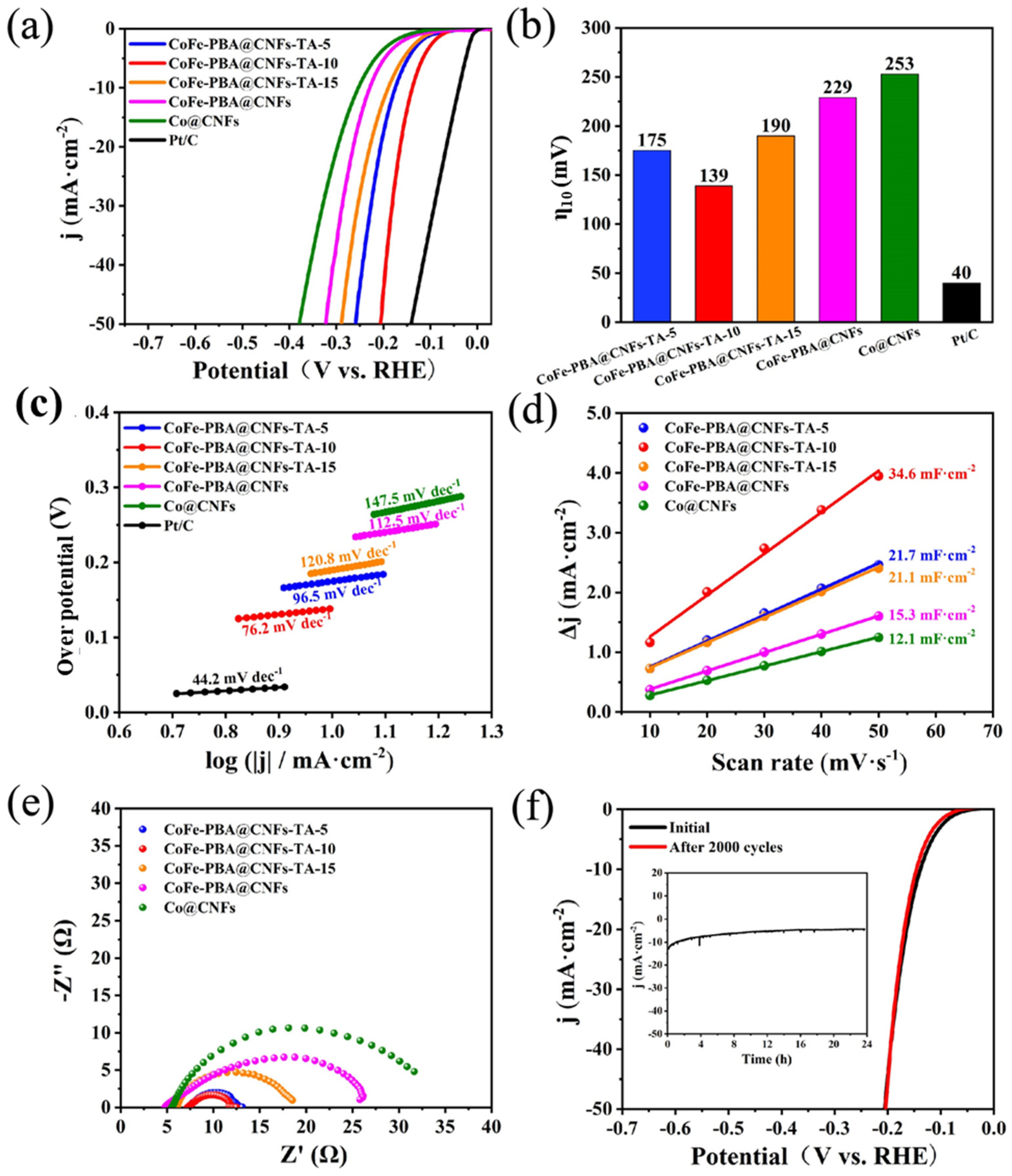
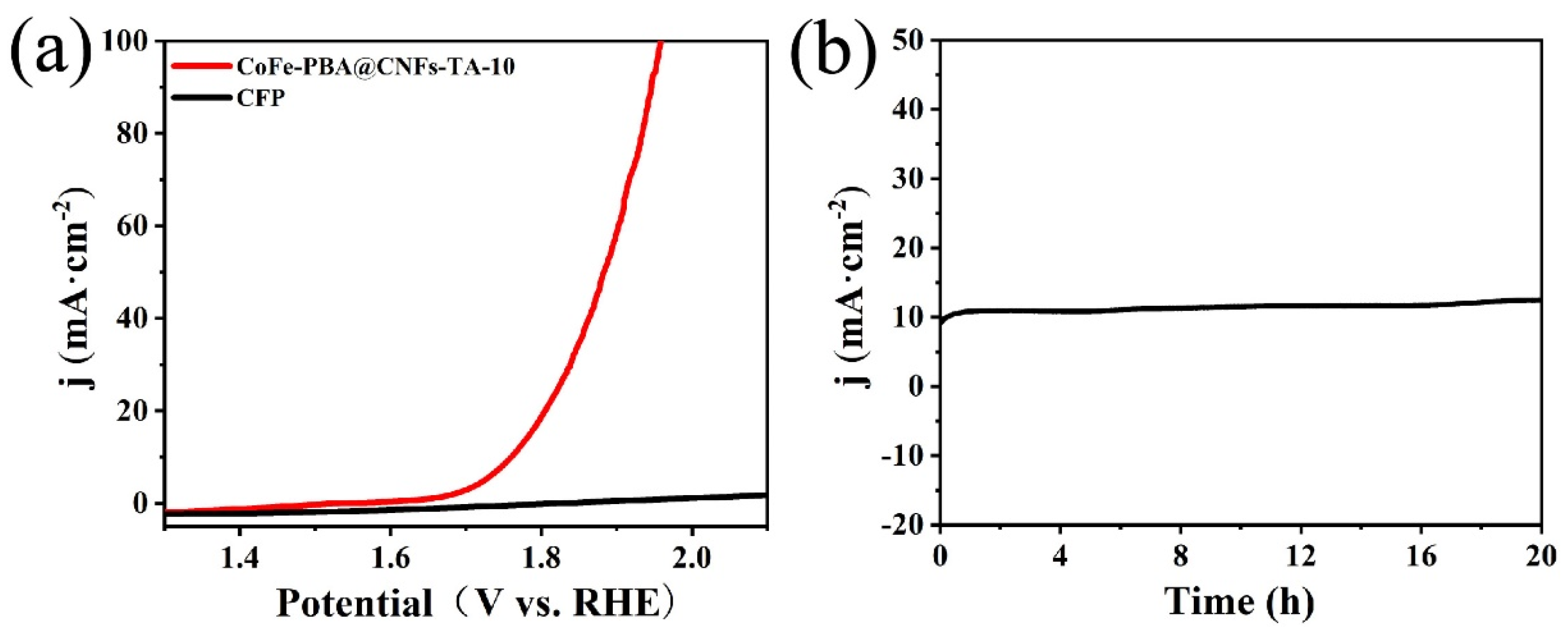
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, J.; Chang, X.; Li, L.; Zhang, M. Synthesis of CoMoO4 nanofibers by electrospinning as efficient electrocatalyst for overall water splitting. Molecules 2024, 29, 7. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Jiang, Z.; Deng, B.; Xin, Y.; Jiang, Z.-J. Defect engineering promoted ultrafine Ir nanoparticle growth and Sr single-atom adsorption on TiO2 nanowires to achieve high-performance overall water splitting in acidic media. Adv. Energy Mater. 2024, 2303987. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, J. Single-atom catalysts supported on two-dimensional tetragonal transition metal chalcogenides for hydrogen and oxygen evolution. iScience 2024, 27, 108788. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.; Huang, X.; Xu, G.; Wang, X. One-step fabrication of vanadium-doped CoFe PBA nanosheets for efficient oxygen evolution reaction. Dalton Trans. 2023, 52, 11297–11302. [Google Scholar] [CrossRef] [PubMed]
- Ghorui, U.K.; Show, B.; Roy, D.; Basak, A.; Adhikary, B.; Mondal, A. Strategically designed Pd-induced changes in alkaline hydrogen evolution reaction and oxygen evolution reaction performances of electrochemical water oxidation by the galvanically synthesized MoO2/MoO3 composite thin film. ACS Appl. Mater. Interfaces 2024, 16, 3460–3475. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-based electrocatalysts for cost-effective ultrahigh-current-density water splitting in anion exchange membrane electrolyzer cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hua, W.; Guo, Y.; Liang, S.; Li, B.; Wang, L.; Wang, J.G. Surface-Fe enriched trimetallic (oxy)hydroxide engineered by S-incorporation and ligand anchoring toward efficient water oxidation. J. Colloid Interface Sci. 2022, 617, 391–398. [Google Scholar] [CrossRef]
- Acedera, R.A.; Dumlao, A.T.; Matienzo, D.D.; Divinagracia, M.; Paraggua, J.A.; Chuang, P.Y.A.; Ocon, J. Templated synthesis of transition metal phosphide electrocatalysts for oxygen and hydrogen evolution reactions. J. Energy Chem. 2024, 89, 646–669. [Google Scholar] [CrossRef]
- Mei, J.; Deng, Y.; Cheng, X.; Wang, X.; Wu, Q. Recent advances in iron-based sulfides electrocatalysts for oxygen and hydrogen evolution reaction. Chin. Chem. Lett. 2024, 35, 108900. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, M.; Song, N.; Wang, C.; Lu, X. General synthesis of Pt and Ni co-doped porous carbon nanofibers to boost HER performance in both acidic and alkaline solutions. Chin. Chem. Lett. 2023, 34, 107359. [Google Scholar] [CrossRef]
