Reuse and Valorization of Solid Digestate Ashes from Biogas Production
Abstract
1. Introduction
- Digestate characteristics, management, and current issues
- Ash formation by thermochemical processes of the digestate
- by combustion
- by pyrolysis
- by gasification
- Prospective reuse of ashes
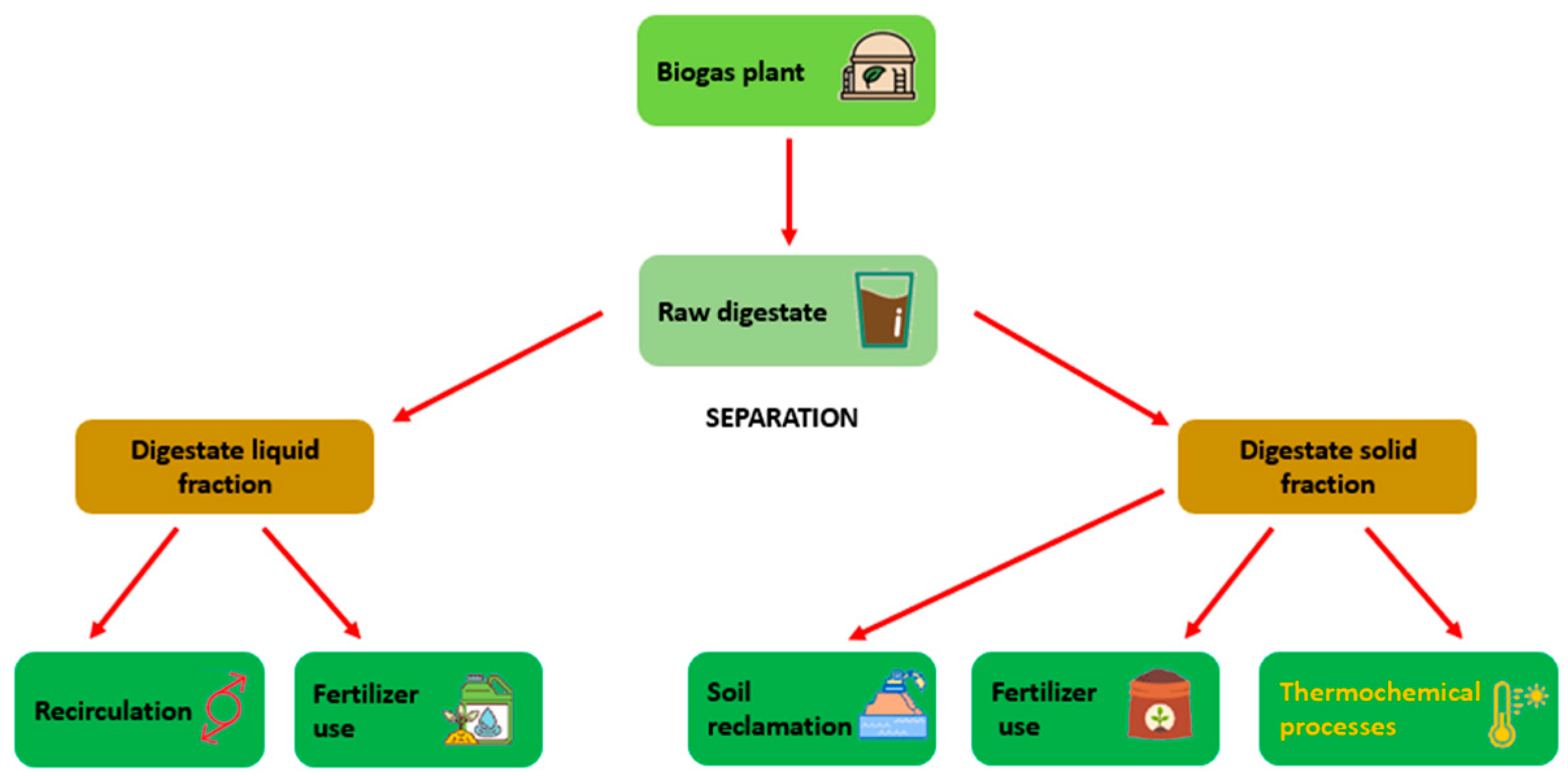
2. Digestate Characteristics and Current Issues
2.1. Digestate Characteristics
2.2. Digestate Management and Current Issues
3. Ash Formation by Thermochemical Processes of the Digestate
3.1. Ash Formation by Combustion Process
3.2. Ash Formation by Pyrolysis Process
3.2.1. Ash Formation by Pyrolysis from Food Waste and OFMSW Digestate
3.2.2. Ash Formation by Pyrolysis from Animal Manure and Agricultural Waste Digestate
3.2.3. Ash Formation by Pyrolysis Digestates Obtained from Sewage Sludge and a Mixture of Different Feedstocks
3.3. Ash Formation by Gasification Process
4. Prospectives for the Reuse of Ashes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as Potential Feedstock for the Production of Biofuels and Value-Added Products: Opportunities and Challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and Utilization of Carbon Dioxide by Chemical and Biological Methods for Biofuels and Biomaterials by Chemoautotrophs: Opportunities and Challenges. Bioresour. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid Waste Issue: Sources, Composition, Disposal, Recycling, and Valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Montt, G.; Fraga, F.; Harsdorff, M. The Future of Work in a Changing Natural Environment: Climate Change, Degradation and Sustainability; Publications of the International Labour Office: Geneva, Switzerland, 2018; ISBN 9789220312070. [Google Scholar]
- Ignatowicz, K.; Filipczak, G.; Dybek, B.; Wałowski, G. Biogas Production Depending on the Substrate Used: A Review and Evaluation Study—European Examples. Energies 2023, 16, 798. [Google Scholar] [CrossRef]
- Vilakazi, S.; Onyari, E.; Nkwonta, O.; Bwapwa, J.K. Reuse of Domestic Sewage Sludge to Achieve a Zero Waste Strategy & Improve Concrete Strength & Durabilit—A Review. S. Afr. J. Chem. Eng. 2023, 43, 122–127. [Google Scholar] [CrossRef]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting Processes for Agricultural Waste Management: A Comprehensive Review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Dutta, S.; He, M.; Xiong, X.; Tsang, D.C.W. Sustainable Management and Recycling of Food Waste Anaerobic Digestate: A Review. Bioresour. Technol. 2021, 341, 125915. [Google Scholar] [CrossRef] [PubMed]
- Baus, D. Overpopulation and the Impact on the Environment; CUNY Academic Works: New York, NY, USA, 2017. [Google Scholar]
- Ogunmoroti, A.; Liu, M.; Li, M.; Liu, W. Unraveling the Environmental Impact of Current and Future Food Waste and Its Management in Chinese Provinces. Resour. Environ. Sustain. 2022, 9, 100064. [Google Scholar] [CrossRef]
