Abstract
Recent literature highlights the crucial role of deadwood in forests, emphasizing its contribution to biodiversity conservation, soil fertility, climate change mitigation, and bioenergy production. However, managing deadwood presents challenges as decision-makers must balance trade-offs and synergies between these ecological benefits. A participatory approach, incorporating user opinions, can support effective decision-making. This study surveyed 1207 university students from Iran, Italy, and Türkiye to explore their perceptions of deadwood’s role and the potential trade-offs among climate change mitigation, biodiversity conservation, and bioenergy production. Results indicate a high level of awareness among students regarding deadwood’s ecological functions, but preferences vary significantly across cultural and regional contexts. Results show that for students of all three countries, the most important function related to the deadwood in forests is the provision of microhabitats for wildlife, while in second place for Iranian students, there is bioenergy production, and for Turkish and Italian students, soil fertilization. In addition, results highlight that students prefer the management strategies based on leaving both standing dead trees and lying deadwood in the forest. This study reinforces existing literature on deadwood’s importance for biodiversity and underscores the need for informed policies that balance ecological values with practical management considerations.
1. Introduction
1.1. The Ecological Significance and Functions of Deadwood in Forest Ecosystems
In recent decades, the scientific community has increasingly recognized the diverse roles of deadwood in forest ecosystems. Deadwood is defined as all woody materials in forests, such as stems, branches, twigs, roots, coarse woody debris, and stumps left after clear-cutting [1,2]. Deadwood includes standing or ground dead trees, branches, and wood fragments of natural origin or resulting from forest management interventions. The quantity and decomposition rate of deadwood are influenced by intrinsic factors like tree species and extrinsic factors such as climate, site conditions, and disturbances [2]. Deadwood plays a crucial role in fundamental functions within forest ecosystems. Firstly, it is considered essential for biodiversity conservation [1,3]. Moreover, deadwood serves as a vital resource for numerous plant and animal species by providing food, building materials, and habitats for refuge and reproduction [4]. This is especially true for saproxylic organisms [5,6], as well as relict, rare, and protected species [7] and various threatened species [8]. Deadwood is also involved in nutrient and carbon cycling, natural regeneration, and geomorphological and soil hydrological processes [9,10]. It releases nutrients into the soil, acting as a natural fertilizer [11]. Additionally, deadwood serves as a significant carbon reservoir, contributing to climate change mitigation, as emphasized by several authors [12,13]. It can also be considered an indicator of naturalness in forests [14], as managed forests typically have smaller amounts of deadwood compared to those left to self-development [15]. Consequently, deadwood is increasingly recognized as a crucial component in forest ecosystem functioning [16] and is considered integral to sustainable forest management [17].
1.2. From Waste to Resource: The Bioenergy Potential of Deadwood and Its Ecological Implications
Deadwood, once considered a waste or by-product, is now recognized as an important component of both renewable energy production and biodiversity conservation [18,19]. The extraction of forest biomass for bioenergy serves as an important economic activity and contributes to the reduction in greenhouse gas (GHG) emissions compared to fossil fuels [20,21,22]. In addition, the production of bioenergy from forests provides economic benefits at the local level by supplying district heating plants [23]. However, increasing demand for bioenergy poses potential conflicts with biodiversity conservation efforts. The removal of deadwood, which is important for maintaining species habitats and forest resilience, can lead to biodiversity loss and undermine the ecosystem services that deadwood provides, such as temporary carbon storage [24,25,26,27,28].
The literature addresses the tension between the use of deadwood for bioenergy and its ecological importance. For example, Russell et al. [26] investigated the biomass and carbon attributes associated with deadwood, while Schuck et al. [3] explored its role in biodiversity conservation. Venier et al. [29] proposed models to effectively integrate deadwood into forest management, emphasizing its essential role in biodiversity conservation. Other researchers, such as Hiron et al. [21] and Giuntoli et al. [30], highlighted the negative impacts of extensive deadwood harvesting on wildlife habitats and the importance of sustainable practices to mitigate these impacts. Repo et al. [31] analyzed different deadwood management solutions and their potential impacts on biodiversity conservation and climate change mitigation in forests.
Despite these environmental concerns, deadwood also has a role to play in supporting the bioeconomy. The European Green Deal emphasizes the restoration of biodiversity while promoting sustainable forest bioenergy practices. Managed forests contribute more effectively to climate change mitigation through their ability to sequester carbon and replace fossil fuels than unmanaged forests, where the decomposition of deadwood releases stored carbon [32]. Coniferous species in managed forests have been found to provide greater climate change benefits than broadleaved species [33]. With the demand for bioenergy expected to triple by 2050, it is crucial to develop guidelines for sustainable biomass harvesting that minimize biodiversity loss, particularly for species dependent on deadwood habitats [27,28,29].
