Key Issues of Salt Cavern Flow Battery
Abstract
1. Introduction
2. Key Issues on Salt Caverns
2.1. Tightness of Salt Cavern
2.2. Conductivity of Interlayers
2.3. Ions in Salt Caverns
2.4. Temperature in Salt Caverns
3. Key Issues on Electrolytes
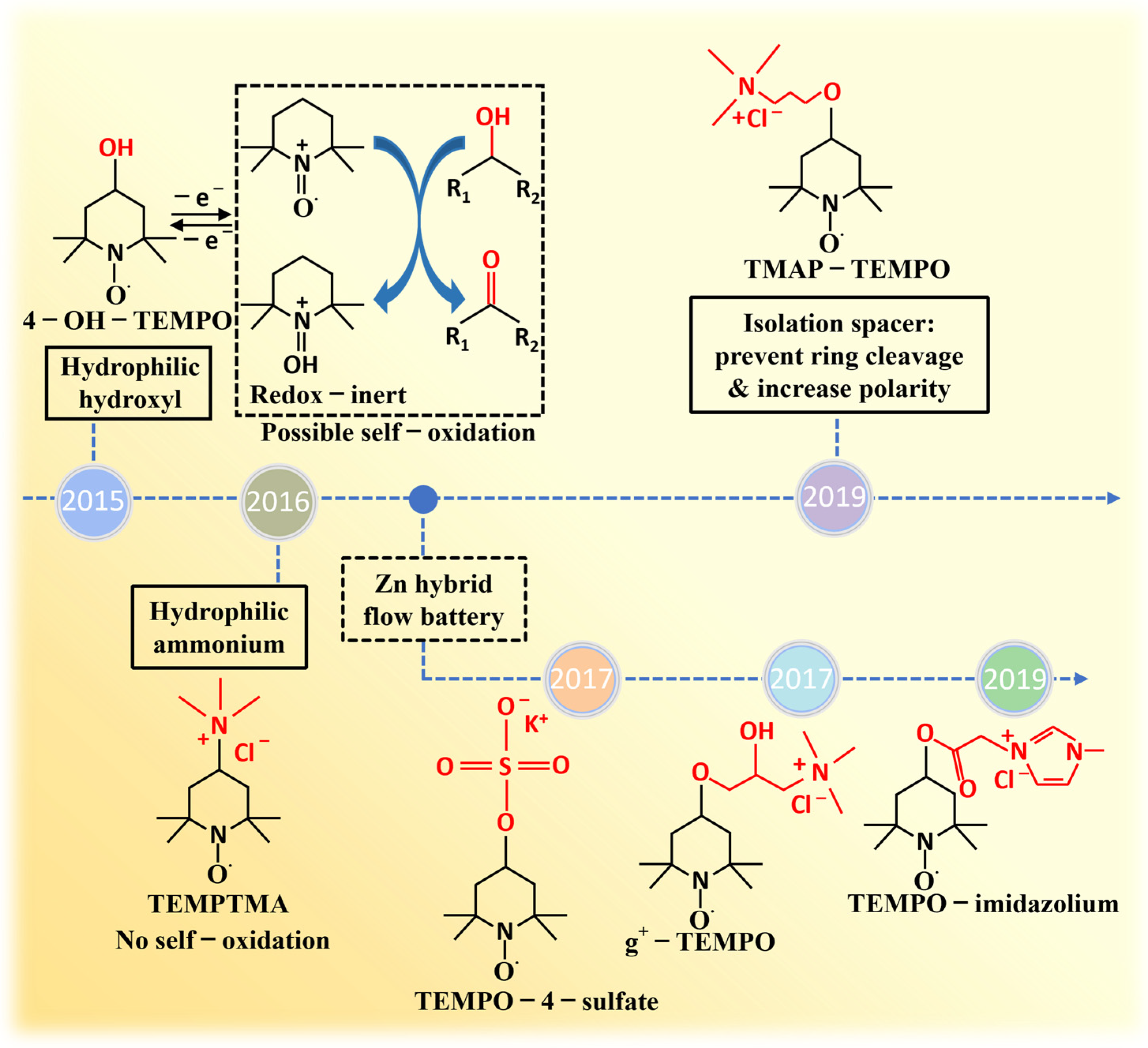
3.1. Selection of Electrolytes
3.2. Permeability of Electrolytes in Surrounding Rock
3.3. Electrolytes Corrosion on Casing Tube
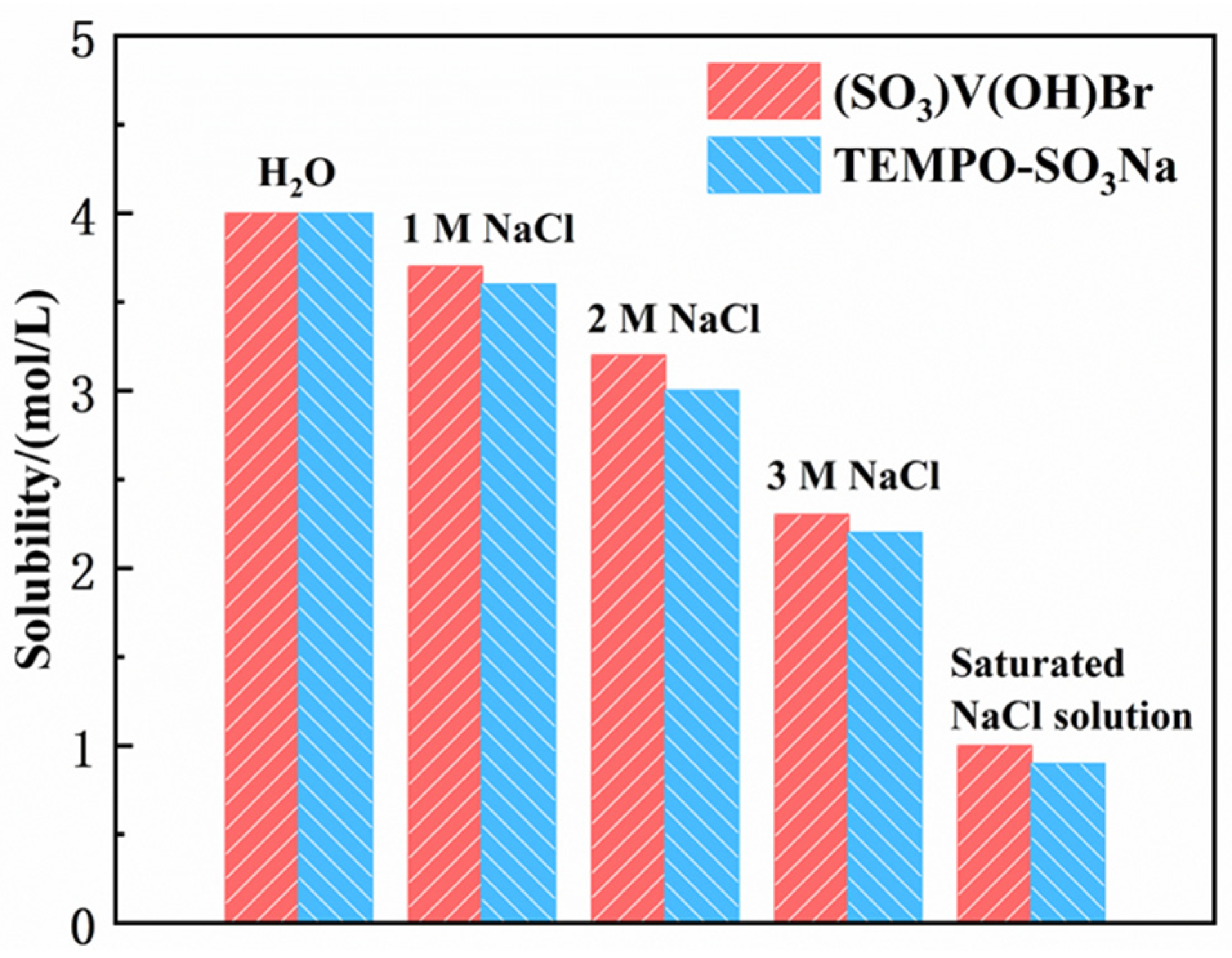
3.4. Concentration of Active Materials
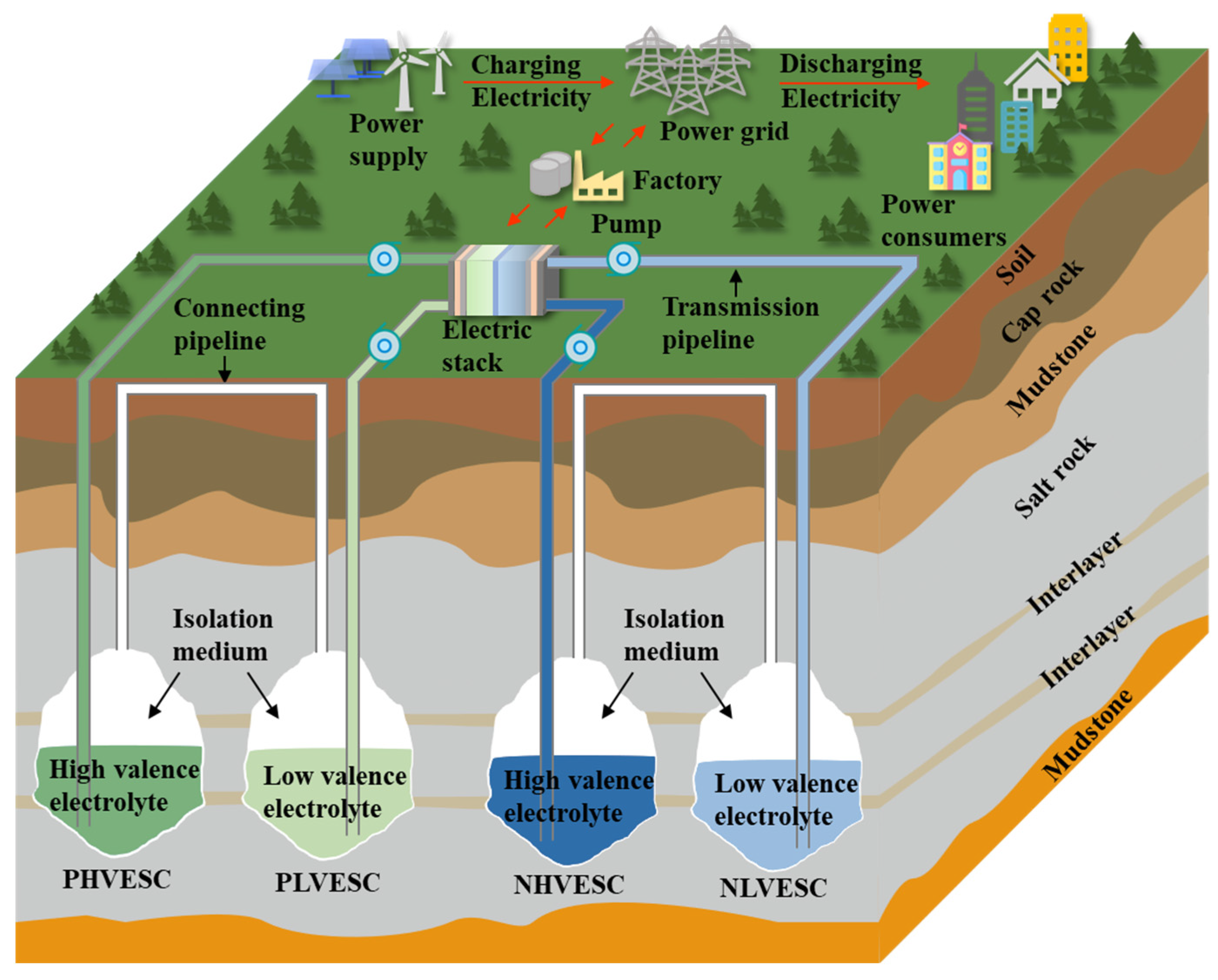
4. A Novel System of SCFB
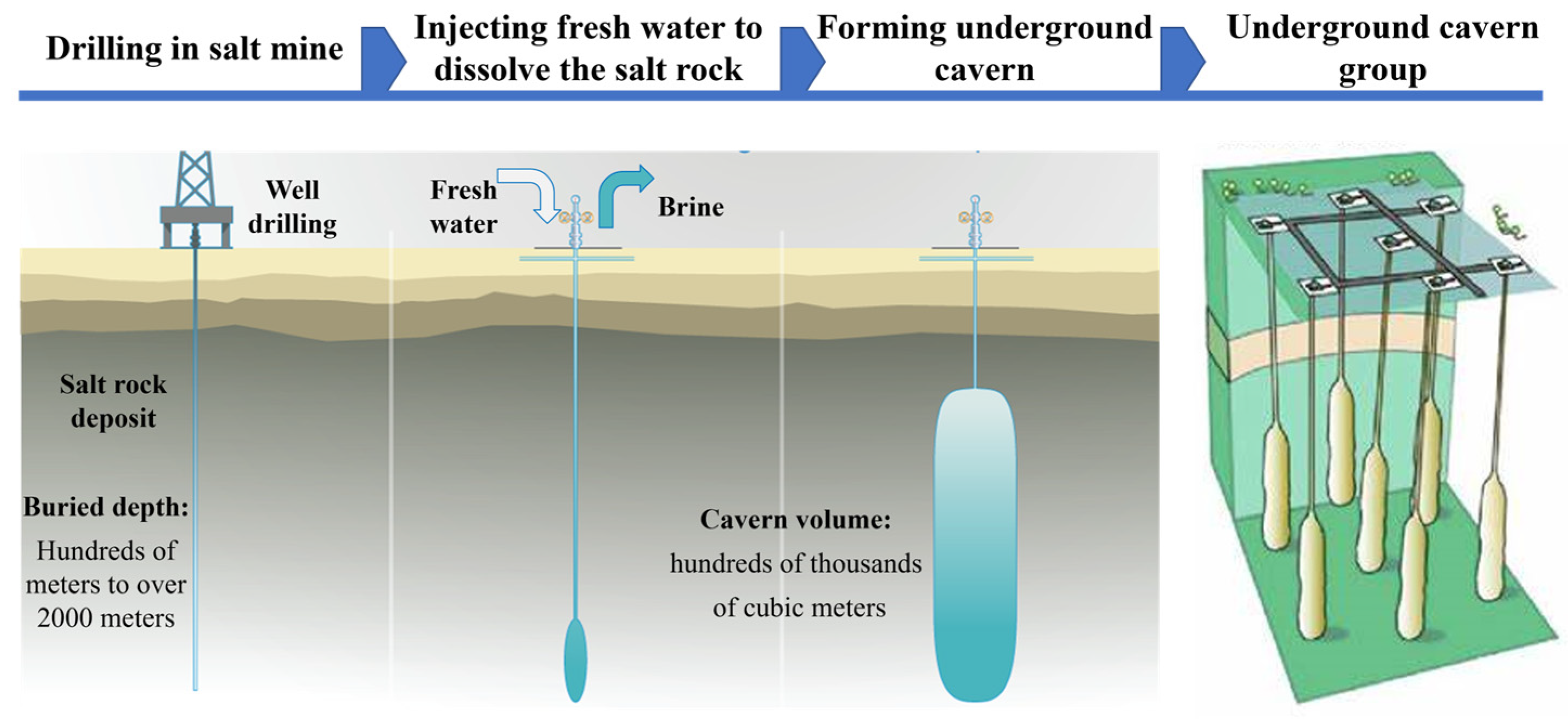
4.1. Operation Process of the Novel SCFB System
4.2. Advantages of Novel SCFB
| Classification of Energy Storage | Energy Storage Technology | Applicable Energy Storage Cycle | Response Time | Operating Life | Comprehensive Efficiency | Advantages | Disadvantages | Maturity Level | Application Scenario |
|---|---|---|---|---|---|---|---|---|---|
| Mechanical energy storage | Pumped hydro storage | Hours to months | Minute level | 40–60 years | 65–85% | Higher efficiency, higher capacity, longer life, lower operating costs | Long construction period, site constraints, large investment | Comparatively mature | Built along the river, peak shaving and valley filling, etc. |
| Compressed air energy storage | Hours to months | Minute level | 20–40 years | 50–65% | High capacity, long life | Lower energy efficiency conversion, large equipment investment, limited site selection | Comparatively mature | Peak shaving and valley filling, etc. | |
| Flywheel energy storage | Seconds to minutes | Minute level | 10–20 years | 80–90% | High efficiency, stability | Short life, high cost, short energy storage time | Generally mature | System frequency modulation, etc. | |
| Hydrogen energy storage | Hydrogen energy storage | Hours to quarters | Second level | 10–25 years | <50% | Long energy storage time, clean, non-polluting | Inefficient, costly, explosive | Immature | Fuel cells, heating, etc.. |
| Electromagnetic energy storage | Supercapacitor energy storage | Seconds to hours | Minute level | 10–30 years | 85–95% | Highly efficient, environmentally friendly, responsive | Short storage time, high cost, lower energy density | Immature | Peak shaving and valley filling, system frequency regulation, etc. |
| Electrochemical energy storage | Lithium-ion batteries | Minutes to days | Hundred millisecond level | 5–15 years | 85–90% | High efficiency, fast response, high energy density | High cost, limited lithium resources, safety risks | Comparatively mature | Distributed energy storage, electric vehicles, etc. |