- Surendra, K.C.; Takara, D.; Hashimoto, A.G.; Khanal, S.K. Biogas as a Sustainable Energy Source for Developing Countries: Opportunities and Challenges. Renew. Sustain. Energy Rev. 2014, 31, 846–859. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, J.; Liu, Y.; Mancl, K. Biogas as a Sustainable Energy Source in China: Regional Development Strategy Application and Decision Making. Renew. Sustain. Energy Rev. 2014, 35, 294–303. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.-H.; Vikrant, K. Value-Added Chemicals from Food Supply Chain Wastes: State-of-the-Art Review and Future Prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Biological Treatment of Organic Materials for Energy and Nutrients Production—Anaerobic Digestion and Composting. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–181. [Google Scholar]
- Kaur, G.; Wong, J.W.C.; Kumar, R.; Patria, R.D.; Bhardwaj, A.; Uisan, K.; Johnravindar, D. Value Addition of Anaerobic Digestate from Biowaste: Thinking Beyond Agriculture. Curr. Sustain. Renew. Energy Rep. 2020, 7, 48–55. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Al-Wahaibi, A.; Osman, A.I.; Al-Muhtaseb, A.H.; Alqaisi, O.; Baawain, M.; Fawzy, S.; Rooney, D.W. Techno-Economic Evaluation of Biogas Production from Food Waste via Anaerobic Digestion. Sci. Rep. 2020, 10, 15719. [Google Scholar] [CrossRef] [PubMed]
- Plana, P.V.; Noche, B. A Review of the Current Digestate Distribution Models: Storage and Transport. WIT Trans. Ecol. Environ. 2016, 202, 345–357. [Google Scholar]
- Thiruselvi, D.; Kumar, P.S.; Kumar, M.A.; Lay, C.-H.; Aathika, S.; Mani, Y.; Jagadiswary, D.; Dhanasekaran, A.; Shanmugam, P.; Sivanesan, S.; et al. A Critical Review on Global Trends in Biogas Scenario with Its Up-Gradation Techniques for Fuel Cell and Future Perspectives. Int. J. Hydrogen Energy 2021, 46, 16734–16750. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, P.S.; Vo, D.-V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas Upgrading, Economy and Utilization: A Review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Gao, M.; Wang, D.; Wang, Y.; Wang, X.; Feng, Y. Opportunities and Challenges for Biogas Development: A Review in 2013–2018. Curr. Pollut. Rep. 2019, 5, 25–35. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The Future of Anaerobic Digestion and Biogas Utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Heerenklage, J.; Rechtenbach, D.; Atamaniuk, I.; Alassali, A.; Raga, R.; Koch, K.; Kuchta, K. Development of a Method to Produce Standardised and Storable Inocula for Biomethane Potential Tests—Preliminary Steps. Renew. Energy 2019, 143, 753–761. [Google Scholar] [CrossRef]
- Stolze, Y.; Bremges, A.; Maus, I.; Pühler, A.; Sczyrba, A.; Schlüter, A. Targeted In Situ Metatranscriptomics for Selected Taxa from Mesophilic and Thermophilic Biogas Plants. Microb. Biotechnol. 2018, 11, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Piech, K.; Kuczera, M. Zagadnienia Aktualnie Poruszane Przez Młodych Naukowców; Creativetime: Krakow, Poland, 2015; Volume 2, ISBN 9788363058470. [Google Scholar]
- Available online: https://energy.ec.europa.eu/topics/renewable-energy/bioenergy/biomethane_en (accessed on 20 December 2023).