In examining the role of deadwood, it is also vital to consider the perceptions of various stakeholder groups. Research has highlighted the importance of understanding these perspectives to inform management strategies. Chambers et al. [34] highlighted the role of dead trees in Central Amazon in the carbon cycle based on the decomposition rate and mortality, while Palace et al. [35] emphasized the importance of the mass of fallen and standing necromass in undisturbed forests in Brazil. In addition, several studies have examined perceptions of deadwood from the perspective of various stakeholder groups. Many of these studies have focused on the opinions of forest visitors, including research by Pelyukh et al. [36] in Ukraine, Kovács et al. [37] in Japan, Rathmann et al. [38] in Germany, as well as Pastorella et al. [39] and Paletto et al. [40] in Italy. Only a few studies have explored the perspectives of other stakeholder categories. For instance, Deuffic and Lyser [19] investigated the opinions and attitudes of French foresters regarding biodiversity, particularly in relation to deadwood conservation. Additionally, Mutz et al. [41] examined the perception of deadwood in streams and rivers among German students with expertise in water management. Their study revealed a positive attitude towards deadwood among these young individuals, who are prospective natural resource managers.
Despite the increasing recognition of the ecological importance of deadwood in forest ecosystems, research investigating the perceptions of younger generations in different cultural contexts remains remarkably scarce. This study aims to fill this gap by exploring university students’ knowledge, opinions, and experiences of the multiple roles of deadwood, particularly in relation to biodiversity conservation, climate change mitigation, and bioenergy production. Using a comparative analytical framework, the research is conducted in three different countries: Iran, Italy, and Türkiye. An online questionnaire serves as the primary data collection tool, allowing for a systematic exploration of students’ perspectives. Specifically, this study seeks to address two key research questions:
(Q1) How does geographical context shape university students’ attitudes toward deadwood in forest ecosystems?
(Q2) What are the perceived trade-offs and synergies associated with the role of deadwood as understood by younger generations?
By adopting a bottom–up approach, this research not only contributes to the existing literature on the ecological functions of deadwood but also highlights the importance of integrating the perspectives of younger generations into forest management strategies. Understanding the ecological awareness of these emerging stakeholders is crucial for developing culturally sensitive communication and education initiatives that promote sustainable forest management.
2. Materials and Methods
This study employed a bottom–up approach, utilizing a questionnaire survey, and was structured in three key phases: (i) development and pre-testing of a structured questionnaire; (ii) sampling and online administration of the questionnaire; (iii) data collection, statistical analysis, and comparative evaluation.
2.1. Questionnaire Construction
The online survey method was chosen primarily for its effectiveness in reaching geographically dispersed respondents. This allowed students from Italy, Türkiye, and Iran to easily access the questionnaire. Furthermore, young respondents, like those in this study, are generally more familiar with and inclined to use online tools [42]. Additionally, the online survey method offers efficiency and cost-effectiveness, allowing for large data collection with minimal resources and in a short time frame [43].
Research teams from Italy, Türkiye, and Iran first developed the questionnaire in English and then translated it into Turkish, Persian, and Italian to ensure accessibility for participants. According to Chen et al. [44], a pre-test phase was conducted to ensure the accuracy and appropriateness of the questionnaire across all three countries. In each country, four university students completed the questionnaire in their respective languages and provided feedback on any difficulties or uncertainties. Based on the pre-test feedback, two questions from the preliminary version were revised to improve clarity and relevance.
During the pre-test phase, respondents completed the questionnaire on a tablet under the supervision of project staff. Researchers recorded any doubts or issues encountered by the respondents [45]. To enhance respondent willingness, the final questionnaire was kept concise with only six closed-ended questions, designed to be completed in about three minutes. All three versions of the questionnaire (in Italian, Persian, and Turkish) used the same set of questions to facilitate the comparison of responses.
The first three questions explored students’ perceptions and opinions regarding the role of deadwood in forests and its management, particularly in addressing the trade-offs between climate change mitigation and bioenergy production.
The first question (Q1) focused on students’ opinions concerning the effect of deadwood in forests. Respondents were presented with six positive and two negative effects associated with the presence of deadwood. They were asked to evaluate the importance of each effect using a 5-point Likert scale, ranging from 0 (not important) to 5 (very important). The Likert scale is a rating scale used as a measurement tool in social sciences research in order to understand personal opinions [46]. The effects presented to the students were as follows: (1) bioenergy production (firewood, woodchips); (2) microhabitat for wildlife (shelter); (3) food for wildlife (nourishment); (4) soil protection from water erosion and landslides; (5) soil fertilization due to deadwood decomposition; (6) climate change mitigation through the temporary carbon storage; (7) increase in harmful insects in the forest; (8) increased risk of forest fires. An ‘Other’ option was included to allow respondents to mention any effects not listed in the provided alternatives. Among the various options, numbers 7 (increase in pests’ disease) and 8 (increased risk of fire) are the negative effects that can be produced by the presence of deadwood in the forest.
The second question (Q2) investigated students’ opinions on the possibility of using deadwood for bioenergy production. Respondents could choose from the following options: (a) Yes, always. It is a good way to economically enhance an otherwise worthless component of the forest; (b) Yes, but only when the technical conditions for getting trunks out of the forest allow it to be conducted easily; (c) No, never because dead trees serve to maintain an ecological balance in the forest.
The third question (Q3) of the section investigated students’ opinions on deadwood management strategies (Table 1). Respondents were asked to rate the effectiveness of four potential strategies for enhancing the ecosystem services provided by deadwood in forests. The effectiveness of each strategy was evaluated using a 5-point Likert scale, ranging from 0 (not efficient) to 5 (very efficient).

Table 1.