| Flow batteries | Hours to months | Hundred millisecond level | 15–20 years | 65–85% | Highly secure, flexible, responsive | Higher cost, lower energy density, large footprint | Generally mature | Peak shaving and valley filling, system frequency regulation, etc. | |
| Lead-acid batteries | Minutes to days | Second level | 5–15 years | 70–90% | Low cost, easy maintenance | Lower energy density, poor low temperature performance, lower lifetime | Comparatively mature | Peak shaving and valley filling, system frequency regulation, etc. | |
| Sodium-ion batteries | Minutes to days | Hundred millisecond level | 5–10 years | 70–85% | Low cost, high security, resourceful | Lower energy density, slower charging speed, larger size | Comparatively mature | Peak shaving and valley filling, system frequency regulation, etc. | |
| Salt cavern flow batteries | Hours to quarters | Hundred millisecond level | 20–30 years | 80–90% | Low cost, small footprint, large capacity | Lower energy density, limited siting | Generally mature | Built along the salt mine, peak shaving and valley filling, etc. |
5. Conclusions
- (1)
- There are four key issues regarding the geologic characterization of salt caverns, namely, tightness, conductivity, ions, and ground temperature. Special attention should be given to ions other than Cl− and Na+ ions in the salt caverns because of the possibility of these ions affecting the electrochemical performance of the SCFB. In addition, it is recommended that the depth of the salt caverns should not exceed 1500 m, depending on the temperature at which the electrolyte operates.
- (2)
- There are four key issues regarding electrolytes, namely, selection, permeability, corrosion, and concentration. Of them, the concentration of electrolytes in salt caverns is the most critical issue. The concentration of active substances is closely linked to the electrochemical performance during discharge processes, thereby determining the feasibility of engineering applications for SCFB systems.
- (3)
- A novel SCFB system that guarantees a high concentration of active materials in the salt caverns has been proposed. This novel system consists of both aboveground and underground components. In the underground system, two or more salt caverns are deployed at the positive or negative electrode end to store electrolytes before and after redox reactions, thereby ensuring high concentrations of active materials within the caverns.
- (4)
- The SCFB energy storage technology has the advantages of an ultra-large capacity, high efficiency, low cost, and fast response, making it suitable for storing GWh-level super backup power. It can provide a long-term stable power source for major engineering projects or military facilities.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wegener, L. Can the Paris Agreement Help Climate Change Litigation and Vice Versa? Transnatl. Environ. Law 2020, 9, 17–36. [Google Scholar] [CrossRef]
