Fatty Acids as Phase Change Materials for Building Applications: Drawbacks and Future Developments
Abstract
1. Introduction
2. Energy Consumption
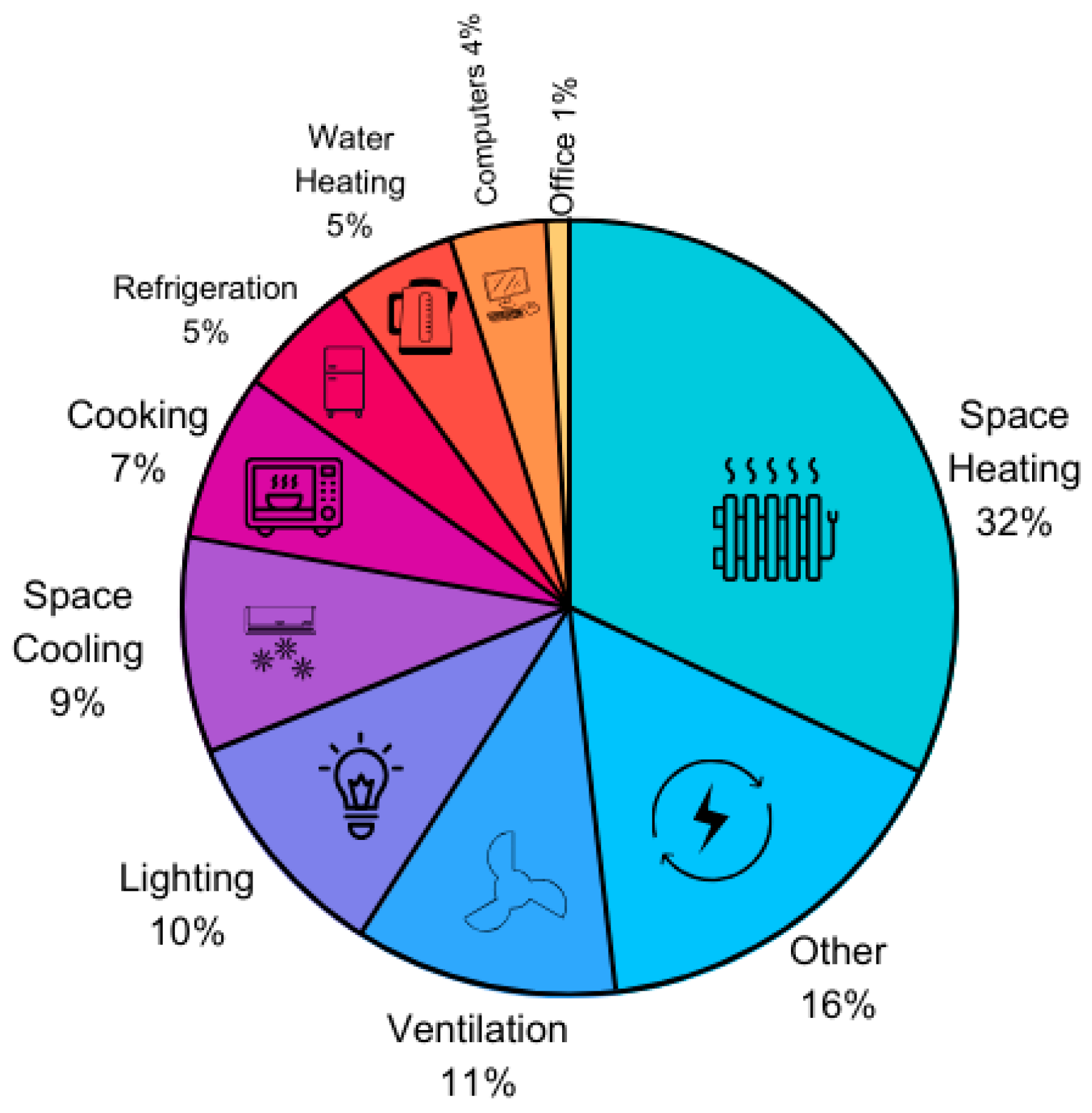
2.1. Building Energy Usage
2.2. PCMs for Building Applications
3. Organic PCMs
3.1. Types of Organic PCMs
3.1.1. Paraffins
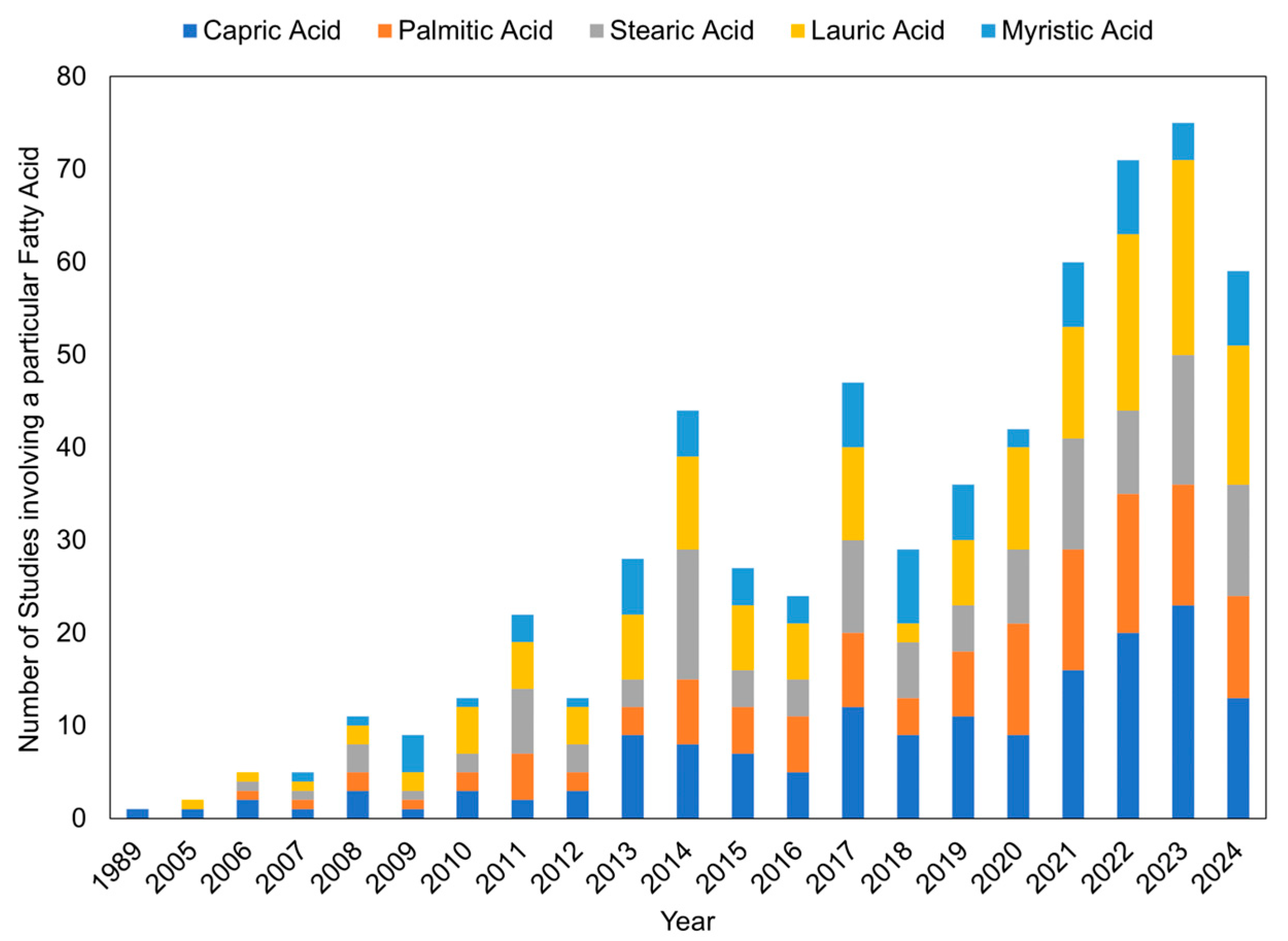
3.1.2. Fatty Acids
3.2. Sustainable Sources and Processes for Obtaining Fatty Acids
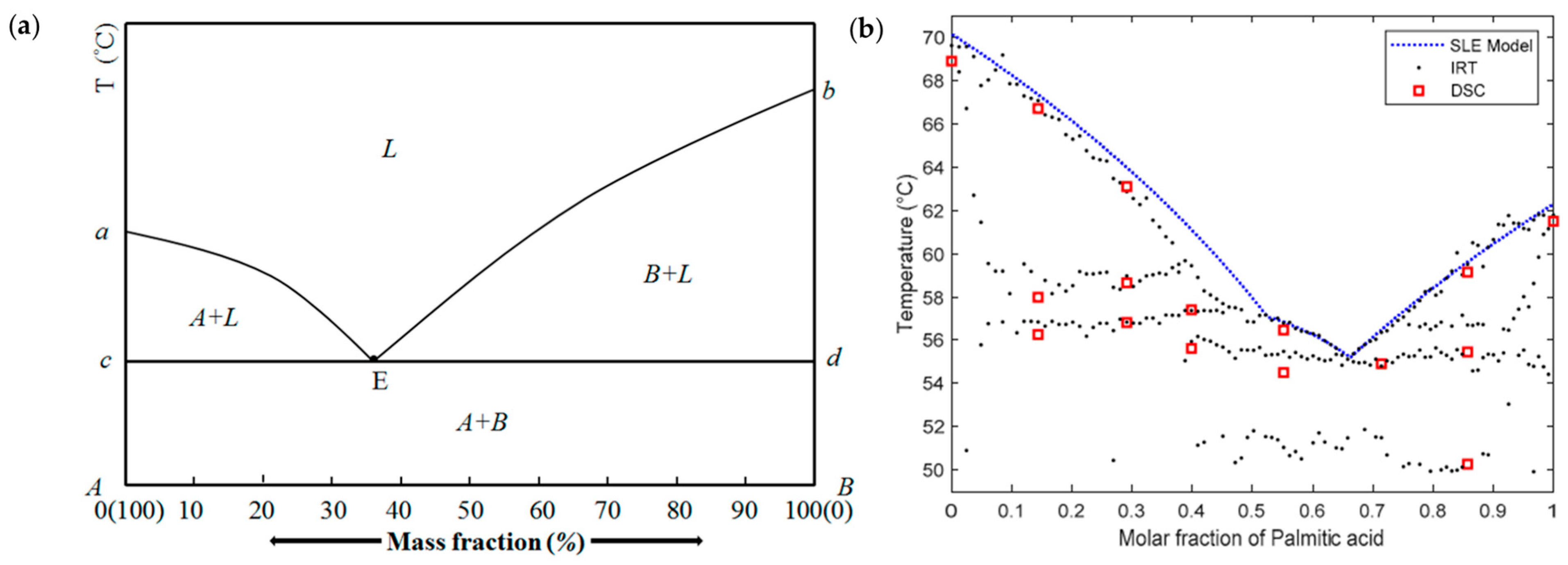
3.3. Fatty Acid Eutectic Mixtures
4. Properties of Fatty Acid-Based PCMs
4.1. Stability
4.2. Thermal Conductivity
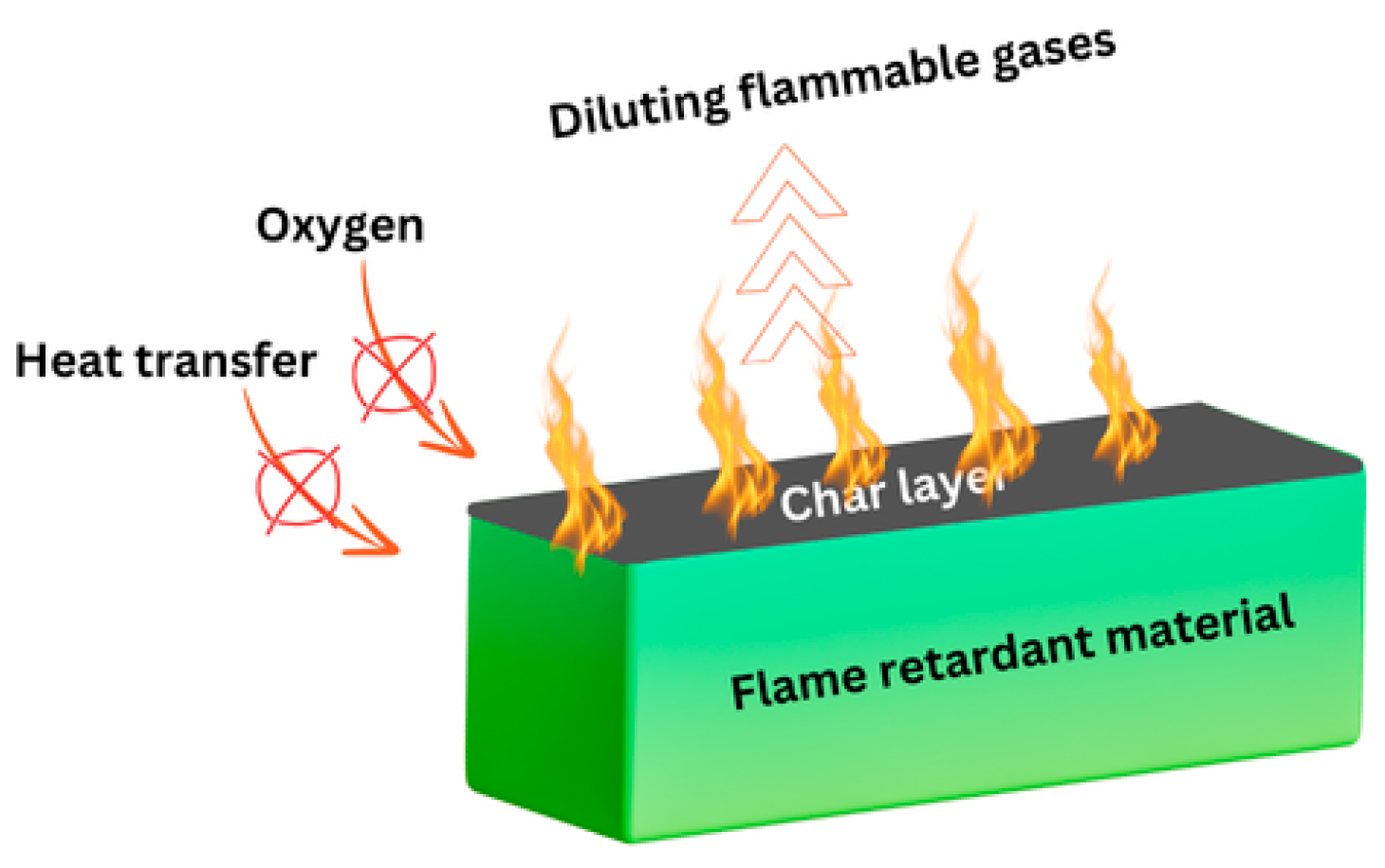
4.3. Flammability