Description of deadwood management strategies considered in the survey.
The final three questions (Q4, Q5, and Q6) of the questionnaire collected personal information from respondents, including gender, age (categorized into three groups: under 25 years old, 25–34 years old, and over 35 years old), and membership in environmental associations. This variable was included with the assumption that members of environmental associations might possess greater knowledge of environmental issues and more information about the role of deadwood in forests compared to non-members.
2.2. Sampling and Administration of Questionnaire
The questionnaire was spread using a snowball sampling method to expand the participant pool and achieve a larger sample size. The snowball sampling method is a non-probability sampling method based on referrals from initial subjects to generate additional subjects [48]. The questionnaire was distributed to Italian students from October to December 2020, to Turkish students from May to July 2021, and to Iranian students from December 2022 to February 2023. A dissemination period of three months was established in each country during the academic year.
Concerning the sample, in Italy, students from the University of Florence and Trento were first involved, enrolled in “Engineering Sciences” and “Economic and Management Sciences”. In Türkiye, students from Istanbul University-Cerrahpaşa enrolled in “Landscape Architecture”, “Architecture and Urban Planning”, and “Social Sciences”. In Iran, students from the University of Mohaghegh Ardabili enrolled in “Landscape Architecture and Forestry Related Sciences” and “Social Sciences”.
After the initial sampling, the distribution was extended using the snowball method. The survey link was shared with personal contacts and posted on social media platforms such as Facebook, Instagram, and Twitter. At the end of the dissemination period, a convenience sample of 1207 students from Italy, Türkiye, and Iran was collected.
2.3. Data Collection and Statistical Processing
Data collected from the questionnaire were stored in a database and analyzed using XLStat 2020 software to generate key descriptive statistics.
For Q1 and Q3, students used a five-point Likert scale to assess the importance of different effects of deadwood in forests and to rate the effectiveness of potential management deadwood strategies. The symmetric Likert scale allowed respondents to choose responses freely in either direction, providing a balanced range of options.
Data from the Likert scale were analyzed to produce descriptive statistics, including mean, median, and standard deviation. For the other questions, frequency distribution percentages (%) were calculated.
Data from the Likert scale were compared using Kruskal–Wallis non-parametric tests to evaluate how respondents’ country of origin (Italy, Iran, Türkiye) influenced their answers. The Kruskal–Wallis test (α = 0.01) is a non-parametric method used to assess differences among three or more independent groups on a single non-normally distributed continuous variable [49].
The Chi-square (χ2) test was applied to investigate students’ opinions on using deadwood for bioenergy production. This test examined the association between the three countries regarding deadwood utilization. The level of significance was set at p < 0.01. The null hypothesis posited that there were no differences among the three countries regarding the use of deadwood, whereas the alternative hypothesis suggested that differences did exist.
Finally, the Principal Component Analysis (PCA) was used to identify key groups of students based on their perceptions of deadwood functions in forests and their preferred management strategies. The PCA method, originally developed by Pearson and later adapted for principal component analysis by Hotelling [50], is a mathematical procedure that transforms several correlated variables (e.g., forest features) into a set of uncorrelated variables known as principal components (e.g., groups of people). The PCA is a procedure suitable for the analysis of quantitative variables, but in some cases, it can be applied to data collected using a Likert scale format, as was conducted in the present study.
3. Results
3.1. Description of the Student Sample
The 1207 university students who completed the survey were distributed as follows in the three countries: 512 respondents from Iran (42.4% of total respondents), 210 from Italy (17.4%), and 485 from Türkiye (40.2%).
Regarding gender, the sample comprised 61.0% females and 39.0% males. Age distribution was as follows: 59.1% of respondents were under 25 years old, 31.0% were between 25 and 34 years old, and 9.9% were over 34 years old. Additionally, 17.0% of respondents were members of environmental associations, while 83.0% were not.
The main characteristics of the respondents by country are summarized in Table 2.

Table 2.
Characteristics of the sample of students involved in the survey by country (% distribution).
3.2. Perceived Importance of Deadwood in Forests
According to students’ opinions, the three most important effects of deadwood in forests were the provision of microhabitats for wildlife (mean value of 3.28 ± 0.91), soil fertilization (3.22 ± 0.97), and soil protection (2.88 ± 1.11). Conversely, the two negative effects were considered less important by the sample of respondents: increased risk of forest fires (2.92 ± 1.13) and increased risk of pests (2.60 ± 1.14).
Observing the data by country, the results revealed that for Turkish and Italian respondents, the most important effect related to the presence of deadwood in forests was soil fertilization, followed by the provision of microhabitats and food for wildlife. Conversely, Iranian respondents prioritized the provision of microhabitats for wildlife, followed by bioenergy production and soil fertilization. Additionally, it is noteworthy that for respondents from all three countries, the main negative effect of deadwood is the increased risk of forest fires, albeit with statistically significant differences between countries. In fact, the non-parametric Kruskal–Wallis test (α = 0.01) highlighted statistically significant differences among the three countries for all positive and negative effects related to the presence of deadwood in forests.
The results about the perceived importance of the effects of deadwood in forests by country are shown in Table 3.

Table 3.
Perceived importance of the effects of deadwood in forests (mean ± SD) by country.
