Effects of Water Injection in Diesel Engine Emission Treatment System—A Review in the Light of EURO 7
Abstract
1. Introduction
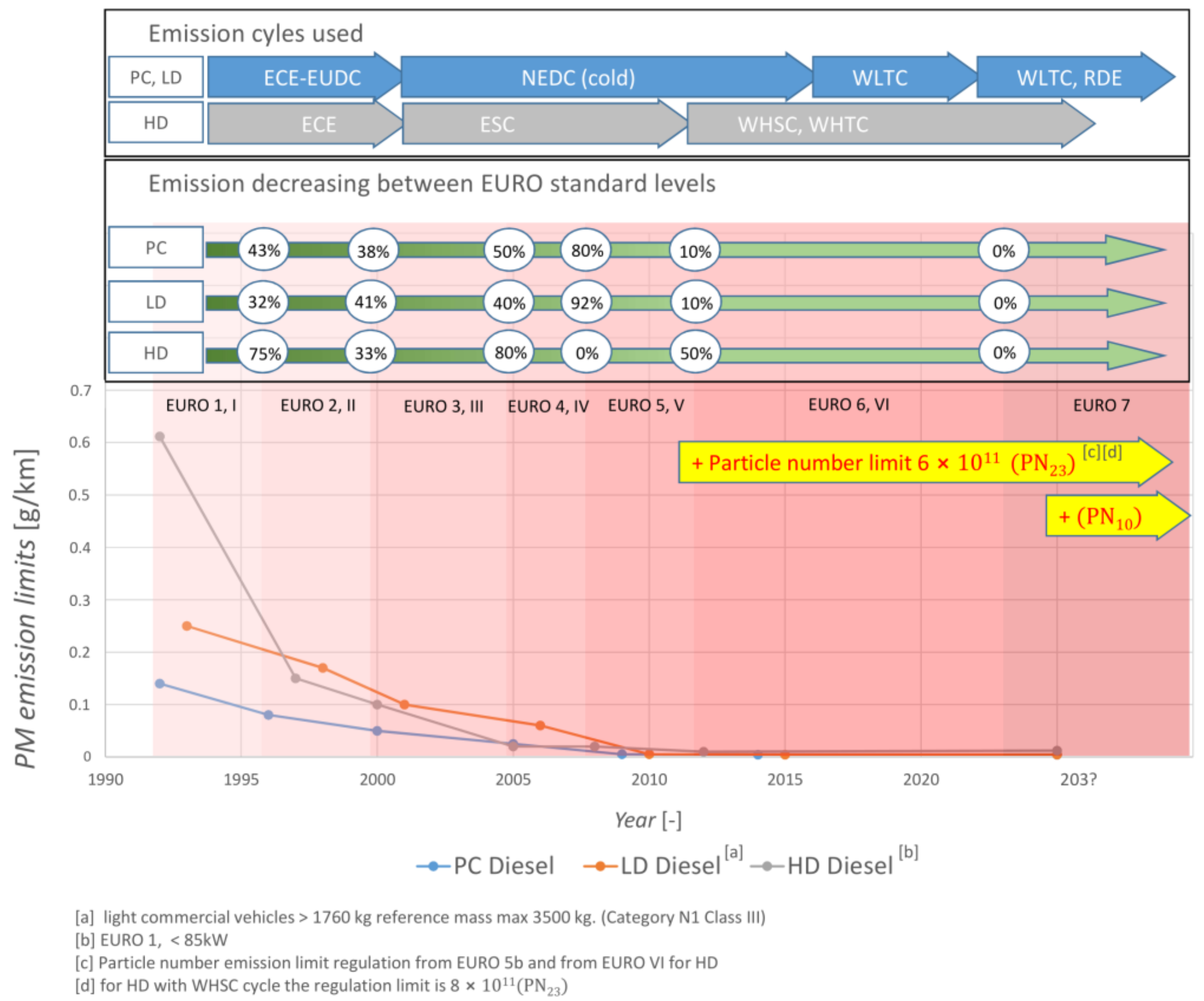
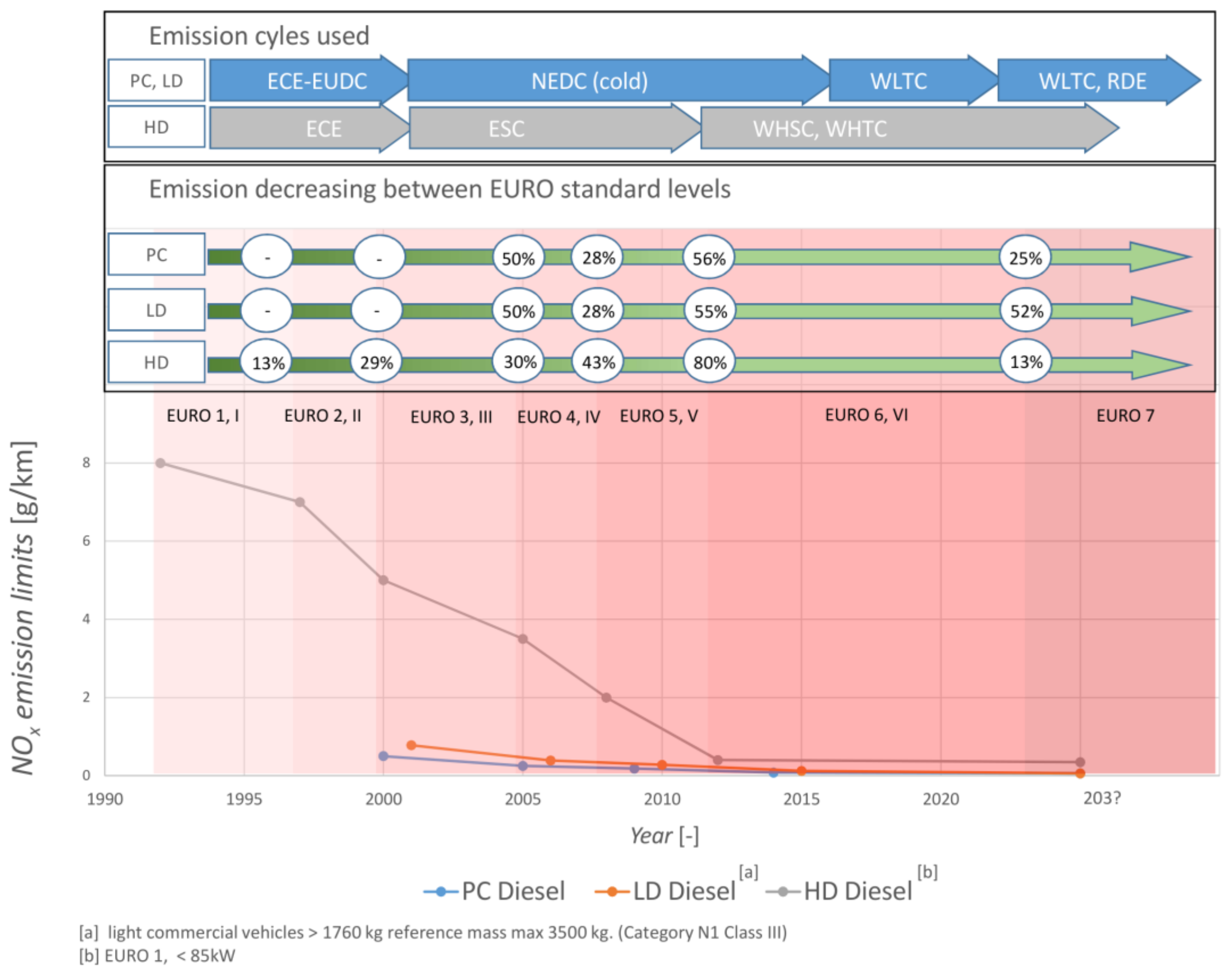
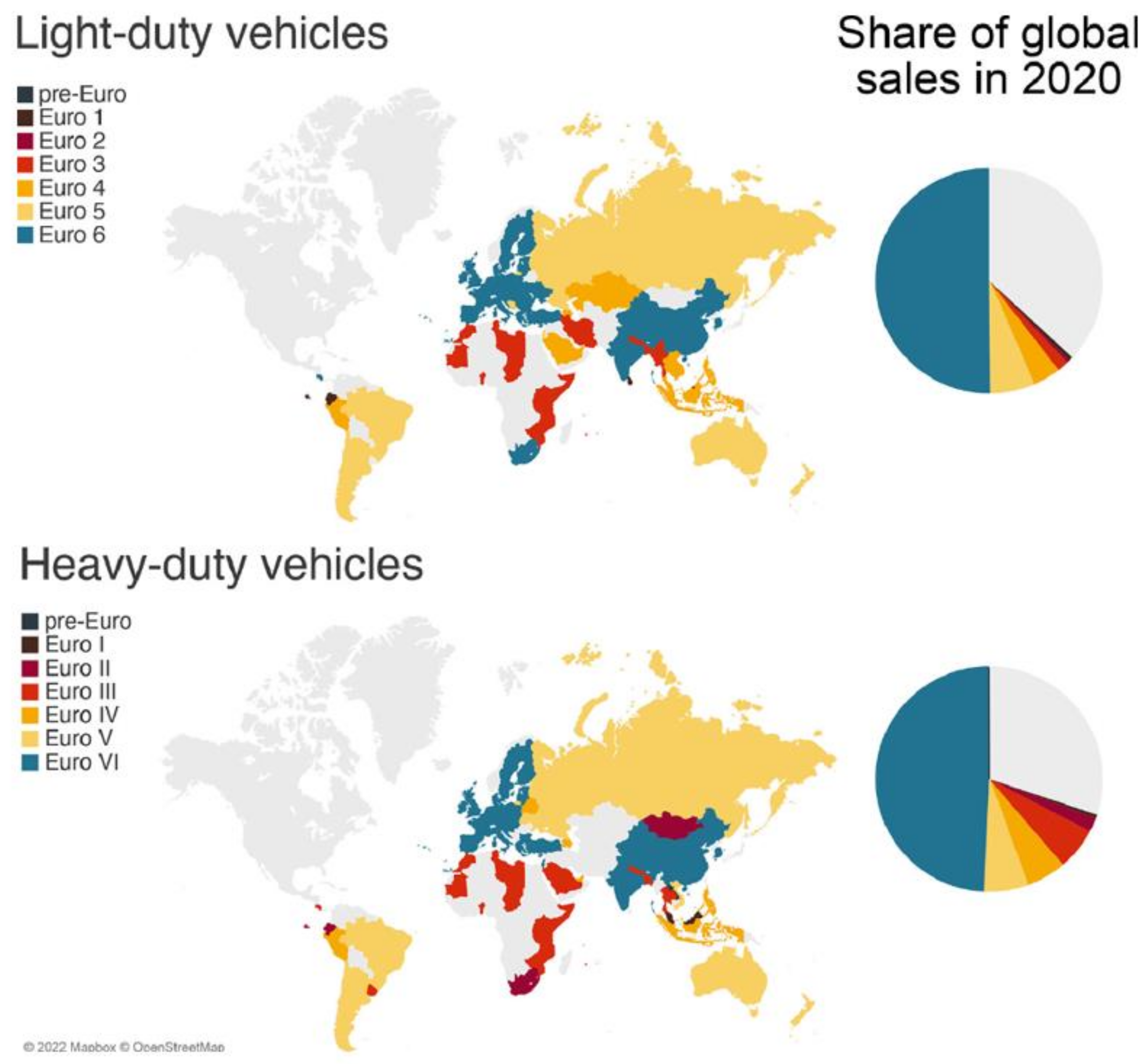
2. Emission Regulations
2.1. On Road Regulations
2.2. Non-Road Regulations
3. Water in the Combustion
- Pre-engine processes (different fuel/fuels introduced into the engine);
- Processes implemented in the engine (EGR, optimized engine control);
- Post-engine systems (catalysts, filters, after-treatment systems) [53].
3.1. Water in Combustion, Past and Present
3.2. Water Effect on NOx Emission
3.3. Effects of Water on Particulates
- carbon;
- ash from fuel and lubricating oil;
- metal particles from engine wear.