4.4. Life Cycle Assessment
5. Techniques to Incorporate PCMs into Building Materials
5.1. Direct Incorporation
5.2. Immersion
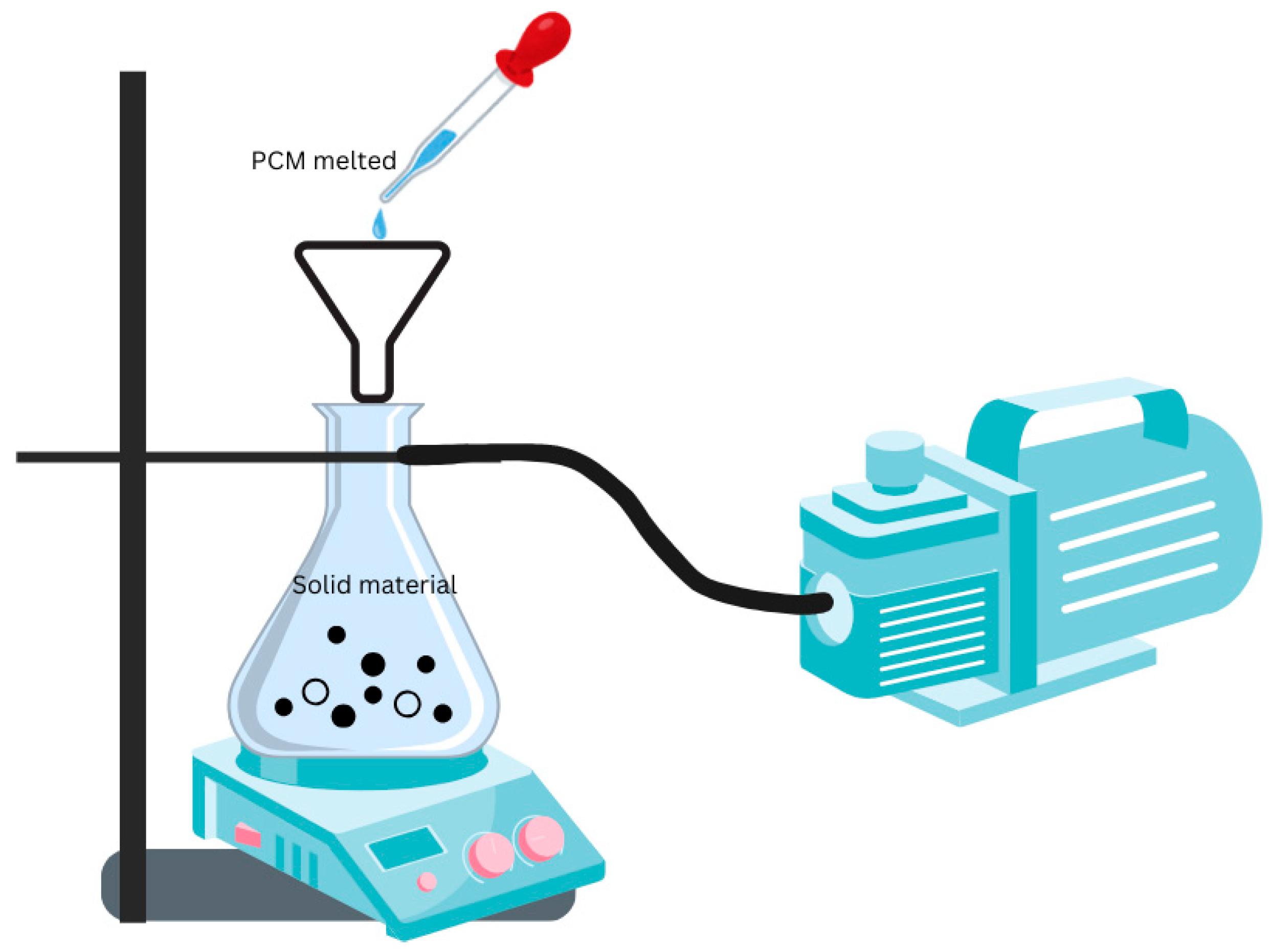
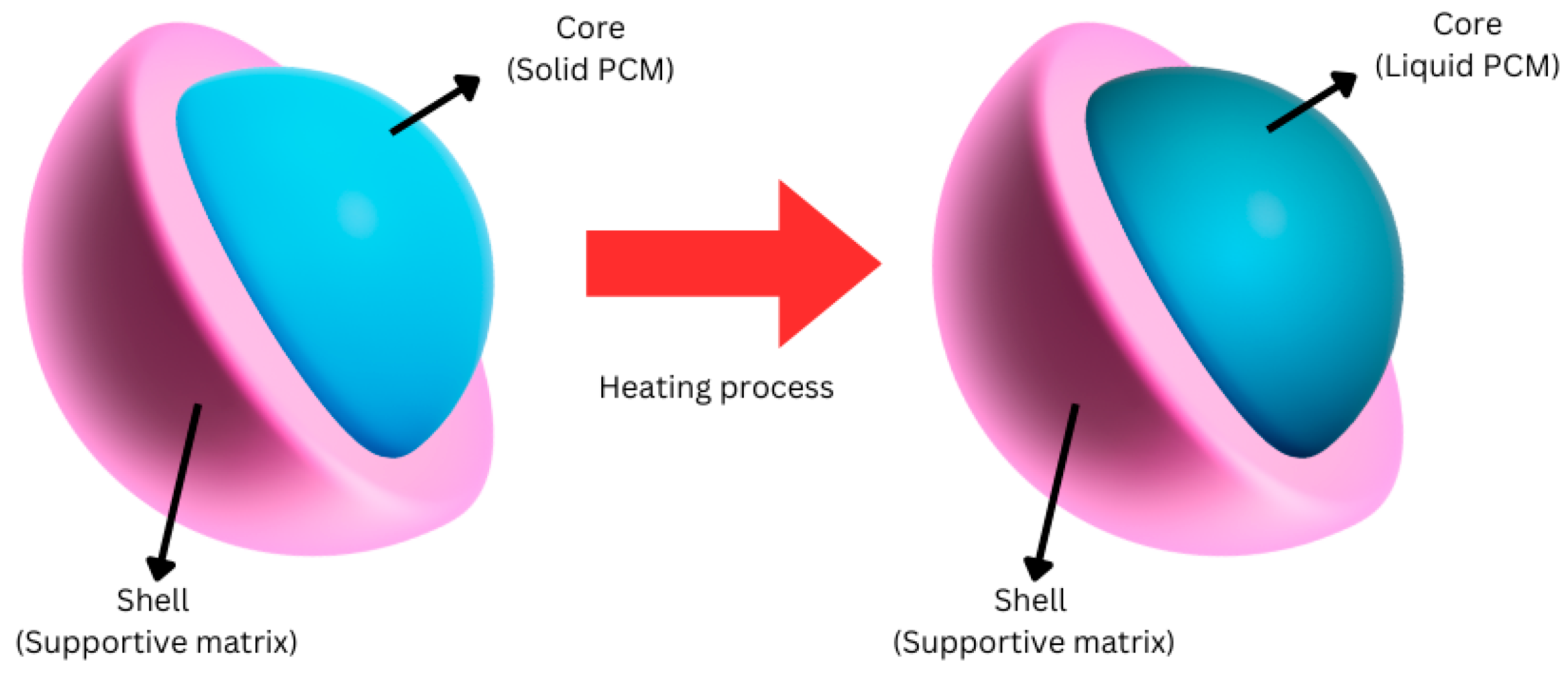
5.3. Encapsulation
5.4. Shape Stabilization
6. Perspectives on Future Research Directions
7. Conclusions
- -
- Obtaining pure fatty acids from more sustainable bio-sources remains a critical area of study, alongside LCAs aimed at assessing the sustainability of these novel technologies.
- -
- Incorporating novel additives has been shown to enhance the properties and performance of these composites. However, a deeper understanding of the synergistic effects on all relevant properties is still needed.
- -
- Reducing the flammability of fatty acid-based PCMs is especially important to mitigate fire risks in building construction materials. Additional research is crucial to fully harness the potential of fatty acid-based PCMs, advancing sustainable building practices and supporting global efforts in energy management. Future studies should focus on understanding the mechanisms of flame-retardancy compounds on pure and complex matrices of fatty acids, as well as on the evaluation of different incorporation techniques and their effect on the long-term performance of the novel PCMs.
