Energy Production in Microbial Fuel Cells (MFCs) during the Biological Treatment of Wastewater from Soilless Plant Cultivation
Abstract
1. Introduction
2. Materials and Methods
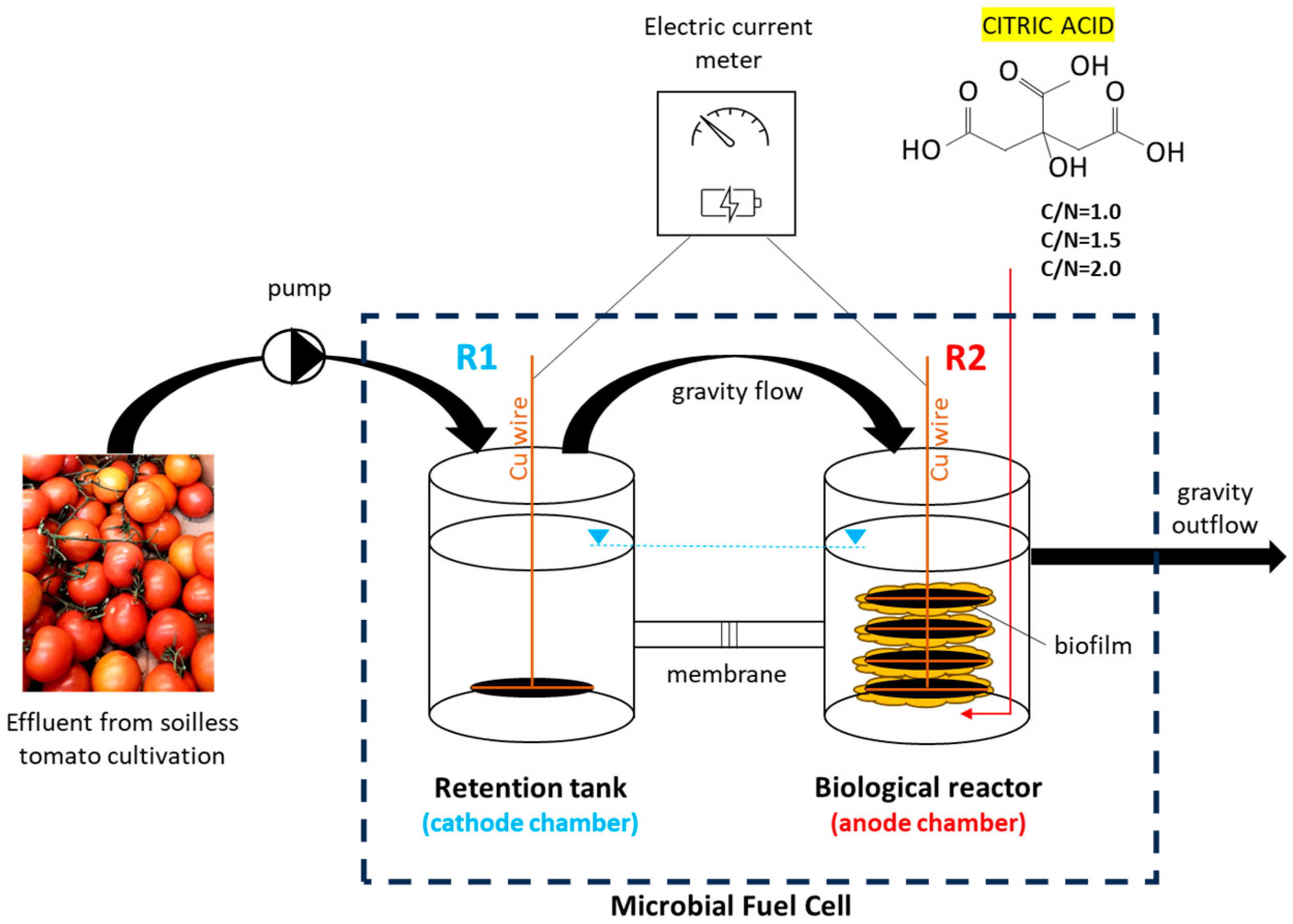
2.1. Microbial Fuel Cell (MFC)
2.2. Drainage Water (DW)
2.3. Physicochemical Analyses
2.4. Computation Methods
- P—electric current power [W],
- U—electric current voltage [V],
- I—electric current intensity [A].
