Abstract
In this study, we synthesized pure and cobalt-doped NiTiO3 perovskite nanostructures using a sol–gel method and characterized them to investigate the impact of cobalt incorporation on their photocatalytic hydrogen production under UV light. XRD analysis confirmed the formation of the hexagonal ilmenite structure, with lattice parameters increasing with cobalt doping, indicating the substitution of larger Co2+ ions onto smaller Ni2+ sites. Raman spectroscopy revealed a decrease in the intensity of active modes, suggesting crystal structure distortion and oxygen vacancy generation. UV-vis spectroscopy showed a decrease in bandgap energy from 2.24 to 2.16 eV with cobalt doping up to 5%, enhancing UV light absorption. SEM and TEM images revealed nanoparticle agglomeration, while cobalt doping did not significantly alter particle size up to 5% doping. Photoluminescence spectroscopy revealed an initial increase in PL intensity for NiTiO3-1%Co, followed by a systematic decrease with higher cobalt concentrations, with NiTiO3-10%Co exhibiting the lowest intensity. Photocatalytic experiments demonstrated a remarkable improvement in hydrogen evolution rate with increasing cobalt doping, with NiTiO3-10%Co exhibiting the highest rate of 940 μmol∙g−1·h−1, a 60.4% increase compared to pure NiTiO3. This enhanced performance is attributed to the substitution of Co2+ on Ni2+ sites, the modification of electronic structure, the suppression of electron–hole recombination, and the creation of surface catalytic sites induced by cobalt incorporation. The proposed mechanism involves the introduction of Co2+/Co3+ energy levels within the NiTiO3 bandgap, facilitating charge separation and transfer, with the Co+/Co2+ redox couple aiding in suppressing electron–hole recombination. These findings highlight the potential of cobalt doping to tune the properties of NiTiO3 perovskite for efficient hydrogen production under UV light.
1. Introduction
The growing energy crisis and climate change concerns have made developing renewable energy technologies critically important [1]. Solar-driven photocatalytic water splitting to produce hydrogen is a promising approach for the sustainable fuel production that could reduce reliance on fossil fuels [2]. When semiconductor photocatalyst materials absorb photons with energy greater than their bandgap, electron–hole pairs are generated. The photogenerated holes in the valence band oxidize water to oxygen, while electrons in the conduction band reduce protons to hydrogen gas [3]. This mimics natural photosynthesis to store solar energy in the chemical bonds of hydrogen fuel [4].
Titanium dioxide was one of the first photocatalysts investigated, but its large bandgap of 3.2 eV limits its solar efficiency [5]. Significant research has since focused on metal oxides, sulfides, nitrides, and oxynitrides with suitable band energies and alignments to drive the hydrogen and oxygen evolution reactions [6]. However, most photocatalysts suffer from rapid recombination of photogenerated carriers, limiting quantum yields [7]. The modification of photocatalysts to suppress recombination and enhance light harvesting through doping, sensitization, and heterostructuring has been a major research thrust [8].
Recent studies have demonstrated the promising potential of perovskite materials for photocatalytic hydrogen production. For instance, Zhang et al. reported an Al-doped SrTiO3 perovskite achieving a hydrogen evolution rate of 550 μmol g−1 h−1 under 16 W white LED [9]. Karthink et al. developed a BaTiO3/g-C3N4 heterostructure with 26 μmol g−1 of hydrogen generation within 5 h [10]. Further advancements include Li et al.’s phosphated 2D MoS2 nanosheets and 3D NiTiO3 nanorods, achieving 337.82 μmol of H2 in 5 h under visible light [11]. Xie et al. found that 2 at% Mo-doped BaTiO3 with Pt deposition doubled the hydrogen evolution rate compared to pure BaTiO3 [12]. Mishra et al. reported 3% Co-doped SrTiO3 nanostructures exhibiting a photocurrent density of 3.45 mA/cm2 [13]. Yan et al. developed a ternary NiTiO3-CuI-graphdiyne system with a hydrogen evolution rate of 509.03 μmol h−1 g−1 [14]. These examples highlight ongoing research to enhance perovskite performance through strategies like doping, heterostructure formation, and novel material combinations.
The perovskite nickel titanate (NiTiO3) has recently emerged as a promising visible-light photocatalyst. It is reported that the bandgap of 2.1–2.4 eV allows for utilizing a larger portion of the solar spectrum compared to UV-responsive materials like TiO2 [15]. NiTiO3 has been shown to photocatalytically degrade organic pollutants and reduce CO2 under visible light [16,17]. However, like other photocatalysts, NiTiO3 exhibits high electron–hole recombination rates that impede its activity. The strategies to address this have included doping NiTiO3 with cobalt (Co) or iron (Fe) and making composites with reduced graphene oxide (rGO) or graphitic carbon nitride (g-C3N4) [18,19,20].
Transition metal ion doping introduces new energy levels in the bandgap of NiTiO3, which can enhance the light absorption, facilitate charge carrier separation, and provide catalytic sites for the surface redox reactions [21]. Cobalt oxides in particular are efficient co-catalysts for the hydrogen evolution reaction [22]. The synergistic effect between cobalt and nickel oxides has both chemical and physical origins for improving the photocatalytic properties of NiTiO3 [23,24]. Chemically, cobalt oxides are efficient co-catalysts for the hydrogen evolution reaction, providing active sites for proton reduction [25].
The reaction mechanism preferentially occurs on Co centers rather than Ni. DFT calculations predict that Co dopants in NiTiO3 lower the Gibbs free energy of hydrogen adsorption, facilitating hydrogen generation [26]. Electronically, the hybridized Co 3d and O 2p energy levels introduce intermediate bands in the bandgap of NiTiO3, enabling visible-light absorption and multiple exciton generation [27,28]. Optically, Co2+ substitution for Ni2+ enhances light harvesting by inducing a bathochromic shift of the absorption edge [29,30]. The narrowed bandgap also improves conductivity and charge carrier mobility. Additionally, Co doping curbs electron–hole recombination in NiTiO3 by promoting charge transfer to the Co sites, confirmed by PL and EPR studies [31,32]. The higher photocurrents are measured for Co-doped NiTiO3 films, consistent with enhanced separation and transportation of photogenerated carriers. Thus, the incorporated cobalt synergistically interacts with the nickel and titanium to modulate the electronic structure and surface chemistry to boost photocatalytic activity. The synergistic effect between cobalt and nickel oxides also improves the redox capability and conductivity of NiTiO3. However, excessive doping can increase the recombination centers or cause thermal instability. The optimal doping concentration must be determined [33].
