Gas Flow Blockage Treatment in Shale Gas: Case Study of Qusaiba Hot Shale, Saudi Arabia
Abstract
1. Introduction
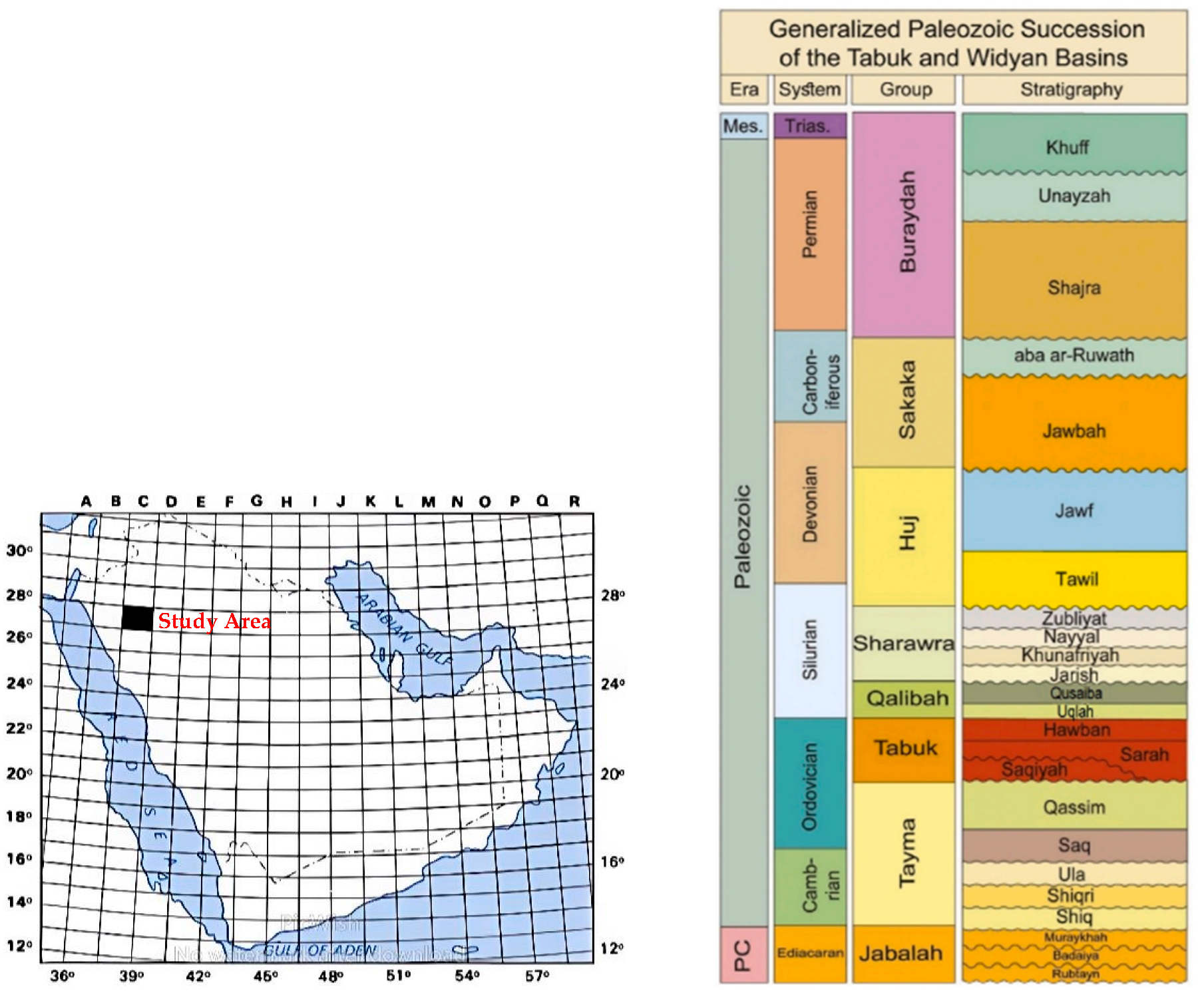
2. Qusaiba Shale Geological Setting