- In the emission, liquid particulate matter (soluble organic fraction (SOF)) also exists in the form of:
- ○
- unburnt or partially burnt hydrocarbon;
- ○
- water;
- ○
- sulfates (which is negligible in modern ultra-low Sulfur diesel (ULSD))
3.4. Effects of Water on Engine Efficiency
3.5. Wear and Corrosion Effects of Water
- improved material qualities, the use of plastics,
- precise injection systems, high injection pressures,
- the possible use of ULSD fuels and, on the other hand, the negligible proportion of engine conditions that favor Sulfurous and Sulfuric acid formation [36].
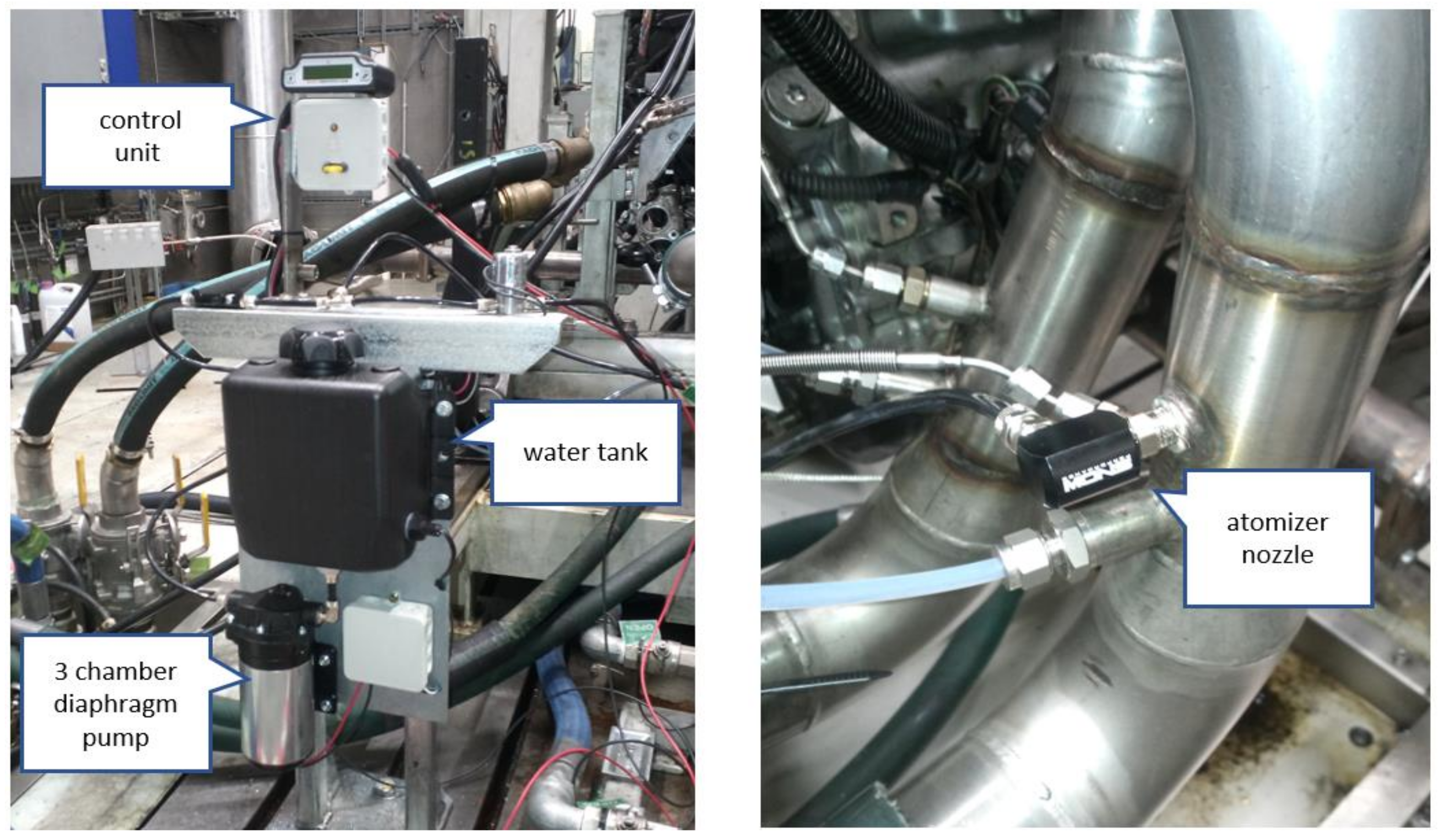
3.6. Types of W.I.
- Intake side fumigation (port water injection):
- water,
- steam,
- (emulsions).
