Synergetic Effect of FeTi in Enhancing the Hydrogen-Storage Kinetics of Nanocrystalline MgH2
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructural Characterization
2.2.1. Scanning Electron Microscopy
2.2.2. X-ray Diffraction
2.3. Thermal Characterization
2.4. Hydrogen-Storage Experiments
3. Results and Discussion
Characterization of the As-Milled Powders
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Key World Energy Statistics. 2020. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 15 January 2024).
- Jackson, R.B.; Friedlingstein, P. Persistent fossil fuel growth threatens the Paris Agreement and planetary health. Environ. Res. Lett. 2019, 14, 12001. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A Comprehensive Review of the Promising Clean Energy Carrier: Hydrogen Production, Transportation, Storage, and Utilization (HPTSU) Technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 Economy Realizable in the Foreseeable Future? Part III: H2 Usage Technologies, Applications, and Challenges and Opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef] [PubMed]
- US DOE Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 15 January 2024).
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Muthukumar, P.; Kumar, A.; Afzal, M.; Bhogilla, S.; Sharma, P.; Parida, A.; Jana, S.; Kumar, E.A.; Pai, R.K.; Jain, I.P. Review on Large-Scale Hydrogen Storage Systems for Better Sustainability. Int. J. Hydrogen Energy 2023, 48, 33223–33259. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen Storage and Delivery: Review of the State of the Art Technologies and Risk and Reliability Analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Schlabach, L.; Zuttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Lin, H.-J.; Lu, Y.-S.; Zhang, L.-T.; Liu, H.-Z.; Edalati, K.; Révész, Á. Recent Advances in Metastable Alloys for Hydrogen Storage: A Review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Drawer, C.; Lange, J.; Kaltschmitt, M. Metal Hydrides for Hydrogen Storage—Identification and Evaluation of Stationary and Transportation Applications. J. Energy Storage 2024, 77, 109988. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A Review on Metal Hydride Materials for Hydrogen Storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of Magnesium Hydride-Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and Kinetics of Hydriding and Dehydriding Reactions in Mg-Based Hydrogen Storage Materials. J. Magnes. Alloys 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Li, Q.; Peng, X.; Pan, F. Magnesium-Based Materials for Energy Conversion and Storage. J. Magnes. Alloys 2021, 9, 2223–2224. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. Improved H-Storage Performance of Novel Mg-Based Nanocomposites Prepared by High-Energy Ball Milling: A Review. Energies 2021, 14, 6400. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in Magnesium: New Perspectives toward Functional Stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Pasquini, L. The Effects of Nanostructure on the Hydrogen Sorption Properties of Magnesium-Based Metallic Compounds: A Review. Crystals 2018, 8, 106. [Google Scholar] [CrossRef]
- Gupta, A.; Faisal, M. Magnesium Based Multi-Metallic Hybrids with Soot for Hydrogen Storage. Int. J. Hydrogen Energy 2024, 53, 93–104. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest Research Advances on Magnesium and Magnesium Alloys Worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Botta, W.J.; Estrin, Y.; Floriano, R.; Fruchart, D.; Grosdidier, T.; Horita, Z.; Huot, J.; Li, H.W.; et al. Impact of Severe Plastic Deformation on Kinetics and Thermodynamics of Hydrogen Storage in Magnesium and Its Alloys. J. Mater. Sci. Technol. 2023, 146, 221–239. [Google Scholar] [CrossRef]
