Abstract
The combustion of fossil fuels constitutes a significant catalyst for climate change, resulting in the annual release of about two billion tonnes of carbon dioxide (CO2). The increase in CO2 emission is directly linked to a heightened occurrence of natural calamities and health-related issues. The substitution of fossil fuels with renewable energy sources is a fundamental approach to reduce the negative impacts caused by consumption of these nonrenewable energy resources. The utilisation of biological methodologies to produce environmentally friendly energy from renewable sources holds significant potential for the sustainable production of fuel. However, the cultivation of first- and second-generation biofuel crops presents a challenge, since they compete for limited cropland, hence constraining their overall viability. In contrast, photosynthetic microorganisms such as algae and cyanobacteria exhibit significant potential as third-generation biofuel catalysts, devoid of the limitations associated with contemporary biofuels. Cyanobacteria, a type of photosynthetic prokaryotes, exhibit significant potential for the direct conversion of carbon dioxide (CO2) into biofuels, chemicals, and various other valuable compounds. There has been a growing interest in the concept of utilising biological processes to convert carbon dioxide into fuels and chemicals. The introduction of a limited number of heterologous genes has the potential to confer upon cyanobacteria the capability to convert particular central metabolites into a diverse range of end products. The progress in the field of synthetic biology and genetic manipulation has enabled the manipulation of cyanobacteria to synthesise compounds that are not generally produced by these organisms in their natural environment. This study focuses on recent papers that employ various methodologies to engineer cyanobacteria for the purpose of producing high-value compounds, such as biofuels.
1. Introduction
Cyanobacteria, the only prokaryote which possess the ability of oxygenic photosynthesis, play a critical role in various important biological processes, including nitrogen fixation, oxygen generation, and global carbon sequestration. These organisms have the potential to be employed in the development of a microbial manufacturing system that has the capability to produce valuable products by absorbing carbon dioxide from the environment and harnessing solar energy. According to Pierobon et al. [1], these photosynthetic microorganisms exhibit extensive ecological, metabolic, and chemical properties, which indicates that these organisms are highly promising in various biological applications. Cyanobacteria possess significant quantities of phycocyanin, lipids, and carbohydrates, rendering them promising candidates for the production of commercially valuable compounds. These chemicals are commonly produced via a specialized induction process, which may encompass physical stresses, such as variations in pH, light intensity, temperature variations, or chemicals (as stress parameters), such as nitrogen deprivation, carbon dioxide, and salt concentrations. Cyanobacteria serve as environmentally friendly and sustainable alternatives, offering promising prospects for the production of efficient biofuels, medicines, nutraceuticals, and potential therapeutic applications in the near future [2,3,4]. Earlier, the primary concern was the enhancement of integrative processes and cultural approaches, which included the development of optimised photo-bioreactor designs [5], strategies to minimise energy consumption during downstream processes for cyanobacteria harvesting [6], and approaches for extracting compounds of significant value [7]. Cyanobacteria are a perfect platform for complex metabolic engineering projects because they grow quickly and are relatively simple bacteria. Utilising a more comprehensive array of molecular tools has facilitated the exploration of cyanobacteria’s potential in the domains of metabolic engineering and biotechnology (Figure 1) [8,9,10]. The capacity to manipulate endogenous genetic material has expanded with scientific progress [10,11].
Selection and introduction of targeted genes and random mutagenesis are a few traditional techniques applied to increase the production of a desired compound [12]. A potential disadvantage associated with conventional methods is the substantial time commitment required for both the design and implementation phases. The resolution of these challenges can be accomplished through the application of metabolic engineering and systems biology techniques to boost microbial output. This strategy often involves the utilisation of mathematical models to simulate and forecast the behaviours that arise inside intricate systems [13]. The utilisation of systems biology methodologies, which rely on the implementation of standardised and thoroughly characterised modules or biological components like promoters, RBSs, riboswitches, and terminator libraries, presents a viable approach for the manipulation of organisms to enhance microbial production [6,14].
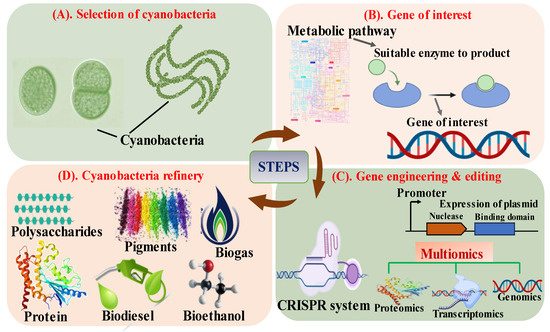

Figure 1.
Schematic representation of steps involved in development of biorefinery in cyanobacteria through genetic approaches; (A) Selection of potentially useful cyanobacteria for the synthesis of biofuel; (B) Identifying the gene of interest for specific metabolite; (C,D) Engineering the target gene into a plasmid and the production of the desirable compound by utilizing various multiomics approaches (modified from Lin et al. [15]).
The CRISPR/Cas9 system, formally referred to as clustered regularly interspaced short palindromic repeats with associated protein 9, has rapidly evolved as a highly efficient and state-of-the-art technology within the past decade [16,17]. The utilization of CRISPR methodology has been extensively utilised to augment cell metabolism, control biosynthetic pathways, and enhance the rates and yields of metabolite production [18]. The application of CRISPR-Cas9 technology to genetically edit genes in the model microalga Chlamydomonas reinhardtii was first demonstrated by Jiang et al. [19]. In contrast, prokaryotic cyanobacteria possess genomes of relatively limited size and have undergone comprehensive sequencing. Several examples of cyanobacteria with desirable characteristics, including increased growth rates and substantial lipid content, are Nostoc sp. PCC 7120, Synechocystis strains PCC 6803 and PCC 7002, as well as Synechococcus strains UTEX 2973 PCC 6301 and PCC 7942. In contrast to eukaryotic microalgae, the operating systems for genetic engineering in these particular organisms are significantly less complex. As a result, a multitude of efforts have been made to enhance the synthesis of fatty acids and other environmentally friendly substances, as demonstrated by the investigations carried out by Santos-Merino et al. [20] and Eungrasamee et al. [21]. Shabestary et al. [22] limited the optimal cell density of biofuel producing Synechocystis cultures utilising inducible CRISPR interference technology.
In recent years, researchers have developed various strategies to enhance the rate of biochemical production. These methodologies encompass either the upregulation of proteins or the genetic manipulation of microorganisms. The primary aim of this study is to present a succinct overview of the possible uses of cyanobacteria in the field of biorefinery, with a specific focus on their potential in the synthesis of various biofuels (such as hydrogen, ethanol, butanol, biodiesel, and lipids), bulk chemical compounds, pigments, and valuable compounds. The subsequent discussion will explicitly address the utilization of multi-omics technology, encompassing genomes, proteomics, and metabolomics, in conjunction with the biorefinery idea for bio-products across various cyanobacterial strains. The inclusion of genetic engineering and genome editing through the utilisation of the innovative CRISPR technology will also be encompassed.
2. Editing the Green E. coli: Cyanobacteria
2.1. Understanding the Metabolism of Cyanobacteria
Cyanobacteria serve as valuable model organisms for comprehending the metabolic processes of higher plants owing to their advantageous traits, including (1) a substantial lipid content primarily found in thylakoids, (2) heightened levels of photosynthetic activity and cellular growth rates compared to algae and higher plants, and (3) facile growth requirements, encompassing essential elements, such as atmospheric carbon dioxide (CO2) and nitrogen (N2), water, mineral salts, and light [23]. In cyanobacteria, both photosynthesis and respiration take place within their cellular and intracytoplasmic membranes. With the exception of Gloeobacter genus members, which possess only the cytoplasmic membrane, respiration is observed in both the cytoplasmic and thylakoid membranes, but photosynthesis occurs specifically within the thylakoids [24].
According to Furbank et al. [25], the enzyme RubisCO assumes a crucial function in constraining the carbon acquisition process within plant photosynthesis. The CBB cycle, utilises ATP and NADPH-reducing power derived from photosynthetic electron transport. This pathway is responsible for the biological fixation of CO2, accounting for an estimated annual fixation of 260 gigatons tonnes [26]. The first or most discussed enzyme that is involved in the generation of two molecules of 3PGA is RubisCO, which is a three-carbon sugar. RubisCO facilitates the incorporation of one molecule of carbon dioxide (CO2) into one molecule of RuBP, a five-carbon sugar. The study conducted by Satagopan et al. [27] employed a purple bacteria strain, Rhodobacter capsulatus, which lacked its own RubisCO enzyme, as a host for selecting Synechococcus PCC 6301 RubisCO variants. The objective was to identify variants that exhibited a higher level of CO2-dependent growth activity compared to the wild-type enzyme.
One of these mutations showed a nearly two-fold augmentation in the carboxylase activity of RubisCO. Cyanobacteria have developed effective CCMs to enhance photosynthetic CO2-fixation. These mechanisms involve the utilization of several active and assisted absorption systems for inorganic carbon (Ci, HCO3−, CO2) [28]. Cyanobacterial CCMs have been observed to enhance the cytoplasmic Ci pool through various mechanisms, including the activation of bicarbonate transporters, CO2-uptake complexes, and auxiliary and regulatory proteins. These mechanisms primarily result in the accumulation of bicarbonate ions, which are less permeable across the cellular membrane. The carboxysome, which is a micro-compartment containing RubisCO, is able to uptake cytoplasmic bicarbonate through a protein shell that selectively allows the passage of this molecule [11,29]. Once inside, the bicarbonate is converted to CO2, being incorporated into 3PGA. The PEPC enzyme not only facilitates the production of RubisCO, but also catalyses the irreversible carboxylation of PEP, a three-carbon metabolite. This carboxylation process involves the utilisation of bicarbonate (HCO3−) ions, leading to the formation of oxaloacetate, a four-carbon metabolite of the citric acid cycle, along with the release of inorganic phosphate. The enzyme known as PEPC, which is present in high quantities in cyanobacteria, has been identified as playing a critical role in aiding the photoautotrophic growth and development of both Synechocystis PCC 6803 [30] and Synechococcus PCC 7942 [31]. According to a study carried out by Takeya et al. [30], it was determined that PEPC accounted for 25% of the carbon dioxide fixation in Synechocystis PCC 6803 [31].
The major metabolic network of cyanobacteria encompasses various metabolic pathways, including glycolysis, the PP pathway, the CBB cycle, a segment of the TCA cycle, and C1 metabolism, as depicted in Figure 2. Cyanobacteria exhibit the presence of many glycolytic variant pathways, namely the EMP, OPP, PK, and ED pathways, as documented by Chen et al. [32]. All of these organisms generate ATP, NAD(P)H, and carbon precursors that are utilised in the biosynthesis of amino acids, nucleotides, fatty acids, and metabolites [32,33]. In the glycolytic process known as the EMP pathway, the enzyme PFK catalyses the phosphorylation of glucose on two separate occasions, resulting in the production of FBP. Subsequently, the process of FBP cleavage results in the production of GAP and DHAP, which are subsequently employed in the generation of ATP via the process of phosphorylation. In the EMP pathway, glucose undergoes a single phosphorylation event to generate G6P. Subsequently, G6P is enzymatically converted into 6PGA and further metabolised to KDPG. Notably, KDPG is a metabolite that is uniquely associated with the EMP system. KDPG is subsequently hydrolysed into a single pyruvate molecule and one GAP molecule, which are utilised in the generation of ATP [34].

Figure 2.