Focusing on the role of deadwood in bioenergy production, the results indicated that in all three countries, the majority of respondents preferred to remove deadwood only when it is technically feasible to extract the trunks from the forest. Specifically, 54.8% of respondents in Italy, 51.1% in Türkiye, and 42.6% in Iran supported this approach (Table 4). However, the results revealed notable differences in the second most preferred option: Iranian and Turkish respondents favored not removing deadwood from forests (34.0% and 29.5%, respectively), whereas Italian respondents preferred to always remove it, viewing it as a valuable opportunity to economically enhance an otherwise worthless component of the forest (26.7%). The results of the Chi-square (χ2) test showed significant differences among the respondents of the three countries regarding the use of deadwood (p < 0.0001).

Table 4.
Distribution of respondents (%) on the use of deadwood by country.
3.3. Preferred Deadwood Management Strategies
The results about the deadwood management strategies in forests highlighted that for most respondents, the preferred strategy is Strategy 1 (mean value of 2.72 ± 1.06), followed by Strategy 2 (2.39 ± 1.01) and Strategy 3 (2.36 ± 1.07). Observing the data by country, Turkish and Iranian respondents showed a similar preference order for the strategies, whereas Italian respondents valued Strategy 3 higher than Strategy 2 (Table 3). In addition, it is interesting to highlight that Iranian respondents assigned higher values to all four strategies compared to Turkish and Italian respondents. Among these, Strategy 4 received the lowest score from both Turkish and Iranian students.
The results of the non-parametric Kruskal–Wallis test (α = 0.01) revealed statistically significant differences among the three countries for all strategies, as shown in Table 5.

Table 5.
Distribution of respondents (%) on the deadwood management strategy by country.
The Principal Component Analysis (PCA) identified two main groups of respondents based on their values assigned to the functions of deadwood and preferences for deadwood management strategies (Figure 1). The first group consists of students who perceive deadwood negatively, assigning high values to the risks of forest fires and pests. This group supports the use of deadwood for bioenergy production and considers Strategy 3 or, alternatively, Strategy 4 as the most efficient management strategy. Conversely, the second group consists of students with a positive perception of deadwood in forests. They view Deadwood as beneficial and consider Strategy 1 to be the most effective. This group aligns with the “ecological” perspective, valuing the ecological roles of deadwood and supporting its retention in forests, except for large standing dead trees. The second group includes “conservationists”, who perceive deadwood primarily for the conservation of biodiversity and secondarily for other services such as climate change mitigation, soil protection, and fertilization.
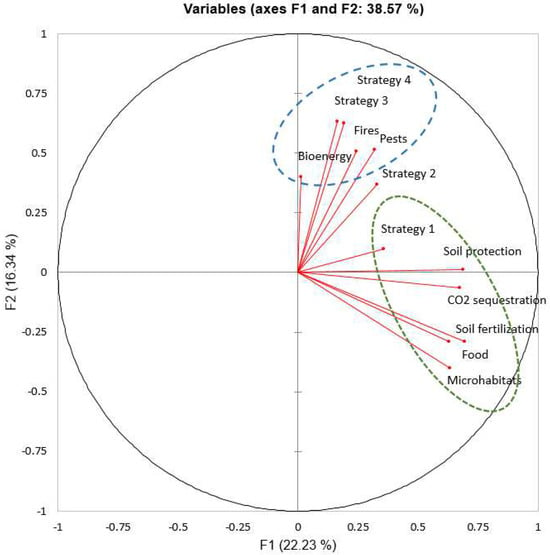
Figure 1.
The Principal Component Analysis (PCA) considers the perceived importance of the effects related to the presence of deadwood in forests and preferred deadwood management strategies.
4. Discussion
The results of the present study show that the new generations with a university level of education have an ecological awareness. In fact, our students view the provision of microhabitats for wildlife, soil fertilization, and soil protection as the primary benefits of deadwood in forests. Conversely, the increased risk of forest fires and pests is seen as less significant by the students. Regarding the perceived importance of deadwood for bioenergy production, our results show a difference between respondents from the three countries, presumably due to the importance of wood energy use. In this sense, Iranian students consider bioenergy production more important than other ecosystem services, such as soil protection and the provision of food for wildlife, compared to Turkish and Italian students. The ecological awareness of our respondents is confirmed by the preferred management strategy based on leaving the standing dead trees and lying deadwood in forests for ecological reasons.
This study contributes to research on Generation Z—individuals born between the late 1990s and early 2000s—and their relationship with the environment. Despite being the first Internet generation, there is limited research on Generation Z [51]. Other authors [52] show that Generation Z is more sensitive to environmental issues than previous generations. Studies focusing on their perceptions and preferences in natural resource management represent a novel and intriguing research area.
Our results confirm that as the importance of biodiversity in forest management increases, people are recognizing the role of deadwood as a key component. As Pelyukh et al. [36] have demonstrated, deadwood is progressively regarded as a vital element for enhancing forest biodiversity and supporting biodiversity conservation. This awareness is also becoming a common sentiment, particularly among professionals in natural resource management.
The students’ prior knowledge and sensitivity about deadwood, gained through university education, helps explain their perception of its crucial role in forest biodiversity. These findings align with Paletto et al. [47], which highlight young people’s awareness of deadwood’s importance for biodiversity conservation, especially in providing food and microhabitats for wildlife like saproxylic organisms.