In this work, we synthesize pure and cobalt-doped nickel titanate (NiTiO3) nanostructures via a sol–gel method. The crystallinity, morphology, and optoelectronic properties of the photocatalysts are characterized by various techniques including XRD, Raman spectroscopy, FTIR, UV-vis spectroscopy, SEM, and TEM. The photocatalytic activity for hydrogen production is evaluated by performing photocatalytic water splitting experiments.
2. Materials and Methods
2.1. Materials
2-Propanol, nickel(II) nitrate hexahydrate, titanium(IV) isopropoxide, cobalt(II) nitrate hexahydrate, and citric acid were purchased from Merck Company (Darmstadt, Germany). All the reagents used in this research were of analytical grade and were used without further purification.
2.2. Pure and Co-Doped NiTiO3 Synthesis
Nickel titanate (NiTiO3) was synthesized at room temperature (25 °C) by first dissolving the following solutions in parallel: (A) 0.5 M nickel nitrate hexahydrate (Ni(NO3)2∙6H2O) in 21.7 mL of 2-propanol, and (B) 0.625 g of citric acid in 4 mL of 2-propanol, for at least 30 min. After complete dissolution, solution A was added to solution B under constant stirring for at least 2 h. Then, 0.5 M titanium isopropoxide (C12H28O4Ti) was added to the resulting solution and stirred constantly until a homogeneous viscous green gel formed. The gel was dried at 40 °C for at least 2 days. The obtained xerogel was ground in an agate mortar and analyzed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to determine the calcination temperature. Once these analyses were performed, the sample underwent a calcination process to obtain NiTiO3 nanoparticles.
To dope the NiTiO3 with cobalt, stoichiometric amounts of nickel were varied by Co, in different concentrations (1, 3, 5, and 10%), with the remainder of the procedure being similar to that mentioned before.
2.3. Characterization
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out from room temperature to 800 °C at a heating rate of 20 °C/min using a simultaneous thermal analyzer (SDT 650, TA Instruments, New Castle, DE, USA). The crystal structure and phase composition of pure and Co-doped NiTiO3 were characterized by X-ray diffraction (XRD) using a 600 W Cu-Kα radiation source (AERIS Research, PANalitycal, Almelo, The Netherlands). The diffraction patterns were collected from 20° to 80° 2θ angles at a scan step of 0.002°. The Rietveld refinement of the XRD data enabled the determination of crystallite sizes and structural parameters. Raman spectra were acquired using a preconfigured Raman spectrometer (MAYA 2000-Pro, Ocean Optics, Dunedin, FL, USA) equipped with a 532 nm laser and 100 mW power to study vibrational modes. The Raman frequency range is 100–900 cm−1 with an integration time of 2 s. Surface chemical bonding information was obtained from an Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) spectrometer (Invenio R, Bruker, Saarbrucken, Germany) over the range of 400–4000 cm−1. The optical properties were investigated by UV-vis diffuse reflectance spectroscopy (DRS) (Evolution 220, Thermo Scientific, San Jose, CA, USA) from 200 to 1100 nm using a 10 cm integrating sphere attachment and with Spectralon as the blank. The morphology of the samples was determined with both field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) (Thermo Scientific Co., Eindhoven, The Netherlands). The FE-SEM was equipped with an energy-dispersive X-ray spectroscopy (EDS) detector to provide elemental analysis. Photoluminescence spectra were measured using a FluoroMax Plus spectrometer (Horiba Scientific, Irvine, CA, USA). The photocatalytic production of hydrogen was monitored in situ using a calibrated microsensor detection system.
2.4. Hydrogen Evolution Test
The hydrogen production capacity of the synthesized NiTiO3 photocatalysts was evaluated using a two-port jacketed photocatalytic cell with methanol as a sacrificial electron donor. Half a liter of aqueous solution containing 10% methanol was prepared as the reaction medium; 250 mg of the photocatalyst powder was dispersed in the solution, which was continuously stirred. Additionally, the temperature of the reactor was controlled at 25 °C using a 1400 W chiller (Vevor, CA, USA).
Prior to illumination, the cell (Figure S2) was purged with ultra-high-purity argon (Ar) gas (99.999%) to remove any residual air and create an inert atmosphere. The argon flow was stopped once the hydrogen sensor showed a sufficiently low baseline voltage, indicative of only trace hydrogen levels. After sealing the cell and allowing 5 min for equilibration, illumination was commenced using a high-intensity UV lamp (Ultra-Vitalux UV-A, OSRAM, Garching, Germany) providing 13.6 W UVA and 3.0 W UVB, and hydrogen generation was monitored using data acquisition software V3.3 (Sensor Trace Basic, Unisense, Aarhus, Denmark).
The evolved hydrogen gas was measured using a Clark-type amperometric sensor (H2-NP, Unisense, Aarhus, Denmark). The sensor transduces a hydrogen oxidation current to a proportional voltage signal.
3. Results and Discussion
3.1. Thermogravimetric and Differential Scanning Calorimetry
The thermal behavior of NiTiO3 and cobalt-doped NiTiO3 was analyzed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Figure 1 shows the results for 10% cobalt-doped NiTiO3 compared to pure NiTiO3.
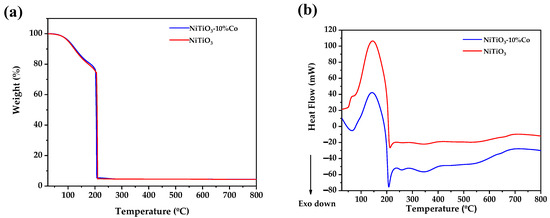
Figure 1.
(a) Thermogravimetric and (b) differential scanning calorimetry of pure and 10%Co-doped NiTiO3.
The TGA data (Figure 1a) indicate the several stages of weight loss. The initial 13% weight loss at ~143 °C is attributed to residual moisture evaporation from the 2-propanol. An additional 10% weight loss at 199 °C corresponds to the removal of coordinated water molecules from the metal salts. The major 70% weight loss is tributed to the decomposition of citric acid, which is the main component of the xerogel. Further minor weight losses occur until 800 °C, with a total loss of 95.79%.
The DSC curve (Figure 1b) displays an exothermic process at 65 °C and five endothermic peaks at 164, 226 290, 408, and 817 °C. The exotherm is likely from spontaneous water and 2-propanol evaporation. The endotherms can be assigned to the elimination of coordinated water, citric acid decomposition, nickel oxidation, titanium oxidation, and nickel titanate formation, respectively.
The onset of the final process at 552 °C indicates the start of the nickel titanate phase transformation. Hence, 600 °C was chosen as the calcination temperature to obtain the NiTiO3 phase.