The figure represents the crosstalk of various pathways involved in the central carbon metabolic network in cyanobacteria, including the Embden-Meyerhof-Parnas (EMP; orange) pathway, the Entner-Doudoroff (ED; red) pathway, the oxidative pentose phosphate (OPP; green), the Calvin-Benson-Bassham (CBB; green) pathway, the phosphoketolase (PK; sky blue) pathway, and the tricarboxylic acid (TCA; purple) cycle. ADP-glucose (ADPG), bisphosphoglycerate (BPGA), acetyl-CoA (Ac-CoA), acetyl phosphate (Ac-P), dihydroxyacetone phosphate (DHAP), fructose-6-phosphate (F6P), glucose-1-phosphate (G1P), glucose-6-phosphate (G6P), glyceraldehyde-3-phosphate (GAP), pyruvate (Pyr), ribose-5-phosphate (R5P), ribulose-5-phosphate (Ru5P), ribulose-1,5-bisphosphate (RuBP), sedoheptulose-7-phosphate (S7P), sedoheptulose-1,7-bisphosphate (SBP), keto-3-deoxy-6-phospho-gluconate (KDPG), phosphoenolpyruvate (PEP), Xylulose-5-phosphate (Xu5P), 2-phosphoglycerate (2PGA), 3-phosphoglycerate (3PGA), 6-phosphogluconolactone (6PGL), 6-phosphogluconate (6PGA), and erythrose-4-phosphate (E4P) are the various intermediates in the pathway. Edda, a KDPG aldolase; Edd, a 6PGA dehydratase; FBPase, a fructose-1,6-bisphosphatase; fructose-1,6-bisphosphate aldolase (FBA); glyceraldehyde-3-phosphate dehydrogenase 1 or 2 (GAP1-2); glucose dehydrogenase (GDH); gluconate kinase (GK); glucokinase (GLK); malic enzyme (ME); pyruvate dehydrogenase (PDH); PEP carboxylase (PEPC); phosphofructokinase (PFK); phosphoglucose isomerase (PGI); phosphoglycerate kinase (PGK); poly-β-hydroxybutyrate (PHB); phosphoketolase (Pk); pyruvate kinase (PK); phosphoribulokinase (PRK); R5P isomerase (Rpi); Ru5P epimerase (Rpe); ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO); transaldolase (Tal); transketolase (Tkt); glucose-6-phosphate dehydrogenase (Zwf); glutamate dehydrogenase (GDH); 6-phosphogluconate dehydrogenase (6PGD); fumarase (FumC); GABA aminotransferase (GABT); glutamate decarboxylase (GAD); isocitrate lyase (ICL); isocitrate dehydrogenase (IDH); malate dehydrogenase (MDH); malic enzyme (ME); malate synthase (MS); pyruvate dehydrogenase (PDH); PEP carboxylase (PEPC); succinate dehydrogenase (SDH); and succinic semialdehyde dehydrogenase (SSADH) are the enzymes involved in the central carbon metabolism of cyanobacteria (modified from Veaudor et al. [34]).
In order to achieve an equivalent level of glycolytic flux as the EMP pathways, the ED pathway requires a significantly reduced quantity of enzyme proteins, approximately 3.5 times smaller. The catabolism of a single glucose molecule by the EMP pathway yields two NADH molecules, while the ED pathway produces one ATP molecule, one NADH molecule, and one NADPH molecule. Cyanobacteria frequently employ the ED pathway as opposed to the EMP pathway, due to their limited access to nutrients rather than a limitation in ATP availability [34]. The conversion of 6PGA to pentose phosphates and the generation of extra NADPH occur through the action of 6PGAD in the OPP pathway. Subsequently, the enzymatic activities of transketolase and transaldolase facilitate the conversion of pentose phosphates into triose phosphates. The regulation of the pentose phosphate pathway is stringent due to its ability to function in two distinct modes: oxidative mode (OPP pathway) for carbohydrate oxidation and reductive mode (Calvin cycle) for carbon dioxide fixation.
In contrast to the aforementioned procedures, the pentose phosphate pathway (PK pathway) circumvents the oxidation of triose to acetyl compounds by cleaving phosphorylated sugars (glucose 6-phosphate or xylulose 5-phosphate) to generate C2 units in the form of acetyl phosphate. The quantity of ATP and NAD(P)H molecules generated per glucose molecule catabolized in cyanobacteria exhibits variation across different glycolytic routes, with ATP production ranging from 1 to 2.33 molecules and NAD(P)H production ranging from 0 to 5.33 molecules [32,33].
The prevailing consensus in the scientific community is that cyanobacteria possess an incomplete TCA cycle due to the absence of 2-oxoglutarate dehydrogenase, which consequently hinders their ability to convert 2-oxoglutarate into succinyl-coenzyme A. The genes responsible for producing a hitherto unidentified 2-oxoglutarate decarboxylase and succinic semialdehyde dehydrogenase have been discovered in the cyanobacterium Synechococcus sp. PCC 7002. Collectively, these two enzymes facilitate the conversion of 2-oxoglutarate into succinate, effectively serving as substitutes for the enzymatic activities of 2-oxoglutarate dehydrogenase and succinyl-CoA synthetase. The presence of these genes has been observed in all cyanobacterial genomes, with the exception of Prochlorococcus and marine Synechococcus species [34,35]. The enzymes isocitrate lyase (Icl) and malate synthase (MS) are involved in the glyoxylate pathway in cyanobacteria, as described by Sarkar and Shimizu [36]. The Mez and PEP synthase (Pps) enzymes play a crucial role in the gluconeogenic pathway, particularly in Synechocystis, where the PEP carboxy-kinase (Pck) enzyme is not present. The CBB cycle is known to operate in both mixotrophic and autotrophic environments, but the pentose phosphate (PP) route mostly functions in heterotrophic conditions for glucose catabolism. The Calvin cycle, OPP, and EMP pathways of Synechocystis PCC 6803, a cyanobacterium capable of growth under many metabolic settings, including photoautotrophic, mixotrophic, and heterotrophic conditions, are likely to regulate their metabolic fluxes in response to different growth environments. In contrast, the TCA cycle exhibits continuous activity at relatively modest levels. In low-light or dark conditions, the metabolic process of oxidative phosphorylation is utilised by organisms to generate ATP from NADPH. Moreover, Wan et al. [37] observed a minimal flux across the ED pathway in heterotrophic conditions.
2.2. Tools and Techniques Implied for the Editing of Cyanobacteria
2.2.1. CRISPR-Technology
The efficiency of the homologous recombination methodology was found to be suboptimal, prompting the development of the CRISPR-assisted method as a means to enhance the effectiveness of genome editing. The method employs a range of tools, including the CRISPR/Cas9 system, as well as the CRISPR/deactivated Cas9 (dCas9), CRISPR/Cpf1, and Cas9-ribonucleoprotein (RNP). Initially, a 20-nucleotide guide RNA (gRNA) target sequence was selected in close proximity to a 2–6 base pair DNA region, taking into consideration the presence of PAM. The Cas9-related system traditionally identified the PAM site as 5′-NGG-3′. However, the Cpf1 system exhibited recognition of a PAM sequence that is rich in thymine (T), specifically 5′ -TTTTN- 3′ [15]. The verification of the clones would be accomplished by the utilisation of DNA sequencing techniques. In a recent study conducted by Ungerer and Pakrasi [38], it was demonstrated that both Cas9 and Cpf1 were effectively employed for genome modifications in different cyanobacterial species. However, it was observed that Cas9 exhibited a higher level of toxicity compared to Cpf1. One potential option could involve the provision of Cas9 within a non-replicable plasmid lacking the homologous region. Behler et al. [39] reported that the cell doubling period of cyanobacteria ranged from 6 to 12 h. This duration allowed for the completion of Cas9 expression, facilitating genome editing and the generation of marker-free mutant strains. According to Li et al. [40], the Cpf1 nuclease has the potential to decrease the size of the homologous arm in cyanobacteria to 400 bp. In the study conducted by Wendt et al. [41], it was shown that the marker-less and scar-less transformation of cells was achieved through a series of cell inoculations in a medium lacking the selection conditions for a total of 10 generations. This process resulted in a plasmid cure rate of roughly 75%. According to Niu et al. [42], Cpf1 is a more suitable choice than Cas9 for synthetic regulation, large DNA fragment deletion, or editing in cyanobacteria due to its practicality in a broader spectrum of hosts.
2.2.2. Multi-Omics
The rapid advancement of biotechnology has been facilitated by the integration of advanced gene engineering techniques and multi-omics analysis. This convergence has played a pivotal role in the emergence of novel concepts, such as synthetic biology and metabolic engineering. Synthetic biology seeks to identify fundamental biological components with the aim of expeditiously devising novel applications within the field of biology. The biological component of a functional protein has been extracted through the process of sequence analysis, encompassing multiple organisms. Furthermore, when combined with transcriptome analysis utilising the genome sequence, it becomes possible to predict the strength and vulnerability of promoters. The objective of metabolic engineering is to establish a dependable biological system capable of achieving substantial chemical synthesis through the use of genome technology. This involves the strategic design and manipulation of genetic and editing circuits to redirect the flow of carbon, as well as the incorporation of proteomics and metabolomics in certain contexts.
Recently, single cell genomics has been utilised for the identification of microbes residing symbiotically inside or on the surface of living host. The cells of the novel cyanobacterial symbiont, dinoflagellate Ornithocercus magnificus, were identified utilising this approach. The study reveals that this undiscovered, but globally widespread, cyanobacterium has undergone significant genomic reduction independent of its closely related organisms, including Synechococcus and Prochlorococcus [43]. The research conducted by Prabha et al. [44] involved a comprehensive analysis of around 41 cyanobacterial genomes to determine the presence and patterns of codon usage bias. The findings revealed that T-terminal codons are largely distributed across the genome, irrespective of the GC concentration. Furthermore, the study demonstrated the absence of any discernible codon usage bias among the genes examined. In addition to the comparison of DNA aspects, the comprehensive study of the entire genome provided evidence for the prediction of proteomes and metabolomes. The accurate anticipation of protein–protein interactions (PPI) in Synechocystis sp. PCC6803 facilitated the investigation of photosynthesis and DNA repair. In a study conducted by Lv et al. [45], the proteins ssl3451, sll1252, and ssl0822 were effectively characterised. These proteins are known to play significant roles in photosynthesis, organic ion trans-membrane transport, and sigma factor activity, respectively. The investigation of cyanobacteria through comparative genome analysis has revealed their capacity to host several natural processes involved in the production of secondary metabolites. These pathways produce intermediate or final compounds that exhibit interesting therapeutic potentials [46]. Reports have indicated the occurrence of notable growth inhibition in Synchocystis sp. PCC6803, with initial attribution of this phenomenon to the toxic effects of lactate. Nevertheless, an alternative hypothesis rooted in the field of proteomics was proposed, suggesting that the observed redox pair imbalance could be attributed to the overexpression of a specific protein involved in the process of dehydrogenation [47]. The identification of columbamide A, a novel secondary metabolite, was achieved through the utilisation of metabolomics coupled with nuclear magnetic resonance in the highly productive cyanobacteria Moorea, as reported by Kleigrewe et al. [48].
