Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules
Abstract
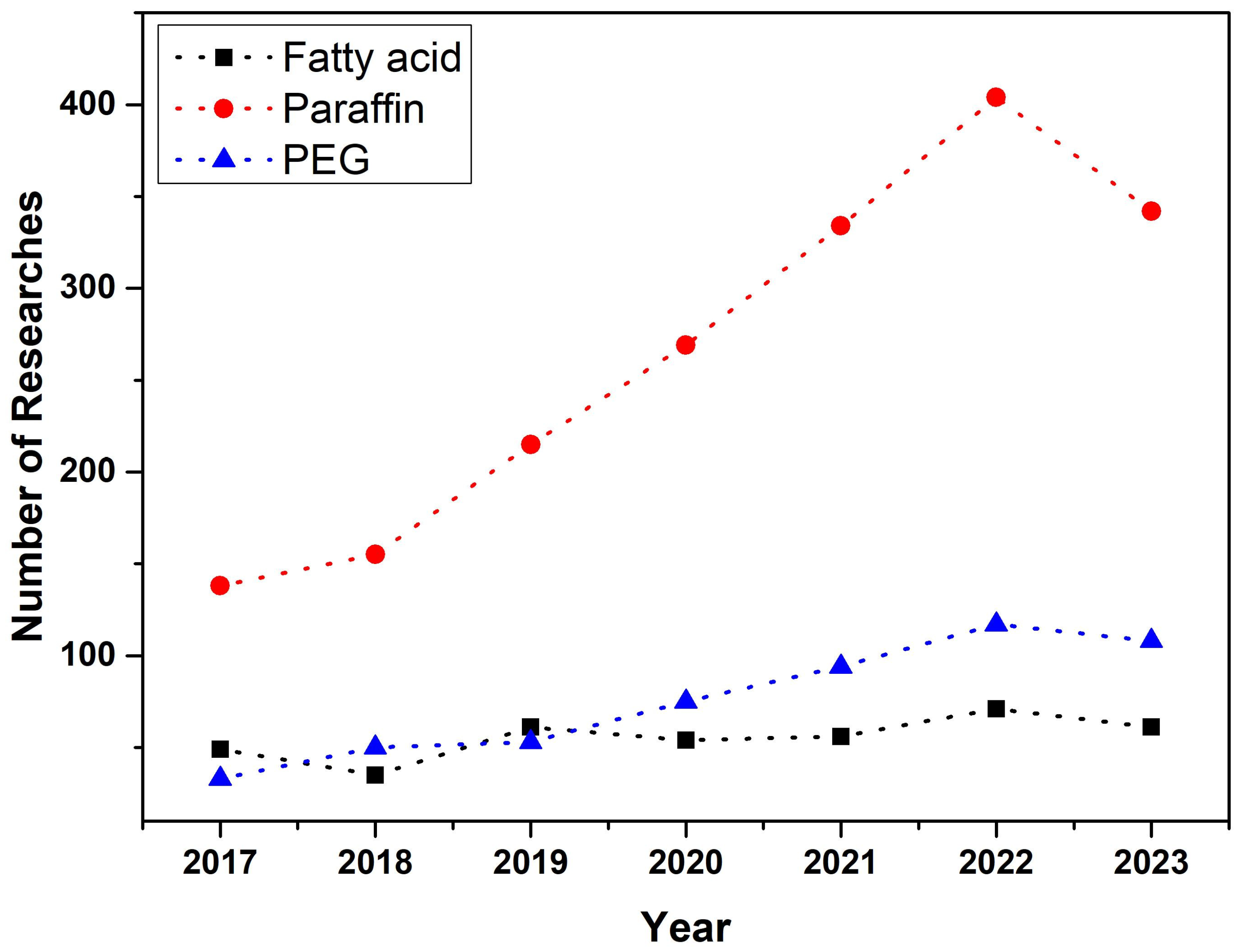
1. Introduction
2. Solid–Liquid Organic PCMs as Core Materials of Micro/Nanocapsules
2.1. Paraffin-Based PCMs
2.2. Fatty Acid
2.3. Other Organic Solid–Liquid PCMs
2.4. Challenges of Solid–Liquid Organic PCMs
3. Micro/Nanoencapsulation of Solid–Liquid Organic PCMs
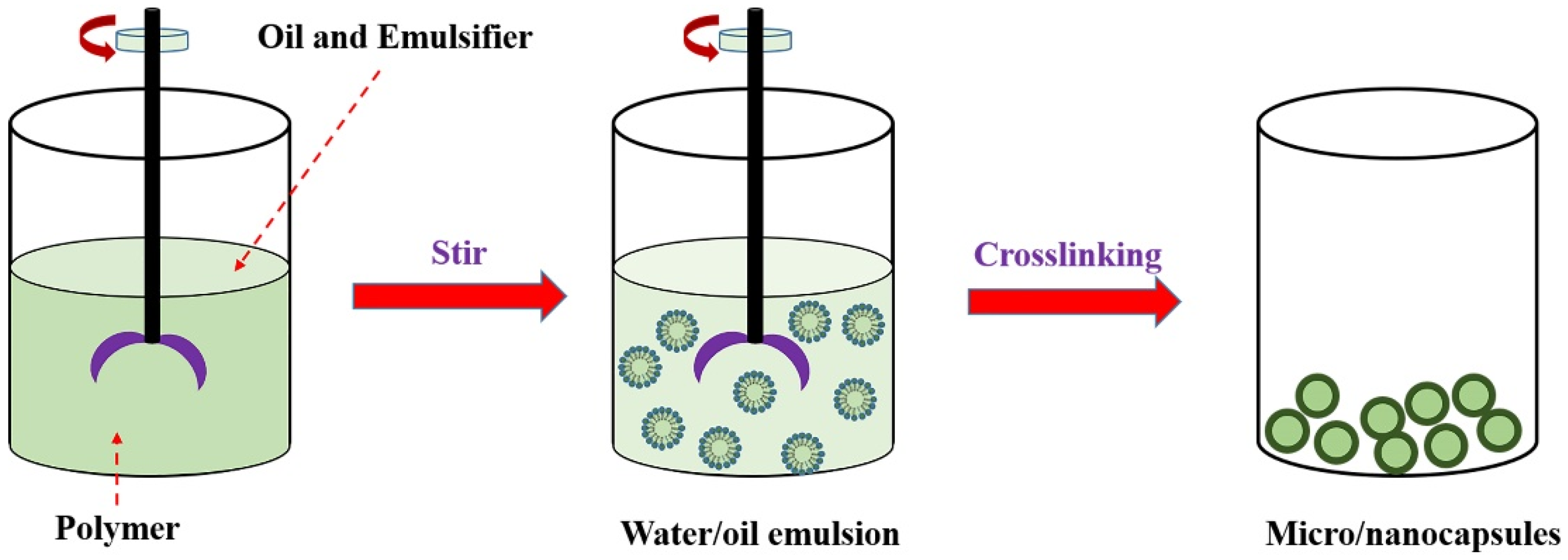
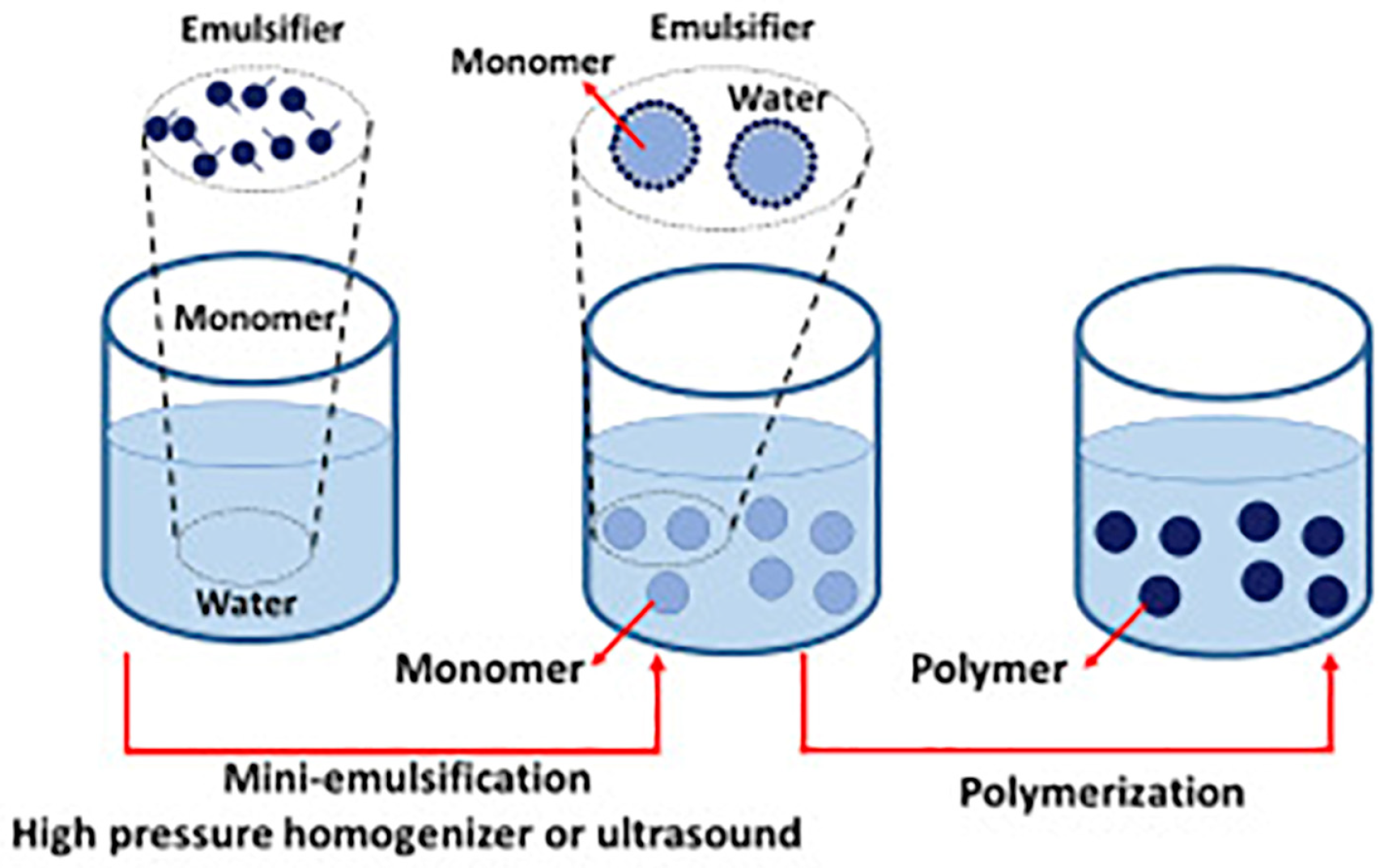
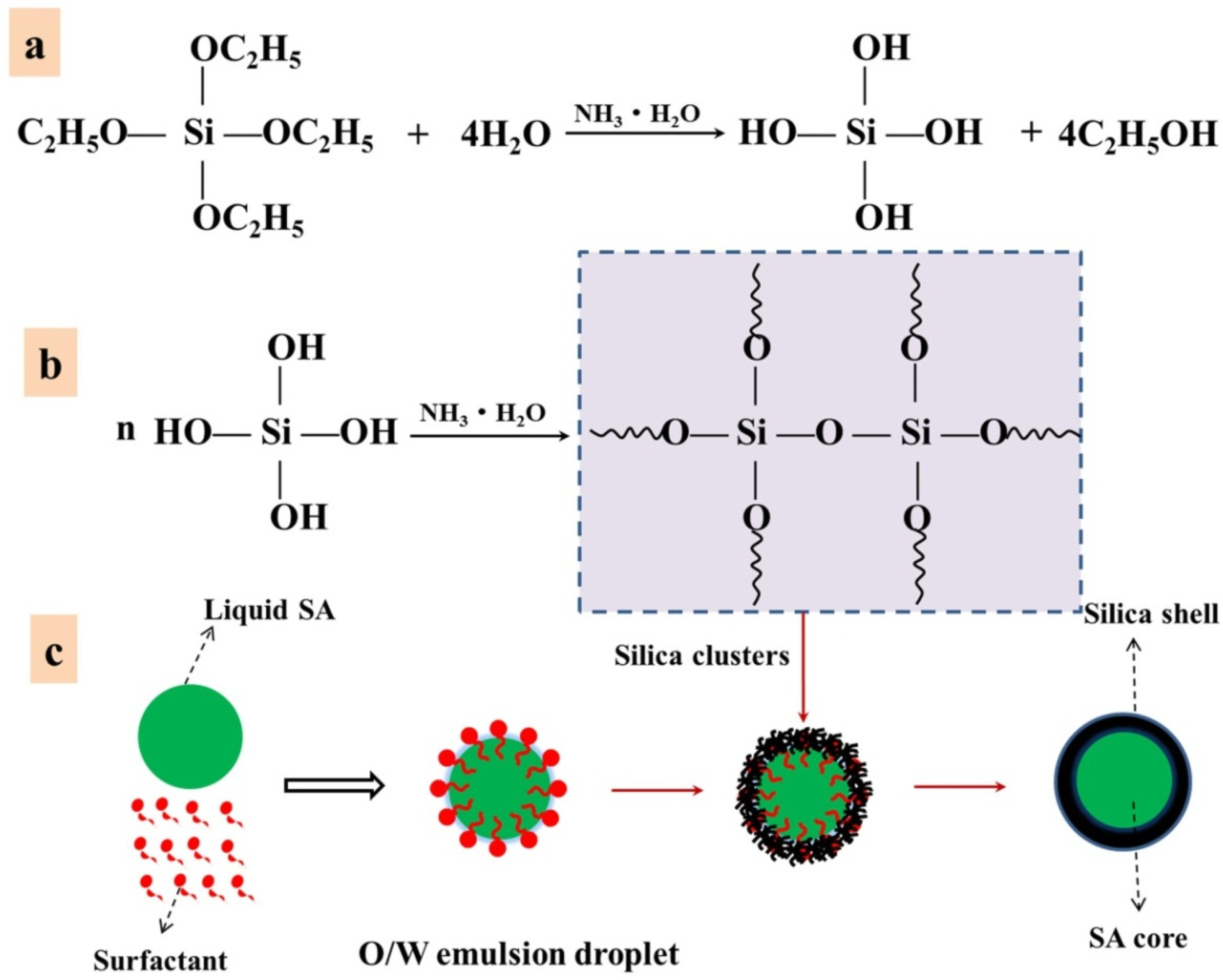
3.1. Encapsulation Methods of Solid–Liquid Organic PCMs
3.1.1. In Situ Polymerization
3.1.2. Interfacial Polymerization
3.1.3. Suspension Polymerization
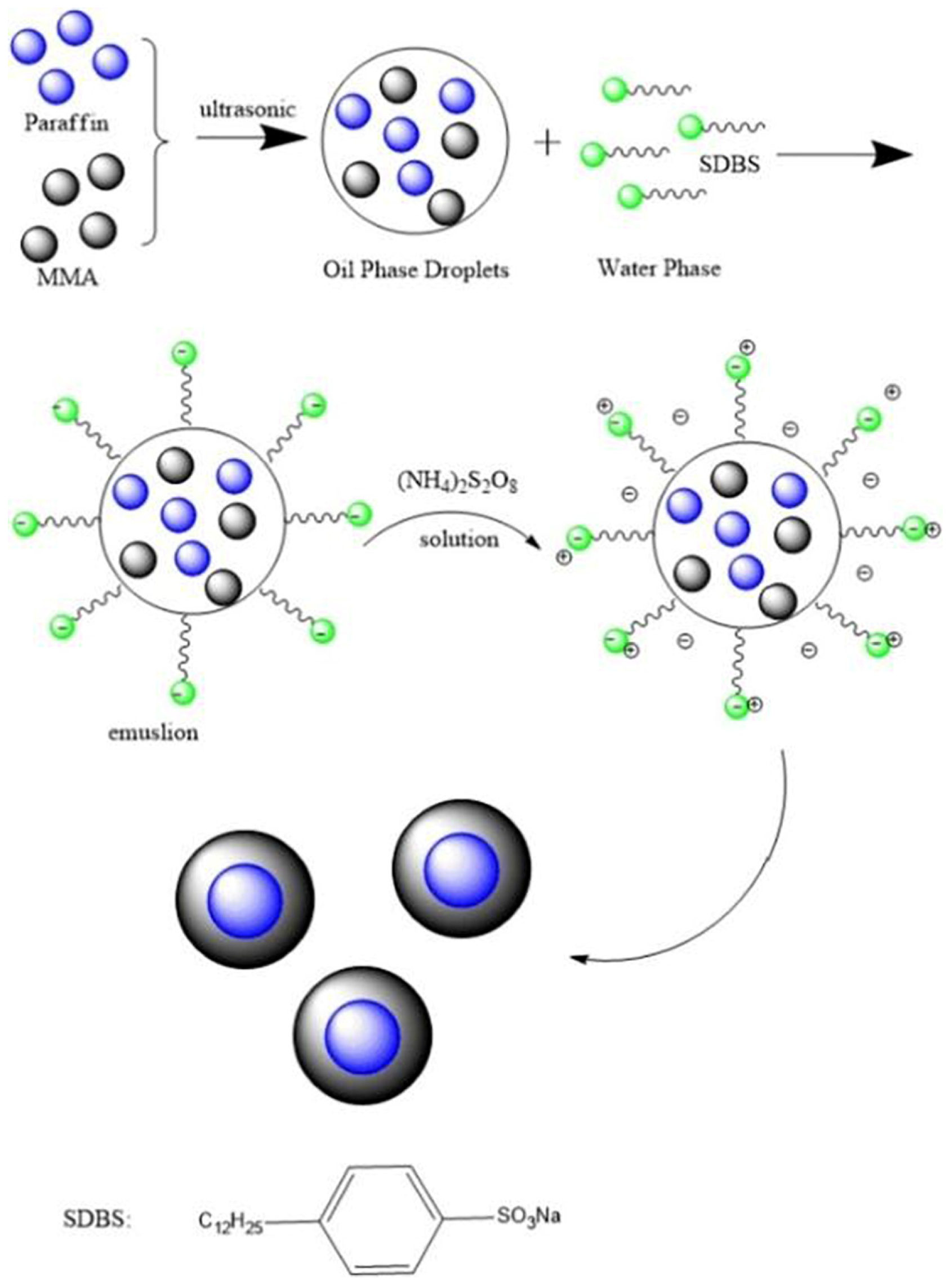
3.1.4. Emulsion Polymerization
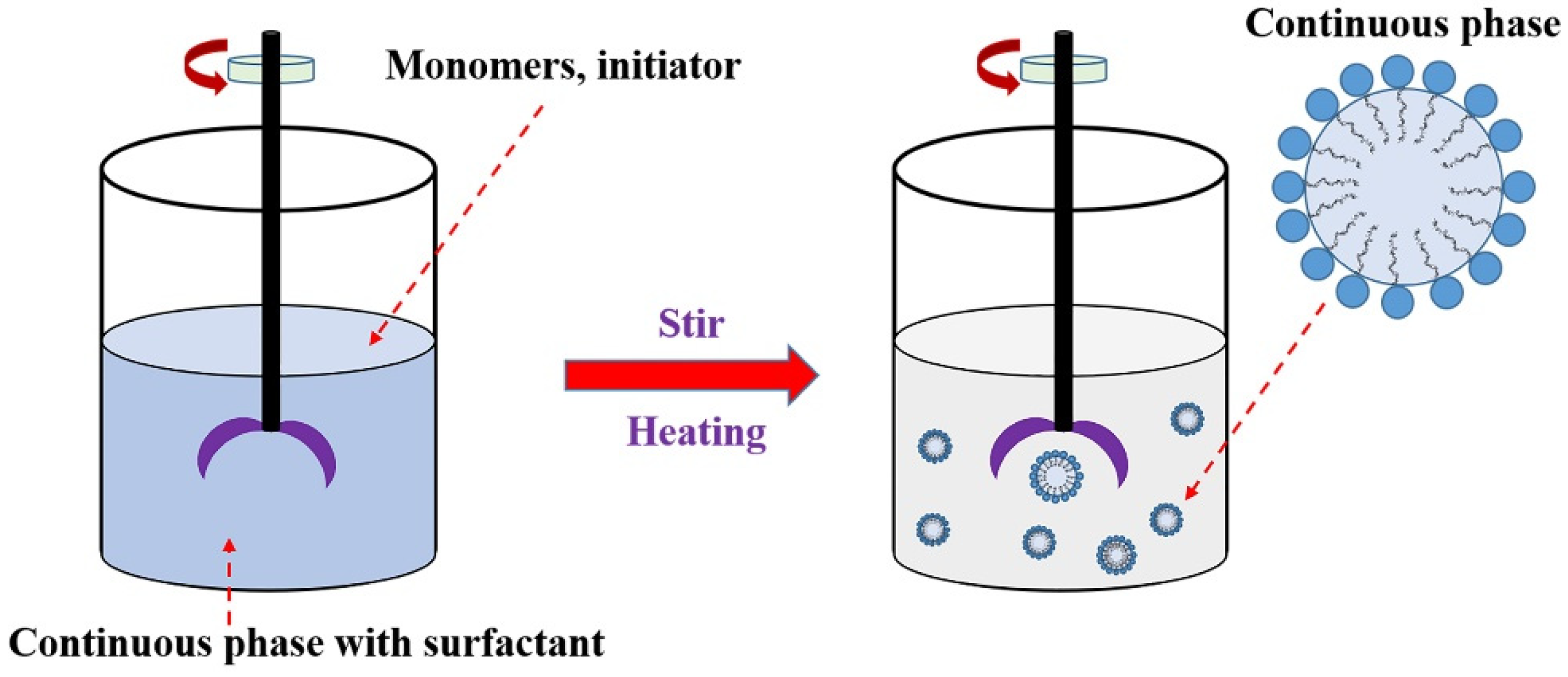
3.1.5. Miniemulsion Polymerization
3.1.6. Sol-Gel
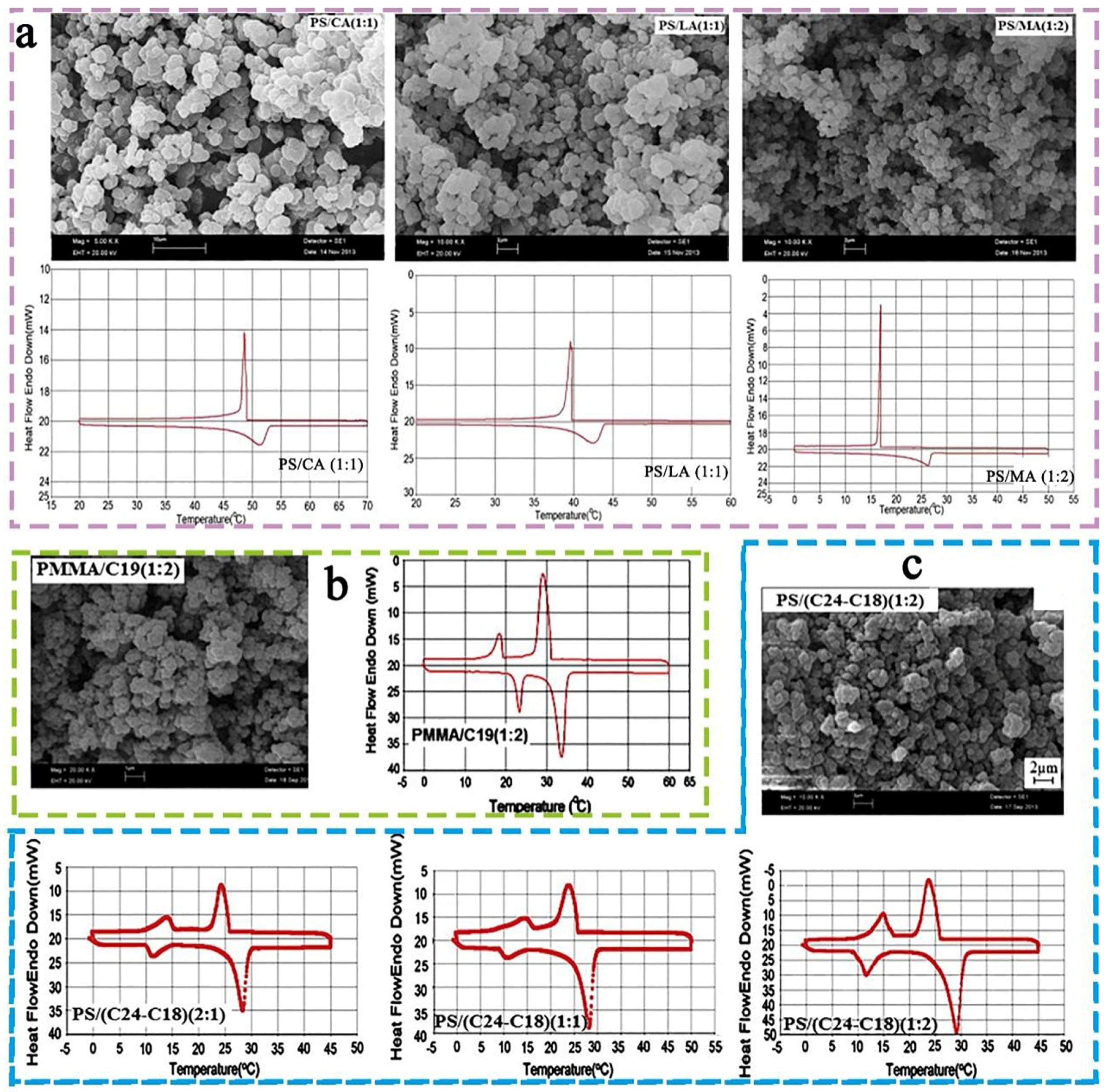
3.2. Analysis of the Encapsulation Methods of Solid–Liquid Organic PCMs
4. Thermal Properties of Solid–Liquid Organic Phase-Change Micro/Nanocapsules
4.1. Thermal Storage Properties Overview of Various Micro/Nanocapsules
4.2. Thermal Conductivity Overview of Solid–Liquid Micro/Nanocapsules
4.2.1. Thermal Conductivity Enhancement by Additives
4.2.2. Thermal Conductivity Enhancement by Inorganic Shells
4.2.3. Thermal Conductivity Enhancement by Metal Shell
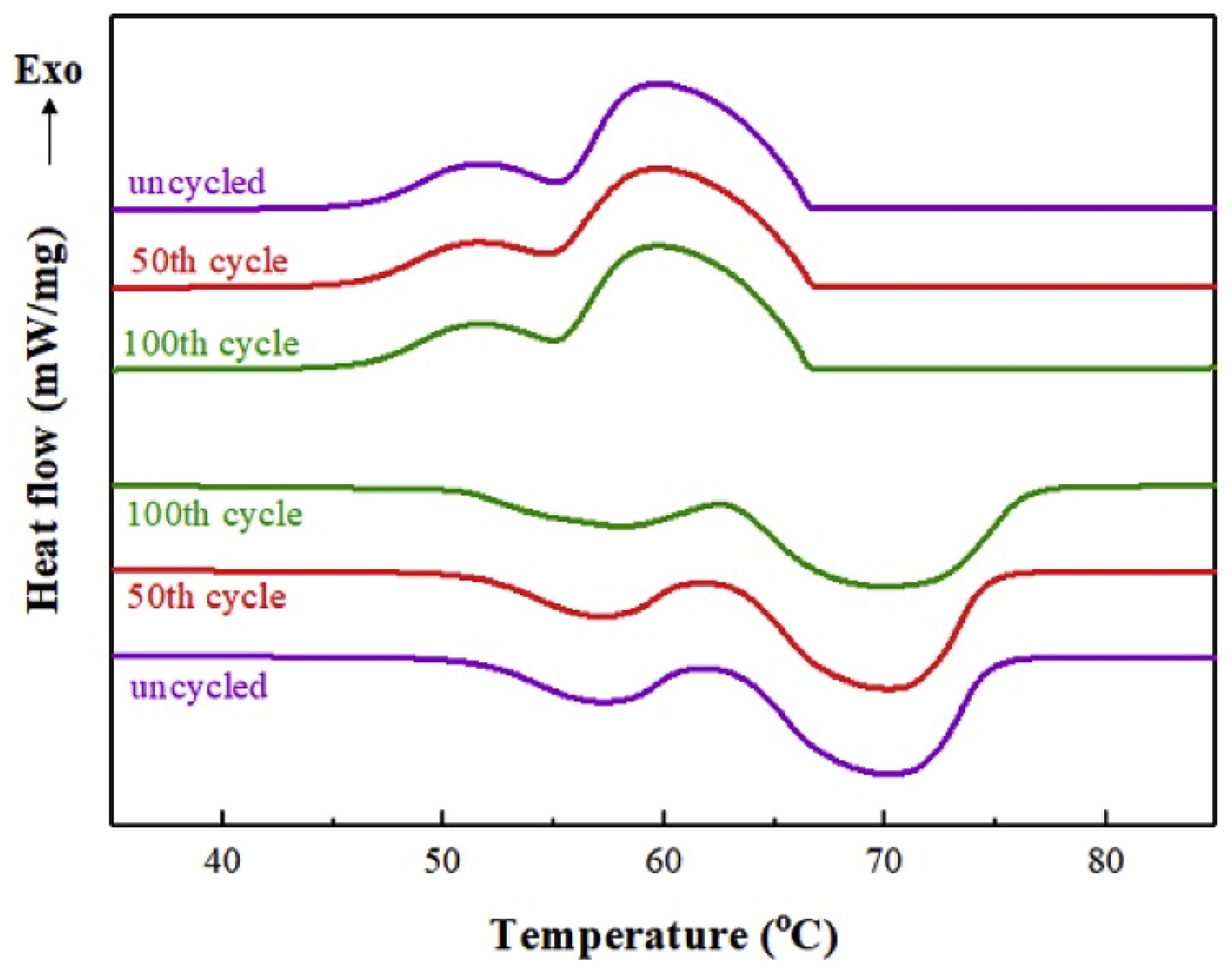
4.3. Thermal Reliability Overview of Solid–Liquid Micro/Nanocapsules
5. Influence Factors on the Thermal Properties of Micro/Nanocapsules