- Yun, G. China’s response to climate change issues after Paris Climate Change Conference. Adv. Clim. Chang. Res. 2016, 7, 235–240. [Google Scholar]
- Shu, Z.; Jin, J.; Zhang, J.; Wang, G.; Lian, Y.; Liu, Y.; Bao, Z.; Guan, T.; He, R.; Liu, C.; et al. 1.5 °C and 2.0 °C of global warming intensifies the hydrological extremes in China. J. Hydrol. 2024, 635, 131229. [Google Scholar] [CrossRef]
- Preston, B.J. The Influence of the Paris Agreement on Climate Litigation: Causation, Corporate Governance and Catalyst (Part II). J. Environ. Law 2020, 33, 227–256. [Google Scholar] [CrossRef]
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Strategies to achieve a carbon neutra1 society a review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Andrew, R.M.; Rogelj, J.; Peters, G.P.; Canadell, J.G.; Knutti, R.; Luderer, G.; Raupach, M.R.; Schaeffer, M.; Van Vuuren, D.P. Persistent growth of CO2 emissions and implications for reaching climate targets. Nat. Geosci. 2014, 7, 709–715. [Google Scholar] [CrossRef]
- Guan, D.; Liu, Z.; Geng, Y.; Lindner, S.; Hubacek, K. The gigatonne gap in China’s carbon dioxide inventories. Nat. Clim. Chang. 2012, 2, 672–675. [Google Scholar] [CrossRef]
- He, L.; Wang, B.; Xu, W.; Cui, Q.; Chen, H. Could China’s long-term low-carbon energy transformation achieve the double dividend effect for the economy and environment? Energy Policy 2021, 183, 20128–20144. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Li, J. Industrial structural transformation and carbon dioxide emissions in China. Energy Policy 2013, 57, 43–51. [Google Scholar] [CrossRef]
- Bloch, H.; Rafiq, S.; Salim, R. Coal consumption, CO2 emission and economic growth in China: Empirical evidence and policy responses. Energy Econ. 2012, 34, 518–528. [Google Scholar] [CrossRef]
- Sun, J.; Li, G.; Wang, Z. Optimizing China’s Energy Consumption Structure under Energy and Carbon Constraints. Struct. Chang. Econ. Dyn. 2018, 47, 57–72. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, K.P.; Xu, X.M.; Zhang, Y.R. Transport energy consumption and saving in China. Renew. Sustain. Energy Rev. 2014, 29, 641–655. [Google Scholar] [CrossRef]
- Suo, C.; Li, Y.P.; Nie, S.; Lv, J.; Ma, Y. Analyzing the effects of economic development on the transition to cleaner production of China’s energy system under uncertainty. J. Clean. Prod. 2020, 279, 123725. [Google Scholar] [CrossRef]
- Beh, E.S.; De Porcellinis, D.; Gracia, R.L.; Xia, K.T.; Gordon, R.G.; Aziz, M.J. A Neutral pH Aqueous Organic–Organometallic Redox Flow Battery with Extremely High Capacity Retention. ACS Energy Lett. 2017, 2, 639–644. [Google Scholar] [CrossRef]