- Huang, G.; Hu, M.; Xu, X.; Alothman, A.A.; Mushab, M.S.S.; Ma, S.; Shen, P.K.; Zhu, J.; Yamauchi, Y. Optimizing heterointerface of Co2P–CoxOy nanoparticles within a porous carbon network for deciphering superior water splitting. Small Struct. 2023, 4, 2200235. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Wang, X.; Dong, J.; Li, H.; Yin, S.; Xia, J. Ru anchored on Co(OH)2 nanowire arrays as highly effective electrocatalyst for full water splitting. Int. J. Hydrogen Energy 2024, 51, 769–776. [Google Scholar] [CrossRef]
- Murugan, N.; Thangarasu, S.; Seo, S.B.; Mariappan, A.; Choi, Y.R.; Oh, T.H.; Kim, Y.A. N-doped defect-rich porous carbon nanosheets framework from renewable biomass as efficient metal-free bifunctional electrocatalysts for HER and OER application. Renew. Energy 2024, 222, 119801. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, S.; Wang, M.; Hu, Z.; Chen, H.; Han, Y.; Yuan, D. Construction of 3D hierarchical Co3O4@CoFe-LDH heterostructures with effective interfacial charge redistribution for rechargeable liquid/solid Zn-air batteries. Inorg. Chem. 2023, 62, 2826–2837. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Lim, H.; Son, S.; Cho, Y.; Park, J.B.; Cho, H.-S.; Jang, A.R.; Lee, Y.-W. Bimetallic metal organic framework-derived Co9S8-MoS2 nanohybrids as an efficient dual functional electrocatalyst towards the hydrogen and oxygen evolution reactions. J. Ind. Eng. Chem. 2024, 130, 317–323. [Google Scholar] [CrossRef]
- Xu, S.; Sun, X.; Cui, W.; Bai, J.; Li, C. Embedding Co6Mo6C2-Mo2C heterostructure nanoparticles in carbon nanofibers as highly efficient electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2024, 49, 309–321. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Zhang, H.; Wang, Y. N-doped carbon wrapped CoFe alloy nanoparticles with MoS2 nanosheets as electrocatalyst for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2023, 48, 22032–22043. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, J.; Liu, Y.; Hou, L.; Deng, J.; Yuan, C. Construction of hetero-phase Mo2C-CoO@N-CNFs film as a self-supported Bi-functional catalyst towards overall water splitting. Chem. Eng. J. 2023, 451, 139025. [Google Scholar] [CrossRef]