Funding
Conflicts of Interest
References
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Muhd, M.Z. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- GaneshKumar, P.; Sivalingam, V.; Divya, S.; Oh, T.H.; Vigneswaran, V.S.; Velraj, R. Thermophysical exploration: State-of-the-art review on phase change materials for effective thermal management in lithium-ion battery systems. J. Energy Storage 2024, 87, 111412. [Google Scholar] [CrossRef]
- Taj, S.A.; Khalid, W.; Nazir, H.; Khan, A.; Sajid, M.; Waqas, A.; Hussain, A.; Ali, M.; Zaki, S.A. Experimental investigation of eutectic PCM incorporated clay brick for thermal management of building envelope. J. Energy Storage 2024, 84, 110838. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Marzhan, K.; Tastambek, K.T.; Sherelkhan, D.K.; Qiao, X. Microbial Co-processing and Beneficiation of Low-rank Coals for Clean Fuel Production: A Review. Eng. Sci. 2023, 25, 942. [Google Scholar] [CrossRef]
- Prieto, C.; Cabeza, L.F. Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl. Energy 2019, 254, 113646. [Google Scholar] [CrossRef]
- Yogev, R.; Kribus, A. Operation strategies and performance of solar thermal power plants operating from PCM storage. Solar Energy 2013, 95, 170–180. [Google Scholar] [CrossRef]
- Huang, L.; Piontek, U.; Zhuang, L.; Zheng, R.; Zou, D. Retrofitting of a solar cooling and heating plant by employing PCM storage and adjusting control strategy. Appl. Energy 2024, 368, 123462. [Google Scholar] [CrossRef]
- Uribe, D.; Vera, S.; Perino, M. Development and validation of a numerical heat transfer model for PCM glazing: Integration to EnergyPlus for office building energy performance applications. J. Energy Storage 2024, 91, 112121. [Google Scholar] [CrossRef]
- Pirasaci, T.; Sunol, A. Potential of phase change materials (PCM) for building thermal performance enhancement: PCM-composite aggregate application throughout Turkey. Energy 2024, 292, 130589. [Google Scholar] [CrossRef]
- Nicolalde, J.F.; Cabrera, M.; Martínez-Gómez, J.; Salazar, R.B.; Reyes, E. Selection of a phase change material for energy storage by multi-criteria decision method regarding the thermal comfort in a vehicle. J. Energy Storage 2022, 51, 104437. [Google Scholar] [CrossRef]
- Socaciu, L.; Giurgiu, O.; Banyai, D.; Simion, M. PCM Selection Using AHP Method to Maintain Thermal Comfort of the Vehicle Occupants. Energy Procedia 2016, 85, 489–497. [Google Scholar] [CrossRef]
- Nasimi, S.; Fakhroleslam, M.; Zarei, G.; Sadrameli, S.M. Passive energy-efficiency optimization in greenhouses using phase change materials; a comprehensive review. J. Energy Storage 2024, 90, 111762. [Google Scholar] [CrossRef]
- Guan, Y.; Meng, Q.; Ji, T.; Hu, W.; Li, W.; Liu, T. Experimental study of the thermal characteristics of a heat storage wall with micro-heat pipe array (MHPA) and PCM in solar greenhouse. Energy 2023, 264, 126183. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, G. Numerical investigation on thermal performance of a solar greenhouse with synergetic energy release of short- and long-term PCM storage. Solar Energy 2024, 269, 112313. [Google Scholar] [CrossRef]
- Badji, A.; Benseddik, A.; Bensaha, H.; Boukhelifa, A.; Bouhoun, S.; Nettari, C.; Kherrafi, M.; Lalmi, D. Experimental assessment of a greenhouse with and without PCM thermal storage energy and prediction their thermal behavior using machine learning algorithms. J. Energy Storage 2023, 71, 108133. [Google Scholar] [CrossRef]
- Farzaneh, F.; Zhang, Q.; Jung, S. Enhancing electric vehicle battery safety and performance: Aluminum tubes filled with PCM. J. Energy Storage 2024, 97, 112922. [Google Scholar] [CrossRef]
- Li, K.; Yao, X.; Li, Z.; Gao, T.; Zhang, W.; Liao, Z.; Ju, X.; Xu, C. Thermal management of Li-ion batteries with passive thermal regulators based on composite PCM materials. J. Energy Storage 2024, 89, 111661. [Google Scholar] [CrossRef]
- Lokhande, I.K.; Tiwari, N. A numerical investigation of novel segmented PCM blocks filled with different phase change material cooling for Lithium-Ion battery. Appl. Therm. Eng. 2024, 252, 123673. [Google Scholar] [CrossRef]
- Wagh, V.A.; Saha, S.K. Optimising extended fin design and heat transfer coefficient for improved heat transfer and PCM recover time in thermal management of batteries. Appl. Therm. Eng. 2024, 255, 123964. [Google Scholar] [CrossRef]
- Anisur, M.R.; Mahfuz, M.H.; Kibria, M.A.; Saidur, R.; Metselaar, I.H.S.C.; Mahlia, T.M.I. Curbing global warming with phase change materials for energy storage. Renew. Sustain. Energy Rev. 2013, 18, 23–30. [Google Scholar] [CrossRef]
- Calati, M.; Hooman, K.; Mancin, S. Thermal storage based on phase change materials (PCMs) for refrigerated transport and distribution applications along the cold chain: A review. Int. J. Thermofluids 2022, 16, 100224. [Google Scholar] [CrossRef]
- Liu, G.; Li, Q.; Wu, J.; Xie, R.; Zou, Y.; Scipioni, A.; Manzardo, A. Improving system performance of the refrigeration unit using phase change material (PCM) for transport refrigerated vehicles: An experimental investigation in South China. J. Energy Storage 2022, 51, 104435. [Google Scholar] [CrossRef]
- Lachheb, M.; Younsi, Z.; Youssef, N.; Bouadila, S. Enhancing building energy efficiency and thermal performance with PCM-Integrated brick walls: A comprehensive review. Build. Environ. 2024, 256, 111476. [Google Scholar] [CrossRef]
- Zhan, H.; Mahyuddin, N.; Sulaiman, R.; Khayatian, F. Phase change material (PCM) integrations into buildings in hot climates with simulation access for energy performance and thermal comfort: A review. Constr. Build. Mater. 2023, 397, 132312. [Google Scholar] [CrossRef]
- Liu, L.; Hammami, N.; Trovalet, L.; Bigot, D.; Habas, J.P.; Malet-Damour, B. Description of phase change materials (PCMs) used in buildings under various climates: A review. J. Energy Storage 2022, 56, 105760. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Khanmohammadi, F.; Darzi, A.A.R. Magnetic influence on phase change materials for optimized thermal energy storage: A comprehensive review and prospective insights. J. Energy Storage 2024, 89, 111625. [Google Scholar] [CrossRef]
- Simonsen, G.; Ravotti, R.; O’Neill, P.; Stamatiou, A. Biobased phase change materials in energy storage and thermal management technologies. Energy Rev. 2023, 184, 113546. [Google Scholar] [CrossRef]
- Rozanna, D.; Chuah, T.G.; Salmiah, A.; Choong, T.S.Y.; Sa’ari, M. Fatty Acids as Phase Change Materials (PCMs) for Thermal Energy Storage: A Review. Int. J. Green Energy 2005, 1, 495–513. [Google Scholar] [CrossRef]
- Png, Z.M.; Soo, X.Y.D.; Chua, M.H.; Ong, P.J.; Suwardi, A.; Tan, C.K.I.; Xu, J.; Zhu, Q. Strategies to reduce the flammability of organic phase change Materials: A review. Solar Energy 2022, 231, 115–128. [Google Scholar] [CrossRef]
- Palacios, A.; De Gracia, A.; Haurie, L.; Cabeza, L.F.; Fernández, A.I.; Barreneche, C. Study of the thermal properties and the fire performance of flame retardant-organic PCM in bulk form. Materials 2018, 11, 117. [Google Scholar] [CrossRef]
- Li, D.; Cheng, X.; Li, Y.; Zou, H.; Yu, G.; Li, G.; Huang, Y. Effect of MOF derived hierarchical Co3O4/expanded graphite on thermal performance of stearic acid phase change material. Solar Energy 2018, 171, 142–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Yang, B.; Cai, S. A review of battery thermal management systems using liquid cooling and PCM. J. Energy Storage 2024, 76, 109836. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.K.; Makki, E.; Giri, J.; Sathish, T. A comprehensive review of critical analysis of biodegradable waste PCM for thermal energy storage systems using machine learning and deep learning to predict dynamic behavior. Heliyon 2024, 10, e25800. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Gopi, S.; Karthik, K.; Nangan, S.; Kanagaraj, K.; Rajendran, S. Nano-PCM materials: Bridging the gap in energy storage under fluctuating environmental conditions. Process. Saf. Environ. Prot. 2024, 189, 1003–1021. [Google Scholar] [CrossRef]
- Baylis, C.; Cruickshank, C.A. Review of bio-based phase change materials as passive thermal storage in buildings. Renew. Sustain. Energy Rev. 2023, 186, 113690. [Google Scholar] [CrossRef]
- Zahir, M.H.; Irshad, K.; Shafiullah; Ibrahim, N.I.; Islam, A.K.; Mohaisen, K.O.; Sulaiman, F.A. Challenges of the application of PCMs to achieve zero energy buildings under hot weather conditions: A review. J. Energy Storage 2023, 64, 107156. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Ji, J.; Lv, Y. A review of the application of hydrated salt phase change materials in building temperature control. J. Energy Storage 2022, 56, 106157. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Wang, B.; Liu, C.; Arıcı, M. Optical and thermal performance of glazing units containing PCM in buildings: A review. Constr. Build. Mater. 2020, 233, 117327. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Potential of macroencapsulated pcm for thermal energy storage in buildings: A comprehensive review. Constr. Build. Mater. 2019, 225, 723–744. [Google Scholar] [CrossRef]
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-mortar based construction materials for energy efficient buildings: A review on research trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Kalbasi, R.; Samali, B.; Afrand, M. Taking benefits of using PCMs in buildings to meet energy efficiency criteria in net zero by 2050. Chemosphere 2023, 311, 137100. [Google Scholar] [CrossRef] [PubMed]
- Hekimoğlu, G.; Sarı, A. A review on phase change materials (PCMs) for thermal energy storage implementations. Mater. Today Proc. 2022, 58, 1360–1367. [Google Scholar] [CrossRef]
- Home|Statistical Review of World Energy. Available online: https://www.energyinst.org/statistical-review (accessed on 12 July 2024).