- Pollutant load:
- L—pollutant load [mg/d],
- C—concentration of pollutants [mg/L],
- Q—daily wastewater flow [L/d].
- Pollutant removal efficiency:
- η—effectiveness of pollutant removal [%],
- Lin.—pollutant load in the inflowing wastewater [mg/d],
- Leff.—pollutant load in the effluent [mg/d].
3. Results and Discussion
3.1. pH, EC, Dissolved Oxygen, Redox Potential
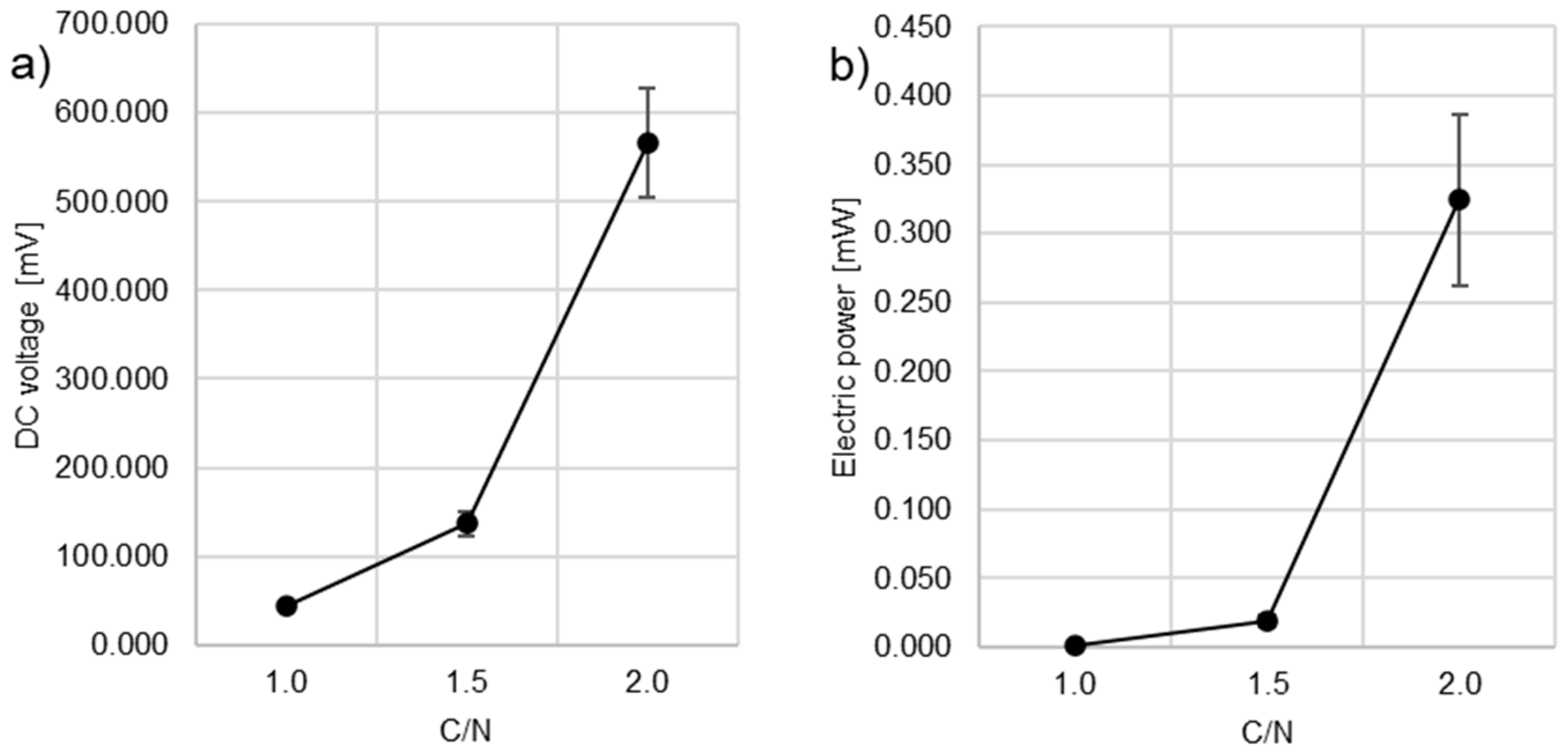
3.2. Electrical Energy Production
3.3. Efficiency of Citric Acid Consumption