- Shen, K.; Tang, Y.; Zhou, Q.; Zhang, Y.; Ge, W.; Shai, X.; Deng, S.; Yang, P.; Deng, S.; Wang, J. Metal-organic framework-derived S-NiFe PBA coupled with NiFe layered double hydroxides as mott-schottky electrocatalysts for efficient alkaline oxygen evolution reaction. Chem. Eng. J. 2023, 471, 144827. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Liang, T.; Song, X.; Li, Z.; Wen, J.; Peng, M.; Zeng, X.; Huang, H.; Wu, H. MOF-derived ultrasmall Ru@RuO2 heterostructures as bifunctional and pH-universal electrocatalysts for 0.79 V asymmetric amphoteric overall water splitting. Chem. Eng. J. 2023, 460, 141672. [Google Scholar] [CrossRef]
- Fang, W.; Wu, Y.; Xin, S.; Hu, Y.; Dang, J.; Li, M.; Chen, B.; Zhao, H.; Li, Z. Fe and Mo dual-site single-atom catalysts for high-efficiency wide-pH hydrogen evolution and alkaline overall water splitting. Chem. Eng. J. 2023, 468, 143605. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Wang, R.; Zhang, G.; Xu, X.; Liu, J.; Sun, Z.; Liu, M.; Wang, C.; Meng, X.; et al. Activating and stabilizing Co sites in CoP for triggering oxygen electrocatalysis in zinc-air battery. Chem. Eng. J. 2023, 475, 146154. [Google Scholar] [CrossRef]
- Cui, X.; Lin, L.; Xu, T.; Liu, J.; Tang, M.; Wang, Z. In-situ fabrication of MOF@CoP hybrid bifunctional electrocatalytic nanofilm on carbon fibrous membrane for efficient overall water splitting. Int. J. Hydrogen Energy 2024, 49, 1446–1457. [Google Scholar] [CrossRef]
- Liao, Y.; Xiao, Y.; Li, Z.; Zhou, X.; Liu, J.; Guo, F.; Li, J.; Li, Y. Structural engineering of Co-metal-organic frameworks via Ce incorporation for improved oxygen evolution. Small 2023, 2307685. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Zhang, Y.; Deng, X.Y.; Ma, Z.Z.; Cheng, R.T.; Wan, Z.H.; Li, J.P.; Wang, X.G. Rational construction of loosely packed nickel nanoparticulates with residual HCOO ligands derived from a Ni-MOF for high-efficiency electrocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 5222–5232. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Aulia, S.; Yeh, M.-H.; Hsiao, L.-Y.; Tarigan, A.M.; Ho, K.-C. Graphene quantum dots induced defect-rich NiFe Prussian blue analogue as an efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2023, 648, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huo, W.; Feng, H.; Xie, Z.; Shang, J.K.; Formo, E.V.; Camargo, P.H.C.; Fang, F.; Jiang, J. Enhancing oxygen evolution reaction performance in prussian blue analogues: Triple-play of metal exsolution, hollow interiors, and anionic regulation. Adv. Mater. 2023, 35, 2304494. [Google Scholar] [CrossRef]