5.1. Influence Factors on the Thermal Storage Properties
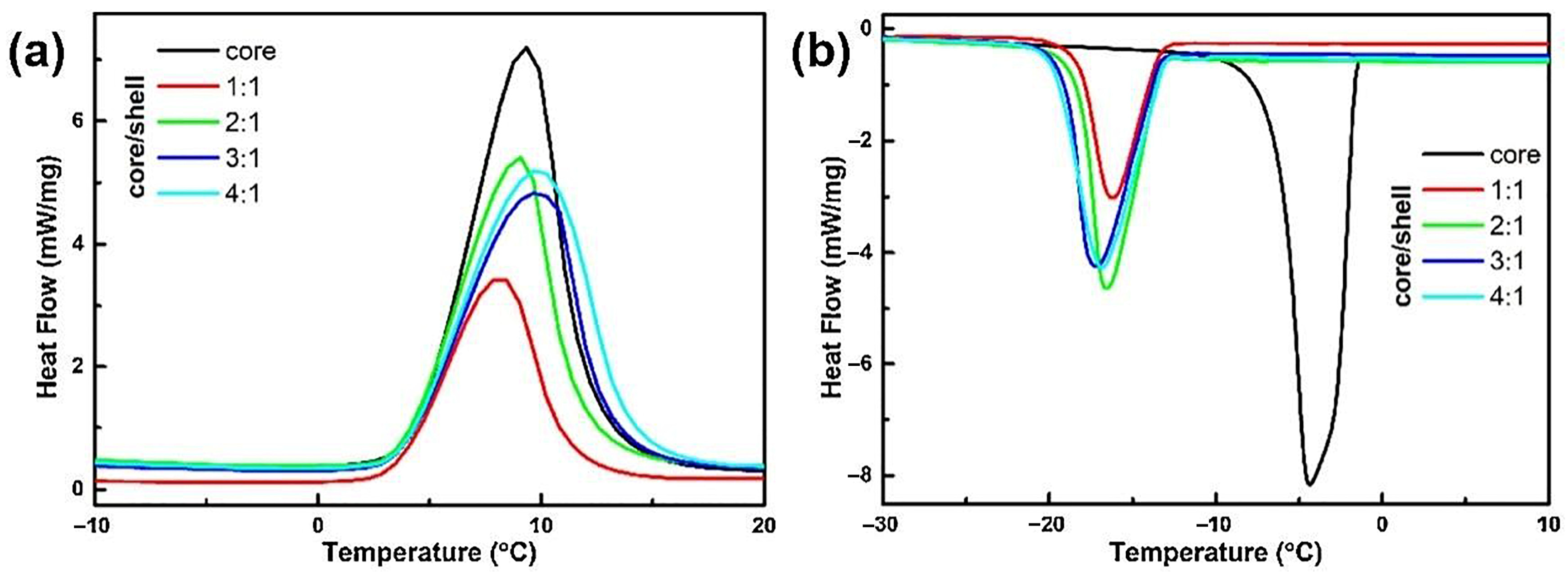
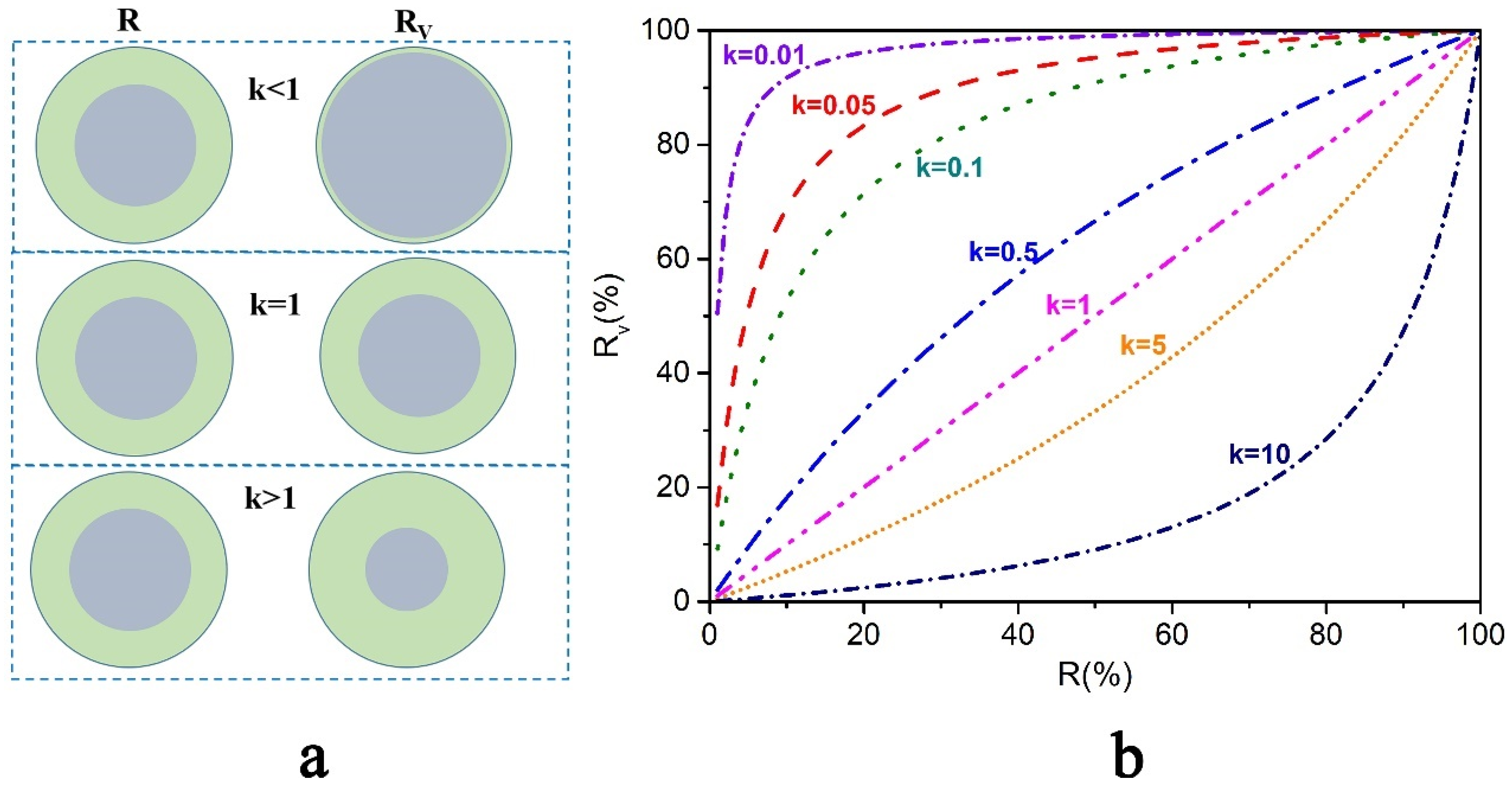
5.1.1. Mass Ratio of Core/Shell
5.1.2. pH
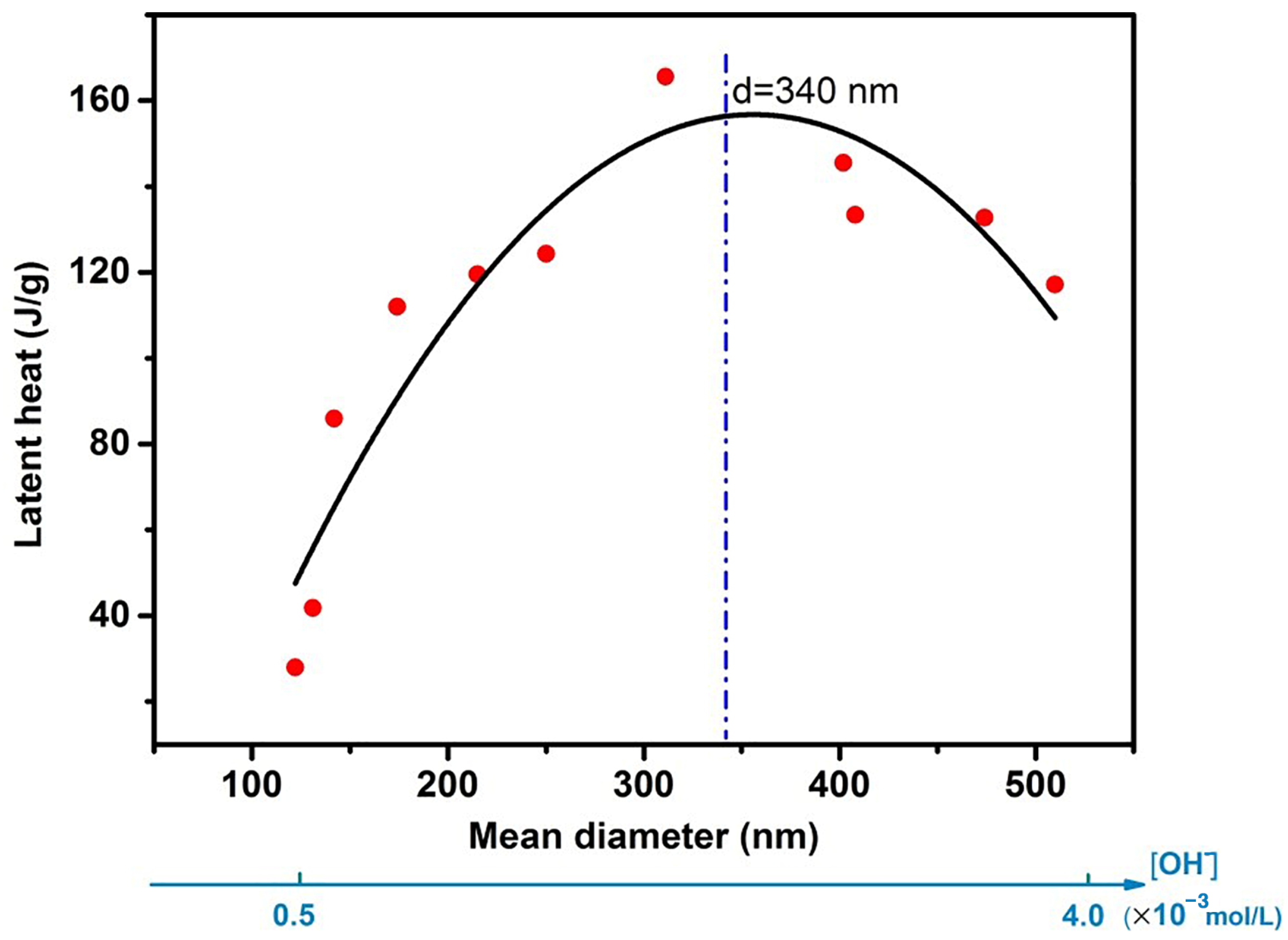
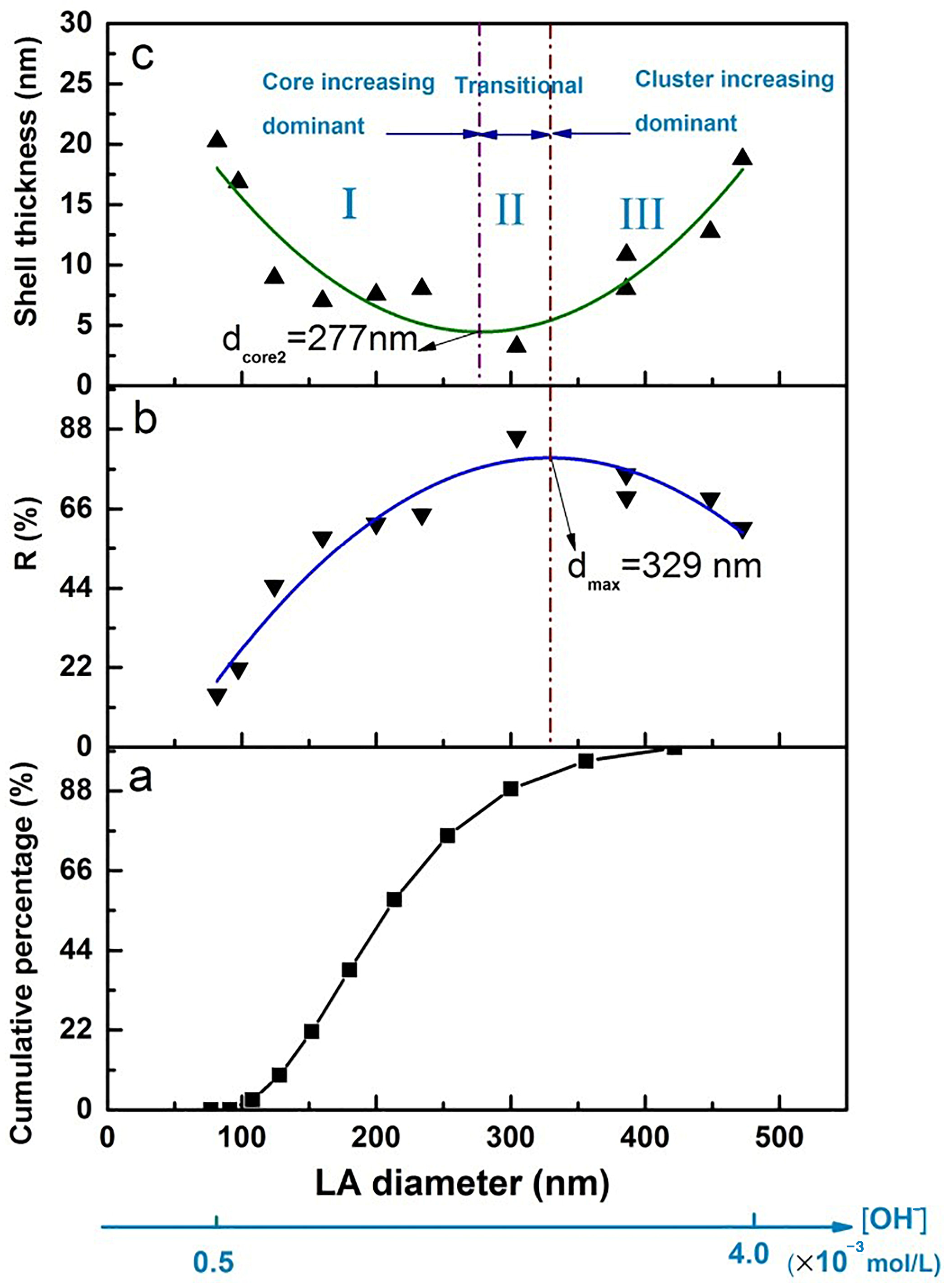
5.1.3. Emulsion Droplet Size
5.1.4. Summary of the Influence Factors on Thermal Storage Capacity
5.2. Influence Factors on Thermal Conductivity
5.2.1. Thermal Conductive Nanoparticle Content
5.2.2. The Distribution and Interface Interaction of Particles in the Shell
5.2.3. High Thermal Conductivity Shell
5.2.4. Mass Ratio of Core/Shell
5.2.5. Summary of the Influence Factors on Thermal Conductivity
5.3. Influence Factors on Thermal Reliability
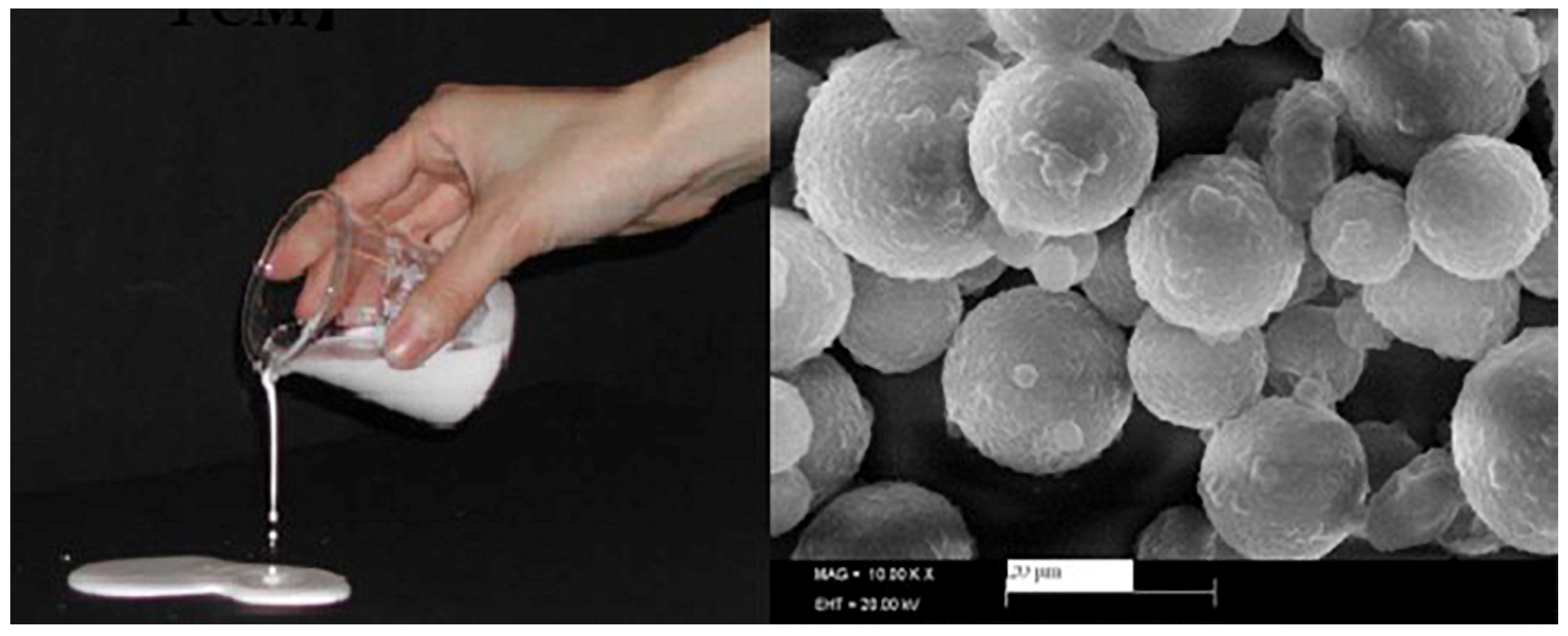
5.3.1. Compactness of the Shell Material
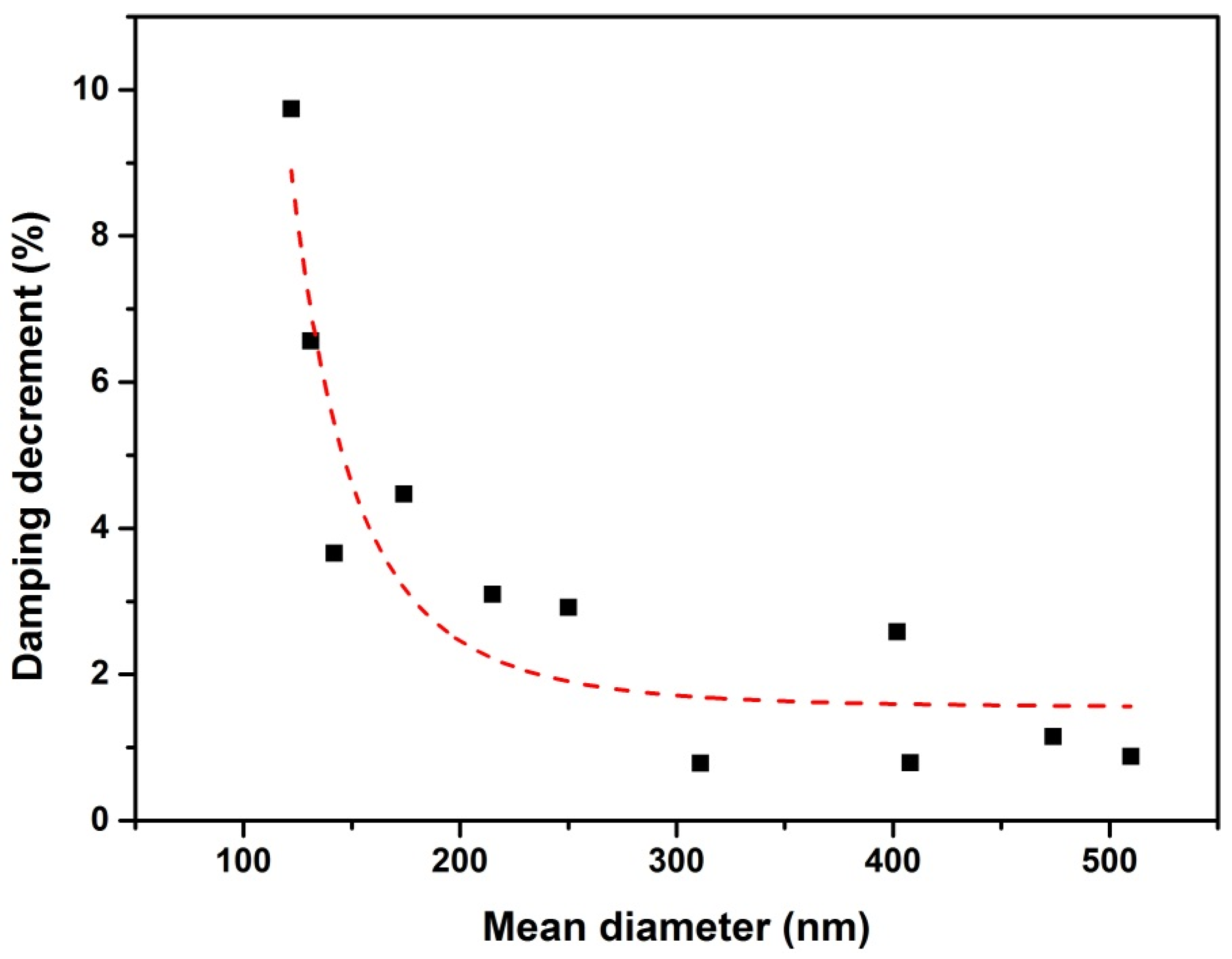
5.3.2. The Size and Shell Thickness
5.3.3. Summary of the Influence Factors on Thermal Reliability
6. Application Prospects of Solid–Liquid Organic Phase-Change Micro/Nanocapsules
6.1. Applications in Working Fluids
6.1.1. Air Conditioning, Ceiling Cooling, and Energy Storage
6.1.2. Thermal Management of Electronic Devices
6.2. Applications in Bulk Form
6.2.1. Green Energy-Saving Building
6.2.2. Food Industry
6.2.3. Temperature-Controlled Textiles
6.2.4. Solar System
7. Further Outlook
- ■
