Abstract
In order to implement timely sustainability solutions, road transportation is gradually transitioning to electric power. However, the maritime sector faces challenges in finding ways to shift towards more sustainable fuel. From the perspective of long-distance shipping, electric transport is economically impractical. Therefore, alternative solutions or proposals contributing to the global reduction of pollutant gas emissions in maritime transport are vitally important. This investigation aims to find solutions that enhance the ecological efficiency of intercontinental cargo ships. In this study, an assessment of a magnesium hydride coating was conducted as it is a prospective coating capable of reducing hydrodynamic resistance to save fuel. Due to MgH2’s ability to release hydrogen at higher temperatures or during a reaction with water, it is expected that this could contribute to an enhancement of the Leidenfrost effect, maintaining a vapor layer on the surface. Samples prepared in situ via reactive magnetron sputtering were submitted to thermal analysis for dehydrogenation range evaluation and the experimental rig for critical (Leidenfrost) temperature identification. In conclusion, thermogravimetric (TG) analysis indicated that the volatile content, primarily hydrogen, in the sample reached approximately 13% by mass. The TG curve exhibited variations in MgH2 mass, with the most significant mass loss occurring at 300 °C. After conducting critical temperature experiments, the potential of MgO coating was observed to be greater than anticipated when compared to the main material, MgH2.
1. Introduction
As the world’s transportation gradually turns electric or otherwise sustainable, the commercial marine transport sector appears to be lagging behind. However, marine transportation is highly efficient in terms of fuel used per mass of goods transported, due to its sheer scale marine transportation accounts for up to 15% of global pollutant gas emissions []. Considering that electric alternatives for long distance routes are unavailable, other avenues are pursued for further improving the efficiency of transcontinental marine vessels. Quite a few of such pursuits include hydrodynamic hull design [], surface coatings [], and gas lubrication []. Currently utilized surface coatings focus on preventing foaling (the aggregation of mollusks and marine flora on the ship), and, in order to be applicable, hydro- or super-hydrophobic coatings would be required to fulfill this function as well. As progressively stricter regulations on anti-fouling agents force more and more biocidal solutions out of the market on grounds of legitimate environmental concerns, increasingly more research is devoted to passive or bio-inert anti-fouling coatings. Some of said coatings can be modified to serve as hydrophobic drag reduction measures. A paper from 2013 [] reports using gold nanostructures and thiol-based coatings with hydrophobic alkyl tails to obtain a super-hydrophobic surface. This study claims figures of drag reduction of up to 38.5% over a considerable range of velocities. A more recent study [] published in 2017 discusses a simple paint coating containing titanium dioxide used to reduce hydrodynamic drag and increase buoyancy. The underlining point being the simplicity of application of such coating, as well as mechanical and abrasive resistance. One common trait between all such coatings is that they are mechanically passive. Changing the conditions of operation or creating metastable constructions such as vapor films could lead a to vastly increased effectiveness of resistance-reducing films and coatings. In pursuit of such measures, the Leidenfrost effect was considered for this purpose. Although, until recently, most research [] was focused on inhibiting the Leidenfrost effect as its presence may be detrimental to systems where cooling is of the utmost importance, such as in the nuclear energetics sector. A study [] focused on Leidenfrost effect-based gas lubrication in an effort to facilitate hydrodynamic drag reduction has claimed figures that approach the physical limits of drag reduction achievable by gas lubrication. Usually, when analyzing the Leidenfrost effect, vapor films, and related phenomena, the concept of the Leidenfrost droplet is broached, as it is the most commonly observed manifestation of the Leidenfrost effect. However, in said study, the inverse geometry is proposed: a heated body (a metal sphere) immersed in a relatively cooler fluid and the system’s hydrodynamic mechanics are observed. The results suggest that objects shrouded by a vapor layer have a greater terminal velocity when falling through a liquid medium, indicating reduced hydrodynamic resistance. The properties of Leidenfrost effect occurrence and vapor film formation depend