Moreover, the application of mass spectrometry for isotope analysis can be employed to authenticate the distribution of metabolic fluxes. In a study conducted by Kanno et al. [49], genetic modifications were introduced to Synechococcus elongatus PCC7942 in order to enhance the production of 2,3-butanediol, facilitate carbon dioxide fixing, and enable glucose utilisation. In their study, Kanno et al. [49] employed metabolomics analysis in combination with C13 isotope supply to demonstrate the significance of RuBP availability in cyanobacteria for CO2 fixation and chemical synthesis. The cellular behaviour of 3-hydroxypropionate-producing Synechocystis sp. PCC6803 [50] and ethanol-producing Synechococcus sp. PCC7002 [51] has been comprehensively investigated using a technique that integrates genomes, proteomics, and metabolomics. Recent years have revealed a strong synergistic relationship between the gene expression patterns and various metabolic states, indicating the viability of gene expression-led metabolic remodelling in cyanobacteria. These datasets have grown increasingly large and have been combined with stress conditions [52].
A current challenge in industrial applications and investigations of the cyanobacterial environmental competence is population heterogeneity. Certain conditions, such as the uneven distribution of media composition and inconsistency of physical parameters like pH and temperature, as evidenced in large-sized bioreactors, can cause a significant loss of yield, as not all cells persist in the same optimal productive state [53]. Also, the advantageous attributes of cyanobacteria, such as high metabolic activity and the presence of gas vesicles, grant them ecological dominance. The transcriptome profiling of individual cells, utilising the single-cell RNA-seq integrated with a FACS (fluorescence-activated cell sorting) technique, has emerged as a possible solution, although the extensive work related to this technique is yet to be realized in the field of cyanobacterial biology [54].
3. Engineering Cyanobacteria as a Source of Value-Added Chemicals
In the past few years, there has been a notable endeavour to employ molecular techniques from many species with the aim of enhancing the capabilities of cyanobacteria and facilitating cellular reprogramming to achieve greater yields of valuable compounds [55].The careful choice of chassis cells is of utmost importance when constructing efficient and dependable facilities for the cultivation of photosynthetic cells, given the intricate nature of metabolic conversions and the anticipated advancements in engineering applications [56]. The ongoing discourse over the manipulation of cyanobacteria is situated within the wider context of the progressively advanced methodologies that have emerged in the domains of metabolic engineering and synthetic biology. These developments hold the potential to pave the way for cyanobacteria to make substantial contributions as crop species in the realm of next-generation biofuels.
There exist multiple strains of cyanobacteria that exhibit a high degree of susceptibility to genetic modification. Modifications of this nature can be executed via cis (chromosome editing) or trans (plasmid addition) mechanisms, both of which have been employed in synthetic biology experiments. The aforementioned approach has been employed in cyanobacteria, along with other bacterial species, in order to broaden the range of substances they can synthesise and to enhance overall production efficacy [30,57]. Cyanobacteria-derived biofuels have been recognised as an alternative resource and have been classified as third-generation biofuels. Cyanobacteria possess the ability to convert carbon dioxide and water into lipids that are rich in carbon content. These lipids serve as a suitable substrate for the synthesis of biodiesel with higher efficiency in comparison to agricultural crops; thus, they are preferred over the traditional oil-seed agricultural crops [58]. In contrast to microalgae, cyanobacteria have a higher tolerance potential for foreign genes when the latter are introduced into them. They have also established a good genetic framework, and their genes are much more resilient when manipulated; moreover, they have a well-regulated photosynthetic mechanism [59]. The study of multiple species of cyanobacteria for the generation of ethanol and the development of photosynthetic innovative biofuels using metabolic processes is made possible through the regulation of the genetic transformation procedures. The marine cyanobacteria Synechococcus sp. PCC 7002 and the two freshwater species Synechocystis sp. PCC 6803 and Synechococcus elongatus sp. PCC 7942 have been extensively researched for their ability to synthesise various fuel molecules.
3.1. The Production of Alcohol and Aldehydes
The utilisation of photosynthetic organisms in the organic synthesis of alcohol is a promising solution to address the constraints associated with petrochemical manufacturing. In a recent study conducted by Deng and Coleman [60], it was observed that Synechococcus 7942, a genetically modified strain of cyanobacteria, exhibited the ability to convert pyruvate into ethanol. This conversion resulted in a concentration of 450 nmol/L of ethanol over a period of 7 days. The process involved the utilisation of heterologous enzymes, namely pyruvate decarboxylase and alcohol dehydrogenase. In a research investigation focused on Synechococcus 7002, it was observed that a decrease in the ratio of NADPH to ATP within the intracellular membrane led to an augmented synthesis of ethanol. This phenomenon was attributed to alterations in intracellular carbon allocation, subsequently resulting in the accumulation of glycogen and soluble sugars. The aforementioned process led to the synthesis of 1-butanol [61]. In the research-based study carried out by Mishra et al. [62], Synechococcus 7942 was utilised as a model organism to showcase the CoA-dependent photoautotrophic synthesis. To do this, the butanol pathway genes from Clostridium acetobutylicum were introduced into Synechococcus 7942. Specifically, the EtfAB and tlh genes were replaced with the Treponema denticola and autoB genes (Thiolase) from E. coli. In contrast, the introduction of the mature culture into typical photosynthetic conditions resulted in a limited production of butanol. Conversely, subjecting the culture to prolonged periods of darkness and oxygen deprivation led to a subsequent increase in butanol accumulation, reaching a level of 14.5 mg L−1 within a span of 7 days [63].
3.1.1. Ethanol Pathway
Alcohol dehydrogenase (Adh) and pyruvate decarboxylase (Pdc) are integral enzymes within the ethanol biosynthesis process. The initial enzyme uses a non-oxidative decarboxylation process to convert pyruvate into acetaldehyde and carbon dioxide, whereas the subsequent enzyme utilises acetaldehyde to generate ethanol. The original experiment for ethanol production was the utilisation of Synechococcus 7942 as the host organism, in which the pdc and adhII genes from Zymomonas mobilis, a known ethanol producer, were heterologously expressed. This expression was carried out under the control of the rbcLS promoter of Synechococcus 7942, despite its inhibitory effect. A quantity of ethanol corresponding to 5 mM (0.23 g L−1) was generated following a cultivation period of 4 weeks, as reported by Deng and Coleman [60]. Additionally, endogenous alcohol dehydrogenase (Adh) and Pdc from Z. mobilis were also co-overexpressed in the Synechocystis 6803 strain. The expression of all four CBB cycle genes was regulated by the psbA2 promoter, while the expression of ethanol biosynthesis genes was controlled by the PnrsB promoter. Following a development period of 7 days at a light intensity of 65 µmol protons m−2 s−1 and a concentration of 2.5 µM Ni2+, a notable rise in ethanol accumulation was detected in the strain that co-expressed Fba together with Pdc and Adh. The ethanol accumulation in this strain was around 750 mgL−1, while the strain expressing only Pdc and Adh had an ethanol accumulation of approximately 400 mgL−1. According to Liang et al. [64], strains that overexpress RuBisCO, FBP/SBPase, and TK enzymes produced ethanol within the range of 600 to 750 mg−1 L−1. According to Roussou et al. [65], the strain that co-overexpressed FBA and TK exhibited a nine-fold increase in ethanol production compared to the strain that only overexpressed FBA, and a four-fold upregulation in comparison to the selective strain that only expressed TK.
3.1.2. CoA Pathway-Dependent Alcohols
The fermentation processes involved in the production of isopropanol and butanol (specifically 1-butanol) proceed via the acetone/butanol/ethanol (ABE) pathway. While isopropanol does possess a higher energy yield than ethanol, its applicability as a long-term substitute for petrol is limited. On the contrary, butanol possesses characteristics that closely resemble those of petrol and can be employed as a direct fuel source in petrol engines [66,67]. The genes associated with the butanol pathway derived from C. acetobutylicum were successfully expressed in Synechococcus 7942. This particular organism holds the distinction of being the first to demonstrate CoA-dependent photoautotrophic synthesis of 1-butanol. The ter gene from T. denticola and the atoB gene (Thiolase) from E. coli were utilised instead of EtfAB and tlh, respectively. Nevertheless, in typical photosynthetic conditions, the presence of butanol was observed in only a minimal amount. However, when the culture was subjected to both darkness and an anoxic environment, a further increase in butanol accumulation (approximately 15 mg L−1 in a week) was observed [63]. The initial reversible decarboxylation reaction involved in the synthesis of acetoacetyl coA potentially diminished the driving force necessary to channel the metabolic flow towards the desired product. Consequently, this may have led to a decrease in the size of the NADH pool, as NADPH is generated during photosynthesis. This reduction in NADH availability could have contributed to the observed decrease in the final concentration of the desired compound. The elimination of limitations arising from the reversible nature of the initial stage in the Clostridium butanol pathway and the presence of NADH in Synechococcus 7942 was achieved through the introduction of novel genes. The conversion of acetyl-CoA to acetoacetyl-CoA was achieved by considering the ATP-driven irreversible condensation of malonyl-CoA in the process of fatty acid biosynthesis. This was accomplished through the introduction of an acetoacetyl-CoA synthase (encoded by NphT7) derived from the Streptomyces sp. strain, as demonstrated by Lan and Liao [63]. The NADPH-dependent acetoacetyl-CoA reductase (PhaB) from Ralstonia eutropha and the (R)-specific enoyl-CoA hydratase (PhaJ) from Aeromonas caviae were substituted for Hbd and Crt, respectively. In order to circumvent the utilisation of AdhE2 and compensate for its bifunctional nature, the substitution of AdhE2 was carried out by expressing two additional enzymes, namely CoA-acylating butyraldehyde dehydrogenase (Bldh) and NADPH-dependent alcohol dehydrogenase (YqhD) from E. coli. Bldh is responsible for the conversion of butyryl-CoA to butyraldehyde, while YqhD serves as an alternative enzyme. In the study conducted by Kusakabe et al. [68], Synechococcus 7942 was selected as the initial subject to investigate the purpose of synthesising isopropanol. In this study, the IPTG-inducible promoter PLlacO1 was employed to enhance the transcription of the genes thl and adc derived from Clostridium acetobutylicum ATCC 824, ctfAB (encoded by atoAD) from E. coli K-12 MG1655, and adh from C. beijerinckii. Propanol was not detected under the standard growth conditions. Nevertheless, the introduction of potassium acetate into the medium resulted in the detection of 21.7 mg L−1 isopropanol after a period of one week. This finding suggests that there were constraints on the carbon flow for acetyl-CoA. It is widely recognized that numerous species of cyanobacteria have the capability to synthesize acetate through the utilization of glycogen stores under conditions of inadequate oxygen and light availability. The strain responsible for isopropanol production was then cultivated in a controlled environment, lacking phosphorus, nitrogen, light, and oxygen. This experimental condition led to the production of 26.5 mg L−1 of isopropanol [68]. Hirokawa et al. [69] observed a significant enhancement in propanol yield (146 mg L−1) by the manipulation of growth phase duration, production phase duration, and the transition from dark and oxygen deficient to light and oxygen efficient circumstances. This modification resulted in a six-fold increase in propanol production.
3.2. Carotenoid Production
Carotenoids, tetraterpenoids, are found in all photosynthetic organisms, and they enhance light absorption and energy dissipation during photosynthesis. In addition to well-known compounds such as β-carotene, zeaxanthin, astaxanthin, and echinenone, cyanobacteria also synthesise specialised carotenoids, such as myxoxanthophyll and orange carotenoid proteins, which function as mechanisms for protecting against photodamage [70,71]. The commercial significance of astaxanthin and zeaxanthin in nutraceuticals stems from their antioxidant characteristics, while β-carotene is utilised as a pro-vitamin A supplement. The limited carotenoid content of cyanobacteria presents a challenge for their competitiveness in the market. However, the application of genetic engineering holds promises for enhancing carotenoid production in cyanobacteria [71]. The process of carotenogenesis is a multifaceted, yet extensively studied, metabolic pathway that enables cyanobacteria to produce a diverse array of terpenoids. The production of these chemicals is initiated with the precursor geranylgeranyl pyrophosphate. The genes responsible for encoding the synthases, desaturases, hydroxylases, and cyclases involved in the biosynthesis of carotenoids have been identified and documented in a study conducted by Sugiyama et al. [72].