- Although most of the preparation of micro/nanocapsules has mentioned the thermal reliability test, the thermal cycling performance only involved representing the evaluation under a certain number of thermal cycles, whereas the actual service life of the micro/nanocapsules cannot be estimated based on the data. Therefore, finding a unified standard to evaluate the thermal reliability of different phase-change micro/nanocapsules is a problem that needs to be solved.
- ■
- The preparation technology for micro/nanocapsules is mostly concentrated in the laboratory stage; realizing large-scale production and promoting technology requires further research.
- ■
- In addition, micro/nanoencapsulation of PCM has several limitations, such as the selection of the correct material and the high cost of the process. Therefore, simplification of the production methods, improvement in stability, and reduction in encapsulation costs are crucial.
- ■
- In addition, the development of bifunctional/multifunctional phase-change micro/nanocapsules will significantly expand their scope of application beyond the traditional application field.
- ■
- Finally, a database of PCMs must be established to facilitate the selection of production methods as well as shell materials.
8. Conclusions
- ✧
- The thermal properties of solid–liquid PCMs with paraffin-based PCMs, fatty acids, and other PCMs are summarized. In the past years, n-octadecane, n-eicosane, n-hexaoxane, and n-octadecane have been the most commonly used PCMs among paraffins, with SA, PA, and LA being the most commonly studied fatty acid PCMs.