3. Materials and Experimental Methodology
3.1. Fluids
3.2. Porous Medium
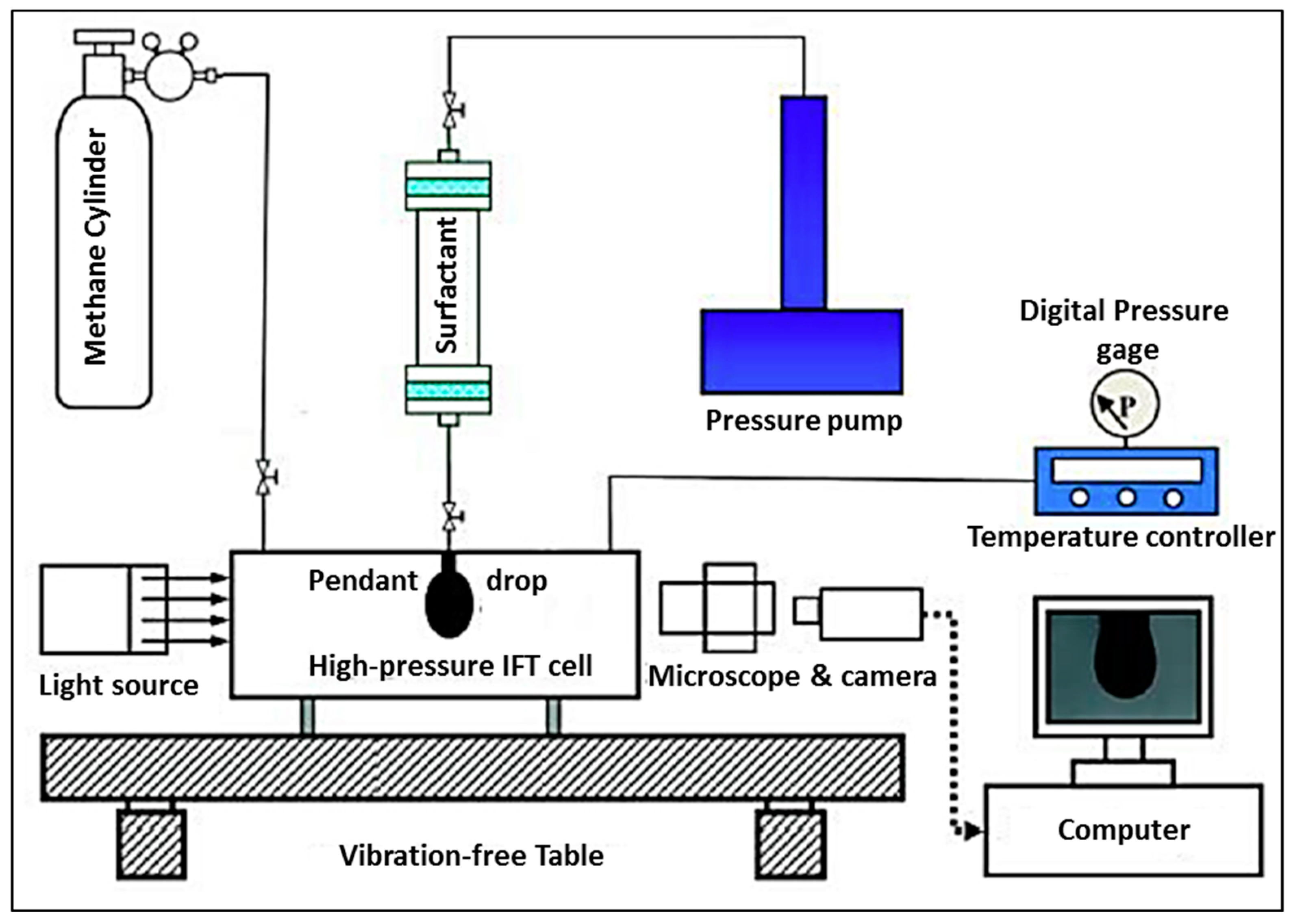
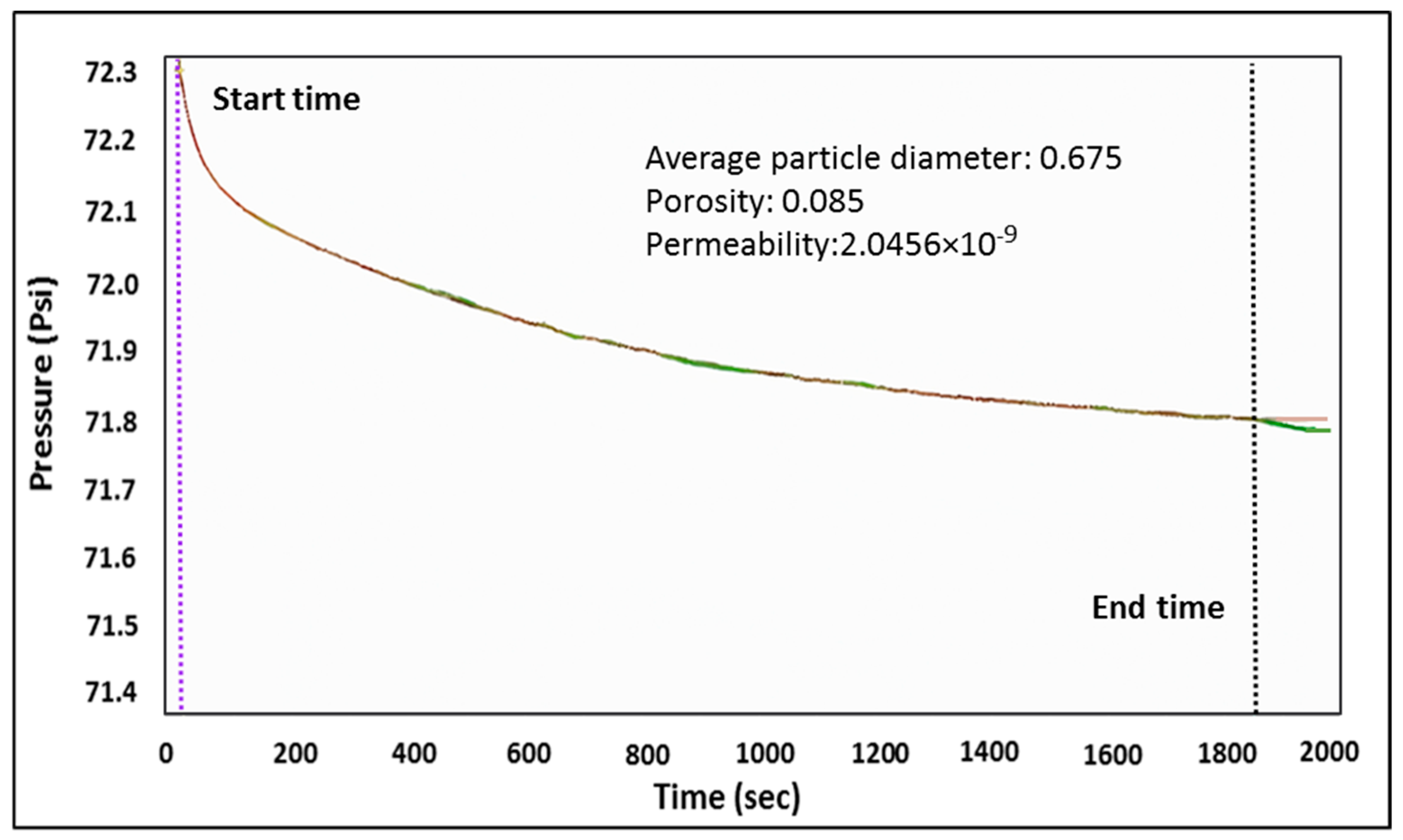
3.3. Instruments and Methodology