The biosynthesis route initiates with the condensation of two molecules of geranylgeranyl pyrophosphate by phytoene synthase (CrtB), resulting in the formation of phytoene. Subsequently, phytoene desaturase converts phytoene into ζ-carotene (CrtP), which is further transformed into lycopene by carotene desaturase (CrtQ). In addition to the production of the typical phytoene desaturase (CrtP), certain genera, such as Anabaena and Nostoc, possess the capacity to directly convert phytoene into lycopene through the action of a specific phytoene desaturase (CrtI). The production of α- and β-carotene, which are the two principal carotenoids, is derived from lycopene. The conversion of α-carotene is facilitated by the enzyme lycopene cyclase (CrtL or CruA), while the production of β-carotene involves a two-step process by the same enzyme (CrtL or CruA), starting from γ-carotene [72,73]. According to Pagels et al. [71], a significant proportion of carotenoids consist of a hydrocarbon chain composed of 40 carbon atoms (C40), which encompasses eight isoprenoid units and several double bond conjugations.
Astaxanthin, a carotenoid known for its powerful antioxidant properties, has been recognised for its potential health advantages [74]. The strain of Synechococcus 7002 has been genetically modified to include the genes β-carotene hydroxylase CrtZ and CrtW from Brevundimonas sp. SD212. As a result, this strain exhibits a significant astaxanthin concentration of 3 mg/g dry cell weight. According to Hasunuma et al. [75], the stimulation of photosynthetic central metabolism in astaxanthin-producing cells seems to compensate for the limited availability of β-carotene as a light-harvesting pigment. The conversion of astaxanthin to β-carotene is the most probable mechanism for achieving this outcome. The study conducted by Hasunuma et al. [75] demonstrated that this method leads to a higher yield of astaxanthin compared to the natural synthesis process by H. pluvialis. Furthermore, cyanobacteria utilise the MEP pathway to synthesise carotenoids, which is critical for enhancing the production of desired chemical compounds. This enhancement is achieved through the process of carbon partitioning, facilitated by the incorporation of CO2 [76]. The authors of this study modified the Synechocystis 6803 strain using genetic engineering techniques to enhance the biosynthesis pathway and reconfigure the intracellular metabolism. By these modifications, they successfully developed a far more efficient astaxanthin anabolic pathway, resulting in a remarkable 500-fold increase in its output. The study conducted by Diao et al. [77] involved the implementation of several key procedures to enhance the production of astaxanthin. These procedures encompassed the introduction of two vital enzymes, namely β-carotenoid ketolase and hydroxylase, along with the optimisation of screening techniques and carbon flux enhancements to augment the availability of precursors in the native MEP pathway. The outcomes of these interventions were observed to positively impact both photosynthesis and central metabolism, potentially attributable to the increased flux of astaxanthin, which reached a level of 29.6 mg g−1 cell dry weight. Furthermore, in their study, Gao et al. [78] demonstrated an augmentation in the synthesis of canthaxanthin (8.8 mg L) and echinenone (16%) through the overexpression of the crtO gene sourced from Nostoc flagelliforme in Nostoc sp. PCC 7120. The cyanobacterium Synechocystis was genetically modified to incorporate the MEP α-branch, resulting in the buildup of lutein. A Synechocystis strain deficient in the native cyanobacterial lycopene cyclase cruA was genetically modified by introducing a cassette containing four genes from Arabidopsis thaliana. These genes encode two lycopene cyclases (AtLCYe and AtLCYb) and two hydroxylases (AtCYP97A and AtCYP97C). The observed synlut strain exhibited normal growth characteristics similar to the wild-type strain, along with minor alterations in pigment production. Certain carotenoids are also effective in preventing several chronic diseases in humans. Changes in salinity and nutrients cause variations in carotenoid composition, which offers an effective way to boost specific targeted carotenoids [79]. These findings indicate that the absence of the cruA gene can be compensated for by the presence of Arabidopsis lycopene cyclases [80].
3.3. Terpenes and Isoprenes (Isoprenoid Pathway)
Terpenoids are a class of organic chemicals that possess notable industrial utility, including several sectors, such as food production, fragrance manufacturing, cosmetic formulation, chemical synthesis, and the development of biofuels. According to Schempp et al. [81] and Mata-Gomez et al. [82], these substances possess diverse chemical characteristics and can serve as colourants, perfumes, feedstock, or precursors. According to Ko et al. [83], these compounds have the potential to serve as advanced fuels supplementing gasoline, jet fuel, and diesel. Isoprene acts as a viable precursor for the generation of second-order drop-in biofuel molecules via the process of oligomerization. Additionally, cyclic monoterpenes, such as limonene, exhibit greater energy density and possess physical features that closely resemble those of Jet A-1 aviation fuel [84]. DMAPP and IPP, consisting of five carbon atoms each, serve as the fundamental constituents for the synthesis of all naturally occurring terpenoids. According to Pattanaik and Lindberg [85], prenyl transferases are enzymes that facilitate the condensation of two C5 units, leading to the formation of hydrocarbons with varying carbon chain lengths and functional groups.
Cyanobacteria have the capability to synthesise terpenoids via the MEP route, which involves the utilisation of G3P and pyruvate derived from photosynthesis. Terpenoids fulfil diverse functions in cellular physiology, encompassing light absorption, modulation of membrane fluidity, and defence against elevated light intensity and oxidative stress [86,87]. Extensive research has been conducted on cyanobacteria as prospective solar-powered cellular systems aimed at the conversion of atmospheric carbon dioxide into terpene-based molecules. Multiple methodologies have yielded cyanobacterial platforms that exhibit the ability to synthesise diverse chemicals, such as isoprene [88], β-phellandrene [89], and limonene [90].
Tracy et al. [91] and Chuck and Donnelly [92], demonstrated that certain terpenoids, including farnesene, myrcene, and limonene, as well as their hydrogenated derivatives, exhibit compatibility with both diesel and aviation fuels. Isoprenoid compounds frequently exhibit a diverse array of structural characteristics and molecular masses, hence presenting the potential for using these terpenoids as viable substitutes for conventional fuels [93,94].
3.4. Limonene
Limonene is basically the oil extracted from the peels of citrus fruit. It is a cyclic monoterpene and has countless applications in corporate sectors. It has a potential of replacing fossil fuels, such as jet fuel blending, and thus has become an important source of next-generation biofuels derived from sustainable sources [92,93,95]. The nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120 produces limonene, making it an essential source for the generation of biofuel, as well as the production of high value chemicals such as limonene. The natural synthesis of limonene could be upregulated via genome modification to increase its yield.
The heterologous expression of limonene synthase (Lms) from Schizonepeta tenuifolia allowed the synthesis of volatile monoterpene limonene in Synechocystis 6803. Three native MEP pathway genes (dxs, crtE, and ipi) were co-expressed with a codon-optimized lms under the control of the Ptrc promoter. Limonene was produced at a rate of 41 g L−1 d−1 by strains that just expressed lms, while 56 g L−1 d−1 was produced by strains that also expressed three additional MEP pathway genes [96]. A group of researchers has successfully engineered a filamentous cyanobacterium, namely Anabaena sp. PCC 7120, to produce and release limonene. This was achieved by expressing the limonene synthase gene from Sitka spruce plant into the cyanobacterium. Limonene is actively transported across the cellular membrane and then released into the surrounding headspace, facilitating its efficient separation from the biomass present in the culture. The coexpression of a synthetic DXP operon (dxs-ipphp-gpps), which includes three rate-limiting enzymes of the MEP pathway, was employed to redirect carbon flux from the Calvin cycle towards the production of limonene, in the context of limonene synthesis. When exposed to increasing levels of light, the yield of limonene increased by a factor of 6.8, while the maximum rate of synthesis increased by a factor of 8.8. The methodology described in this study has the potential to be utilised in the synthesis of a wide range of commodity chemicals and drop-in-fuels [97].
By modifying the ribosome binding sites (RBS) in Synechococcus 7942, Wang et al. [50] altered the trc promoter’s initial RBS to boost limonene production. Consequently, strain L1113 demonstrated limonene production of 32.8 g/L/OD/d. Similar to this, a synthetic RBS inserted into the psbA promoter enhanced the production of limonene to 885.1 g/L/OD/d. However, before a process can become economically viable, production needs to be significantly boosted [95,97].
Acoording to Lin et al., [98] the genetic modification in Synechococcus elongatus UTEX 2973 enabled the production of limonene, a terpenoid compound widely utilised in commercial applications. A mutation was discovered in the gene responsible for producing geranylgeranyl pyrophosphate synthase crtE, which led to a significant 2.5-fold augmentation in the production of limonene. The strain exhibited a limonene production of 16.4 mg L−1 at a rate of 8.2 mg L−1 day−1, which is an eight-fold improvement compared to the prior limonene productivity observed in other cyanobacterial species. The investigation employed a combinatorial metabolic engineering strategy to optimise the genes implicated in the biosynthesis of limonene. The findings of the study indicate that augmenting the expression levels of enzymes in the MEP pathway plays a pivotal role in facilitating the production of limonene.
3.5. Fatty Metabolites (Fatty Alkanes, Fatty Alcohols, Fatty Acids)
Due to increasing apprehensions regarding global warming, biofuels have garnered recognition as potentially viable substitutes for fossil fuels due to their renewable nature and generally reduced carbon emissions [99]. Oleochemicals encompass a diverse range of fatty acid derivatives, including fatty acids, fatty alcohols, fatty alkanes, fatty acid methyl/ethyl esters, and waxes [100]. According to Marella et al. [101], these substances have many applications, such as biodiesels, lubricants, surfactants, and other purposes. Lipid-derived biodiesels have gained recognition as superior biofuel molecules compared to ethanol, primarily due to their high energy density and compatibility with the pre-existing liquid fuel infrastructure [102].
Alkanes are the major constituents of conventional petroleum; thus, alkane could be produced by several strains of cyanobacteria, and biosynthesis could be enhanced through genome modifications to create less environmental pollution, which is essential for the sustainable production of biofuels [103,104]. Firstly, the FAR enzyme converts fatty acyl-ACP to fatty aldehyde. Secondly, the polyketide synthase enzyme synthesises alkenes [104]. The optimization of fatty alkanes production was carried out in Synechococcus sp. NKBG15041c, which produces α-olefin through the expression of the aar and ado genes. Thus, alkane was synthesized from fatty acids and fatty aldehyde substrates through the action of ADO and AAR [105,106]. Overexpressing both aldehyde-deformylating oxygenase and acyl-acyl carrier protein reductase, Wang and colleagues created a number of mutant strains of Synechocystis 6803, with a maximum yield of 1.3% of DCW [107]. Some cyanobacterial strains under salt stress can also synthesize alkanes. Subsequently, alkane production was reported to be 1200 µg−1 DCW when Anabaena sp. PCC 7120 was cultivated under salt stress (nitrogen deficiency) conditions [108].
