Review of AEM Electrolysis Research from the Perspective of Developing a Reliable Model
Abstract
1. Introduction
1.1. Electrolyzer Market
1.2. Hydrogen Production
1.3. Chemical and Industrial Processes
1.4. Energy Storage
1.5. Grid Balancing
1.6. Global Energy Transition
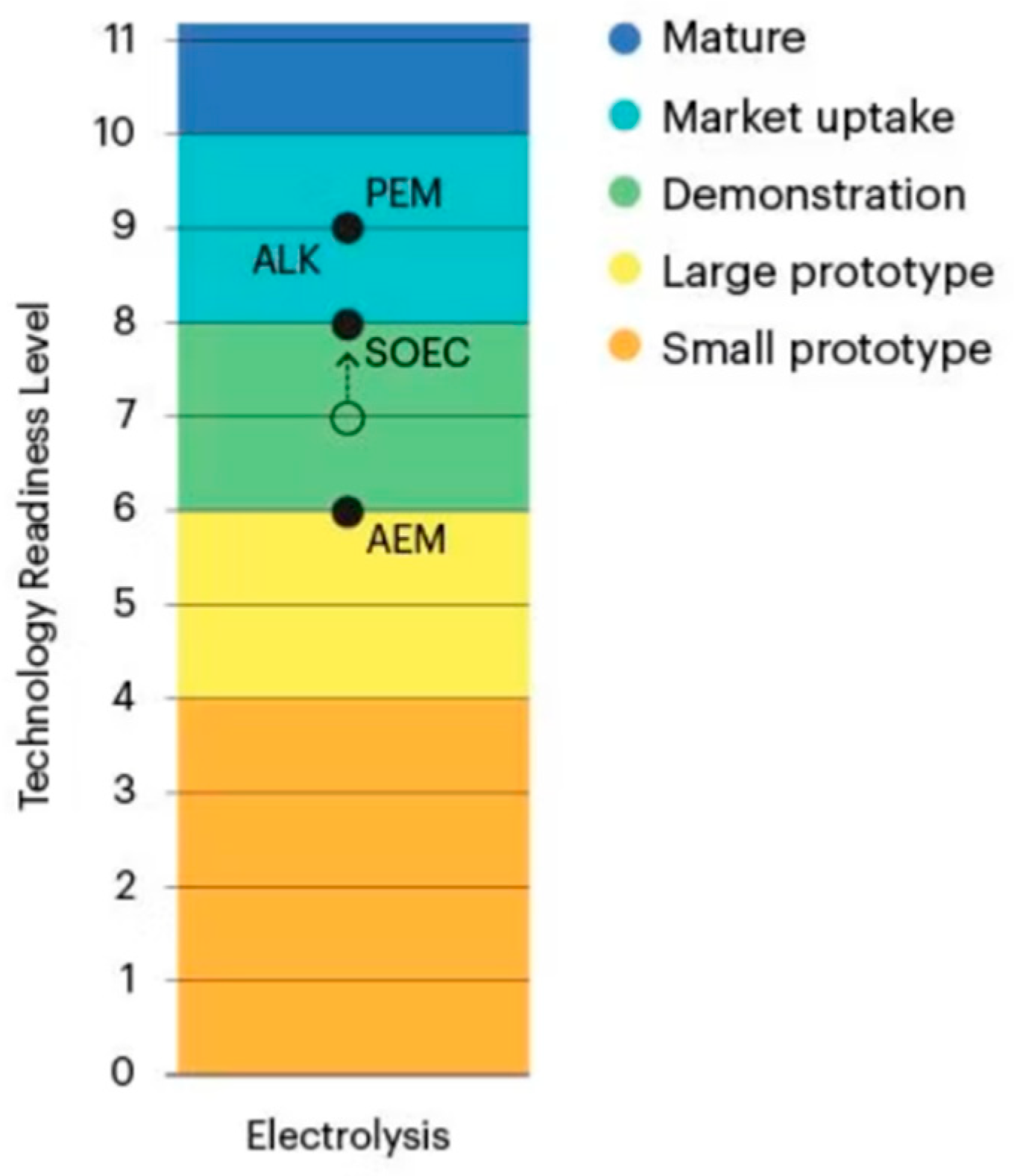
1.7. Technology Description
1.8. Research Overview
1.9. Electrolyzer Efficiency
2. An Overview of Laboratory Investigation Techniques for AEM Membrane-Based Electrolysis
- Research on Improved Anion-Conductive Polymer Materials (Membranes and Ionomers): This area focuses on developing new materials that enhance the functionality and efficiency of anion-exchange membranes and associated ionomers. The goal is to create polymers that provide better chemical stability and improved ion exchange capacities.
- Research on Improving Hydroxide Ion Conductivity: This research aims to enhance the ionic conductivity of hydroxide ions within the electrolyte systems, which is crucial for increasing the efficiency of electrochemical reactions in systems like fuel cells and electrolyzers.
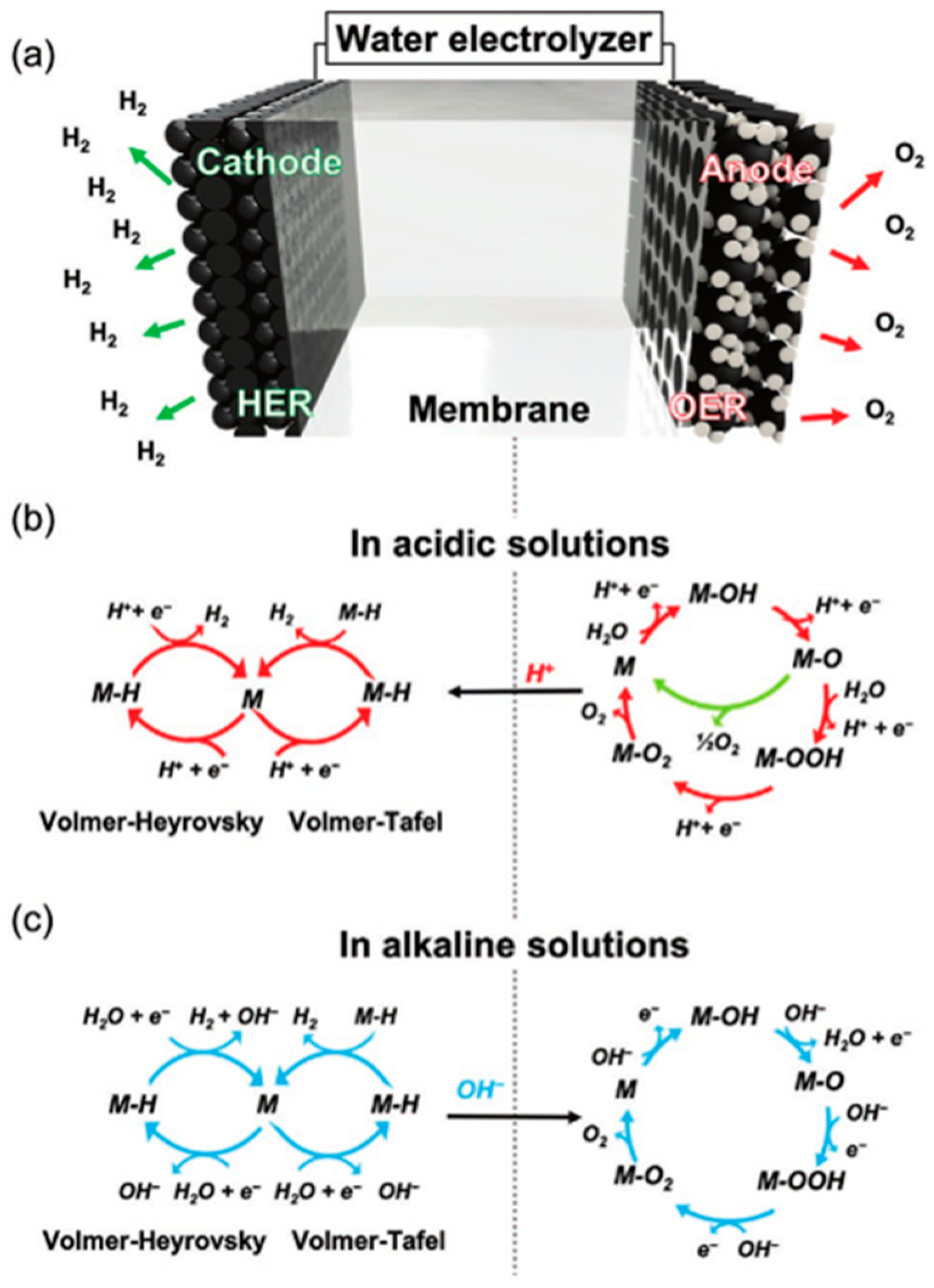
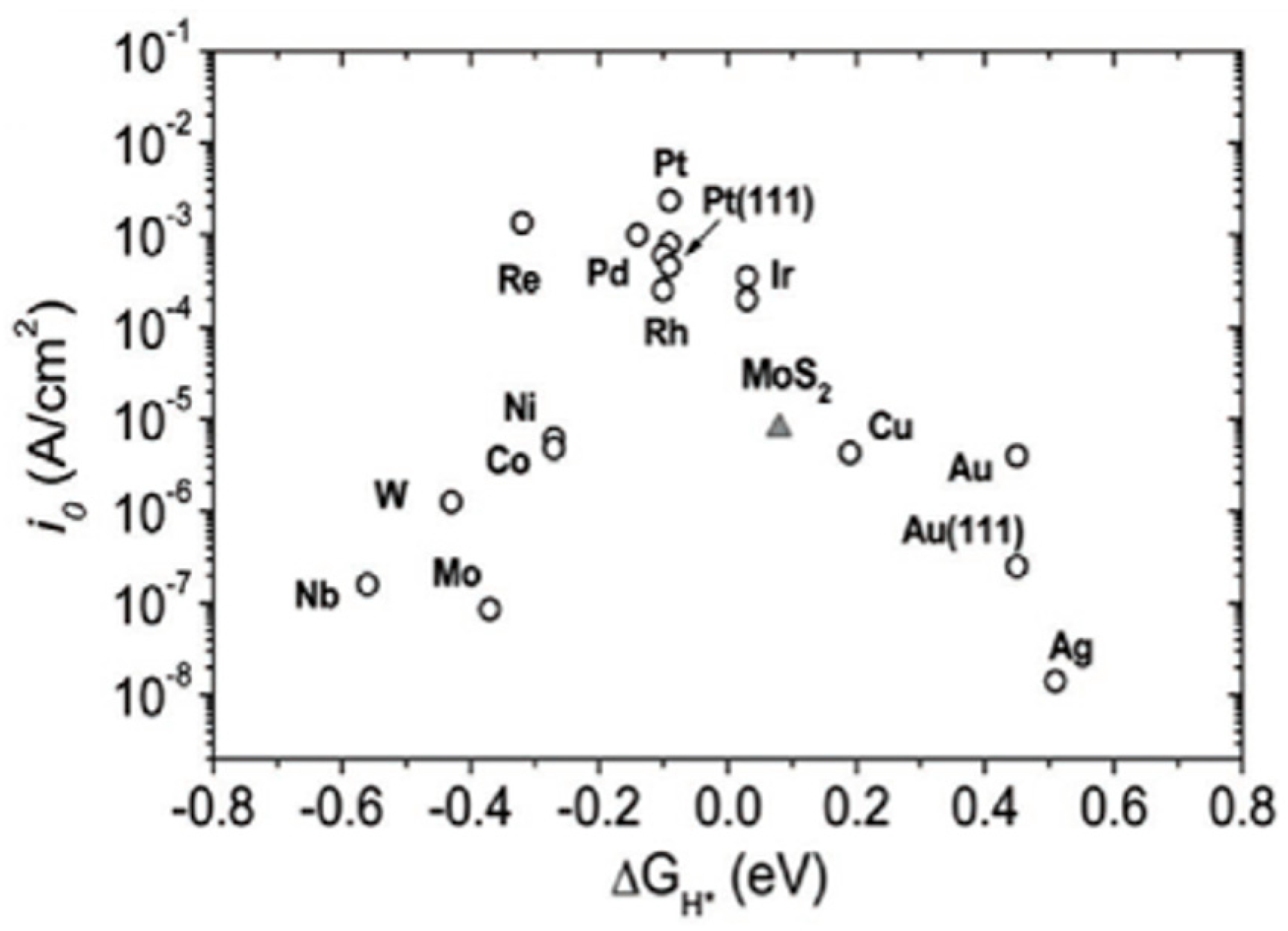
- Research on the Phenomena of Oxygen Evolution Reaction (OER) and Hydrogen Evolution Reaction (HER): Investigating these fundamental electrochemical reactions is essential for optimizing the efficiency of water electrolysis. Understanding the kinetics and mechanisms of OER and HER can lead to significant improvements in energy conversion rates.
- Physical and Electrochemical Characterization of MEA (Membrane Electrode Assembly): This research involves detailed studies of the MEA’s properties, focusing on aspects such as durability, ion transport, and electrochemical stability. Physical and electrochemical techniques are employed to evaluate how different materials and construction techniques affect the performance of the MEA.
- Implementation and Scaling up through the Commercialization of this Electrolysis Method: The final step involves taking the research from the lab to the market, which includes scaling up the production processes and implementing the technology in commercial applications. This phase is critical for assessing the viability of the electrolysis methods and ensuring they meet industry standards for commercial use.