4. Results
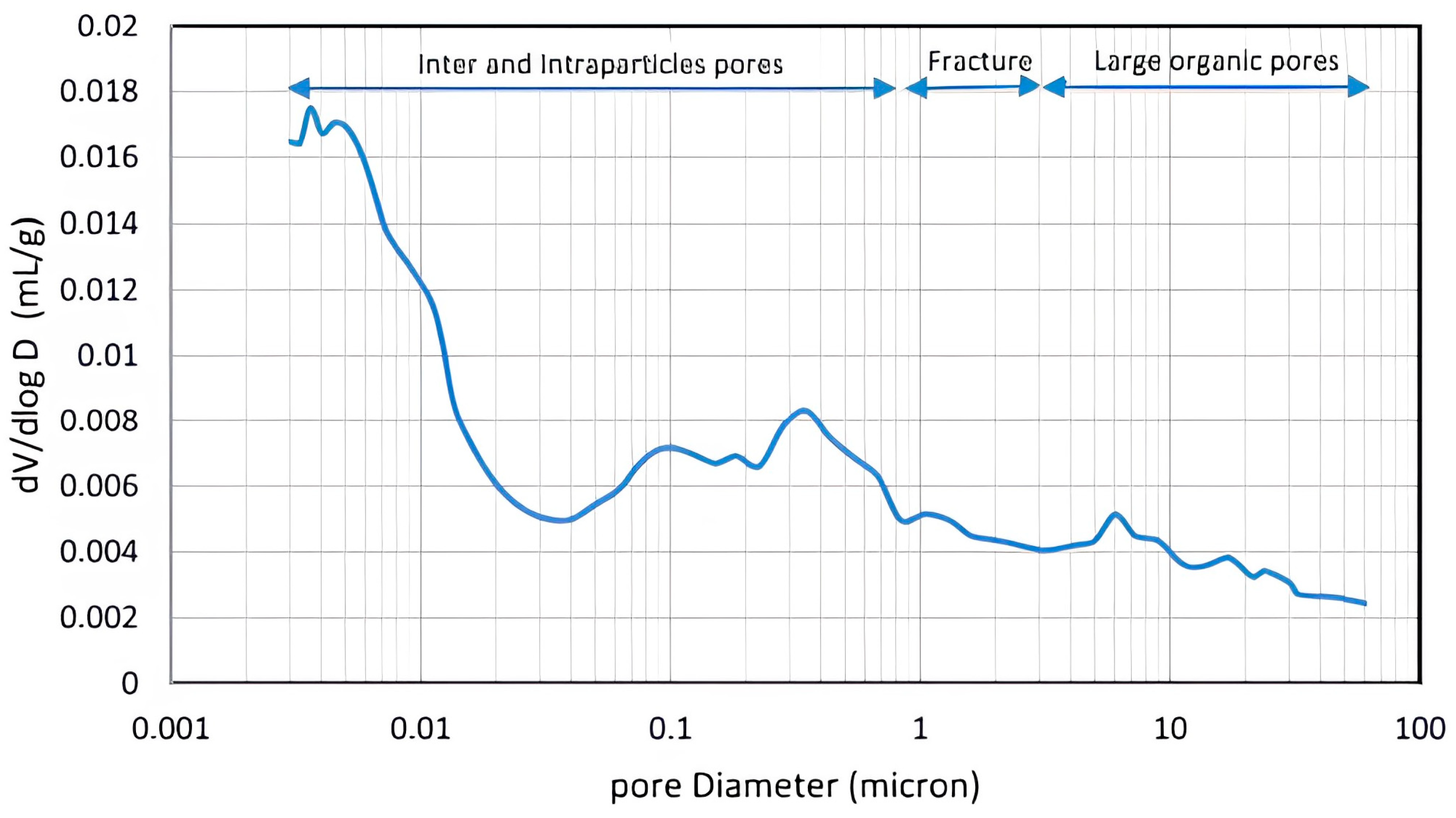
4.1. Shale Characterization
4.2. Fluids Characterization
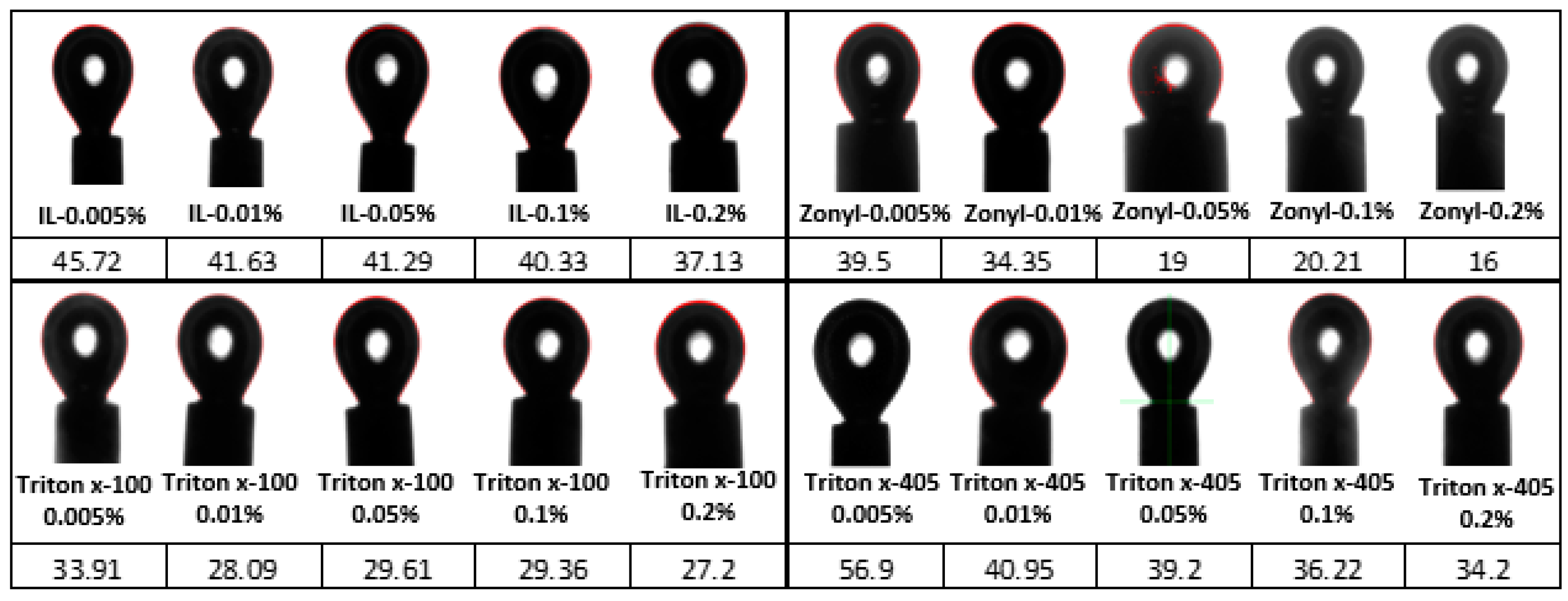