- ✧
- The methods of micro/nanoencapsulation of organic solid–liquid PCMs and their effects on the shell material, size, and properties of the micro/nanocapsules were analyzed. The sol-gel method is used to prepare micro/nanocapsules with organic shells, whereas other methods are mostly used to prepare organic shells. However, all methods can be used to prepare micro/nanocapsules with high thermal storage capacity because they can be adjusted by the preparation conditions. Recently, other synthesis methods have gradually occupied a larger proportion of micro/nanocapsules, indicating that researchers are working on expanding the research on shell materials to further strengthen the performance of micro/nanocapsules.
- ✧
- The thermal properties, including the thermal storage capacity, thermal reliability, and thermal conductivity, of various micro/nanocapsules and their influencing factors were reviewed.
- ✧
- The core/shell mass ratio, shell-forming conditions, and core-formation conditions during the preparation process are the main factors affecting the thermal storage properties. In addition, considering the density difference between the core and shell materials, Rv should be used to fairly assess the encapsulation effect of the micro/nanocapsules.
- ✧
- Nanoparticle content, interface interaction, and dispersion state are key factors affecting the enhanced λ of micro/nanocapsules with additives, whereas a continuous shell with a high λ is better. However, the additive amount of conductive particles is negatively correlated to the thermal storage capacities; this problem requires attention, including the deposition problem when used in the working fluid.
- ✧
- The compactness of the shell, mesopores in the shell structure, and size of the micro/nanocapsule particles are important factors that affect the thermal reliability of micro/nanocapsules.
- ✧
- Finally, the application prospects were presented for phase-change micro/nanocapsules with good thermal performance, which are crucial to the applications of solar systems, working fluids, and thermal management of electronics.
Funding
Data Availability Statement
Conflicts of Interest
References
- da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Al-Sulaiman, F.A.; Rahman, S.; Yilbas, B.S.; Sahin, A.Z. Heat transfer enhancement of phase change materials for thermal energy storage applications: A critical review. Renew. Sustain. Energy Rev. 2017, 74, 26–50. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Shchukina, E.; Graham, M.; Zheng, Z.; Shchukin, D. Nanoencapsulation of phase change materials for advanced thermal energy stor-age systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Vadhera, J.; Sura, A.; Nandan, G.; Dwivedi, G. Study of Phase Change materials and its domestic application. Mater. Today Proc. 2018, 5, 3411–3417. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Meng, J.-Y.; Tang, X.-F.; Zhang, Z.-L.; Zhang, X.-X.; Shi, H.-F. Fabrication and properties of poly (polyethylene glycol octadecyl ether methacrylate). Thermochim. Acta 2013, 574, 116–120. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Yen, T.Q.; Leitch, M.A.; Wilson, G.R.; Brown, E.A.; Rider, D.A.; Reddy, C.M. Alkenones as renewable phase change materials. Renew. Energy 2019, 134, 89–94. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Ahmed, T.; Bhouri, M.; Groulx, D.; White, M.A. Passive Thermal Management of Tablet PCs Using Phase Change Materials: Intermittent Operation. Appl. Sci. 2019, 9, 902. [Google Scholar] [CrossRef]
- Ali, H.M.; Arshad, A.; Janjua, M.M.; Baig, W.; Sajjad, U. Thermal performance of LHSU for electronics under steady and transient oper-ations modes. Int. J. Heat Mass Transfer 2018, 127, 1223–1232. [Google Scholar] [CrossRef]
- Setoh, G.; Tan, F.; Fok, S. Experimental studies on the use of a phase change material for cooling mobile phones. Int. Commun. Heat Mass Transf. 2010, 37, 1403–1410. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Sasaki, K.; Kuroda, A.; Takeda, R.; Kaito, Y. Experimental and numerical study on phase change material (PCM) for thermal management of mobile devices. Appl. Therm. Eng. 2016, 98, 320–329. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in ther-mal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.-R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Li, P.; Zhao, X.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and build-ing applications. Renew. Sustain. Energy Rev. 2017, 77, 246–262. [Google Scholar] [CrossRef]
- Serale, G.; Cascone, Y.; Capozzoli, A.; Fabrizio, E.; Perino, M. Potentialities of a Low Temperature Solar Heating System Based on Slurry Phase Change Materials (PCS). Energy Procedia 2014, 62, 355–363. [Google Scholar] [CrossRef]
- Emam, M.; Ahmed, M. Cooling concentrator photovoltaic systems using various configurations of phase-change material heat sinks. Energy Convers. Manag. 2018, 158, 298–314. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Shu, L. Experimental study of using phase change material cooling in a solar tracking concentrated photovolta-ic-thermal system. Solar Energy 2018, 159, 777–785. [Google Scholar] [CrossRef]
- Al Shannaq, R.; Farid, M. Microencapsulation of phase change materials (PCMs) for thermal energy storage systems. In Advances in Thermal Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 247–284. [Google Scholar] [CrossRef]
- Liu, L.; Alva, G.; Huang, X.; Fang, G. Preparation, heat transfer and flow properties of microencapsulated phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2016, 66, 399–414. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, D.; Ling, X.; Li, Y.; Wang, Y.; Yu, Q.; She, X.; Li, Y.; Ding, Y. Yulong n-Alkanes Phase Change Materials and Their Microencapsulation for Thermal Energy Storage: A Critical Review. Energy Fuels 2018, 32, 7262–7293. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent Heat Thermal Energy Storage Systems with Solid–Liquid Phase Change Materials: A Review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Rodriguez-Cumplido, F.; Pabon-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nano-encapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced thermal systems driven by paraffin-based phase change materials—A review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Singh, J.; Parvate, S.; Vennapusa, J.R.; Maiti, T.K.; Dixit, P.; Chattopadhyay, S. Facile method to prepare 1-dodecanol@ poly (mela-mine-paraformaldehyde) phase change energy storage microcapsules via surfactant-free method. J. Energy Storage 2022, 49, 104089. [Google Scholar] [CrossRef]
- Ali, M.A.; Fayaz; Viegas, R.F.; Kumar, M.B.S.; Kannapiran, R.K.; Feroskhan, M. Enhancement of heat transfer in paraffin wax PCM using nano graphene composite for industrial helmets. J. Energy Storage 2019, 26, 100982. [Google Scholar] [CrossRef]
- Vakhshouri, A.R. Paraffin as phase change material. Paraffin Overv. 2020, 1–23. [Google Scholar] [CrossRef]
- Ohlrogge, J.B.; Jaworski, J.G. Regulation of fatty acid synthesis. Annu. Rev. Plant Biol. 1997, 48, 109–136. [Google Scholar] [CrossRef]
- Harwood, J.L. Fatty acid biosynthesis. In Plant Lipids; Blackwell: Tokyo, Japan, 2020; pp. 27–66. [Google Scholar]
- Feldman, D.; Shapiro, M.; Banu, D.; Fuks, C. Fatty acids and their mixtures as phase-change materials for thermal energy storage. Sol. Energy Mater. 1989, 18, 201–216. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.; Huang, M.; Sarwar, J.; Norton, B. Increased photovoltaic performance through temperature regulation by phase change materials: Materials comparison in different climates. Sol. Energy 2015, 115, 264–276. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Fatty acid esters-based composite phase change materials for thermal energy storage in buildings. Appl. Therm. Eng. 2012, 37, 208–216. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Gbabode, G.; Negrier, P.; Mondieig, D.; Moreno Calvo, E.; Calvet, T.; Cuevas-Diarte, M.À. Structures of the High-Temperature Solid Phases of the Odd-Numbered Fatty Acids from Tridecanoic Acid to Tricosanoic Acid. Chemistry 2007, 13, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Suppes, G.; Goff, M.; Lopes, S. Latent heat characteristics of fatty acid derivatives pursuant phase change material applications. Chem. Eng. Sci. 2003, 58, 1751–1763. [Google Scholar] [CrossRef]
- Costa, M.C.; Sardo, M.; Rolemberg, M.P.; Coutinho, J.A.; Meirelles, A.J.; Ribeiro-Claro, P.; Krähenbühl, M.A. The solid–liquid phase diagrams of binary mixtures of consecutive, even saturated fatty acids. Chem. Phys. Lipids 2009, 160, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Verma, K.; Mishra, V.; Chaudhary, B. A review on carbon-based phase change materials for thermal energy storage. J. Energy Storage 2022, 50, 104166. [Google Scholar] [CrossRef]
- A Talja, R.; Roos, Y.H. Phase and state transition effects on dielectric, mechanical, and thermal properties of polyols. Thermochim. Acta 2001, 380, 109–121. [Google Scholar] [CrossRef]
- Kaizawa, A.; Maruoka, N.; Kawai, A.; Kamano, H.; Jozuka, T.; Senda, T.; Akiyama, T. Thermophysical and heat transfer properties of phase change material candidate for waste heat transportation system. Heat Mass Transf. 2007, 44, 763–769. [Google Scholar] [CrossRef]
- Sari, A.; Eroglu, R.; Biçer, A.; Karaipekli, A. Synthesis and Thermal Energy Storage Properties of Erythritol Tetrastearate and Erythritol Tetrapalmitate. Chem. Eng. Technol. 2010, 34, 87–92. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Eroğlu, R.; Biçer, A. Erythritol Tetra Myristate and Erythritol Tetra Laurate as Novel Phase Change Materials for Low Temperature Thermal Energy Storage. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 1285–1295. [Google Scholar] [CrossRef]
- Nikolić, R.; Marinović-Cincović, M.; Gadžurić, S.; Zsigrai, I. New materials for solar thermal storage—solid/liquid transitions in fatty acid esters. Sol. Energy Mater. Sol. Cells 2003, 79, 285–292. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Electrospun phase change fibers based on polyethylene glycol/cellulose acetate blends. Appl. Energy 2011, 88, 3133–3139. [Google Scholar] [CrossRef]
- Constantinescu, M.; Dumitrache, L.; Constantinescu, D.; Anghel, E.M.; Popa, V.T.; Stoica, A.; Olteanu, M. Latent heat nano composite building materials. Eur. Polym. J. 2010, 46, 2247–2254. [Google Scholar] [CrossRef]
- Mathur, S.; Moudgil, B.M. Adsorption Mechanism(s) of Poly(Ethylene Oxide) on Oxide Surfaces. J. Colloid Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A poly(ethylene glycol)-based smart phase change material. Sol. Energy Mater. Sol. Cells 2008, 92, 1260–1268. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Karaipekli, A. Synthesis, characterization, thermal properties of a series of stearic acid esters as novel solid–liquid phase change materials. Mater. Lett. 2009, 63, 1213–1216. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Zare, P.; Perera, N.; Lahr, J.; Hasan, R. Solid-liquid phase change materials for the battery thermal management systems in electric vehicles and hybrid electric vehicles—A systematic review. J. Energy Storage 2022, 52, 105026. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Palanikkumaran, M.; Gupta, K.K.; Agrawal, A.K.; Jassal, M. Highly stable hexamethylolmelamine microcapsules containing n-octadecane prepared by in situ encapsulation. J. Appl. Polym. Sci. 2009, 114, 2997–3002. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. The manufacture of microencapsulated phase change materials suitable for the design of thermally enhanced fabrics. Thermochim. Acta 2007, 452, 149–160. [Google Scholar] [CrossRef]
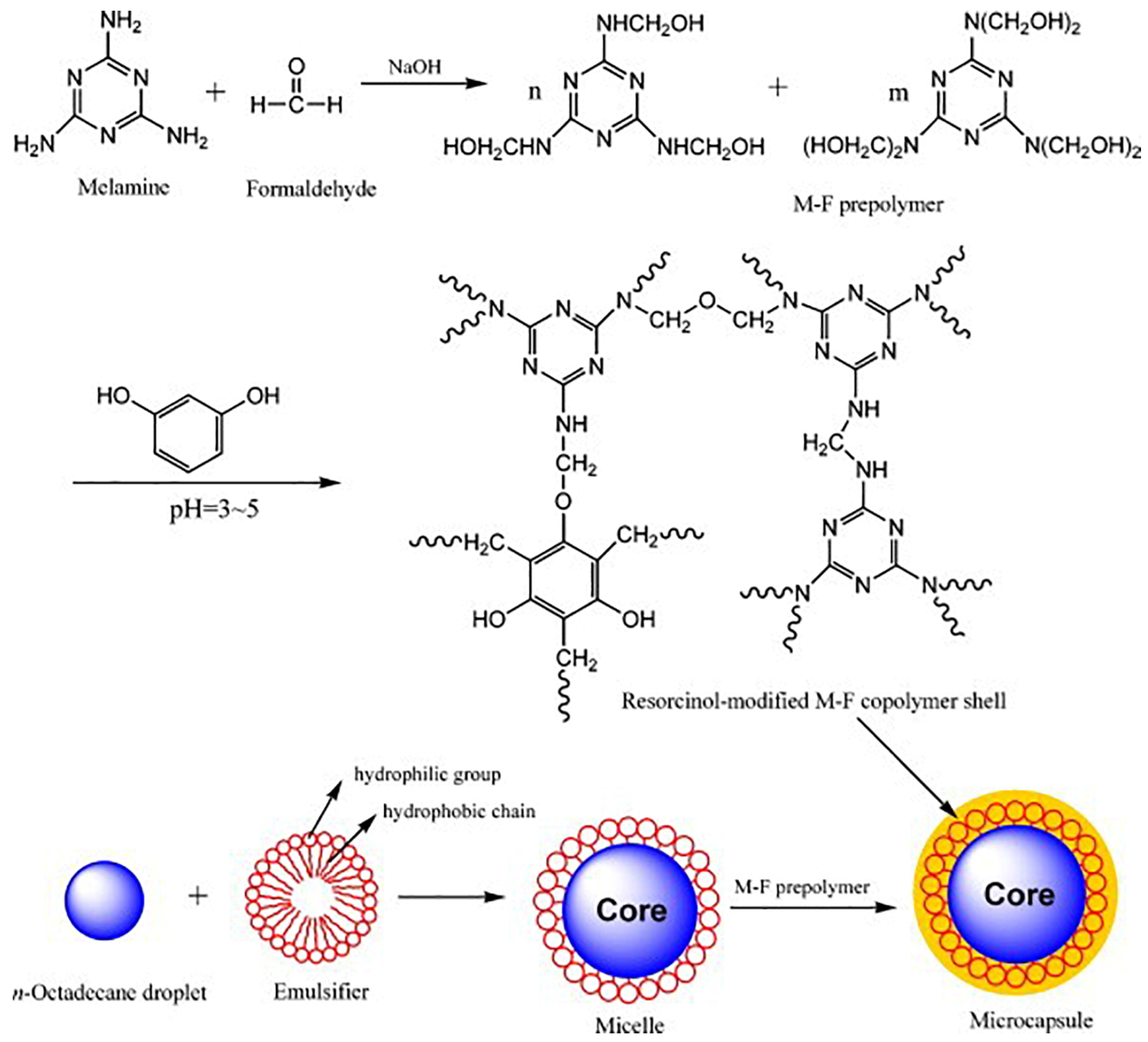