It is possible to create energy-rich fatty alcohol molecules from FAA and its active forms, such as fatty acyl-ACPs and fatty acyl-CoAs, which have the potential to be used directly as biofuel. The enzymes known as FAR were first discovered in the jojoba plant before being found in bacteria, plants, insects, and mammals. Different pathways are used in eukaryotic and prokaryotic systems to produce fatty alcohol, with fatty acyl-CoA reductase (Acr) converting fatty acyl-CoA to fatty aldehyde, which is then converted to alcohol by fatty aldehyde reductase (Ahr) [93,109].
Fatty alcohols can be readily used as an alternative to fuels since they are molecules that possesses high energy. The pathway for making fatty alcohols in cyanobacteria is similar to that for making fatty alkanes, in which the intermediate aldehyde serves as the substrate for the corresponding alcohol. In cyanobacteria, biosynthesis of fatty alcohols can occur when acyl-ACP or acyl-CoA is reduced. The production of fatty alcohols was enhanced in Synechocystis 6803 when a fatty acyl-CoA reductase was expressed, obtained from Simmondsia chinensis. Moreover, when the poly-3-hydroxybutyrate (poly-3HB) and glycogen were prevented from carbon partitioning, an improved production of fatty alcohol was observed [110,111]. When comparing cells treated with the chemical molecule butylated hydroxyanisole (CBHA) to the control (Co; 163.62 ± 1.57 mgL−1d−1), the biomass and lipid productivities increased by 11% (181.60 ± 1.94 mgL−1d−1) and 48% (18.71 ± 0.20 mgL−1d−1), respectively. The biomass yields in CBHA-treated cells could reach up to 2 g L−1 [112]. Additionally, enhancement of fatty alcohol production could also be achieved in Synechocystis 6803, when the fatty aldehyde reductase obtained from Marinobacter aquaolei VT8 was expressed, followed by the elimination of reductase enzymes from alkane synthesis pathways [113].
Fatty acids have gained immense recognition for the biological synthesis of biofuels owing to their ability to produce compounds similar to diesel. They are crucial for hydrocarbon-based biofuel production, and converting cyanobacteria into cell factories through metabolic engineering can be beneficial for the next generation. Cell-free fatty acids (FFAs) are a beneficial method, as they eliminate the time and cost of cell recovery or biofuel extraction, leading to an increased FFA production and secretion in cyanobacteria [114]. Although there are limited instances of genetic transformation for microalgae, successful examples of genetic manipulation that improve plant oil production (and/or change the fatty acid composition) are reported in organisms such as Brassica napus and Arabidopsis thaliana [115]. FFAs are used as starting materials for biodiesel and substrates for fatty alcohol, alkene, and alkane synthesis. Fatty acids are a promising biosynthesis target due to their easy conversion into diesel-like compounds. The biological production of FFAs through metabolic engineering could meet heavy transportation fuel needs [116].
A thorough understanding of lipid metabolism and the molecular mechanisms underlying the increased triacylglycerol accumulation is needed for the efficient production of triacylglycerols. Genetic modifications of lipid pathways are possible for cyanobacteria by upregulating fatty acid biosynthesis or by downregulating β-oxidation. To increase the production of mono-unsaturated lipids, knocking out or modifying the enzymes responsible for the synthesis of polyunsaturated lipids in the cell can be performed [117]. As shown in Figure 3, the enzyme ACC, encoded by accDACB, catalyses the carboxylation of acetyl-CoA to malonyl-CoA. This process produces fatty acyl-ACP through the action of the enzyme fatty acid synthesis II (FAS II). An intermediate of fatty acid synthesis, acyl-ACP, or fatty acyl-ACP, is a key precursor for phospholipid production [118]. In Synechocystis, the biochemical balance of fatty acyl-ACP is gained through the recycling of free fatty acids into fatty acyl-ACP. This reaction involves the use of an enzyme, AAS, encoded by aas. Similarly, this balance is reduced by the production of hydrocarbons such as alkane and alkene. This conversion is catalysed by acyl-ACP reductase and aldehyde dehydrogenase encoded by the aar and ado genes, respectively [106,119]. A set of genes, PlsX, PlsY, and PlsC is responsible for the direct conversion of fatty acyl-ACP to phospholipids, whereas lipA converts the phospholipids into free fatty acids [120]. Changing the expression pattern of these genes affects the production of lipids in Synechocystis. For example, three Synechocystis 6803 strains having aas-overexpression, aas-overexpression with aar gene interruption, and accDACB-overexpression with the lipA gene showed a significant increase in lipid production. Whereas overexpression of aar/ado results in increased production of alkanes, especially heptadecane (Figure 3; Eungrasamee et al. [21]).

Figure 3.
The fatty acid biosynthesis and the cross-linking pathways that either increase or decrease the biochemical balance of fatty acyl-ACP in Synechocystis sp. PCC 6803. Key enzyme genes include accABCD, multi-subunit acetyl-CoA carboxylase gene; aar, acyl-ACP reductase gene; aas, acyl-ACP synthetase; ado, aldehyde oxidase; fabD, malonyl coenzyme A-acyl carrier protein transacylase; lipA, lipolytic enzyme genes; plsX, plsY, plsC, putative phosphate acyl-transferases; phaA, polyhydroxyalkanoates specific beta-ketothiolase gene. The green arrows represent the overexpression of that gene, whereas the red arrows represent the knockout of that gene (modified from Eungrasamee et al. [21]).
AAS is one of the primary targets to be eliminated for FAA synthesis in cyanobacteria because it may recycle the FFA into ACP. Kaczmarzyk et al. [119] inactivated AAS, thereby increasing FFA production in Synechocystis 6803 and Synechococcus 7942. They catalysed the esterification of FFA to ACP and used the released fatty acids as raw materials to create complex lipids by recycling them.
Kato et al. [121] found that the balance between FFA production and exportation is crucial for cyanobacteria FFA productivity. They manipulated the Synechococcus elongatus mutant (dAS1T) by inactivating the wzt gene, which effectively exports O-antigen in the outer membrane of cyanobacteria. This inactivation increased FFA secretion by removing the O-antigen layer from the dAS1T cells. In another experiment, the expression of GPD1 from Saccharomyces cerevisiae and DGAT from Rhodococcus opacus increased the lipid production of Synechocystis 6803. The genes encode glycerol-3-phosphate dehydrogenase and diacylglycerol acyltransferase essential for cyanobacteria’s lipid metabolism [114]. Therefore, several species of cyanobacteria, including Synechococcus 7942, Synechocystis 6803, and Synechococcus 7002, have all been genetically modified to produce fatty acids. Biosynthesis of lipid occurs when the hydrocarbon chains are elongated, which results from the condensation of the acyl-acyl carrier protein. The thioesterase enzyme causes the cleavage of acyl-ACP bond, producing free fatty acids FFA. Thus, acyl-ACP synthetase elimination promotes FFA buildup in the said microbial species by preventing FFA recycling [122,123]. Several genetically modified cyanobacteria along with their byproducts are listed in Table 1.

Table 1.
Genetically modified cyanobacteria and their byproducts.
4. Optimization of the Biofuel Production Pathway
Fossil fuels can be replaced by photosynthetic microorganisms that directly transform solar energy into liquid fuel. These organisms can be used to settle a potential conflict between using land for the production of food or biofuels [132]. The ability of photosynthetic microbes for biofuel production integrates two-step procedures, including the production of plant biomass and the synthesis of biofuel from biomass by using heterotrophs, into one scheme. The intermediates of the CBB cycle are directed into fermentative pathways to produce biofuel using photosynthesis as a substitute for conventional methods [133]. Along with heterologous pathway gene expression, several other factors are taken into consideration, as well as modified in the enhancement of biofuel pathways in cyanobacteria in order to boost the productivity of the targeted compound. For instance, changing driving forces, balancing co-factors, and improving the capacity to fix carbon are some of the strategies that are crucial [31]. The exploration of the photosynthetic platform makes it desirable to consider an effective method of fixing atmospheric CO2. By modifying solar energy absorption and utilization capacities, synthetic biology and metabolic engineering techniques have made some improvements towards enhancing the efficiency of photosynthesis [59]. The Synechocystis 6803 strain produced 55%, 67%, 37%, and 69% ethanol, respectively, after overexpressing four CBB cycle enzymes: RuBisCo, SBPase, aldolase, Fba, and Tk. Additionally, it was found that strains with the CBB cycle genes were associated with a higher ethanol-to-total biomass ratio, indicating that improved carbon fixation may result in increased product synthesis [64]. Increased productivity is frequently mentioned as a suitable driving force for pathway flux. In S. elongatus 7942, for example, the irreversible condensation by ATP-driven acetoacetyl-CoA synthase from the Streptomyces sp. strain was used to increase 1-butanol synthesis in order to overcome the thermodynamically undesirable condensation of two moles of acetyl-CoA to acetoacetyl-CoA, catalysed by thiolase in a reversible reaction [63]. The balanced combination of co-factors is an additional approach used to increase the effectiveness of product synthesis. Due to the production of NADPH during photosynthetic processes, the NADPH/NADP+pool in cyanobacteria is larger than the NADH/NAD+pool. As a result, the pathway involving these enzymes produces more products when NADPH-dependent enzymes are utilised instead of NADH-dependent enzymes [31]. The productivity of 1-butanol synthesis in S. elongatus 7942 increased by shifting NADH-dependent aldehyde/ alcohol dehydrogenase (AdhE2) to NADPH-dependent alcohol dehydrogenase (YqhD). Overexpressing the OPP Zwf gene increased the NADPH pool, causing the ethanolic strain Synechocystis 6803 with the yqhD gene to produce 33% more ethanol than the strain lacking the Zwf gene [134].
5. Economic Status and Global Response
A requirement for the widespread implementation of bioenergy is the economical mobilization and conversion of biomass, along with the availability and sustainability of feedstock. To optimize the financial performance of a bioenergy supply chain, important decisions may be made about production capacity, supply chain structure, transportation options, and conversion facility location [135]. The trade-off between economies of scale and transport costs is a crucial aspect of designing a supply chain that is cost-effective. Although larger production scales enable cost reductions due to economies of scale, they also increase the need to transport biomass over greater distances, which raises the cost of upstream transport [136]. The use of biofuels instead of fossil fuels may have a number of advantages. Since biofuels can be produced domestically, the import of fossil fuels may decline. The price of petroleum can be decreased by minimizing demand, which would be beneficial to domestic consumers economically but may ultimately result in an increase in petroleum consumption abroad [137]. At the present time, ethanol facilitates up to about 80% of the world’s production of liquid biofuels. The United States and Brazil, the two largest ethanol producers in the world, produce about 75% of the total amount. In comparison with ethanol, the production of biodiesel is comparatively less concentrated. With 12 billion litres or 43% of the total output, the European Union continues to dominate the world’s biodiesel production. The production of biofuels is expected to increase globally over the next ten years, though at a slower rate than over the previous five years (United States). France, Germany, China, Canada, and Italy are closely behind the biggest two consumers of bioethanol and biodiesel, which are the United States and Brazil. In spite of the global crisis caused by the COVID-19 pandemic, the United States achieved a notable milestone in 2020 by attaining a record-breaking level of energy generation from renewable sources. This accomplishment serves as evidence of the nation’s prominent role as a global frontrunner in the field of renewable energy production [138]. In order to enhance the market penetration of blended fuels in India, the Indian government has implemented several strategic measures, including the establishment of the National Biodiesel Mission, the Biodiesel Blending Programme, and the Ethanol Blended Petrol (EBP) Programme. By 2030, gasoline and diesel are expected to be supplied with 20% ethanol and 5% biodiesel, according to the National Biofuel Policy (NBP) 2018. The study by Gozgor et al. [139] raises an important question for governments and policymakers in nations like the OECD countries: to what extent is globalization necessary for the growth of the demand for renewable energy? [139]. Now, it is critical to investigate how economic globalization has affected particular renewable energy sources, such as biofuel, as it is relevant to their research question and the demand for renewable energy. Thus, economic globalisation is a dynamic procedure that involves expanding cross-border economic activity through various pathways of global trade, foreign direct investment, funding flows, and technology diffusion.