- Zhang, H.; Diao, J.; Ouyang, M.; Yadegari, H.; Mao, M.; Wang, M.; Henkelman, G.; Xie, F.; Riley, D.J. Heterostructured core–shell Ni–Co@Fe–Co nanoboxes of prussian blue analogues for efficient electrocatalytic hydrogen evolution from alkaline seawater. ACS Catal. 2023, 13, 1349–1358. [Google Scholar] [CrossRef]
- Shreyanka, N.S.; Theerthagiri, J.; Lee, S.J.; Yu, Y.; Choi, M.Y. Multiscale design of 3D metal–organic frameworks (M−BTC, M: Cu, Co, Ni) via play enabling bifunctional electrocatalysts for robust overall water splitting. Chem. Eng. J. 2022, 446, 137045. [Google Scholar] [CrossRef]
- Jiao, C.; Hu, M.; Hu, T.; Zhang, J. Enhanced proton conductivity and overall water splitting efficiency of dye@MOF by post-modification of MOF. J. Solid State Chem. 2023, 322, 123978. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Empirical analysis and recent advances in metal-organic framework-derived electrocatalysts for oxygen reduction, hydrogen and oxygen evolution reactions. Mater. Chem. Phys. 2022, 289, 126438. [Google Scholar] [CrossRef]
- Le, H.T.; Lee, J.E.; Yun, S.Y.; Kwon, O.; Park, J.K.; Jeong, Y.K. Plasma-induced oxygen vacancies in N-doped hollow NiCo PBA nanocages derived from prussian blue analogue for efficient OER in alkaline media. Int. J. Mol. Sci. 2023, 24, 9246. [Google Scholar] [CrossRef]
- Diao, F.; Mikkel, R.K.; Cao, H.; Yan, X.; Liu, P.; Christian, E.; Xiao, X. Moderate heat treatment of CoFe Prussian blue analogues for enhanced oxygen evolution reaction performance. J. Energy Chem. 2023, 78, 476–486. [Google Scholar] [CrossRef]
- Shi, W.; Nie, P.; Zhu, G.; Hu, B.; Yang, J.; Liu, J. Self-supporting prussian blue@CNF based battery-capacitor with superhigh adsorption capacity and selectivity for potassium recovery. Chem. Eng. J. 2020, 388, 124162. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Chen, Z.; Wang, R.; Fei, B.; Liu, H.; Guo, Y.; Wu, R. Unconventional bi-vacancies activating inert prussian blue analogues nanocubes for efficient hydrogen evolution. Chem. Eng. J. 2021, 420, 127671. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Liu, T.; Peng, N.; Yu, H.; Zheng, R.; Zhang, L.; Shui, M.; Shu, J. Copper hexacyanoferrate as ultra-high rate host for aqueous ammonium ion storage. Chem. Eng. J. 2021, 421, 127671. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, L.; Li, J.; Shi, J.; Liu, Z.; Fan, L.; Cai, W. Zeolitic imidazolate framework induced CoFe-PBA/Carbides hollow cube toward robust bi-functional electrochemical oxygen reaction. Chem. Eng. J. 2023, 472, 144818. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Chen, L.; Gao, L.; Tian, W.; Yuan, Z. Binary FeNi phosphides dispersed on N,P-doped carbon nanosheets for highly efficient overall water splitting and rechargeable Zn-air batteries. Chem. Eng. J. 2020, 389, 124408. [Google Scholar] [CrossRef]