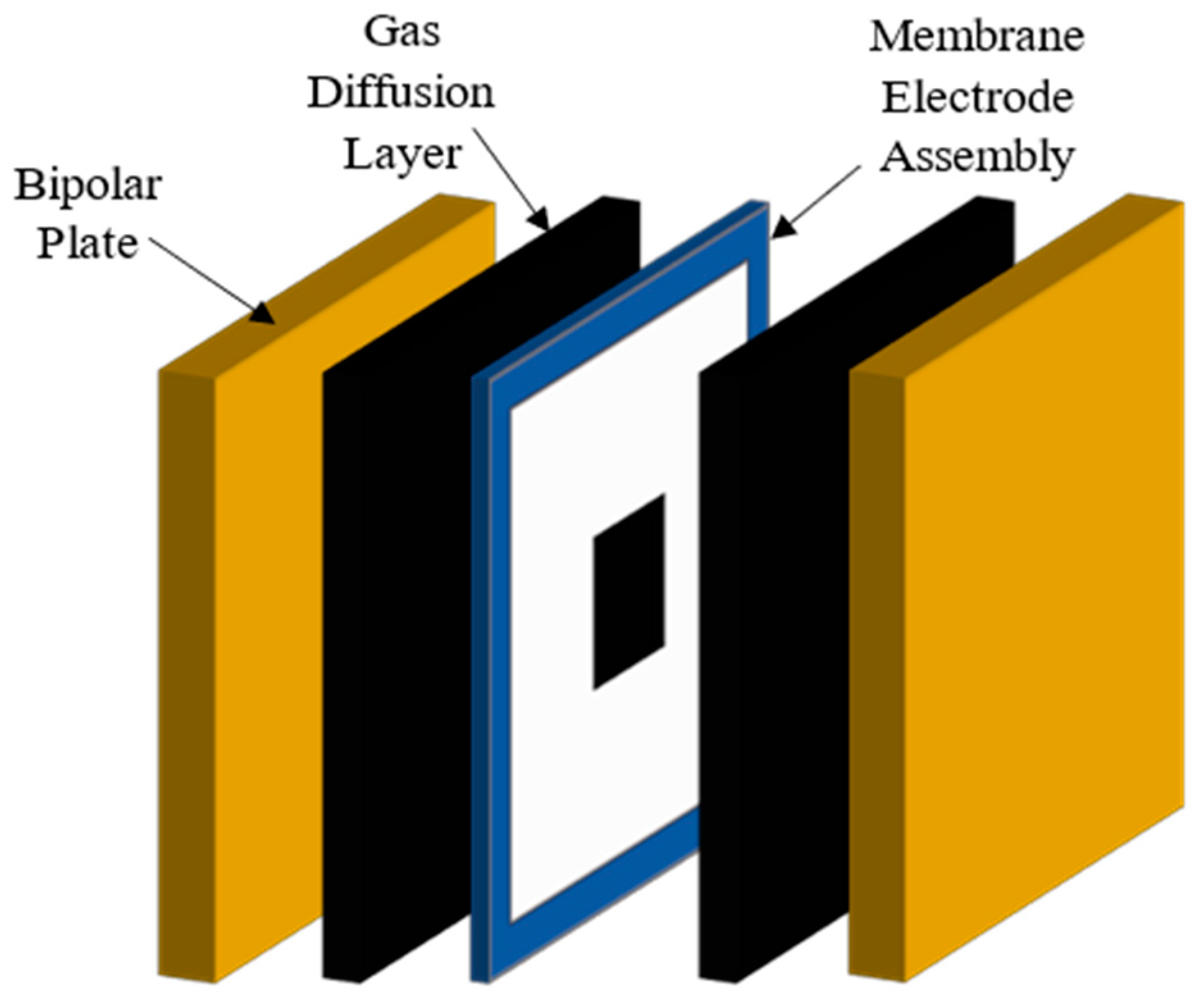
3. Cell Components
3.1. Electrolyte Handling in Anion-Exchange Membrane Electrolyzers
3.2. Materials
3.3. Membrane
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. Available online: https://www.iea.org/ (accessed on 3 April 2024).
- Jin, H.; Ruqia, B.; Park, Y.; Kim, H.J.; Oh, H.S.; Choi, S.I.; Lee, K. Nanocatalyst Design for Long-Term Operation of Proton/Anion Exchange Membrane Water Electrolysis. Adv. Energy Mater. 2021, 11, 2003188. [Google Scholar] [CrossRef]
- Cho, M.K.; Lim, A.; Lee, S.Y.; Kim, H.J.; Yoo, S.J.; Sung, Y.E.; Park, H.S.; Jang, J.H. A Review on Membranes and Catalysts for Anion Exchange Membrane Water Electrolysis Single Cells. J. Electrochem. Sci. Technol. 2017, 8, 183–196. [Google Scholar] [CrossRef]
- Lim, A.; Kim, H.J.; Henkensmeier, D.; Jong Yoo, S.; Young Kim, J.; Young Lee, S.; Sung, Y.E.; Jang, J.H.; Park, H.S. A Study on Electrode Fabrication and Operation Variables Affecting the Performance of Anion Exchange Membrane Water Electrolysis. J. Ind. Eng. Chem. 2019, 76, 410–418. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, S.Y.; Oh, S.H.; Kim, J.K.; Lim, M.S.; Ahn, C.Y.; Cho, Y.H.; Sung, Y.E. High-performance anion-exchange membrane water electrolysis. Electrochim. Acta 2019, 295, 99–106. [Google Scholar] [CrossRef]
- Pavel, C.C.; Cecconi, F.; Emiliani, C.; Santiccioli, S.; Scaffidi, A.; Catanorchi, S.; Comotti, M. Highly Efficient Platinum Group Metal Free Based Membrane-Electrode Assembly for Anion Exchange Membrane Water Electrolysis. Angew. Chemie 2014, 126, 1402–1405. [Google Scholar] [CrossRef]
- Spa, A. Acta Spa Products 2024. Available online: https://www.enapter.com/aem-electrolysis/ (accessed on 3 April 2024).