- Direct water injection:
- With separate injectors:
- ○
- water,
- ○
- (emulsions).
- With the own injectors of the engine:
- ○
- emulsions.
- Emulsions can be:
- injection of water and fuel together (as non-stable emulsions),
3.7. Effects of Water on Exhaust Gas Recirculation
3.8. Effects of Excess Water after Combustion on the Internal Combustion Engine’s Emission Aftertreatment System
- reduced exhaust gas temperature,
- increased number of water and its related compounds, radicals after combustion.
3.8.1. Effect on DOC
3.8.2. Effect of DPF
3.8.3. Effect of SCR
4. Conclusions
- It can be concluded that much more emission reduction can be achieved through retrofitting than through further tightening of emission standards:
- ○
- Depending on the exact emission limit values of PM and NOx, since the introduction of EURO 1/I and NRMM regulations, the tightening of emission levels has reached at least 95%.
- ○
- With the current emission regulations, new car sales were around 72.5 million worldwide in 2023. By contrast, the number of used cars in the world is estimated at 1.5 billion. The volume of used cars between Euro 3-5/III-IV is expected to be the largest, for which the greatest results could be achieved globally from a retrofitting point of view and for which modifications could also be solved economically.
- ○
- The fuel requirement for NRMM equipment is much less stringent. As most of these machines are not registered, it is difficult to quantify their volume, but their emissions are likely significantly higher than for on-road. Sulfur content causes catalyst poisoning through CeO2 deactivation. In case of high sulfur content in fuel, the retrofitting with conventional catalytic converters is not feasible.
- ○
- Non-road machinery has much higher lifespan compared to PC vehicles, where the depreciation is much less and retrofitting can be a cost-effective solution.
- Based on the literature review, it can be concluded that W.I. can be used effectively in different ATSs, causing further emission reductions at the tailpipe:
- ○
- EGR—EGR is unable to considerably reduce NOx emissions at higher loads and significantly decreases the maximum performance. For this reason, during high power vehicle operating situations, EGR rates are often lowered or shut off entirely. However, intake fumigation can reduce NOx emissions under high load operating conditions without significantly increasing PM emissions, as the main thermal effect still successfully suppresses the cylinder temperature. Intake water fumigation would be the best match with EGR, for NOx and particulate emission reduction across the entire operating range of the engine, with further emission reductions.
- ○
- DOC—Pd-coated DOC is preferable in the case of excess water in the emission system. At low temperatures, enhanced CO and hydrocarbon absorption, and reduced light off temperatures can be seen.
- ○
- DPF—Water can enhance passive regeneration with increased oxidation at lower temperatures. It can result in fuel savings in the system with lower backpressures and extended periods between active regenerations.
- ○
- SCR—During high loads, temperature continuously increases along the DOC, DPF and SCR, with the highest temperature at the SCR, where water has a positive effect on the SCR’s denitrification activity through the Eley-Rideal mechanism. However, because H2O has a greater adsorption capacity than NH3 at lower temperatures, when the temperature drops, so does the denitrification effectiveness of SCR in the presence of water.
- Conclusions on full emission system tailpipe emissions:
- ○
- In vehicles without emission treatment systems (even without EGR), intake fumigation can be a universal and a cost-effective retrofitting option.
- ○
- Adding intake fumigation to vehicles with EGR, the two techniques can complement each other effectively across the full power range of the internal combustion engine. This means that with easily applicable engine intake water fumigation, we can reach further emission reduction without even modifying the engine control.
- ○
- After a typical Euro 5/V system with DOC and DPF, at least 96% of the particles are filtered out on a number basis. DPF filters out the solid particulates, while DOC removes the SOF in HC form. In such ATS, only NOx remains, which W.I. and EGR combined can further decrease.
- -
- No research is available on the effects of W.I. on the PN10 particle size range. Studies with intake charge moisture content suggest that the use of water may have potential benefits in this area
- -
- From a practical point of view, an important achievement is that the cooling effect of W.I. does not depend on traffic conditions. This cooling effect typically comes from the high air flow of the moving vehicle, which decreases the temperature of the intake charge and the EGR cooler. W.I.’s cooling effect can continue to cool the charge air during city conditions, even when there is no significant airflow during idling or hard accelerations.
- -
- Due to downsizing, the load on internal combustion engines is increasing, which could make the use of intake water fumigation increasingly beneficial.
Future Directions
- At the end of the exhaust system, the Ammonia Slip Catalyst (ASC) is responsible for removing the remaining NH3. The new EURO 7 establishes emission limits for NH3. The effect of water in this context also requires further research.
- To verify findings mentioned above, intake water fumigation tests are planned with EURO 5/V aftertreatment systems, along with synchronized EGR and water fumigation. This will include measuring particles below 23 nanometers to provide information in relation to EURO 7.
- Meanwhile, investigating the potential corrosive effects in the planned EURO 5/V system, that may arise in individual components due to the presence of excess water could represent a significant contribution to the existing body of research.