- Edalati, K.; Bachmaier, A.; Beloshenko, V.A.; Beygelzimer, Y.; Blank, V.D.; Botta, W.J.; Bryła, K.; Čížek, J.; Divinski, S.; Enikeev, N.A.; et al. Nanomaterials by Severe Plastic Deformation: Review of Historical Developments and Recent Advances. Mater. Res. Lett. 2022, 10, 163–256. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Plocinski, T. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles. Int. J. Hydrogen Energy 2008, 33, 1859–1867. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Fátay, D.; Spassov, T.; Delchev, P.; Ribárik, G.; Révész, Á. Microstructural development in nanocrystalline MgH2 during H-absorption/desorption cycling. Int. J. Hydrogen Energy 2007, 32, 2914–2919. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Révész, Á.; Fátay, D. Microstructural evolution of ball-milled MgH2 during a complete dehydrogenation–hydrogenation cycle. J. Power Sources 2010, 195, 6997–7002. [Google Scholar] [CrossRef]
- Novakovic, J.G.; Novakovic, N.; Kurko, S.; Govedarovic, S.M.; Pantic, T.; Mamula, B.P.; Batalovic, K.; Radakovic, J.; Rmus, J.; Shelyapina, M.; et al. Influence of defects on the stability and hydrogen-sorption behavior of Mg-based hydrides. Chem. Phys. Chem. 2019, 20, 1216–1247. [Google Scholar] [CrossRef]
- Révész, Á.; Kánya, Z.; Verebélyi, T.; Szabó, P.J.; Zhilyaev, A.P.; Sapssov, T. The effect of high-pressure torsion on the microstructure and hydrogen absorption kinetics of ball-milled Mg70Ni30. J. Alloys Compd. 2010, 504, 83–88. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, L.; Zhang, W.; Liu, H.; Huang, Z.; Yang, L.; Gu, C.; Sun, W.; Gao, M.; et al. Recent Advances in Catalyst-Modified Mg-Based Hydrogen Storage Materials. J. Mater. Sci. Technol. 2023, 163, 182–211. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-Based Materials for Hydrogen Storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Khan, D.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of nanocrystalline Mg2Ni prepared from compressed 2MgH2–Ni powder. Int. J. Hydrogen Energy 2018, 43, 22391–22400. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, L.; Huang, Y.; Gao, P.; Jiao, L.; Yuan, H.; Wang, Y. Improved hydrogen storage properties of MgH2 with Ni-based compounds. Int. J. Hydrogen Energy 2017, 42, 24247–24255. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Spassov, T. Microstructural evolution of ball-milled Mg–Ni powder during hydrogen sorption. Int. J. Hydrogen Energy 2013, 38, 8342–8349. [Google Scholar] [CrossRef]
