Effect of Dropping Speed of Reducing Agent on the Preparation of LA/Ag Phase-Change Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process
2.3. Characterisation
3. Results and Discussion
3.1. Morphology of LA/Ag Nanocapsules
3.2. Microstructures and Chemical Compositions
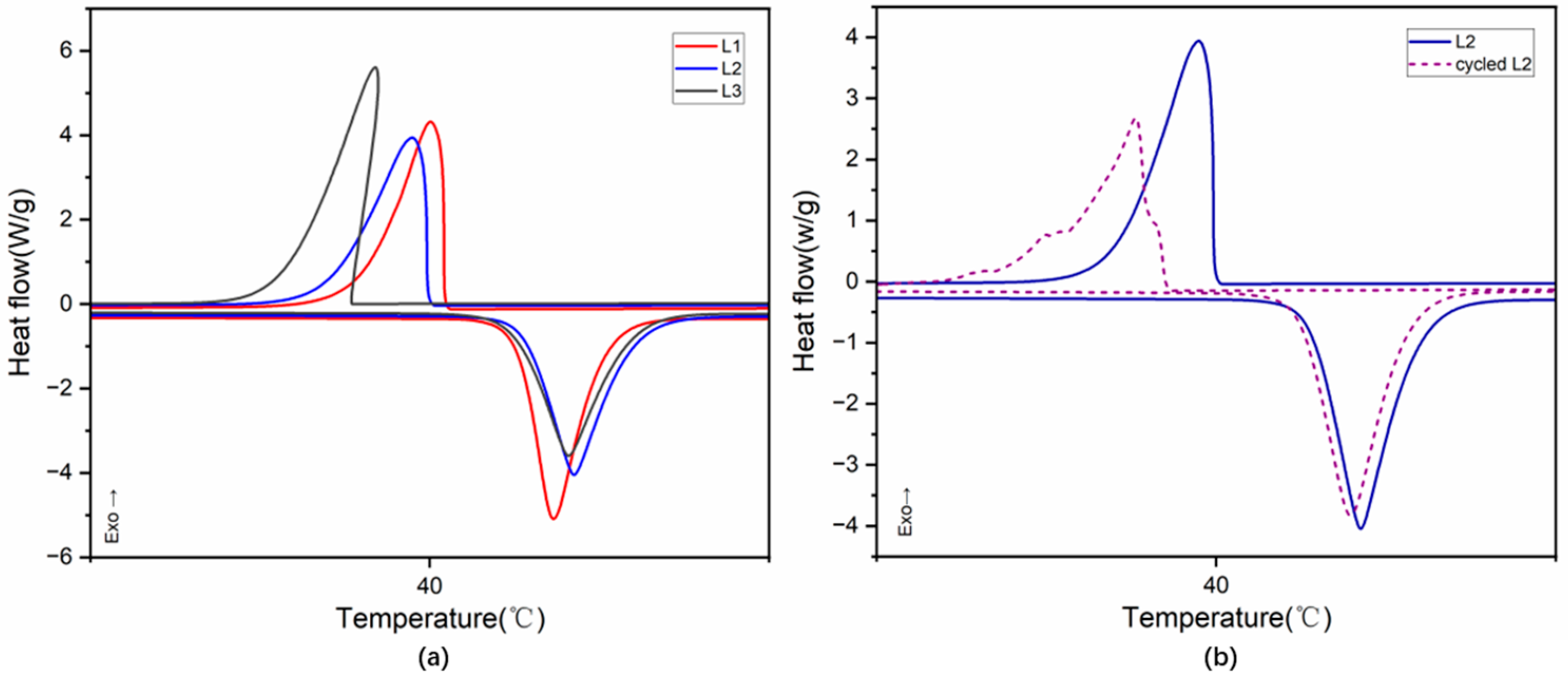
3.3. Phase-Change Properties of LA/Ag Nanocapsules
3.4. Impact of Dropping Speed of Reducing Agent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Zhang, H.; Baeyens, J.; Caceres, G.; Degreve, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 14, 329–337. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.; Gschwander, S.; Haussmann, T. Micro-encapsulated phase-change materials integrated into construction materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.; Ding, L.; Chen, L.; Zhu, X.; Mao, Z.; Feng, X.; Sui, X.; Wang, B. Flexible textiles with polypyrrole deposited phase change microcapsules for efficient photothermal energy conversion and storage. Sol. Energy Mater. Sol. Cells 2021, 224, 110985. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Wan, H.; Liu, H.; Wu, D.; Wang, X. Construction of polyaniline/carbon nanotubes-functionalized phase-change microcapsules for thermal management application of supercapacitors. Chem. Eng. J. 2020, 396, 125317. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–64205. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–48391. [Google Scholar] [CrossRef]
- Reddy, K.-S.; Mudgal, V.; Mallick, T.K. Review of latent heat thermal energy storage for improved material stability and effective load management. J. Energy Storage 2018, 15, 205–15227. [Google Scholar] [CrossRef]
- Sharma, S.-D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Lu, X.; Zhao, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol. Energy 2019, 185, 374–386. [Google Scholar] [CrossRef]