- Consider integrating W.I. and pressure swirl techniques in future projects to further optimize emission reduction strategies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AECC | Automotive Edge Computing Consortium |
| ATS | Aftertreatment system |
| BEV | Battery Electric Vehicles |
| BTE | Brake thermal efficiency |
| BSFC | Brake specific fuel consumption |
| CARB | Californian Air Research Board |
| CeO2 | Cerium oxide |
| CO | Carbon-monoxide |
| CO2 | Carbon-dioxide |
| DOC | Diesel Oxidation Catalyst |
| DPF | Diesel Particulate Filter |
| EGR | Exhaust Gas Recirculation |
| FeO | Iron oxide |
| HC | Hydrocarbon |
| HD | Heavy duty |
| HO2 | Hydroperoxyl |
| H2O | Water |
| ICE | Internal combustion engine |
| LD | Light duty |
| NEDC | New European Driving Cycle |
| NH3 | Ammonia |
| NH4 | Ammonium |
| NRMM | Non-road Mobile Machinery |
| NRSC | Non-road Steady-State Cycle |
| NRTC | Non-road Transient Cycle |
| NO | Nitric oxide |
| NOx | Nitrogen oxides |
| N2O | Nitrous oxide |
| N2 | Nitrogen |
| O | Oxygen |
| OEM | Original equipment manufacturer |
| OH | Hydroxyls |
| OSC | Oxigen storage capacity |
| PEMS | Portable emission measurement system |
| PGM | Platinum group materials |
| PM | Particulate mass |
| PM2.5 | Particulate mass size distribution larger than 2.5 µm |
| PC | Personal car |
| Pd | Palladium |
| PN | Particle number |
| PN23 | Particle number size distribution larger than 23 nm |
| PN10 | Particle number size distribution larger than 10 nm |
| Pt | Platinum |
| RDE | Real Driving Emission |
| REC | Retrofit Emission Control |
| SCR | Selective Catalytic Reduction |
| SiC | Silicon carbide |
| SOF | Soluble Organic Fraction |
| ULSD | Ultra low sulfur diesel |
| VCR | Variable Compression Ratio |
| V2O5 | Vanadium oxide |
| W.I. | Water introducing to combustion |
| ZEV | Zero Emission Vehicles |
References
- Lee, T.; Park, J.; Kwon, S.; Lee, J.; Kim, J. Variability in operation-based NOx emission factors with different test routes, and its effects on the real-driving emissions of light diesel vehicles. Sci. Total Environ. 2013, 461–462, 377–385. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A. NOx Pollutants from Diesel Vehicles and Trends in the Control Technologies. In Diesel and Gasoline Engines; IntechOpen: London, UK, 2020; p. 16. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Hirn, S.; Schleh, C. Nanoparticles in the lung. Nat. Biotechnol. 2010, 28, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.F.; Mudway, I.S.; Brown, J.L.; Stenfors, N.; Helleday, R.; Duggan, S.T.; Wilson, S.J.; Boman, C.; Cassee, F.R.; Frew, A.J.; et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur. Respir. J. 2006, 27, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Törnqvist, H.; Newby, D.E.; Robinson, S.D.; Gonzalez, M.; Darnley, K.; MacNee, W.; Boon, N.A.; Donaldson, K.; Blomberg, A.; et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005, 112, 3930–3936. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tian, L.; Ahmadi, G. Numerical assessment of respiratory airway exposure risks to diesel exhaust particles. Exp. Comput. Multiph. Flow 2019, 1, 51–59. [Google Scholar] [CrossRef]
- Baumüller, J.; Löbel, J.; Koch, E.; Thiel, W.R. Emission, Umwandlung, Imission. In Stadtklima und Luftreinhaltung; Springer: Berlin/Heidelberg, Germany, 1988; pp. 151–273. [Google Scholar] [CrossRef]
- Karagulian, F.; Van Dingenen, R.; Belis, C.A.; Janssens-Maenhout, G.; Crippa, M.; Guizzardi, D.; Dentener, F. Attribution of Anthropogenic PM2.5 to Emission Sources; European Commision, Joint Research Centre: Brussels, Belgium, 2017; p. 49. ISBN 978-92-79-66599-8. [Google Scholar] [CrossRef]
- EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008L0050-20150918 (accessed on 21 August 2023).
- European Environment Agency. Particulate Matter from Natural Sources and Related Reporting under the EU Air Quality Directive in 2008 and 2009; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar] [CrossRef]
- Viana, M.; Pey, J.; de Leeuw, F.; Querol, X.; Alastuey, A.; dall’Osto, M.; Moreno, T. Reporting on Natural Events in the EU Member States under Directive 2008/50/EC: Years 2008–2009, ETC/ACM Technical Paper 2011/17. 2011, p. 76. Available online: https://google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiLrczPi4KJAxUY7QIHHdO8EAEQFnoECBwQAQ&url=https%3A%2F%2Fwww.eionet.europa.eu%2Fetcs%2Fetc-atni%2Fproducts%2Fetc-atni-reports%2Fetcacm_tp_2011_17_naturalevents2008-09%2F%40%40download%2Ffile%2FETCACM_TP_2011_17_natural_events2008-2009.pdf&usg=AOvVaw2qTWKt755whcGlKsRQ-XV8&opi=89978449 (accessed on 16 March 2024).
- Järlskog, I.; Jaramillo-Vogel, D.; Rausch, J.; Gustafsson, M.; Strömvall, A.-M.; Andersson-Sköld, Y. Concentrations of tire wear microplastics and other traffic-derived non-exhaust particles in the road environment. Environ. Int. 2022, 170, 107618. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 2491–2504. [Google Scholar] [CrossRef]
- Grigoratos, T.; Mathissen, M.; Vedula, R.; Mamakos, A.; Agudelo, C.; Gramstat, S.; Giechaskiel, B. Interlaboratory Study on Brake Particle Emissions—Part I: Particulate Matter Mass Emissions. Atmosphere 2023, 14, 498. [Google Scholar] [CrossRef]
- WHO European Centre for Environment and Health. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project Technical Report. 2013. Available online: https://iris.who.int/bitstream/handle/10665/341712/WHO-EURO-2013-4101-43860-61757-eng.pdf?sequence=1 (accessed on 29 March 2024).
- European Environment Agency. Emissions of the Main Air Pollutants in Europe. 2023. Available online: https://www.eea.europa.eu/ims/emissions-of-the-main-air (accessed on 16 March 2024).
- European Environment Agency. Emissions of Air Pollutants from Transport in Europe. 2023. Available online: https://www.eea.europa.eu/en/analysis/indicators/emissions-of-air-pollutants-from?activeAccordion=ecdb3bcf-bbe9-4978-b5cf-0b136399d9f8 (accessed on 16 March 2024).
- McDonald, B.C.; Dallmann, T.R.; Martin, E.W.; Harley, R.A. Long-term trends in nitrogen oxide emissions from motor vehicles at national, state, and air basin scales. J. Geophys. Res. 2012, 117, 11. [Google Scholar] [CrossRef]
- Kalghatgi, G. Development of Fuel/Engine Systems—The Way Forward to Sustainable Transport. Engineering 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Ning, L.; Duan, Q.; Chen, Z.; Kou, H.; Liu, B.; Yang, B.; Zeng, K. A comparative study on the combustion and emissions of a non-road common rail diesel engine fueled with primary alcohol fuels (methanol, ethanol, and n-butanol)/diesel dual fuel. Fuel 2020, 266, 117034. [Google Scholar] [CrossRef]
- Biró, N.; Kiss, P. Emission Quantification for Sustainable Heavy-Duty Transportation. Sustainability 2023, 15, 7483. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Bautista, F.M.; Romero, A.A.; Luna, D. Internal Combustion Engines and Carbon-Neutral Fuels: A Perspective on Emission Neutrality in the European Union. Energies 2024, 17, 1172. [Google Scholar] [CrossRef]
- Milojević, S.; Glišović, J.; Savić, S.; Bošković, G.; Bukvić, M.; Stojanović, B. Particulate Matter Emission and Air Pollution Reduction by Applying Variable Systems in Tribologically Optimized Diesel Engines for Vehicles in Road Traffic. Atmosphere 2024, 15, 184. [Google Scholar] [CrossRef]
- Milojević, S.; Savić, S.; Maric, D.; Stopka, O.; Krstić, B.; Stojanovic, B. Correlation between Emission and Combustion Characteristics with the Compression Ratio and Fuel Injection Timing in Tribologically Optimized Diesel Engine. Teh. Vjesn. 2022, 29, 1210–1219. [Google Scholar] [CrossRef]
- Council of the European Union. Proposal for a Regulation of the European Parliament and of the Council on Type-Approval of Motor Vehicles and Engines and of Systems, Components and Separate Technical Units Intended for Such Vehicles, with Respect to Their Emissions and Battery Durability (Euro 7) and Repealing Regulations (EC) No 715/2007 and (EC) No 595/2009. 2023. Available online: https://data.consilium.europa.eu/doc/document/ST-16960-2023-REV-1/en/pdf (accessed on 6 April 2024).