- Ignatowicz, K.; Piekarski, J.; Kogut, P. Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions. Energies 2021, 14, 5948. [Google Scholar] [CrossRef]
- Sica, D.; Esposito, B.; Supino, S.; Malandrino, O.; Sessa, M.R. Biogas-Based Systems: An Opportunity towards a Post-Fossil and Circular Economy Perspective in Italy. Energy Policy 2023, 182, 113719. [Google Scholar] [CrossRef]
- Sobczak, A.; Chomać-Pierzecka, E.; Kokiel, A.; Różycka, M.; Stasiak, J.; Soboń, D. Economic Conditions of Using Biodegradable Waste for Biogas Production, Using the Example of Poland and Germany. Energies 2022, 15, 5239. [Google Scholar] [CrossRef]
- Refai, S. Development of Efficient Tools for Monitoring and Improvement of Biogas Production. Ph.D. Thesis, Universitäts-und Landesbibliothek Bonn, Bonn, Germany, 2017. [Google Scholar]
- Czajka, K.; Kawalec, W.; Król, R.; Sówka, I. Modelling and Calculation of Raw Material Industry. Energies 2022, 15, 5035. [Google Scholar] [CrossRef]
- Alghashm, S.; Song, L.; Liu, L.; Ouyang, C.; Zhou, J.L.; Li, X. Improvement of Biogas Production Using Biochar from Digestate at Different Pyrolysis Temperatures during OFMSW Anaerobic Digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Al Seadi, T. Good Practice in Quality Management of AD Residues from Biogas Production: Task 24 Og AEA Technology Environment; IEA Bioenergy: Esbjerg, Denmark, 2001. [Google Scholar]
- Wellinger, A.; Murphy, J.D.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 0857097415. [Google Scholar]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic Assessment of Pyrolysed Food Waste Digestate for Sandy Soil Management. J. Environ. Manage. 2017, 187, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of Biochar from Food Waste Digestate: Pyrolysis Behavior and Product Properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Fang, Y.Y.; Cheng, P.H.; Lin, Y.Q. Characterization of Mesoporous Biochar Produced from Biogas Digestate Implemented in an Anaerobic Process of Large-Scale Hog Farm. Biomass Convers. Biorefin. 2018, 8, 945–951. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tsai, W.T.; Chen, J.W.; Lin, Y.Q.; Chang, Y.M. Characterization of Biochar Prepared from Biogas Digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Tayibi, S.; Monlau, F.; Marias, F.; Thevenin, N.; Jimenez, R.; Oukarroum, A.; Alboulkas, A.; Zeroual, Y.; Barakat, A. Industrial Symbiosis of Anaerobic Digestion and Pyrolysis: Performances and Agricultural Interest of Coupling Biochar and Liquid Digestate. Sci. Total Environ. 2021, 793, 148461. [Google Scholar] [CrossRef]
- Feng, Y.; Bu, T.; Zhang, Q.; Han, M.; Tang, Z.; Yuan, G.; Chen, D.; Hu, Y. Pyrolysis Characteristics of Anaerobic Digestate from Kitchen Waste and Availability of Phosphorus in Pyrochar. J. Anal. Appl. Pyrolysis. 2022, 168, 105729. [Google Scholar] [CrossRef]
- Basinas, P.; Rusín, J.; Chamrádová, K.; Kaldis, S.P. Pyrolysis of the Anaerobic Digestion Solid By-Product: Characterization of Digestate Decomposition and Screening of the Biochar Use as Soil Amendment and as Additive in Anaerobic Digestion. Energy Convers. Manag. 2023, 277, 116658. [Google Scholar] [CrossRef]
- Kratzeisen, M.; Starcevic, N.; Martinov, M.; Maurer, C.; Müller, J. Applicability of Biogas Digestate as Solid Fuel. Fuel 2010, 89, 2544–2548. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of Residence Time during Hydrothermal Carbonization of Biogas Digestate on the Combustion Characteristics of Hydrochar and the Biogas Production of Process Water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and Activated Carbon Production by Hydrothermal Carbonization of Digestate. Biomass Convers. Biorefin. 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy Metals in Source-Separated Compost and Digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, A.; Ahlborn, J.; Hamscher, G. Simultaneous Determination of 14 Sulfonamides and Tetracyclines in Biogas Plants by Liquid-Liquid-Extraction and Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2014, 406, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Govasmark, E.; Stäb, J.; Holen, B.; Hoornstra, D.; Nesbakk, T.; Salkinoja-Salonen, M. Chemical and Microbiological Hazards Associated with Recycling of Anaerobic Digested Residue Intended for Agricultural Use. Waste Manag. 2011, 31, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B.; Fuchs, W.; Al, T.; Madsen, S.M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; IEA Bioenergy: Esbjerg, Denmark, 2015. [Google Scholar]
- WOOD, Wood Environment & Infrastructure Solutions UK Limited. Digestate and Compost as Fertilizers: Risk Assessment and Risk Management Options; Final Report; European Commission: London, UK, 2019. [Google Scholar]