on the Leidenfrost point, which is a temperature at which (in a given set of conditions) a vapor layer is formed spontaneously. Additionally, Leidenfrost point temperature depends on many various factors, one of which is surface properties of the interfacing solids. Numerous studies [] have been conducted in order to analyze the effect of surface properties on vapor film formation and its stability, but the common consensus concludes that hydrophobic properties have a positive impact on the Leidenfrost effect. Based on the analyzed thermal-physical mechanics of the Leidenfrost effect, it was theorized that additional gas release from the submerged object (in cases of situations involving inverted Leidenfrost geometry, such as described in a previously mentioned study []) could reduce the Leidenfrost temperature, allowing for vapor film formation in circumstances involving lower temperature gradients. Considering the requirements for such materials to be metallic or ceramic and able to facilitate gas release, metal hydrides fall well into the bracket. Such materials can undergo thermal decomposition, releasing hydrogen in the process. However, due attention must be paid to some undesired hydride properties, such as instability at atmospheric pressure or violent reactions with water or tendency to be contaminated by air. These boundaries root out many candidates, leaving just a few, with magnesium hydride among them. Due to research [] being conducted on magnesium hydride as a potential compound for hydrogen storage applications, MgH2 is commercially available, as well as able to facilitate tame hydrogen release during reactions with water. Additionally, it does not readily react with air and is able to undergo thermal decomposition at reasonable temperatures (~300 °C). Hence, this innovative research will aim to determine the thermo-physical cooling properties of magnesium hydride-coated samples immersed in water in order to determine the influence of the simultaneous release of volatile gases on the formation of a vapor layer in the case of stationary bodies. This research will lead to the future application of various combinations to moving objects, although the first step is to understand the processes on a stationary surface, which is what this research is aimed at.
2. Materials and Methods
2.1. Materials
Spherical samples of diameter of D = 19.05 mm and plates with a diameter of D = 15 mm and a thickness of 1.2 mm were taken from high purity aluminum (99.99%) to conduct experiments. For the observation of critical temperature variation and different conditions investigation, the main attention was focused on the surface impact. Spherical samples’ surface was covered/prepared with different materials: magnesium hydride (the main investigation material) and magnesium oxide (the additional material that was selected to evaluate the differences between oxide and hydride). A polished aluminum surface was chosen as the reference surface/coating. Several studies have been conducted [,], proving that this surface allows to reach the low critical temperatures at which the Leidenfrost phenomena occurs. The surfaces of reference samples were smoothed with 3M Hookit abrasive discs with a roughness of P-1000, P-3000, and P-4000. The final surface of the specimens was polished using a paraffin-based abrasive compound. Magnesium hydride (MgH2) was deposited using DC sputtering magnetron deposition system and 1 A magnetron current, 400 V ± 20 V magnetron supply voltage. The chamber was pressurized to 5.5 mTorr with argon, and then to 120 mTorr with hydrogen gas. The deposition took 1 h. The physical vapor deposition system with a circular magnetron was employed to deposit magnesium oxide (MgO) films. A magnesium target (95 mm, 99.99% purity) served as the primary cathode material in the sputtering process. Prior to deposition, the chamber was brought to a base vacuum pressure of 3 mTorr using rotary and diffusion pumps. The magnetron sputtering process occurred under a pressure of 40 mTorr, utilizing a mixture of O2 and Ar gases introduced into the vacuum chamber. The Ar gas was introduced up to 8 mTorr, followed by injection of O2 up to 40 mTorr. The samples were positioned beneath the magnetron, keeping a distance of 7 cm between the target and the sample holder. Power for the magnetron was supplied by a pulsed DC power source with a current of 1 A and a voltage of 160 V ± 20 V. The duration of the deposition process was 5 h. The thickness of the MgH2 film was approximately 2 µm, while the thickness of the MgO film was about 150 nm. The surfaces of MgH2 and MgO coatings replicated the surfaces of the specimens, although the roughness of the coatings was not assessed. The examples of samples used in experiments are shown in Figure 1.
Figure 1.
Experimental samples.