Gundersen and Frivold [53] examined Swedish forestry students’ evaluations of managing forests with dead and wind-felled trees at three different times: 1978, 1980, and 1988. Results showed that while most respondents recommended removing wind-felled trees in 1978 and 1980, by 1988, a majority supported retaining these trees. These results are in line with the present research, highlighting the growing recognition of the biological functions of deadwood over time.
The importance of soil fertilization and protection linked to deadwood is consistent with Merganičová et al. [2] and Kraigher et al. [54], who highlight deadwood’s role in improving soil stability and reducing erosion and runoff.
In our study, the role of deadwood in bioenergy production was emphasized only by some students, especially Iranian ones. However, it is important to highlight that wood is a sustainable and ecological material for the building industry with positive impacts on the climate thanks to long-term carbon storage [55]. Furthermore, all residues from the wood industry, in addition to forest biomass, can be valorized for bioenergy production. The use of forest biomass to produce bioenergy is part of a logic of joint valorization of both high-added-value products and low-added-value products. This strategy is of key importance, especially in countries with low Gross Domestic Product (GDP) and rural areas.
Country-specific data reveal that Turkish and Italian respondents consider soil fertilization the most important effect of deadwood. In contrast, Iranian students prioritize providing microhabitats for wildlife, followed by bioenergy production and soil fertilization. These results align with Paletto et al. [47], which shows differences in perceptions of deadwood functions among students from different countries. These variations are likely attributable to cultural factors and differing levels of environmental awareness. Those authors highlighted that Turkish university students have a more positive perception of deadwood compared to their Italian counterparts. Conversely, Italian students view managed forests—where the deadwood is removed during silvicultural treatments—more favorably [40,47].
Regarding deadwood management strategies, this study indicates that the most preferred approach is removing both standing dead trees and lying deadwood (Strategy 1). This strategy is typical of production forests, where significant attention is given to minimizing the risks of forest fires and insect pollution. Conversely, the least effective strategy, according to respondents, is creating deadwood islands or îlot de senescence (Strategy 4). Deadwood islands are small, permanently unmanaged patches intended to offer sustainable habitats—including microhabitat trees, lying deadwood, standing dead trees, and old stumps—for biodiversity within production forests [56]. Some studies have examined deadwood management strategies for biodiversity conservation without considering trade-offs with other ecosystem services like bioenergy production and climate mitigation. In a recent study aimed at identifying factors associated with management practices that increase deadwood amounts, Bače and Svoboda [57] found that the most effective practice is to retain live trees or, alternatively, to retain standing dead trees. Conversely, the authors emphasized that other practices—such as retaining felled-lying logs, wind-thrown trees, high tree stumps, and targeted tree killing—are less effective when implemented individually [58,59]. It is also important to note that living tree species diversity is necessary for deadwood diversity, and current forest management practices affect the ecological conditions that influence deadwood [60].
This research shows that the public prioritizes both biodiversity conservation efforts and the importance of deadwood. Given that the study sample consists of university students, it seems the message about aligning deadwood retention with intrinsic values and ethical obligations for forest biodiversity has been effectively communicated [61]. Indeed, recognizing the importance of deadwood and its dependent organisms (e.g., saproxylic species) is complex, and this diversity is not always seen as positive [62,63]. Thus, public information is essential for shifting and deepening perceptions of deadwood. Equally, it is crucial to incorporate information about the ecological functions of deadwood into broader public relations strategies for managing forest biodiversity.
These results contrast with other research where participants either lack awareness or deliberately overlook the ecological link between deadwood and forest biodiversity despite scientific evidence [28,64].
5. Conclusions
The significance of studies like the present, which explore preferences and opinions regarding various forest ecosystem services, lies in their contribution to optimizing forest management. Such research helps balance forest ecosystem services by identifying potential trade-offs. Therefore, it is emphasized that such studies can be a valuable asset in the planning of multiple-use forest management. They provide a robust combination of consistency, validity, and legitimacy, particularly when different methodologies yield similar results.
From a methodological point of view, the main weakness of this study is that the sample of students (snowball samples) is biased and cannot provide the basis for valid generalizations to the population (i.e., university and post-university students of three countries). A second weakness of this study is that it considered only a segment of society, such as university and post-university students. Therefore, our results should be considered as a preliminary overview of a larger study involving more students to be selected in a stratified manner by country and faculty, as well as other segments of society such as adults and the elderly.
Finally, it is important to recognize that preferences and opinions are fundamentally shaped by rational cognitive processes and individual subjective characteristics, making them challenging to categorize. However, education and information—such as those influencing the student sample in this study—play a crucial role in shaping forest management measures.