- Laustsen, M.J. Energy Efficiency Requirements in Building Codes, Energy Efficiency Policies for New Buildings. 2008. Available online: https://origin.iea.org/reports/energy-efficiency-requirements-in-building-codes-policies-for-new-buildings (accessed on 12 July 2024).
- Energy Efficiency in Existing Buildings. Available online: https://natural-resources.canada.ca/energy-efficiency/buildings/existing-buildings/20682 (accessed on 12 July 2024).
- U.S. Energy Information Administration. Office of Energy Statistics 2018 Commercial Buildings Energy Consumption Survey. 2022. Available online: https://www.eia.gov/consumption/commercial/ (accessed on 22 July 2024).
- Togun, H.; Sultan, H.S.; Mohammed, H.I.; Sadeq, A.M.; Biswas, N.; Hasan, H.A.; Homod, R.Z.; Abdulkadhim, A.H.; Yaseen, Z.M.; Talebizadehsardari, P. A critical review on phase change materials (PCM) based heat exchanger: Different hybrid techniques for the enhancement. J. Energy Storage 2024, 79, 109840. [Google Scholar] [CrossRef]
- Zhi, M.; Yue, S.; Zheng, L.; Su, B.; Fu, J.; Sun, Q. Recent developments in solid-solid phase change materials for thermal energy storage applications. J. Energy Storage 2024, 89, 111570. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Stritih, U.; Tyagi, V.V.; Stropnik, R.; Paksoy, H.; Haghighat, F.; Joybari, M.M. Integration of passive PCM technologies for net-zero energy buildings. Sustain. Cities Soc. 2018, 41, 286–295. [Google Scholar] [CrossRef]
- Imafidon, O.J.; Ting, D.S.K. Energy consumption of a building with phase change material walls–The effect of phase change material properties. J. Energy Storage 2022, 52, 105080. [Google Scholar] [CrossRef]
- Baylis, C.; Cruickshank, C.A. Parametric analysis of phase change materials within cold climate buildings: Effects of implementation location and properties. Energy Build. 2024, 303, 113822. [Google Scholar] [CrossRef]
- Ibrahim, Z.; Newby, S.; Hassani, V.; Ya’akub, S.R.; Abu Bakar, S.; Razlan, Z.; Khairunizam, W. A review of the application and effectiveness of heat storage system using phase change materials in the built environment. AIP Conf. Proc. 2021, 2339, 020131. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.U.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Navya, S.; Lund, I. Inorganic PCMs applications in passive cooling of buildings-A review. J. Phys. Conf. Ser. 2021, 2116, 12103. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Lian, P.; Yan, R.; Wu, Z.; Wang, Z.; Chen, Y.; Zhang, L.; Sheng, X. Thermal performance of novel form-stable disodium hydrogen phosphate dodecahydrate-based composite phase change materials for building thermal energy storage. Adv. Compos. Hybrid. Mater. 2023, 6, 74. [Google Scholar] [CrossRef]
- Zhou, K.; Sheng, Y.; Guo, W.; Wu, L.; Wu, H.; Hu, X.; Xu, Y.; Li, Y.; Ge, M.; Du, Y.; et al. Biomass porous carbon/polyethylene glycol shape-stable phase change composites for multi-source driven thermal energy conversion and storage. Adv. Compos. Hybrid. Mater. 2023, 6, 34. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Guo, Z.; Bin Xu, B.; Cao, Y.; Ren, J.; Hou, H.; Xiao, Y.; Elashiry, M.; El-Bahy, Z.M.; et al. Hydrophobic Multilayered PEG@PAN/MXene/PVDF@SiO2 Composite Film with Excellent Thermal Management and Electromagnetic Interference Shielding for Electronic Devices. Small 2024. early view. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, J.; Li, T.; Sheng, X.; Chen, Y. A ‘net-ball’ structure fiber membrane with electro-/photo-thermal heating and phase change synchronous temperature regulation capacity via electrospinning. Sol. Energy Mater. Sol. Cells 2024, 276, 113078. [Google Scholar] [CrossRef]
- Yan, R.; Huang, Z.; Zhang, L.; Chen, Y.; Sheng, X. Cellulose-reinforced foam-based phase change composites for multi-source driven energy storage and EMI shielding. Compos. Commun. 2024, 51, 102047. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid. Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Kumar Singh Rathore, P.; Kumar Shukla, S. Enhanced thermophysical properties of organic PCM through shape stabilization for thermal energy storage in buildings: A state of the art review. Energy Build. 2021, 236, 110799. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Dash, L.; Mahanwar, P.A. A Review on Organic Phase Change Materials and Their Applications. 2021. Available online: http://www.ijeast.com (accessed on 14 July 2024).
- Al-Yasiri, Q.; Szabó, M. Paraffin As a Phase Change Material to Improve Building Performance: An Overview of Applications and Thermal Conductivity Enhancement Techniques. Renew. Energy Environ. Sustain. 2021, 6, 38. [Google Scholar] [CrossRef]
- Chen, C.; Chen, A.; Li, L.; Peng, W.; Weber, R.; Liu, J. Distribution and Emission Estimation of Short- And Medium-Chain Chlorinated Paraffins in Chinese Products through Detection-Based Mass Balancing. Environ. Sci. Technol. 2021, 55, 7335–7343. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.W.F.; De Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.; Silva, M.P.; Da Costa, W.A.; Pinto, R.H.; Cordeiro, R.M.; Da Cruz, J.N.; Neto, A.C.; et al. Extraction of bioactive compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Supercritical Carbon Dioxide as Green Solvent; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–167. [Google Scholar] [CrossRef]
- Melgosa, R.; Sanz, M.T.; Beltrán, S. Supercritical CO2 processing of omega-3 polyunsaturated fatty acids–Towards a biorefinery for fish waste valorization. J. Supercrit. Fluids 2021, 169, 105121. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, H.; Dai, H.; Kou, Y.; Yan, X.; Tian, Y.; Shi, Q. Heat capacity study of fatty acids as phase change materials for thermal energy storage. J. Chem. Thermodyn. 2024, 197, 107338. [Google Scholar] [CrossRef]
- El Majd, A.; Sair, S.; Ousaleh, H.A.; Bouhaj, Y.; Belouaggadia, N.; Younsi, Z.; El Bouari, A. Advancing tent thermoregulation: Integrating shape-stabilized PCM into fabric design. J. Energy Storage 2024, 95, 112681. [Google Scholar] [CrossRef]
- Çankırı, A.K.; Üniversitesi, K.; Sari, A. Capric Acid and Palmitic Acid Eutectic Mixture Applied in Building Wallboard for Latent Heat Thermal Energy Storage. 2014. Available online: https://www.researchgate.net/publication/237378416 (accessed on 13 July 2024).