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Rao, Y.; Lin, G.; Luo, Y.; Chen, S.; Wang, L. Preparation and thermal properties of microencapsulated phase change material for en-hancing fluid flow heat transfer. Heat Transf. Asian Res. 2007, 36, 28–37. [Google Scholar] [CrossRef]
- Du, X.; Fang, Y.; Cheng, X.; Du, Z.; Zhou, M.; Wang, H. Fabrication and Characterization of Flame-Retardant Nanoencapsulated n-Octadecane with Melamine–Formaldehyde Shell for Thermal Energy Storage. ACS Sustain. Chem. Eng. 2018, 6, 15541–15549. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Peng, H.; Wang, J.; Zhang, X.; Ma, J.; Shen, T.; Li, S.; Dong, B. A review on synthesis, characterization and application of nanoencapsulated phase change materials for thermal energy storage systems. Appl. Therm. Eng. 2020, 185, 116326. [Google Scholar] [CrossRef]
- Bryant, Y.G. Melt spun fibers containing microencapsulated phase change material. In ASME International Mechanical Engineer-ing Congress and Exposition; American Society of Mechanical Engineers: Nashville, TN, USA, 1999; pp. 225–234. [Google Scholar]
- Shi, J.; Wu, X.; Sun, R.; Ban, B.; Li, J.; Chen, J. Nano-encapsulated phase change materials prepared by one-step interfacial polymeriza-tion for thermal energy storage. Mater. Chem. Phys. 2019, 231, 244–251. [Google Scholar] [CrossRef]
- Zou, G.L.; Tan, Z.C.; Lan, X.Z.; Sun, L.X.; Zhang, T. Preparation and characterization of microencapsulated hexadecane used for thermal energy storage. Chin. Chem. Lett. 2004, 15, 729–732. [Google Scholar]
- Yuan, H.; Kalfas, G.; Ray, W. Suspension polymerization. J. Macromol. Sci. Part C Polym. Rev. 1991, 31, 215–299. [Google Scholar] [CrossRef]
- You, M.; Zhang, X.; Wang, J.; Wang, X. Polyurethane foam containing microencapsulated phase-change materials with styrene–divinybenzene co-polymer shells. J. Mater. Sci. 2009, 44, 3141–3147. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Xu, L.; Xie, H.; Guo, Z. Preparation and thermal characterization of n-octadecane/pentafluorostyrene nanocap-sules for phase-change energy storage. J. Energy Storage 2021, 35, 102327. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Alkan, C.; Özcan, A.N. Thermal energy storage characteristics of myristic acid-palmitic eutectic mixtures encapsu-lated in PMMA shell. Sol. Energy Mater. Sol. Cells 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Alay, S.; Göde, F.; Alkan, C. Preparation and characterization of poly (methylmethacrylate-coglycidyl methacrylate)/n-hexadecane nanocapsules as a fiber additive for thermal energy storage. Fibers Polym. 2010, 11, 1089–1093. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Rezvanpour, M.; Hasanzadeh, M.; Azizi, D.; Rezvanpour, A.; Alizadeh, M. Synthesis and characterization of micro-nanoencapsulated n-eicosane with PMMA shell as novel phase change materials for thermal energy storage. Mater. Chem. Phys. 2018, 215, 299–304. [Google Scholar] [CrossRef]
- Fonseca, G.E.; McKenna, T.F.; Dubé, M.A. Miniemulsion vs. conventional emulsion polymerization for pressure-sensitive adhesives production. Chem. Eng. Sci. 2010, 65, 2797–2810. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Synthesis and characterization of stearic acid/silicon dioxide nanoencapsules for solar energy storage. Sol. Energy 2018, 173, 42–52. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Tsai, P.-S.; Yang, Y.-M. Preparation of silica microspheres encapsulating phase-change material by sol-gel method in O/W emulsion. J. Microencapsul. 2006, 23, 3–14. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoen-capsulated phase change materials via sol-gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Afifi, A.B.M.; Mahlia, T.M.I.; Akhiani, A.R.; Metselaar, H.S.C. Facile synthesis and thermal performances of stea-ric acid/titania core/shell nanocapsules by sol–gel method. Energy 2015, 85, 635–644. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, D. Design and fabrication of dual-functional microcapsules containing phase change material core and zirconium oxide shell with fluorescent characteristics. Sol. Energy Mater. Sol. Cells 2015, 133, 56–68. [Google Scholar] [CrossRef]
- Laghari, I.A.; Samykano, M.; Pandey, A.; Said, Z.; Kadirgma, K.; Tyagi, V. Thermal conductivity and Thermal properties enhancement of Paraffin/Titanium Oxide based Nano enhanced Phase change materials for Energy storage. In Proceedings of the 2022 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 21–24 February 2022; pp. 1–5. [Google Scholar]
- Yuan, H.; Bai, H.; Chen, H.; Zhang, Z.; An, H.; Tian, W. Synthesis and properties of high thermal conductivity Ag shell-coated phase change materials. Renew. Energy 2021, 179, 395–405. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Altıntaş, A. Preparation, characterization and latent heat thermal energy storage properties of mi-cro-nanoencapsulated fatty acids by polystyrene shell. Appl. Therm. Eng. 2014, 73, 1160–1168. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Biçer, A.; Altuntaş, A.; Bilgin, C. Micro/nanoencapsulated n-nonadecane with poly (methyl methacrylate) shell for thermal energy storage. Energy Convers. Manag. 2014, 86, 614–621. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Döğüşcü, D.K.; Kızıl, Ç. Micro/nano encapsulated n-tetracosane and n-octadecane eutectic mixture with polystyrene shell for low-temperature latent heat thermal energy storage applications. Sol. Energy 2015, 115, 195–203. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Chai, Y.; Wang, L. Preparation and Characterization of High Content Paraffin Wax Microcapsules and Mi-cro/Nanocapsules with Poly Methyl Methacrylate Shell by Suspension-Like Polymerization. Chin. J. Chem. 2017, 35, 497–506. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.-H.; Zeng, X.-R. Preparation, characterization, and thermal properties of microPCMs containing n-dodecanol by us-ing different types of styrene-maleic anhydride as emulsifier. Colloid Polym. Sci. 2009, 287, 549–560. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Yang, F.; Liu, X.; Wu, S. Preparation and characterization of nano-encapsulated n-tetradecane as phase change mate-rial for thermal energy storage. Chem. Eng. J. 2009, 153, 217–221. [Google Scholar] [CrossRef]
- Nan, G.-H.; Wang, J.-P.; Wang, Y.; Wang, H.; Li, W.; Zhang, X.-X. Preparation and properties of nanoencapsulated phase change materials containing polyaniline. Acta Phys. Chim. Sin. 2014, 30, 338–344. [Google Scholar]
- Niu, X.; Xu, Q.; Zhang, Y.; Zhang, Y.; Yan, Y.; Liu, T. Fabrication and Properties of Micro-Nanoencapsulated Phase Change Materials for Internally-Cooled Liquid Desiccant Dehumidification. Nanomaterials 2017, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Z.; Yu, X.; Li, B. Preparation and Thermal Energy Storage of Carboxymethyl Cellulose-Modified Nanocapsules. BioEnergy Res. 2013, 6, 1135–1141. [Google Scholar] [CrossRef]
- Han, S.; Lyu, S.; Wang, S.; Fu, F. High-intensity ultrasound assisted manufacturing of melamine-urea-formaldehyde/paraffin nanocapsules. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 75–83. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Ramachandran, T.; Shanmugasundaram, O. Synthesis, characterization, and development of thermally enhanced cotton fabric using nanoencapsulated phase change materials containing paraffin wax. J. Text. Inst. 2014, 105, 1279–1286. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, J.; Zhang, X. Preparation of double-shell nanoencapsulated phase change materials by interfacial polymeriza-tion in an emulsion system. New Chem. Mater. 2011, 39, 45–49. [Google Scholar]
- Kook, J.; Cho, W.; Koh, W.; Cheong, I.W.; Kim, J.H. Preparation and characterization of octadecane/polyurea nanocapsule-embedded poly(ethylene oxide) nanofibers. J. Appl. Polym. Sci. 2015, 132, 42539. [Google Scholar] [CrossRef]
- Barlak, S.; Sara, O.N.; Karaipekli, A.; Yapıcı, S. Thermal Conductivity and Viscosity of Nanofluids Having Nanoencapsulated Phase Change Material. Nanoscale Microscale Thermophys. Eng. 2016, 20, 85–96. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Fu, X.; Sun, R. Synthesis and thermal properties of a novel nanoencapsulated phase change material with PMMA and SiO2 as hybrid shell materials. Thermochim. Acta 2015, 617, 90–94. [Google Scholar] [CrossRef]
- Pan, L.; Tao, Q.; Zhang, S.; Wang, S.; Zhang, J.; Wang, S.; Wang, Z.; Zhang, Z. Preparation, characterization and thermal properties of mi-cro-encapsulated phase change materials. Sol. Energy Mater. Sol. Cells 2012, 98, 66–70. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A.; Karaipekli, A.; Uzun, O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 143–147. [Google Scholar] [CrossRef]
- Li, H.; Jiang, M.; Li, Q.; Li, D.; Huang, J.; Hu, W.; Dong, L.; Xie, H.; Xiong, C. Facile preparation and thermal performances of hexadecanol/crosslinked poly-styrene core/shell nanocapsules as phase change material. Polym. Compos. 2014, 35, 2154–2158. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.O.; Unal, M. Nanoencapsulation of n-alkanes with poly(styrene-co-ethylacrylate) shells for thermal energy storage. Appl. Energy 2015, 150, 335–340. [Google Scholar] [CrossRef]
- Alay, S.; Alkan, C.; Göde, F. Synthesis and characterization of poly (methyl methacrylate)/n-hexadecane microcapsules using dif-ferent cross-linkers and their application to some fabrics. Thermochim. Acta 2011, 518, 1–8. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers. Manag. 2011, 52, 687–692. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Konuklu, Y.; Fernández, A. Preparation and exhaustive characterization of paraffin or palmitic acid microcapsules as novel phase change material. Sol. Energy 2015, 112, 300–309. [Google Scholar] [CrossRef]
- Li, M.G.; Zhang, Y.; Xu, Y.H.; Zhang, D. Effect of different amounts of surfactant on characteristics of nanoencapsulated phase-change materials. Polym. Bull. 2011, 67, 541–552. [Google Scholar] [CrossRef]
- Sirohi, S.; Singh, D.; Nain, R.; Parida, D.; Agrawal, A.K.; Jassal, M. Electrospun composite nanofibres of PVA loaded with nanoencap-sulated n-octadecane. RSC Adv. 2015, 5, 34377–34382. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compositions, morphologies, and properties. Energy Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Du, X.; Wang, S.; Du, Z.; Cheng, X.; Wang, H. Preparation and characterization of flame-retardant nanoencapsulated phase change materials with poly(methylmethacrylate) shells for thermal energy storage. J. Mater. Chem. A 2018, 6, 17519–17529. [Google Scholar] [CrossRef]
- Zhang, G.H.; Bon, S.A.; Zhao, C.-Y. Synthesis, characterization and thermal properties of novel nanoencapsulated phase change ma-terials for thermal energy storage. Solar Energy 2012, 86, 1149–1154. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-encapsulated organic phase change material based on copolymer nanocomposites for thermal energy storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Fang, Y.; Kuang, S.; Gao, X.; Zhang, Z. Preparation and characterization of novel nanoencapsulated phase change materials. Energy Convers. Manag. 2008, 49, 3704–3707. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, H.; Wan, W.; Gao, X.; Zhang, Z. Preparation and thermal performance of polystyrene/n-tetradecane composite nanoen-capsulated cold energy storage phase change materials. Energy Convers. Manag. 2013, 76, 430–436. [Google Scholar] [CrossRef]
- Fu, W.; Liang, X.; Xie, H.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermophysical properties of n-tetradecane@ polystyrene-silica compo-site nanoencapsulated phase change material slurry for cold energy storage. Energy Build. 2017, 136, 26–32. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Liang, X.; Liu, H.; Gao, X.; Zhang, Z. Ultrasonic synthesis and characterization of polystyrene/n-dotriacontane com-posite nanoencapsulated phase change material for thermal energy storage. Appl. Energy 2014, 132, 551–556. [Google Scholar] [CrossRef]