6. Challenges and Future Perspectives
The increased demand for energy on a global scale has influenced society’s main focus on biofuels made from renewable biomass. The edible nature of feedstocks based on oil or starch and the requirements of subsequent bioprocesses limit their use in the production of biofuels [140]. The ability of cyanobacteria to use CO2 for the production of biofuels and their precursor molecules makes them a very promising resource for biofuel production as a substitute for these materials [141]. However, cyanobacteria lack an efficient synthetic mechanism for the synthesis of biofuels. For example, triglycerides, which are produced naturally by algae, lead to the accumulation of more lipids, whereas membrane-related lipids obtained from cyanobacteria include MGDG, SQDG, PG, and DGDG. Consequently, cyanobacteria have less potential for producing biodiesel because they do not possess storage lipids; however, the direct release of extracellular free fatty acids has a benefit in that it helps to avoid energy-intensive processes [104]. Moreover, engineering cyanobacteria is also a challenging factor due to a lack of sufficient genomic resources, restricting the development of genetic engineering in cyanobacteria. Rigidity and robustness of the cell wall cause problems in transformation, leading to a low efficiency of transformation methods. By adjusting a few parameters, the permeability of the cell can be enhanced, but the low recovery after the procedure leads to a decrease in efficiency. Even though cyanobacteria are capable of directly converting CO2 into chemicals, the concentration of the desired product is currently low in the industry. For instance, cyanobacteria can only produce 5.5 g/L of ethanol at their maximum, but E. coli can easily produce 10 g/L. Glycogen synthesis was disrupted, which led to a decrease in glycogen utilisation. This improved the carbon flux to the targeted chemical production but had an adverse effect on cell growth. In the future, it will be crucial to strike a balance between the production of desirable molecules and the utilisation of glycogen [15].
Although biofuel can emerge as a sustainable and environmentally friendly alternative to petroleum and diesel, this shift is not economically feasible, considering the high production cost of biodiesel. According to the reports from the U.S. Department of Energy (2018), the cost of diesel was $3.24 per gallon, whereas the cost of biodiesel was $3.55 per gallon. The reason underlying this high cost is the greater production cost of biodiesel, at $2.29/kg, compared to $1.08/kg for petroleum. These high operational and production costs are major bottlenecks that are preventing the commercialization of microalgae-based biodiesel. The large capital investment ($55.6 million) had a discounted rate of 7.34%, a negative net present value of $26 million, and an internal return rate (IRR) of 0.38%. Despite the poor profit, revenues from biodiesel, animal feed, and power yielded an annual benefit of $4.82 million [142]. When it comes to using biofuels instead of fossil fuels, the majority of developing nations suffer from challenging situations. Communities in developing countries are using less biofuel as a result of the manufacturing procedure becoming more expensive and inefficient. This lessens the appeal of the industry to producers and encourages a reduction in biofuel consumption. So, the primary concern is the elevated cost of biofuel, which makes it a less affordable form of energy than fossil fuels. There are several factors, such as a lack of infrastructure, a scarcity of feedstock, poor funding options, and ineffective conversion technologies, that contribute to the rising prices of biofuel production in developing countries [143]. The type of strain used may determine how well the PBRs (Photobioreactors) function, but there are still certain limitations to this system, including excessive heating, cleaning problems, biofouling, and excessive operating and capital expenses [144]. To improve domestic energy security, promote economic growth, and lower emissions of greenhouse gases (GHG) and other pollutants, a number of countries support the production and application of liquid biofuels for transportation. Therefore, in order to lessen environmental impacts resulting from carbon dioxide emissions, it is crucial that researchers should focus more on methods to promote the production of biofuel and its application. All cyanobacteria-based biofuel synthesis research is currently restricted to laboratory environments, and prior to commercialization, many problems must be resolved, including the development of strains with high production and improved abilities to cope with challenging conditions. The productivity of the synthetic cyanobacterial systems can be enhanced by optimising and engineering more robust and high-efficient cyanobacterial chassis modifications involving photosynthesis, CO2 uptake and fixation, products exporting, tolerance, and cell regulation. To reduce the production cost, researchers can work on developing strategies that can reduce the overall production cost. Microalgae can be cultured under specific conditions, wherein, along with the primary role of producing biofuel, several secondary roles can be fulfilled. Various secondary value-added products, including vitamins, proteins, etc., can be obtained, which will contribute to the overall cost reduction. Microalgae can also be cultivated in wastewater, where, along with the production of biofuel, they can efficiently remove contaminants from wastewater. Also, microalgae can be grown in aquaculture, where they can help the fauna already present in the waterbody by regulating the gaseous flow in it. All this effort can lower the production costs, thus terminating the major bottleneck in the biofuel production process involving microalgae.
7. Conclusions
In conclusion, cyanobacteria have proven to be promising biocatalysts for the long-term synthesis of chemicals and biofuels. The ability of these organisms to perform photosynthesis, using carbon dioxide and sunlight to produce energy and organic compounds, distinguishes them as environmentally friendly alternatives to traditional fuel and chemical production techniques. By harnessing the metabolic pathways of cyanobacteria, researchers have been able to optimize their capabilities for synthesizing various biofuels and valuable chemicals. Furthermore, cyanobacteria can be engineered and manipulated to increase their productivity and yield of target biofuels and chemicals, making them highly versatile and customizable biocatalysts. However, challenges such as scalability, cost-efficiency, and maintaining consistent productivity need to be explored to utilise the full potential of cyanobacteria-based bioproduction on an industrial scale. Continued research, technological advancements, and investment in bioprocess optimization are essential to overcoming these challenges and establishing cyanobacteria as a sustainable and economically viable platform for the production of biofuels and chemicals in the future. Overall, the use of cyanobacteria as biocatalysts holds great promise in the pursuit of a greener and more sustainable energy and chemical industry.
Author Contributions
G.S., V.K.S., S.J., P.R., R.S., P.K.S. and R.L. assisted in the writing and editing of the manuscript, including figures and table. G.S. and R.P.S. designed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Garvita Singh and Renu Soni are thankful to the Department of Botany, Gargi College, University of Delhi, Delhi, for providing support during the preparation of this manuscript. Varsha K. Singh (09/0013(12862)/2021-EMR-1) and Palak Rana (09/0013 (16603)/2023-EMR-I) are thankful to the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for financial assistance in the form of a Junior Research Fellowship (JRF). Sapana Jha (No. R/Dev./Sch./UGC Non-NET Fello./2022-23/52561) is thankful to BHU for providing institutional fellowship. The incentive grant received by Rajeshwar Sinha from IoE (Scheme no. 6031), Banaras Hindu University, Varanasi, India, is highly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Ribosome binding sites (RBSs); D-ribulose-1,5- bisphosphate carboxylase/oxygenase (RubisCO); Calvin-Benson-Bassham (CBB); 3-phosphoglycerate (3PGA); 1,5-ribulose bisphosphate (RuBP); carbon-concentrating mechanisms (CCMs); phosphoenolpyruvate carboxylase (PEPC); phosphoenolpyruvate (PEP); tricarboxylic acid (TCA); Embden-Meyerhof-Parnas (EMP); oxidative pentose phosphate (OPP); pentose phosphate (PP); phosphoketolase (PK); Entner-Doudoroff (ED); phosphofructokinase (PFK); fructose bisphosphate (FBP); glyceraldehyde-3-phosphate (GAP); dihydroxyacetone phosphate (DHAP); 6-phosphogluconate (6PGA); 6-phosphogluconate dehydrogenase (6PGAD); 2-keto-3-deoxy-6-phosphogluconate (KDPG); protospacer adjacent motifs (PAM); methylerythritol 4-phosphate (MEP); dimethylallyl-pyrophosphate (DMAPP); isopentenyl-pyrophosphate (IPP); fatty acyl-ACP reductase (FAR); aldehyde deformylating oxygenase (ADO); acyl-ACP reductase (AAR); free fatty acids (FFAs); acetyl-CoA carboxylase (ACC); acyl-acyl carrier protein synthetase (AAS); acyl carrier protein (ACP); sedoheptulose-1,7-bisphosphatase (SBPase); aldolase (Fba); transketolase (Tk); monogalactosyldiacylglycerol (MGDG); sulfoquinovosyldiacylglycerol (SQDG); phosphotidylglycerol (PG); digalactosyldiacylglycerol (DGDG).