3.4. Nitrogen Removal Efficiency
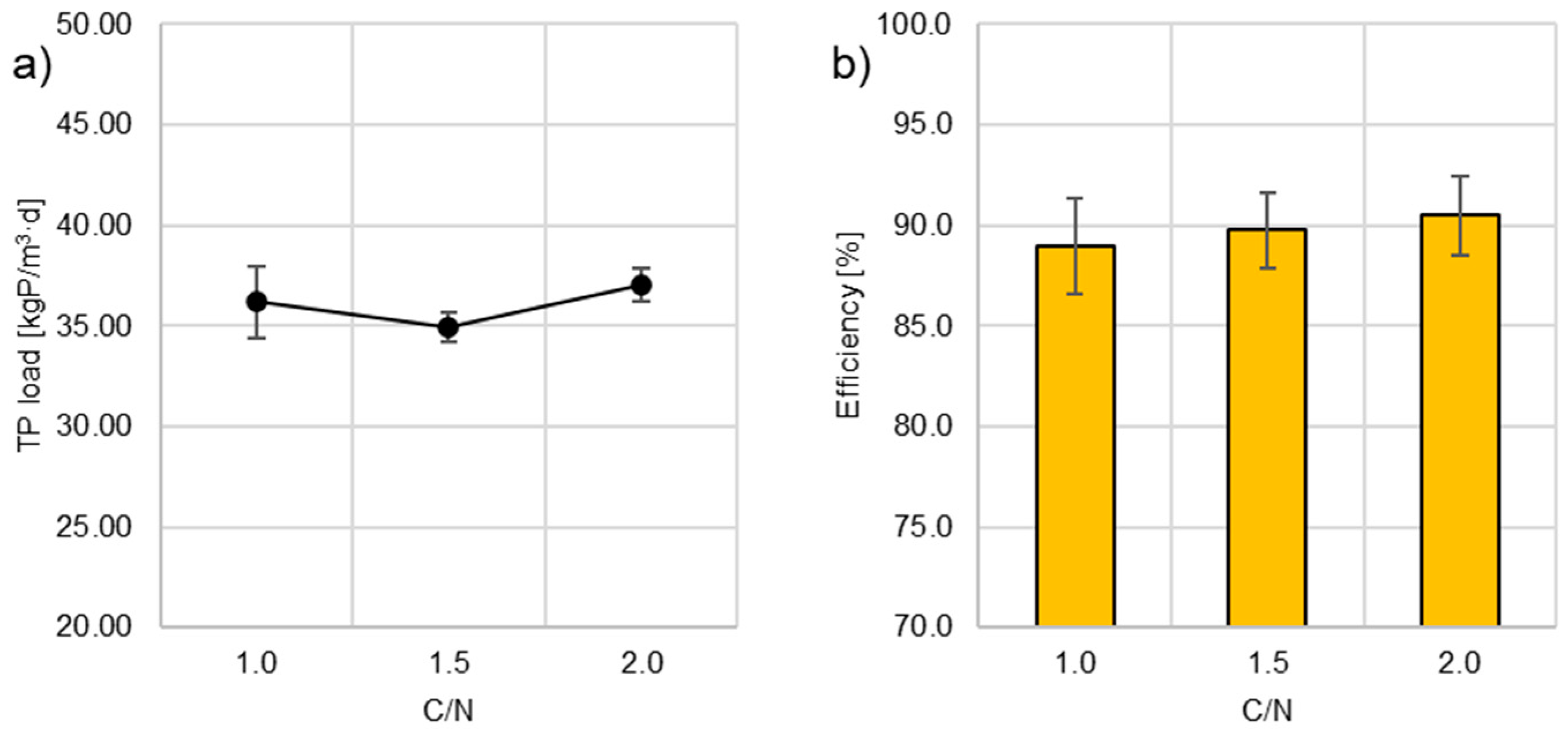
3.5. Phosphorus, Calcium and Magnesium
4. Conclusions
- Citric acid is an efficient organic substrate for both aiding biological treatment and for generating electrical energy in a microbial fuel cell.
- With the increase in the C/N ratio from 1.0 to 2.0, the voltage of the electric current increased from 44.34 ± 60.92 mV to 566.06 ± 2.47 mV, corresponding to the electric current power increase from 0.0020 ± 0.0002 mW to 0.3241 ± 0.0623 mW.