- Turns, S.R. An Introduction to Combustión. Concepts and Applications, 2nd ed.; Holman, J.P., Lloyd, J., Eds.; McGraw Hill: New York, NY, USA, 2000; pp. 168–171. [Google Scholar]
- Lin, S.-L.; Lee, W.-J.; Lee, C.-F.F.; Wu, Y.-P. Reduction in emissions of nitrogen oxides, particulate matter, and polycyclic aromatic hydrocarbon by adding water-containing butanol into a diesel-fueled engine generator. Fuel 2012, 93, 364–372. [Google Scholar] [CrossRef]
- Fiebig, M.; Wiartalla, A.; Holderbaum, B.; Kiesow, S. Particulate emissions from diesel engines: Correlation between engine technology and emissions. J. Occup. Med. Toxicol. 2014, 9, 6. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, R.; Ni, J.; Chen, Q. Potential Improvement in PM-NOx Trade-Off in a Compression Ignition Engine by n-Octanol Addition and Injection Pressure. Processes 2021, 9, 310. [Google Scholar] [CrossRef]
- Ashwin, J.; Ashok, B.; Vignesh, R.; Saravanan, B.; Avinash, A. Chapter 3—NOx and PM trade-off in IC engines. In NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 2022; pp. 69–93. [Google Scholar] [CrossRef]
- Aminjan, K.K.; Sedaghat, M.; Heidari, M.; Khashehchi, M.; Mohammadzadeh, K.; Salahinezhad, M.; Bina, R. Numerical investigation of the impact of fuel temperature on spray characteristics in a pressure-swirl atomizer with spiral path. Exp. Comput. Multiph. Flow 2024, 1–8. [Google Scholar] [CrossRef]
- Chybowski, L.; Laskowski, R.; Gawdzińska, K. An overview of systems supplying water into the combustion chamber of diesel engines to decrease the amount of nitrogen oxides in exhaust gas. J. Mar. Sci. Technol. 2015, 20, 393–405. [Google Scholar] [CrossRef]
- Wróblewski, A.; Langer, A.; Szczyglak, P.; Rekúč, A. The influence of added water on fuel injector wear in a diesel engine. Tribologia 2018, 279, 153–158. [Google Scholar] [CrossRef]
- Holtbecker, R.; Geist, M. Exhaust Emissions Reduction Technology for Sulzer Marine Diesel Engines: General Aspects; Wärtsilä NSD Switzerland Ltd.: Winterthur, Switzerland, 1998. [Google Scholar]
- Vollenweider, J.; Geist, M.; Schaub, M. Residual fuels in emission-controlled diesel engines-Background, developments and operational results. In Proceedings of the 21st CIMAC Congress, Interlaken, Switzerland, 15–18 May 1995. [Google Scholar]
- May, J.; Bosteels, D.; Favre, C. A comparison of light-duty vehicle emissions over different test cycles and in real driving conditions. In Proceedings of the FISITA 2014 World Automotive Congress, Maastricht, The Netherlands, 2–6 June 2014; Association for Emissions Control by Catalysts (AECC): Brussels, Belgium, 2014. [Google Scholar]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Biró, N.; Szőllősi, D.; Kiss, P. Particle Counter Design Upgrade for Euro 7. Atmosphere 2023, 14, 1411. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Gruzieva, O.; Elihn, K.; Juárez-Facio, A.T.; Steimer, S.S.; Kuhn, J.; Silvergren, S.; Portugal, J.; Piña, B.; Olofsson, U.; et al. Toxicity and health effects of ultrafine particles: Towards an understanding of the relative impacts of different transport modes. Environ. Res. 2023, 231 Pt 2, 116186. [Google Scholar] [CrossRef]
- Marval, J.; Tronville, P. Ultrafine particles: A review about their health effects, presence, generation, and measurement in indoor environments. Build. Environ. 2022, 216, 108992. [Google Scholar] [CrossRef]
- Szőllősi, D.; Kiss, P. Effects of humidity on the emissions of the diesel engines. In Proceedings of the 16th European-African Regional Conference of the ISTVS, Lublin, Poland, 11–13 October 2023; International Society for Terrain-Vehicle Systems (ISTVS): Durham, NC, USA, 2023. [Google Scholar] [CrossRef]
- Gui, R.; Yan, Q.; Xue, T.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. The promoting/inhibiting effect of water vapor on the selective catalytic reduction of NOx. J. Hazard. Mater. 2022, 439, 129665. [Google Scholar] [CrossRef]
- Europian Comission. Proposal for a Regulation of the European Parliament and of the Council on Type-Approval of Motor Vehicles and Engines and of Systems, Components and Separate Technical Units Intended for Such Vehicles, with Respect to Their Emissions and Battery Durability (Euro 7) and Repealing Regulations (EC) No 715/2007 and (EC) No 595/2009. 2022. Available online: https://www.europarl.europa.eu/RegData/docs_autres_institutions/commission_europeenne/com/2022/0586/COM_COM(2022)0586_EN.pdf (accessed on 30 April 2024).
- Mulholland, E.; Joshua, M.; Yoann, B.; Kaylin, L.; Felipe, R. The role of NOx emission reductions in Euro 7/VII vehicle emission standards to reduce adverse health impacts in the EU27 through 2050. Transp. Eng. 2022, 9, 100133. [Google Scholar] [CrossRef]
- Ayetor, G.K.; Mbonigaba, I.; Sackey, M.N.; Andoh, P. Vehicle regulations in Africa: Impact on used vehicle import and new vehicle sales. Transp. Res. Interdiscip. Perspect. 2021, 10, 100384. [Google Scholar] [CrossRef]
- Deloitte. Deloitte Africa Automotive Insights: Navigating the African Automotive Sector: Ethiopia, Kenya and Nigeria. 2016. Available online: https://www2.deloitte.com/content/dam/Deloitte/za/Documents/deloitteafrica/ZA_Deloitte-Africa-automotive-insights-Ethiopia-Kenya-Nigeria-Apr16-2017.pdf (accessed on 30 April 2004).
- ACEA. Economic and Market Report Global and EU Auto Industry: Full Year 2023. 2024. Available online: https://www.acea.auto/files/Economic_and_Market_Report-Full_year_2023.pdf (accessed on 1 May 2004).
- Hagan, R.; Markey, E.; Clancy, J.; Keating, M.; Donnelly, A.; O’Connor, D.J.; Morrison, L.; McGillicuddy, E.J. Non-Road Mobile Machinery Emissions and Regulations: A Review. Air 2023, 1, 14–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Ma, C.; Zhang, Y.; Wu, L.; Mao, H. Emission characteristics and chemical composition of particulate matter emitted by typical non-road construction machinery. Atmos. Pollut. Res. 2020, 11, 679–685. [Google Scholar] [CrossRef]
- Lončarević, Š.; Ilinčić, P.; Šagi, G.; Lulić, Z. Problems and Directions in Creating a National Non-Road Mobile Machinery Emission Inventory: A Critical Review. Sustainability 2022, 14, 3471. [Google Scholar] [CrossRef]
- Luo, T.; Gorte, R.J. Characterization of SO2-poisoned ceria-zirconia mixed oxides. Appl. Catal. B Environ. 2004, 53, 77–85. [Google Scholar] [CrossRef]
- Corbett, J.J.; Fischbeck, P.S. Commercial Marine Emissions and Life-Cycle Analysis of Retrofit Controls in a Changing Science and Policy Environment. Nav. Eng. J. 2002, 114, 93–106. [Google Scholar] [CrossRef]
- Gunston, B. Jane’s Fighting Aircraft of WW II; Military Press: Sussex, UK, 1989; p. 318. ISBN 1851704930. [Google Scholar]
- Berni, F.; Breda, S.; Lugli, M.; Cantore, G. A Numerical Investigation on the Potentials of Water Injection to Increase Knock Resistance and Reduce Fuel Consumption in Highly Downsized GDI Engines. Energy Procedia 2015, 81, 826–835. [Google Scholar] [CrossRef]
- Raut, A.A.; Mallikarjuna, J.M. Effect of in-cylinder air-water interaction on water evaporation and performance characteristics of a direct water injected GDI engine. Eng. Sci. Technol. Int. J. 2021, 24, 480–492. [Google Scholar] [CrossRef]
- Imahashi, T.; Hashimoto, K.; Hayashi, J.I.; Yamada, T. Research on NOx Reduction for Large Marine Diesel Engines; ISME: Yokohama, Japan, 1995. [Google Scholar]
- Senčić, T.; Mrzljak, V.; Blecich, P.; Bonefačić, I. 2D CFD Simulation of Water Injection Strategies in a Large Marine Engine. J. Mar. Sci. Eng. 2019, 7, 296. [Google Scholar] [CrossRef]
- Andrews, G.E.; Bartle, K.; Pang, S.; Nurein, A.; Williams, P. The Reduction in Diesel Particulate Emissions Using Emulsified Fuels; SAE Technical Paper, 880348; SAE International: Warrendale, PA, USA, 1988. [Google Scholar] [CrossRef]
- Ishida, M.; Chen, Z. An Analysis of the Added Water Effect on NO Formation in D.I. Diesel Engines; SAE Technical Paper, 941691; SAE International: Warrendale, PA, USA, 1994. [Google Scholar] [CrossRef]
- Wan, J.; Zhuang, Y.; Huang, Y.; Qian, Y.; Qian, L. A review of water injection application on spark-ignition engines. Fuel Process. Technol. 2021, 221, 106956. [Google Scholar] [CrossRef]
- Prasad, H.S.; Gonsalvis, J.; Vijay, V.S. Effect of Introduction of Water into Combustion Chamber of Diesel Engines—A Review. Energy Power 2015, 5, 28–33. [Google Scholar]
- Sun, X.; Ning, J.; Liang, X.; Jing, G.; Chen, Y.; Chen, G. Effect of direct water injection on combustion and emissions characteristics of marine diesel engines. Fuel 2022, 309, 122213. [Google Scholar] [CrossRef]
- Quader, A.A. Why Intake Charge Dilution Decreases Nitric Oxide Emission from Spark Ignition Engines. SAE Trans. 1971, 80, 20–30. Available online: https://www.jstor.org/stable/44731349 (accessed on 3 May 2024).