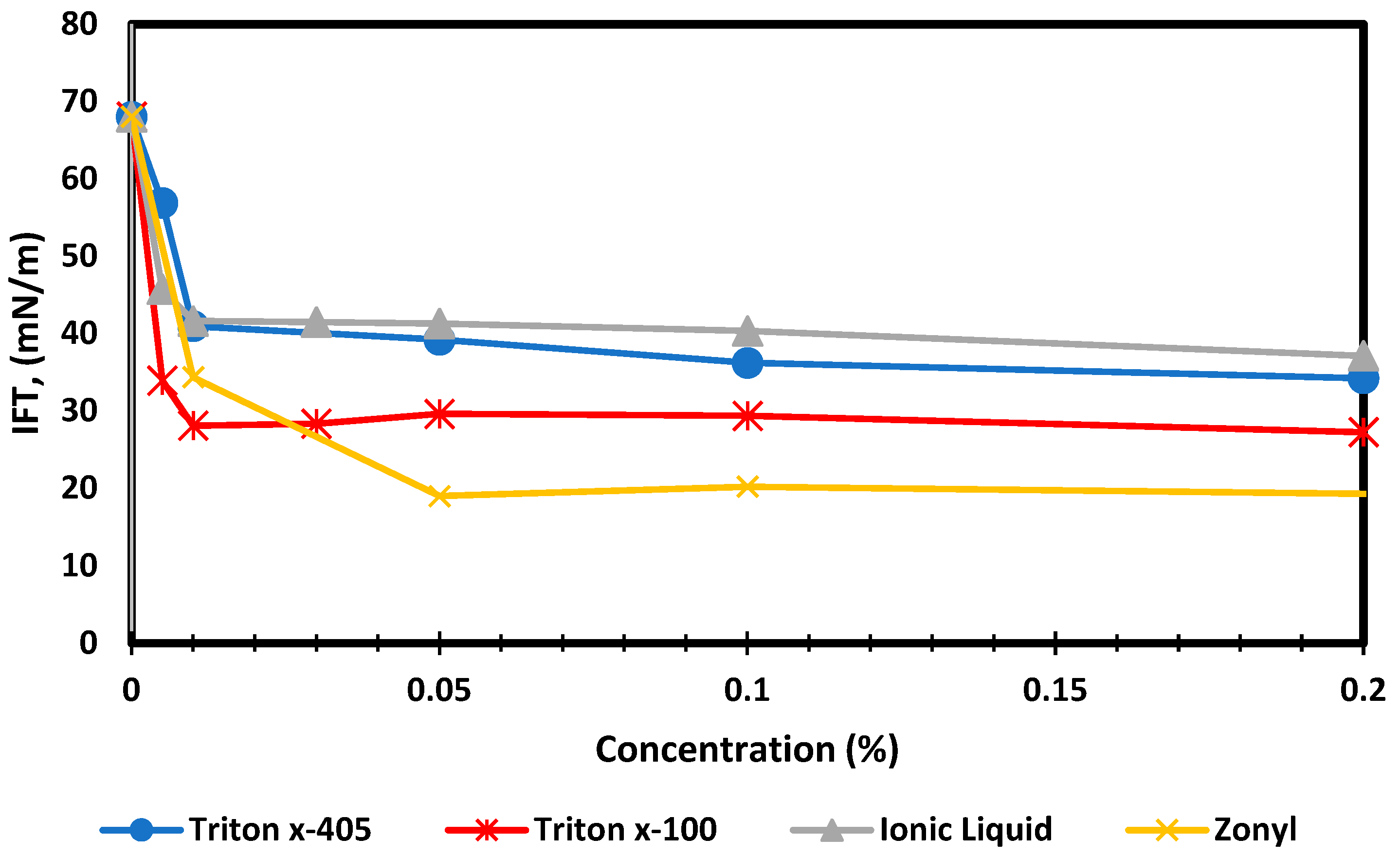
4.2.1. Chemical Solutions—Methane Gas Surface Tension
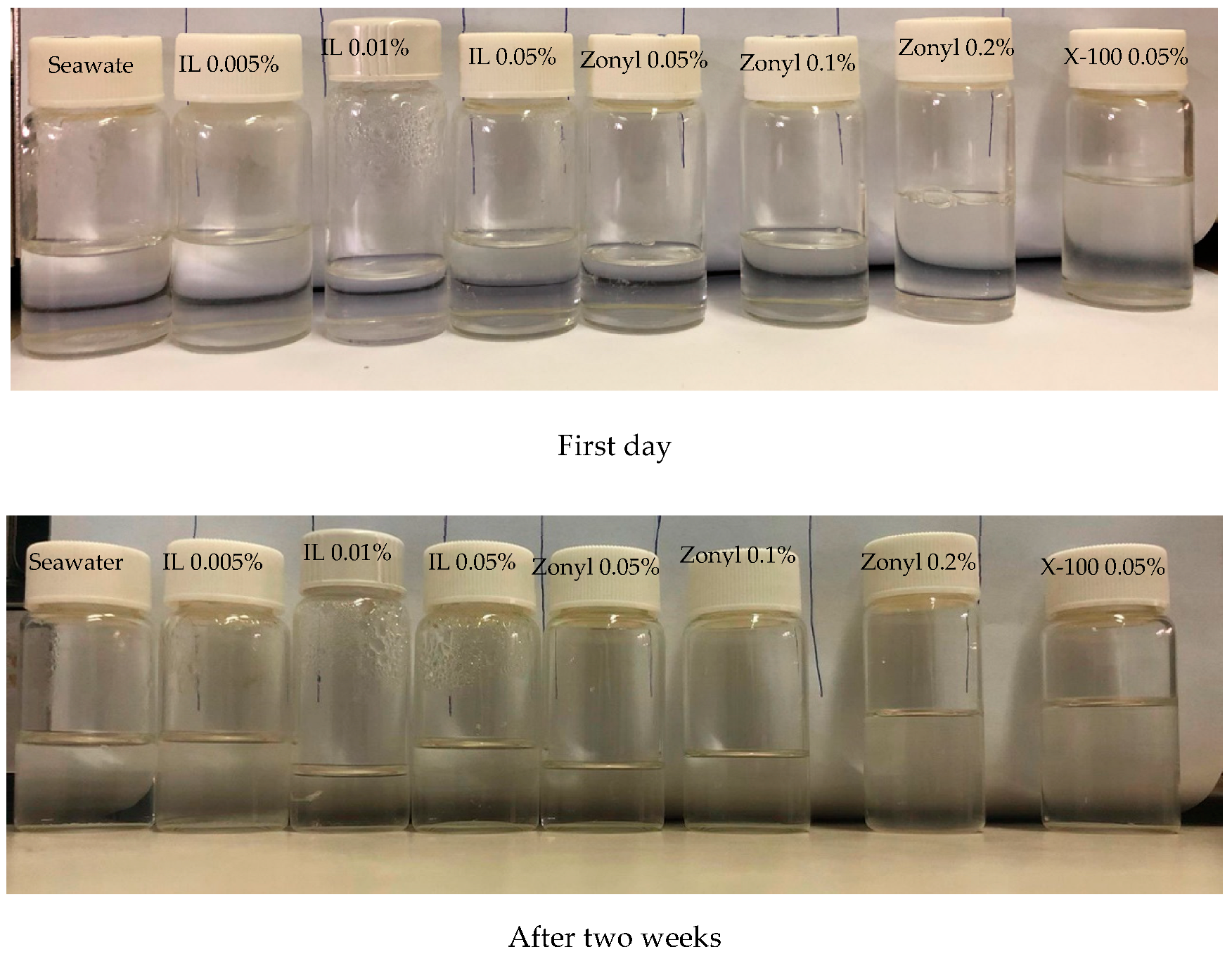
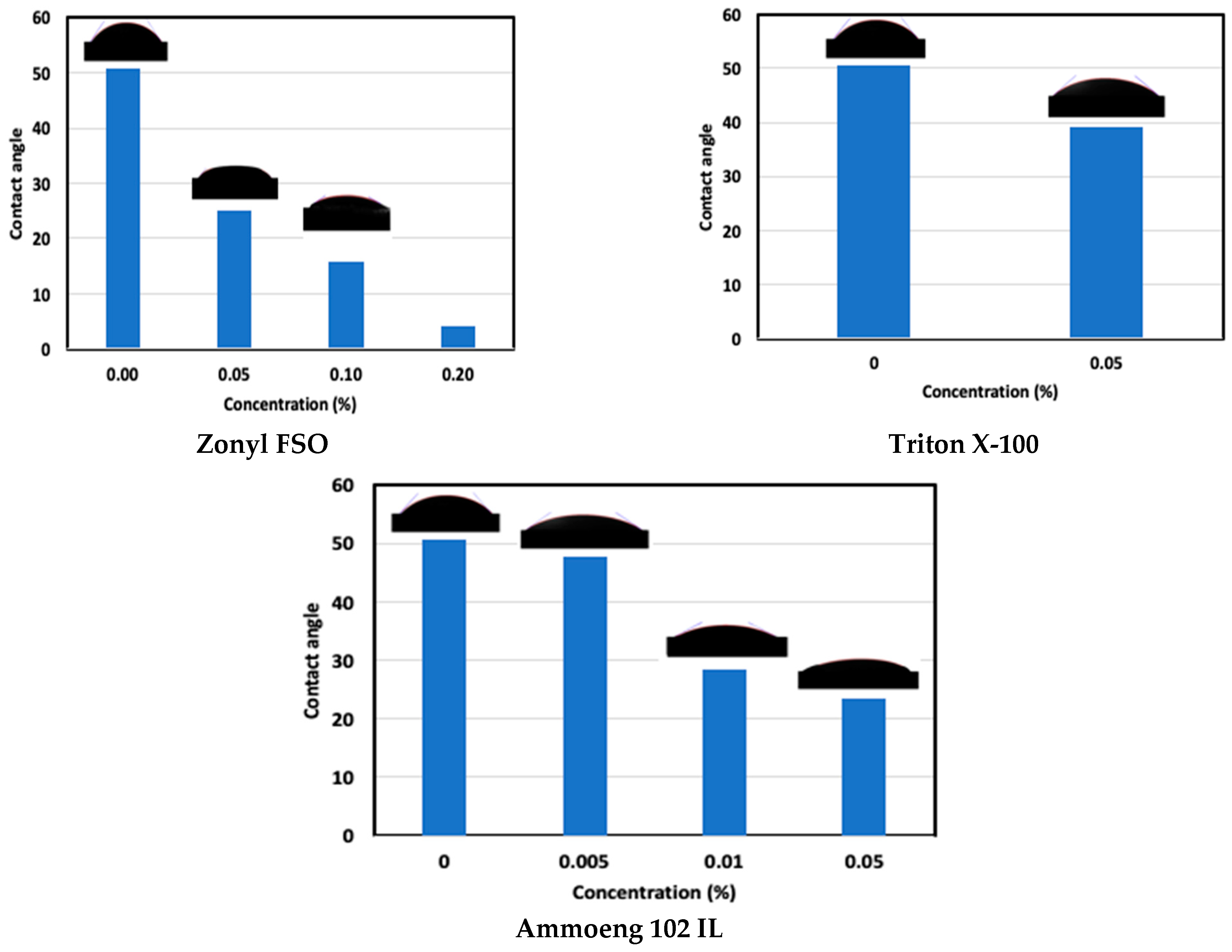
4.2.2. Wettability Alteration
5. Discussion
6. Conclusions
- The chemicals tested in this work showed excellent tolerance to salinity up to seawater salinity of 40 K ppm and temperature as high as 70 °C.