- The electric current’s power per 1 mg of consumed organic carbon is contingent on the operating parameters of the Microbial Fuel Cell (MFC). A greater disparity in redox potentials between the anode and cathode chambers results in higher current power generated per 1 mg of organic carbon.
- The best performance of MFC is achieved after depletion of the oxidized forms of pollutants, when the redox potential decreases in the anode chamber in the presence of an organic substrate.
- Citric acid consumption efficiency was 58.3 ± 5.5%, 65.8 ± 4.0%, and 61.0 ± 5.6% at C/N ratios of 1.0, 1.5, and 2.0, respectively. Simultaneously, the substrate facilitated the formation of a stable biofilm on the filling, which served as both the anode in the MFC. The chelating properties of citric acid, along with its sludge-dissolving ability, contributed to a decrease in ion-exchange membrane contamination.
- The denitrification efficiency increased with higher citric acid doses, reaching 51.47 ± 7.57%, 80.18 ± 9.84%, and 95.60 ± 1.99% at C/N ratios of 1.0, 1.5, and 2.0, respectively. Simultaneously, there was no rise in ammonia nitrogen concentration in the effluent, and nitrites accounted for 1.12%, 1.73%, and 0.82% of the total nitrogen, respectively.
- Regardless of the organic substrate dose applied, the efficiency of dephosphatation was high and reached 88.97 ± 2.41; 89.75 ± 1.90 and 90.48 ± 1.99% at C/N 1.0, 1.5 and 2.0, respectively. This was due to the removal of phosphates by precipitation with calcium and magnesium ions upon the increased alkalinity of the treated DW caused by nitrate reduction.
- The MFC constructed based on a retention tank for untreated DW and a biological reactor for DW treatment fed with an external carbon source seems to represent a promising source of sustainable, renewable energy, allowing for its further diversification.
- Future research should focus on assessing the influence of substrate type and technological parameters, including hydraulic retention time and pollutant load, on the effectiveness of treating DW and generating energy in MFCs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.H. Progress in Microbial Fuel Cell Technology for Wastewater Treatment and Energy Harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Asadnia, M. A Review on the Contribution of Electron Flow in Electroactive Wetlands: Electricity Generation and Enhanced Wastewater Treatment. Chemosphere 2020, 254, 126926. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. The Integrated Processes for Wastewater Treatment Based on the Principle of Microbial Fuel Cells: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A Mini Review on Biofouling on Air Cathode of Single Chamber Microbial Fuel Cell; Prevention and Mitigation Strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Gao, C.; Liu, L.; Yang, F. Development of a Novel Proton Exchange Membrane-Free Integrated MFC System with Electric Membrane Bioreactor and Air Contact Oxidation Bed for Efficient and Energy-Saving Wastewater Treatment. Bioresour. Technol. 2017, 238, 472–483. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of Water Transport Characteristics on Membrane Internal Conductive Structure in Forward Osmosis Microbial Fuel Cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Lovley, D.R. Bug Juice: Harvesting Electricity with Microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef]
- Xu, L.; Yu, W.; Graham, N.; Zhao, Y.; Qu, J. Application of Integrated Bioelectrochemical-Wetland Systems for Future Sustainable Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 1741–1743. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent Advancement in Scaling-up Applications of Microbial Fuel Cells: From Reality to Practicability. Sustain. Energy Technol. Assess. 2021, 45, 101226. [Google Scholar] [CrossRef]
- An, J.; Lee, Y.S.; Kim, T.; Chang, I.S. Significance of Maximum Current for Voltage Boosting of Microbial Fuel Cells in Series. J. Power Sources 2016, 323, 23–28. [Google Scholar] [CrossRef]