- Liu, P.; Gu, X.; Bian, L.; Cheng, X.; Peng, L.; He, H. Thermal Properties and Enhanced Thermal Conductivity of Capric Acid/Diatomite/Carbon Nanotube Composites as Form-Stable Phase Change Materials for Thermal Energy Storage. ACS Omega 2019, 4, 2964–2972. [Google Scholar] [CrossRef]
- Zuo, P.; Liu, Z.; Zhang, H.; Dai, D.; Fu, Z.; Corker, J.; Fan, M. Formulation and phase change mechanism of Capric acid/Octadecanol binary composite phase change materials. Energy 2023, 270, 126943. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Zhao, Y.M.; Li, G.D.; Pan, W.G. Preparation and thermal properties of palmitic acid @ZnO/Expanded graphite composite phase change material for heat storage. Energy 2022, 242, 122972. [Google Scholar] [CrossRef]
- Liu, S.; Xin, S.; Jiang, S. Study of Capric-Palmitic Acid/Clay Minerals as Form-Stable Composite Phase-Change Materials for Thermal Energy Storage. ACS Omega 2021, 6, 24650–24662. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Kaygusuz, K. Capric acid and stearic acid mixture impregnated with gypsum wallboard for low-temperature latent heat thermal energy storage. Int. J. Energy Res. 2008, 32, 154–160. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.S.; Singh, J.K. Microencapsulation of stearic acid with SiO2 shell as phase change material for potential energy storage. Sci. Rep. 2020, 10, 15023. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhao, Y.; Liu, X.; Shi, Y.; Jiang, D. Thermal Properties and Reliabilities of Lauric Acid-Based Binary Eutectic Fatty Acid as a Phase Change Material for Building Energy Conservation. ACS Omega 2022, 7, 16097–16108. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Q.; Wu, M.; Du, P.; Liu, C.; Rao, Z. Thermal properties optimization of lauric acid as phase change material with modified boron nitride nanosheets-sodium sulfate for thermal energy storage. J. Energy Storage 2023, 61, 106781. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef]
- Fang, G.; Zhao, M.; Sun, P. Experimental study of the thermal properties of a fatty acid-modified graphite composite phase change material dispersion system. J. Energy Storage 2022, 53, 105108. [Google Scholar] [CrossRef]
- Parsons, S.; Raikova, S.; Chuck, C.J. The viability and desirability of replacing palm oil. Nat. Sustain. 2020, 3, 412–418. [Google Scholar] [CrossRef]
- Nawkarkar, P.; Singh, A.K.; Abdin, M.Z.; Kumar, S. Life cycle assessment of Chlorella species producing biodiesel and remediating wastewater. J. Biosci. 2019, 44, 89. [Google Scholar] [CrossRef]
- Baena, A.; Orjuela, A.; Rakshit, S.K.; Clark, J.H. Enzymatic hydrolysis of waste fats, oils and greases (FOGs): Status, prospective, and process intensification alternatives. Chem. Eng. Process.-Process Intensif. 2022, 175, 108930. [Google Scholar] [CrossRef]
- Kumaran, M.; Palanisamy, K.M.; Bhuyar, P.; Maniam, G.P.; Rahim, M.H.A.; Govindan, N. Agriculture of microalgae Chlorella vulgaris for polyunsaturated fatty acids (PUFAs) production employing palm oil mill effluents (POME) for future food, wastewater, and energy nexus. Energy Nexus 2023, 9, 100169. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.J.; Zhang, Y.; Li, Y.-F.; Liu, G.-L.; Jiang, H.; Hu, Z.; Chi, Z.-M. Fatty acids from oleaginous yeasts and yeast-like fungi and their potential applications. Crit. Rev. Biotechnol. 2018, 38, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Lecomte, J.; Barouh, N.; Sahaka, M.; Lafont, D.; Rodier, J.-D.; Parsiegla, G.; Demarne, F.; Gontero, B.; Villeneuve, P.; et al. Using Enzymes to Harvest Fatty Acids from Galactosyldiacylglycerols, the Most Abundant Lipids in Plant Biomass. ACS Sustain. Chem. Eng. 2024, 12, 4103–4113. [Google Scholar] [CrossRef]
- Zenevicz, M.C.P.; Jacques, A.; Furigo, A.F.; Oliveira, J.V.; de Oliveira, D. Enzymatic hydrolysis of soybean and waste cooking oils under ultrasound system. Ind. Crops Prod. 2016, 80, 235–241. [Google Scholar] [CrossRef]
- Neves, A.M.D.; Visioli, L.J.; Enzweiler, H.; Paulino, A.T. Lipase from Candida rugosa incorporated in pectin hydrogel via immobilization for hydrolysis of lipids in dairy effluents and production of fatty acids. J. Water Process Eng. 2024, 58, 104821. [Google Scholar] [CrossRef]
- Yahya, A.B.; Usaku, C.; Daisuk, P.; Shotipruk, A. Enzymatic hydrolysis as a green alternative for glyceride removal from rice bran acid oil before γ-oryzanol recovery: Statistical process optimization. Biocatal. Agric. Biotechnol. 2023, 50, 102727. [Google Scholar] [CrossRef]
- Machado, S.A.; Da Rós, P.C.M.; de Castro, H.F.; Giordani, D.S. Hydrolysis of vegetable and microbial oils catalyzed by a solid preparation of castor bean lipase. Biocatal. Agric. Biotechnol. 2021, 37, 102188. [Google Scholar] [CrossRef]
- Yong, Q.; Yuan, G.; Li, H. Extraction and separation of unsaturated fatty acids from sunflower oil. IOP Conf. Ser. Earth Environ. Sci. 2021, 680, 012063. [Google Scholar] [CrossRef]
- Timms, R.E. Fractional crystallisation-The fat modification process for the 21st century. Eur. J. Lipid Sci. Technol. 2005, 107, 48–57. [Google Scholar] [CrossRef]
- Maeda, K.; Naito, Y.; Kuramochi, H.; Arafune, K.; Itoh, K.; Taguchi, S.; Yamamoto, T. High-Pressure crystallization of binary unsaturated fatty acids in cylindrical cell. J. Cryst. Growth 2021, 576, 126380. [Google Scholar] [CrossRef]
- Sun, M.; Liu, T.; Sha, H.; Li, M.; Liu, T.; Wang, X.; Chen, G.; Wang, J.; Jiang, D. A review on thermal energy storage with eutectic phase change materials: Fundamentals and applications. J. Energy Storage 2023, 68, 107713. [Google Scholar] [CrossRef]
- Mailhé, C.; Duquesne, M.; del Barrio, E.P.; Azaiez, M.; Achchaq, F. Phase diagrams of fatty acids as biosourced phase change materials for thermal energy storage. Appl. Sci. 2019, 9, 1067. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. [Google Scholar] [CrossRef]
- Smallman, R.E.; Bishop, R.J. Chapter 3 Structural phases: Transitions their formation and transitions. In Modern Physical Metallurgy and Materials Engineering, 6th ed.; Smallman, R.E., Bishop, R.J., Eds.; Butterworth-Heinemann: Oxford, UK, 1999; Chapter 3; pp. 42–83. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Martin, V.; Chiu, J.N. Phase equilibrium in the design of phase change materials for thermal energy storage: State-of-the-art. Renew. Sustain. Energy Rev. 2017, 73, 558–581. [Google Scholar] [CrossRef]
- Ke, H. Phase diagrams, eutectic mass ratios and thermal energy storage properties of multiple fatty acid eutectics as novel solid-liquid phase change materials for storage and retrieval of thermal energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Ali, M.; Kannan, A.M. Fatty acids based eutectic phase change system for thermal energy storage applications. Appl. Therm. Eng. 2018, 142, 466–475. [Google Scholar] [CrossRef]
- Jebasingh, E.B.; Arasu, V.A. A Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. J. Energy Storage 2020, 31, 101708. [Google Scholar] [CrossRef]
- Zhao, P.; Yue, Q.; He, H.; Gao, B.; Wang, Y.; Li, Q. Study on phase diagram of fatty acids mixtures to determine eutectic temperatures and the corresponding mixing proportions. Appl. Energy 2014, 115, 483–490. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Han, J.; Bai, R.; Gao, W.; Zhou, M. A novel capric-stearic acid/expanded perlite-based cementitious mortar for thermal energy storage. Solar Energy 2024, 273, 112501. [Google Scholar] [CrossRef]
- Dai, J.; Ma, F.; Fu, Z.; Sangiorgi, C.; Tataranni, P.; Tarsi, G.; Li, C.; Hou, Y.; Guo, Y. Binary eutectic phase change materials application in cooling asphalt: An assessment for thermal stability and durability. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134790. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, R.; Zhang, N.; Chen, L.; Chen, D.; Li, X. Performance improvement of lauric acid-1-hexadecanol eutectic phase change material with bio-sourced seashell powder addition for thermal energy storage in buildings. Constr. Build. Mater. 2023, 366, 130223. [Google Scholar] [CrossRef]