- Yin, T.; Zhou, X.; Wu, A.; Yan, H.; Feng, Q.; Tian, C. CoP particles embedded in N-doped two-dimensional carbon sheets as efficient electrocatalyst for water splitting. Electrochim. Acta 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Xiang, R.; Duan, Y.; Tong, C.; Peng, L.; Wang, J.; Shah, S.S.A.; Najam, T.; Huang, X.; Wei, Z. Self-standing FeCo prussian blue analogue derived FeCo/C and FeCoP/C nanosheet arrays for cost-effective electrocatalytic water splitting. Electrochim. Acta 2019, 302, 45–55. [Google Scholar] [CrossRef]
- Niu, H.; Lin, S.; Chen, Y.; Feng, J.; Zhang, Q.; Wang, A. Hydrogel derived FeCo/FeCoP embedded in N, P-codoped 3D porous carbon framework as a highly efficient electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2021, 536, 147950. [Google Scholar] [CrossRef]
- Liang, X.; Wang, G.; Gu, W.; Ji, G. Prussian blue analogue derived carbon-based composites toward lightweight microwave absorption. Carbon 2021, 177, 97–106. [Google Scholar] [CrossRef]
- Lu, X.; Ye, K.H.; Zhang, S.; Zhang, J.; Yang, J.; Huang, Y.; Ji, H. Amorphous type FeOOH modified defective BiVO4 photoanodes for photoelectrochemical water oxidation. Chem. Eng. J. 2022, 428, 131027. [Google Scholar] [CrossRef]
- Ullah, N.; Xie, M.; Chen, L.; Yaseen, W.; Zhao, W.; Yang, S.; Xu, Y.; Xie, J. Novel 3D graphene ornamented with CoO nanoparticles as an efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Mater. Chem. Phys. 2021, 261, 124237. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3395–J3404. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, Y.; Xu, W.; Wang, D.; Zhao, Y.; Chen, W.; Xiang, X.; Pang, X.; Zhang, B.; Shang, H. Highly active and durable nitrogen-doped CoP/CeO2 nanowire heterostructures for overall water splitting. Chem. Eng. J. 2023, 460, 141119. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, N.; Shen, Y.; Li, C.; Liu, R. Enhancing U(VI) adsorptive removal via amidoximed polyacrylonitrile nanofibers with hierarchical porous structure. Colloid Polym. Sci. 2020, 299, 25–35. [Google Scholar] [CrossRef]