- Reductions in the surface tension and contact angle were observed at different levels for all chemicals tested; however, a significant reduction was observed for Zonyl FSO.
- An effective capillary pressure drop of 66% was observed for Zonyl FSO at CMC. Such a drop can induce a lower invasion depth during the fracking stage and promote liquid blockage removal during the flowback stage.
- An increase in Zonyl FSO concentration to 0.2% had a minimal effect on surface tension but drastically decreased the contact angle, rendering shale very strongly water-wet. Such a wettability shift induced a smaller capillary pressure drop (54%); however, this could be effective in promoting gas desorption during extended production stage.
- Triton X-100 at CMC of 0.05% was the second most effective surfactant, as it was able to reduce the capillary pressure by 47%, which is good enough to elevate any liquid blockage throughout the flowback stage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eseme, E.; Urai, J.L.; Krooss, B.M.; Littke, R. Review of Mechanical Properties of Oil Shales: Implications for Exploitation and Basin Modelling. Oil Shale 2007, 24, 159–174. [Google Scholar] [CrossRef]
- Pagels, M.; Willberg, D.M.; Edelman, E.; Zagorski, W.; Frantz, J. Quantifying fracturing fluid damage on reservoir rock to optimize production. In Proceedings of the Unconventional Resources Technology Conference, Denver, CO, USA, 12–14 August 2013. Paper URTEC-1578948-MS. [Google Scholar]
- Gdanski, R.D.; Fulton, D.D.; Shen, C. Fracture face skin evolution during cleanup. In Proceedings of the Annual Technical Conference and Exhibition, Society of Petroleum Engineers, San Antonio, TX, USA, 24–27 September 2006. Paper SPE-101083-MS. [Google Scholar]
- Environmental Resources Management (ERM). Recovered Water Management Study in Shale Wells. 2014. Available online: http://www.iogp.org/wp-content/uploads/2016/10/water-mgmt_OGP_Final_Report_22.pdf (accessed on 7 August 2016).
- Bennion, D.B.; Thomas, F.B.; Bietz, R.F. Low Permeability Gas Reservoirs: Problems, Opportunities and Solutions for Drilling, Completion, Stimulation and Production. In Proceedings of the Gas Technology Conference, Calgary, AB, Canada, 28 April–1 May 1996. SPE 35577. [Google Scholar]
- Dehghanpour, H.; Lan, Q.; Saeed, Y.; Fei, H.; Qi, Z. Spontaneous imbibition of brine and oil in gas shales: Effect of water adsorption and resulting microfractures. Energy Fuel 2013, 27, 3039. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, B.; Wei, M. Microfracture and Surfactant Impact on Linear Cocurrent Brine Imbibition in Gas- Saturated Shale. Energy Fuel 2015, 29, 1438. [Google Scholar] [CrossRef]
- Phan, T.; Kazempour, M.; Nguyen, D.; Champion, N. Treating liquid banking problem to increase shale gas wells productivity. In Proceedings of the International Conference and Exhibition on Formation Damage Control, Society of Petroleum Engineers, Lafayette, LA, USA, 7–9 February 2018. Paper SPE-189523-MS. [Google Scholar]
- Kim, J.; Zhang, H.; Sun, H.; Li, B.; Carman, P. Choosing surfactants for the Eagle Ford shale formation: Guidelines for maximizing flowback and initial oil recovery. In Proceedings of the Low Perm Symposium, Society of Petroleum Engineers, Denver, CO, USA, 5–6 May 2016. Paper SPE-180227-MS. [Google Scholar]
- Kumar, V.; Pope, G.A.; Sharma, M.M. Improving the gas and condensate relative permeability using chemical treatments. In Proceedings of the Gas Technology Symposium, Calgary, AB, Canada, 15–18 May 2006. Paper SPE-100529-MS. [Google Scholar]
- Wijaya, N.; Sheng, J.J. Effect of desiccation on shut-in benefits in removing water blockage in tight water-wet cores. Fuel 2019, 244, 314–323. [Google Scholar] [CrossRef]
- Paktinat, J.; Williams, C.; Pinkhouse, J.A.; Clark, G.A.; Penny, G.S. Case histories: Damage preventions by leak off control of fracturing fluids in Appalachian gas reservoirs. In Proceedings of the International Symposium and Exhibition on Formation Damage Control, Society of Petroleum Engineers, Lafayette, LA, USA, 15–17 February 2006. Paper SPE-98145-MS. [Google Scholar]
- Xu, L.; Fu, Q. Ensuring better well stimulation in unconventional oil and gas formations by optimizing surfactant additives. In Proceedings of the Western Regional Meeting, Society of Petroleum Engineers, Bakersfield, CA, USA, 21–23 March 2012. Paper SPE-154242-MS. [Google Scholar]