- Abhijith, M.T.; Sreekumar, A. A Development of palmitic acid-lauryl alcohol as binary eutectic for cold thermal energy storage in buildings indoor thermal comfort application-Thermophysical studies and discharging characteristics. J. Energy Storage 2023, 64, 107002. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Mariappan, V.; Kalidoss, P.; Anish, R.; Sarafoji, P.; Reddy, J.V.; Satpathy, T.K. Preparation and thermal characterization of capric-myristic acid binary eutectic mixture with silver–antimony tin oxide and silver-graphane nanoplatelets hybrid-nanoparticles as phase change material for building applications. Mater. Lett. 2022, 328, 133086. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Nas, M.; Ouikhalfan, M.; Sarı, A.; Tyagi, V.; Sharma, R.; Kurbetci, Ş.; Saleh, T.A. Silica fume/capric acid-stearic acid PCM included-cementitious composite for thermal controlling of buildings: Thermal energy storage and mechanical properties. Energy 2021, 219, 119588. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Duquesne, M.; Mailhé, C.; Doppiu, S.; Dauvergne, J.-L.; Santos-Moreno, S.; Godin, A.; Fleury, G.; Rouault, F.; del Barrio, E.P. Characterization of fatty acids as biobased organic materials for latent heat storage. Materials 2021, 14, 4707. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Ok, Y.S.; Kua, W.; Kim, S. Thermal properties of composite organic phase change materials (PCMs): A critical review on their engineering chemistry. Appl. Therm. Eng. 2020, 181, 115960. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro-Rivero, M.E.; Zou, B.; Jiang, Z.; Harrison, M.T.; Ding, Y. A perspective on Phase Change Material encapsulation: Guidance for encapsulation design methodology from low to high-temperature thermal energy storage applications. J. Energy Storage 2023, 72, 108597. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Mazumder, M.A.J.; Salhi, B.; Sari, A.; Afzaal, M.; Al-Sulaiman, F.A. Effects of carbon-based fillers on thermal properties of fatty acids and their eutectics as phase change materials used for thermal energy storage: A Review. J. Energy Storage 2021, 35, 102329. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Zhang, N.; Xiang, B.; Zhang, Z.; Qian, B. Thermal reliability of typical fatty acids as phase change materials based on 10,000 accelerated thermal cycles. Sustain. Cities Soc. 2019, 46, 101380. [Google Scholar] [CrossRef]
- Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.I.; Barreneche, C. Degradation of fatty acid phase-change materials (PCM): New approach for its characterization. Molecules 2021, 26, 982. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kant, K.; Shukla, A.; Chen, C.R.; Sharma, A. Thermal stability and reliability test of some saturated fatty acids for low and medium temperature thermal energy storage. Energies 2021, 14, 4509. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, G.; Jia, Z.; Gao, Q.; Li, Y.; Gu, Z. Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials 2022, 15, 6856. [Google Scholar] [CrossRef]
- Cellat, K.; Beyhan, B.; Güngör, C.; Konuklu, Y.; Karahan, O.; Dündar, C.; Paksoy, H. Thermal enhancement of concrete by adding bio-based fatty acids as phase change materials. Energy Build. 2015, 106, 156–163. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, P.; Rathore, S.; Sharma, R.K.; Gupta, N.K. Integration of lauric acid/zeolite/graphite as shape stabilized composite phase change material in gypsum for enhanced thermal energy storage in buildings. Appl. Therm. Eng. 2023, 224, 120088. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Liu, C. Thermal storage performance of paraffin and fatty acid mixtures used in walls and floors. Mater. Res. Express 2019, 6, 105522. [Google Scholar] [CrossRef]
- Yan, Q.; Fan, Q.; Jing, Z. Study on Thermal Storage Performance of Industrial Paraffin and Fatty Acid Binary Mixture. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012030. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, D.; Chen, J.; Li, W.; Chen, Z.; Gibb, S.W.; Long, Y. Ultrathin graphite sheets stabilized stearic acid as a composite phase change material for thermal energy storage. Energy 2018, 166, 246–255. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Q.; Luo, H.; Luo, S. Three-dimensional graphitic hierarchical porous carbon/stearic acid composite as shape-stabilized phase change material for thermal energy storage. Appl. Energy 2019, 260, 114278. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Xie, B.; Ma, H.; Chen, J. Enhanced properties of diatomite-based composite phase change materials for thermal energy storage. Renew. Energy 2019, 147, 265–274. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Sarı, A.; Mazumder, M.A.J.; Salhi, B.; Hekimoğlu, G.; Al-Sulaiman, F.A.; Inamuddin. Thermal energy storage and thermal conductivity properties of fatty acid/fatty acid-grafted-CNTs and fatty acid/CNTs as novel composite phase change materials. Sci. Rep. 2020, 10, 15388. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.B.; Montazer, M. In situ incorporation and loading of copper nanoparticles into a palmitic–lauric phase-change material on polyester fibers. J. Appl. Polym. Sci. 2019, 136, 46951. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.; Min, X.; Gao, W.; Huang, S. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 273–279. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, D.; Zhu, G. Preparation and characterization of form-stable phase-change materials with enhanced thermal conductivity based on nano-Al2O3 modified binary fatty acids and expanded perlite. Energy Build. 2022, 271, 112330. [Google Scholar] [CrossRef]
- José, J.S.; Sanz-Tejedor, M.A.; Arroyo, Y. Effect of fatty acid composition in vegetable oils on combustion processes in an emulsion burner. Fuel Process. Technol. 2015, 130, 20–30. [Google Scholar] [CrossRef]
- McLaggan, M.S.; Hadden, R.M.; Gillie, M. Flammability assessment of phase change material wall lining and insulation materials with different weight fractions. Energy Build. 2017, 153, 439–447. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Res. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Van Der Veen, I.; De Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Y.; Zhu, Z.; Xie, Z.; Sun, X.; Jia, C.; Liu, F.; Wang, J.; Yang, J. Preparation of a halogen-free flame retardant and its effect on the poly(L-lactic acid) as the flame retardant material. Polymer 2021, 229, 124027. [Google Scholar] [CrossRef]
- Pielichowska, K.; Paprota, N.; Pielichowski, K. Fire Retardant Phase Change Materials—Recent Developments and Future Perspectives. Materials 2023, 16, 4391. [Google Scholar] [CrossRef]
- Diaconu, B.; Cruceru, M.; Anghelescu, L. Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review. Fire 2023, 6, 175. [Google Scholar] [CrossRef]
- Palacios, A.; De Gracia, A.; Cabeza, L.F.; Julià, E.; Fernández, A.I.; Barreneche, C. New formulation and characterization of enhanced bulk-organic phase change materials. Energy Build. 2018, 167, 38–48. [Google Scholar] [CrossRef]
- Alkhazaleh, A.H.; Almanaseer, W.; Alkhazali, A. Experimental investigation on thermal properties and fire performance of lauric acid/diphenyl phosphate/expanded perlite as a flame retardant phase change material for latent heat storage applications. Sustain. Energy Technol. Assess. 2023, 56, 103059. [Google Scholar] [CrossRef]
- Han, K.T.; Lhosupasirirat, S.; Srikhirin, P.; Houngkamhang, N.; Srikhirin, T. Development of Flame Retardant Stearic Acid Doped Graphite Powder and Magnesium Hydroxide Nanoparticles, Material for Thermal Energy Storage Applications. J. Phys. Conf. Ser. 2022, 2175, 012043. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, B.; Wang, L.; Lu, R.; Zhao, D.; Zhang, S. Novel hybrid form-stable polyether phase change materials with good fire resistance. Energy Storage Mater. 2017, 6, 46–52. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, P.; Wang, Y.; Zhou, C.; Lei, J. Form-stable phase change materials with enhanced thermal stability and fire resistance via the incorporation of phosphorus and silicon. Mater. Des. 2018, 160, 763–771. [Google Scholar] [CrossRef]
- Fernandes, J.; Peixoto, M.; Mateus, R.; Gervásio, H. Life cycle analysis of environmental impacts of earthen materials in the Portuguese context: Rammed earth and compressed earth blocks. J. Clean. Prod. 2019, 241, 118286. [Google Scholar] [CrossRef]
- Ji, R.; Li, X.; Lv, C. Synthesis and evaluation of phase change material suitable for energy-saving and carbon reduction of building envelopes. Energy Build. 2023, 278, 112603. [Google Scholar] [CrossRef]
- EnergyPlusTM. 30 September 2017, United States: 00. Available online: https://www.osti.gov/servlets/purl/1395882 (accessed on 24 July 2024).