- Hu, M.; Ju, Y.; Liang, K.; Suma, T.; Cui, J.W.; Caruso, F. Void engineering in metal-organic frameworks via synergistic etching and surface functionalization. Adv. Funct. Mater. 2016, 26, 5827–5834. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Y.; Hu, Y.X.; Kong, B.; Zhang, J.; Gu, Q.F.; Tong, Y.P.; Wang, X.B.; Jiang, S.P.; Wang, H.T. Hydrothermal synthesis of metal-polyphenol coordination crystals and their derived metal/N-doped carbon composites for oxygen electrocatalysis. Angew. Chem. Int. Ed. 2016, 55, 12470–12474. [Google Scholar] [CrossRef]
- Ma, W.; Li, D.; Liao, L.; Zhou, H.; Zhang, F.; Zhou, X.; Mo, Y.; Yu, F. High-performance bifunctional porous iron-rich phosphide/nickel nitride heterostructures for alkaline seawater splitting. Small 2023, 19, e2207082. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, Z.; Hao, W.; Xu, H.; Guo, Y.; Wu, R. Catalyzing overall water splitting at an ultralow cell voltage of 1.42 V via coupled Co-doped NiO nanosheets with carbon. Appl. Catal. B 2019, 252, 214–221. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, K.; Yu, Z.; Sun, C.; Wang, Z.; Feng, N.; Mai, L.; Wang, Y.; Xia, Y. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 2020, 13, 2200–2208. [Google Scholar] [CrossRef]
- Yuan, Y.; Adimi, S.; Guo, X.; Thomas, T.; Zhu, Y.; Guo, H.; Priyanga, G.S.; Yoo, P.; Wang, J.; Chen, J.; et al. A surface-oxide-rich activation layer (soal) on Ni2Mo3N for a rapid and durable oxygen evolution reaction. Angew. Chem. Int. Ed. 2020, 59, 18036–18041. [Google Scholar] [CrossRef]
- Ren, A.; Yu, B.; Huang, M.; Liu, Z. Encapsulation of cobalt prussian blue analogue-derived ultra-small CoP nanoparticles in electrospun N-doped porous carbon nanofibers as an efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2024, 51, 490–502. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Zhu, X.; Bai, L.; Liu, Z. Porous Prussian Blue Analogs Decorated Electrospun Carbon Nanofibers as Efficient Electrocatalyst for Overall Water Splitting. Energies 2024, 17, 1154. https://doi.org/10.3390/en17051154
Xiao Z, Zhu X, Bai L, Liu Z. Porous Prussian Blue Analogs Decorated Electrospun Carbon Nanofibers as Efficient Electrocatalyst for Overall Water Splitting. Energies. 2024; 17(5):1154. https://doi.org/10.3390/en17051154
Chicago/Turabian StyleXiao, Zhiqing, Xiubin Zhu, Lu Bai, and Zhicheng Liu. 2024. "Porous Prussian Blue Analogs Decorated Electrospun Carbon Nanofibers as Efficient Electrocatalyst for Overall Water Splitting" Energies 17, no. 5: 1154. https://doi.org/10.3390/en17051154
APA StyleXiao, Z., Zhu, X., Bai, L., & Liu, Z. (2024). Porous Prussian Blue Analogs Decorated Electrospun Carbon Nanofibers as Efficient Electrocatalyst for Overall Water Splitting. Energies, 17(5), 1154. https://doi.org/10.3390/en17051154