- Park, J.H.; Yoon, H.C.; Kim, J.N.; Jeong, C.H.; Jeong, E.Y.; Yun, D.S.; Yoon, H.; Park, S.H.; Han, M.H.; Yoo, C.Y. Anion-Exchange-Membrane-Based Electrochemical Synthesis of Ammonia as a Carrier of Hydrogen Energy. Korean J. Chem. Eng. 2018, 35, 1620–1625. [Google Scholar] [CrossRef]
- Cho, M.K.; Park, H.Y.; Lee, H.J.; Kim, H.J.; Lim, A.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; et al. Alkaline Anion Exchange Membrane Water Electrolysis: Effects of Electrolyte Feed Method and Electrode Binder Content. J. Power Sources 2018, 382, 22–29. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A Review of the Synthesis and Characterization of Anion Exchange Membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Ito, H.; Kawaguchi, N.; Someya, S.; Munakata, T.; Miyazaki, N.; Ishida, M.; Nakano, A.; Kozmai, A.; Pismenskaya, N.; Nikonenko, V.; et al. Experimental Investigation of Electrolytic Solution for Anion Exchange Membrane Water Electrolysis. Int. J. Hydrogen Energy 2018, 39, 1728. [Google Scholar] [CrossRef]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Zhang, J.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Anion Exchange Membrane Water Electrolyzer: Electrode Design, Lab-Scaled Testing System and Performance Evaluation. EnergyChem 2022, 4, 100087. [Google Scholar] [CrossRef]
- Milewski, J.; Wejrzanowski, T.; Fung, K.Z.; Szczśniak, A.; Ćwieka, K.; Tsai, S.Y.; Dybiński, O.; Skibiński, J.; Tang, J.Y.; Szabłowski, Ł. Supporting ionic conductivity of Li2CO3/K2CO3 molten carbonate electrolyte by using yttria stabilized zirconia matrix. Int. J. Hydrogen Energy 2021, 46, 14977–14987. [Google Scholar] [CrossRef]
- Cossar, E.; Barnett, A.O.; Seland, F.; Baranova, E.A. The Performance of Nickel and Nickel-Iron Catalysts Evaluated as Anodes in Anion Exchange Membrane Water Electrolysis. Catalysts 2019, 9, 814. [Google Scholar] [CrossRef]
- Faid, A.Y.; Xie, L.; Barnett, A.O.; Seland, F.; Kirk, D.; Sunde, S. Effect of Anion Exchange Ionomer Content on Electrode Performance in AEM Water Electrolysis. Int. J. Hydrogen Energy 2020, 45, 28272–28284. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gil-Rostra, J.; Escudero, C.; Villar-García, I.J.; Yubero, F.; de Lucas Consuegra, A.; González-Elipe, A.R. Active Sites and Optimization of Mixed Copper-Cobalt Oxide Anodes for Anion Exchange Membrane Water Electrolysis. J. Power Sources 2021, 485, 229217. [Google Scholar] [CrossRef]
- Xu, J.; Amorim, I.; Li, Y.; Li, J.; Yu, Z.; Zhang, B.; Araujo, A.; Zhang, N.; Liu, L. Stable Overall Water Splitting in an Asymmetric Acid/Alkaline Electrolyzer Comprising a Bipolar Membrane Sandwiched by Bifunctional Cobalt-Nickel Phosphide Nanowire Electrodes. Carbon Energy 2020, 2, 646–655. [Google Scholar] [CrossRef]
- Park, Y.S.; Yang, J.; Lee, J.; Jang, M.J.; Jeong, J.; Choi, W.S.; Kim, Y.; Yin, Y.; Seo, M.H.; Chen, Z.; et al. Superior Performance of Anion Exchange Membrane Water Electrolyzer: Ensemble of Producing Oxygen Vacancies and Controlling Mass Transfer Resistance. Appl. Catal. B Environ. 2020, 278, 119276. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Raja Sulaiman, R.R.; Wong, W.Y.; Loh, K.S. Recent Developments on Transition Metal–Based Electrocatalysts for Application in Anion Exchange Membrane Water Electrolysis. Int. J. Energy Res. 2022, 46, 2241–2276. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. A Review of Alkaline Solid Polymer Membrane in the Application of AEM Electrolyzer: Materials and Characterization. Int. J. Energy Res. 2021, 45, 18337–18354. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, Y.S.; Woo, S.; Yang, J.; Jang, M.J.; Jeong, J.; Kwon, N.; Lim, B.; Han, J.W.; et al. High-Efficiency Anion-Exchange Membrane Water Electrolyzer Enabled by Ternary Layered Double Hydroxide Anode. Small 2021, 17, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Park, J.E.; Jang, G.Y.; Kim, O.H.; Kwon, O.J.; Cho, Y.H.; Sung, Y.E. High-Performance and Durable Water Electrolysis Using a Highly Conductive and Stable Anion-Exchange Membrane. Int. J. Hydrogen Energy 2022, 47, 9115–9126. [Google Scholar] [CrossRef]