- Frahat, N.B.; Ustaoglu, A.; Gencel, O.; Sarı, A.; Hekimoğlu, G.; Yaras, A.; Díaz, J.J.d.C. Fuel, cost, energy efficiency and CO2 emission performance of PCM integrated wood fiber composite phase change material at different climates. Sci. Rep. 2023, 13, 7714. [Google Scholar] [CrossRef] [PubMed]
- Hawes, D.W.; Feldman, D.; Banu, D. Latent heat storage in building materials Objectives of research in thermal storage building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Wang, R.; Ren, M.; Gao, X.; Qin, L. Preparation and properties of fatty acids based thermal energy storage aggregate concrete. Constr. Build. Mater. 2018, 165, 1–10. [Google Scholar] [CrossRef]
- Jahangiri, A.; Forouhi, N.; Jamekhorshid, A.; Farid, M.M.; Kamgar, R. Performance Evaluation of Concrete Containing Fatty Acids as a Medium of Thermal Energy Storage. J. Mater. Civ. Eng. 2024, 36, 04024070. [Google Scholar] [CrossRef]
- Suresh, C.; Hotta, T.K.; Saha, S.K. Phase change material incorporation techniques in building envelopes for enhancing the building thermal Comfort-A review. Energy Build. 2022, 268, 112225. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Al-Yasiri, Q.; Szabó, M. Incorporation of phase change materials into building envelope for thermal comfort and energy saving: A comprehensive analysis. J. Build. Eng. 2020, 36, 102122. [Google Scholar] [CrossRef]
- Williams, J.D.; Peterson, G.P. A review of thermal property enhancements of low-temperature Nano-enhanced phase change materials. Nanomaterials 2021, 11, 2578. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Konuklu, Y.; Akar, H.B. Promising palmitic acid/poly(allyl methacrylate) microcapsules for thermal management applications. Energy 2023, 262, 125491. [Google Scholar] [CrossRef]
- Elhamy, A.A.; Mokhtar, M. Phase Change Materials Integrated Into the Building Envelope to Improve Energy Efficiency and Thermal Comfort. Future Cities Environ. 2024, 10, 9. [Google Scholar] [CrossRef]




| Year | Type of PCM | Building Material | Highlights | Reference |
|---|---|---|---|---|
| 2024 | Inorganic, Organic, Eutectic | Brick walls | Investigate how the type of PCM, its location, and quantity integrated into brick walls influence energy efficiency enhancement | [23] |
| 2024 | PCMs derived from waste | Building envelopes | Focus on minimizing greenhouse gas emissions and analyzing the use of machine learning to predict the material’s behavior | [33] |
| 2024 | Nano-PCMs (nanoparticles integrated into PCMs) | Building envelopes (walls, floors, ceilings) | Provide information on the role of nano-PCMs in boosting energy efficiency and identifies the advantages regarding the thermal properties for the application on residential and industrial scales | [34] |
| 2023 | Bio-PCM (plant-based oils) | Building-integrated PCM | Examine the thermophysical properties of biobased PCMs directly using plant-based oils, building integration techniques, and lifetime impacts | [35] |
| 2023 | Inorganic, Organic, Eutectic | Building envelopes | Review passive cooling benefits from PCM integrations in buildings in tropical climates. The study detailed information to simulate access to buildings enhanced with PCMs. | [24] |
| 2023 | Inorganic, Organic, Eutectic | Building materials | Explore the problems associated with the selection of PCMs and techniques to encapsulate them for heating and cooling applications | [36] |
| 2022 | Inorganic: Hydrated salts | Building materials, building envelopes, and air-conditioning systems | Analysis of methods to enhance thermal properties of hydrated salts, as well as encapsulation techniques and their application in thermal energy storage systems | [37] |
| 2020 | Paraffin and Eutectic | Glazing units | Summary of experimental and numerical research focused on PCMs incorporated into glazing units, as well as challenges and future works | [38] |
| 2019 | Macro-encapsulated PCMs | Building envelopes | Review of the available PCMs suitable for macro-encapsulation and their influence in building envelopes | [39] |
| 2018 | Inorganic and Organic | Mortar based-materials | Summary of details regarding various PCM-mortar combinations, their benefits, drawbacks, and application in buildings | [40] |
| PCM | Benefits | Drawbacks | Melting Points | Latent Heat of Fusion |
|---|---|---|---|---|
| Organic |
|
| −12 to 187 °C | 130 to 260 kJ/kg |
| Inorganic |
|
| 11 to 120 °C | 25 to 200 kJ/kg |
| Eutectics |
|
| 4 to 93 °C | 100 to 230 kJ/kg |
| Fatty Acid | Melting Temperature (°C) | Melting Latent Heat (J/g) | Freezing Temperature (°C) | Freezing Latent Heat (J/g) | Reference |
|---|---|---|---|---|---|
| Capric acid | 32.14 | 156.40 | 32.53 | 154.24 | [73] |
| 30.92 | 163.37 | 27.69 | 167.95 | [74] | |
| 30.48 | 169.17 | - | - | [75] | |
| Palmitic acid | 59.40 | 218.53 | 58.23 | 216.46 | [73] |
| 59.66 | 209.35 | 58.90 | 212.48 | [76] | |
| 61.71 | 206.68 | 59.48 | 204.25 | [77] | |
| Stearic acid | 68.86 | 252.72 | 68.91 | 254.12 | [78] |
| 72.09 | 200.9 | 64.65 | 194.42 | [79] | |
| Lauric acid | 43.93 | 178.11 | 40.63 | 178.98 | [80] |
| 42.8 | 172.19 | 41.2 | 170.26 | [81] | |
| Myristic acid | 54.28 | 191.27 | 51.69 | 194.36 | [80] |
| 53.6 | 199.4 | 51.8 | 199.0 | [82] |
| Year | Eutectic Mixture | Highlights | Reference |
|---|---|---|---|
| 2024 | Capric acid—Stearic acid |
| [107] |
| 2024 | Stearic acid—Palmitic acid |
| [108] |
| 2023 | Lauric acid—1 hexadecanol |
| [109] |
| 2023 | Palmitic acid—Lauryl alcohol |
| [110] |
| 2022 | Capric acid—Myristic acid |
| [111] |
| 2021 | Capric acid—Stearic acid |
| [112] |
| 2018 | Methyl palmitate—Lauric acid |
| [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, P.; De la Hoz Siegler, H.; Clarke, M. Fatty Acids as Phase Change Materials for Building Applications: Drawbacks and Future Developments. Energies 2024, 17, 4880. https://doi.org/10.3390/en17194880
Herrera P, De la Hoz Siegler H, Clarke M. Fatty Acids as Phase Change Materials for Building Applications: Drawbacks and Future Developments. Energies. 2024; 17(19):4880. https://doi.org/10.3390/en17194880
Chicago/Turabian StyleHerrera, Paola, Hector De la Hoz Siegler, and Matthew Clarke. 2024. "Fatty Acids as Phase Change Materials for Building Applications: Drawbacks and Future Developments" Energies 17, no. 19: 4880. https://doi.org/10.3390/en17194880
APA StyleHerrera, P., De la Hoz Siegler, H., & Clarke, M. (2024). Fatty Acids as Phase Change Materials for Building Applications: Drawbacks and Future Developments. Energies, 17(19), 4880. https://doi.org/10.3390/en17194880