- Fang, Y.; Kuang, S.; Gao, X.; Zhang, Z. Preparation and characterization of novel nanoencapsulated phase change materials. Energy Convers. Manag. 2008, 49, 3704–3707. [Google Scholar] [CrossRef]
- Konuklu, Y.; Akar, H.B. Promising palmitic acid/poly(allyl methacrylate) microcapsules for thermal management applications. Energy 2023, 262, 125491. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Bilgin, C. Micro/nano encapsulation of some paraffin eutectic mixtures with poly(methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl. Energy 2014, 136, 217–227. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Yang, F.; Liu, X.; Wu, S. Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem. Eng. J. 2009, 153, 217–221. [Google Scholar] [CrossRef]
- Liang, C.; Lingling, X.; Hongbo, S.; Zhibin, Z. Microencapsulation of butyl stearate as a phase change material by interfacial polycondensation in a polyurea system. Energy Convers. Manag. 2009, 50, 723–729. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.I.; Son, K. Development of thermoregulating textile materials with microencapsulated phase change materials (PCM). II. Prep. Appl. PCM Microcapsules. J. Appl. Polym. Sci. 2005, 96, 2005–2010. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Wang, H.; Zhang, K.; Jia, X.; Tian, C.; Zhou, Y.; Wang, J. Morphological control and thermal properties of nanoencapsulated n-octadecane phase change material with organosilica shell materials. Energy Convers. Manag. 2016, 119, 151–162. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Microencapsulated Paraffin Phase-Change Material with Calcium Carbonate Shell for Thermal Energy Storage and Solar-Thermal Conversion. Langmuir 2018, 34, 14254–14264. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zou, T.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Self-assembly Synthesis and Properties of Microencapsulatedn-Tetradecane Phase Change Materials with a Calcium Carbonate Shell for Cold Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 3074–3080. [Google Scholar] [CrossRef]