Author Contributions
Conceptualization, I.D.M., A.P. and C.S.; methodology, I.D.M., A.P. and C.S.; software, A.P.; investigation, A.P., S.B. and K.S.; data curation, I.D.M. and A.P.; writing—original draft preparation I.D.M., A.P. and C.S.; writing—review and editing S.B. and K.S.; supervision, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Löfroth, T.; Birkemoe, T.; Shorohova, E.; Dynesius, M.; Fenton, N.J.; Drapeau, P.; Tremblay, J.A. Deadwood Biodiversity, Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Svoboda, M.; Bače, R.; Šebeň, V. Deadwood in forest ecosystems. In Forest Ecosystems—More than Just Trees; Blanco, J.A., Lo, Y.H., Eds.; InTech: Rijeka, Croatia, 2012; pp. 81–108. [Google Scholar]
- Schuck, A.; Meyer, P.; Menke, N.; Lier, M.; Lindner, M. Forest Biodiversity Indicator: Dead Wood—A Proposed Approach towards Operationalising the MCPFE Indicator. In EFI Proceedings Monitoring and Indicators of Forest Biodiversity in Europe—From Ideas to Operationality; Marchetti, M., Ed.; European Forest Institute: Joensuu, Finland, 2004; pp. 49–77. [Google Scholar]
- Bütler Sauvain, R. Dead Wood in Managed Forests: How Much and How Much Is Enough? Development of a Snag Quantification Method by Remote Sensing & GIS and Snag Targets Based on Three-Toed Woodpeckers’ Habitat Requirements; EPFL: Lausanne, Switzerland, 2003. [Google Scholar]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Mason, F.; Zapponi, L. The forest biodiversity artery: Towards forest management for saproxylic conservation. iForest 2016, 9, 205–216. [Google Scholar] [CrossRef]
- Radu, S. The ecological role of deadwood in natural forests. In Nature Conservation. Concepts and Practice; Gafta, D., Akeroyd, J., Eds.; Springer Link: Berlin/Heidelberg, Germany, 2004; pp. 137–141. [Google Scholar]
- Ranius, T.; Kindvall, O.; Kruys, N.; Jonsson, B.G. Modelling dead wood in Norway spruce stands subject to different management regimes. For. Ecol. Manag. 2003, 182, 13–29. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E.; Piaszczyk, W.; Wiecheć, M. How the deadwood of different tree species in various stages of decomposition affected nutrient dynamics? J. Soils Sediments 2018, 18, 2759–2769. [Google Scholar] [CrossRef]
- Herrmann, S.; Bauhus, J. Nutrient retention and release in coarse woody debris of three important central European tree species and the use of NIRS to determine deadwood chemical properties. For. Ecosyst. 2018, 5, 22. [Google Scholar] [CrossRef]
- Holub, S.M.; Spears, J.D.H.; Lajtha, K. A reanalysis of nutrient dynamics in coniferous coarse woody debris. Can. J. For. Res. 2001, 31, 1894–1902. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Annual carbon flux from woody debris for a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, 8220. [Google Scholar] [CrossRef]
- De Meo, I.; Agnelli, A.E.; Graziani, A.; Kitikidou, K.; Lagomarsino, A.; Milios, E.; Radoglou, K.; Paletto, A. Deadwood volume assessment in Calabrian pine (Pinus brutia Ten.) peri-urban forests: Comparison between two sampling methods. J. Sust. For. 2017, 36, 666–686. [Google Scholar] [CrossRef]
- Winter, S.; Fischer, H.S.; Fischer, A. Relative Quantitative Reference Approach for Naturalness Assessments of forests. For. Ecol. Manag. 2010, 259, 1624–1632. [Google Scholar] [CrossRef]
- Rondeux, J.; Sanchez, C. Review of indicators and field methods for monitoring biodiversity within national forest inventories. Core variable: Deadwood. Environ. Monit. Assess. 2010, 164, 617–630. [Google Scholar] [CrossRef]
- Vandekerkhove, K.; De Keersmaeker, L.; Menke, N.; Meyer, P.; Verschelde, P. When nature takes over from man: Dead wood accumulation in previously managed oak and beech woodlands in North-western and Central Europe. For. Ecol. Manag. 2009, 258, 425–435. [Google Scholar] [CrossRef]
- MCPFE. Ministerial Conference on the Protection of Forests in Europe. Background information. In Proceedings of the MCPFE Expert Level Meeting, Vienna, Austria, 10–11 June 2002. [Google Scholar]
- Verkerk, P.J.; Lindner, M.; Zanchi, G.; Zudin, S. Assessing impacts of intensified biomass removal on deadwood in European forests. Ecol. Indic. 2011, 11, 27–35. [Google Scholar] [CrossRef]
- Deuffic, P.; Lyser, S. Biodiversity or bioenergy: Is deadwood conservation an environmental issue for French forest owners? Can. J. For. Res. 2012, 42, 1491–1502. [Google Scholar] [CrossRef]
- Dahlberg, A.; Thor, G.; Allmér, J.; Jonsell, M.; Jonsson, M.; Ranius, T. Modelled impact of Norway spruce logging residue extraction on biodiversity in Sweden. Can. J. For. Res. 2011, 41, 1220–1232. [Google Scholar] [CrossRef]