- McGlade, C.; Ekins, P. The geographical distribution of fossil fuels unused when limiting global warming to 2 degrees C. Nature 2015, 517, 187–190. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, Y.; Yuan, Z.; Li, X.; Yu, G.; Yang, Z.; Xu, T. Organic Electrolytes for pH-Neutral Aqueous Organic Redox Flow Batteries. Adv. Funct. Mater. 2021, 32, 2108777. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Seshadri, A.K.; Sparks, N.J.; Toumi, R. The role of wind-solar hybrid plants in mitigating renewable energy-droughts %J TERI information digest on energy and environment: TIDEE. Renew. Energy 2022, 21, 277. [Google Scholar]
- Alam, M.J.E.; Muttaqi, K.M.; Sutanto, D. Battery Energy Storage to Mitigate Rapid Voltage/Power Fluctuations in Power Grids Due to Fast Variations of Solar/Wind Outputs. IEEE Access 2021, 9, 12191–12202. [Google Scholar] [CrossRef]
- Schdler, Y.; Renken, V.; Sorg, M.; Gerdes, L.; Fischer, A. Power transport needs for the German power grid for a major demand coverage by wind and solar power. Energy Strategy Rev. 2021, 34, 100626. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Poullikkas, A. A comparative overview of large-scale battery systems for electricity storage. Renew. Sustain. Energy Rev. 2013, 27, 778–788. [Google Scholar] [CrossRef]
- Wu, F.; Gao, R.; Li, C.; Liu, J. A comprehensive evaluation of wind-PV-salt cavern-hydrogen energy storage and utilization system: A case study in Qianjiang salt cavern, China. Energy Convers. Manag. 2023, 277, 116633. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Yang, C.; Shi, X.; Wan, J.; Jurado, M.J.; Li, Y.; Jiang, D.; Chen, J.; Qiao, W.; et al. The role of underground salt caverns for large-scale energy storage: A review and prospects. Energy Storage Mater. 2023, 63, 103045. [Google Scholar] [CrossRef]
- Hongwei, Y.; Jinhui, D.; Wei, D.; Song, X.; Jinghui, S. China’s energy storage industry: Develop status, existing problems and countermeasures. Renew. Sustain. Energy Rev. 2017, 71, 767–784. [Google Scholar]
- DeBruler, C.; Hu, B.; Moss, J.; Liu, X.; Luo, J.; Sun, Y.; Liu, T.L. Designer Two-Electron Storage Viologen Anolyte Materials for Neutral Aqueous Organic Redox Flow Batteries. Chem 2017, 3, 961–978. [Google Scholar] [CrossRef]
- Duan, Z.N.; Qu, Z.G.; Wang, Q.; Wang, J.J. Structural modification of vanadium redox flow battery with high electrochemical corrosion resistance. Appl. Energy 2019, 250, 1632–1640. [Google Scholar] [CrossRef]
- Ha, S.; Gallagher, K.G. Estimating the system price of redox flow batteries for grid storage. J. Power Sources 2015, 296, 122–132. [Google Scholar] [CrossRef]