- de Cortazar, M.G.; Rodríguez, R. Thermal storage nanocapsules by miniemulsion polymerization. J. Appl. Polym. Sci. 2013, 127, 5059–5064. [Google Scholar] [CrossRef]
- Fuensanta, M.; Paiphansiri, U.; Romero-Sánchez, M.D.; Guillem, C.; López-Buendía, M.; Landfester, K. Thermal properties of a novel nanoencapsulated phase change material for thermal energy storage. Thermochim. Acta 2013, 565, 95–101. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Yu, F.; Zeng, X.-R.; Zhang, Z.-G. Preparation, characterization and thermal properties of nanocapsules containing phase change material n-dodecanol by miniemulsion polymerization with polymerizable emulsifier. Appl. Energy 2012, 91, 7–12. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.-H.; Zeng, X.-R.; Gao, X.-N.; Zhang, Z.-G. Poly(methyl methacrylate) copolymer nanocapsules containing phase-change material (n-dodecanol) prepared via miniemulsion polymerization. J. Appl. Polym. Sci. 2015, 132, 42334. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Nan, G.; Wang, H.; Li, W.; Zhang, X. A Novel Method for the Preparation of Narrow-Disperse Nanoencapsulated Phase Change Materials by Phase Inversion Emulsification and Suspension Polymerization. Ind. Eng. Chem. Res. 2015, 54, 9307–9313. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Kong, W.; Zong, C. Properties of n-octadecane nanocapsules prepared with anionic polyurethane. Polym. Mater. Als Sci. Eng. 2014, 30, 172–176+181. [Google Scholar]
- Lu, J.; Yang, L.I.; Han, Y.; Qian, F. Preparation and properties characterization of microencapsulated phase change materials using acrylic resin copolymers/n-dodecanol. Chin. J. Process Eng. 2019, 19, 617–622. [Google Scholar]
- Zhu, Y.; Liang, S.; Wang, H.; Zhang, K.; Jia, X.; Tian, C.; Zhou, Y.; Wang, J. Morphological control and thermal properties of nanoencapsulated n-octadecane phase change material with organosilica shell materials. Energy Convers. Manag. 2016, 119, 151–162. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, Y.; Wang, H.; Wu, T.; Tian, C.; Wang, J.; Bai, R. Preparation and Characterization of Thermoregulated Rigid Polyurethane Foams Containing Nanoencapsulated Phase Change Materials. Ind. Eng. Chem. Res. 2016, 55, 2721–2730. [Google Scholar] [CrossRef]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Chen, K.; Gao, X.; Chang, P.; Tian, C.; Wang, J.; Huang, Y. Preparation and properties of nanoencapsulated n-octadecane phase change material with organosilica shell for thermal energy storage. Energy Convers. Manag. 2015, 105, 908–917. [Google Scholar] [CrossRef]
- Kalaiselvam, S. Bifunctional nanoencapsulated eutectic phase change material core with SiO2/SnO2 nanosphere shell for ther-mal and electrical energy storage. Mater. Des. 2018, 154, 291–301. [Google Scholar]
- Yuan, H.; Bai, H.; Lu, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Size controlled lauric acid/silicon dioxide nanocapsules for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 191, 243–257. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Lu, X.; Zhao, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol. Energy 2019, 185, 374–386. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, Y.F.; Tao, X.; Yick, K.L. Fabrication and properties of microcapsules and nanocapsules containing n-octadecane. Mater. Chem. Phys. 2004, 88, 300–307. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Carmona, M.; Romero, A.; Sánchez, P. Thermal and morphological stability of polystyrene micro-capsules containing phase-change materials. J. Appl. Polym. Sci. 2011, 120, 291–297. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, J.; Wang, H.; Song, G.; Liu, Y.; Tang, G. Enhancement in thermal property of phase change microcapsules with modi-fied silicon nitride for solar energy. Sol. Energy Mater. Sol. Cells 2016, 151, 89–95. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcap-sules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhao, T. Fabrication and characterization of poly (melamine-formaldehyde)/silicon carbide hybrid microencap-sulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol. Energy Mater. Sol. Cells 2018, 183, 82–91. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.G. Magnetic nanoparticle-embedded PCM nanocapsules based on par-affin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Wu, Z. Enhancement in thermal property and mechanical property of phase change microcapsule with modified carbon nanotube. Appl. Energy 2014, 127, 166–171. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium di-oxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 143, 29–37. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and cal-cium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, D. Microencapsulation of n-dodecane into zirconia shell doped with rare earth: Design and synthesis of bifunctional microcapsules for photoluminescence enhancement and thermal energy storage. Energy 2016, 97, 113–126. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Devanuri, J.K. Containers for Thermal Energy Storage. In Micro-and Nano-Containers for Smart Applications; Springer: Singapore, 2022; pp. 289–307. [Google Scholar]
- Sharma, S.; Sagara, K. Latent Heat Storage Materials and Systems: A Review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Ferrer, G.; Solé, A.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Review on the methodology used in thermal stability characterization of phase change materials. Renew. Sustain. Energy Rev. 2015, 50, 665–685. [Google Scholar] [CrossRef]
- Lan, W.; Shang, B.; Wu, R.; Yu, X.; Hu, R.; Luo, X. Thermally-enhanced nanoencapsulated phase change materials for latent function-ally thermal fluid. Int. J. Therm. Sci. 2021, 159, 106619. [Google Scholar] [CrossRef]
- Nikpourian, H.; Bahramian, A.R.; Abdollahi, M. On the thermal performance of a novel PCM nanocapsule: The effect of core/shell. Renew. Energy 2020, 151, 322–331. [Google Scholar] [CrossRef]
- Dhivya, S.; Hussain, S.I.; Kalaiselvam, S. Novel metal coated nanocapsules of ethyl esters fatty acid eutectic mixture as phase change material with enhanced thermal conductivity for energy storage applications. Thermochim. Acta 2020, 687, 178581. [Google Scholar] [CrossRef]
- Wang, H.; Ma, W.; Zhang, J.; Yang, Z.; Zong, D. Novel synthesis of silica coated palmitic acid nanocapsules for thermal energy storage. J. Energy Storage 2020, 30, 101402. [Google Scholar] [CrossRef]
- He, L.; Mo, S.; Lin, P.; Jia, L.; Chen, Y.; Cheng, Z. D-mannitol@silica/graphene oxide nanoencapsulated phase change material with high phase change properties and thermal reliability. Appl. Energy 2020, 268, 115020. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, S.; Hao, S.; Zhang, Z.; An, H.; Tian, W.; Chan, M.; Bai, H. Fabrication and properties of nanoencapsulated stearic acid phase change materials with Ag shell for solar energy storage. Sol. Energy Mater. Sol. Cells 2022, 239, 111653. [Google Scholar] [CrossRef]
- Trivedi, G.; Parameshwaran, R. Micro/nanoencapsulation of dimethyl adipate with melamine formaldehyde shell as phase change material slurries for cool thermal energy storage. Chem. Thermodyn. Therm. Anal. 2022, 6, 100037. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, X.; Zhong, Z.; Zhang, X.; Peng, H. Nanoencapsulated n-tetradecane phase change materials with melamine–urea–formaldehyde–TiO2 hybrid shell for cold energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128162. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Gómez, M.A.; Jaramillo, F.; Fernández, A.G.; Cabeza, L.F. Thermal reliability of organic-organic phase change materials and their shape-stabilized composites. J. Energy Storage 2021, 40, 102661. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Z.; Ma, W. Fabrication and characterization of a novel polyurethane microencapsulated phase change material for thermal energy storage. Prog. Org. Coatings 2021, 151, 106006. [Google Scholar] [CrossRef]
- Liu, C.; Cao, H.; Jin, S.; Bao, Y.; Cheng, Q.; Rao, Z. Synthesis and characterization of microencapsulated phase change material with phenol-formaldehyde resin shell for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 243, 111789. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cao, Z.; Yang, D.W.; Xu, F.; Sun, L.X.; Zhang, X.F.; Zhang, L. Effects of MWNTs on phase change enthalpy and thermal conductivity of a solid-liquid organic PCM. J. Therm. Anal. Calorim. 2009, 95, 507–512. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Z.; Guo, Q.; Wu, G. Preparation and thermal properties of short carbon fibers/erythritol phase change materials. Energy Convers. Manag. 2017, 136, 220–228. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells 2019, 192, 72–80. [Google Scholar] [CrossRef]
- Liu, H.; Niu, J.; Wang, X.; Wu, D. Design and construction of mesoporous silica/n-eicosane phase-change nanocomposites for su-percooling depression and heat transfer enhancement. Energy 2019, 188, 116075. [Google Scholar] [CrossRef]
- Sukhorukov, G.; Fery, A.; Möhwald, H. Intelligent micro- and nanocapsules. Prog. Polym. Sci. 2005, 30, 885–897. [Google Scholar] [CrossRef]
- Pretzl, M.; Neubauer, M.; Tekaat, M.; Kunert, C.; Kuttner, C.; Leon, G.; Berthier, D.; Erni, P.; Ouali, L.; Fery, A. Formation and Mechanical Characterization of Aminoplast Core/Shell Microcapsules. ACS Appl. Mater. Interfaces 2012, 4, 2940–2948. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for en-ergy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.; Wang, R. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Wang, X.; Niu, J.; van Paassen, A. Raising evaporative cooling potentials using combined cooled ceiling and MPCM slurry storage. Energy Build. 2008, 40, 1691–1698. [Google Scholar] [CrossRef]
- Wang, X.; Niu, J. Performance of cooled-ceiling operating with MPCM slurry. Energy Convers. Manag. 2009, 50, 583–591. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z. An overview of fundamental studies and applications of phase change material slurries to secondary loop re-frigeration and air conditioning systems. Renew. Sustain. Energy Rev. 2012, 16, 5021–5058. [Google Scholar] [CrossRef]
- Pathak, L.; Trivedi, G.; Parameshwaran, R.; Deshmukh, S.S. Microencapsulated phase change materials as slurries for thermal ener-gy storage: A review. Mater. Today Proc. 2021, 44, 1960–1963. [Google Scholar] [CrossRef]
- Ye, J.; Mo, S.; Jia, L.; Chen, Y. Experimental performance of a LED thermal management system with suspended microencapsulated phase change material. Appl. Therm. Eng. 2022, 207, 118155. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Wang, Y.; Lian, C.; Li, Q.; Wang, Z. Performance investigation of a battery thermal management system with microen-capsulated phase change material suspension. Appl. Therm. Eng. 2020, 180, 115795. [Google Scholar] [CrossRef]
- Deng, X.; Wang, S.; Wang, J.; Zhang, T. Analytical modeling of microchannel heat sinks using microencapsulated phase change ma-terial slurry for chip cooling. Procedia Eng. 2017, 205, 2704–27011. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Yu, Q.; Li, Y.; Feng, Z.; Ding, Y. Investigation of thermal management for lithium-ion pouch battery module based on phase change slurry and mini channel cooling plate. Energy 2019, 167, 561–574. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, X.; Li, G.; Li, J. Thermodynamic assessment of active cooling/heating methods for lithium-ion batteries of electric vehicles in extreme conditions. Energy 2014, 64, 1092–1101. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, M.; Bahrampoury, R.; Ranjbar, A.; Borhani, S. Cylindrical battery thermal management based on microencapsulated phase change slurry. J. Energy Storage 2021, 40, 102602. [Google Scholar] [CrossRef]