By comparing the pure and 10% cobalt-doped samples, the weight loss percentages are similar, indicating that cobalt doping does not significantly impact decomposition processes. However, the lower intensity endothermic peaks are observed from 25 to 206 °C for the Co-doped sample, suggesting that less energy is required to remove coordinated solvent molecules. Furthermore, more intense exothermic peaks from 206 to 800 °C indicate easier formation of the nickel oxide, titanium oxide, and nickel titanate phases compared to undoped NiTiO3.
3.2. X-ray Diffraction
Figure 2 depicts the result of X-ray diffraction (XRD) of pure and cobalt-doped NiTiO3. Detailed structural characterization of pure and cobalt-doped NiTiO3 was undertaken using Rietveld refinement of X-ray diffraction data using the PANAlytical Highscore Plus software V4.9. The Rietveld analysis results are shown in Table 1.
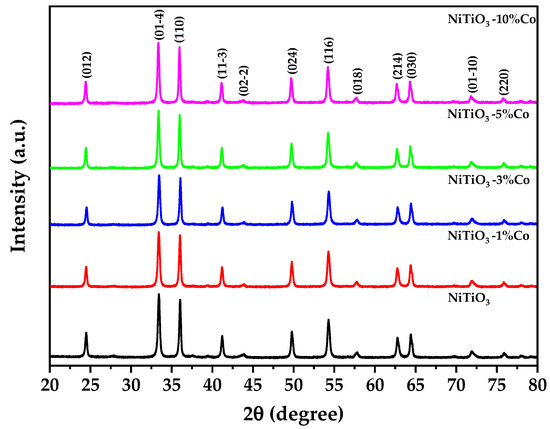
Figure 2.
X-ray diffraction patterns of pure and cobalt-doped NiTiO3.

Table 1.
Structural parameters of pure and cobalt-doped NiTiO3.
The results display that all samples exhibited diffraction patterns consistent with the prevailing hexagonal ilmenite structure of NiTiO3 (space group R-3, no. 148) [34], which is in good agreement with the 96-900-7390 COD database standard [35]. The lattice parameters showed a systematic increase with cobalt doping, with a = b and c constants expanding from 5.0276 Å and 13.8124 Å for undoped NiTiO3 to 5.0306 Å and 13.8212 Å for 10% Co-doped NiTiO3. Using Shannon’s tabulated ionic radii [36], these changes can be attributed to the substitution of larger Co2+ ions (0.745 Å) onto smaller Ni2+ sites (0.69 Å).
The gradual decline in calculated density from 5.1 g/cm3 for undoped NiTiO3 to 5.08 g/cm3 for 10% Co-doped NiTiO3 provides further evidence for the replacement of the higher-atomic-mass nickel with lower-mass cobalt ions. The Rietveld refinement also shows a rise in average crystallite size from 39 to 45 nm with increasing cobalt content from 0 to 10%. This suggests that cobalt incorporation helps to promote crystallization, possibly by facilitating cation diffusion and the accommodation of defects [37], which is consistent with DSC analysis.
3.3. Raman Spectroscopy
NiTiO3 has an ilmenite crystal structure with R-3 space group symmetry. The oxygen atoms occupy sites with D3d symmetry and are coordinated tetrahedrally to two Ti4+ cations and two Ni2+ cations (Figure 3). Based on group theory predictions, the normal vibrational modes at the Brillouin zone center have the following symmetries: 5Ag + 5Eg + 4Au + 4Eu (in addition to zero-frequency acoustic modes Au + Eu) [38]. Therefore, there are ten Raman active modes (5Ag + 5Eg), with each Eg mode doubly degenerate (Eg = E1g + E2g), along with eight IR active modes (4Au + 4Eu) [39].
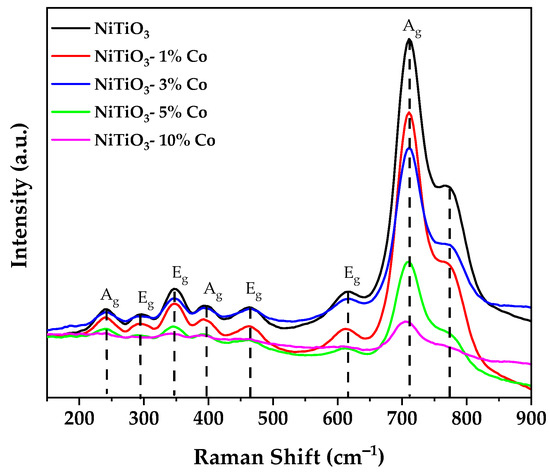
Figure 3.
Raman spectrum of pure and cobalt-doped NiTiO3.
The Raman analysis results clearly showed that all Raman active modes exhibit decreased intensity related to the amount of cobalt doping in the sample. The undoped nickel titanate was the least affected, while the sample with 10% doping showed the greatest intensity decrease, with the main peak diminishing to only 10.8% of the undoped intensity. This effect is primarily attributed to distortion of the crystal structure due to substitutional doping of Co ions on Ni sites and the generation of oxygen vacancies [24,40].
The incorporation of cobalt distorts the NiTiO3 lattice, modifying bond lengths and angles. This disrupts the symmetry, leading to relaxation of Raman selection rules and broader, less intense vibrational peaks. Furthermore, charge compensation for aliovalent Co2+ doping may induce oxygen vacancies, which also perturb the lattice vibrations [41]. The systematic intensity decrease associated with higher cobalt doping levels correlates with increasing structural disorder.
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
Figure 4 shows the FTIR spectra of undoped NiTiO3 and cobalt-doped NiTiO3 samples. Several characteristic peaks are observed, with intensities that vary systematically with the cobalt doping level.
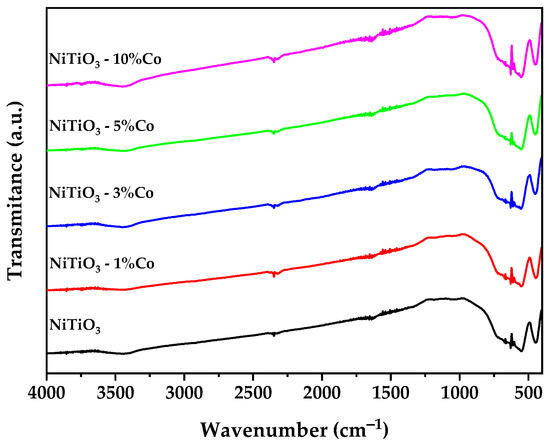
Figure 4.
FTIR spectra of pure and Co-doped NiTiO3.