- Li, C.; He, G.; Yan, H.; Yu, H.; Song, Y. Synthesis of microencapsulated stearic acid with amorphous TiO2 as shape-stabilized PCMs for thermal energy storage. Energy Procedia 2018, 152, 390–394. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and Performances of Phase Change Materials Microcapsules with a Polymer/BN/TiO2 Hybrid Shell for Thermal Energy Storage. Energy Fuels 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, J.; Wang, H.; Song, G.; Liu, Y.; Tang, G. Enhancement in thermal property of phase change microcapsules with modified silicon nitride for solar energy. Sol. Energy Mater. Sol. Cells 2016, 151, 89–95. [Google Scholar] [CrossRef]
- Lan, W.; Shang, B.; Wu, R.; Yu, X.; Hu, R.; Luo, X. Thermally-enhanced nanoencapsulated phase change materials for latent functionally thermal fluid. Int. J. Therm. Sci. 2021, 159, 106619. [Google Scholar] [CrossRef]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, S.; Hao, S.; Zhang, Z.; An, H.; Tian, W.; Chan, M.; Bai, H. Fabrication and properties of nanoencapsulated stearic acid phase change materials with Ag shell for solar energy storage. Sol. Energy Mater. Sol. Cells 2022, 239, 111653. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Applied Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Chen, H.; Zhang, Z.; An, H.; Tian, W. Synthesis and properties of high thermal conductivity Ag shell-coated phase change materials. Renew. Energy 2021, 179, 395–405. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Alumina based doped templated carbons: A comparative study with zeolite and silica gel templates. Microporous Mesoporous Mater. 2018, 257, 241–252. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]



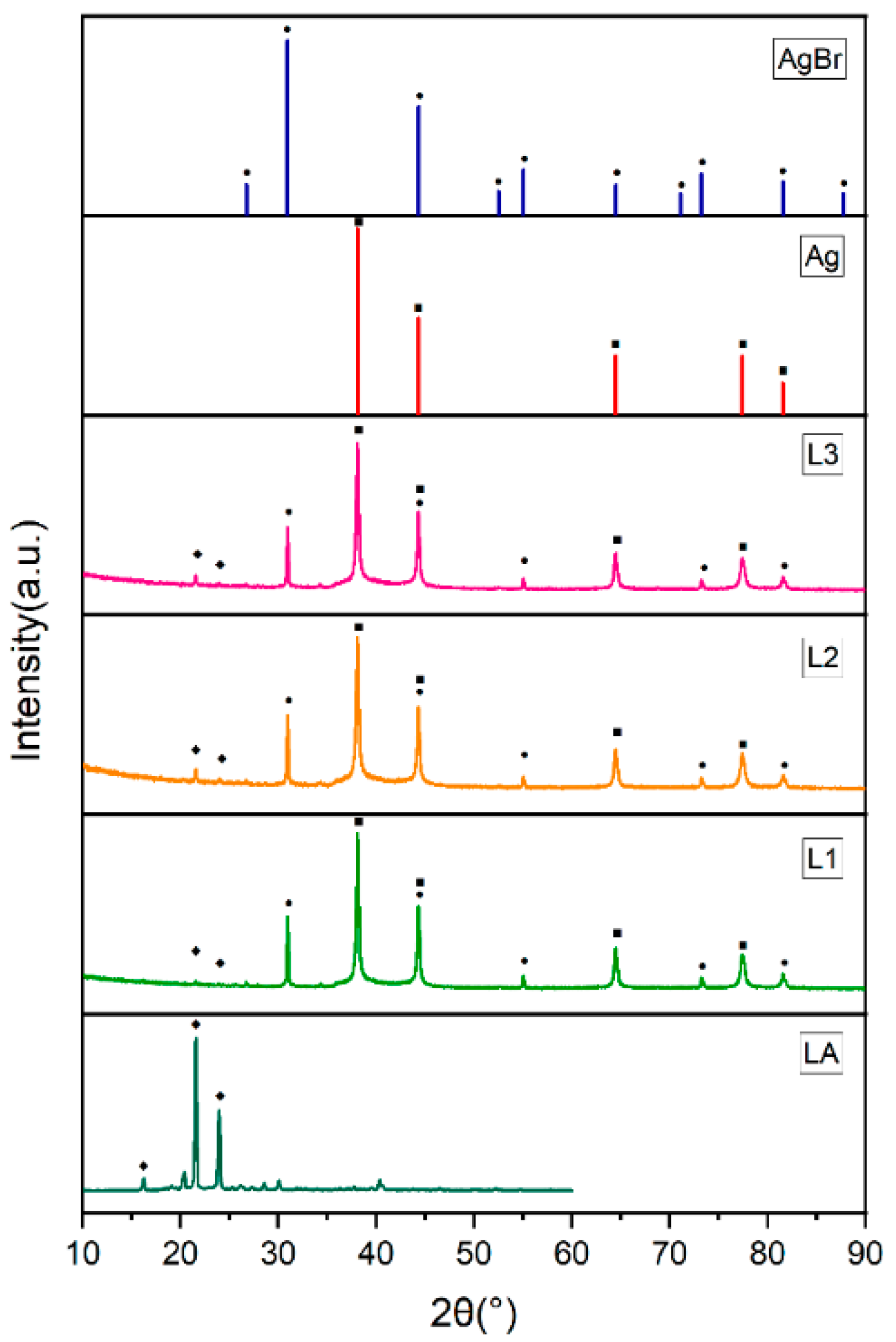
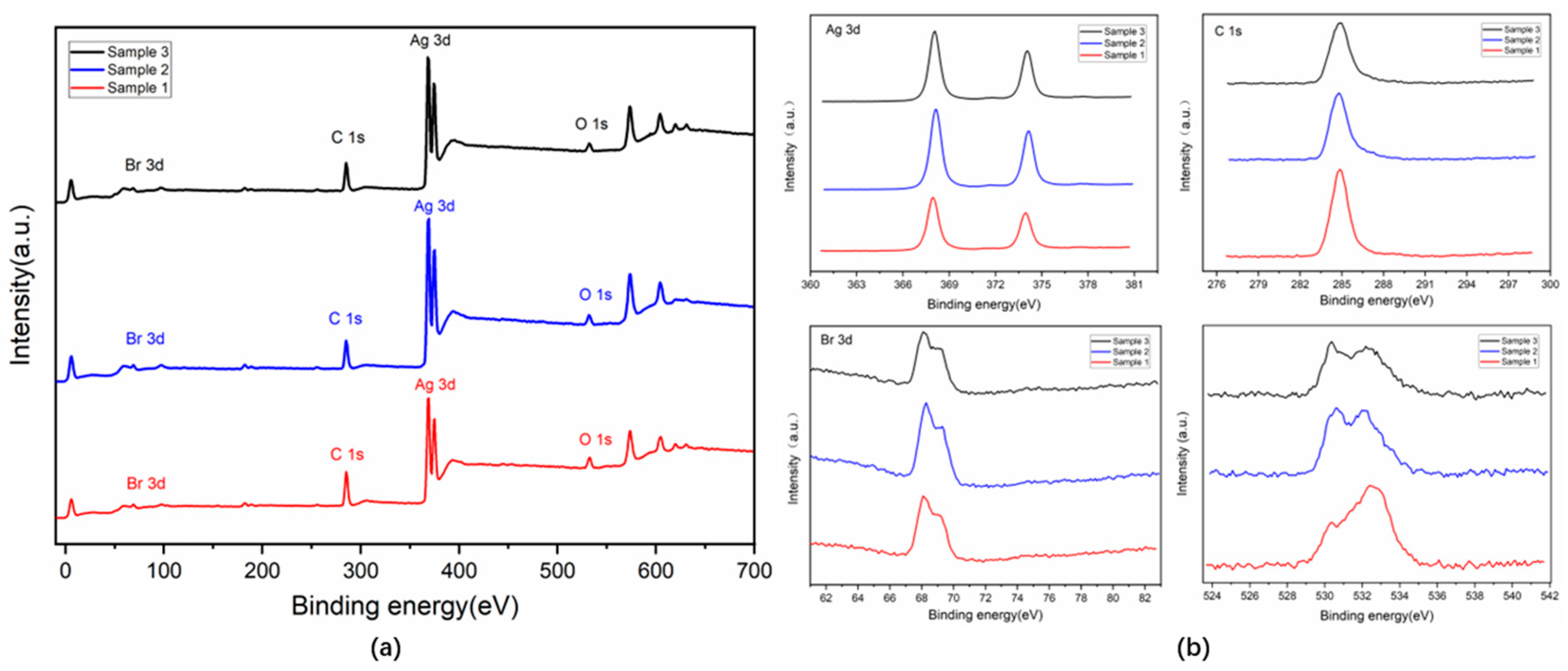