- Park, J.E.; Choi, H.J.; Kang, S.Y.; Jang, G.Y.; Kim, O.H.; Karuppannan, M.; Sung, Y.E.; Kwon, O.J.; Cho, Y.H. Effect of Pore Structures in Nickel-Based Porous Transport Layers for High-Performance and Durable Anion-Exchange Membrane Water Electrolysis. Int. J. Energy Res. 2022, 46, 16670–16678. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Jang, M.J.; Habibpour, S.; Chen, Z.; Fowler, M. NiFeOx and NiFeCoOx Catalysts for Anion Exchange Membrane Water Electrolysis. Electrochem 2022, 3, 843–861. [Google Scholar] [CrossRef]
- Zeng, L.; Yuan, W.; Ma, X.; He, Q.; Zhang, L.; Wang, J.; Wei, Z. Dual-Cation Interpenetrating Polymer Network Anion Exchange Membrane for Fuel Cells and Water Electrolyzers. Macromolecules 2022, 55, 4647–4655. [Google Scholar] [CrossRef]
- Khataee, A.; Shirole, A.; Jannasch, P.; Krüger, A.; Cornell, A. Anion Exchange Membrane Water Electrolysis Using AemionTM Membranes and Nickel Electrodes. J. Mater. Chem. A 2022, 10, 16061–16070. [Google Scholar] [CrossRef]
- Sankar, S.; Roby, S.; Kuroki, H.; Miyanishi, S.; Tamaki, T.; Anilkumar, G.M.; Yamaguchi, T. High-Performing Anion Exchange Membrane Water Electrolysis Using Self-Supported Metal Phosphide Anode Catalysts and an Ether-Free Aromatic Polyelectrolyte. ACS Sustain. Chem. Eng. 2023, 11, 854–865. [Google Scholar] [CrossRef]
- Khalid, H.; Najibah, M.; Park, H.S.; Bae, C.; Henkensmeier, D. Properties of anion exchange membranes with a focus on water electrolysis. Membranes 2022, 12, 989. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative study of anion exchange membranes for low-cost water electrolysis. Int. J. Hydrogen Energy 2020, 45, 26070–26079. [Google Scholar] [CrossRef]
- Lei, C.; Yang, K.; Wang, G.; Wang, G.; Lu, J.; Xiao, L.; Zhuang, L. Impact of catalyst reconstruction on the durability of anion exchange membrane water electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 16725–16733. [Google Scholar] [CrossRef]
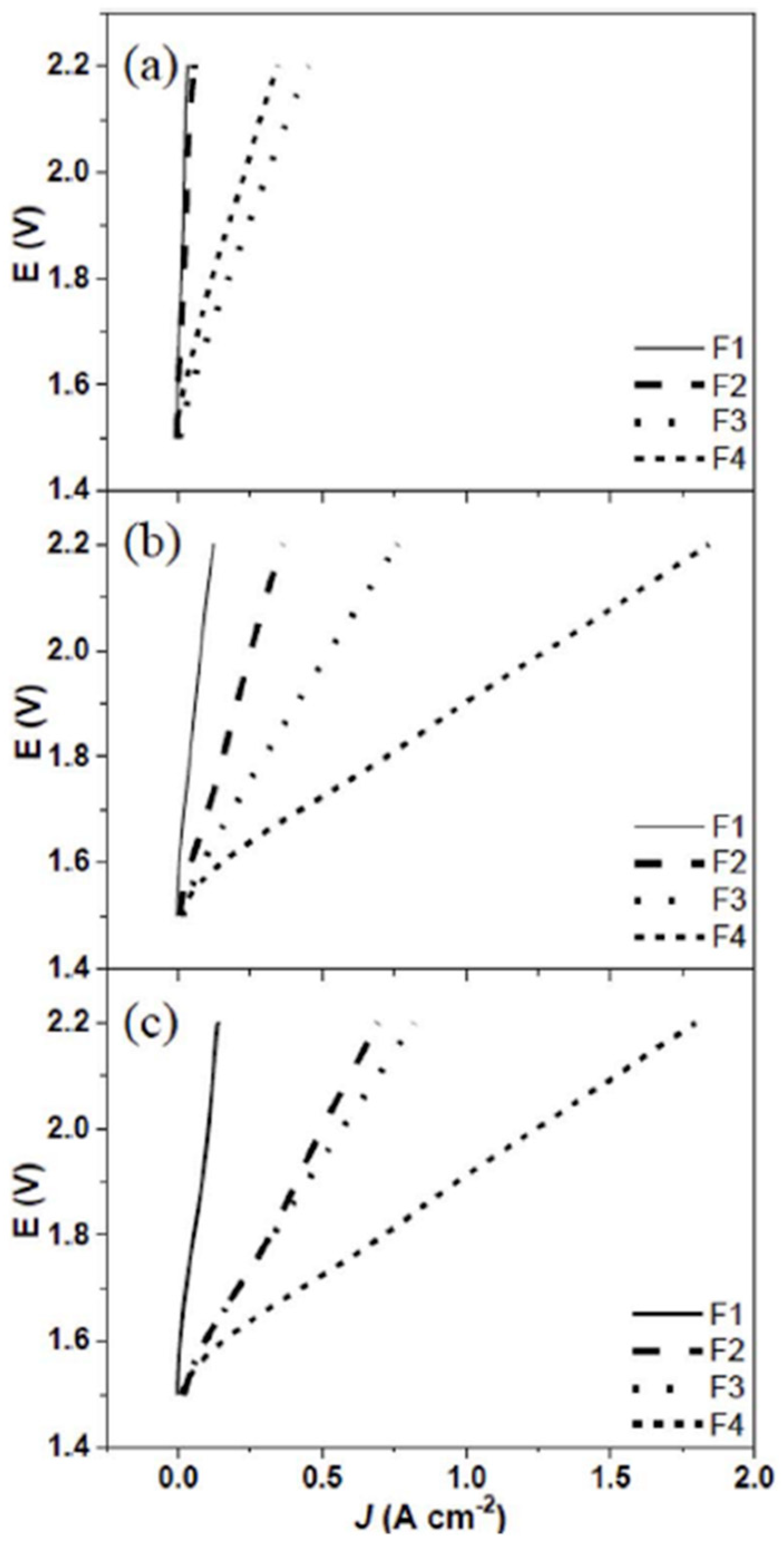
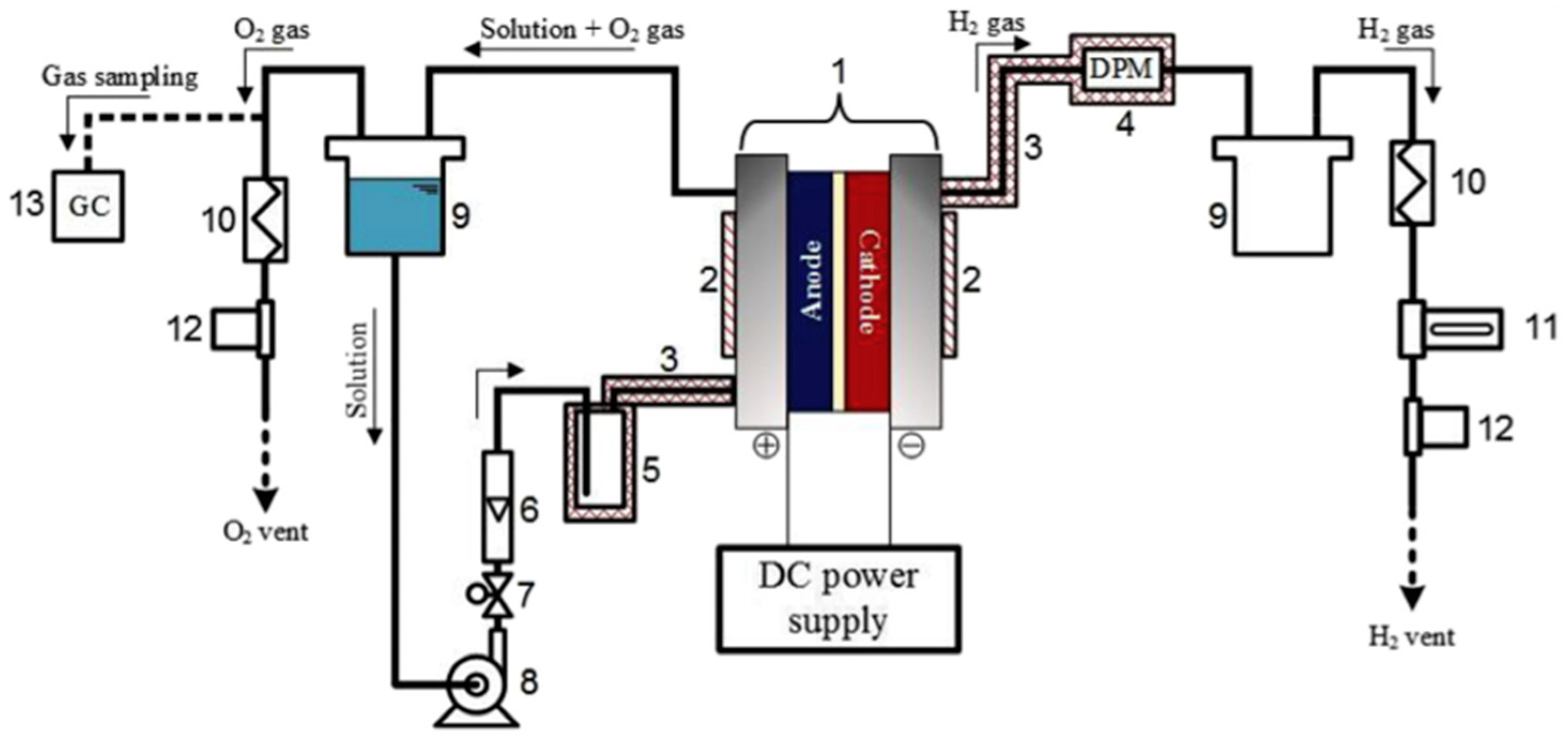
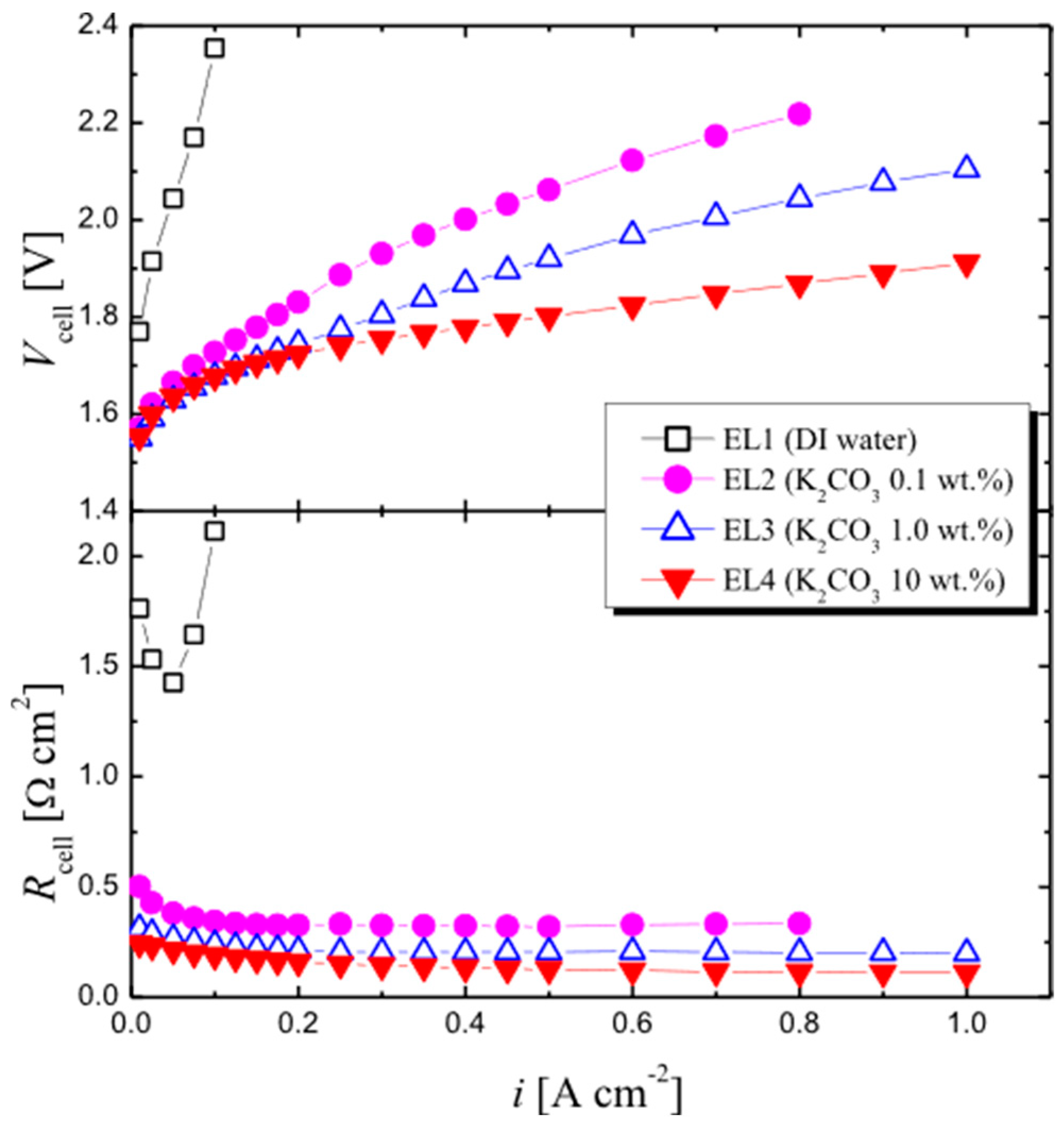
| Notation | Electrolyte Solution | Catalyst Loading | |||
|---|---|---|---|---|---|
| Solute | Concentration | pH | Anode (CuCoOx) | Cathode (Pt) | |
| EL1 | DI water | DI water | 10.8 | 4.2 mg cm−2 | 1.8 mg cm−2 |
| EL2 | K2CO3 | 0.1 wt% (7.2 mM) | 10.8 | 4.2 mg cm−2 | 1.7 mg cm−2 |
| EL3 | K2CO3 | 1.0 wt% (72 mM) | 11.3 | 4.0 mg cm−2 | 1.7 mg cm−2 |
| EL4 | K2CO3 | 10 wt% (720 mM) | 11.8 | 4.1 mg cm−2 | 1.7 mg cm−2 |
| EL5 | KOH | 10 mM | 12.0 | 4.1 mg cm−2 | 1.7 mg cm−2 |
| Sample | Feed Type | Electrodes | ||||
|---|---|---|---|---|---|---|
| Initial Feed | Operating Feed | Binder Content wt% | ||||
| Anode OER | Cathode HER | Anode OER | Cathode HER | Anode OER | Cathode HER | |
| F1 | 0.5 M KOH | DI water | 0.5 M KOH | DI water | 20 | 9 |
| F2 | 0.5 M KOH | DI water | 0.5 M KOH | None | ||
| F3 | 0.5 M KOH | 0.5 M KOH | 0.5 M KOH | DI water | ||
| F4 | 0.5 M KOH | 0.5 M KOH | 0.5 M KOH | None | ||
| BC5 | 0.5 M | DI water | 0.5 M | None | 5 | 9 |
| BC9 | KOH | KOH | 9 | 9 | ||
| BC15 | 15 | 9 | ||||
| BC20 | 20 | 9 | ||||
| Parameters\Source | No | [8] | [22] | [18] | [18] | [18] | ||
|---|---|---|---|---|---|---|---|---|
| material | anode | surface | 1 | 7.4 cm2 | ||||
| thickness | 2 | |||||||
| conductivity | 3 | |||||||
| porosity | 4 | |||||||