2.2. Thermogravimetric Method (Method 1)
Thermogravimetric analysis (TGA) was conducted using the NETZSCH-Gerätebau GmbH (Selb, Germany) STA 449 F3 Jupiter analyzer with silicon carbide heater. For this analysis, magnesium hydride powder was obtained by mechanically removing it from aluminum plates, onto which the material was previously deposited using reactive magnetron sputtering. A sample of approximately 1.87 mg of magnesium hydride powder was loaded post-calibration into Al2O3 crucible, placed in a S-type thermocouple-equipped chamber and heated at 20 K/min from 40 °C to 510 °C under 40 mL/min Ar gas flow and 20 mL/min N2 flow when using mixed gases. The differential thermogravimetric (DTG) curves were produced by numerically deriving the thermogravimetric (TG) curves. For some TGA analysis steps, the material adhered to prepared aluminum samples (plates) was used. The sample with aluminum plate was loaded post-calibration into another Al2O3 crucible, placed in a S-type thermocouple-equipped chamber and heated at 20 K/min from 40 °C to 280 °C, followed by 5 K/min from 280 °C to 400 °C under 40 mL/min Ar gas flow and 20 mL/min N2 flow when using mixed gases, or 60 mL/min argon flow when using only argon as carrier gas. If steam was used during thermogravimetry, the relative gas humidity was set to 50%. The differential thermogravimetric (DTG) curves were produced by numerically deriving the TG curves. This method was used to identify the starting point of the dehydrogenation process of MgH2, and mass loss peaks were also evaluated (Figure 2).
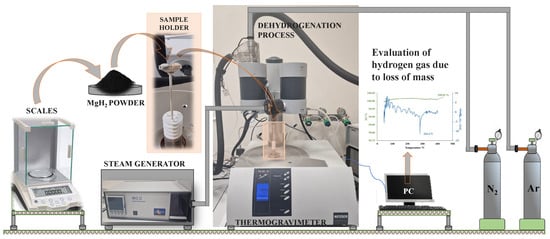
Figure 2.
Investigation of the dehydrogenation process of MgH2.
2.3. Research of Critical Temperature Investigations for Vapor Film Formation (Method 2)
Experiments with spherical samples were conducted on the experimental bench, which was designed and constructed in the laboratory (Figure 3). Experimental bench consists of four main parts: (1) tube furnace, (2) water tank, (3) water heater, and (4) data collection system. The investigations of critical temperature points were performed in two stages. In the first step, the prepared spherical sample was heated up until the temperature required for the experiment was reached, while, in the second step, the sample cooling dynamics was recorded. Before starting the experimental investigation, a tube furnace was switched on and a desired sample temperature was set (200 °C, 300 °C, 450 °C). During the experiment, the nitrogen gas was fed from the bottom of the furnace at the rate of 2 L/min. When the sample reached the set temperature in the tube furnace, the sample was lowered down into the water tank. The sample surface temperature was captured and registered by an E-type thermocouple paired with data logger TC-08 system. A water heater was used to maintain the temperature of water tank at fixed temperatures: 20 °C, 40 °C, and 60 °C. The choice of water temperature was based on both the possibilities of Leidenfrost formation and the realistic temperature of the water in the environment. Three repetitions were conducted under identical conditions to obtain accurate results and determine the deviation of the obtained experimental data. At the observed Leidenfrost point temperature, the deviation ranged from 2 to 4%, while in the temperature range from 450 °C to 200 °C, the deviation fluctuated between 2 and 10%. The aim of these investigations was to identify the critical temperature formation boundaries (Leidenfrost temperatures) when investigating different samples, whose surface properties have an impact when reaching critical temperatures.
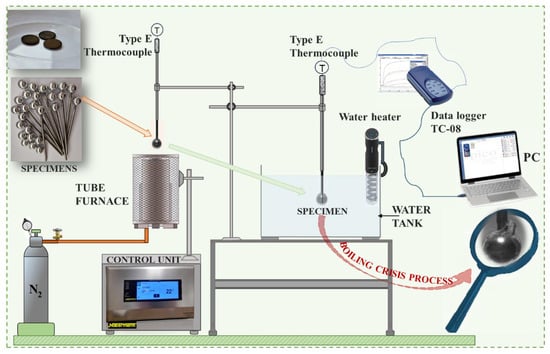
Figure 3.