- Sabbah, R.; Farid, M.M.; Al-Hallaj, S. Micro-channel heat sink with slurry of water with micro-encapsulated phase change material: 3D-numerical study. Appl. Therm. Eng. 2009, 29, 445–454. [Google Scholar] [CrossRef]
- Sivanathan, A.; Dou, Q.; Wang, Y.; Li, Y.; Corker, J.; Zhou, Y.; Fan, M. Phase change materials for building construction: An overview of nano-/micro-encapsulation. Nanotechnol. Rev. 2020, 9, 896–921. [Google Scholar] [CrossRef]
- Aguayo, M.; Das, S.; Maroli, A.; Kabay, N.; Mertens, J.C.; Rajan, S.D.; Sant, G.; Chawla, N.; Neithalath, N. The influence of microencapsulated phase change material (PCM) characteristics on the microstructure and strength of cementitious composites: Experiments and finite element simulations. Cem. Concr. Compos. 2016, 73, 29–41. [Google Scholar] [CrossRef]
- Arıcı, M.; Bilgin, F.; Nižetić, S.; Karabay, H. PCM integrated to external building walls: An optimization study on maximum activation of latent heat. Appl. Therm. Eng. 2020, 165, 114560. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.-M.; Gschwander, S.; Haussmann, T. Micro-encapsulated phase-change materials integrated into construction materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Arce, P.; Castellón, C.; Castell, A.; Cabeza, L.F. Use of microencapsulated PCM in buildings and the effect of adding awnings. Energy Build. 2012, 44, 88–93. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Arivazhagan, R.; Geetha, N.; Sivasamy, P.; Kumaran, P.; Gnanamithra, M.K.; Sankar, S.; Loganathan, G.B.; Arivarasan, A. Review on performance assessment of phase change materials in buildings for thermal management through passive approach. Mater. Today Proc. 2020, 22, 419–431. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Phase change materials for advanced cooling packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Rubio, A.; Lagarón, J.M. Development of zein-based heat-management structures for smart food packaging. Food Hydrocoll. 2013, 30, 182–191. [Google Scholar] [CrossRef]
- Ünal, M.; Konuklu, Y.; Paksoy, H. Thermal buffering effect of a packaging design with microencapsulated phase change material. Int. J. Energy Res. 2019, 43, 4495–4505. [Google Scholar] [CrossRef]
- Geng, X.; Li, W.; Wang, Y.; Lu, J.; Wang, J.; Wang, N.; Li, J.; Zhang, X. Reversible thermochromic microencapsulated phase change materials for thermal energy storage application in thermal protective clothing. Appl. Energy 2018, 217, 281–294. [Google Scholar] [CrossRef]
- Iqbal, K.; Sun, D. Development of thermo-regulating polypropylene fibre containing microencapsulated phase change materials. Renew. Energy 2014, 71, 473–479. [Google Scholar] [CrossRef]
- Pause, B. Nonwoven Protective Garments with Thermo-Regulating Properties. J. Ind. Text. 2003, 33, 93–99. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.; Jang, J. Molecular weight effect on antimicrobial activity of chitosan treated cotton fabrics. J. Appl. Polym. Sci. 2001, 80, 2495–2501. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.; Son, K. Development of thermoregulating textile materials with microencapsulated phase change materials (PCM). II. Preparation and application of PCM microcapsules. J. Appl. Polym. Sci. 2005, 96, 2005–2010. [Google Scholar] [CrossRef]
- Sánchez, P.; Sánchez-Fernandez, M.V.; Romero, A.; Rodríguez, J.F.; Sánchez-Silva, L. Development of thermo-regulating textiles using paraffin wax microcapsules. Thermochim. Acta 2010, 498, 16–21. [Google Scholar] [CrossRef]
- Koo, K.; Park, Y.; Choe, J.; Kim, E. The application of microencapsulated phase-change materials to nylon fabric using direct dual coating method. J. Appl. Polym. Sci. 2008, 108, 2337–2344. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Silakhori, M.; Metselaar, H.; Mahlia, T.; Fauzi, H. Preparation and characterisation of microencapsulated paraffin wax with polyani-line-based polymer shells for thermal energy storage. Mater. Res. Innov. 2014, 18, S6-480–S6-484. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Development of microencapsulated phase change material for solar thermal energy storage. Appl. Therm. Eng. 2017, 112, 1205–1212. [Google Scholar] [CrossRef]


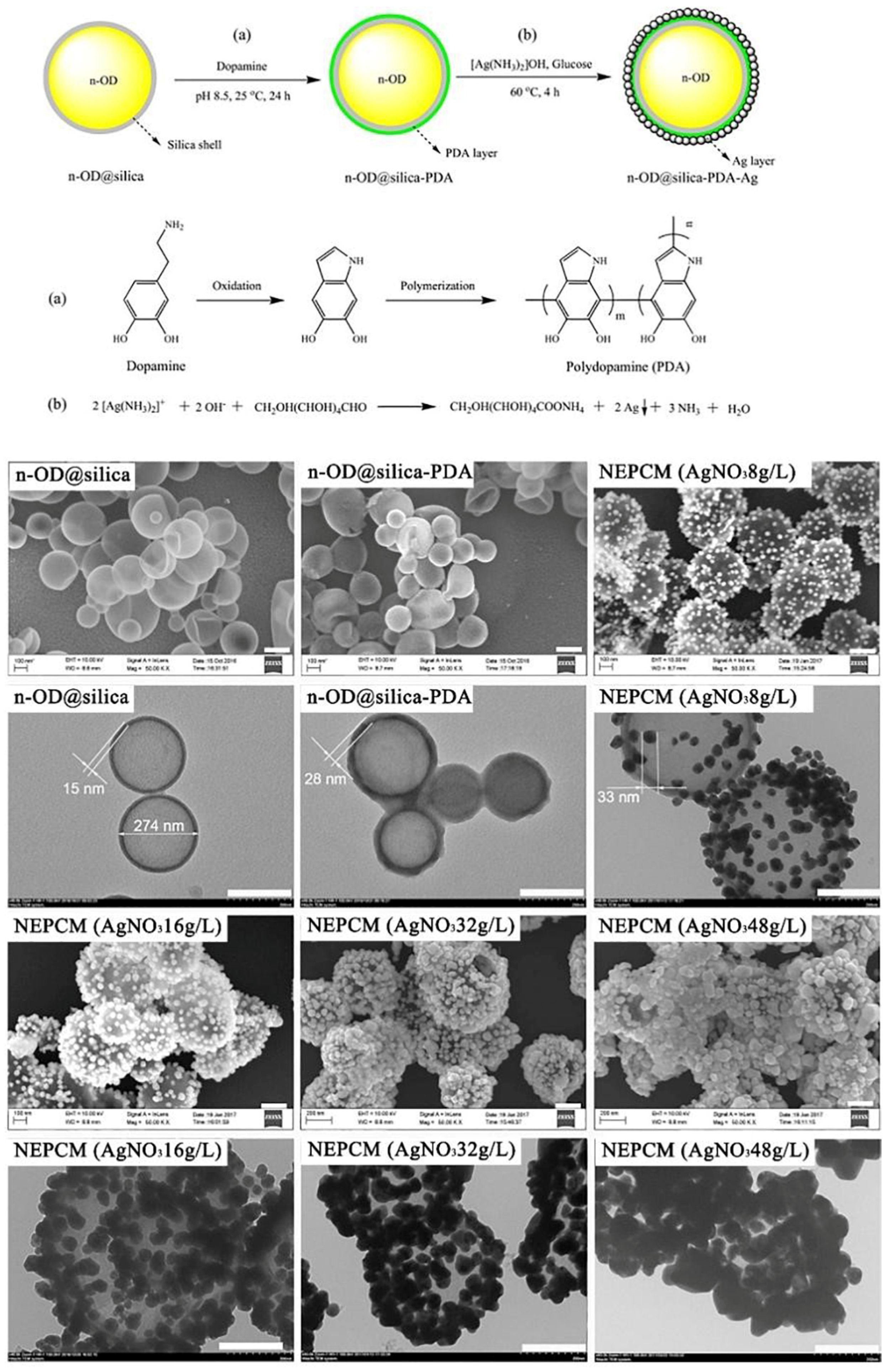
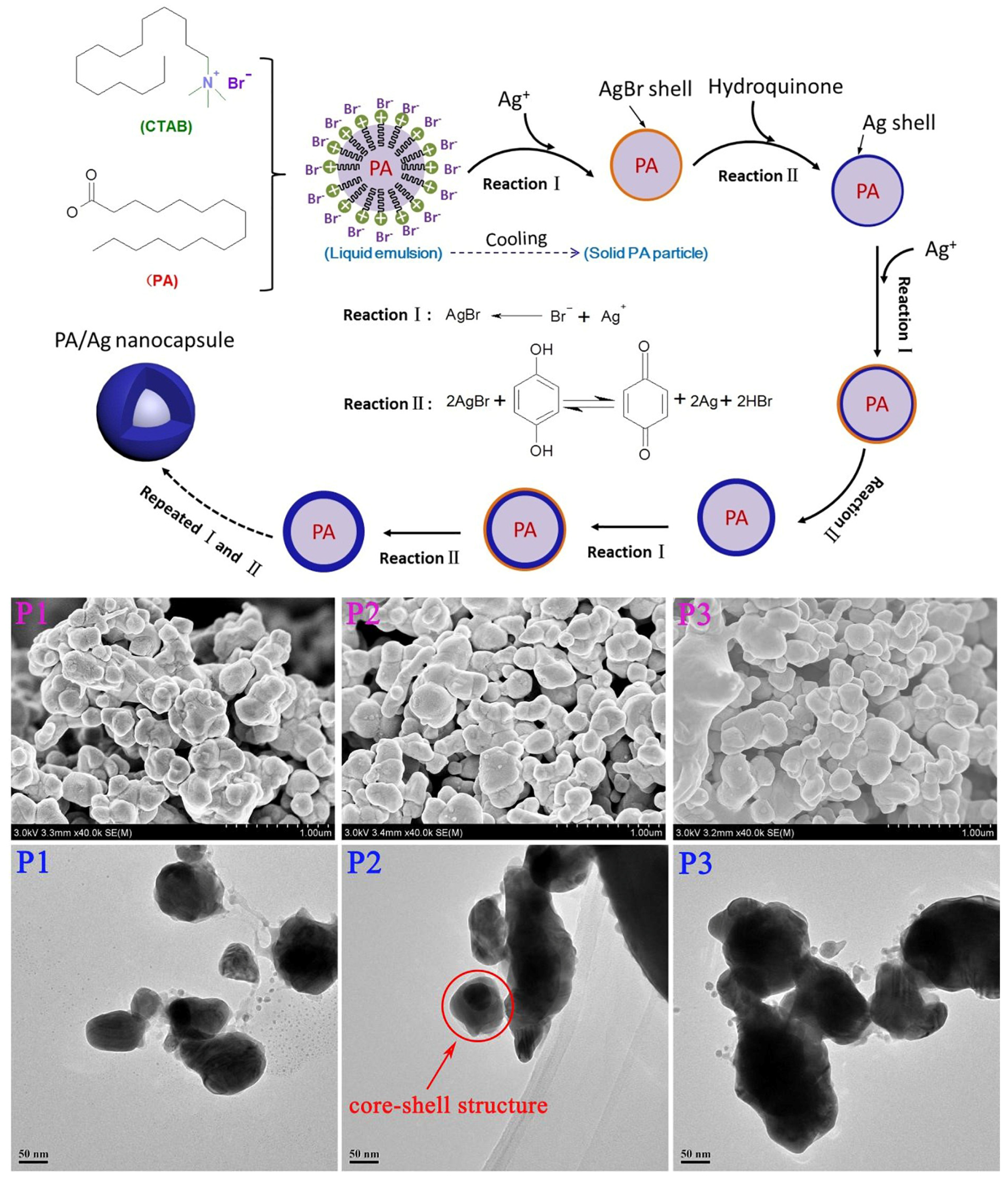

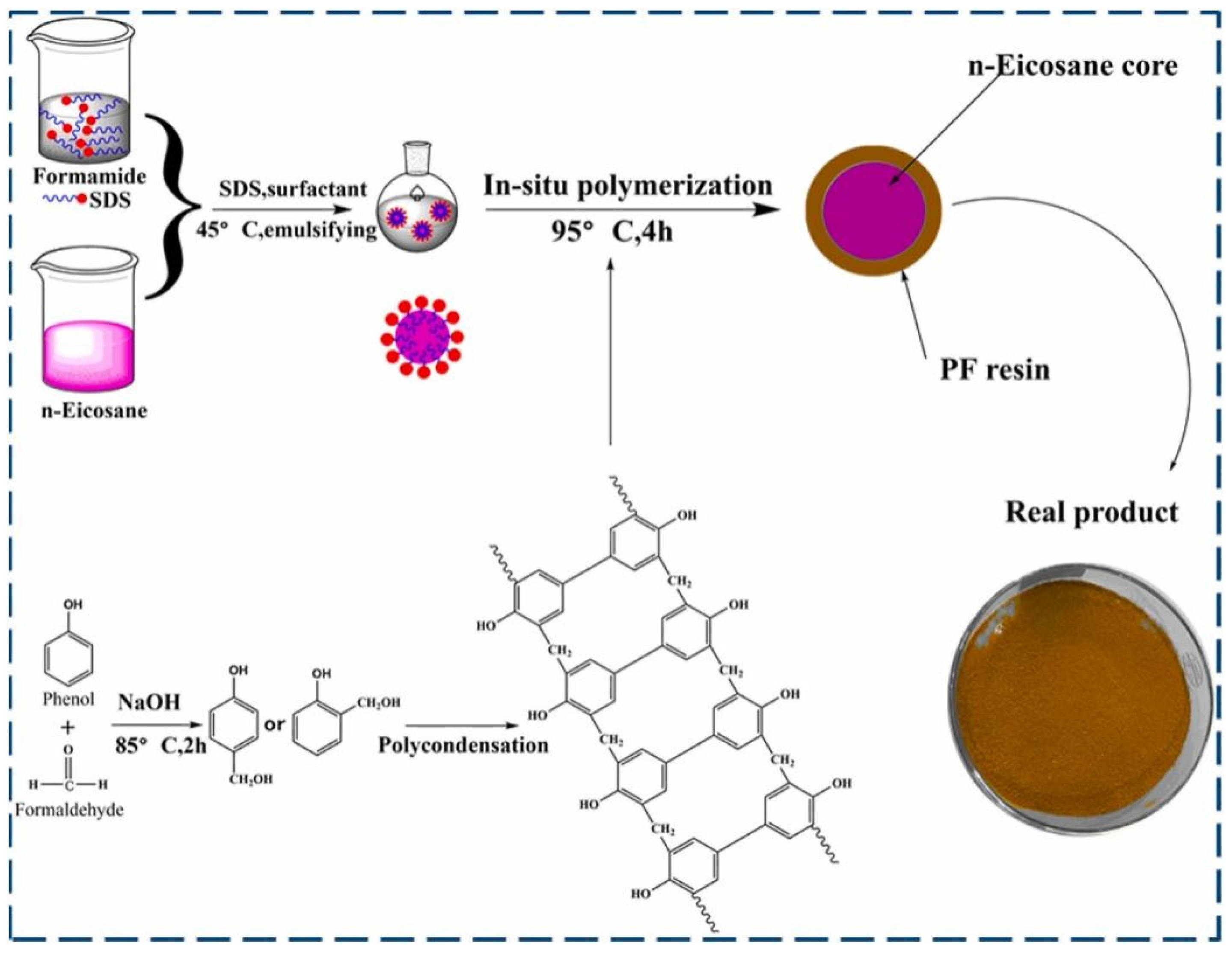
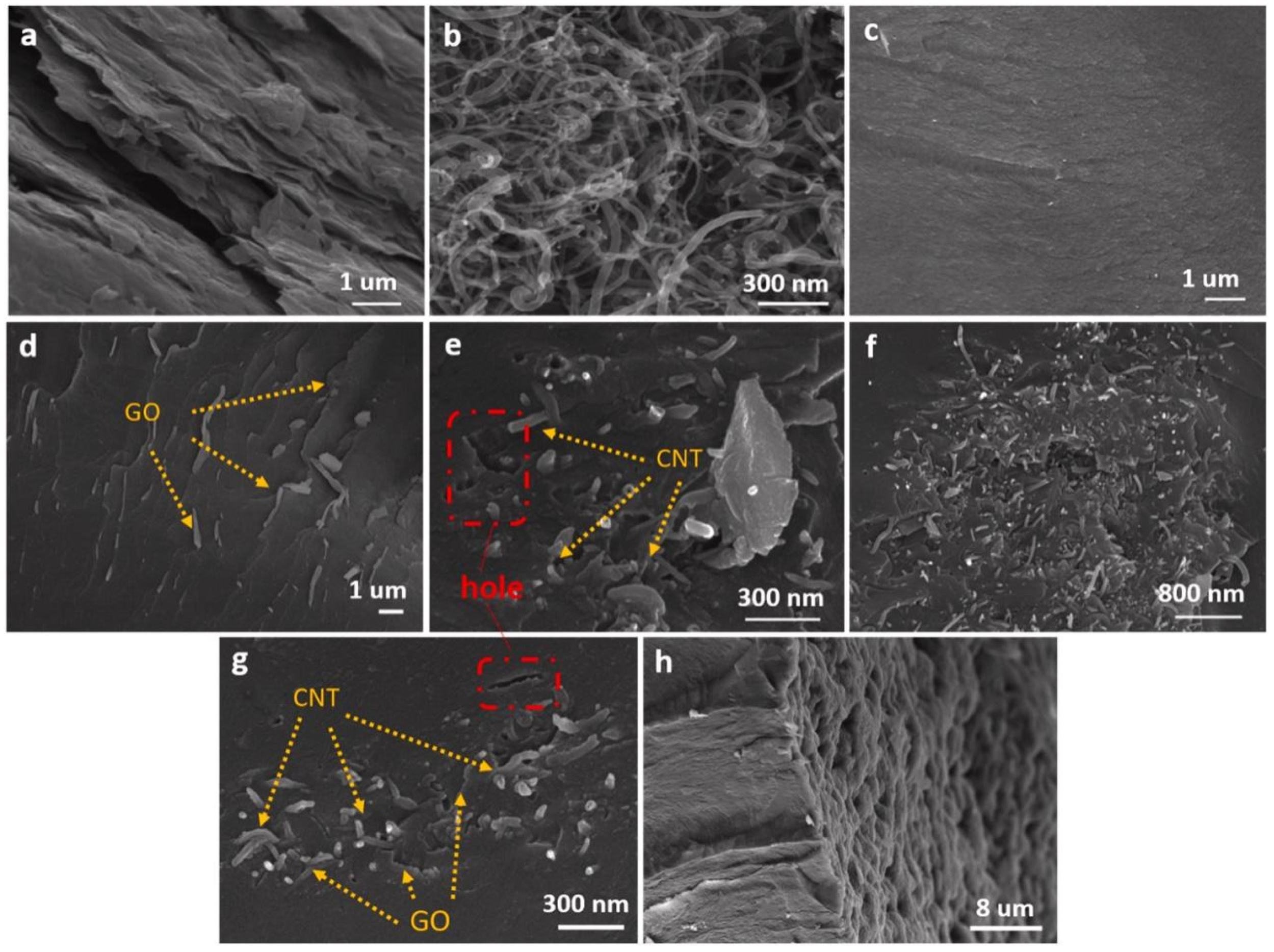


| Paraffins | Tm (°C) | ΔHm (J/g) | λ (W/m·K) |
|---|---|---|---|
| n-Tetradecane (C14) | 6 | 228–230 | 0.14 |
| n-Pentadecane (C15) | 10 | 205 | 0.2 |
| n-Hexadecane (C16) | 18 | 237 | 0.2 |
| n-Heptadecane (C17) | 22 | 213 | 0.145 |
| n-Octadecane (C18) | 28 | 245 | 0.148 |
| n-Nonadecane (C19) | 32 | 222 | 0.22 |
| n-Eicosane (C20) | 37 | 246 | |
| n-Henicosane (C21) | 40 | 200, 213 | |
| n-Docosane (C22) | 44.5 | 249 | 0.2 |
| n-Tricosane (C23) | 47.5 | 232 | |
| n-Tetracosane (C24) | 52 | 255 | |
| n-Pentacosane (C25) | 54 | 238 | |
| n-Hexacosane (C26) | 56.5 | 256 | |
| n-Heptacosane (C27) | 59 | 236 | |
| n-Octacosane (C28) | 64.5 | 253 | |
| n-Nonacosane (C29) | 65 | 240 | |
| n-Triacontane (C30) | 66 | 251 | |
| n-Hentriacontane (C31) | 67 | 242 | |
| n-Dotriacontane (C32) | 69 | 170 | |
| n-Triatriacontane (C33) | 71 | 268 | 0.2 |
| Paraffin C16-C18 | 20–22 | 152 | |
| Paraffin C13-C24 | 22–24 | 189 | 0.21 |
| RT 35 HC | 35 | 240 | 0.2 |
| Paraffin C16-C28 | 42–44 | 189 | |
| Paraffin C20-C33 | 48–50 | 189 | |
| Paraffin C22-C45 | 58–60 | 189 | 0.2 |
| Paraffin C21-C50 | 66–68 | 189 | |
| RT 70 HC | 69–71 | 260 | 0.2 |
| Paraffin natural wax 811 | 82–86 | 85 | 0.72 |
| Fatty Acid | Tm (°C) | ΔHm (J/g) | λ (W/m·K) |
|---|---|---|---|
| Enanthic | −7.4 | 107 | |
| Butyric | −5.6 | 126 | |
| Caproic | −3 | 131 | |
| Propyl palmiate | 10 | 186 | |
| Pelargonic | 12.3 | 127 | |
| Isopropyl stearate | 14–18 | 140–142 | |
| Phenylacetic | 16.7 | 102 | |
| Caprylic | 16 | 149 | 0.149 |
| Butyl stearate | 19 | 140 | |
| Dimethyl sabacate | 21 | 120~135 | |
| Undecylenic | 24.6 | 141 | |
| Vinyl stearate | 27~29 | 122 | |
| Undecylic | 28.4 | 139 | |
| Capric | 31.5 | 153 | 0.153 |
| Tridecylic | 41.8 | 157 | |
| Methyl-12 hydroxy-stearate | 42~43 | 120~126 | |
| Lauric | 45 | 193 | 0.150 |
| Elaidic | 47 | 218 | |
| Myristic | 56 | 212 | 0.150 |
| Pentadecanoic | 52~53 | 178 | |
| Margaric | 60 | 172 | |
| Palmitic acid | 62 | 218 | 0.162 |
| Stearic acid | 71 | 237 | 0.172 |
| Nonadecylic | 67 | 192 | |
| Arachidic | 74 | 227 | |
| Heneicosylic | 73~74 | 193 | |
| Acetamide | 81 | 241 |
| Tm (°C) | ΔHm (J/g) | λ (W/m·K) | Reference | |
|---|---|---|---|---|
| Erythritol | 118.4 | 379.6 | [52,53] | |
| Butyl stearate | 23.7 | 121 | 0.230 | [60] |
| Isopropyl stearate | 22.1 | 113.1 | 0.150 | |
| Glycerol tristearate | 63.5 | 149.4 | 0.170 | |
| Erythritol tetrapalmitate | 21.9 | 201.1 | 0.250 | [44] |
| Erythritol tetrastearate | 30.4 | 208.8 | 0.260 | |
| PEG400 | 3.2 | 91.4 | [59] | |
| PEG1000 | 34.8 | 154.4 | 0.280 | [61] |
| PEG1500 | 47.2 | 161.4 | 0.310 | |
| PEG2000 | 50.8 | 165.4 | 0.310 | |
| PEG4000 | 56.0 | 173.6 | 0.330 | |
| PEG6000 | 59.5 | 179.7 | 0.340 | |
| PEG8000 | 59.7 | 177.5 | 0.330 | |
| PEG10000 | 58.0 | 182.9 | 0.330 | |
| PEG12000 | 60.9 | 173.4 | 0.320 | |
| PEG20000 | 62.3 | 168.5 | 0.320 |
| EPCMs | R (%) | ΔHm, PCMs (J/g) | (g/cm3) | ΔHv (J/cm3) | Reference |
|---|---|---|---|---|---|
| CA/PS | 45.40 | 86.45 | 0.97 | 83.86 | [93] |
| LA/PS | 43.56 | 87.21 | 0.96 | 83.72 | |
| MA/PS | 48.70 | 98.26 | 0.94 | 92.36 | |
| C19/PMMA | 60.30 | 139.20 | 0.91 | 126.67 | [94] |
| PS/(C24-C18)(2:1) | 29.60 | 71.73 | 0.96 | 68.86 | [95] |
| PS/(C24-C18)(1:1) | 48.0 | 116.32 | 0.91 | 105.95 | |
| PS/(C24-C18)(1:2) | 64.4 | 156.39 | 0.87 | 136.06 | |
| Paraffin wax/PMMA (94 μm) | 89.5 | 137.20 | 0.92 | 126.22 | [96] |
| Paraffin wax/PMMA (0.1–19 μm) | 80.2 | 123.00 | 0.94 | 115.62 | |
| n-dodecanol/MF | 93.1 | 187.50 | 0.85 | 159.38 | [97] |
| C12/MF | 60.0 | 150.00 | 0.87 | 130.50 | [67] |
| C14/UF | 61.8 | 134.16 | 0.88 | 118.06 | [98] |
| Shell | Core | Melting | Solidifying | R (%) | D (nm) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |||||
| UF | C14 | 9.01 | 134.16 | 2.81 | 134.50 | 61.80 | 100.00 | [98] |
| 8.49 | 131.09 | 2.19 | 129.83 | 60.30 | ||||
| 7.19 | 103.57 | 1.78 | 103.48 | 47.70 | ||||
| 5.57 | 66.01 | 2.40 | 66.39 | 30.40 | ||||
| PNDA (modified MF resin) | C18 | 32.60 | 126.10 | 18.90 | 124.30 | 87.00 | 109.60 | [68] |
| 32.50 | 125.40 | 19.40 | 123.50 | 86.40 | 109.30 | |||
| 32.00 | 126.50 | 19.70 | 123.70 | 86.90 | 108.80 | |||
| 32.40 | 124.50 | 19.50 | 123.40 | 86.10 | 110.20 | |||
| 32.10 | 125.90 | 19.70 | 124.10 | 86.80 | 111.70 | |||
| P (MMA-co-AMA) | C18 | 24.70 | 129.00 | 23.80 | 155.00 | 58.11 | 692.80 | [99] |
| 24.60 | 140.00 | 25.3 | 165.00 | 63.06 | ||||
| 25.20 | 140.00 | 25.7 | 156.00 | 63.06 | 614.00 | |||
| 27.40 | 151.00 | 27.2 | 166.00 | 68.02 | ||||
| 26.50 | 141.00 | 26.6 | 161.00 | 63.51 | 577.50 | |||
| MF resin | C18 | 34.70 | 195.00 | 32.10 | 182.00 | 87.00 | 840.00 | [99] |
| 34.40 | 97.00 | 15.50 | 101.00 | 45.00 | ||||
| MF resin | C18 | 28.06 | 137.62 | 25.82 | 137.93 | 59.58 | 484.00 | [100] |
| 28.14 | 145.26 | 25.32 | 147.01 | 62.88 | 636.00 | |||
| 28.22 | 137.07 | 25.26 | 137.86 | 59.34 | 630.00 | |||
| 27.88 | 117.12 | 25.51 | 115.51 | 50.70 | 684.00 | |||
| 27.62 | 111.36 | 25.15 | 109.78 | 48.21 | 723.00 | |||
| PNDA (modified MF resin) | Paraffin | 28.00 | 83.46 | NA | NA | 62.00 | 50.00 | [101] |
| MUF | Paraffin | 27.50 | 49.40 | 30.10 | 46.00 | 22.80 | 413.30± 1.30 | [102] |
| 27.40 | 69.20 | 29.90 | 67.50 | 31.60 | 340.40 ± 3.20 | |||
| 27.10 | 80.30 | 30.20 | 75.10 | 36.70 | 370.40 ± 6.40 | |||
| 27.30 | 62.00 | 29.90 | 56.90 | 28.30 | 368.00 ± 2.30 | |||
| 28.30 | 24.90 | 29.70 | 24.50 | 14.90 | 455.70 ± 25.10 | |||
| UF | Paraffin | 64.30 | 74.20 | NA | NA | 48.12 | 256.00 | [103] |
| PU and PS | C18 | 28.00 | 122.00 | 22.00 | 125.00 | 54.00 | 410.00 | [104] |
| PU | C18 | 23.70 | 123.40 | 28.20 | 124.10 | 78.10 | 200.00 | [105] |
| PU | C19 | 30.54 | 92.85 | 30.78 | 91.86 | 44.70 | 103.00 | [106] |
| PMMA SiO2 | Paraffin | 26.80 | 69.90 | 19.80 | 71.00 | 57.40 | 120.00 | [107] |
| 26.60 | 57.50 | 19.60 | 57.50 | 46.90 | ||||
| 27.10 | 53.50 | 19.50 | 53.50 | 43.60 | ||||
| 28.00 | 62.10 | 19.80 | 62.40 | 50.70 | ||||
| AlOOH | PA | 12.70 | 19.00 | NA | NA | 10.19 | 200.00 | [108] |
| 13.80 | 22.60 | NA | NA | 12.12 | ||||
| 16.00 | 27.80 | NA | NA | 14.91 | ||||
| PMMA | C22 | 41.00 | 54.60 | 40.60 | 48.70 | 28.00 | 160.00 | [109] |
| P (MMA-co-GMA) | C16 | 17.23 | 148.05 | 14.85 | 147.63 | 62.46 | 260.00 | [80] |
| PS | Hexadecanol | 52.68 | 124.85 | NA | NA | c48.72 | 120.00 | [110] |
| PS-co-EA | C14 | 7.97 | 182.68 | −0.18 | 184.94 | 79.26 | 50–200 | [111] |
| C15 | 11.60 | 121.83 | 5.20 | 127.89 | 69.16 | |||
| C16 | 20.38 | 196.09 | 12.78 | 201.70 | 87.09 | |||
| C17 | 24.04 | 140.51 | 17.23 | 149.60 | 81.59 | |||
| PMMA | C16 | 17.34 | 145.61 | 14.85 | 128.19 | 61.42 | 220 | [112] |
| PMMA | C20 | 35.20 | 84.20 | 34.90 | 87.50 | 35 | 700 | [113] |
| PS-co-EA | Paraffin | 42.39 | 49.03 | 37.41 | 49.05 | 32.12 | 165 | [114] |
| PA | 62.66 | 97.93 | 56.22 | 97.21 | 47.79 | 265 | ||
| PMMA | C19 | 31.23 | 139.20 | 31.03 | 142.39 | 60.30 | [94] | |
| PS | C24-C18 | 25.96 | 156.39 | 26.04 | 152.83 | 64.40 | [95] | |
| UF | C16 | 16.15 | 143.70 | 16.04 | 134.30 | 68.90 | 253.00 | [115] |
| 16.26 | 121.00 | 16.32 | 112.50 | 58.06 | 259.00 | |||
| 16.27 | 119.40 | 15.88 | 112.30 | 57.29 | 268.00 | |||