- Perry, M.L.; Weber, A.Z. Advanced Redox-Flow Batteries: A Perspective. J. Electrochem. Soc. 2016, 163, A5064–A5067. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, X.; Liu, J.; Huang, Z.; Chen, Y.; Zhou, M.; Liu, W.; Zhou, H. Towards a high efficiency and low-cost aqueous redox flow battery: A short review. J. Power Sources 2024, 601, 234242. [Google Scholar] [CrossRef]
- Prieto-Díaz, P.A.; Trovò, A.; Marini, G.; Rugna, M.; Vera, M.; Guarnieri, M. Experiment-supported survey of inefficient electrolyte mixing and capacity loss in vanadium flow battery tanks. Chem. Eng. J. 2024, 492, 152137. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y. Electrochemical analysis of electrolyte temperature and composition for all-iron redox flow battery. Int. J. Green Energy 2022, 19, 1285–1289. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Shi, X.; Wenjie, X.; Chunhe, Y. Modeling the construction of energy storage salt caverns in bedded salt. Appl. Energy 2019, 255, 113866. [Google Scholar] [CrossRef]
- Jinlong, L.; Xilin, S.; Shuai, Z. Construction modeling and parameter optimization of multi-step horizontal energy storage salt caverns. Energy 2020, 203, 117840. [Google Scholar]
- Hudec, M.R.; Jackson, M.P.A. Terra infirma: Understanding salt tectonics. Earth-Sci. Rev. 2007, 82, 1–28. [Google Scholar] [CrossRef]
- Cuevas, C. Pore structure characterization in rock salt. Eng. Geol. 1997, 47, 17–30. [Google Scholar] [CrossRef]
- Alkan, H. Percolation model for dilatancy-induced permeability of the excavation damaged zone in rock salt. Int. J. Rock Mech. Min. Sci. 2009, 46, 716–724. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Hesse, M.A.; Prodanovic, M.; Gardner, J. Deformation-assisted fluid percolation in rock salt. Science 2015, 350, 1069–1072. [Google Scholar] [CrossRef]
- Koelemeijer, P.J.; Peach, C.J.; Spiers, C.J. Surface diffusivity of cleaved NaCl crystals as a function of humidity: Impedance spectroscopy measurements and implications for crack healing in rock salt. J. Geophys. Res. Solid Earth 2012, 117. [Google Scholar] [CrossRef]
- Li, S.Y. Rheology of rock salt for salt tectonics modeling. Pet. Sci. 2016, 13, 712–724. [Google Scholar] [CrossRef]
- Liu, X.; Shi, X.; Li, Y.; Li, P.; Zhao, K.; Ma, H.; Yang, C. Maximum gas production rate for salt cavern gas storages. Energy 2021, 234, 121211. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Sun, P.; Li, Y.; Xu, T. Organic Electrolytes for Aqueous Organic Flow Batteries. Mater. Today Energy 2021, 20, 100634. [Google Scholar] [CrossRef]
- Available online: https://newatlas.com/brine4power-largest-redox-flow-battery/50405/ (accessed on 10 July 2017).