Schematic diagram of the experimental rig for critical (Leidenfrost) temperature.
2.4. Optical Microscopy Examination of the Surface of Samples (Method 3)
The surface of the samples after exposure to high temperature and water vapor was examined using optical microscope OLYMPUS® BX51TF (Manufacturer: Olympus Corporation, Tokyo, Japan, Serial number: 7E18497). QCapture Pro Optical Microscope software (version 6.0.0.605) was used to capture the images. After selecting the area to be observed, the objective was moved to the desired area of the samples, the area was illuminated, and the image was focused. Images with optical magnifications of ×100 and ×500 were taken.
3. Results
3.1. Thermogravimetric Method
The aim of this investigation was to test the MgH2 material, which is believed to be promising due to its ability to store hydrogen and therefore is hoped to be able to be used as a friction-reducing coating. MgH2 exhibits dehydrogenation at a temperature range of 200–400 °C and can accumulate hydrogen to about 8% by mass []. Usually, metal hydrides are accompanied by exothermic heat release []. This would allow us to accelerate thermal processes when generating vapor film during the boiling crisis. Due to these reasons, the aim was to investigate hydrogen separation trends (of prepared samples with MgH2 coatings) and to evaluate the thermal stability when using thermogravimetric (TG) analysis (Figure 4). For these experiments, a thin layer of MgH2 was mechanically removed from plates and the obtained powder was used for investigation, raising the dehydrogenation environment temperature at a rate of 20 K/min to 510 °C.
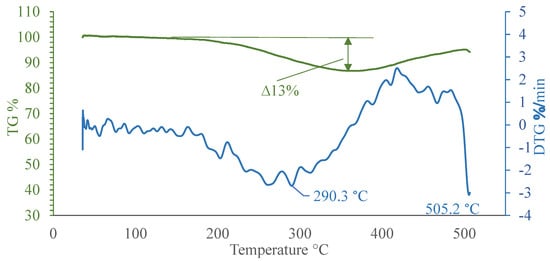
Figure 4.
Changes in the mass of MgH2 sample with increasing environment temperature up to 510 °C.
Thermogravimetric (TG) analysis using Method 1 revealed that volatile content [] (most of the hydrogen) in the sample reached around 13% by mass. In addition, the TG curve shows the variations of MgH2 mass, starting from 200 °C and continuing to 350 °C. The most intense mass loss was observed at a temperature of 300 °C. After reaching 350 °C, the mass of the sample started to increase, which shows a possible formation of oxides on the surface []. The curves of MgH2’s mass loss rate (Figure 4, DTG curve) confirm that hydrogen separation took place in the first step, while the formation of oxides and hydroxides took place in next stage. The conditionally thermally stable MgH2 sample could be stored up to 200 °C, which determinates the initial temperature value when applying Method 2.
Additional experimental tests were carried out on MgH2-coated sample plates in order to select the temperature range that will serve as the working range for Method 2. Given that the mass of the aluminum sample were significantly higher than that of the thin-film coating, the results are therefore valid only if they show the most significant changes, such as a sudden change in the mass loss rate of the DTG curve (Figure 5).
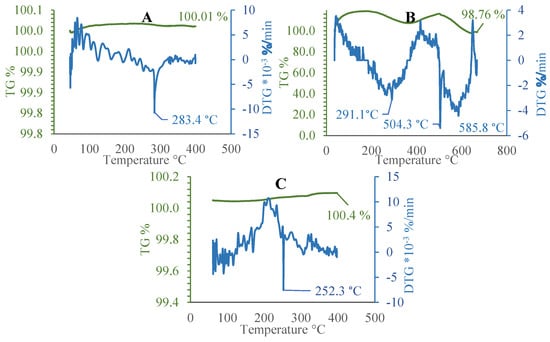
Figure 5.