- European Commission; Joint Research Centre; Institute for Environment and Sustainability. Biochar Application to Soils: A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Czekała, W.; Nowak, M.; Piechota, G. Sustainable Management and Recycling of Anaerobic Digestate Solid Fraction by Composting: A Review. Bioresour. Technol. 2023, 375, 128813. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing Amendment Properties of Digestate by Studying the Organic Matter Composition and the Degree of Biological Stability during the Anaerobic Digestion of the Organic Fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing Potential of Separated Biogas Digestates in Annual and Perennial Biomass Production Systems. Front. Sustain. Food Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Catenacci, A.; Boniardi, G.; Mainardis, M.; Gievers, F.; Farru, G.; Asunis, F.; Malpei, F.; Goi, D.; Cappai, G.; Canziani, R. Processes, Applications and Legislative Framework for Carbonized Anaerobic Digestate: Opportunities and Bottlenecks. A Critical Review. Energy Convers. Manag. 2022, 263, 115691. [Google Scholar] [CrossRef]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, Nitrous Oxide and Ammonia Emissions during Storage and after Application of Dairy Cattle Slurry and Influence of Slurry Treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Tiwary, A.; Williams, I.D.; Pant, D.C.; Kishore, V.V.N. Emerging Perspectives on Environmental Burden Minimisation Initiatives from Anaerobic Digestion Technologies for Community Scale Biomass Valorisation. Renew. Sustain. Energy Rev. 2015, 42, 883–901. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and Odor Characterization of Gas Emissions Released during Composting of Solid Wastes and Digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Manu, M.K.; Li, D.; Liwen, L.; Jun, Z.; Varjani, S.; Wong, J.W.C. A Review on Nitrogen Dynamics and Mitigation Strategies of Food Waste Digestate Composting. Bioresour. Technol. 2021, 334, 125032. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A. The Valorization of the Anaerobic Digestate from the Organic Fractions of Municipal Solid Waste: Challenges and Perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef] [PubMed]
- Montusiewicz, A.; Szaja, A.; Musielewicz, I.; Cydzik-Kwiatkowska, A.; Lebiocka, M. Effect of Bioaugmentation on Digestate Metal Concentrations in Anaerobic Digestion of Sewage Sludge. PLoS ONE 2020, 15, e0235508. [Google Scholar] [CrossRef] [PubMed]
- Khakbaz, A.; De Nobili, M.; Mainardis, M.; Contin, M.; Aneggi, E.; Mattiussi, M.; Cabras, I.; Busut, M.; Goi, D. Monitoring of Heavy Metals, Eox and Las in Sewage Sludge for Agricultural Use: A Case Study. Detritus 2020, 12, 160–168. [Google Scholar] [CrossRef]
- Lin, W.Y.; Ng, W.C.; Wong, B.S.E.; Teo, S.L.M.; Sivananthan, G.d/o.; Baeg, G.H.; Ok, Y.S.; Wang, C.H. Evaluation of Sewage Sludge Incineration Ash as a Potential Land Reclamation Material. J. Hazard. Mater. 2018, 357, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Li, G.; Luo, W.; Sun, Y. Manure Digestate Storage under Different Conditions: Chemical Characteristics and Contaminant Residuals. Sci. Total Environ. 2018, 639, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Al Seadi, T.; Drosg, B.; Fuchs, W.; Rutz, D.; Janssen, R. Biogas Digestate Quality and Utilization. In The Biogas Handbook; Elsevier: Amsterdam, The Netherlands, 2013; pp. 267–301. [Google Scholar]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage Sludge in Agriculture—The Effects of Selected Chemical Pollutants and Emerging Genetic Resistance Determinants on the Quality of Soil and Crops—A Review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Delzeit, R.; Kellner, U. The Impact of Plant Size and Location on Profitability of Biogas Plants in Germany under Consideration of Processing Digestates. Biomass Bioenergy 2013, 52, 43–53. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Research and Innovation. Bioeconomy: The European Way to Use Our Natural Resources: Action Plan 2018; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. The Pyrolysis and Gasification of Digestate from Agricultural Biogas Plant. Arch. Environ. Prot. 2015, 41, 70–75. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life Cycle Assessment of Biogas Digestate Processing Technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- González-Arias, J.; Gil, M.V.; Fernández, R.Á.; Martínez, E.J.; Fernández, C.; Papaharalabos, G.; Gómez, X. Integrating Anaerobic Digestion and Pyrolysis for Treating Digestates Derived from Sewage Sludge and Fat Wastes. Environ. Sci. Pollut. Res. 2020, 27, 32603–32614. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a Functional Integration of Anaerobic Digestion and Pyrolysis for a Sustainable Resource Management. Comparison between Solid-Digestate and Its Derived Pyrochar as Soil Amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Li, H.; Lindmark, J.; Nordlander, E.; Thorin, E.; Dahlquist, E.; Zhao, L. Using the Solid Digestate from a Wet Anaerobic Digestion Process as an Energy Resource. Energy Technol. 2013, 1, 94–101. [Google Scholar] [CrossRef]