The absorbance peaks at 449, 549, and 621 cm−1, attributed to the Ti–O–Ni, Ni–O, and Ti–O lattice vibrations in NiTiO3, respectively [42,43,44], increase in intensity with higher cobalt concentration. This indicates that cobalt doping enhances the polarizability of the Ti–O bonds. Conversely, the peak at 670 cm−1, associated with bending vibration of O–Ti–O bonds [45]. The sharp band at 2347 cm−1, assigned to atmospheric CO2, also decreases with more cobalt doping due to improved sample purity or structural changes that alter surface basicity [46].
The band around 3400 cm−1 is typically attributed to O-H stretching vibrations, likely from adsorbed water molecules on the surface of the nanoparticles [47].
An anomalous noisy feature appears between 1373 and 1776 cm−1 for the doped samples. This may arise from organic residues or structural hydroxyl groups [48] associated with oxygen vacancies generated to charge-compensate the aliovalent cobalt doping.
3.5. UV–Visible Spectroscopy
The UV-vis absorption spectra (Figure 5a) of pure and cobalt-doped NiTiO3 exhibit distinct regions related to the electronic structure. The fundamental absorption edge from 200 to 550 nm corresponds to the bandgap transition, while higher wavelength humps arise from intraband transitions associated with the d-orbital manifold [49].
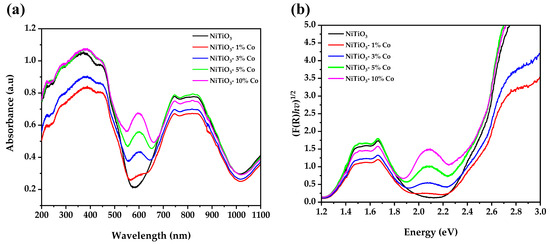
Figure 5.
(a) UV-vis spectra of pure and Co-doped NiTiO3 and (b) Kubelka–Munk plot for the band gap calculation.
The bandgap energies (Figure 5b) systematically decrease from 2.24 eV to 2.16 eV with cobalt doping levels from 0 to 5%, before recovering slightly to 2.23 eV at 10% doping. This bandgap narrowing can be attributed to substitution of Co2+ ions into the Ni2+ sites in the crystal lattice [24].
In the ilmenite structure, both Ni2+ and Ti4+ adopt octahedral coordination with oxygen. The introduction of high-spin Co2+ ions causes splitting of the original Ni 3d bands due to exchange interactions with the Co 3d electrons. This splits the conduction band and introduces intermediate states that reduce the bandgap [23,50].
At higher doping levels (>5%), bandgap widening may occur due to increased structural disorder from cobalt incorporation exceeding the NiTiO3 solubility limit. This disrupts the lattice periodicity that causes bandgap narrowing [51].
The new sub-bandgap absorption feature from 550 to 650 nm likely arises from d–d transitions of tetrahedrally coordinated Co2+ ions occupying interstitial sites. Tetrahedral crystal field splitting is less than octahedral, shrinking the d-orbital energy spacing and allowing lower energy d–d transitions [52,53].
3.6. Scanning and Transmission Electron Microscopy
The scanning electron microscopy (SEM) images (Figure 6a,c,e,g,i) reveal that the synthesized pure and cobalt-doped NiTiO3 nanoparticles exhibit significant agglomeration, forming irregularly shaped clusters. This agglomeration can be attributed to the high surface energy of the nanoparticles [54] and their tendency to minimize the overall surface area.
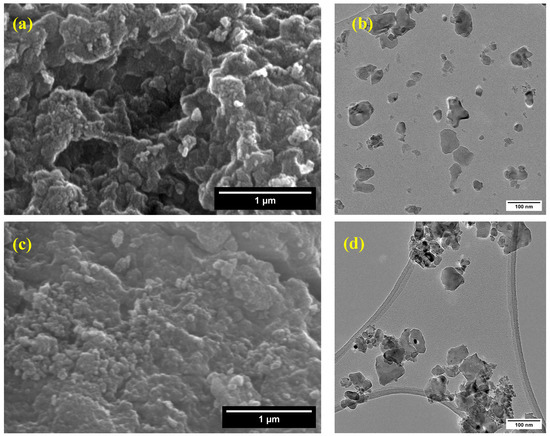
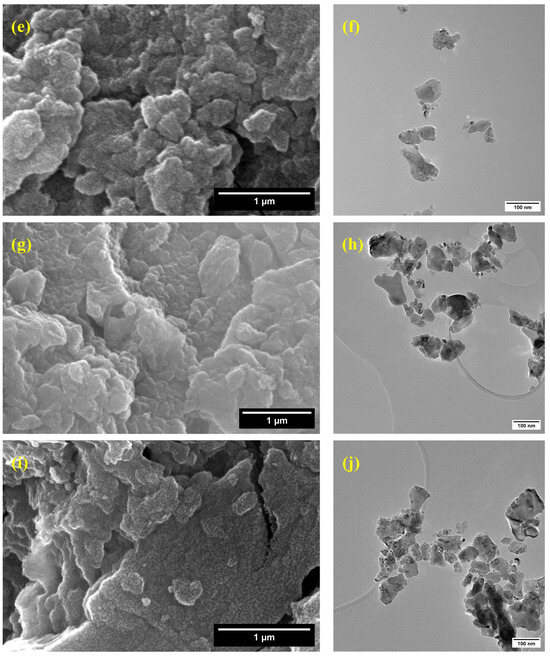
Figure 6.
Scanning and transmission electron microscopy images of (a,b) NiTiO3, (c,d) NiTiO3-1%Co, (e,f) NiTiO3-3%Co, (g,h) NiTiO3-5%Co, and (i,j) NiTiO3-10%Co.
Transmission electron microscopy (TEM) was employed to gain further insights into the morphology and size distribution of the nanoparticles. The TEM images (Figure 6b,d,f,h,j) show that the nanoparticles have a relatively uniform size distribution. The particle size analysis, as presented in Figure S1, indicates that the average particle sizes are 38.94 ± 18.55 nm for pure NiTiO3, 36.73 ± 20.83 nm for NiTiO3-1%Co, 36.24 ± 20.83 nm for NiTiO3-3%Co, 36.79 ± 22.47 nm for NiTiO3-5%Co, and 42.13 ± 19.66 nm for NiTiO3-10%Co. These results suggest that cobalt doping does not significantly alter the particle size of NiTiO3 up to 5% doping concentration. However, a slight increase in particle size is observed for the 10% cobalt-doped sample, which may be due to the influence of cobalt on the crystallization and growth of NiTiO3 nanoparticles.