| material | 5 | 20% platinum on carbon surface 0.5 mg/cm2 | CuCo-oxide on nickel foam + IrO2 catalyst in the amount of 4 mg/cm2 on nickel PTFE foam | 5% Nafion as an ionic binder with a nickel channel | form of NiFe2O4 on nickel fiber paper | 5% Nafion as an ionic binder with a nickel channel | ||
| electrolyte/membrane | surface | 6 | ||||||
| thickness | 7 | |||||||
| conductivity | 8 | 0.32 mS/dm | ||||||
| porosity | 9 | |||||||
| material | 10 | AEM anion-exchange membrane, X37-50 grade T | KOH electrolyte in various concentrations | |||||
| cathode | surface | 11 | 4.9 cm2 | |||||
| thickness | 12 | |||||||
| conductivity | 13 | |||||||
| porosity | 14 | 40% wt | ||||||
| material | 15 | 20% platinum on carbon surface of 0.5 mg/cm2 | 1 mg/cm2 of Pt/C, on carbon-coated paper | 5% Nafion as an ionic binder with a nickel channel | NiFeCo on stainless steel fiber paper | 5% Nafion as an ionic binder with a nickel channel | ||
| thermal flow | temperature | 16 | 20 °C | 45 °C | 20 °C | |||
| working pressure | 17 | 1 atm | 1 atm | |||||
| flow | anode | 18 | water | KOH 3–5 mL/min | deionized water | KOH 3–5 mL/min | ||
| cathode | 19 | 100 mL/min Nitrogen | KOH 3–5 mL/min | KOH 3–5 mL/min | ||||
| experimental data | U-I curve | 20 | + | + | ||||
| EIS | 21 | + | + | |||||
| L.P. | [6] | [9] | [25] | [25] | [25] |
|---|---|---|---|---|---|
| 1 | 100 m2/g | 2.5 cm × 2.5 cm | 5 cm2 | 5 cm2 | 1.8 cm × 1.8 cm. |
| 2 | the thickness of catalyst layer 4.79, 10.11, 12.52 µm | the thickness of catalyst layer 4.79, 10.11, 12.52 µm | final thickness of the electrode was 0.75 mm on 1.7 mm nickel foam | ||
| 3 | |||||
| 4 | 0.35 cm3/g | ||||
| 5 | ACTA 3030, Cuco Ox, synthesized by co-precipitation of Co (NO3)2·6H2O and CuSO4·5H2O | IrO2 (Premion®, Alfa Aesar, Haverhill, MA, USA) + polytetrafluoroethylene (PTFE) binder (60 wt% PTFE dispersion in H2O, Aldrich) | The 40 wt% Pt/C with iridium oxide (IrO2) with a loading amount of 1.0, 2.0, 3.0 mg cm−2 (Premion®, Alfa Aesar, USA) | The 40 wt% Pt/C with iridium oxide (IrO2) with a loading amount of 1.0, 2.0, and 3.0 mg cm−2 (Premion®, Alfa Aesar, USA) | NiO, NiFeOx, and NiFeCoOx catalysts deposited on the nickel foam (thickness of 1.7 mm), amount of 25 mg/cm2 |
| 6 | |||||
| 7 | 28 micro m (in dry form) | 50 µm | 30 µm | ||
| 8 | 12 mS/cm (HCO3− form) | 1.70 mohm/cm2 | 1.30 mohm/cm2 | ||
| 9 | |||||
| 10 | Membrane: A-201 from Tokuyama Corporation, Electrolyte: diluted carbonate/bicarbonate aqueous solution, such as 1 wt% K2CO3 or 1 wt% K2CO3/KHCO3 (0.67% and 0.33%, respectively) | A201, Tokuyama | FAA-3 (Fumatech Co., Bietigheim-Bissingen, Germany) | Orion TM1 (Orion Polymer, Cohoes, NY, USA) | Fumasep FAA-3-50 or dioxide membrane X-37-50 grade T), soaked in a 1.0 M KOH solution overnight (about 24 h) and rinsed with distilled water |
| 11 | 180 m2/g | 2.5 cm × 2.5 cm | 5 cm2 | 5 cm2 | 1.8 cm × 1.8 cm. |
| 12 | 250 μm thick titanium papers | ||||
| 13 | |||||
| 14 | 0.59 cm3/g | ||||
| 15 | ACTA 4030, nickel-based nanostructured material with the transition metal deposited on CeO2-La2O3/carbon support | Pt/C (Pt 46.5 wt%, Tanaka K.K.), PTFE contents of 5, 9, 15, and 20 wt% (with respect to the total solid weight) were used, and 250 μm thick titanium papers | The 40 wt% Pt/C with 0.2, 0.4, and 0.6 mgPt cm−2 of metal loading amount | The 40 wt% Pt/C with 0.2, 0.4, and 0.6 mgPt cm−2 of metal loading amount | Pt/C (40 wt%, HISPEC 4000, Johnson Matthey, London, UK) catalyst was used for HER at the cathode, and 1 mg of Pt was deposited using drop casting method on the carbon paper (Sigracet 29BC) which has a thickness of 235 micro m |
| 16 | 50 °C | 50/60/70/80 | 50/60/70/80 | 45–70 °C | |
| 17 | |||||
| 18 | H2O | 1 M of KOH 1 mL/min | 1 M of KOH 1 mL/min | 90 mL/min | |
| 19 | 1 M of KOH 1 mL/min | 1 M of KOH 1 mL/min | |||