- Ogwang, I.; Kasedde, H.; Nabuuma, B.; Kirabira, J.B.; Lwanyaga, J.D. Characterization of Biogas Digestate for Solid Biofuel Production in Uganda. Sci. Afr. 2021, 12, e00735. [Google Scholar] [CrossRef]
- Unyay, H.; Piersa, P.; Perendeci, N.A.; Wielgosinski, G.; Szufa, S. Valorization of Anaerobic Digestate: Innovative Approaches for Sustainable Resource Management and Energy Production—Case Studies from Turkey and Poland. Int. J. Green Energy 2023, 11. [Google Scholar] [CrossRef]
- Freda, C.; Nanna, F.; Villone, A.; Barisano, D.; Brandani, S.; Cornacchia, G. Air Gasification of Digestate and Its Co-Gasification with Residual Biomass in a Pilot Scale Rotary Kiln. Int. J. Energy Environ. Eng. 2019, 10, 335–346. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Illi, L.; Oechsner, H.; Kruse, A. Valorization of Maize Silage Digestate from Two-Stage Anaerobic Digestion by Hydrothermal Carbonization. Energy Convers. Manag. 2020, 222, 113218. [Google Scholar] [CrossRef]
- Akarsu, K.; Duman, G.; Yilmazer, A.; Keskin, T.; Azbar, N.; Yanik, J. Sustainable Valorization of Food Wastes into Solid Fuel by Hydrothermal Carbonization. Bioresour. Technol. 2019, 292, 121959. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of Hydrochars Obtained by Hydrothermal Carbonization of Manure-Based Digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical Conversion of Sewage Sludge: A Critical Review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Li, F.; Wang, L.; Hu, C.; Feng, J.; Li, W. Pathway of Biomass-Potassium Migration in Co-Gasification of Coal and Biomass. Fuel 2019, 239, 365–372. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Yuan, H.; Zhuang, X.; Yuan, S.; Yin, X.; Wu, C. Release and Transformation Pathways of Various K Species during Thermal Conversion of Agricultural Straw. Part 1: Devolatilization Stage. Energy Fuels 2018, 32, 9605–9613. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Williams, A. Observations on the Release of Gas-Phase Potassium during the Combustion of Single Particles of Biomass. Fuel 2016, 182, 110–117. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Wang, Y.; Li, S.; Wei, X. Release of Alkali Metals during Co-Firing Biomass and Coal. Renew. Energy 2016, 96, 91–97. [Google Scholar] [CrossRef]
- Li, F.; Yu, B.; Li, J.; Wang, Z.; Guo, M.; Fan, H.; Wang, T.; Fang, Y. Exploration of Potassium Migration Behavior in Straw Ashes under Reducing Atmosphere and Its Modification by Additives. Renew. Energy 2020, 145, 2286–2295. [Google Scholar] [CrossRef]
- Tchoffor, P.A.; Moradian, F.; Pettersson, A.; Davidsson, K.O.; Thunman, H. Influence of Fuel Ash Characteristics on the Release of Potassium, Chlorine, and Sulfur from Biomass Fuels under Steam-Fluidized Bed Gasification Conditions. Energy Fuels 2016, 30, 10435–10442. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, W.; Song, Q.; Zhuo, J.; Yao, Q. Effect of Steam and SiO2 on the Release and Transformation of K2CO3 and KCl during Biomass Thermal Conversion. Energy Fuels 2018, 32, 9633–9639. [Google Scholar] [CrossRef]
- Reinmöller, M.; Schreiner, M.; Guhl, S.; Neuroth, M.; Meyer, B. Ash Behavior of Various Fuels: The Role of the Intrinsic Distribution of Ash Species. Fuel 2019, 253, 930–940. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of Sewage Sludge Management: Standards, Regulations and Analytical Methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal Carbonization of Biogas Digestate: Effect of Digestate Origin and Process Conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Obernberger, I.; Biedermann, F. Fractionated Heavy Metal Separation in Biomass Combustion Plants-Possibilities, Technological Approach, Experiences. In Impact of Mineral Impurities in Solid Fuel Combustion; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Anonymous. Düngemittelverordnung—DüMV. 2008. Available online: https://www.buzer.de/gesetz/10396/index.htm (accessed on 25 November 2023).
- Dahl, J.; Obernberger, I.; Dahl, J.; Obernberger, I. Thermodynamic and Experimental Investigations on the Possibilities of Heavy Metal Recovery from Contaminated Biomass Ashes by Thermal Treatment. 1998. Available online: https://www.academia.edu/20099072/THERMODYNAMIC_AND_EXPERIMENTAL_INVESTIGATIONS_ON_THE_POSSIBILITIES_OF_HEAVY_METAL_RECOVERY_FROM_CONTAMINATED_BIOMASS_ASHES_BY_THERMAL_TREATMENT?uc-g-sw=72032245 (accessed on 25 November 2023).