- Ding, B.; Chen, J.; He, Y.; Liu, W.; Chen, L.; Xu, J.; Rui, Y. Feasibility analysis of underground flow battery storage in bedded salt rocks of China. J. Energy Storage 2023, 68, 107520. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Bai, W.; Lu, J.; Fu, X.; Fu, Y.; Shi, X. Mechanical Behavior of Sediment-Type High-Impurity Salt Cavern Gas Storage during Long-Term Operation. Energies 2024, 17, 3983. [Google Scholar] [CrossRef]
- Liang, X.; Ma, H.; Cai, R.; Zhao, K.; Wang, X.; Zheng, Z.; Shi, X.; Yang, C. Study of Impact of Sediment on the Stability of Salt Cavern Underground Gas Storage. Energies 2023, 16, 7825. [Google Scholar] [CrossRef]
- Portarapillo, M.; Di Benedetto, A. Risk Assessment of the Large-Scale Hydrogen Storage in Salt Caverns. Energies 2021, 14, 2856. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Liu, W.; Li, L.; Qiao, W.; Chen, J.; Li, D.; Li, Z.; Fan, J. Construction simulation of large-spacing-two-well salt cavern with gas blanket and stability evaluation of cavern for gas storage. J. Energy Storage 2022, 48, 103932. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Xu, J.; Wu, Y.; Cui, Y.; Chen, L. An unsymmetrical two-electron viologens anolyte for salt cavern redox flow battery. J. Power Sources 2021, 492, 229659. [Google Scholar] [CrossRef]
- Wei, X.; Ban, S.; Shi, X.; Li, P.; Li, Y.; Zhu, S.; Yang, K.; Bai, W.; Yang, C. Carbon and energy storage in salt caverns under the background of carbon neutralization in China. Energy 2023, 272, 127120. [Google Scholar] [CrossRef]
- Wang, H.; Ke, W.; Wei, Z.; Li, D.; Chen, L. An In-situ Brine Solution in Salt-cavern Supported Redox-flow Battery Using Iron/Organic Materials. Chem. Lett. 2021, 50, 389–391. [Google Scholar] [CrossRef]
- Wei, X.; Shi, X.; Li, Y.; Li, P.; Ban, S.; Zhao, K.; Ma, H.; Liu, H.; Yang, C. A comprehensive feasibility evaluation of salt cavern oil energy storage system in China. Appl. Energy 2023, 351, 121807. [Google Scholar] [CrossRef]
- Shi, X.; Liu, W.; Chen, J.; Jiang, D.; Wu, F.; Zhang, J.; Jinyang, F. Softening model for failure analysis of insoluble interlayers during salt cavern leaching for natural gas storage. Acta Geotech. 2018, 13, 801–816. [Google Scholar] [CrossRef]
- Wei, L.; Jie, C.; Deyi, J.; Xilin, S.; Yinping, L.; Daemen, J.J.K.; Chunhe, Y. Tightness and suitability evaluation of abandoned salt caverns served as hydrocarbon energies storage under adverse geological conditions (AGC). Appl. Energy 2016, 178, 703–720. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Chen, J.; Jiang, D.; Wu, F.; Fan, J.; Li, Y. Feasibility evaluation of large-scale underground hydrogen storage in bedded salt rocks of China: A case study in Jiangsu province. Energy 2020, 198, 117348. [Google Scholar] [CrossRef]
- Wang, J.; An, G.; Shan, B.; Wang, W.; Jia, J.; Wang, T.; Zheng, X. Parameter optimization of solution mining under nitrogen for the construction of a gas storage salt cavern. J. Nat. Gas Sci. Eng. 2021, 91, 103954. [Google Scholar] [CrossRef]
- Fatah, A.; Al-Yaseri, A.; Theravalappil, R.; Radwan, O.A.; Amao, A.; Al-Qasim, A.S. Geochemical reactions and pore structure analysis of anhydrite/gypsum/halite bearing reservoirs relevant to subsurface hydrogen storage in salt caverns. Fuel 2024, 371, 131857. [Google Scholar] [CrossRef]
- Toda, K.; Watanabe, J.; Sato, M. Synthesis and ionic conductivity of new layered perovskite compound, Ag2La2Ti3O10. Solid State Ion. Diffus. React. 1996, 90, 15–19. [Google Scholar] [CrossRef]
- Qiu, Z.-F.; Wang, J.-J. Experimental Study on the Anisotropic Hydraulic Conductivity of a Sandstone–Mudstone Particle Mixture. J. Hydrol. Eng. 2015, 20, 04015029. [Google Scholar] [CrossRef]
- Sun, W.; Dai, L.; Li, H.; Hu, H.; Wu, L.; Jiang, J. Electrical conductivity of mudstone (before and after dehydration at high P-T) and a test of high conductivity layers in the crust. Am. Mineral. 2017, 102, 2450–2456. [Google Scholar] [CrossRef]
- Wei, X.; Shi, X.; Li, Y.; Ma, H.; Ban, S.; Liu, X.; Liu, H.; Yang, C. Analysis of the European energy crisis and its implications for the development of strategic energy storage in China. J. Energy Storage 2024, 82, 110522. [Google Scholar] [CrossRef]