Mass changes (TGA and DTG) at different environments: (A) with argon; (B) with argon and nitrogen mixture; and (C) with argon and steam.
Conducted thermogravimetric analysis results (Figure 5) suggest that the analyzed material undergoes a slight relative increase in mass, along with well-pronounced, albeit minor, differential peaks at well-defined points, signifying gas evolution and mass loss. Several characteristic peaks between the range of 252 and 504 °C represent the highest activity of magnesium hydride and allow to limit the experiments of this research. The obtained data (Figure 5C) indicates that the presence of steam significantly lowers the temperature of the most intense point of hydrogen evolution, likely due to the reaction of magnesium hydride with water at slightly elevated temperatures. Introducing nitrogen to the carrier gas also has a notable difference, changing the peak point by approx. 3 K. The experiments showed considerable consistency across multiple iterations. On the basis of the TGA study and considering the physical properties of magnesium hydride and the conditions for the onset of the boiling crisis, the experimental temperatures of the samples were defined at 200, 300, and 450 °C. We decided to use only argon as a protective gas, which could create the most inert environment possible during the experiments according to Method 2.
The surface analysis with magnesium hydride (MgH2) as the main material in this study and using Method 3 (Figure 6) was carried out under three different sets of conditions: the surface was evaluated immediately after deposition via the magnetron sputtering technique; the surface passed the heating cycle to the set temperature in an argon environment; and the surface passed the heating cycle to the set temperature in an argon and steam environment. The surface evaluation was performed using optical microscopy in ×100 and ×500 magnifications.
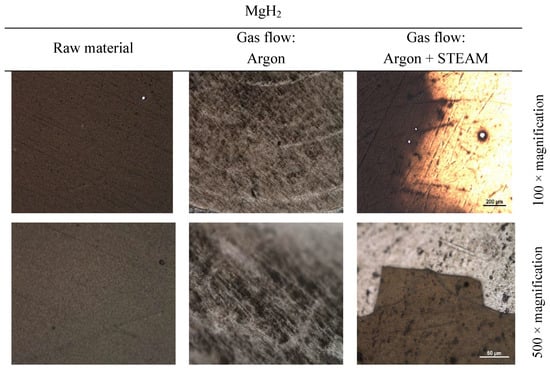
Figure 6.
Magnesium hydride sample optical microscopy images.
As is usual for such types of thin films, an optical analysis of untreated deposited coating gives a good handle on deposition, and in turn, surface quality. As seen in Figure 6, the untreated films (left column) are uniform and appear plain even at a higher order of magnification. Additionally, a more reactive treatment environment, such as steam, yields more contrasting structures (right column), namely flaking of the film, as observed in the image at the lower right. Inert processing environment produces surfaces that are roughened (as observed in the middle column) but lack any distinguishable features, for instance, flaking. Similar surface finishes are commonly observed in sintered materials [], which can be expected as the processing conditions are similar to those used for sintering ceramics. Likewise, thermogravimetry analysis provides a valuable description on how the coating material behaves after being exposed to temperature and how moisture content in the reaction atmosphere affects this behavior.
3.2. Research of Critical Temperature Investigations for Vapor Film Formation
The main objective of this investigation was to identify critical temperatures (Leidenfrost temperature) at points where continuous vapor generation process takes place in the case of non-stationary heat transfer. In order to evaluate the impact of the MgH2 coating for Leidenfrost formation, both MgO and polished Al surface samples were tested under the same conditions when recording cooling dynamics according to Method 2. Experiments were conducted for different water temperatures: 20 °C, 40 °C, 60 °C. The initial temperatures of samples were set at 200, 300, and 450 °C, considering the dehydrogenation process discussed in Section 3.1.