- Liang, T.; Achour, S.H.; Longoria, R.A.; DiCarlo, D.A.; Nguyen, Q.P. Flow physics of how surfactants can reduce water blocking caused by hydraulic fracturing in low permeability reservoirs. J. Pet. Sci. Eng. 2017, 157, 631–642. [Google Scholar] [CrossRef]
- Zhou, L.; Das, S.; Ellis, B.R. Effect of surfactant adsorption on the wettability alteration of gas-bearing shales. Environ. Eng. Sci. 2016, 33, 766–777. [Google Scholar] [CrossRef]
- Hussien, O.S.; Elraies, K.A.; Almansour, A.; Husin, H.; Shuhili, J.A. Beyond fracking: Enhancement of shale gas desorption via surface tension reduction and wettability alteration. J. Nat. Gas Sci. Eng. 2018, 57, 322–330. [Google Scholar] [CrossRef]
- Hussien, O.S.; Elraies, K.A.; Almansour, A.; Husin, H.; Belhaj, A.; Ern, L. Experimental study on the use of surfactant as a fracking fluid additive for improving shale gas productivity. J. Pet. Sci. Eng. 2019, 183, 106426. [Google Scholar] [CrossRef]
- Paktinat, J.; Pinkhouse, J.A.; Stoner, W.P.; Williams, C.; Carder, G.A.; Penny, G.S. Case histories: Post-frac fluid recovery improvements of Appalachian Basin gas reservoirs. In Proceedings of the Eastern Regional Meeting, Society of Petroleum Engineers, Morgantown, WV, USA, 14–16 September 2005. Paper SPE-97365-MS. [Google Scholar]
- Zelenev, A.S.; Ellena, L. Microemulsion technology for improved fluid recovery and enhanced core permeability to gas. In Proceedings of the 8th European Formation Damage Conference, Society of Petroleum Engineers, Scheveningen, The Netherlands, 27–29 May 2009. Paper SPE-122109-MS. [Google Scholar]
- Agee, D.; Yudho, A.; Schafer, L.; Thouvenin, E.; Grant, G.; Garnier, A.; Wijanarko, A. Post-fracturing fluid recovery enhancement with microemulsion. In Proceedings of the International Symposium and Exhibition on Formation Damage Control, Society of Petroleum Engineers, Lafayette, LA, USA, 10–12 February 2010. Paper SPE-128098-MS. [Google Scholar]
- Sharma, M.K.; Shah, D.O. Introduction to Macro and Microemulsions; ACS Publications: Washington, DC, USA, 1985. [Google Scholar] [CrossRef]
- Katz, C.A.; Calzola, Z.J.; Mbindyo, J.K. Structure and solvent properties of microemulsions. J. Chem. Educ. 2008, 85, 263. [Google Scholar] [CrossRef]
- Rostami, A.; Nasr-El-Din, H.A. Microemulsion vs. surfactant assisted gas recovery in low permeability formations with water blockage. In Proceedings of the Western North American and Rocky Mountain Joint Meeting, Society of Petroleum Engineers, Denver, CO, USA, 16–18 April 2014. Paper SPE-169582-MS. [Google Scholar]
- Dong, B.; Meng, M.; Qiu, Z.; Lu, Z.; Zhang, Y.; Zhong, H. Formation damage prevention using microemulsion in tight sandstone gas reservoir. J. Pet. Sci. Eng. 2019, 173, 101–111. [Google Scholar] [CrossRef]
- Penny, G.S.; Pursley, J.T. Field studies of drilling and completion fluids to minimize damage and enhance gas production in unconventional reservoirs. In Proceedings of the European Formation Damage Conference, Society of Petroleum Engineers, Scheveningen, The Netherlands, 30 May–1 June 2007. Paper SPE-107844-MS. [Google Scholar]
- Wijaya, P.N.; Sheng, J.J. Mitigating near-fracture blockage and enhancing oil recovery in tight reservoirs by adding surfactants in hydraulic fracturing fluid. J. Pet. Sci. Eng. 2020, 185, 106611. [Google Scholar] [CrossRef]
- Jadwa Investment Report. Natural Gas and The Vision 2030. 2016. Available online: http://argaamplus.s3.amazonaws.com/0dad8cce-b3f5-4f13-a7f5-7f292e7f8f1b.pdf (accessed on 1 October 2016).
- Holditch, S.A. Tight Gas Sands. J. Pet. Technol. 2006, 58, 86–93. [Google Scholar] [CrossRef]
- Rogner, H. An assessment of world hydrocarbon resources. Annu. Rev. Energy Environ. 1996, 22, 217–262. [Google Scholar] [CrossRef]
- Hakimi, M.H.; Wan, H.A.; Alqudah, M.; Makeen, Y.M.; Mustapha, K.A. Organic geochemical and petrographic characteristics of the oil shales in the Lajjun area, Central Jordan: Origin of organic matter input and preservation conditions. Fuel 2016, 181, 34–45. [Google Scholar] [CrossRef]
- Vaslet, D.; Janjou, D.; Robelin, C.; Al-Muallem, M.S.; Halawani, M.A.; Brosse, J.M.; Brecton, J.P.; Courbouleix, S.; Roobol, M.J.; Dagain, J. Explanatory Notes of the Geologic Map of the Tayma Quadrangle, Sheet 27C, International Index NG-37-2; Saudi Geological Survey: Jeddah, Saudi Arabia, 1994. [Google Scholar]
- Cole, G.A.; Abu-Ali, M.A.; Aoudeh, S.M.; Carrigan, W.J.; Chen, H.H.; Colling, E.L.; Gwathney, W.J.; Al-Hajji, A.A.; Halpern, H.I.; Jones, P.J.; et al. Organic geochemistry of the Paleozoic petroleum system of Central Saudi Arabia. Energy Fuels 1994, 8, 1425–1442. [Google Scholar] [CrossRef]