- Sawa, N.; Kajitani, S. Physical Properties of Emulsion Fuel (Water/Oil-Type) and Its Effect on Engine Performance under Transient Operation; SAE Technical Paper, 920198; SAE International: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Mortellaro, F.; Silvestri, N.; Rolando, L.; Medda, M.; Corrigan, D. Water Injection Contribution to Enabling Stoichiometric Air-to-Fuel Ratio Operation at Rated Power Conditions of a High-Performance DISI Single Cylinder Engine; SAE Technical Paper, 2019-24-0173; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Miyamoto, N.; Ogawa, H.; Wang, J.; Ohashi, H. Significant NOx Reductions with Direct Water Injection into the Sub-Chamber of an IDI Diesel Engine; SAE Technical Paper, 950609; SAE International: Warrendale, PA, USA, 1995. [Google Scholar] [CrossRef]
- Schihl, P.; Tasdemir, J.; Bryzik, W. Determination of Laminar Flame Speed of Diesel Fuel for Use in a Turbulent Flame Spread Premixed Combustion Model. In Transformational Science and Technology for the Current and Future Force; World Scientific: Singapore, 2006; pp. 291–298. [Google Scholar] [CrossRef]
- Hountalas, D.; Mavropoulos, G.; Zannis, T.; Mamalis, S. Use of Water Emulsion and Intake Water Injection as NOx Reduction Techniques for Heavy Duty Diesel Engines; SAE Technical Paper, 2006-01-1414; SAE International: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Wang, X.; Zhou, J. Combustion and emission analysis of heavy-duty vehicle diesel engine. AIP Conf. Proc. 2017, 1820, 050003. [Google Scholar] [CrossRef]
- Neshat, E.; Bajestani, A.V.; Honnery, D. Advanced numerical analyses on thermal, chemical and dilution effects of water addition on diesel engine performance and emissions utilizing artificial inert species. Fuel 2019, 242, 596–606. [Google Scholar] [CrossRef]
- Fenimore, C.P. Formation of nitric oxide in premixed hydrocarbon flames. Symp. (Int.) Combust. 1971, 13, 373–380. [Google Scholar] [CrossRef]
- Bowman, C.T. Control of combustion-generated nitrogen oxide emissions: Technology driven by regulation. Symp. (Int.) Combust. 1992, 24, 859–878. [Google Scholar] [CrossRef]
- Dempsey, A.B.; Zeman, J.; Wall, M. A System to Enable Mixing Controlled Combustion with High Octane Fuels Using a Prechamber and High-Pressure Direct Injector. Front. Mech. Eng. 2021, 7, 637665. [Google Scholar] [CrossRef]
- Rente, T. Injection Strategies for Heavy Duty DI Diesel Engines. Ph.D Thesis, Chalmers University of Technology, Göteborg, Sweden, 2004. Available online: https://research.chalmers.se/publication/4323 (accessed on 4 May 2024).
- Glarborg, P. Detailed Kinetic Mechanisms of Pollutant Formation in Combustion Processes. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 11; Volume 45, pp. 603–645. ISBN 9780128195796. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Chen, S.; Zheng, C.; Li, J. Physical and chemical effects of CO2 and H2O additives on counterflow diffusion flame burning methane. Energy Fuels 2013, 27, 7602–7611. [Google Scholar] [CrossRef]
- Meng, S.; Sun, S.; Xu, H.; Guo, Y.; Feng, D.; Zhao, Y.; Wang, P.; Qin, Y. The effects of water addition on the laminar flame speeds of CO/H2/O2/H2O mixtures. Int. J. Hydrogen Energy 2016, 41, 10976–10985. [Google Scholar] [CrossRef]
- Blocquet, M.; Schoemaecker, C.; Amedro, D.; Herbinet, O.; Battin-Leclerc, F.; Fittschen, C. Quantification of OH and HO2 radicals during the low-temperature oxidation of hydrocarbons by Fluorescence Assay by Gas Expansion technique. Proc. Natl. Acad. Sci. USA 2013, 110, 20014–20017. [Google Scholar] [CrossRef]
- Srinivasan, N.K.; Michael, J.V. The thermal decomposition of water. Int. J. Chem. Kinet. 2006, 38, 211–219. [Google Scholar] [CrossRef]
- Dryer, F.L. Water addition to practical combustion systems—Concepts and applications. Symp. (Int.) Combust. 1977, 16, 279–295. [Google Scholar] [CrossRef]
- Konstandopoulos, A.G.; Kostoglou, M. Reciprocating flow regeneration of soot filters. Combust. Flame 2000, 121, 488–500. [Google Scholar] [CrossRef]
- Suarez-Corredor, A.F.; Bäbler, M.U.; Olsson, L.; Skoglundh, M.; Westerberg, B. Understanding the NH3 adsorption mechanism on a vanadium-based SCR catalyst: A data-driven modeling approach. Chem. Eng. Sci. 2022, 262, 117975. [Google Scholar] [CrossRef]
- Jasper, A.W. Predicting third-body collision efficiencies for water and other polyatomic baths. Faraday Discuss. 2022, 238, 68–86. [Google Scholar] [CrossRef]
- Ghaly, A.; Eldrainy, Y.; El-Maghlany, W.; Yousef, A. Novel thermal throttling model in spark ignition engines: A way to replace a mechanical one. Therm. Sci. Eng. Prog. 2017, 4, 223–230. [Google Scholar] [CrossRef]
- Ladommatos, N.; Abdelhalim, S.; Zhao, H.; Hu, Z. The Effects on Diesel Combustion and Emissions of Reducing Inlet Charge Mass Due to Thermal Throttling with Hot EGR; SAE Technical Paper 980185; SAE International: Warrendale, PA, USA, 1998; p. 11. [Google Scholar] [CrossRef]
- Ladommatos, N.; Abdelhalim, S.; Zhao, H. The Effects of Exhaust Gas Recirculation on Diesel Combustion and Emissions. Int. J. Engine Res. 2000, 1, 107–126. [Google Scholar] [CrossRef]
- Odaka, M.; Koike, N.; Tsukamoto, Y.; Narusawa, K.; Yoshida, K. Effects of EGR with a Supplemental Water Injection to Control Exhaust Emissions from Heavy-Duty Diesel Powered Vehicles; SAE Technical Paper 910739; SAE International: Warrendale, PA, USA, 1991. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Li, D.D.; Wang, C.; Chan, Q.N.; Yeoh, G.H. Soot: A review of computational models at different length scales. Exp. Comput. Multiph. Flow 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Ushakov, S.; Valland, H.; Nielsen, B.J.; Hennie, E. Particle size distributions from heavy-duty diesel engine operated on low-sulfur marine fuel. Fuel Process. Technol. 2013, 106, 350–358. [Google Scholar] [CrossRef]
- Liati, A.; Schreiber, D.; Dasilva, Y.A.R.; Eggenschwiler, P.D. Ultrafine particle emissions from modern Gasoline and Diesel vehicles: An electron microscopic perspective. Environ. Pollut. 2018, 239, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Rossomando, B.; Meloni, E.; De Falco, G.; Sirignano, M.; Arsie, I.; Palma, V. Experimental characterization of ultrafine particle emissions from a light-duty diesel engine equipped with a standard DPF. Proc. Combust. Inst. 2021, 38, 5695–5702. [Google Scholar] [CrossRef]
- Bedford, F.; Rutland, C.; Dittrich, P.; Raab, A.; Wirbeleit, F. Effects of Direct Water Injection on DI Diesel Engine Combustion; SAE Technical Paper, 2000-01-2938; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Tauzia, X.; Maiboom, A.; Shah, S.R. Experimental study of inlet manifold water injection on combustion and emissions of an automotive direct injection Diesel engine. Energy 2010, 35, 3628–3639. [Google Scholar] [CrossRef]
- Rounce, P.; Tsolakis, A.; York, A.P. E Speciation of particulate matter and hydrocarbon emissions from biodiesel combustion and its reduction by aftertreatment. Fuel 2012, 96, 90–99. [Google Scholar] [CrossRef]
- Worm, J. The Impact of Water Injection on Spark Ignition Engine Performance under High Load Operation. Ph.D. Dissertation, Michigan Technological University, Houghton, MI, USA, 2017. Available online: https://digitalcommons.mtu.edu/etdr/552 (accessed on 6 May 2024).