Energy-dispersive X-ray spectroscopy (EDX) analysis was performed to confirm the elemental composition of the pure and cobalt-doped NiTiO3 samples (Table 2). The presence of Ni, Ti, and O in all samples verifies the formation of NiTiO3. The atomic percentages of Ni and Ti are relatively close to the expected stoichiometric ratio of 1:1. The detection of Co in the doped samples confirms the successful incorporation of cobalt into the NiTiO3 lattice. The Co atomic percentage increases with the nominal doping concentration, reaching 2.37% for the NiTiO3-10%Co sample. These results are consistent with the XRD and Raman analyses, supporting the effective doping of cobalt into the NiTiO3 structure.

Table 2.
Energy-dispersive X-ray spectroscopy (EDX) analysis of pure and Co-doped NiTiO3.
3.7. Photoluminiscence Spectroscopy
The photoluminescence (PL) spectra of pure and cobalt-doped NiTiO3 samples (Figure 7) provide valuable insights into their charge carrier dynamics. Upon excitation at 350 nm, all samples exhibit broad emission bands in the visible region (450–700 nm), characteristic of defect-related emissions in NiTiO3. Interestingly, the NiTiO3-1%Co sample shows an increase in PL intensity compared to pure NiTiO3, which may be attributed to the introduction of new radiative recombination centers at low doping concentrations [55]. However, as cobalt doping increases beyond 1%, the PL intensity systematically decreases, with NiTiO3-10%Co exhibiting the lowest intensity. This trend suggests that higher cobalt concentrations effectively suppress radiative recombination of photogenerated electron–hole pairs [56]. The reduced PL intensity at higher doping levels correlates well with the enhanced photocatalytic activity observed in hydrogen evolution experiments, particularly for the 10% Co-doped sample. The quenching effect can be attributed to the introduction of Co2+/Co3+ energy levels within the NiTiO3 bandgap, which act as electron traps and facilitate charge separation [57]. Additionally, the slight red-shift observed in the emission peaks of doped samples indicates a narrowing of the bandgap [56], consistent with our UV-vis spectroscopy results.
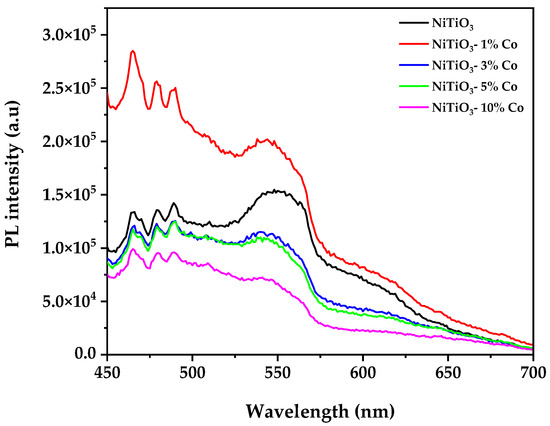
Figure 7.
Photoluminescence spectra of pure and Co-doped NiTiO3.
3.8. Hydrogen Generation Evaluation
The photocatalytic hydrogen generation performance of pure NiTiO3 and cobalt-doped NiTiO3 samples was evaluated under UV light irradiation (Figure 8) to investigate the influence of cobalt doping on the photocatalytic activity. The use of UV light is particularly relevant for NiTiO3-based photocatalysts, as NiTiO3 has a wide bandgap and is primarily active in the UV region. The hydrogen evolution rates, expressed in μmol·g−1·h−1, provide valuable insights into the effectiveness of the photocatalysts under UV light irradiation.
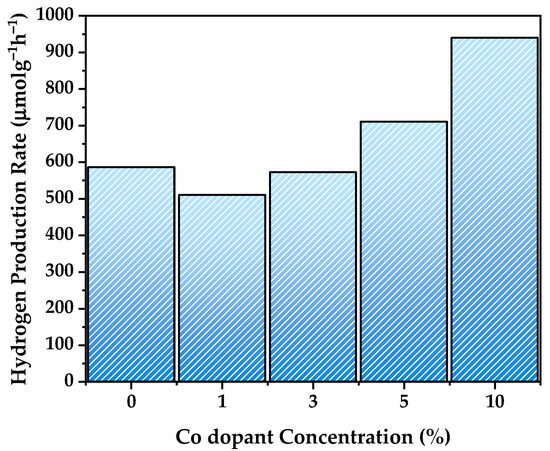
Figure 8.
Hydrogen production rate of pure and Co-doped NiTiO3.
Pure NiTiO3 exhibited a hydrogen evolution rate of 586 μmol·g−1·h−1, establishing a baseline for comparison. The slightly lower rate of 510 μmol·g−1·h−1 observed for the NiTiO3-1%Co sample may be attributed to the low cobalt doping concentration, which might not be sufficient to significantly enhance the UV light absorption and charge carrier dynamics.
However, as the cobalt doping concentration increased, a notable improvement in the hydrogen evolution rate was observed. The NiTiO3-3%Co and NiTiO3-5%Co samples achieved rates of 572 and 710 μmol·g−1·h−1, respectively, surpassing that of pure NiTiO3. This enhancement can be attributed to the modification of the electronic structure induced by cobalt doping [58]. As evidenced by the UV-vis analysis, cobalt doping introduced new energy levels within the bandgap of NiTiO3, potentially enhancing the absorption of UV light and promoting the generation of photogenerated charge carriers [32]. Additionally, cobalt doping may have suppressed electron–hole recombination, facilitating efficient charge separation and transfer under UV light irradiation [23]. The incorporation of cobalt into the NiTiO3 lattice, as confirmed by XRD and Raman analyses, suggests the creation of additional surface catalytic sites [31] that can actively participate in the photocatalytic hydrogen evolution reaction.
Remarkably, the NiTiO3-10%Co sample demonstrated the highest hydrogen evolution rate of 940 μmol·g−1·h−1, representing a substantial 60.4% increase compared to pure NiTiO3. This significant enhancement can be attributed to the synergistic effects of cobalt doping on the photophysical and photochemical properties of NiTiO3 under UV light irradiation [32]. The higher cobalt doping concentration likely led to a more pronounced modification of the electronic structure, resulting in enhanced UV light absorption and efficient charge carrier generation. Moreover, the increased density of surface catalytic sites provided by the cobalt dopants may have facilitated the adsorption and reduction of protons [59], promoting hydrogen evolution.
The observed trend in hydrogen evolution rates highlights the critical role of cobalt doping concentration in optimizing the photocatalytic performance of NiTiO3 under UV light irradiation. The enhanced UV light absorption, efficient charge separation, and increased surface catalytic sites collectively contribute to improved hydrogen generation activity. These results demonstrate the potential of cobalt doping as an effective strategy to tune the photophysical and photochemical properties of NiTiO3, making it a promising photocatalyst for efficient hydrogen production under UV light irradiation. The utilization of UV light takes advantage of the intrinsic UV absorption properties of NiTiO3 while leveraging the benefits of cobalt doping to further enhance its photocatalytic performance.