During the cooling process on different sample surfaces, a tendency of temperature variation could be observed; however, the temperature variation rate in all (Al, MgH2, MgO) cases is different. The performed investigations showed that nano-layer thin film could have different impacts, inhibit cooling dynamics, or accelerate them in various cases. It was observed that vapor flux generation is strongly affected by the surface physical properties of the material, one of which is hydrophilicity/hydrophobicity. In cases of MgH2 hydrides (Figure 7(a2,b2,c2)), it could be emphasized that the material is more hydrophilic; therefore, the Leidenfrost effect was not observed at initially, except in the 450 ° C sample. It was observed that the hydrate coating creates specific conditions during the subsequent cooling process. Initially, the temperature change rate is more sudden than in other cases. This means that instantaneous water contact occurs with a sample, leading to better heat transfer. In the case of low-temperature samples, no significant influence of released hydrogen gas on the Leidenfrost effect was observed. However, as it was expected, this coating is capable to form secondary vapor/gas generation when the water makes contact with the surface, likely due to thermochemical processes (Figure 7(c2), MgH2). These processes are led by exothermic reactions. Therefore, after a layer of vapor bubbles forms (as insulator) and water is pushed off from the surface, a temperature variation of surface is observed. This temperature variation effect could be observed near the higher sample temperatures, as well as when the water temperature is higher. The most surprising finding was that the curves of cooling dynamics for magnesium hydride converged immediately after the thermochemical process, as for example with MgH2 450 °C and 60 °C. This lead to the conclusion that thermochemical processes on the surface can be used to modify the cooling dynamics in the direction desired.
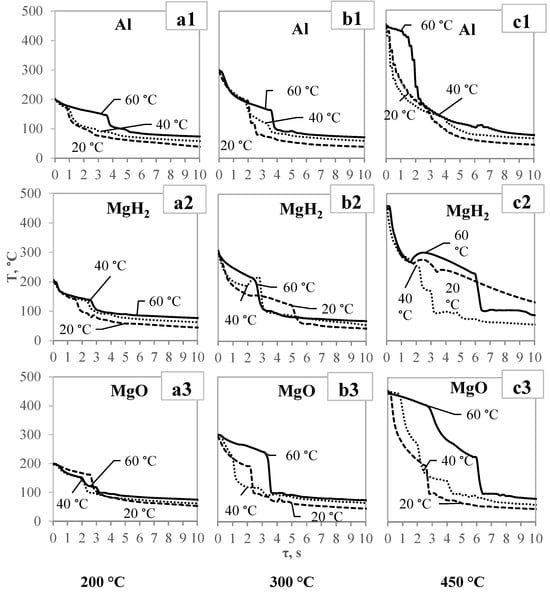
Figure 7.
Cooling dynamics of spherical specimens after immersion in 20 °C, 40 °C, and 60 °C water (the initial temperatures of the tested specimens were as follows: (a1–a3) at 200 °C; (b1–b3) at 300 °C; (c1–c3) at 450 °C.).
Other experiment results also have showed noteworthy trends (Table 1). Table 1 shows the critical temperatures (Leidenfrost temperatures) of different samples, which indicates whether the vapor point has been reached, and, if so, at which temperature the effect occurred.

Table 1.
Leidenfrost temperature (critical temperature).
According to Figure 7 and Table 1, it could be stated that samples covered with the MgO coating were the most favorable (from the three investigated surfaces) for the formation of the Leidenfrost effect. Even at lower temperatures of samples and water, it was observed that vapor film forms in all cases, but only with a difference in the sustained duration. The results of the polished aluminum surface could be considered as average because the Leidenfrost effect was not lower than the sample temperature of 300 °C and a water temperature of 40 °C.
In order to compare the results, the performed experiments were fixed by recording equipment, and the processed material was matched with the obtained curves (Figure 7). The compared curves are provided in Figure 8 for different coating cases (450 °C samples, 60 °C water).
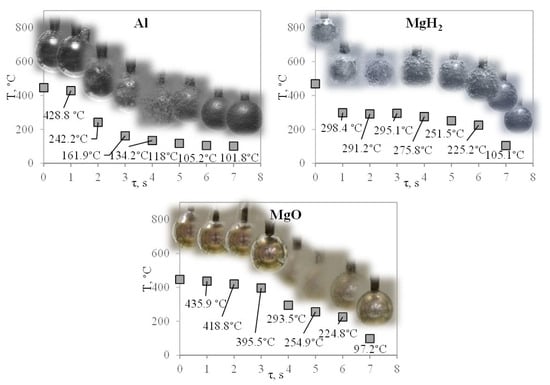
Figure 8.