- Manohar, A.K.; Mansfeld, F. The Internal Resistance of a Microbial Fuel Cell and Its Dependence on Cell Design and Operating Conditions. Electrochim. Acta 2009, 54, 1664–1670. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Membrane Cation Transport on PH and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, P.; Huang, X. Recent Progress in Electrodes for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and Operation of a Novel Mediator- and Membrane-Less Microbial Fuel Cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Li, S.; Duan, L.; Zhang, H.; Li, M.; Zhao, Y.; Xing, F. Inhibition Strategies of Reverse Solute Flux in Osmotic Microbial Fuel Cells: Take Forward Osmosis as Reference. ACS ES T Water 2023, 3, 2835–2848. [Google Scholar] [CrossRef]
- Cristiani, P.; Carvalho, M.L.; Guerrini, E.; Daghio, M.; Santoro, C.; Li, B. Cathodic and Anodic Biofilms in Single Chamber Microbial Fuel Cells. Bioelectrochemistry 2013, 92, 6–13. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A Review of the Substrates Used in Microbial Fuel Cells (MFCs) for Sustainable Energy Production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K. Factors Affecting Current Production in Microbial Fuel Cells Using Different Industrial Wastewaters. Bioresour. Technol. 2011, 102, 5105–5112. [Google Scholar] [CrossRef]
- Prystay, W.; Lo, K.V. Treatment of Greenhouse Wastewater Using Constructed Wetlands. J. Environ. Sci. Health Part B 2001, 36, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dobrowolski, A. Analysis of Wastewater Generated in Greenhouse Soilless Tomato Cultivation in Central Europe. Water 2019, 11, 2538. [Google Scholar] [CrossRef]
- Saxena, P.; Bassi, A. Removal of Nutrients from Hydroponic Greenhouse Effluent by Alkali Precipitation and Algae Cultivation Method. J. Chem. Technol. Biotechnol. 2013, 88, 858–863. [Google Scholar] [CrossRef]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent Advances and Perspectives in the Treatment of Hydroponic Wastewater: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Bugajski, P.; Kaczor, G.; Bergel, T. The Removal of Reliability Nitrogen in Wastewater Treatment Plant with Sequencing Biological Reactor. Acta Sci. Pol. Form. Circumiectus 2015, 14, 19–27. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Sukias, J.P.S. Removal of Nitrate and Phosphorus from Hydroponic Wastewater Using a Hybrid Denitrification Filter (HDF). Bioresour. Technol. 2009, 100, 3175–3179. [Google Scholar] [CrossRef]
- Kwon, M.J.; Hwang, Y.; Lee, J.; Ham, B.; Rahman, A.; Azam, H.; Yang, J.S. Waste Nutrient Solutions from Full-Scale Open Hydroponic Cultivation: Dynamics of Effluent Quality and Removal of Nitrogen and Phosphorus Using a Pilot-Scale Sequencing Batch Reactor. J. Environ. Manag. 2021, 281, 111893. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Rosli, N.S.M.; Abdullah, R.; Yaacob, J.S.; Qi, N.C.; Loke, S.P. Resource Recovery from Hydroponic Wastewaters Using Microalgae-Based Biorefineries: A Circular Bioeconomy Perspective. J. Biotechnol. 2022, 360, 11–22. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, J.; Lie, Z.; Lai, D.Y.F. Effects of Applying Different Carbon Substrates on Nutrient Removal and Greenhouse Gas Emissions by Constructed Wetlands Treating Carbon-Depleted Hydroponic Wastewater. Bioresour. Technol. 2022, 357, 127312. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Jóźwiak, T.; Struk-Sokołowska, J.; Bryszewski, K. The Share of Electrochemical Reduction, Hydrogenotrophic and Heterotrophic Denitrification in Nitrogen Removal in Rotating Electrobiological Contactor (REBC) Treating Wastewater from Soilless Cultivation Systems. Sci. Total Environ. 2019, 683, 21–28. [Google Scholar] [CrossRef]
- Mielcarek, A.; Bryszewski, K.Ł.; Rodziewicz, J.; Janczukowicz, W. Single-Stage or Two-Stages Bio-Electrochemical Treatment Process of Drainage from Soilless Tomato Cultivation with Alternating Current. Sep. Purif. Technol. 2022, 299. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Bryszewski, K.; Janczukowicz, W.; Kłobukowska, K. Energy Consumption for Nutrient Removal from High-Nitrate and High-Phosphorus Wastewater in Aerobic and Anaerobic Bioelectrochemical Reactors. Energies 2022, 15, 7251. [Google Scholar] [CrossRef]