| 16.36 | 114.60 | 16.07 | 98.99 | 54.99 | 285.00 | |||
| PS | C18 | 27.00 | 44.00 | 23.00 | 43.00 | 20.47 | 31.00 | [116] |
| SiO2 | C18 | 27.50 | 108.60 | 5.60 | 99.80 | 53.80 | 335.00 | [117] |
| PHEMA-SiO2 | 27.90 | 104.30 | 8.60 | 104.80 | 51.60 | 449.00 | ||
| PS-SiO2 | 27.90 | 101.10 | 7.00 | 97.30 | 50.10 | 289.00 | ||
| PMMA | C18 | 32.60 | 110.10 | 19.50 | 108.30 | 91.00 | 104.00 | [118] |
| 33.50 | 111.20 | 18.30 | 110.00 | 92.20 | 101.00 | |||
| 32.70 | 108.90 | 18.80 | 107.20 | 90.10 | 109.00 | |||
| 33.20 | 110.50 | 19.00 | 108.60 | 91.30 | 112.00 | |||
| 32.00 | 109.10 | 18.80 | 107.20 | 90.10 | 106.00 | |||
| PEMA | C18 | 32.20 | 198.50 | 29.80 | 197.10 | 89.50 | 140 | [119] |
| PMMA | 31.90 | 208.7 | 30.2 | 205.9 | 94.7 | 119 | ||
| St-MMA | C18 | 30.20 | 97.90 | 25.90 | 93.80 | 40.90 | 152.00 ± 12.00 | [120] |
| 28.90 | 74.40 | 25.60 | 73.80 | 31.10 | 236.00 ± 13.00 | |||
| 28.40 | 85.90 | 25.30 | 82.90 | 35.90 | 146.00 ± 51.00 | |||
| 29.50 | 107.90 | 24.60 | 104.90 | 45.10 | 102.00 ± 11.00 | |||
| 30.00 | 117.30 | 26.70 | 113.80 | 49.00 | 127.00 ± 30.00 | |||
| PS | C18 | 20–25 | 124.40 | NA | NA | 53.55 | 100–123 | [121] |
| PS | C14 | 4.04 | 98.71 | −3.43 | 91.27 | 89.00 | 132.00 | [122] |
| PS-SiO2 | C14 | 2.13 | 83.38 | 0.39 | 79.37 | 42.56 | 120.00 | [123] |
| PS | 2.59 | 82.35 | 0.008 | 81.46 | 42.04 | 80.00 | ||
| PMMA | C20 | 34.66 | 124.70 | 32.92 | 119.13 | 62.00 | 135.00 | [82] |
| PS | C32 | 70.90 | 174.80 | 61.80 | 177.1 | 61.23 | 168.00 | [124] |
| St-co-MMA | Paraffin | 61.50 | 140.30 | NA | NA | 60.70 | 439.40 | [125] |
| 60.40 | 79.75 | NA | NA | 34.50 | 274.80 | |||
| 60.40 | 44.21 | NA | NA | 19.10 | 118.80 | |||
| 61.40 | 17.20 | NA | NA | 7.50 | 74.00 | |||
| Styrene–butyl | RT80 | 78.40 | 4.90 | 54.10 | 6.50 | 3.04 | 52.00 | [126] |
| acrylate | 81.80 | 16.60 | 55.00 | 18.90 | 10.3 | 58.00 | ||
| copolymer | 79.80 | 12.00 | 54.40 | 14.60 | 7.45 | 62.00 | ||
| PMMA | n-dodecanol | 18.20 | 98.80 | NA | NA | 82.20 | 150.00 | [127] |
| PMMA | n-dodecanol | 18.40 | 109.3 | NA | NA | 44.07 | 50–100 | [128] |
| MMA | C18 | 33.20 | 116.00 | 15.70 | 112.00 | 52.90 | 466.00 | [129] |
| PUT | C18 | 31.30 | 35.20 | 19.50 | 34.80 | 55.00 | 601.00 | [130] |
| 30.10 | 67.70 | 20.00 | 65.40 | 74.00 | 698.00 | |||
| 30.80 | 81.80 | 19.90 | 80.10 | 70.00 | 723.00 | |||
| 28.40 | 36.40 | 18.10 | 35.40 | 70.00 | 432.00 | |||
| 29.50 | 48.20 | 19.30 | 47.70 | 62.00 | 598.00 | |||
| 30.10 | 73.70 | 20.30 | 72.80 | 71.00 | 654.00 | |||
| 25.30 | 18.50 | 15.40 | 18.20 | 72.00 | 401.00 | |||
| 29.00 | 39.60 | 18.30 | 38.60 | 76.00 | 432.00 | |||
| 31.90 | 58.50 | 17.90 | 57.50 | 75.00 | 497.00 | |||
| Acrylic resin copolymers | n-dodecanol | 22.26 | 93.31 | NA | NA | 43.00 | 933.91 | [131] |
| Organosilica | C18 | 28.44 | 113.30 | 23.76 | 108.80 | 55.40 | NA | [132] |
| SiO2 | C18 | 27.35 | 116.00 | 24.17 | 109.00 | NA | 563.00 | [133] |
| Ag-coated SiO2 | C18 | 28.51 | 126.90 | 22.19 | 120.60 | 62.83 | 274.00 | [134] |
| 28.27 | 106.58 | 22.13 | 104.80 | 52.77 | ||||
| 28.03 | 71.95 | 22.30 | 71.65 | 35.62 | ||||
| 28.05 | 49.30 | 22.03 | 50.60 | 24.41 | ||||
| 27.77 | 33.19 | 22.21 | 34.00 | 16.43 | ||||
| 27.51 | 26.13 | 22.26 | 26.87 | 12.93 | ||||
| Organosilica | C18 | 27.92 | 107.50 | 24.58 | 102.00 | 51.26 | 385.00 | [135] |
| 27.92 | 98.41 | 24.94 | 92.32 | 46.93 | 346.00 | |||
| 27.69 | 105.50 | 25.12 | 102.40 | 50.30 | 624.00 | |||
| 27.35 | 93.20 | 24.16 | 91.73 | 44.44 | 693.00 | |||
| 26.51 | 95.18 | 23.31 | 92.98 | 45.39 | 200.00 | |||
| SiO2 | PEG | 3.19 | 62.27 | 2.61 | 57.53 | 55.20 | 528.00 | [136] |
| SiO2 | PA | 61.60 | 180.91 | 57.08 | 181.22 | 89.55 | 722.50 | [88] |
| 60.92 | 172.16 | 56.80 | 173.40 | 85.22 | 466.40 | |||
| 61.06 | 168.16 | 57.62 | 170.23 | 83.25 | 183.70 | |||
| SiO2 | LA | 36.70 | 165.60 | 34.30 | 152.50 | 85.90 | 210–460 | [137] |
| SiO2 | LA | 34.50 | 40.60 | 29.40 | 32.50 | 21.10 | 60.00 | [138] |
| 37.30 | 90.40 | 32.80 | 82.30 | 46.90 | 104.00 | |||
| 36.80 | 116.00 | 34.80 | 94.00 | 60.20 | 221.00 | |||
| 38.80 | 160.00 | 32.90 | 154.80 | 83.00 | 357.00 | |||
| 36.70 | 120.50 | 34.30 | 119.40 | 62.50 | 615.00 | |||
| 38.80 | 7.20 | 30.20 | 4.70 | 3.70 | 750.00 | |||
| 40.60 | 4.30 | 28.80 | 2.10 | 2.20 | 828.00 | |||
| TiO2 | SA | 59.14 | 123.96 | 50.54 | 109.43 | 64.76 | 946.40 | [89] |
| 58.72 | 99.02 | 50.20 | 84.93 | 51.73 | 620.10 | |||
| 58.46 | 87.95 | 49.97 | 74.76 | 45.94 | 583.40 | |||
| 58.23 | 58.12 | 49.80 | 50.59 | 30.36 | 317.60 | |||
| Core | λPCM (W/(m·K)) | Shell | D | R (%) | λmicro/nanocapsules (W/(m·K)) | Enhancement (%) | Reference |
|---|---|---|---|---|---|---|---|
| n-octadecane | PMF | 77.00 | About 0.1300 | [146] | |||
| PMF + nano-SiC (3 wt%) | 72.90 | 18.31 | |||||
| PMF + nano-SiC (5 wt%) | 72.80 | 41.36 | |||||
| PMF + nano-SiC (7 wt%) | 72.30 | 60.34 | |||||
| n-dodecanol | PMF | 0.1157 | 0 | [147] | |||
| PMF+GO (0.2 wt%) | 0.1297 | 12.1 | |||||
| PMF+GO (0.4 wt%) | 0.1396 | 20.65 | |||||
| PMF+GO (0.6 wt%) | 0.1586 | 37.07 | |||||
| PMF+GO (0.8 wt%) | 0.1663 | 43.73 | |||||
| PMF+GO (1 wt%) | 0.1738 | 50.21 | |||||
| PMF+GO (2 wt%) | 0.1897 | 63.95 | |||||
| PMF+GO (3 wt%) | 0.1918 | 65.77 | |||||
| PMF+GO (4 wt%) | 0.1924 | 66.29 | |||||
| Paraffin | 0.220 | Polyurea | 400~600 nm | 66.56 | 0.2250 | 102 | [148] |
| Polyurea + nano-Fe3O4 (3.1 wt%) | 62.11 | 0.2330 | 106 | ||||
| Polyurea + nano-Fe3O4 (5.7 wt%) | 56.42 | 0.3350 | 152 | ||||
| Polyurea + nano-Fe3O4 (6.6 wt%) | 54.83 | 0.3420 | 155 | ||||
| Paraffin | 0.220 | PMMA | 0.5~2 μm | 63.59 | 0.2442 | 111 | [145] |
| PMMA + nano-Al2O3 (5 wt%) | 60.77 | 0.2786 | 127 | ||||
| PMMA + nano-Al2O3 (17 wt%) | 53.81 | 0.3104 | 141 | ||||
| PMMA + nano-Al2O3 (27 wt%) | 48.70 | 0.3409 | 155 | ||||
| PMMA + nano-Al2O3 (33 wt%) | 43.92 | 0.3591 | 163 | ||||
| PMMA + nano-Al2O3 (38 wt%) | 43.43 | 0.3816 | 173 | ||||
| n-octadecane | 0.150 | PMMA | 4~11 μm | 73.14 | [144] | ||
| PMMA + Si3N4 (5 wt%) | 88.00 | 0.2315 | 154 | ||||
| PMMA + Si3N4 (10 wt%) | 81.92 | 0.2753 | 184 | ||||
| PMMA + Si3N4 (15 wt%) | 73.78 | 0.2918 | 195 | ||||
| PMMA + Si3N4 (20 wt%) | 66.13 | 0.2997 | 200 | ||||
| PMMA + Si3N4 (30 wt%) | 63.61 | 0.3630 | 242 | ||||
| Paraffin | UFR | 13.08 μm | 37.38 | 0.0820 | [149] | ||
| UFR + CNT (1 wt%) | 0.15 μm | 23.14 | 0.0950 | ||||
| UFR + CNT (2 wt%) | 0.1170 | ||||||
| UFR + CNT (3 wt%) | 0.1330 | ||||||
| UFR + CNT (4 wt%) | 0.1460 | ||||||
| n-octadecane | 0.150 | SiO2 | 7~16 μm | 0.6213 | 414 | [87] | |
| n-octadecane | 0.150 | CaCO3 | 5 μm | 21.89 | 1.6740 | 1120 | [150] |
| n-eicosane | 0.161 | TiO2 | 1.5~2 μm | 49.90 | 0.8650 | 540 | [151] |
| n-octadecane | 0.153 | SiO2 | 274 nm | 62.83 | 0.2460 | 161 | [134] |
| SiO2 + PDA | 300 nm | 52.77 | 0.1980 | 129 | |||
| SiO2 + PDA + Ag (8 g/L) | 500 nm | 35.62 | 0.5360 | 350 | |||
| SiO2 + PDA + Ag (16 g/L) | 550 nm | 24.41 | 0.5180 | 339 | |||
| SiO2 + PDA + Ag (32 g/L) | 650 nm | 16.43 | 0.8400 | 549 | |||
| SiO2 + PDA + Ag (48 g/L) | 600 nm | 12.94 | 1.3460 | 161 | |||
| Paraffin | 0.300 | SiO2 | 30 μm | 50.80 | 1.0310 | 344 | [152] |
| SiO2 + GO | 49.60 | 1.1620 | 387 | ||||
| RT28 | 0.289 | CaCO3 | 0.7590 | 262 | [153] | ||
| RT28+RT42 | CaCO3 | 0.7390 | |||||
| RT42 | 0.388 | CaCO3 | 0.9360 | 241 | |||
| n-docecane | 0.152 | ZrO2 | 1~1.5 μm | 64.4 | 0.906 | 596 | [154] |
| PA | 0.226 | Ag | 100~600 nm | 34.30 | 4.0720 | 1800 | [92] |
| Core | Shell | D | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | N | Reference |
|---|---|---|---|---|---|---|---|---|
| ES-EP | SiO2-Ag | 510~580 nm | 20.2 | 108.5 | 15.0 | 107.8 | 0 | [161] |
| 20.1 | 108.4 | 14.9 | 107.7 | 200 | ||||
| 20.1 | 108.4 | 14.9 | 107.6 | 500 | ||||
| 20.0 | 108.3 | 14.8 | 107.6 | 1000 | ||||
| SiO2-Cu | 510~580 nm | 20.6 | 109.7 | 15.6 | 108.8 | 0 | ||
| 20.5 | 109.7 | 15.6 | 108.8 | 200 | ||||
| 20.5 | 109.6 | 15.5 | 108.7 | 500 | ||||
| 20.4 | 109.6 | 15.5 | 108.7 | 1000 | ||||
| LA | SiO2 | 131 nm | 35.9 | 36.8 | 33.7 | 48.6 | 1200 | [137] |
| 142 nm | 37.0 | 81.9 | 33.1 | 76.1 | ||||
| 174 nm | 37.2 | 107.5 | 33.0 | 89.3 | ||||
| 311 nm | 36.9 | 165.1 | 36.1 | 150.5 | ||||
| 408 nm | 35.8 | 133.4 | 33.4 | 117.4 | ||||
| 510 nm | 37.0 | 115.2 | 33.5 | 112.3 | ||||
| 474 nm | 35.1 | 130.7 | 33.2 | 127.4 | ||||
| 250 nm | 36.8 | 120.3 | 34.5 | 112.7 | ||||
| 402 nm | 37.9 | 143.5 | 33.3 | 120.6 | ||||
| 215 nm | 36.5 | 115.5 | 33.0 | 103.8 | ||||
| 122 nm | 37.5 | 27.5 | 31.1 | 25.5 | ||||
| n-octadecane | PPFS | 490 nm | 31.2 | 171.8 | 23.0 | 169.3 | 200 | [76] |
| PA | SiO2 | 474 nm | 62.9 | 109.9 | 57.8 | 100.1 | 0 | [162] |
| 62.8 | 109.2 | 57.9 | 98.6 | 100 | ||||
| PA | Ag | 100–600 nm | 56.40 | 107.87 | 57.46 | 105.22 | 2000 | [92] |
| D-mannitol | silica/GO | 100–400 nm | 166.2 | 216.7 | 122.3 | 174.4 | 1 | [163] |
| 205.6 | 176.7 | 10 | ||||||
| 214.6 | 181.1 | 20 | ||||||
| 196.0 | 166.6 | 30 | ||||||
| 199.5 | 169.9 | 40 | ||||||
| 206.7 | 169.2 | 50 | ||||||
| Paraffin wax | PU | 25~185 nm | 62.4 | 153.9 | 66.9 | 142.3 | 0 | [160] |
| 63.0 | 152.2 | 66.8 | 142.4 | 50 | ||||
| 63.0 | 151.2 | 66.8 | 141.5 | 100 | ||||
| SA | Ag | 167~252 nm | 46.13 | 117.73 | 66.13 | 116.61 | 2000 | [164] |
| SA | TiO2 | 946 nm | 59.14 | 123.95 | 50.54 | 109.43 | 0 | [89] |
| 59.41 | 119.23 | 50.01 | 105.68 | 2500 | ||||
| PA | SiO2 | 221 nm | 38.8 | 160.0 | 32.8 | 154.8 | 0 | [138] |
| 38.9 | 157.3 | 32.9 | 152.5 | 3000 | ||||
| 357 nm | 36.7 | 120.5 | 34.3 | 119.4 | 0 | |||
| 36.8 | 118.4 | 34.3 | 116.7 | 3000 | ||||
| 615 nm | 36.8 | 116.0 | 34.8 | 94.0 | 0 | |||
| 36.9 | 114.0 | 34.7 | 92.8 | 3000 | ||||
| Dimethyl adipate | MF | 900 nm | 6.4 | 80.2 | 100 | [165] | ||
| C14 | MUF | 209 nm | 7.7 | 140.3 | −0.76 | 142.2 | 100 | [166] |
| MUF-TiO2 | 156.2 nm | 8.4 | 156.2 | −4.32 | 156.0 | 100 | ||
| n-octadecane | SiO2 | 346 nm | 27.92 | 98.4 | 24.94 | 92.3 | 500 | [135] |
| 99.1 | 92.3 | 100 | ||||||
| 101.6 | 94.4 | 300 | ||||||
| 101.6 | 94.9 | 500 | ||||||
| n-octadecane | SiO2 | 624 nm | 27.69 | 105.5 | 25.12 | 102.4 | 0 | [135] |
| 107.6 | 104.9 | 100 | ||||||
| 105.1 | 102.9 | 300 | ||||||
| 102.7 | 100.7 | 500 | ||||||
| n-octadecane | ST-MMA | 102 nm | 24.9 | 107.9 | 27.3 | 104.9 | 0 | [120] |
| 24.8 | 106.7 | 27.3 | 105.9 | 90 | ||||
| 24.4 | 105.1 | 27.5 | 100.5 | 180 | ||||
| 24.9 | 105.4 | 27.8 | 101.4 | 270 | ||||
| 24.1 | 105.1 | 27.1 | 103.7 | 360 | ||||
| n-nonadecane | PMMA | 8.18 μm | 31.2 | 139.2 | 31.0 | 142.4 | 0 | [94] |
| 31.2 | 132.7 | 5000 | ||||||
| CA | PS | 7.7 μm | 21.89 | 80.56 | 0 | [93] | ||
| 17.22 | 80.28 | 5000 | ||||||
| LA | PS | 13.2 μm | 21.89 | 80.56 | 0 | |||
| 17.22 | 80.28 | 5000 | ||||||
| MA | PS | 42.0 μm | 47.12 | 92.12 | 0 | |||
| 48.74 | 88.29 | 5000 | ||||||
| n-octadecane | SiO2 | 563 nm | 27.35 | 109.5 | 24.17 | 98.85 | 0 | [167] |
| 109.4 | 99.09 | 1 | ||||||
| 118.2 | 107.3 | 100 | ||||||
| 115.3 | 103.4 | 300 | ||||||
| 114.4 | 102.4 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Liu, S.; Li, T.; Yang, L.; Li, D.; Bai, H.; Wang, X. Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules. Energies 2024, 17, 604. https://doi.org/10.3390/en17030604
Yuan H, Liu S, Li T, Yang L, Li D, Bai H, Wang X. Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules. Energies. 2024; 17(3):604. https://doi.org/10.3390/en17030604
Chicago/Turabian StyleYuan, Huanmei, Sitong Liu, Tonghe Li, Liyun Yang, Dehong Li, Hao Bai, and Xiaodong Wang. 2024. "Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules" Energies 17, no. 3: 604. https://doi.org/10.3390/en17030604
APA StyleYuan, H., Liu, S., Li, T., Yang, L., Li, D., Bai, H., & Wang, X. (2024). Review on Thermal Properties with Influence Factors of Solid–Liquid Organic Phase-Change Micro/Nanocapsules. Energies, 17(3), 604. https://doi.org/10.3390/en17030604