Trend of temperature change depending on cooling time.
According to the images (Figure 8), it is possible to clearly see the formation of a vapor flux and a “lifetime” duration by relating the images to the experimental curves. As it was noticed, the sample covered with the MgO coating facilitates the formation of the Leidenfrost effect with the most stable and the longest lasting vapor film in the case of non-stationary heat transfer.
One common tendency (Figure 9) that can be observed during the analysis of surfaces using Method 3 is as follows: the temperature of water is not the most influential factor, whereas a higher temperature of the sample leads to thinner and thinner film remaining upon an interaction with water. In some cases, upon heating the film, it visibly changed after interacting with water. In these cases, it is very likely that due to various temperatures and reactions with water, the hydride undergoes dehydrogenation (up to 300 °C). The film remains intact in rapid reactions with water at lower temperatures of the samples (200 °C, 300 °C), and then slowly reacts with water. However, high temperatures of samples (450 °C) and sudden cooling indicate that the film is vulnerable and begins to flake off in pieces. Flaking indicates a difference in thermal expansion coefficients during rapid temperature changes, the thermal contraction is also very quick, which also negatively affects film adhesion.
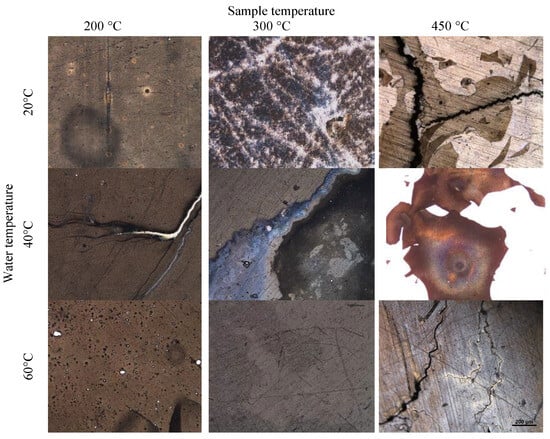
Figure 9.
Microscopy images of magnesium hydride samples; ×100 optical magnification.
4. Conclusions
In this work, a thermal analysis of magnesium hydride was carried out to determine the conditions of the vapor layer generation process on the surface under Leidenfrost conditions. Due to the ability of magnesium hydride to release hydrogen during the reaction with water, it was expected that it could contribute to the generation of a vapor layer. The results obtained revealed the specific character of the hydride and provided insight into the behavior of this material during the thermochemical processes with variations in both ambient and sample temperatures. The conducted experiments indicate that only a temperature of 450 °C enabled the formation of vapor films and, in turn, the Leidenfrost effect. The film on the surface of the specimen at 450 °C with an MgH2 coating lasted only 0.3 s, while it persisted for approximately 2 s on a polished aluminum surface and even 3.3 s on MgO under the same conditions. On the basis of the visual information recorded, the vapor film that formed on the MgH2 coating cannot be considered as a stable film boiling, but rather as a vapor–gas lubrication system. Determining its effect on friction would require separate studies, despite the fact that the literature suggests that a loss of contact with water can significantly reduce friction. Another finding is that the production of hydrogen gas is very rapid during its reaction with water, and the entire coating reacts almost instantly. This thermochemical reaction between the surface and the water is likely to lead to an increase in temperature during the cooling process. During the thermochemical reaction at a sample temperature of 450 °C, the magnesium hydride film broke into pieces, and could no longer be used in repeated tests. Notwithstanding the results obtained, an additional comparative analysis between the surfaces of the different samples showed that the Leidenfrost effect is more favorable for magnesium oxides than for magnesium hydrides. A vapor film was fixed on the magnesium oxide surface in all test conditions, both at the lowest water temperature of 20 °C and at the lowest test temperature of the 200 °C samples. In regard to the polished aluminum, the results are average, and the fastest cooling dynamics were found immediately after the disappearance of the vapor layer, compared to other cases.