3.9. Proposed Mechanism of Photocatalytic H2 Evolution
Based on our experimental findings and established photocatalytic principles, we propose the following mechanism (Figure 9) for enhanced hydrogen production in Co-doped NiTiO3. Upon UV light absorption, electron–hole pairs are generated:
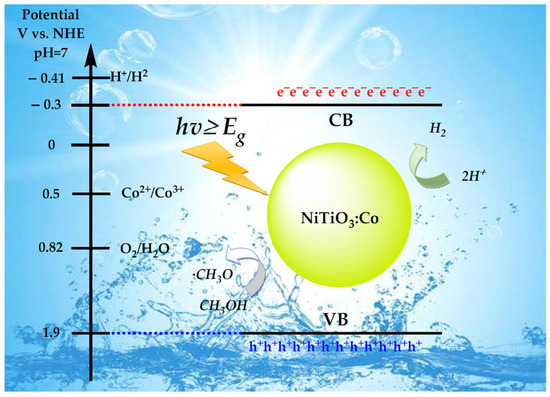
Figure 9.
Schematic representation of the mechanism of pure and Co-doped NiTiO3.
Co2+ dopants introduce energy levels within the NiTiO3 bandgap, acting as electron traps and facilitating charge separation:
Photogenerated electrons reduce water to hydrogen at the catalyst surface:
Holes oxidize the methanol sacrificial agent through a series of steps:
The overall methanol oxidation potential is E0 = 0.02 V vs. NHE. The Co+/Co2+ redox couple aids in charge separation and transfer, suppressing electron–hole recombination:
This mechanism elucidates the observed enhancement in hydrogen evolution with increasing cobalt doping up to 10%, as cobalt introduces beneficial intermediate energy levels and catalytic sites. The conduction band of NiTiO3 (−0.3 V vs. NHE) is sufficiently negative to drive the hydrogen evolution reaction, while the methanol oxidation potential ensures efficient hole scavenging [2,3,60,61].
4. Conclusions
Pure and cobalt-doped NiTiO3 perovskite nanostructures were successfully synthesized using a sol–gel method and thoroughly characterized. XRD analysis confirmed the formation of the hexagonal ilmenite structure, with lattice parameters increasing with cobalt doping, indicating the substitution of larger Co2+ ions onto smaller Ni2+ sites. Raman spectroscopy revealed a decrease in the intensity of active modes, suggesting crystal structure distortion and oxygen vacancy generation. UV-vis spectroscopy showed a decrease in bandgap energy from 2.24 to 2.16 eV with cobalt doping up to 5%, enhancing UV light absorption. SEM and TEM images revealed nanoparticle agglomeration, while cobalt doping did not significantly alter particle size up to 5% doping.
Photoluminescence spectroscopy provided further insights, revealing an initial increase in PL intensity for NiTiO3-1%Co, followed by a systematic decrease with higher cobalt concentrations. NiTiO3-10%Co showed the lowest PL intensity, suggesting effective suppression of radiative recombination at higher doping levels.
Photocatalytic experiments demonstrated a remarkable improvement in hydrogen evolution rate with increasing cobalt doping, with NiTiO3-10%Co exhibiting the highest rate of 940 μmol∙g−1·h−1, a 60.4% increase compared to pure NiTiO3. The proposed mechanism elucidates these observations: cobalt doping introduces Co2+/Co3+ energy levels within the NiTiO3 bandgap, acting as electron traps and facilitating charge separation. Upon UV light absorption, photogenerated electrons reduce water to hydrogen, while holes oxidize methanol. The Co+/Co2+ redox couple aids in suppressing electron–hole recombination, leading to more efficient utilization of charge carriers for hydrogen production.
This enhanced performance is attributed to the substitution of Co2+ on Ni2+ sites, the modification of the electronic structure, the suppression of electron–hole recombination, and the creation of surface catalytic sites induced by cobalt incorporation. These findings highlight the potential of cobalt doping to tune the properties of NiTiO3 perovskite for efficient hydrogen production under UV light.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en17153704/s1: Figure S1: Particle size distribution of (a) NiTiO3, (b) NiTiO3-1%Co, (c) NiTiO3-3%Co, (d) NiTiO3-5%Co and (e) NiTiO3-10%Co; Figure S2: Jacketed photocatalytic cell.
Author Contributions
Conceptualization, A.B.Q.C. and E.J.S.S.; methodology, A.B.Q.C., E.J.S.S., R.V.M. and S.R.; software, E.J.S.S. and W.O.L.R.; validation, A.B.Q.C. and E.J.S.S.; formal analysis, A.B.Q.C., W.O.L.R. and E.J.S.S.; investigation, A.B.Q.C., E.J.S.S., W.O.L.R., R.M.T.C., F.G.G. and J.P.M.S.; resources, A.B.Q.C., F.G.G. and J.P.M.S.; data curation, A.B.Q.C. and E.J.S.S.; writing—original draft preparation, A.B.Q.C., E.J.S.S., R.V.M. and S.R.; writing—review and editing, A.B.Q.C., E.J.S.S., R.V.M. and S.R.; visualization, R.V.M. and S.R.; supervision, A.B.Q.C. and E.J.S.S.; project administration, A.B.Q.C.; funding acquisition, A.B.Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Nacional Jorge Basadre Grohmann through “Fondos del canon sobrecanon y regalias mineras”, approved by rectoral resolution Nº 9155-2021-UNJBG with the project “Photocatalytic and photo-electrocatalytic generation of hydrogen in the Tacna region using pure and doped NiTiO3 nanoparticles”.
Data Availability Statement
The results of the current research are contained within the article, as well as Supplementary Information. The data are not public due to ethical and legal reasons, but would be available upon reasonable request to the corresponding author.