- Antiqueira, F.J.; Leiva, D.R.; Zepon, G.; de Cunha, B.F.R.F.; Figueroa, S.J.A.; Botta, W.J. Fast Hydrogen Absorption/Desorption Kinetics in Reactive Milled Mg-8 Mol% Fe Nanocomposites. Int. J. Hydrogen Energy 2020, 45, 12408–12418. [Google Scholar] [CrossRef]
- Wan, H.; Fang, D.; Zhou, S.; Yang, X.; Dai, Y.; Ran, L.; Chen, Y.; Pan, F. Enhanced Dehydrogenation Properties and Mechanism Analysis of MgH2 Solid Solution Containing Fe Nano-Catalyst. Int. J. Hydrogen Energy 2023, 48, 34180–34191. [Google Scholar] [CrossRef]
- Song, M.; Zhang, L.; Yao, Z.; Zheng, J.; Shang, D.; Chen, L.; Li, H. Unraveling the Degradation Mechanism in Hydrogen Storage Property of Fe Nanocatalysts Modified MgH2. Inorg. Chem. Front. 2022, 9, 3874–3884. [Google Scholar] [CrossRef]
- Malahayati; Nurmalita; Ismail; Machmud, M.N.; Jalil, Z. Sorption Behavior of MgH2-Ti for Hydrogen Storage Material Prepared by High Pressure Milling. J. Phys. Conf. Ser. 2021, 1882, 012005. [Google Scholar] [CrossRef]
- Shahi, R.; Tiwari, A.; Shaz, M.; Srivastava, O.N. Studies on de/Rehydrogenation Characteristics of Nanocrystalline MgH2 Co-Catalyzed with Ti, Fe and Ni. Int. J. Hydrogen Energy 2013, 38, 2778–2784. [Google Scholar] [CrossRef]
- Yun, H.; Wang, H.; Bai, J.; Wang, X.; Dai, X.; Hou, X.; Xu, Y. Activation and Hydrogen Storage Properties of Mg-Based Composites Synthesized by Catalytic Mechanochemical Hydrogenation Strategy. Int. J. Hydrogen Energy 2024, 50, 1025–1039. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kis, V.K.; Schafler, E.; Révész, Á. Microstructural and Morphological Investigations on Mg-Nb2O5-CNT Nanocomposites Processed by High-Pressure Torsion for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2020, 45, 7917–7928. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwon, I.H.; Kwon, S.N.; Park, C.G.; Park, H.R.; Bae, J.-S. Preparation of hydrogen-storage alloy Mg–10wt% Fe2O3 under various milling conditions. Int. J. Hydrogen Energy 2006, 31, 43–47. [Google Scholar] [CrossRef]
- Campos, R.B.V.; Camargo, S.A.S., Jr.; Brum, M.C.; Dos Santos, D.S. Hydrogen uptake enhancement by the use of a magnesium hydride and carbon nanotubes mixture. Mater. Res. 2017, 20, 85–88. [Google Scholar] [CrossRef]
- Varin, R.; Zaranski, Z.; Czujko, T.; Polanski, M.; Wronski, Z. The Composites of Magnesium Hydride and Iron-Titanium Intermetallic. Int. J. Hydrogen Energy 2011, 36, 1177. [Google Scholar] [CrossRef]
- Guoxian, L.; Erde, W.; Shoushi, F. Hydrogen absorption and desorption characteristics of mechanically milled Mg-35wt.% FeTi1.2 powders. J. Alloys Compd. 1995, 223, 111–114. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; He, J.; Zheng, J.; Wu, F.; Chen, L. Achieving Superior Hydrogen Storage Properties of MgH2 by the Effect of TiFe and Carbon Nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Vijay, R.; Sundaresan, R.; Maiya, M.P.; Murthy, S.; Fu, Y.; Klein, H.-P.; Groll, M. Characterisation of Mg–x Wt.% FeTi (x = 5–30) and Mg–40wt.% FeTiMn Hydrogen Absorbing Materials Prepared by Mechanical Alloying. J. Alloys Compd. 2004, 384, 283–295. [Google Scholar] [CrossRef]
- Révész, Á.; Paramonov, R.; Spassov, T.; Gajdics, M. Microstructure and Hydrogen Storage Performance of Ball-Milled MgH2 Catalyzed by FeTi. Energies 2023, 16, 1061. [Google Scholar] [CrossRef]
- Kondo, T.; Shindo, K.; Sakurai, Y. Dependence of hydrogen storage characteristics of Mg–TiFe0.92Mn0.08 composite on amount of TiFe0.92Mn0.08. J. Alloys Compd. 2005, 404, 511–514. [Google Scholar] [CrossRef]
- Neto, R.M.L.; de Araújo Silva, R.; Floriano, R.; Coutinho, G.C.S.; Falcão, R.B.; Leiva, D.R.; Botta Filho, W.J. Synthesis by High-Energy Ball Milling of MgH2-TiFe Composites for Hydrogen Storage. Mater. Sci. Forum 2017, 899, 13–18. [Google Scholar] [CrossRef]