- Prasad, H.S.; Vijay, V.S.; Gonsalvis, J. Effect of direct water injection at different crank angles on diesel engine emission and performance. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012039. [Google Scholar] [CrossRef]
- Pamminger, M.; Wang, B.; Hall, C.M.; Vojtech, R.; Wallner, T. The impact of water injection and exhaust gas recirculation on combustion and emissions in a heavy-duty compression ignition engine operated on diesel and gasoline. Int. J. Engine Res. 2020, 21, 1555–1573. [Google Scholar] [CrossRef]
- Tsukahara, M.; Yoshimoto, Y.; Murayama, T. W/O Emulsion Realizes Low Smoke and Efficient Operation of DI Engines without High Pressure Injection; SAE Technical Paper 890449; SAE International: Warrendale, PA, USA, 1989. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Y.; Xu, G.; Duan, H.; Jia, M. Exploring the potential of water injection (WI) in a high-load diesel engine under different fuel injection strategies. Energy 2022, 243, 123074. [Google Scholar] [CrossRef]
- Sahin, Z.; Tuti, M.; Durgun, O. Experimental investigation of the effects of water adding to the intake air on the engine performance and exhaust emissions in a DI automotive diesel engine. Fuel 2014, 115, 884–895. [Google Scholar] [CrossRef]
- Kim, M.-S.; Akpudo, U.E.; Hur, J.-W. A Study on Water-Induced Damage Severity on Diesel Engine Injection System Using Emulsified Diesel Fuels. Electronics 2021, 10, 2285. [Google Scholar] [CrossRef]
- Rao, X.; Sheng, C.; Guo, Z.; Xu, C.; Dai, L.; Yuan, C. Corrosion behaviors of cylinder liner in marine diesel engine burning low sulfur fuel oil: An experimental and molecular dynamics simulation study. Tribol. Int. 2022, 171, 107575. [Google Scholar] [CrossRef]
- Karvounis, N.; Pang, K.M.; Mayer, S.; Walther, J.H. Numerical simulation of condensation of sulfuric acid and water in a large two-stroke marine diesel engine. Appl. Energy 2018, 211, 1009–1020. [Google Scholar] [CrossRef]
- Nockert, J.; Nyborg, L.; Norell, M. Corrosion of stainless steels in simulated diesel exhaust environment with urea. Mater. Corros. 2012, 63, 388–395. [Google Scholar] [CrossRef]
- Hashimoto, R.; Mori, G.; Yasir, M.; Tröger, U.; Wieser, H. Impact of Condensates Containing Chloride and Sulphate on the Corrosion in Automotive Exhaust Systems. Berg Hüttenmännische Monatshefte 2013, 158, 377–383. [Google Scholar] [CrossRef]
- Canfield, C.A. Effects of Diesel—Water Emulsion Combustion on Diesel Engine NOx Emissions. Master’s Thesis, University of Florida, Gainesville, FL, USA, 1999. Available online: https://apps.dtic.mil/sti/pdfs/ADA366907.pdf (accessed on 29 July 2024).
- Zhu, S.; Hu, B.; Akehurst, S.; Copeland, C.; Lewis, A.; Yuan, H.; Kennedy, I.; Bernards, J.; Branney, C. A review of water injection applied on the internal combustion engine. Energy Convers. Manag. 2019, 184, 139–158. [Google Scholar] [CrossRef]
- Subramanian, K.A. A comparison of water–diesel emulsion and timed injection of water into the intake manifold of a diesel engine for simultaneous control of NO and smoke emissions. Energy Convers. Manag. 2011, 52, 849–857. [Google Scholar] [CrossRef]
- Serrano, J.; Jiménez-Espadafor, F.I.; Lora, A.; Modesto-López, L.; Gañán-Calvo, A.; López-Serrano, J. Experimental analysis of NOx reduction through water addition and comparison with exhaust gas recycling. Energy 2019, 168, 737–752. [Google Scholar] [CrossRef]
- Cook, D.H.; Law, C.K. A preliminary study on the utilization of water-in-oil emulsions in Diesel engines. Combust. Sci. Technol. 1978, 18, 217–221. [Google Scholar] [CrossRef]
- Lif, A.; Holmberg, K. Water-in-diesel emulsions and related systems. Adv. Colloid Interface Sci. 2006, 123–126, 231–239. [Google Scholar] [CrossRef]
- Park, J.W.; Huh, K.Y.; Lee, J.H. Reduction of NOx, smoke and brake specific fuel consumption with optimal injection timing and emulsion ratio of water-emulsified diesel. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2001, 215, 83–93. [Google Scholar] [CrossRef]
- Fromager, M.; Schmelzle, P.; Schulz, P. Practical Experience Using a Diesel/Water Emulsion. In Proceedings of the 2nd International Symposium on Fuels and Lubricants, Symposium Papers, New Delhi, India, 10–12 March 2000; Volume 2, p. 435. [Google Scholar]
- Tsao, K.C.; Wang, C.L. Puffing and Micro-Explosion Phenomena of Water Emulsion Fuels. SAE Trans 1986, 95, 308–320. Available online: https://www.jstor.org/stable/44722629 (accessed on 29 July 2024).
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A.; Nissar, Z.; Sazhin, S. S Puffing/micro-explosion in composite fuel/water droplets heated in flames. Combust. Flame 2021, 233, 111599. [Google Scholar] [CrossRef]
- Rostampour, A.; Shojaeefard, M.H.; Molaeimanesh, G.R. Role of water micro-explosion on fuel droplet size distribution, engine performance, and emissions in a water-diesel emulsified engine: A comprehensive numerical investigation. Int. J. Engine Res. 2023, 24, 1110–1120. [Google Scholar] [CrossRef]
- Szőllősi, D.; Kiss, P. The combination of exhaust gas recirculation and water injection in a modern diesel engine. In Proceedings of the 16th European-African Regional Conference of the ISTVS, International Society for Terrain-Vehicle Systems (ISTVS), Yokohama, Japan, 11–13 October 2023. accepted for publication. [Google Scholar]
- Park, J.; Lee, K.-H.; Park, S. Comprehensive Spray Characteristics of Water in Port Fuel Injection Injector. Energies 2020, 13, 396. [Google Scholar] [CrossRef]
- Naik, G.G.; Dharmadhikari, H.M. Methods for reducing NOx and PM emissions in compression ignition engine: A review. Mater. Today Proc. 2023, 72 Pt 3, 1406–1412. [Google Scholar] [CrossRef]
- Mobility Engineering, Society of Automotive Engineering (SAE). 1 August 2015. Available online: https://www.mobilityengineeringtech.com/component/content/article/42141-sae-ma-01342 (accessed on 11 August 2024).