Acknowledgments
We are grateful to the Universidad Nacional Jorge Basadre Grohmann and their projects “Study of the application of nanotechnology for the purification of water with arsenic in the Tacna region”, “Study of ferroelectric materials (BiFeO3 and Bi2FeCrO6) and their application in solar cells” and “Determination of the optical fingerprints of solid, liquid and organic materials using visible and infrared spectroscopy”, approved by rectoral resolutions No. 3780-2014-UN/JBG, 4516-2018-UN/JBG and No. 5854-2019-UN/JBG, respectively, for their facilities for material characterization. The author Elisban Juani Sacari Sacari gratefully acknowledges the financial support provided by CONCYTEC through the PROCIENCIA program under the "Becas en programas de doctorado en alianzas interinstitucionales" competition, according to contracts N°PE501088673-2024-PROCIENCIA-BM and N°PE501084296-2023-PROCIENCIA-BM for undertaking a Doctoral program in Physics at the Universidad Nacional de Ingeniería, Peru.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, L.; Lin, J.; Wu, N.; Xie, S.; Meng, C.; Zheng, Y.; Wang, X.; Zhao, Y. Review and outlook on the international renewable energy development. Energy Built Environ. 2022, 3, 139–157. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Mazhar, F.; Kausar, A.; Iqbal, M. Photocatalytic hydrogen generation using TiO2: A state-of-the-art review. Zeitschrift Phys. Chem. 2022, 236, 1697–1728. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Do, H.H.; Le Nguyen, D.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Zhang, M.; Salvador, P.A.; Rohrer, G.S. Influence of particle size and shape on the rate of hydrogen produced by Al-doped SrTiO3 photocatalysts. J. Am. Ceram. Soc. 2022, 105, 5336–5346. [Google Scholar] [CrossRef]
- Karthik, K.V.; Reddy, C.V.; Reddy, K.R.; Ravishankar, R.; Sanjeev, G.; Kulkarni, R.V.; Shetti, N.P.; Raghu, A.V. Barium titanate nanostructures for photocatalytic hydrogen generation and photodegradation of chemical pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 20646–20653. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Gong, H.; Jin, Z. Phosphated 2D MoS2 nanosheets and 3D NiTiO3 nanorods for efficient photocatalytic hydrogen evolution. ChemCatChem 2020, 12, 5492–5503. [Google Scholar] [CrossRef]
- Xie, P.; Yang, F.; Li, R.; Ai, C.; Lin, C.; Lin, S. Improving hydrogen evolution activity of perovskite BaTiO3 with Mo doping: Experiments and first-principles analysis. Int. J. Hydrogen Energy 2019, 44, 11695–11704. [Google Scholar] [CrossRef]
- Mishra, A.; Parangusan, H.; Bhadra, J.; Ahmed, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Synthesis and photoelectrochemical performance of Co doped SrTiO3 nanostructures photoanode. Environ. Prog. Sustain. Energy 2023, 42, e14186. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Jin, Z. Graphdiyne Based Ternary GD-CuI-NiTiO3 S-Scheme Heterjunction Photocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 24896–24906. [Google Scholar] [CrossRef]
- Diab, K.R.; El-Maghrabi, H.H.; Nada, A.A.; Youssef, A.M.; Hamdy, A.; Roualdes, S.; Abd El-Wahab, S. Facile fabrication of NiTiO3/graphene nanocomposites for photocatalytic hydrogen generation. J. Photochem. Photobiol. A Chem. 2018, 365, 86–93. [Google Scholar] [CrossRef]
- Guo, H.; Wan, S.; Wang, Y.; Ma, W.; Zhong, Q.; Ding, J. Enhanced photocatalytic CO2 reduction over direct Z-scheme NiTiO3/g-C3N4 nanocomposite promoted by efficient interfacial charge transfer. Chem. Eng. J. 2021, 412, 128646. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Younis, S.A.; Ali, H.R.; Nada, A.A. Solar-driven photocatalytic transformation of toluene to benzoic acid over perovskite-type NiTiO3 decorated with reduced GO and g-C3N4 nanosheets. J. Environ. Chem. Eng. 2023, 11, 109477. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, J.; Jiang, Y.; Kiani, M.; Li, B.; Wang, R. Fabrication of high-activity hybrid NiTiO3/g-C3N4 heterostructured photocatalysts for water splitting to enhanced hydrogen production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, P.; Peng, T.; Liu, Q.; Chen, W.; Liu, B.; Yuan, Y.; Zhang, W.; Song, F.; Gu, J.; et al. Mechanically alloyed NiTiO3/transition metal heterostructures: Introducing oxygen vacancies for exceptionally enhanced hydrogen evolution reaction activity. J. Mater. Chem. A 2020, 8, 14908–14914. [Google Scholar] [CrossRef]
- Zan, L.; Zhang, H.; Bo, X.; Zhao, Y.; Tian, H.; Chen, H.; Wei, Q.; Tang, H.; Fu, F. Investigation of the synergistic effect on cobalt oxide modified silver surface for electrocatalytic hydrogen evolution reaction. J. Alloys Compd. 2021, 869, 159324. [Google Scholar] [CrossRef]
- Dat, T.T. Effects of Co doping on properties of ilmenite NiTiO3 ceramics. Vietnam J. Sci. Technol. 2018, 56, 119. [Google Scholar] [CrossRef]
- Jiang, K.; Pham, T.-T.; Kang, S.G.; Men, Y.; Shin, E.W. Modification of the structural properties of NiTiO3 materials by transition metal dopants: The dopant size effect. J. Alloys Compd. 2018, 739, 393–400. [Google Scholar] [CrossRef]
- Wu, M.; Ke, S.; Chen, W.; Zhang, S.; Zhu, M.; Zhang, Y.; Foo, M.L.; Tang, L. Optimization of the facet structure of cobalt oxide catalysts for enhanced hydrogen evolution reaction. Catal. Sci. Technol. 2020, 10, 1040–1047. [Google Scholar] [CrossRef]
- Hang, R.; Liu, S.; Liu, Y.; Zhao, Y.; Bai, L.; Jin, M.; Zhang, X.; Huang, X.; Yao, X.; Tang, B. Preparation, characterization, corrosion behavior and cytocompatibility of NiTiO3 nanosheets hydrothermally synthesized on biomedical NiTi alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 715–722. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, B.; Li, R.; Fan, C.; Liang, Z.; Han, P. Structural, electronic and optical properties of Ilmenite ATiO3(A=Fe, Co, Ni). Mater. Sci. Semicond. Process. 2015, 39, 6–16. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.; Ha, C.A.; Hoang, T.C.; van Nguyen, T.T.; Nguyen, D.T.; Luu, C.L. Exceptional photodecomposition activity of heterostructure NiTiO3–TiO2 catalyst. J. Sci. Adv. Mater. Devices 2022, 7, 100407. [Google Scholar] [CrossRef]