- Silva, R.; Lean Neto, R.L.; Leiva, D.R.; Ishikawa; Kiminami, C.S.; Jorge, A.M., Jr.; Botta, W.J. Room temperature hydrogen absorption by Mg and Mg TiFe nanocomposites processed by high-energy ball milling. Int. J. Hydrogen Energy 2018, 43, 12251–12259. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. An Overview of TiFe Alloys for Hydrogen Storage: Structure, Processes, Properties, and Applications. J. Energy Storage 2023, 68, 107772. [Google Scholar] [CrossRef]
- Shang, H.; Sheng, P.; Li, J.; Zhang, W.; Zhang, X.; Guo, S.; Li, Y.; Zhang, Y. Characteristics of Hydrogen Storage of As-Milled TiFe-Based Alloys. Int. J. Hydrogen Energy 2023, 50, 190–200. [Google Scholar] [CrossRef]
- Falcão, R.B.; Dammann, E.D.; Rocha, C.J.; Durazzo, M.; Ichikawa, R.U.; Martinez, L.G.; Botta, W.J.; Neto, R.M.L. An alternative route to produce easily activated nanocrystalline TiFe powder. Int. J. Hydrogen Energy 2018, 43, 16107–16116. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Arita, M.; Daio, T.; Akiba, E.; Horita, Z. Mechanism of activation of TiFe intermetallics for hydrogen storage by severe plastic deformation using high-pressure torsion. Appl. Phys. Lett. 2013, 103, 143902. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Beatrice, C.A.G.; Neto, R.M.L.; Silva, W.B.; Pessan, L.A.; Botta, W.J.; Leiva, D.R. Hydrogen Absorption/Desorption Behavior of a Cold-Rolled TiFe Intermetallic Compound. Mater. Res. 2021, 24, e20210204. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion: Similarities and differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Sandrock, G.; Suda, S.; Schlapbach, L. Applications. In Hydrogen in Intermetallic Compunds II; Topics in Applied Physics; Schlapbach, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 67, pp. 197–258. [Google Scholar] [CrossRef]
- Guinet, P.; Perroud, P.; Rebiere, J. Technological aspects and characteristics of industrial hydride reservoirs. Int. J. Hydrogen Energy 1980, 5, 609–618. [Google Scholar] [CrossRef]