- Falfari, S.; Bianchi, G.M.; Cazzoli, G.; Forte, C.; Negro, S. Basics on Water Injection Process for Gasoline Engines. Energy Procedia 2018, 148, 50–57. [Google Scholar] [CrossRef]
- BMW Group. Pressclub Global, Article. 14 April 2016. Available online: https://www.press.bmwgroup.com/global/article/detail/T0236962EN/the-new-bmw-m4-gts (accessed on 28 March 2024).
- Bosch Media Service, Press Release, Mobility Solutions. 31 August 2016. Available online: https://www.boschmediaservice.hu/en/press_release/water-injection-107.html (accessed on 11 August 2024).
- Hountalas, D.T.; Mavropoulos, G.C.; Zannis, T. Comparative Evaluation of EGR, Intake Water Injection and Fuel/Water Emulsion as NOx Reduction Techniques for Heavy Duty Diesel Engines; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Bae, C.; Choi, M.; Kwak, Y. Effects of water direct injection on the torque enhancement and fuel consumption reduction of a gasoline engine under high-load conditions. Int. J. Engine Res. 2016, 17, 795–808. [Google Scholar] [CrossRef]
- Charlton, S.J. Developing Diesel Engines to Meet Ultra-Low Emission Standards; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Jacobs, T.; Assanis, D.; Filipi, Z. The Impact of Exhaust Gas Recirculation on Performance and Emissions of a Heavy-Duty Diesel Engine; SAE Technical Paper 2003-01-1068; SAE International: Warrendale, PA, USA, 2003. [Google Scholar] [CrossRef]
- De Serio, D.; De Oliveira, A.; Sodré, J.R. Effects of EGR rate on performance and emissions of a diesel power generator fueled by B7. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 1919–1927. [Google Scholar] [CrossRef][Green Version]
- Yutaka, T.; Takada, N.; Iida, N. Transient NOx Characteristics of Freight Vehicles with EGR System in Real Traffic Conditions. SAE Trans 2005, 114, 1129–1138. Available online: http://www.jstor.org/stable/44722067 (accessed on 15 August 2024).
- Vojtisek-Lom, M.; Fenkl, M.; Dufek, M.; Mareš, J. Off-Cycle, Real-World Emissions of Modern Light Duty Diesel Vehicles; SAE Technical Paper 2009-24-0148; SAE International: Warrendale, PA, USA, 2009. [Google Scholar] [CrossRef]
- Ladommatos, N.; Adelhalim, S.M.; Zhao, H.; Hu, Z. The effects of carbon dioxide in exhaust gas recirculation on diesel engine emissions. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 1998, 212, 25–42. [Google Scholar] [CrossRef]
- Szőllősi, D.; Kiss, P. Reactions on the typical temperatures of the diesel aftertreatment system. Mech. Eng. Lett. R D Res. Dev. 2023, 23, 179–198. [Google Scholar]
- Manufacturers of Emission Controls Association. Reports. December 2007. Available online: https://www.meca.org/wp-content/uploads/galleries/default-file/MECA%20Diesel%20White%20Paper%2012-07-07%20final.pdf (accessed on 16 August 2024).
- Caporali, R.; Chansai, S.; Burch, R.; Delgado, J.J.; Goguet, A.; Hardacre, C.; Mantarosie, L.; Thompsett, D. Critical role of water in the direct oxidation of CO and hydrocarbons in diesel exhaust after treatment catalysis. Appl. Catal. B Environ. 2014, 147, 764–769. [Google Scholar] [CrossRef]
- Karre, A.V.; Garlapalli, R.K.; Jena, A.; Tripathi, N. State of the art developments in oxidation performance and deactivation of diesel oxidation catalyst (DOC). Catal. Commun. 2023, 179, 106682. [Google Scholar] [CrossRef]
- Al-Aqtash, O.; Farkas, F.; Sápi, A.; Szenti, I.; Boldizsár, T.; Ábrahámné, K.B.; Kukovecz, Á.; Kónya, Z. Differently shaped Al2O3-based Pd catalysts loaded catalytic converter for novel non-road mobile machinery exhaust systems. React. Kinet. Mech. Catal. 2023, 136, 149–161. [Google Scholar] [CrossRef]
- Christensen, J.M.; Grunwaldt, J.D.; Jensen, A.D. Effect of NO2 and water on the catalytic oxidation of soot. Appl. Catal. B Environ. 2017, 205, 182–188. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Lietti, L. Oxidation of model soot by NO2 and O2 in the presence of water vapor. Chem. Eng. Sci. 2017, 173, 560–569. [Google Scholar] [CrossRef]
- Cho, S.M. Properly apply selective catalytic reduction for NOx removal. Chem. Eng. Prog. 1994, 90, 39–45. [Google Scholar]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction Pathways in the Selective Catalytic Reduction Process with NO and NO2 at Low Temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Raimondi, F.; Wokaun, A. Enhanced Reoxidation of Vanadia by NO2 in the Fast SCR Reaction. J. Catal. 2002, 209, 159–165. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Bregani, F.; Forzatti, P. Fourier Transform-Infrared Study of the Adsorption and Coadsorption of Nitric Oxide, Nitrogen Dioxide and Ammonia on Vanadia-Titania and Mechanism of Selective Catalytic Reduction. Appl. Catal. 1990, 64, 259–278. [Google Scholar] [CrossRef]
- Zhang, B.; Liebau, M.; Suprun, W.; Liu, B.; Zhang, S.; Gläser, R. Suppression of N2O formation by H2O and SO2 in selective catalytic reduction of NO with NH3 over Mn/Ti–Si catalyst. Catal. Sci. Technol. 2019, 9, 4759–4770. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Tan, D.; Ye, Y.; Zhang, B.; Huang, B.; Zhong, W.; Zhang, J.; Hu, J. Overview of mechanisms of promotion and inhibition by H2O for selective catalytic reduction denitrification. Fuel Process. Technol. 2023, 252, 107956. [Google Scholar] [CrossRef]




| EGR | W.I. | |
|---|---|---|
| NOx removal | xxx | xxx |
| Particulates removal | -x | - |
| Dilution effect | xxx | xx |
| Thermal effect | - | xxx |
| Chemical effect | x | x |
| Thermal throttling | -x | x |
| Inlet temp. effect | -x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szőllősi, D.; Kiss, P. Effects of Water Injection in Diesel Engine Emission Treatment System—A Review in the Light of EURO 7. Energies 2024, 17, 5107. https://doi.org/10.3390/en17205107
Szőllősi D, Kiss P. Effects of Water Injection in Diesel Engine Emission Treatment System—A Review in the Light of EURO 7. Energies. 2024; 17(20):5107. https://doi.org/10.3390/en17205107
Chicago/Turabian StyleSzőllősi, Dániel, and Péter Kiss. 2024. "Effects of Water Injection in Diesel Engine Emission Treatment System—A Review in the Light of EURO 7" Energies 17, no. 20: 5107. https://doi.org/10.3390/en17205107
APA StyleSzőllősi, D., & Kiss, P. (2024). Effects of Water Injection in Diesel Engine Emission Treatment System—A Review in the Light of EURO 7. Energies, 17(20), 5107. https://doi.org/10.3390/en17205107