References
- Pierobon, S.C.; Cheng, X.; Graham, P.J.; Nguyen, B.; Karakolis, E.G.; Sinton, D. Emerging microalgae technology: A review. Sustain. Energy Fuels 2018, 2, 13–38. [Google Scholar] [CrossRef]
- Li, X.; Shen, C.R.; Liao, J.C. Isobutanol production as an alternative metabolic sink to rescue the growth deficiency of the glycogen mutant of Synechococcus elongatus PCC 7942. Photosynth. Res. 2014, 120, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Jha, S.; Rana, P.; Mishra, S.; Kumari, N.; Singh, S.C.; Sinha, R.P. Resilience and mitigation strategies of cyanobacteria under ultraviolet radiation stress. Int. J. Mol. Sci. 2023, 24, 12381. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural sun-screening compounds and DNA-repair enzymes: Photoprotection and photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Shin, Y.S.; Choi, H.I.; Choi, J.W.; Lee, J.S.; Sung, Y.J.; Sim, S.J. Multilateral approach on enhancing economic viability of lipid production from microalgae: A review. Bioresour. Technol. 2018, 258, 335–344. [Google Scholar] [CrossRef]
- Cheng, A.A.; Lu, T.K. Synthetic biology: An emerging engineering discipline. Ann. Rev. Biomed. Eng. 2012, 14, 155–178. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Song, X.; Diao, J.; Chen, L.; Zhang, W. Toolboxes for cyanobacteria: Recent advances and future direction. Biotechnol. Adv. 2018, 36, 1293–1307. [Google Scholar] [CrossRef]
- Carroll, A.L.; Case, A.E.; Zhang, A.; Atsumi, S. Metabolic engineering tools in model cyanobacteria. Metab. Eng. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Veaudor, T.; Chauvat, F. Comparative genomics of DNA recombination and repair in cyanobacteria: Biotechnological implications. Front. Microbiol. 2016, 7, 1809. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.; Blazeck, J. High throughput mutagenesis and screening for yeast engineering. J. Biol. Eng. 2022, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, T.Y.; Jang, Y.S.; Choi, S.; Lee, S.Y. Systems metabolic engineering for chemicals and materials. Trends Biotechnol. 2011, 29, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Pakrasi, H.B.; Wangikar, P.P. Recent advances in synthetic biology of cyanobacteria. Appl. Microbiol. Biotechnol. 2018, 102, 5457–5471. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.R.; Tan, S.I.; Hsiang, C.C.; Sung, P.K.; Ng, I.S. Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresour. Technol. 2019, 291, 121932. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2013, 346, 1258096. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Gupta, A.; Singh, A.P.; Kumari, N.; Mishra, S.; Singh, P.R.; Jaiswal, J.; Sinha, R.P. Application of synthetic biology approaches to high-yield production of mycosporine-like amino acids. Fermentation 2023, 9, 669. [Google Scholar] [CrossRef]
- Jiang, W.; Brueggeman, A.J.; Horken, K.M.; Plucinak, T.M.; Weeks, D.P. Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot. Cell 2014, 13, 1465–1469. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Garcillán-Barcia, M.P.; de la Cruz, F. Engineering the fatty acid synthesis pathway in Synechococcus elongatus PCC 7942 improves omega-3 fatty acid production. Biotechnol. Biofuels 2018, 11, 239. [Google Scholar] [CrossRef]
- Eungrasamee, K.; Miao, R.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Shabestary, K.; Anfelt, J.; Ljungqvist, E.; Jahn, M.; Yao, L.; Hudson, E.P. Targeted repression of essential genes to arrest growth and increase carbon partitioning and biofuel titers in cyanobacteria. ACS Synth. Biol. 2018, 7, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model microorganisms and beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef]
- Mimuro, M.; Tomo, T.; Tsuchiya, T. Two unique cyanobacteria lead to a traceable approach of the first appearance of oxygenic photosynthesis. Photosynth. Res. 2008, 97, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Quick, W.P.; Sirault, X.R.R. Improving photosynthesis and yield potential in cereal crops by targeted genetic manipulation: Prospects, progress and challenges. Field Crop. Res. 2015, 182, 19–29. [Google Scholar] [CrossRef]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef]
- Satagopan, S.; Huening, K.A.; Robert Tabita, F. Selection of Cyanobacterial (Synechococcus sp. Strain PCC 6301) RubisCO variants with improved functional properties that confer enhanced CO2-dependent growth of Rhodobacter capsulatus, a photosynthetic bacterium. mBio 2019, 10, e01537-19. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen proterozoic oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef]
- Narainsamy, K.; Farci, S.; Braun, E.; Junot, C.; Cassier-Chauvat, C.; Chauvat, F. Oxidative-stress detoxification and signalling in cyanobacteria: The crucial glutathione synthesis pathway supports the production of ergothioneine and ophthalmate. Mol. Microbiol. 2016, 100, 15–24. [Google Scholar] [CrossRef]
- Takeya, M.; Hirai, M.Y.; Osanai, T. Allosteric inhibition of phosphoenolpyruvate carboxylases is determined by a single amino acid residue in cyanobacteria. Sci. Rep. 2017, 7, 41080. [Google Scholar] [CrossRef]
- Angermayr, S.A.; Rovira, A.G.; Hellingwerf, K.J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015, 33, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schreiber, K.; Appel, J.; Makowka, A.; Fähnrich, B.; Roettger, M.; Hajirezaei, M.R.; Sönnichsen, F.D.; Schönheit, P.; Martin, W.F. The Entner-Doudoroff pathway is an overlooked glycolytic route in cyanobacteria and plants. Proc. Natl. Acad. Sci. USA 2016, 113, 5441–5446. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Cano, M.; Wang, B.; Douchi, D.; Yu, J. The plasticity of cyanobacterial carbon metabolism. Curr. Opin. Chem. Biol. 2017, 41, 12–19. [Google Scholar] [CrossRef]
- Veaudor, T.; Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Sassi, J.F.; Cassier-Chauvat, C.; Chauvat, F. Recent Advances in the Photoautotrophic Metabolism of Cyanobacteria: Biotechnological Implications. Life 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bryant, D.A. The tricarboxylic acid cycle in cyanobacteria. Science 2011, 334, 1551–1553. [Google Scholar] [CrossRef]
- Sarkar, D.; Shimizu, K. An overview on biofuel and biochemical production by photosynthetic microorganisms with understanding of the metabolism and by metabolic engineering together with efficient cultivation and downstream processing. Bioresour. Bioprocess. 2015, 2, 17. [Google Scholar] [CrossRef]
- Wan, N.; DeLorenzo, D.M.; He, L.; You, L.; Immethun, C.M.; Wang, G.; Baidoo, E.E.K.; Hollinshead, W.; Keasling, J.D.; Moon, T.S. Cyanobacterial carbon metabolism: Fluxome plasticity and oxygen dependence. Biotechnol. Bioeng. 2017, 114, 1593–1602. [Google Scholar] [CrossRef]
- Ungerer, J.; Pakrasi, H.B. Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria. Sci. Rep. 2016, 6, 39681. [Google Scholar] [CrossRef]
- Behler, J.; Vijay, D.; Hess, W.R.; Akhtar, M.K. CRISPR-Based technologies for metabolic engineering in Cyanobacteria. Trends Biotechnol. 2018, 36, 996–1010. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.R.; Huang, C.H.; Sung, L.Y.; Wu, M.Y.; Hu, Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab. Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Wendt, K.E.; Ungerer, J.; Cobb, R.E.; Zhao, H.; Pakrasi, H.B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb. Cell Fact. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.C.; Lin, G.M.; Xie, L.R.; Wang, Z.Q.; Xing, W.Y.; Zhang, J.Y.; Zhang, C.C. Expanding the potential of CRISPR-Cpf1-based genome editing technology in the Cyanobacterium Anabaena PCC 7120. ACS Synth. Biol. 2018, 8, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T. Single-cell genomics unveiled a cryptic cyanobacterial lineage with a worldwide distribution hidden by a dinoflagellate host. Proc. Natl. Acad. Sci. USA 2019, 116, 15973–15978. [Google Scholar] [CrossRef] [PubMed]
- Prabha, R.; Singh, D.P.; Sinha, S.; Ahmad, K.; Rai, A. Genome-wide comparative analysis of codon usage bias and codon context patterns among cyanobacterial genomes. Mar. Genom. 2017, 32, 31–39. [Google Scholar] [CrossRef]
- Lv, Q.; Ma, W.; Liu, H.; Li, J.; Wang, H.; Lu, F.; Zhou, C.; Shi, T. Genome-wide protein-protein interactions and protein function exploration in cyanobacteria. Sci. Rep. 2015, 5, 15519. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Borirak, O.; de Koning, L.J.; van der Woude, A.D.; Hoefsloot, H.C.; Dekker, H.L.; Roseboom, W.; de Koster, C.G.; Hellingwerf, K.J. Quantitative proteomics analysis of an ethanol-and a lactate-producing mutant strain of Synechocystis sp. PCC6803. Biotechnol. Biofuels 2015, 8, 111. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Almaliti, J.; Tian, I.Y.; Kinnel, R.B.; Korobeynikov, A.; Monroe, E.A.; Duggan, B.M.; Di Marzo, V.; Sherman, D.H.; Dorrestein, P.C.; et al. Combining mass spectrometric metabolic profiling with genomic analysis: A powerful approach for discovering natural products from cyanobacteria. J. Nat. Prod. 2015, 78, 1671–1682. [Google Scholar] [CrossRef]
- Kanno, M.; Carroll, A.L.; Atsumi, S. Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat. Commun. 2017, 8, 14724. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhang, W. Proteomic and metabolomic analyses reveal metabolic responses to 3-hydroxypropionic acid synthesized internally in cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 209. [Google Scholar] [CrossRef]
- Kopka, J.; Schmidt, S.; Dethloff, F.; Pade, N.; Berendt, S.; Schottkowski, M.; Martin, N.; Dühring, U.; Kuchmina, E.; Enke, H.; et al. Systems analysis of ethanol production in the genetically engineered cyanobacterium Synechococcus sp. PCC 7002. Biotechnol. Biofuels 2017, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Varshney, R.K.; Shukla, P. Sigma factor modulation for cyanobacterial metabolic engineering. Trends Microbiol. 2021, 29, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Rey, G. Understanding gradients in industrial bioreactors. Biotechnol. Adv. 2021, 46, 107660. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Shukla, P. Emerging tools and strategies in cyanobacterial omics. Trend Biotechnol. 2022, 40, 4–7. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, A.M.; Jensen, T.J.; Strickland, L.M. Genetic tools for the advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis. Microb. Cell Factories 2016, 15, 190. [Google Scholar] [CrossRef]
- Lai, M.C.; Lan, E.I. Advances in metabolic engineering of cyanobacteria for photosynthetic biochemical production. Metabolites 2015, 5, 636–658. [Google Scholar] [CrossRef]
- Agarwal, P.; Soni, R.; Kaur, P.; Madan, A.; Mishra, R.; Pandey, J.; Singh, G. Cyanobacteria as a promising alternative for sustainable environment: Synthesis of biofuel and biodegradable plastics. Front. Microbiol. 2022, 13, 939347. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Lu, X. Engineering cyanobacteria chassis cells toward more efficient photosynthesis. Curr. Opin. Biotechnol. 2020, 62, 1–6. [Google Scholar] [CrossRef]
- Deng, M.D.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef]
- Fathima, A.M.; Chuang, D.; Laviña, W.A.; Liao, J.; Putri, S.P.; Fukusaki, E. Iterative cycle of widely targeted metabolic profiling for the improvement of 1-butanol titer and productivity in Synechococcus elongatus. Biotechnol. Biofuels 2018, 11, 188. Available online: https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-018-1187-8. (accessed on 14 November 2023). [CrossRef] [PubMed]
- Mishra, S.; Kumari, N.; Singh, V.; Sinha, R. Cyanobacterial Biofuel: A Platform for Green Energy. Adv. Environ. Eng. Res. 2023, 5, 1–42. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 2011, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef]
- Roussou, S.; Albergati, A.; Liang, F.; Lindblad, P. Engineered cyanobacteria with additional overexpression of selected Calvin-Benson-Bassham enzymes show further increased ethanol production. Metab. Eng. Commun. 2021, 12, e00161. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef]
- Walther, T.; François, J.M. Microbial production of propanol. Biotechnol. Adv. 2016, 34, 984–996. [Google Scholar] [CrossRef]