| Core | Shell | Mean Diameter (μm) | R (%) | λ (W/(m·K)) | Ref. |
|---|---|---|---|---|---|
| Paraffin | Polyurea | 0.4–0.6 | 66.56 | 0.225 | [36] |
| n-eicosane | TiO2 | 1.5–2 | 49.90 | 0.865 | [37] |
| PA | Ag | 0.1–0.6 | 34.30 | 4.072 | [38] |
| Samples | LA Amount (g) | CCTAB (mmol/L) | CKBr (mmol/L) | CAgNO3-1 (mmol/L) | CAgNO3-2 (g) | Creductant (mol/L) | Vreductant (mL/s) |
|---|---|---|---|---|---|---|---|
| L1 | 0.4 | 2.0 | 18.0 | 80.0 | 1.5 | 0.1603 | 0.03 |
| L2 | 0.06 | ||||||
| L3 | 0.09 |
| Samples | Weight Percentage (%) | Atomic Percentage (%) |
|---|---|---|
| L1 | 20.91 | 5.06 |
| L2 | 24.52 | 6.34 |
| L3 | 27.35 | 7.91 |
| Samples | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | R (%) | Q (%) |
|---|---|---|---|---|---|---|
| LA | 43.86 | 168.35 | 41.59 | 166.97 | 100 | |
| L1 | 42.59 | 47.78 | 40.42 | 44.27 | 28.38 | 82.53 |
| L2 | 42.78 | 45.44 | 39.93 | 45.03 | 26.99 | 81.51 |
| L3 | 42.51 | 43.69 | 38.00 | 44.09 | 25.95 | 80.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yuan, H.; Hu, D.; Li, T.; Bai, H. Effect of Dropping Speed of Reducing Agent on the Preparation of LA/Ag Phase-Change Nanocapsules. Energies 2024, 17, 933. https://doi.org/10.3390/en17040933
Liu S, Yuan H, Hu D, Li T, Bai H. Effect of Dropping Speed of Reducing Agent on the Preparation of LA/Ag Phase-Change Nanocapsules. Energies. 2024; 17(4):933. https://doi.org/10.3390/en17040933
Chicago/Turabian StyleLiu, Sitong, Huanmei Yuan, Dengti Hu, Tonghe Li, and Hao Bai. 2024. "Effect of Dropping Speed of Reducing Agent on the Preparation of LA/Ag Phase-Change Nanocapsules" Energies 17, no. 4: 933. https://doi.org/10.3390/en17040933
APA StyleLiu, S., Yuan, H., Hu, D., Li, T., & Bai, H. (2024). Effect of Dropping Speed of Reducing Agent on the Preparation of LA/Ag Phase-Change Nanocapsules. Energies, 17(4), 933. https://doi.org/10.3390/en17040933