- Minougou, J.D.; Gholami, R.; Poirier, S. A one-dimensional diffusive transport model to evaluate H2S generation in salt caverns hydrogen storage sites. Gas Sci. Eng. 2024, 126, 205336. [Google Scholar] [CrossRef]
- Lai, Y.Y.; Li, X.; Liu, K.; Tung, W.Y.; Zhu, Y. Stable Low-Cost Organic Dye Anolyte for Aqueous Organic Redox Flow Battery. ACS Appl. Energy Mater. 2020, 3, 2290–2295. [Google Scholar] [CrossRef]
- Briot, L.; Petit, M.; Cacciuttolo, Q.; Pera, M.C. Aging phenomena and their modelling in aqueous organic redox flow batteries: A review. J. Power Sources 2022, 536, 231427. [Google Scholar] [CrossRef]
- Huang, J.; Dong, X.; Guo, Z.; Wang, Y. Progress of Organic Electrodes in Aqueous Electrolyte for Energy Storage and Conversion. Angew. Chem. 2020, 59, 18322–18333. [Google Scholar] [CrossRef]
- Han, Y.; Cui, H.; Ma, H.; Chen, J.; Liu, N. Temperature and pressure variations in salt compressed air energy storage (CAES) caverns considering the air flow in the underground wellbore. J. Energy Storage 2022, 52, 104846. [Google Scholar] [CrossRef]
- Xie, D.; Wang, T.; Li, L.; He, T.; Chai, G.; Wang, D.; Zhang, H.; Ma, T.; Zhang, X. Temperature distribution of brine and gas in the tubing during debrining of a salt cavern gas storage. J. Energy Storage 2022, 50, 104236. [Google Scholar] [CrossRef]
- Liu, T.; Wei, X.; Nie, Z.; Sprenkle, V.; Wang, W. A Total Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4-HO-TEMPO Catholyte. Adv. Energy Mater. 2016, 6, 1501449. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Hager, M.D.; Schubert, U.S. An Aqueous Redox-Flow Battery with High Capacity and Power: The TEMPTMA/MV System. Angew. Chem. Int. Ed. Engl. 2016, 55, 14427–14430. [Google Scholar] [CrossRef]
- Liu, Y.; Goulet, M.-A.; Tong, L.; Liu, Y.; Ji, Y.; Wu, L.; Gordon, R.G.; Aziz, M.J.; Yang, Z.; Xu, T. A Long-Lifetime All-Organic Aqueous Flow Battery Utilizing TMAP-TEMPO Radical. Chem 2019, 5, 1861–1870. [Google Scholar] [CrossRef]
- Hu, B.; DeBruler, C.; Rhodes, Z.; Liu, T.L. Long-Cycling Aqueous Organic Redox Flow Battery (AORFB) toward Sustainable and Safe Energy Storage. J. Am. Chem. Soc. 2017, 139, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, B.; Debruler, C.; Liu, T.L. A pi-Conjugation Extended Viologen as a Two-Electron Storage Anolyte for Total Organic Aqueous Redox Flow Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Yang, C.; Daemen, J.J.K.; Yang, Y.; Zhang, G. Permeability characteristics of mudstone cap rock and interlayers in bedded salt formations and tightness assessment for underground gas storage caverns. Eng. Geol. 2015, 193, 212–223. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Yang, C.; Qu, D. Test Study of Influence of Brine on Tensile Strength of Muddy Intercalation. Chin. J. Rock Mech. Eng. 2009, 28, 2301–2308. (In Chinese) [Google Scholar]
- Montero, J.; da Silva Freitas, W.; Mecheri, B.; Forchetta, M.; Galloni, P.; Licoccia, S.; D’Epifanio, A. A Neutral-pH Aqueous Redox Flow Battery Based on Sustainable Organic Electrolytes. ChemElectroChem 2022, 10, e202201002. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Wang, X.-P.; Shao, M.; Ye, C.-Q.; Dong, S.-G.; Du, R.-G.; Lin, C.-J. Study on effect of chloride ions on corrosion behavior of reinforcing steel in simulated polluted concrete pore solutions by scanning micro-reference electrode technique. J. Electroanal. Chem. 2021, 895, 115454. [Google Scholar] [CrossRef]
- Wan, L.; Wang, B.; Xie, M.; Hu, C.; Wen, Y. Prediction of corrosion life of nitrogen blanket casing in salt cavern gas storage. Energy Sci. Eng. 2022, 10, 3044–3056. [Google Scholar] [CrossRef]
- Koyiit, N.; Gencten, M.; Ahin, M.; Sahin, Y. A novel vanadium/cobalt redox couple in aqueous acidic solution for redox flow batteries. Int. J. Energy Res. 2020, 44, 411–424. [Google Scholar]
- Ruan, R.; Wang, Y.; Hu, C.; Gao, A.; Xu, L. Electrode Potential Regulation of Carbon Fiber Based on Galvanic Coupling and Its Application in Electrochemical Grafting. ACS Appl. Mater. Interfaces 2021, 13, 17013–17021. [Google Scholar] [CrossRef]