- Wang, S. Application of Solid Ash Based Catalysts in Heterogeneous Catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Sahraei, O.A.; Iliuta, M.C. Development of Residue Coal Fly Ash Supported Nickel Catalyst for H2 Production via Glycerol Steam Reforming. Appl. Catal. B 2021, 291, 119958. [Google Scholar] [CrossRef]
- Ferella, F.; Puca, A.; Taglieri, G.; Rossi, L.; Gallucci, K. Separation of Carbon Dioxide for Biogas Upgrading to Biomethane. J. Clean. Prod 2017, 164, 1205–1218. [Google Scholar] [CrossRef]
- Pedrazzi, S.; Allesina, G.; Belló, T.; Rinaldini, C.A.; Tartarini, P. Digestate as Bio-Fuel in Domestic Furnaces. Fuel Process. Technol. 2015, 130, 172–178. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of Sewage Sludge in EU Application of Old and New Methods-A Review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Khiari, B.; Marias, F.; Zagrouba, F.; Vaxelaire, J. Analytical Study of the Pyrolysis Process in a Wastewater Treatment Pilot Station. Desalination 2004, 167, 39–47. [Google Scholar] [CrossRef]
- Sanchez Careaga, F.J.; Porat, A.; Briens, L.; Briens, C. Pyrolysis Shaker Reactor for the Production of Biochar. Can. J. Chem. Eng. 2020, 98, 2417–2424. [Google Scholar] [CrossRef]
- Wen, Y.; Shi, Z.; Wang, S.; Mu, W.; Jönsson, P.G.; Yang, W. Pyrolysis of Raw and Anaerobically Digested Organic Fractions of Municipal Solid Waste: Kinetics, Thermodynamics, and Product Characterization. Chem. Eng. J. 2021, 415, 129064. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, H.; Lü, F.; Shao, L.; He, P. Char Derived from Food Waste Based Solid Digestate for Phosphate Adsorption. J. Clean. Prod. 2021, 297, 126687. [Google Scholar] [CrossRef]
- González, R.; González, J.; Rosas, J.G.; Smith, R.; Gómez, X. Biochar and Energy Production: Valorizing Swine Manure through Coupling Co-Digestion and Pyrolysis. C 2020, 6, 43. [Google Scholar] [CrossRef]
- Weidemann, E.; Buss, W.; Edo, M.; Mašek, O.; Jansson, S. Influence of Pyrolysis Temperature and Production Unit on Formation of Selected PAHs, Oxy-PAHs, N-PACs, PCDDs, and PCDFs in Biochar—A Screening Study. Environ. Sci. Pollut. Res. 2018, 25, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of Pyrolysis Temperature on Production of Digested Sludge Biochar and Its Application for Ammonium Removal from Municipal Wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Inguanzo, M.; Pis, J.J. Microwave-Induced Pyrolysis of Sewage Sludge. Water Res. 2002, 36, 3261–3264. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Suitability of Marginal Biomass-Derived Biochars for Soil Amendment. Sci. Total Environ. 2016, 547, 314–322. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Pan, L.; Zhu, X.; Xie, S.; Yu, G.; Wang, Y.; Pan, X.; Zhu, G.; Angelidaki, I. Treatment of Digestate Residues for Energy Recovery and Biochar Production: From Lab to Pilot-Scale Verification. J. Clean. Prod. 2020, 265, 121852. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of Food Waste Digestate to Ash and Biochar Composites for High Performance Adsorption of Methylene Blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using Sewage Sludge with High Ash Content for Biochar Production and Cu(II) Sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Jansson, S.; Mašek, O. Unexplored Potential of Novel Biochar-Ash Composites for Use as Organo-Mineral Fertilizers. J. Clean. Prod. 2019, 208, 960–967. [Google Scholar] [CrossRef]
- Carchesio, M.; Tatàno, F.; Lancellotti, I.; Taurino, R.; Colombo, E.; Barbieri, L. Comparison of Biomethane Production and Digestate Characterization for Selected Agricultural Substrates in Italy. Environ. Technol. 2014, 35, 2212–2226. [Google Scholar] [CrossRef]
- Zuo, L.; Lin, R.; Shi, Q.; Xu, S. Evaluation of the Bioavailability of Heavy Metals and Phosphorus in Biochar Derived from Manure and Manure Digestate. Water Air Soil Pollut. 2020, 231, 553. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed With Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Rodríguez Alberto, D.; Tyler, A.C.; Trabold, T.A. Phosphate Adsorption Using Biochar Derived from Solid Digestate. Bioresour. Technol. Rep. 2021, 16, 100864. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Calamai, A.; Palchetti, E.; Masoni, A.; Marini, L.; Chiaramonti, D.; Dibari, C.; Brilli, L. The Influence of Biochar and Solid Digestate on Rose-Scented Geranium (Pelargonium graveolens L’Hér.) Productivity and Essential Oil Quality. Agronomy 2019, 9, 260. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in Biofuel Production from Gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2018, 12, 60. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage Sludge Gasification in a Bench Scale Rotary Kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Barisano, D.; Freda, C.; Nanna, F.; Fanelli, E.; Villone, A. Biomass Gasification and In-Bed Contaminants Removal: Performance of Iron Enriched Olivine and Bauxite in a Process of Steam/O2 Gasification. Bioresour. Technol. 2012, 118, 187–194. [Google Scholar] [CrossRef]