- Kusakabe, T.; Tatsuke, T.; Tsuruno, K.; Hirokawa, Y.; Atsumi, S.; Liao, J.C.; Hanai, T. Engineering a synthetic pathway in cyanobacteria for isopropanol production directly from carbon dioxide and light. Metab. Eng. 2013, 20, 101–108. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Suzuki, I.; Hanai, T. Optimization of isopropanol production by engineered cyanobacteria with a synthetic metabolic pathway. J. Biosci. Bioeng. 2015, 119, 585–590. [Google Scholar] [CrossRef]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018, 120, 616–624. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Takaichi, S. Carotenogenesis in cyanobacteria: CruA/CruP-type and CrtL-type lycopene cyclases. J. Gen. Appl. Microbiol. 2020, 66, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Takaichi, S.; Ishikawa, T. Identification and functional analysis of the geranylgeranyl pyrophosphate synthase gene (crtE) and phytoene synthase gene (crtB) for carotenoid biosynthesis in Euglena gracilis. BMC Plant Biol. 2016, 16, 4. [Google Scholar] [CrossRef]
- Li, B.; Lee, J.Y.; Luo, Y. Health benefits of astaxanthin and its encapsulation for improving bioavailability: A review. J. Agri. Food Res. 2023, 14, 100685. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takaki, A.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth. Biol. 2019, 8, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Pade, N.; Erdmann, S.; Enke, H.; Dethloff, F.; Dühring, U.; Georg, J.; Hagemann, M. Insights into isoprene production using the cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Song, X.; Zhang, L.; Cui, J.; Chen, L.; Zhang, W. Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin. Metab. Eng. 2020, 61, 275–287. [Google Scholar] [CrossRef]
- Gao, X.; Xu, H.; Zhu, Z.; She, Y.; Ye, S. Improved production of echinenone and canthaxanthin in transgenic Nostoc sp. PCC 7120 overexpressing a heterologous crtO gene from Nostoc flagelliforme. Microbiol. Res. 2020, 236, 126455. [Google Scholar] [CrossRef]
- Paliwal, C.; Pancha, I.; Ghosh, T.; Maurya, R.; Chokshi, K.; Bharadwaj, S.V.; Mishra, S. Selective carotenoid accumulation by varying nutrient media and salinity in Synechocystis sp. CCNM 2501. Bioresour. Technol. 2015, 197, 363–368. [Google Scholar] [CrossRef]
- Lehmann, M.; Vamvaka, E.; Torrado, A.; Jahns, P.; Dann, M.; Rosenhammer, L.; Rühle, T. Introduction of the carotenoid biosynthesis α-branch into Synechocystis sp. PCC 6803 for lutein production. Front. Plant Sci. 2021, 12, 699424. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food Chem. 2017, 66, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, H.J.; Choi, S.Y.; Choi, J.I.; Woo, H.M. Bio-solar cell factories for photosynthetic isoprenoids production. Planta 2019, 249, 181–193. [Google Scholar] [CrossRef]
- Donoso, D.; Ballesteros, R.; Bolonio, D.; Garcia-Martinez, M.J.; Lapuerta, M.; Canoira, L. Hydrogenated turpentine: A biobased component for jet fuel. Energy Fuels 2020, 35, 1465–1475. [Google Scholar] [CrossRef]
- Pattanaik, B.; Lindberg, P. Terpenoids and their biosynthesis in cyanobacteria. Life 2015, 5, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Zakar, T.; Laczko-Dobos, H.; Toth, T.N.; Gombos, Z. Carotenoids assist in cyanobacterial photosystem II assembly and function. Front. Plant Sci. 2016, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Melnicki, M.R.; Kerfeld, C.A. Structure and functions of Orange Carotenoid Protein homologs in cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth. Res. 2016, 130, 123–135. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Tracy, N.I.; Chen, D.; Crunkleton, D.W.; Price, G.L. Hydrogenated monoterpenes as diesel fuel additives. Fuel 2009, 88, 2238–2240. [Google Scholar] [CrossRef]
- Chuck, C.J.; Onnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91. [Google Scholar] [CrossRef]
- Oliver, N.J.; Rabinovitch-Deere, C.A.; Carroll, A.L.; Nozzi, N.E.; Case, A.E.; Atsumi, S. Cyanobacterial metabolic engineering for biofuel and chemical production. Curr. Opin. Chem. Biol. 2016, 35, 43–50. [Google Scholar] [CrossRef]
- Lin, P.C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, A.; Johnson, T.J.; Esmaeili, N.; Johnson, M.D.; Richardson, J.W.; Muthukumarappan, K.; Gibbons, W.R. Life cycle analysis of a large-scale limonene production facility utilizing filamentous N2-fixing cyanobacteria. Algal Res. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Kiyota, H.; Okuda, Y.; Ito, M.; Hirai, M.Y.; Ikeuchi, M. Engineering of cyanobacteria for the photosynthetic production of limonene from CO2. J. Biotechnol. 2014, 185, 1–7. [Google Scholar] [CrossRef]
- Halfmann, C.; Gu, L.; Zhou, R. Engineering cyanobacteria for the production of a cyclic hydrocarbon fuel from CO2 and H2O. Green Chem. 2014, 16, 3175–3185. [Google Scholar] [CrossRef]
- Lin, P.C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Pfleger, B.F.; Gossing, M.; Nielsen, J. Metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Marella, E.R.; Holkenbrink, C.; Siewers, V.; Borodina, I. Engineering microbial fatty acid metabolism for biofuels and biochemicals. Curr. Opin. Biotechnol. 2018, 50, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. A perspective: Photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, W.; Zhang, W.; Chen, L.; Lu, X. Versatility of hydrocarbon production in cyanobacteria. Appl. Microbiol. Biotechnol. 2017, 101, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.; Rautela, A.; Kumar, S. Approaches in the photosynthetic production of sustainable fuels by cyanobacteria using tools of synthetic biology. World J. Microbiol. Biotechnol. 2021, 37, 201. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; Del Cardayre, S.B. Microbial biosynthesis of alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Liang, Y.; Arai, D.; Maeda, Y.; Honda, T.; Muto, M.; Tanaka, T. Alkane production by the marine cyanobacterium Synechococcus sp. NKBG15041c possessing the α-olefin biosynthesis pathway. Appl. Microbiol. Biotechnol. 2015, 99, 1521–1529. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lu, X. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol. Biofuels 2013, 6, 69. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R.; Sirisattha, S.; Tanaka, Y.; Mahakhant, A.; Takabe, T. Improved alkane production in nitrogen-fixing and halotolerant cyanobacteria via abiotic stresses and genetic manipulation of alkane synthetic genes. Curr. Microbiol. 2015, 71, 115–120. [Google Scholar] [CrossRef]
- Teerawanichpan, P.; Robertson, A.J.; Qiu, X. A fatty acyl-CoA reductase highly expressed in the head of honey bee (Apis mellifera) involves biosynthesis of a wide range of aliphatic fatty alcohols. Insect Biochem. Mol. Biol. 2010, 40, 641–649. [Google Scholar] [CrossRef]
- Love, J. Microbial pathways for advanced biofuel production. Biochem. Soc. Trans. 2022, 50, 987–1001. [Google Scholar] [CrossRef]
- Qi, F.; Yao, L.; Tan, X.; Lu, X. Construction, characterization and application of molecular tools for metabolic engineering of Synechocystis sp. Biotechnol. Lett. 2013, 35, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Jutur, P.P. Dynamic allocation of carbon flux triggered by task-specific chemicals is an effective non-gene disruptive strategy for sustainable and cost-effective algal biorefineries. Chem. Eng. J. 2021, 418, 129413. [Google Scholar] [CrossRef]
- Yao, L.; Qi, F.; Tan, X.; Lu, X. Improved production of fatty alcohols in cyanobacteria by metabolic engineering. Biotechnol. Biofuels 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Xin, C.; Zheng, Y.; Cheng, Y.; Sun, S.; Yuan, J.S. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc. Natl. Acad. Sci. USA 2016, 113, 14225–14230. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.K.; Danielewicz, M.A.; Wong, D.M.; Anderson, L.A.; Boothe, J.R. Phenotypic screening with oleaginous microalgae reveals modulators of lipid productivity. ACS Chem. Biol. 2013, 8, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Lennen, R.M.; Pfleger, B.F. Microbial production of fatty acid-derived fuels and chemicals. Curr. Opin. Biotechnol. 2013, 24, 1044–1053. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Gago, G.; Diacovich, L.; Arabolaza, A.; Tsai, S.C.; Gramajo, H. Fatty acid biosynthesis in actinomycetes. FEMS Microbiol. Rev. 2011, 35, 475–497. [Google Scholar] [CrossRef]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O. Thematic review series: Glycerolipids. Acyltransferases in bacterial glycerophospholipid synthesis. J. Lipids Res. 2008, 49, 1867–1874. [Google Scholar] [CrossRef]
- Kato, A.; Takatani, N.; Ikeda, K.; Matsuura, M.; Kojima, K.; Aichi, M.; Omata, T. Modulation of the balance of fatty acid production and secretion is crucial for enhancement of growth and productivity of the engineered mutant of the cyanobacterium Synechococcus elongatus. Biotechnol. Biofuels 2016, 9, 91. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Jones, H.D. Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol. Bioeng. 2012, 109, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, A.M. Improved free fatty acid production in cyanobacteria with Synechococcus sp. PCC 7002 as host. Front. Bioeng. Biotechnol. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, A.M. Borrowing genes from Chlamydomonas reinhardtii for free fatty acid production in engineered cyanobacteria. J. Appl. Phycol. 2013, 25, 1495–1507. [Google Scholar] [CrossRef]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Varman, A.M.; Xiao, Y.; Pakrasi, H.B.; Tang, Y.J. Metabolic engineering of Synechocystis sp. strain PCC 6803 for isobutanol production. Appl. Environ. Microbiol. 2013, 79, 908–914. [Google Scholar] [CrossRef]
- Yunus, I.S.; Wichmann, J.; Wördenweber, R.; Lauersen, K.J.; Kruse, O.; Jones, P.R. Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel. Metab. Eng. 2018, 49, 201–211. [Google Scholar] [CrossRef]
- Roumezi, B.; Avilan, L.; Risoul, V.; Brugna, M.; Rabouille, S.; Latifi, A. Overproduction of the Flv3B flavodiiron, enhances the photobiological hydrogen production by the nitrogen-fixing cyanobacterium Nostoc PCC 7120. Microb. Cell Factories 2020, 19, 65. [Google Scholar] [CrossRef]
- Peramuna, A.; Morton, R.; Summers, M.L. Enhancing alkane production in cyanobacterial lipid droplets: A modeFl platform for industrially relevant compound production. Life 2015, 5, 1111–1126. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.; Lee, S.M.; Um, Y.; Kim, Y.; Sim, S.J.; Woo, H.M. Direct conversion of CO2 to α-farnesene using metabolically engineered Synechococcus elongatus PCC 7942. J. Agric. Food Chem. 2017, 65, 10424–10428. [Google Scholar] [CrossRef] [PubMed]
- Namakoshi, K.; Nakajima, T.; Yoshikawa, K.; Toya, Y.; Shimizu, H. Combinatorial deletions of glgC and phaCE enhance ethanol production in Synechocystis sp. PCC 6803. J. Biotechnol. 2016, 239, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; de Mattos, M.J.T. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar] [CrossRef]
- Choi, Y.N.; Park, J.M. Enhancing biomass and ethanol production by increasing NADPH production in Synechocystis sp. PCC 6803. Bioresour. Technol. 2016, 213, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; You, F.; Snyder, S.W. Biomass-to-bioenergy and biofuel supply chain optimization: Overview, key issues and challenges. Comput. Chem. Eng. 2014, 66, 36–56. [Google Scholar] [CrossRef]
- Flodén, J. Opportunities and challenges for rail transport of solid wood biofuel. Eur. J. Transp. Infrastruct. Res. 2016, 16, 512–553. [Google Scholar] [CrossRef]
- Huang, H.X.; Khanna, M.; Onal, H.; Chen, X.G. Stacking low carbon policies on the renewable fuels standard: Economic and greenhouse gas implications. Energy Policy 2013, 56, 5–15. [Google Scholar] [CrossRef]
- McLaughlin, K.; Bird, L. The US Set a Record for Renewables in 2020, But More Is Needed [Internet]; World Resources Institute: Washington, DC, USA, 2021. [Google Scholar]
- Gozgor, G.; Mahalik, M.K.; Demir, E.; Padhan, H. The impact of economic globalization on renewable energy in the OECD countries. Energy Policy 2020, 139, 111365. [Google Scholar] [CrossRef]
- Brown, B.; Schoney, R.; Nolan, J. Assessing the food vs. fuel issue: An agent-based simulation. Energy Policy 2021, 159, 112553. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Co-cultivation of two engineered strains of Synechocystis sp. PCC 6803 results in improved bioethanol production. Renew. Energ. 2020, 146, 1124–1133. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to produce cost-effective third-generation biofuel from microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Avinash, A.; Sasikumar, P.; Murugesan, A. Understanding the interaction among the barriers of biodiesel production from waste cooking oil in India-an interpretive structural modeling approach. Renew. Energ. 2018, 127, 678–684. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).