- Ackerman, W.C. Saudi Arabia’s Natural Gas Aspirations: The Domestic Outlook. Middle East Institute, 9 February 2022. [Google Scholar]
- Jones, P.J.; Stump, T.E. Depositional and tectonic setting of the lower Silurian hydrocarbon source rock facies, Central Saudi Arabia. AAPG Bull. 1999, 83, 314–332. [Google Scholar]
- Mahmoud, M.D.; Vaslet, D.; Husseini, M.I. The lower Silurian Qusaiba formation of Saudi Arabia. An important hydrocarbon source rock. Am. Assoc. Petrol. Geol. Bull. 1992, 76, 1491–1506. [Google Scholar]
- Abu-Ali, M.A.; Rudkiewicz, J.-L.L.; McGillivray, J.G.; Behar, F. Paleozoic petroleum system of Central Saudi Arabia. Geo Arab. 1999, 4, 321–336. [Google Scholar] [CrossRef]
- Slatt, R.M.; Rodriguez, N.D. Comparative sequence stratigraphy and organic geochemistry of gas shales: Commonality or coincidence. J. Nat. Gas Sci. Eng. 2012, 8, 68–84. [Google Scholar] [CrossRef]
- Konert, G.; Afifi, A.M.; Al-Hajri, S.A.; Droste, H.J. Paleozoic stratigraphy and hydrocarbon habitat of the Arabian Plate. AAPG Bull. 1999, 83, 1320–1336. [Google Scholar]
- Arouri, R.K.; Van Lear, J.P.; Jenden, J.D.; Carrigan, J.W.; Al-Hajji, A.A. Controls on hydrocarbon properties in Paleozoic petroleum system in Saudi Arabia: Exploration and development implications. AAPG Bull. 2010, 94, 163–188. [Google Scholar] [CrossRef]
- Faqira, M.; Bhullar, A.; Abdelghayoum, A. Silurian Qusaiba Shale Play: Distribution and Characteristics. In Proceedings of the AAPG Conference, Austin, TX, USA, 5–10 December 2010. [Google Scholar]
- AlQuraishi, A.; AlLaboun, A.; AlGhamdi, F.; AlHussinan, S. Silurian Qusaiba shale: Petrophysical, mineralogical and geochemical analysis. J. Pet. Sci. Eng. 2020, 192, 107209. [Google Scholar] [CrossRef]
- Laboun, A.A. Tectono-stratigraphy of the exposed Silurian deposits in Arabia. Arab. J. Geosci. 2009, 2, 119–131. [Google Scholar] [CrossRef]
- Aboulresh, M.O. An integrated characterization of the porosity in Qusaiba shale, Saudi Arabia. J. Petrol. Sci. Eng. 2017, 149, 75–78. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Sun, J.P.; Zhang, H.J.; Liu, L.F. Recovery of origin organic parameters of the outcropping source rocks from Southern China. Energy Explor. Exploit. 2002, 20, 365–370. [Google Scholar] [CrossRef]
- Rossen, W.R. Foams in enhanced oil recovery. In Foams: Theory, Measurements and Applications; Rossen, W.R., Prud'homme, R.K., Khan, S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 413–464. [Google Scholar]

| pH | 7.1 | Turbidity NTU | 2.21 | Ca mg/L | 766 | K mg/L | 810 | NO−3 mg/L | 37 |
| Density gm/cc | 1.0256 | Total Alkalinity | 174 | Mg mg/L | 2648 | Cl mg/L | 36,585 | F mg/L | 2.19 |
| Viscosity cp | 1.08 | HCO−3 mg/L | 212 | Na mg/L | 22,353 | S mg/L | 5015 | TDS mg/L | 68,358 |
| Chemical | Zonyl FSO | Triton X-100 | Triton X-405 | Ammoeng IL |
| Type | Anionic | Nonionic | Nonionic | |
| Molecular Formula | Ammonium bis [2-(perfluoroalkyl) ethyl] phosphate 4 | Octylphenol Ethoxylate | Octylphenol Ethoxylate | Tetra-alkyl ammonium sulfate |
| Chemical Structure |  | 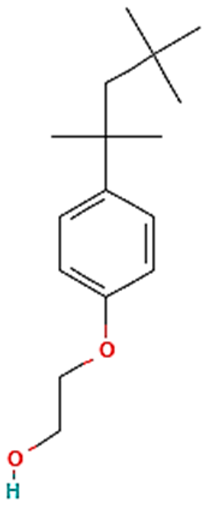 R = octyl (C8) x = 9.5 (avg) | 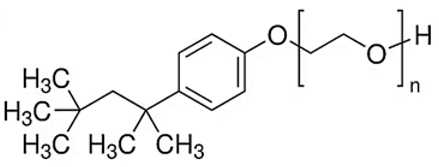 R = octyl (C8) x = 35 (avg) |  |
| Sample | Al2O3 | SiO2 | SO2 | K2O | CaO | TiO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|
| QS1 | 8.67 | 32.74 | 4.85 | 5.68 | 23.31 | 2.65 | 22.10 |
| QS2 | 6.98 | 33.63 | 9.65 | 4.88 | 12.85 | 2.34 | 29.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQuraishi, A.A.; AlMansour, A.O.; AlAwfi, K.A.; Alonaizi, F.A.; AlYami, H.Q.; Ali, A.M.A. Gas Flow Blockage Treatment in Shale Gas: Case Study of Qusaiba Hot Shale, Saudi Arabia. Energies 2024, 17, 5025. https://doi.org/10.3390/en17205025
AlQuraishi AA, AlMansour AO, AlAwfi KA, Alonaizi FA, AlYami HQ, Ali AMA. Gas Flow Blockage Treatment in Shale Gas: Case Study of Qusaiba Hot Shale, Saudi Arabia. Energies. 2024; 17(20):5025. https://doi.org/10.3390/en17205025
Chicago/Turabian StyleAlQuraishi, Abdulrahman A., Abdullah O. AlMansour, Khalid A. AlAwfi, Faisal A. Alonaizi, Hamdan Q. AlYami, and Ali M. AlGhamdi Ali. 2024. "Gas Flow Blockage Treatment in Shale Gas: Case Study of Qusaiba Hot Shale, Saudi Arabia" Energies 17, no. 20: 5025. https://doi.org/10.3390/en17205025
APA StyleAlQuraishi, A. A., AlMansour, A. O., AlAwfi, K. A., Alonaizi, F. A., AlYami, H. Q., & Ali, A. M. A. (2024). Gas Flow Blockage Treatment in Shale Gas: Case Study of Qusaiba Hot Shale, Saudi Arabia. Energies, 17(20), 5025. https://doi.org/10.3390/en17205025