| 20 | + | + | + | + | |
| 21 | +/- | + | + | + |
| L.P. | [17] | [12] | [31] | [29] | [18] |
|---|---|---|---|---|---|
| 1 | 25 cm2 = 50 mm × 50 mm | 5 cm2 | |||
| 2 | Ni-foam-1.4 mm was rolled to a 300 µm thickness using a slip-roll machine | 0.25 mm | varied between 100 and 1700 nm | ||
| 3 | |||||
| 4 | pore volume of 0.35 cm3/g−1, Ni-foam-0.96 (before slip roll), 0.84-after | 75% | |||
| 5 | NiO-synthesis procedure included (Ir as reference). The catalyst loading was kept equal to 5 mg/cm2 for all catalysts | Cuco Ox (catalyst loading 4.0 to 4.2 mg cm−2) +Ni foam as CC | nickel felt (BEKAERT) and immersion in 4 M hydrochloric acid solution for 10 min at room temperature | copper–cobalt mixed oxide films deposited on carbon paper (CP, TGP-H-90, Fuel Cell Earth) gas diffusion layer (GDL). An optimum Co/Cu atomic ratio of 1.8. Catalyst loads of 0.04 and 0.68 mg cm−2 | |
| 6 | |||||
| 7 | 28 µm | 50, 15, 20, 50, 75 µm | |||
| 8 | ion-exchange capacity (IEC) was 1.7 × 103 mol/g | IEC-0.92, 2.35, 2.20, 1.62, 1.65–2.18, 1.23–1.44 mmol/g | |||
| 9 | |||||
| 10 | Nafion (5 wt%, Alfa Aesar) or Fumion FAA-3 (10 wt%, Fumatech, full cell store) N2-saturated alkaline 1 M KOH electrolyte | AEM (A201, Tokuyama) | Fumasep FAA3-50, FAA-PK-75 (Bietigheim-Bissingen, Germany), PiperION 15, PiperION 20 (College Station, TX, USA), Nafion 212, and Orion Polymer’s TM1. All were immersed in 0.5 M NaCl solution for 24 h, followed by immersion in DI water for 24 h to remove any excess salts | electrolyte–potassium hydroxide (KOH, 85%, VWR), AEMION™ AEMs submerged in 1 M of KOH | Fumapem® FAA-3-50 supplied by Fuel Cell Store |
| 11 | 25 cm2 = 50 mm × 50 mm | 5 cm2 | 6.25 cm2 | ||
| 12 | 0.25 mm | ||||
| 13 | |||||
| 14 | 75% | ||||
| 15 | Ni/C-synthesis procedure included (Pt/C as reference). The catalyst loading was kept equal to 5 mg/cm2 for all catalysts | Pt/C (TEC10V50E, TKK), Pt loading at about 1.7 mg/cm2 | nickel felt (BEKAERT) and immersion in 4 M hydrochloric acid solution for 10 min at room temperature | nanostructured metallic Nickel films deposited on carbon paper GDL supports | |
| 16 | ambient | 50 °C | 30–60 | 60 C | room temperature, 30–70 °C, 40 °C |
| 17 | ambient | ||||
| 18 | 1 mol dm3 of KOH solution was circulated at each side of the AEMWE, respectively, at 50 cm3/min | electrolyte solution K2CO3, DI water 0.1 wt% (7.2 mM), pH 10.8, 20 mL/min | 1 M of KOH. A two-channel peristaltic pump (Watson-Marlow) at 10 rpm, equivalent to 2 mL min−1 | 1.0 M of KOH electrolyte solution flow rate of 2 mL min−1 | |
| 19 | 1 mol dm3 of KOH solution was circulated at each side of the AEMWE, respectively, at 50 cm3/min | - | 1 M of KOH. A two-channel peristaltic pump (Watson-Marlow) at 10 rpm, equivalent to 2 mL min−1 | 1.0 M of KOH electrolyte solution flow rate of 2 mL min−1 | |
| 20 | + | + | |||
| 21 | + | + | |||
| L.P. | [32] | [28] | [19] | [33] | [5] |
| 1 | 5 cm2 | 5 cm2, electrolyzer-4 cm2 | 1 cm2 | 4 cm2 | 5 cm2 |
| 2 | |||||
| 3 | 440 S·m−1 at 4 MPa | ||||
| 4 | |||||
| 5 | NiFe2O4 on nickel fiber paper | FC:PtRu/C (Johnson Matthey, 60 wt%, 0.2 mg cm−2), electrolyzer-CoFeP TPA powder (2.0 mg cm−2) was mixed with QPPO ionomer | Co-Ni-P/NF | platinized anticorrosion sintered titanium particles were used as the anode PTL, synthetic NiFeCo was used as the anode catalyst | IrO2 (Surepure Chemetals, Florham Park, NJ, USA) |
| 6 | |||||
| 7 | 50 µm, 38 µm, and 28 µm, respectively | 20 µm | 75 µm | 50 µm | |
| 8 | 160.5 mS cm−1 | ||||
| 9 | |||||
| 10 | Sustainion, AEMION, and A-201 | cPVBMP-3.0 Coppo membrane | FAAM-75-PK; Fumatech, 1.0 M NaOH was used as a single electrolyte | QAPPT, immersed in 1 M of KOH solution for 24 h to replace Cl− with OH− and then washed several times with deionized water until the washing water was neutral | FAA-3-50 (Fumatech, Germany) was soaked in 1 M of potassium hydroxide (KOH) solution for 1 h and then rinsed with deionized water |