- Macrì, D.; Catizzone, E.; Molino, A.; Migliori, M. Supercritical Water Gasification of Biomass and Agro-Food Residues: Energy Assessment from Modelling Approach. Renew Energy 2020, 150, 624–636. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling Anaerobic Digestion with Gasification, Pyrolysis or Hydrothermal Carbonization: A Review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air Gasification of Biogas-Derived Digestate in a Downdraft Fixed Bed Gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; Hazarika, S.; Baruah, D.C. Assessment of By-Products of Bioenergy Systems (Anaerobic Digestion and Gasification) as Potential Crop Nutrient. Waste Manag. 2017, 59, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy Options of Mixed Agricultural Wastes Management: Coupling Anaerobic Digestion with Gasification for Enhanced Energy and Material Recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.K.; Tuli, D.K. Wood Ash as a Potential Heterogeneous Catalyst for Biodiesel Synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Park, J.-E.; Youn, H.-K.; Yang, S.-T.; Ahn, W.-S. CO2 Capture and MWCNTs Synthesis Using Mesoporous Silica and Zeolite 13X Collectively Prepared from Bottom Ash. Catal. Today 2012, 190, 15–22. [Google Scholar] [CrossRef]
| Digestate Origin * | Composition | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash | C | N | P | Na | Mg | K | Ca | Fe | Si | Al | ||
| Food waste (wt% dry basis) | 25.6 | 42.1 | 5.8 | 2.0 | n.a. | 0.3 | 0.6 | 3.2 | 0.9 | n.a. | n.a. | [39] |
| Food waste (%) | 10.2 | 43.5 | 1.9 | 18.8 a | 5.7 a | 2.8 a | 2.8 a | 14.9 a | 4.2 a | 5.6 a | 7.1 a | [40] |
| Pig manure (wt%) | 40.8 | 34.1 | 4.3 | 4.1 | 0.2 | 1.3 | 0.8 | 9.9 | 0.8 | 1.1 | 0.3 | [41] |
| Swine manure (wt%) | 23.0 | 37.2 | 4.6 | 11.5 | 0.1 | 0.9 | 0.1 | 26.3 | 0.5 | 0.7 | 0.2 | [42] |
| Quinoa residue and wastewater sludge (wt%) | 15.9 | 37.0 | 2.8 | 1.7 | 0.2 | 0.4 | 1.3 | 1.7 | 0.5 | n.a. | n.a. | [43] |
| Kitchen waste and domestic waste (% dry basis) | 42.1 | 23.1 | 3.6 | 1.1 a | 0.7 a | n.a. | 1.0 a | 28.6 a | 3.0 a | 3.3 a | 1.2 a | [44] |
| Corn silage and small proportion of cattle slurry (wt% TS) | 20.5 | 40.2 | 3.2 | 0.9 | 0.4 | 0.8 | 4.8 | 2.1 | n.a. | 1.9 | 0.1 | [45] |
| Maize silage, grass and grass silage, Potatoes (% dry basis) | 18.3 | 45.3 | 2.9 | 1.3 | n.a. | n.a. | 1.2 | n.a. | n.a. | n.a. | n.a. | [46] |
| Maize silage, sugar sorghum/sudan grass silage, poultry manure and Corn cob mix (% dry basis) | 14.6 | 43.2 | 1.5 | 1.1 | n.a. | n.a. | 1.1 | n.a. | n.a. | n.a. | n.a. | [46] |
| Energy crop and cow manure (wt% dry basis) | 29.2 | 40.0 | 1.9 | 3.1 a | 0.4 a | 2.2 a | 13.2 a | 6.5 a | 0.8 | 19.6 a | 1.2 a | [47] |
| Agricultural residue (% dry basis) | 16.0 | 44.1 | 3.2 | 1.2 | 0.3 | 0.7 | 1.6 | 1.3 | 2.3 | 1.8 | n.a. | [48] |
| Residual municipal solid waste (% dry basis) | 55.5 | 24.1 | 1.5 | 0.7 | 0.9 | 1.4 | 1.6 | 10.4 | 3.2 | 10.2 | n.a. | [48] |
| Sewage sludge (% dry basis) | 46.9 | 28.6 | 3.4 | 2.7 | 0.5 | 0.9 | 0.7 | 4.6 | 3.3 | 7.6 | n.a. | [48] |
| Vegetable, garden and fruit waste (% dry basis) | 43.8 | 29.5 | 2.0 | 2.6 | 0.5 | 0.8 | 0.7 | 4.3 | 3.0 | 7.1 | n.a. | [48] |
| Maize silage, liquid cattle manure, grass silage (% dry basis) | 27.7 | 50.5 | 3.6 | 1.4 | n.a. | 0.9 | 5.2 | 2.0 | 0.3 | 6.7 | 0.5 | [49] |
| Digestate | Feedstock Components | (%) |
|---|---|---|
| A | Maize silage | 50 |
| Grass and grass silage | 40 | |
| Potatoes | 10 | |
| B | Maize silage | 81 |
| Sugar sorghum/sudan grass silage | 9 | |
| Poultry manure | 7 | |
| Corn cob mix | 3 |
| Digestate | P | K | Mg | Na | Ca | Si | S | Fe | Al | As | Cr | Ni | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg/kg) | ||||||||||||
| A | 20.4 | 8.5 | 2.7 | 3.1 | 17.0 | 18.0 | 3.2 | 22.5 | 3.1 | 0.8 | 76 | 36 | [46] |
| B | 26.7 | 15.5 | 8.4 | 0.8 | 13.6 | 30.4 | 0.9 | 1.8 | 1.2 | 1.1 | 184 | 285 | [46] |
| Energy crop | 3.09 | 13.15 | 2.23 | 0.41 | 6.53 | 19.6 | 0.91 | 0.80 | 1.23 | n.a. | n.a. | n.a. | [47,98] |
| Pinewood with bark | 2.6 | 6.4 | 6.0 | 0.7 | 41.7 | 25.0 | 1.9 | 2.3 | 4.6 | 4.1 | 325.5 | 66 | [46,99] |
| Biochar * | Ash | P | K | Mg | Na | Ca | Si | Fe | Al | As | Cr | Ni | Cu | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | ||||||||||||||
| FWD 300 °C | 35.7 | 2.92 | 0.87 | 0.37 | n.a. | 4.55 | n.a. | 1.37 | n.a. | 9.1 × 10−5 | 0.004 | 0.002 | 0.007 | [39] |
| FWD 400 °C | 49.2 | 4.13 | 1.24 | 0.50 | n.a. | 6.53 | n.a. | 1.97 | n.a. | 1.1 × 10−4 | 0.004 | 0.003 | 0.004 | [39] |
| FWD 500 °C | 55.1 | 4.54 | 1.39 | 0.53 | n.a. | 7.26 | n.a. | 2.18 | n.a. | 9.8 × 10−5 | 0.004 | 0.005 | 0.01 | [39] |