- Fruchart, D.; Jehan, M.; Skryabina, N.; de Rango, P. Hydrogen Solid State Storage on MgH2 Compacts for Mass Applications. Metals 2023, 13, 992. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kovács Kis, V.; Béke, F.; Novák, Z.; Schafler, E.; Révész, Á. Microstructural Investigation of Nanocrystalline Hydrogen-Storing Mg-Titanate Nanotube Composites Processed by High-Pressure Torsion. Energies 2020, 13, 563. [Google Scholar] [CrossRef]
- Ribárik, G.; Jóni, B.; Ungár, T. Global optimum of microstructure parameters in the CMWP line-profile-analysis method by combining Marquardt-Levenberg and Monte-Carlo procedures. J. Mater. Sci. Technol. 2019, 35, 1508–1514. [Google Scholar] [CrossRef]
- Ribárik, G.; Gubicza, J.; Ungár, T. Correlation between strength and microstructure of ball-milled Al–Mg alloys determined by X-ray diffraction. Mater. Sci. Eng. A 2004, 387, 343–347. [Google Scholar] [CrossRef]
- Révész, Á.; Spassov, T.; Kis, V.; Schafler, E.; Gajdics, M. The Influence of Preparation Conditions on the Hydrogen Sorption of Mg-Nb2O5-CNT Produced by Ball Milling and Subsequent High-Pressure Torsion. J. Nanosci. Nanotechnol. 2020, 20, 4587–4590. [Google Scholar] [CrossRef] [PubMed]
- Haraki, T.; Oishi, K.; Uchida, H.; Miyamoto, Y.; Abe, M.; Kokaji, T.; Uchida, S. Properties of Hydrogen Absorption by Nano-Structured FeTi Alloys. Int. J. Mater. Res. 2008, 99, 507–512. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Pergamon: Oxford, UK, 1975. [Google Scholar]
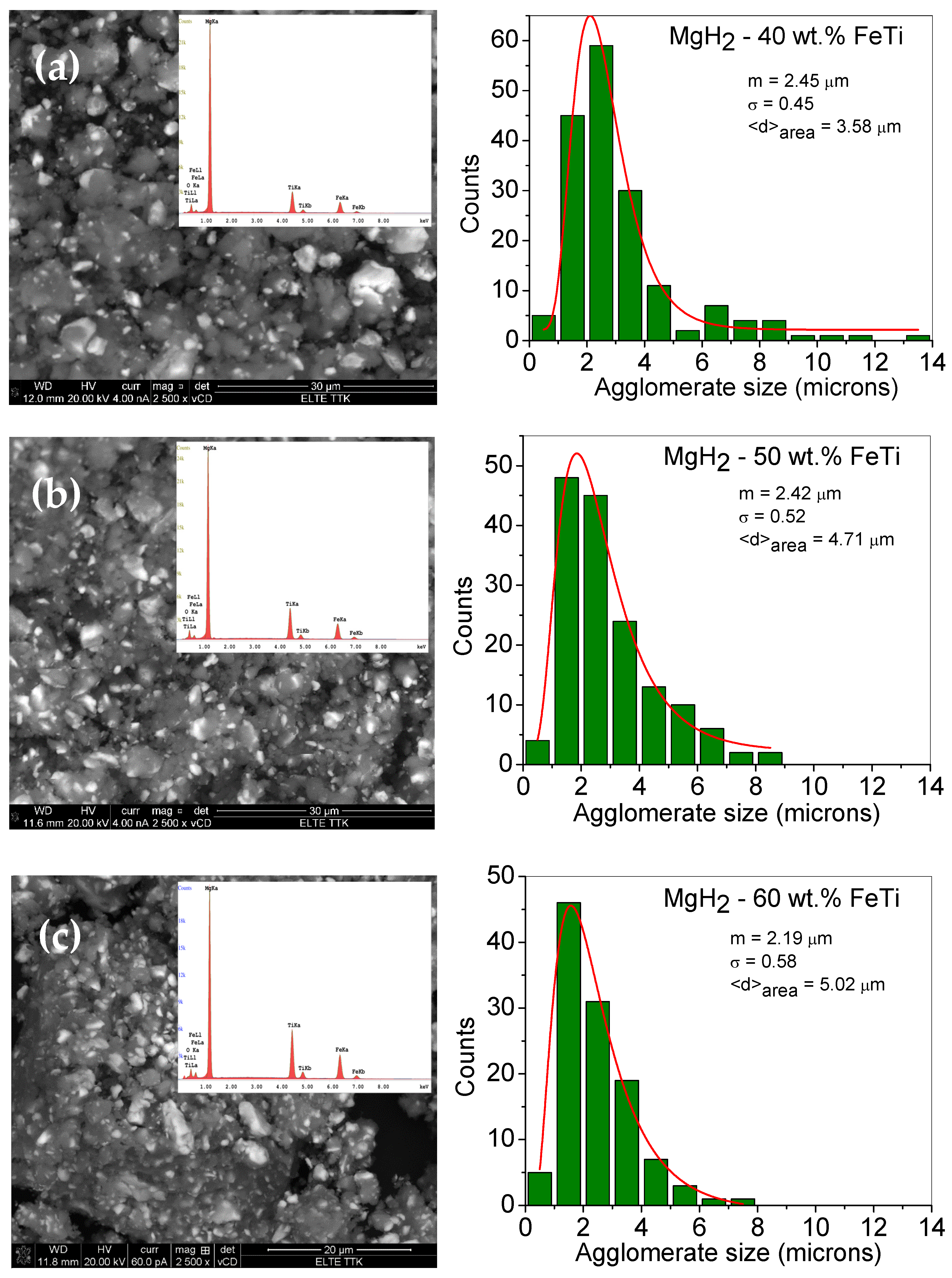



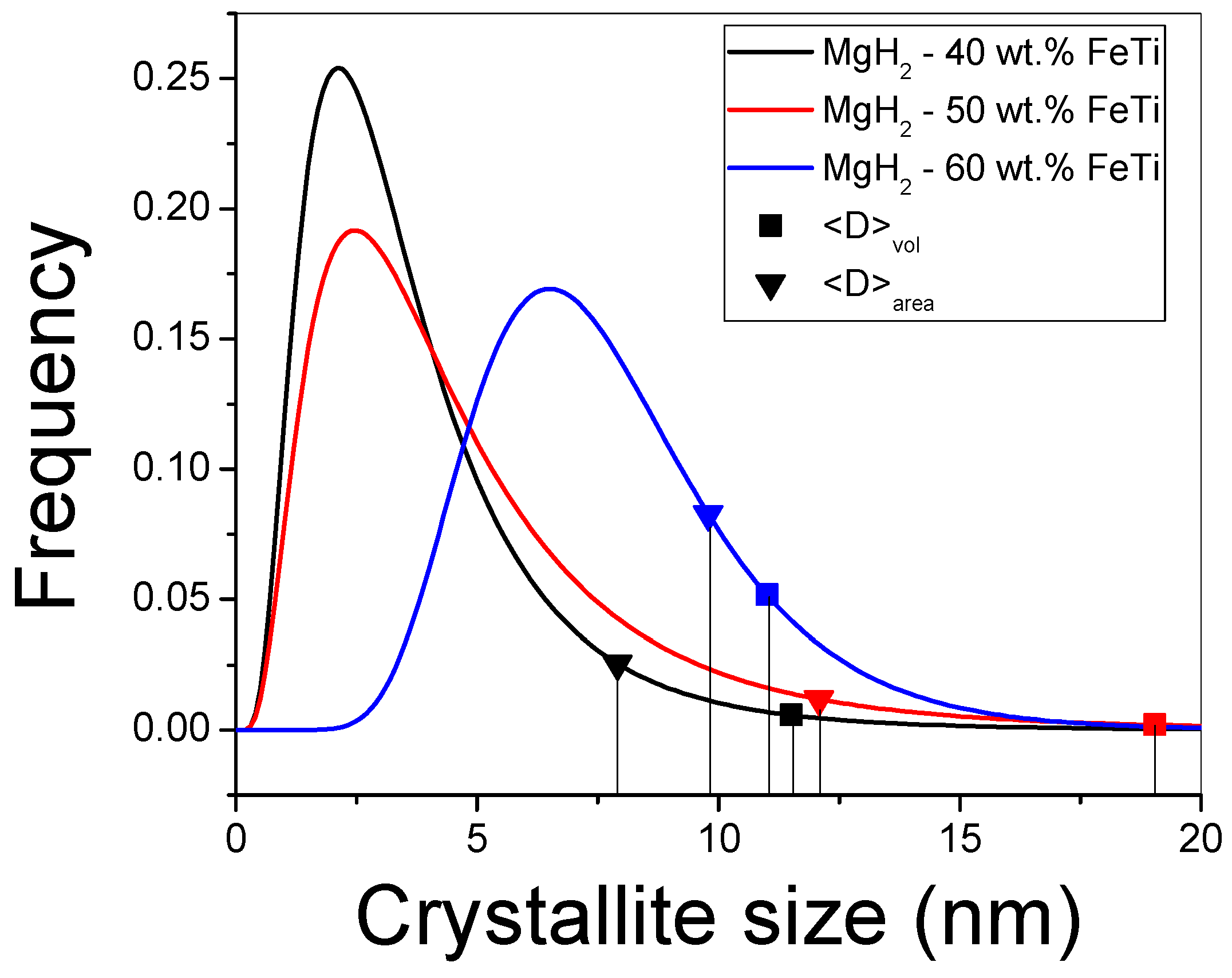
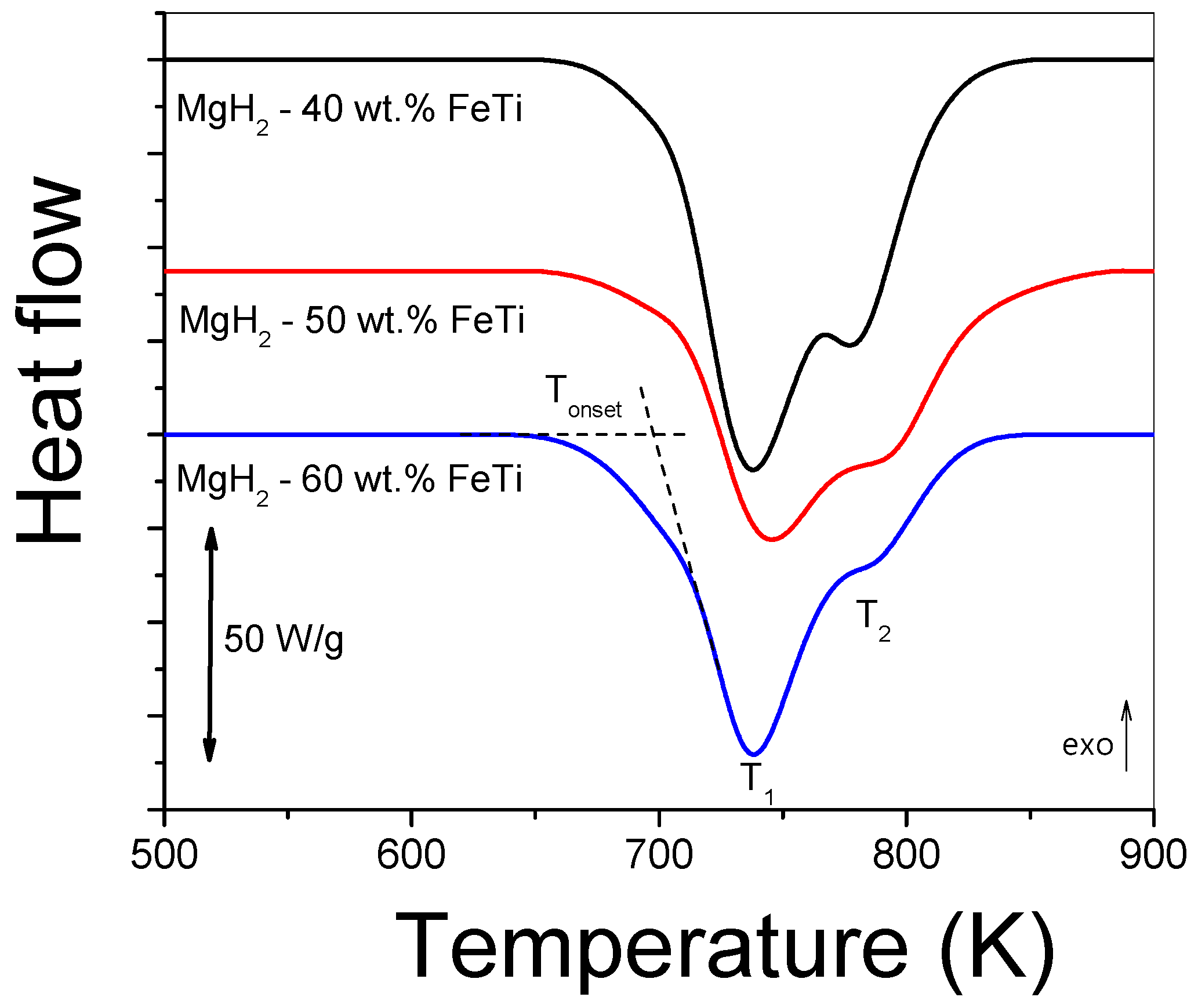

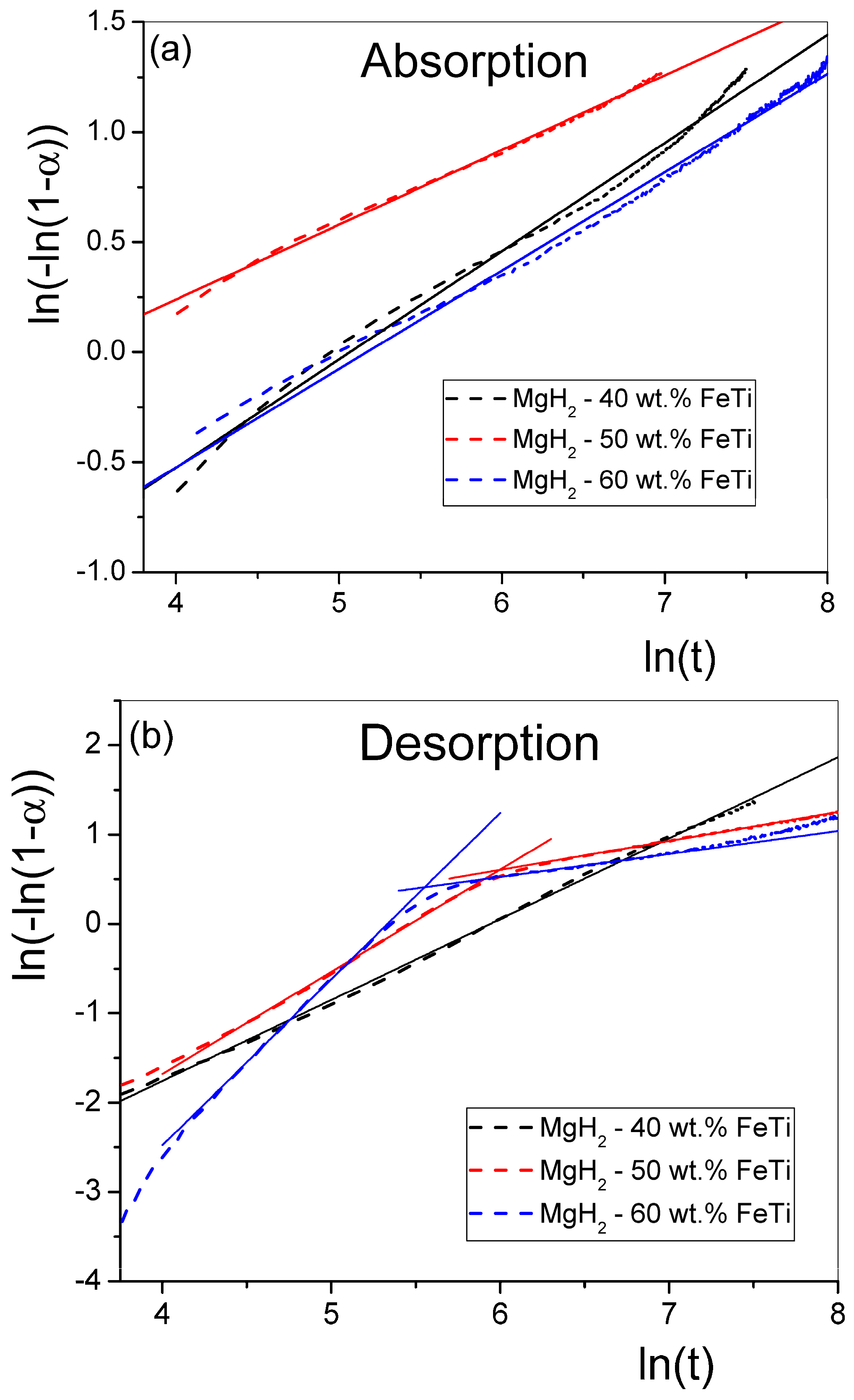
| Elements | MgH2-40 wt.% FeTi | MgH2-50 wt.% FeTi | MgH2-60 wt.% FeTi |
|---|---|---|---|
| Mg | 68.14 | 61.50 | 53.28 |
| Ti | 15.70 | 18.91 | 23.12 |
| Fe | 16.16 | 19.59 | 23.61 |
| Elements, wt.% | A | B | C | D |
|---|---|---|---|---|