- Chanda, A.; Joshi, S.R.; Akshay, V.R.; Varma, S.; Singh, J.; Vasundhara, M.; Shukla, P. Structural and optical properties of multilayered un-doped and cobalt doped TiO2 thin films. Appl. Surf. Sci. 2021, 536, 147830. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Luminescence characteristics of cobalt doped TiO2 nanoparticles. J. Lumin. 2012, 132, 178–184. [Google Scholar] [CrossRef]
- Jiang, K.; Men, Y.; Liu, S.; Wang, J.; An, W.; Yu, H.; Shin, E.W. Highly stable and selective CoxNiyTiO3 for CO2 methanation: Electron transfer and interface interaction. J. CO2 Util. 2021, 53, 101743. [Google Scholar] [CrossRef]
- Dao, D.Q.; Nguyen, T.K.A.; Pham, T.-T.; Shin, E.W. Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation. Catalysts 2020, 10, 1332. [Google Scholar] [CrossRef]
- Böer, K.W. Doping and junction formation. In Survey of Semiconductor Physics; Böer, K.W., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 963–997. ISBN 978-94-010-5293-1. [Google Scholar]
- Thoan, N.H.; Hung, P.P.; Dung, D.D.; Ngoc, T.V.; Bac, L. Effect of copper dopant on microstructural and optical properties of NiTiO3 ilmenite materials synthesized by citrate method. J. Nanostructures 2020, 10, 769–778. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gupta, H.; Chitrarasu, K.; Chellasamy, V.; Thangadurai, P. Influence of Nb ion doping on the electrical properties of nanocrystalline NiTiO3 ceramics and their universal behavior. Ionics 2020, 26, 939–952. [Google Scholar] [CrossRef]
- Hung, P.P.; Dat, T.T.; Dung, D.D.; Trung, N.N.; Hanh, M.H.; Toan, D.N.; Bac, L.H. Effect of Annealing Temperature on Structural, Optical and Visible-Light Photocatalytic Properties of NiTiO3 Nanopowders. J. Electron. Mater. 2018, 47, 7301–7308. [Google Scholar] [CrossRef]
- Qi, W.; Mattursun, A.; Gao, M.; Hushur, A.; Zhang, H. Lattice dynamics of NiTiO3 under high pressure: Raman evidence under two pressure-transmitting mediums. Results Phys. 2022, 43, 106114. [Google Scholar] [CrossRef]
- Stella, C.; Prabhakar, D.; Prabhu, M.; Soundararajan, N.; Ramachandran, K. Oxygen vacancies induced room temperature ferromagnetism and gas sensing properties of Co-doped TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 1636–1644. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781498708166. [Google Scholar]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. Facile one-pot solvothermal approach to produce inorganic binary TiO2@NiTiO3 and ternary Au-TiO2@NiTiO3 yellow nano-pigment for environmental and energy use. Mater. Res. Express 2021, 8, 45016. [Google Scholar] [CrossRef]
- Yang, B.; Bai, X.; Wang, J.; Fang, M.; Wu, X.; Liu, Y.; Huang, Z.; Lao, C.-Y.; Min, X. Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts 2019, 9, 561. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Nithya, V.D.; Fathima, K.S.; Sanjeeviraja, C.; Selvan, G.K.; Arumugam, S.; Selvan, R.K. Investigations on the temperature dependent electrical and magnetic properties of NiTiO3 by molten salt synthesis. Mater. Res. Bull. 2013, 48, 1110–1116. [Google Scholar] [CrossRef]
- Chellasamy, V.; Thangadurai, P. Structural and electrochemical investigations of nanostructured NiTiO3 in acidic environment. Front. Mater. Sci. 2017, 11, 162–170. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chang, Y.-H.; Yang, W.-D.; Tsai, B.-S. Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method. J. Non Cryst. Solids 2006, 352, 789–794. [Google Scholar] [CrossRef]
- Enhessari, M.; Sakhaei, M.; Salehabadi, A.; Etemad, L. CeO2/NiTiO3 nanocomposites; synthesis, photoluminescence and magnetic behavior. Mater. Sci. Pol. 2017, 35, 275–282. [Google Scholar] [CrossRef]
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non Cryst. Solids 1987, 89, 206–216. [Google Scholar] [CrossRef]
- Li, M.-W.; Yuan, J.-P.; Gao, X.-M.; Liang, E.-Q.; Wang, C.-Y. Structure and optical absorption properties of NiTiO3 nanocrystallites. Appl. Phys. A 2016, 122, 725. [Google Scholar] [CrossRef]
- Radtke, G.; Lazar, S.; Botton, G.A. High-resolution EELS investigation of the electronic structure of ilmenites. Phys. Rev. B 2006, 74, 155117. [Google Scholar] [CrossRef]
- Lacz, A.; Lach, R.; Drozdz, E. Effect of nickel addition on the physicochemical properties of SrTiO3-based materials. Ceram. Int. 2022, 48, 24906–24914. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Monllor-Satoca, D.; Vinoth, V.; Anandan, S.; Lana-Villarreal, T. Electrochemical Doping as a Way to Enhance Water Photooxidation on Nanostructured Nickel Titanate and Anatase Electrodes. ChemElectroChem 2017, 4, 1429–1435. [Google Scholar] [CrossRef]
- Ruiz Preciado, M.A.; Kassiba, A.; Morales-Acevedo, A.; Makowska-Janusik, M. Vibrational and electronic peculiarities of NiTiO3 nanostructures inferred from first principle calculations. RSC Adv. 2015, 5, 17396–17404. [Google Scholar] [CrossRef]
- Moghtada, A.; Shahrouzianfar, A.; Ashiri, R. Facile synthesis of NiTiO3 yellow nano-pigments with enhanced solar radiation reflection efficiency by an innovative one-step method at low temperature. Dye. Pigment. 2017, 139, 388–396. [Google Scholar] [CrossRef]
- Huang, H.; Liu, X.; Li, F.; He, Q.; Ji, H.; Yu, C. In situ construction of a 2D CoTiO3/g-C3N4 photocatalyst with an S-scheme heterojunction and its excellent performance for CO2 reduction. Sustain. Energy Fuels 2022, 6, 4903–4915. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Da Pinto Silva, L.; Zegaoui, O.; Da Esteves Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef]
- Fu, C.; Liu, L.; Wei, Y.; Huang, W.; Zhao, G. Linking the Doping-Induced Trap States to the Concentration of Surface-Reaching Photoexcited Holes in Transition-Metal-Doped TiO2 Nanoparticles. J. Phys. Chem. Lett. 2024, 15, 6504–6511. [Google Scholar] [CrossRef]
- Lakshmi, T.; Hajarabeevi, N.; Mishra, T.; Aman, N. Synthesis of cobalt doped nickel titanate nanomaterials for enhanced photocatalytic reduction of chromium. Optik 2024, 298, 171561. [Google Scholar] [CrossRef]
- Alam, U.; Pandey, A.; Verma, N. An anthraquinone-integrated S-scheme-based NiTiO3-g-C3N4 composite with enhanced hydrogen production activity. Int. J. Hydrogen Energy 2023, 48, 2532–2541. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).