Author Contributions
R.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, writing—original draft, and writing—review and editing; J.E.: data curation and writing—review and editing; M.B.: data curation and writing—review and editing; L.V.: investigation, methodology, data curation, formal analysis, software, and visualization; M.U.: resources and writing—review and editing; I.K.: data curation; M.M.: writing—review and editing; N.S.: investigation and methodology; K.Z.: methodology, conceptualization, and resources; V.M.: resources, software, and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the Research Council of Lithuania (LMTLT), agreement No. S-MIP-22-77.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eyring, V.; Köhler, H.W.; van Aardenne, J.; Lauer, A. Emissions from international shipping: 1. The last 50 years. J. Geophys.Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Zou, Z.-J.; Guo, H.-P.; Chen, C.-Z. A study on the interaction among hull, engine and propeller during self-propulsion of a ship. Ocean. Eng. 2023, 286, 115702. [Google Scholar] [CrossRef]
- Schrader, L.-U. Passive Drag Reduction via Bionic Hull Coatings. J. Ship Res. 2019, 63, 206–218. [Google Scholar] [CrossRef]
- Jiang, B.; Ma, Y.; Wang, L.; Guo, Z.; Zhong, X.; Wu, T.; Liu, Y.; Wu, H. Thermal decomposition mechanism investigation of hyperbranched polyglycerols by TGA-FTIR-GC/MS techniques and ReaxFF reactive molecular dynamics simulations. Biomass Bioenergy 2023, 168, 106675. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. A 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- Hwang, G.B.; Patir, A.; Page, K.; Lu, Y.; Allan, E.; Parkin, I.P. Buoyancy increase and drag-reduction through a simple superhydrophobic coating. Nanoscale 2017, 9, 7588–7594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, Y.; Liu, F.; Du, H.; Li, Y.; Zhang, H.; To, S.; Wang, S.; Pan, C.; Yu, J.; et al. Inhibiting the Leidenfrost effect above 1000 °C for sustained thermal cooling. Nature 2022, 601, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Vakarelski, I.U.; Marston, J.O.; Chan, D.Y.C.; Thoroddsen, S.T. Drag Reduction by Leidenfrost Vapor Layers. Phys. Rev. Lett. 2011, 106, 214501. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; De Coninck, J. (Eds.) The Surface Wettability Effect on Phase Change; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Suárez-Alcántara, K.; Tena-Garcia, J.R.; Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. [Google Scholar] [CrossRef] [PubMed]
- Narayan, N.M.; Gopalkrishna, S.B.; Mehdi, B.; Ryll, S.; Specht, E.; Fritsching, U. Multiphase numerical modeling of boiling flow and heat transfer for liquid jet quenching of a moving metal plate. Int. J. Therm. Sci. 2023, 194, 108587. [Google Scholar] [CrossRef]
- Cai, C.; Mudawar, I. Review of the dynamic Leidenfrost point temperature for droplet impact on a heated solid surface. Int. J. Heat Mass Transf. 2023, 217, 124639. [Google Scholar] [CrossRef]
- Rathi, B.; Agarwal, S.; Shrivastava, K.; Miyaoka, H.; Ichikawa, T.; Kumar, M.; Jain, A. An insight into the catalytic mechanism of perovskite ternary oxide for enhancing the hydrogen sorption kinetics of MgH2. J. Alloys Compd. 2024, 970, 172616. [Google Scholar] [CrossRef]
- Delhomme, B.; de Rango, P.; Marty, P.; Bacia, M.; Zawilski, B.; Raufast, C.; Miraglia, S.; Fruchart, D. Large scale magnesium hydride tank coupled with an external heat source. Int. J. Hydrogen Energy 2012, 37, 9103–9111. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. Effect of oxygen on the hydrogen storage properties of TiFe alloys. J. Energy Storage 2022, 55, 105543. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.-X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Boussemghoune, M.; Chikhi, M.; Ozay, Y.; Guler, P.; Unal, B.O.; Dizge, N. The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support. Symmetry 2020, 12, 770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).