- Hiron, M.; Jonsell, M.; Kubart, A.; Thor, G.; Schroeder, M.; Dahlberg, A.; Johansson, V.; Ranius, T. Consequences of bioenergy wood extraction for landscape-level availability of habitat for dead wood-dependent organisms. J. Environ. Manag. 2017, 198, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cowie, A.L.; Berndes, G.; Bentsen, N.S.; Brandão, M.; Cherubini, F.; Egnell, G.; George, B.; Gustavsson, L.; Hanewinkel, M.; Harris, Z.M.; et al. Applying a science-based systems perspective to dispel misconceptions about climate effects of forest bioenergy. GCB Bioenergy 2021, 13, 1210–1231. [Google Scholar] [CrossRef]
- Lindblom, P.G.; Rasmussen, R.O. Bioenergy and Regional Development in the Nordic Countries; Nordregio Working Paper; Nordregio: Stockholm, Sweden, 2008; Volume 5, 96p. [Google Scholar]
- Chisika, S.N.; Park, J.; Yeom, C. Paradox of deadwood circular bioeconomy in Kenya’s public forests. Sustainability 2021, 13, 7051. [Google Scholar] [CrossRef]
- Johansson, V.; Felton, A.; Ranius, T. Long-term landscape scale effects of bioenergy extraction on dead wood-dependent species. For. Ecol. Manag. 2016, 371, 103–113. [Google Scholar] [CrossRef]
- Russell, M.B.; Fraver, S.; Aakala, T.; Gove, J.H.; Woodall, C.W.; D’Amato, A.W.; Ducey, M.J. Quantifying carbon stores and decomposition in dead wood: A review. For. Ecol. Manag. 2015, 350, 107–128. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Sippola, A.-L.; Lempérière, G.; Dodelin, B.; Alexander, K.N.A.; Butler, J.E. Deadwood as an Indicator of Biodiversity in European Forests: From Theory to Operational Guidance. In EFI Proceedings Monitoring and Indicators of Forest Biodiversity in Europe—From Ideas to Operationality; Marchetti, M., Ed.; European Forest Institute: Joensuu, Finland, 2004; pp. 193–206. [Google Scholar]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Venier, L.A.; Hébert, C.; De Grandpré, L.; Arsenault, A.; Walton, R.; Morris, D.M. Modelling deadwood supply for biodiversity conservation: Considerations, challenges and recommendations. For. Chron. 2015, 91, 407–416. [Google Scholar] [CrossRef]
- Giuntoli, J.; Barredo, J.I.; Avitabile, V.; Camia, A.; Cazzaniga, N.E.; Grassi, G.; Jasinevičius, G.; Jonsson, R.; Marelli, L.; Robert, N.; et al. The quest for sustainable forest bioenergy: Win-win solutions for climate and biodiversity. Renew. Sustain. Energy Rev. 2022, 159, 112180. [Google Scholar] [CrossRef]
- Repo, A.; Eyvindson, K.; Halme, P.; Mönkkönen, M. Forest bioenergy harvesting changes carbon balance and risks biodiversity in boreal forest landscapes. Can. J. For. Res. 2020, 50, 1184–1193. [Google Scholar] [CrossRef]
- Schulze, E.D.; Sierra, C.A.; Egenolf, V.; Woerdehoff, R.; Irslinger, R.; Baldamus, C.; Stupak, I.; Spellmann, H. The climate change mitigation effect of bioenergy from sustainably managed forests in Central Europe. GCB Bioenergy 2020, 12, 186–197. [Google Scholar] [CrossRef]
- Schulze, E.D.; Stupak, I.; Hessenmöller, D. The climate mitigation potential of managed versus unmanaged spruce and beech forests in Central Europe. In Bioenergy with Carbon Capture and Storage; Academic Press: Cambridge, MA, USA, 2019; pp. 131–149. [Google Scholar]
- Chambers, J.Q.; Higuchi, N.; Schimel, J.P.; Ferreira, L.V.; Melack, J.M. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia 2000, 122, 380–388. [Google Scholar] [CrossRef]
- Palace, M.; Keller, M.; Asner, G.P.; Silva, J.N.M.; Passos, C. Necromass in undisturbed and logged forests in the Brazilian Amazon. For. Ecol. Manag. 2007, 238, 309–318. [Google Scholar] [CrossRef]
- Pelyukh, O.; Paletto, A.; Zahvoyska, L. People’s attitudes towards deadwood in forest: Evidence from the Ukrainian Carpathians. J. For. Sci. 2019, 65, 171–182. [Google Scholar] [CrossRef]
- Kovács, B.; Uchiyama, Y.; Miyake, Y.; Penker, M.; Kohsaka, R. An explorative analysis of landscape value perceptions of naturally dead and cut wood: A case study of visitors to Kaisho Forest, Aichi, Japan. J. For. Res. 2020, 25, 291–298. [Google Scholar] [CrossRef]
- Rathmann, J.; Sacher, P.; Volkmann, N.; Mayer, M. Using the visitor-employed photography method to analyse deadwood perceptions of forest visitors: A case study from Bavarian Forest National Park, Germany. Eur. J. For. Res. 2020, 139, 431–442. [Google Scholar] [CrossRef]
- Pastorella, F.; Avdagić, A.; Čabaravdić, A.; Mraković, A.; Osmanović, M.; Paletto, A. Tourists’ perception of deadwood in mountain forests. Ann. For. Res. 2016, 59, 311–326. [Google Scholar] [CrossRef]
- Paletto, A.; Becagli, C.; De Meo, I. Aesthetic preferences for deadwood in forest landscape: A case study in Italy. J. Environ. Manag. 2022, 311, 114829. [Google Scholar] [CrossRef] [PubMed]