| FWD 700 °C | 60.2 | 4.78 | 1.53 | 0.55 | n.a. | 7.76 | n.a. | 2.22 | n.a. | N.D. | 0.002 | 0.004 | 0.011 | [39] |
| BC 400 °C | 34.27 | 18.02 | 2.71 | 2.66 | 5.57 | 15.03 | 5.78 | 4.22 | 7.20 | n.a. | n.a. | n.a. | n.a. | [40] |
| BC 500 °C | 36.31 | 18.88 | 2.33 | 2.96 | 5.51 | 15.37 | 5.27 | 4.06 | 7.29 | n.a. | n.a. | n.a. | n.a. | [40] |
| BC 600 °C | 36.94 | 17.70 | 2.58 | 2.78 | 5.31 | 16.15 | 5.85 | 4.34 | 7.19 | n.a. | n.a. | n.a. | n.a. | [40] |
| BC 700 °C | 37.66 | 19.28 | 2.50 | 2.85 | 5.42 | 14.98 | 5.51 | 4.37 | 7.18 | n.a. | n.a. | n.a. | n.a. | [40] |
| BC 800 °C | 37.92 | 18.42 | 2.83 | 2.71 | 5.36 | 15.12 | 5.39 | 4.15 | 7.31 | n.a. | n.a. | n.a. | n.a. | [40] |
| DSD 300 °C | ~55 | 3.48 | 0.58 | 1.02 | 0.92 | 22.30 | n.a. | 0.67 | 0.36 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 400 °C | ~60 | 4.12 | 0.64 | 1.16 | 0.99 | 26.94 | n.a. | 0.71 | 0.40 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 500 °C | ~65 | 3.96 | 0.63 | 1.11 | 0.95 | 25.00 | n.a. | 0.65 | 0.37 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 600 °C | ~65 | 4.58 | 0.73 | 1.30 | 1.10 | 29.36 | n.a. | 0.76 | 0.44 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 700 °C | ~90 | 6.25 | 1.02 | 1.71 | 1.48 | 41.01 | n.a. | 1.04 | 0.61 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 800 °C | ~90 | 6.79 | 1.00 | 1.81 | 1.50 | 40.24 | n.a. | 1.15 | 0.63 | n.a. | n.a. | n.a. | n.a. | [110] |
| DSD 900 °C | ~85 | 6.72 | 0,67 | 1.83 | 1.20 | 41.79 | n.a. | 1.09 | 0.62 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 300 °C | ~50 | 2.77 | 1.07 | 0.43 | 1.36 | 15.70 | n.a. | 0.88 | 1.45 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 400 °C | ~60 | 3.17 | 1.24 | 0.50 | 1.57 | 17.19 | n.a. | 0.82 | 1.67 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 500 °C | ~65 | 3.31 | 1.29 | 0.51 | 1.63 | 18.24 | n.a. | 0.91 | 1.72 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 600 °C | ~65 | 3.45 | 1.37 | 0.53 | 1.69 | 19.18 | n.a. | 1.02 | 1.81 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 700 °C | ~80 | 3.93 | 1.48 | 0.64 | 1.89 | 21.42 | n.a. | 1.43 | 1.98 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 800 °C | ~80 | 4.07 | 1.48 | 0.62 | 1.81 | 21.40 | n.a. | 1.26 | 2.11 | n.a. | n.a. | n.a. | n.a. | [110] |
| PSD 900 °C | ~80 | 4.36 | 1.10 | 0.67 | 1.23 | 23.72 | n.a. | 1.23 | 2.21 | n.a. | n.a. | n.a. | n.a. | [110] |
| Ash * | P | K | Mg | Na | Ca | Si | Al | Reference |
|---|---|---|---|---|---|---|---|---|
| (wt%) | ||||||||
| Digestate gasification | 26.96 | 4.29 | 8.45 | 0.57 | 19.85 | 24.11 | 3.09 | [134] |
| Digestate pyrolysis | 17.70 | 2.58 | 2.78 | 5.31 | 16.15 | 5.85 | 7.19 | [40] |
| WA | 1.15 | 7.3 | 2.9 | 0.6 | 14.7 | 17.6 | 9.0 | [137] |
| WAC500 °C | 0.80 | 6.7 | 2.7 | 0.6 | 13.3 | 15.7 | 8.3 | [137] |
| WAC800 °C | 0.50 | 5.7 | 4.5 | 5.7 | 17.8 | 21.5 | 1.2 | [137] |
| WAC1000 °C | 0.50 | 7.0 | 3.1 | 5.6 | 22.3 | 22.9 | 1.3 | [137] |
| WAC1200 °C | 0.50 | 2.5 | 0.5 | - | 36.6 | 20.1 | 0.2 | [137] |
| AKWAC1 | 0.55 | 36.8 | 3.0 | 8.5 | 10.5 | 31.9 | 1.1 | [137] |
| AKWAC0.5 | 0.47 | 10.8 | 2.6 | 8.3 | 8.5 | 31.9 | 1.3 | [137] |
| ACaWAC0.5 | 0.40 | 3.0 | 2.7 | 1.0 | 44.5 | 30.5 | 1.5 | [137] |
| Material * | K | Mg | Na | Ca | Fe | Si | Al | Reference |
|---|---|---|---|---|---|---|---|---|
| (wt%) | ||||||||
| Digestate ash pyrolysis | 2.33 | 2.96 | 5.51 | 15.37 | 4.06 | 5.27 | 7.29 | [40] |
| Original fly ash | 3.37 | 0.24 | n.a. | 0.25 | 1.96 | 20.36 | 13.45 | [104] |
| Washed fly ash | 3.39 | 0.23 | n.a. | 0.19 | 1.83 | 18.74 | 12.78 | [104] |
| A1 | 1.02 | 0.15 | 6.35 | 0.17 | 1.53 | 11.10 | 7.76 | [104] |
| A2 | 0.81 | 0.18 | 6.90 | 0.20 | 1.52 | 11.34 | 7.83 | [104] |
| A3 | 1.13 | 0.17 | 6.67 | 0.22 | 1.60 | 12.05 | 8.12 | [104] |
| A4 | 0.92 | 0.066 | 7.10 | 0.21 | 1.48 | 13.81 | 9.75 | [104] |
| B1 | 0.81 | 0.0072 | 6.91 | 0.097 | 1.02 | 12.19 | 9.03 | [104] |
| B2 | 0.68 | 0.18 | 7.45 | 0.21 | 1.65 | 12.58 | 9.44 | [104] |
| Zeolite 13X | 0.11 | 0.72 | 5.07 | 0.32 | 0.35 | 11.68 | 6.16 | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammarella, D.; Di Giuliano, A.; Gallucci, K. Reuse and Valorization of Solid Digestate Ashes from Biogas Production. Energies 2024, 17, 751. https://doi.org/10.3390/en17030751
Mammarella D, Di Giuliano A, Gallucci K. Reuse and Valorization of Solid Digestate Ashes from Biogas Production. Energies. 2024; 17(3):751. https://doi.org/10.3390/en17030751
Chicago/Turabian StyleMammarella, Daniel, Andrea Di Giuliano, and Katia Gallucci. 2024. "Reuse and Valorization of Solid Digestate Ashes from Biogas Production" Energies 17, no. 3: 751. https://doi.org/10.3390/en17030751
APA StyleMammarella, D., Di Giuliano, A., & Gallucci, K. (2024). Reuse and Valorization of Solid Digestate Ashes from Biogas Production. Energies, 17(3), 751. https://doi.org/10.3390/en17030751