- Sun, C.; Vezzù, K.; Pagot, G.; Nale, A.; Bang, Y.H.; Pace, G.; Negro, E.; Gambaro, C.; Meda, L.; Zawodzinski, T.A.; et al. Elucidation of the interplay between vanadium species and charge-discharge processes in VRFBs by Raman spectroscopy. Electrochim. Acta 2019, 318, 913–921. [Google Scholar] [CrossRef]
- Shi, S.; Huang, S.; Li, Y.; Ma, H.; Yang, C.; Li, P. Salt Cavern Flow Battery System. U.S. Patent 18/347529, 21 July 2023. [Google Scholar]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.C. Material Design of Aqueous Redox Flow Batteries: Fundamental Challenges and Mitigation Strategies. Adv. Mater. 2020, 32, e2002132. [Google Scholar] [CrossRef] [PubMed]
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Stocks, R.; Blakers, A.; Nadolny, A.; Cheng, C. A global atlas of pumped hydro systems that repurpose existing mining sites. Renew. Energy 2024, 224, 120113. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Gao, Z.; Chen, S.; Xu, Y.; Chen, H. Overview of dynamic operation strategies for advanced compressed air energy storage. J. Energy Storage 2023, 66, 107408. [Google Scholar] [CrossRef]
- Rupp, A.; Baier, H.; Mertiny, P.; Secanell, M. Analysis of a flywheel energy storage system for light rail transit. Energy 2016, 107, 625–638. [Google Scholar] [CrossRef]
- Nagpal, M.; Kakkar, R. An evolving energy solution: Intermediate hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 12168–12188. [Google Scholar] [CrossRef]
- Karandikar, P.B.; Talange, D.B.; Mhaskar, U.P.; Bansal, R. Development, modeling and characterization of aqueous metal oxide based supercapacitor. Energy 2012, 40, 131–138. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Lin, H.; Kim, K.; He, R.; Feng, S.; Liu, H. Research progress on electrolyte key salts for sodium-ion batteries. Prog. Nat. Sci. Mater. Int. 2024, 34, 263–273. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, H.; Wang, J.; Qi, J.; Liu, Y. Engineering of multi-shelled SnO2 hollow microspheres for highly stable lithium-ion batteries. J. Mater. Chem. A 2016, 4, 17673–17677. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Han, W. Recycling and management of waste lead-acid batteries: A mini-review. Waste Manag. Res. 2016, 34, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, W.; Li, X. Progress and Perspectives of Flow Battery Technologies. Electrochem. Energy Rev. 2019, 2, 492–506. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Feng, J. Research progress in preparation of electrolyte for all-vanadium redox flow battery. J. Ind. Eng. Chem. 2022, 118, 33–43. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]






| Name | Capacity Fade (%/Cycle) | Supporting Electrolyte | Capacity (A·h/L) | Energy Densit (W·h/L) | System Energy Efficiency (%) |
|---|---|---|---|---|---|
| (SO3)V(OH)Br/TEMPOSO3Na [50] | 0.05 | 2 M NaCl | 13.4 | 10.8 | 80 |
| MV2+Cl2/4-HO-TEMPO [69] | 0.01 | 1 M NaCl | 2.68 | 3.35 | 87 |
| MV/TEMPTMA [70] | - | 1.5 M NaCl | 54 | 38 | 70 |
| BTMAP-Vi/TMAPTEMPO [71] | 0.007 | 1 M NaCl | 40.2 | 23.9 | 90 |
| BTMAP-Vi/BTMAP-Fc [15] | 0.0057 | 0.5 M NaCl | 26 | 20 | 65 |
| MV/FcNCl [72] | 0.01 | 2 M NaCl | 84 | - | 60 |
| [(NPr)2TTz]Cl4/NMe-TEMPO [73] | 0.03 | 2 M NaCl | 60 | 53.7 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, Y.; Shi, X.; Liu, Y.; Ma, H.; Li, P.; Liu, Y.; Liu, X.; Xu, M.; Yang, C. Key Issues of Salt Cavern Flow Battery. Energies 2024, 17, 5190. https://doi.org/10.3390/en17205190
Huang S, Li Y, Shi X, Liu Y, Ma H, Li P, Liu Y, Liu X, Xu M, Yang C. Key Issues of Salt Cavern Flow Battery. Energies. 2024; 17(20):5190. https://doi.org/10.3390/en17205190
Chicago/Turabian StyleHuang, Si, Yinping Li, Xilin Shi, Yahua Liu, Hongling Ma, Peng Li, Yuanxi Liu, Xin Liu, Mingnan Xu, and Chunhe Yang. 2024. "Key Issues of Salt Cavern Flow Battery" Energies 17, no. 20: 5190. https://doi.org/10.3390/en17205190
APA StyleHuang, S., Li, Y., Shi, X., Liu, Y., Ma, H., Li, P., Liu, Y., Liu, X., Xu, M., & Yang, C. (2024). Key Issues of Salt Cavern Flow Battery. Energies, 17(20), 5190. https://doi.org/10.3390/en17205190