- Mutz, M.; Piégay, H.; Gregory, K.J.; Borchardt, D.; Reich, M.; Schmieder, K. Perception and evaluation of dead wood in streams and rivers by German students. Limnologica 2006, 36, 110–118. [Google Scholar] [CrossRef]
- Reips, U.D. Standards for Internet-based experimenting. Exp. Psychol. 2002, 49, 243. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.B. Researching Internet-based populations: Advantages and disadvantages of online survey research, online questionnaire authoring software packages, and web survey services. J. Comput. Mediat. Commun. 2005, 10, 1083–1091. [Google Scholar] [CrossRef]
- Chen, D.-C.; Hou, J.-C.; Zheng, Q.-D. The Empirical Research on the Impact of Applying VR Technology to Students’ Skill Learning in Machining Processing Courses on Questionnaire Evaluation. In Innovative Technologies and Learning; Cheng, Y.-P., Pedaste, M., Bardone, E., Huang, Y.-M., Eds.; Springer: Cham, Switzerland, 2024; pp. 97–107. [Google Scholar]
- Regmi, P.R.; Waithaka, E.; Paudyal, A.; Simkhada, P.; Van Teijlingen, E. Guide to the design and application of online questionnaire surveys. Nepal J. Epidemiol. 2016, 6, 640. [Google Scholar] [CrossRef]
- Likert, R.A. A technique for the measurement of attitudes. Arch. Psychol. 1932, 40, 5–53. [Google Scholar]
- Paletto, A.; Bayraktar, S.; Becagli, C.; De Meo, I. Young Generations’ Perception of the Role of Deadwood in Forests: Comparison between Italy and Türkiye. Ecologies 2023, 4, 426–441. [Google Scholar] [CrossRef]
- Parker, C.; Scott, S.; Geddes, A. Snowball Sampling. In SAGE Research Methods Foundations; Sage Publications Inc.: Thousand Oaks, CA, USA, 2019. [Google Scholar] [CrossRef]
- McKight, P.; Najab, J. Kruskal-Wallis test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Hotelling, H. Analysis of complex of statistical variables into principal component. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Berkup, S.B. Working with Generations X and Y in Generation Z period: Management of different generations in business life. Med. J. Soc. Sci. 2014, 5, 218–229. [Google Scholar] [CrossRef]
- Malikova, I. Perception of Global Issues of Environment and Circular Economy by Generation Z. In Proceedings of the 20th International Scientific Conference Globalization and its Socio-Economic Consequences, SHS Web of Conferences 92, Zilina, Slovakia, 21–22 October 2020; EDP Sciences: Les Ulis, France, 2021. [Google Scholar]
- Gundersen, V.S.; Frivold, L.H. Public preferences for forest structures: A review of quantitative surveys from Finland, Norway and Sweden. Urban For. Urban Green. 2008, 7, 241–258. [Google Scholar] [CrossRef]
- Kraigher, H.; Jurc, D.; Kalan, P.; Kutnar, L.; Levanic, T.; Rupel, M.; Smolej, I. Beech coarse woody debris characteristics in two virgin forest reserves in southern Slovenia. Wood Sci. Technol. 2002, 69, 91–134. [Google Scholar]
- Fadai, A.; Fuchs, M.; Winter, W. Wood-Based Construction for Multi-story Buildings: Application of Cement Bonded Wood Composites as Structural Element. In Materials and Joints in Timber Structures; Aicher, S., Reinhardt, H.-W., Garrecht, H., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 471–484. [Google Scholar]
- Lachat, T.; Bütler, R. Îlots de sénescence et arbres-habitat pour augmenter la biodiversité en forêt. La. Forêt. 2008, 6, 20–21. [Google Scholar]
- Bače, R.; Svoboda, M. Hodnocení Aspektů Managementu Mrtvého Dřeva v Hospodářských Lesích a Předběžný Návrh Doporučení. 2012. Available online: https://home.czu.cz/storage/507/74451_dwm_f3.2012.pdf (accessed on 15 May 2024).
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Bače, R.; Svoboda, M.; Vítková, L. Deadwood Management in Production Forests. In Management Guidelines for Forest Managers in Central European Temperate Forests; Department of Forest Ecology, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences Prague: Prague, Czech Republic, 2019. [Google Scholar]
- Greenbaum, A. Nature connoisseurship. Environ. Values 2005, 14, 389–407. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Kruys, N.; Ranius, T. Ecology of species living on deadwood-lessons for dead wood management. Silva Fenn. 2005, 39, 289–309. [Google Scholar] [CrossRef]
- Kellert, S.R. Values and perceptions of invertebrates. Biol. Conserv. 1993, 7, 845–855. [Google Scholar] [CrossRef]
- Doerfler, I.; Gossner, M.; Müller, J.; Seibold, S.; Weisser, W. Deadwood enrichment combining integrative and segregative conservation elements enhances biodiversity of multiple taxa in managed forests. Biol. Conserv. 2018, 228, 70–78. [Google Scholar] [CrossRef]
- Sacher, P.; Meyerhoff, J.; Mayer, M. Evidence of the association between deadwood and forest recreational site choices. For. Pol. Econ. 2022, 135, 102638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).