| Mg | 49.58 | 69.24 | 56.51 | 50.92 |
| Ti | 24.89 | 15.64 | 20.69 | 25.29 |
| Fe | 25.53 | 15.12 | 22.80 | 23.79 |
| mCMWP (nm) | σCMWP | 〈D〉area (nm) | 〈D〉vol (nm) | |
|---|---|---|---|---|
| MgH2-40 wt.% FeTi | 3.10 | 0.61 | 7.9 | 11.5 |
| MgH2-50 wt.% FeTi | 3.87 | 0.67 | 12.1 | 19.0 |
| MgH2-60 wt.% FeTi | 7.30 | 0.34 | 9.8 | 11.0 |
| Powder | Tonset (K) | T1 (K) | T2 (K) | ∆Htot (Jg−1) |
|---|---|---|---|---|
| MgH2-40 wt.% FeTi | 702 | 738 | 778 | −9646 |
| MgH2-50 wt.% FeTi | 703 | 746 | 787 | −7471 |
| MgH2-60 wt.% FeTi | 696 | 738 | 781 | −7028 |
| Powder | Total Absorbed Hydrogen (wt.%) | Absorbed Hydrogen at 100 s (wt.%) | Total Desorbed Hydrogen (wt.%) | Desorbed Hydrogen at 200 s (wt.%) |
|---|---|---|---|---|
| MgH2-40 wt.% FeTi | 5.8 | 3.3 | 5.5 | 2.2 |
| MgH2-50 wt.% FeTi | 5.5 | 4.3 | 5.1 | 2.9 |
| MgH2-60 wt.% FeTi | 2.2 | 1.3 | 2.1 | 1.3 |
| Powder | JMA Absorption Exponent [n] | JMA Desorption Exponent [n] | |
|---|---|---|---|
| MgH2-40 wt.% FeTi | 0.49 | 0.95 | |
| MgH2-50 wt.% FeTi | 0.34 | 1.14 | 0.32 |
| MgH2-60 wt.% FeTi | 0.45 | 1.86 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramonov, R.; Spassov, T.; Nagy, P.; Révész, Á. Synergetic Effect of FeTi in Enhancing the Hydrogen-Storage Kinetics of Nanocrystalline MgH2. Energies 2024, 17, 794. https://doi.org/10.3390/en17040794
Paramonov R, Spassov T, Nagy P, Révész Á. Synergetic Effect of FeTi in Enhancing the Hydrogen-Storage Kinetics of Nanocrystalline MgH2. Energies. 2024; 17(4):794. https://doi.org/10.3390/en17040794
Chicago/Turabian StyleParamonov, Roman, Tony Spassov, Péter Nagy, and Ádám Révész. 2024. "Synergetic Effect of FeTi in Enhancing the Hydrogen-Storage Kinetics of Nanocrystalline MgH2" Energies 17, no. 4: 794. https://doi.org/10.3390/en17040794
APA StyleParamonov, R., Spassov, T., Nagy, P., & Révész, Á. (2024). Synergetic Effect of FeTi in Enhancing the Hydrogen-Storage Kinetics of Nanocrystalline MgH2. Energies, 17(4), 794. https://doi.org/10.3390/en17040794