| 11 | 5 cm2 | 5 cm2, electrolyzer-4 cm2 | 1 cm2 | 4 cm2 | 5 cm2 |
| 12 | |||||
| 13 | |||||
| 14 | |||||
| 15 | NiFeCo on stainless steel fiber paper | FC-Pt/C (Johnson Matthey, 60 wt%, 0.2 mg cm−2), or FeNx-CNTs37 (4 mg cm−2), Electrolyzer-Pt/C (Johnson Matthey, 40 wt%) | Co-Ni-P/NF | Carbon paper was used as the cathode porous transport layer (PTL), 60 wt% Pt/C | 40 wt% Pt/C |
| 16 | 40, 50, 60 °C | FC-80 °C, Electrolyzer-60 °C | 80 | 50, 60, 70 | |
| 17 | FC-0.2 MPa backpressure | ||||
| 18 | 3–5 mL min−1, 0.1 M, 0.5 M, and 1 M of KOH | FC-pure H2, 1 L min−1, and 100% RH, electrolyzer-DI water | For commercial electrolyzer, 1.0 M of NaOH, 12.5 mL/min | DI water or KOH solution, 1 or 2.5 mL/min | |
| 19 | 3–5 mL min−1, 0.1 M, 0.5 M, and 1 M of KOH | air (CO2-free), 1 L min−1, and 100% RH | For commercial electrolyzer 0.5 M of H2SO4, 12.5 mL/min | pure water | DI water or KOH solution, 1 or 2.5 mL/min |
| 20 | + | + | + | + | + |
| 21 | + | + |
| L.P. | [24] | [4] | [30] | [26] |
|---|---|---|---|---|
| 1 | 7.4 cm2 | 5 cm2 | 5 cm2 | |
| 2 | foam thickness is known | |||
| 3 | ||||
| 4 | ||||
| 5 | NiFeV layered double hydroxide (LDH) nanosheets supported on the Ni surface by corroding a Ni foam (NF) electrode | Iridium oxide (IrO2, Premion1, Alfa Aesar), Titanium paper (250 mm, Bekaert) | Ni2P−Fe/NF, NF ranging from 220 to 800 μm, or IrO2 | Ti-felt, Ni-felt, and Ni-foam, Ni-Fe alloy black (Sigma Aldrich Co., USA), 1 mg metal cm2 |
| 6 | 4.9 | |||
| 7 | 75 µm | 25 µm | 50 µm | |
| 8 | ||||
| 9 | 46% | |||
| 10 | AEM, X37-50 grade T, Dioxide Materials/50 mL min−1 in 1 M KOH | FAA-3-PK-75 | PE(VBTAC), PFT-C6-TMA ionomer | FAA-3-50 membrane (Fumatech Co., Germany) immersed in a 1.0 M of KOH solution at room temperature for 3 h and rinsed with deionized (DI) water, ionomer (FAA-3-Br, Fumatech Co, Germany) |
| 11 | 4.9 cm2 | 5 cm2 | ||
| 12 | ||||
| 13 | ||||
| 14 | ||||
| 15 | Pt/C | platinum on carbon (Pt/C, Pt 46.5 wt%, Tanaka K.K), carbon paper (TGP-H-120, Toray) | Pt/C | A carbon-based PTL (JNTG40-A3, JNTG Co., Republic of Korea) was used as the cathode PTL, 40 wt% Pt/C (Johnson Matthey Co., Wayne, PA, USA) was used at the cathode, 0.4 mg of metal cm2 |
| 16 | 50 | 50, 60, 70, 80, 90 | 80 | 70 |
| 17 | pressing time was controlled (0, 1, and 3 min) at 50 °C and 395 psi | |||
| 18 | 1 M KOH | 0.5 M of KOH 1 mL/min | 1 M of KOH aqueous solution or pure water, 5 mL/min | 1.0 M of KOH solution at 60 °C with a flow rate of 5 mL/min |
| 19 | 1.0 M of KOH solution at 60 °C with a flow rate of 5 mL/min | |||
| 20 | + | + | + | + |
| 21 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernat, R.; Milewski, J.; Dybinski, O.; Martsinchyk, A.; Shuhayeu, P. Review of AEM Electrolysis Research from the Perspective of Developing a Reliable Model. Energies 2024, 17, 5030. https://doi.org/10.3390/en17205030
Bernat R, Milewski J, Dybinski O, Martsinchyk A, Shuhayeu P. Review of AEM Electrolysis Research from the Perspective of Developing a Reliable Model. Energies. 2024; 17(20):5030. https://doi.org/10.3390/en17205030
Chicago/Turabian StyleBernat, Rafal, Jaroslaw Milewski, Olaf Dybinski, Aliaksandr Martsinchyk, and Pavel Shuhayeu. 2024. "Review of AEM Electrolysis Research from the Perspective of Developing a Reliable Model" Energies 17, no. 20: 5030. https://doi.org/10.3390/en17205030
APA StyleBernat, R., Milewski, J., Dybinski, O., Martsinchyk, A., & Shuhayeu, P. (2024). Review of AEM Electrolysis Research from the Perspective of Developing a Reliable Model. Energies, 17(20), 5030. https://doi.org/10.3390/en17205030