- Dunets, C.S.; Zheng, Y. Removal of Phosphate from Greenhouse Wastewater Using Hydrated Lime. Environ. Technol. 2014, 35, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, A.; Jóźwiak, T.; Rodziewicz, J.; Bryszewski, K.; Janczukowicz, W.; Kalisz, B.; Tavares, J.M.R. Recovery of Phosphorus and Other Minerals from Greenhouse Wastewater Generated during Soilless Tomato Cultivation by Means of Alkalizing Agents. Sci. Total Environ. 2023, 892, 164757. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.J.; Rodrigues, E.; Gaspar, A.R.; Gomes, Á. Energy Performance Factors in Wastewater Treatment Plants: A Review. J. Clean. Prod. 2021, 322, 129107. [Google Scholar] [CrossRef]
- Castellet-Viciano, L.; Torregrossa, D.; Hernández-Sancho, F. The Relevance of the Design Characteristics to the Optimal Operation of Wastewater Treatment Plants: Energy Cost Assessment. J. Environ. Manag. 2018, 222, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An Overview of Microbial Fuel Cell Usage in Wastewater Treatment, Resource Recovery and Energy Production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, H.; Hou, D.; Lobo, F.L.; Xing, D.; Ren, Z.J. Shipboard Bilge Water Treatment by Electrocoagulation Powered by Microbial Fuel Cells. Front. Environ. Sci. Eng. 2019, 13, 53. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A Review of a Recently Emerged Technology: Constructed Wetland–Microbial Fuel Cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Struk-Sokołowska, J. The Impact of Biodegradable Carbon Sources on Nutrients Removal in Post-Denitrification Biofilm Reactors. Sci. Total Environ. 2020, 720, 137377. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dabrowska, D.; Ciesielski, S.; Thornton, A.; Struk-Sokołowska, J. Citric Acid Application for Denitrification Process Support in Biofilm Reactor. Chemosphere 2017, 171, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Romero, C.V. Bioelectricity through Microbial Fuel Cells Using Avocado Waste. Energy Rep. 2022, 8, 376–382. [Google Scholar] [CrossRef]
- Nosek, D.; Cydzik-Kwiatkowska, A. Microbial Structure and Energy Generation in Microbial Fuel Cells Powered with Waste Anaerobic Digestate. Energies 2020, 13, 4712. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Wang, H.; Long, X.; Li, X. Simultaneous Enhancement of Heavy Metal Removal and Electricity Generation in Soil Microbial Fuel Cell. Ecotoxicol. Environ. Saf. 2020, 192, 110314. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Duan, L.; Liu, J.; Zhang, H.; Zhao, Y. Evaluation and Optimization of Reverse Osmosis Pretreatment Technology Using the Modified Intermediate Blocking Model. J. Clean. Prod. 2023, 417, 138029. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients Removal and Recovery in Bioelectrochemical Systems: A Review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fadzli, F.S.; Bhawani, S.A.; Adam Mohammad, R.E. Microbial Fuel Cell: Recent Developments in Organic Substrate Use and Bacterial Electrode Interaction. J. Chem. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of Different Substrates on the Performance, Bacterial Diversity, and Bacterial Viability in Microbial Fuel Cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef]
- Berovic, M.; Legisa, M. Citric Acid Production. Biotechnol. Annu. Rev. 2007, 13, 303–343. [Google Scholar] [CrossRef]
- Sokic-Lazic, D.; Minteer, S.D. Citric Acid Cycle Biomimic on a Carbon Electrode. Biosens. Bioelectron. 2008, 24, 939–944. [Google Scholar] [CrossRef]
- Kargi, F.; Uygur, A.; Başkaya, H.S. Phosphate Uptake and Release Rates with Different Carbon Sources in Biological Nutrient Removal Using a SBR. J. Environ. Manag. 2005, 76, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Thornton, A. The Feasibility of Citric Acid as External Carbon Source for Biological Phosphorus Removal in a Sequencing Batch Biofilm Reactor (SBBR). Biochem. Eng. J. 2015, 93, 102–107. [Google Scholar] [CrossRef]
- Starowicz, A.; Zieliński, M.; Rusanowska, P.; Dębowski, M. Microbial Fuel Cell Performance Boost through the Use of Graphene and Its Modifications—Review. Energies 2023, 16, 576. [Google Scholar] [CrossRef]
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elvanidi, A.; Reascos, C.M.B.; Gourzoulidou, E.; Kunze, A.; Max, J.F.J.; Katsoulas, N. Implementation of the Circular Economy Concept in Greenhouse Hydroponics for Ultimate Use of Water and Nutrients. Horticulturae 2020, 6, 83. [Google Scholar] [CrossRef]
- Park, Y.; Park, S.; Nguyen, V.K.; Yu, J.; Torres, C.I.; Rittmann, B.E.; Lee, T. Complete Nitrogen Removal by Simultaneous Nitrification and Denitrification in Flat-Panel Air-Cathode Microbial Fuel Cells Treating Domestic Wastewater. Chem. Eng. J. 2017, 316, 673–679. [Google Scholar] [CrossRef]
- Venkatramanan, V.; Shah, S.; Prasad, R. A Critical Review on Microbial Fuel Cells Technology: Perspectives on Wastewater Treatment. Open Biotechnol. J. 2021, 15, 131–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Noori, J.S.; Angelidaki, I. Simultaneous Organic Carbon, Nutrients Removal and Energy Production in a Photomicrobial Fuel Cell (PFC). Energy Environ. Sci. 2011, 4, 4340–4346. [Google Scholar] [CrossRef]
- Puig, S.; Serra, M.; Vilar-Sanz, A.; Cabré, M.; Bañeras, L.; Colprim, J.; Balaguer, M.D. Autotrophic Nitrite Removal in the Cathode of Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 4462–4467. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus Immobilization in Water and Sediment Using Iron-Based Materials: A Review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically Mediated Precipitation of Phosphate Minerals for Phosphorus Removal and Recovery: Progress and Perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Mielcarek, A.; Janczukowicz, W.; Rodziewicz, J.; Majkowska-Gadomska, J.; Chojnowska, M. Hydrogel Chitosan Sorbent Application for Nutrient Removal from Soilless Plant Cultivation Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 18484–18497. [Google Scholar] [CrossRef] [PubMed]
- Smoczyński, L.; Muńska, K.T.; Pierożyński, B.; Kosobucka, M. Elektrokoagulacja Ścieków Modelowych Na Elektrodach Żelaznych. Proc. ECOpole 2012, 6, 56. [Google Scholar] [CrossRef]


| Parameter | Series 1 | Series 2 | Series 3 | |
|---|---|---|---|---|
| pH | – | 6.14–6.19 | 6.15–6.31 | 6.14–6.34 |
| Electrolytic conductivity | mS/cm | 6.79 ± 0.03 | 6.98 ± 0.06 | 6.95 ± 0.12 |
| Total organic carbon | mg/L | 3.17 ± 0.52 | 12.68 ± 0.20 | 11.02 ± 1.12 |
| Total nitrogen | 563.46 ± 36.11 | 608.20 ± 13.40 | 590.60 ± 9.10 | |
| N-NO3 | 562.00 ± 27.00 | 607.54 ± 12.50 | 498.54 ± 5.54 | |
| N-NO2 | 0.334 ± 0.216 | 0.267 ± 0.027 | 0.354 ± 0.052 | |
| N-NH4 | 0.025 ± 0.002 | 0.108 ± 0.003 | 0.017 ± 0.016 | |
| Total phosphorus | 79.87 ± 0.17 | 77.80 ± 1.20 | 81.90 ± 1.40 | |
| Ca | 618.33 ± 26.39 | 675.50 ± 27.50 | 325.00 ± 50.00 | |
| Mg | 213.67 ± 17.46 | 251.00 ± 31.00 | 224.00 ± 17.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarek, A.; Bryszewski, K.Ł.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W. Energy Production in Microbial Fuel Cells (MFCs) during the Biological Treatment of Wastewater from Soilless Plant Cultivation. Energies 2024, 17, 548. https://doi.org/10.3390/en17030548
Mielcarek A, Bryszewski KŁ, Kłobukowska K, Rodziewicz J, Janczukowicz W. Energy Production in Microbial Fuel Cells (MFCs) during the Biological Treatment of Wastewater from Soilless Plant Cultivation. Energies. 2024; 17(3):548. https://doi.org/10.3390/en17030548
Chicago/Turabian StyleMielcarek, Artur, Kamil Łukasz Bryszewski, Karolina Kłobukowska, Joanna Rodziewicz, and Wojciech Janczukowicz. 2024. "Energy Production in Microbial Fuel Cells (MFCs) during the Biological Treatment of Wastewater from Soilless Plant Cultivation" Energies 17, no. 3: 548. https://doi.org/10.3390/en17030548
APA StyleMielcarek, A., Bryszewski, K. Ł., Kłobukowska, K., Rodziewicz, J., & Janczukowicz, W. (2024). Energy Production in Microbial Fuel Cells (MFCs) during the Biological Treatment of Wastewater from Soilless Plant Cultivation. Energies, 17(3), 548. https://doi.org/10.3390/en17030548









