1. Introduction
Energy usage and renewable energy deployment have been key concerns over the past two decades, with many researchers working to tackle the energy dilemma. Phase change materials (PCMs) are extensively employed in thermal energy storage systems to alleviate the energy crisis [
1]. Thermal energy storage based on the latent heat of phase change material is a kind of technology that realizes cold storage (solidification process) and cooling release (melting process) by using the transformation of material states at a fixed temperature of phase change. PCMs have the characteristics of high heat storage density and high thermal efficiency, and have attracted people’s attention in many fields such as refrigeration, air conditioning and building energy storage [
2].
In recent years, due to the high latent heat of liquid-solid phase change in water, liquid-solid phase change energy storage systems made of water have been widely used in industrial and residential air conditioning [
1]. Despite the benefit of utilizing phase change’s large latent heat therefore reducing storage space for energy, these systems have a flaw. To continuously make ice using a traditional ice-on-coil refrigeration system, coil tubes where ice forms need to be kept at temperatures as low as 269–263 K. This requirement limits the coefficient of performance (COP) of the refrigerator in the system. To enhance the COPs of refrigerators in such thermal energy storage systems, it is essential to choose a thermal energy-storing material that undergoes a phase change at a temperature close to the desired air-cooling level, i.e., 280–293 K [
3].
Phase change materials play a crucial role in phase change energy storage. They efficiently harness latent heat within a specific temperature range, absorbing or releasing a significant amount of heat during the material state transition for energy storage [
4]. Tetrabutylammonium bromide (TBAB) form ionic semi clathrate hydrates crystals with water molecules [
5] and the crystallization temperature under one atmospheric pressure is within the range of 273–286 K (its undercooling during solidification is 4–6 K). TBAB hydrate is more suitable as a phase change material for cold storage air conditioning systems. Wuttig et al. [
6] studied the preparation of cold storage materials, namely TBAB hydrates, using quantitative measurement of electrical resistance. The coupling effect of the initial concentration (10–30 wt%) and mixing rate (250–550 rpm) of TBAB solution was considered. When the initial concentration of TBAB increased from 10 wt% to 30 wt%, the induction time decreased by 89% and the growth rate of TBAB hydrate increased by more than 5.6 times. Hyunho Kim et al. [
7] used ion chromatography and differential scanning calorimetry (DSC) to study the hydration number and thermal properties of TBAB semi-clathrate hydrate crystals formed from TBAB aqueous solutions with TBAB mole fraction (x) in the range of (0.0060–0.0360). These results indicate that the crystal structure (hydration number) and thermal performance of TBAB hydrate will continue to change with different initial solution mole fractions. Motoi Oshima et al. [
8] conducted experiments to study the phase equilibrium point of a new type of hydrate cold storage material and simulated the performance of the new type of hydrate cold storage material in cold storage air conditioning systems under different operating modes.
Although phase change materials composed of a single substance have a long service life, they have problems with low thermal conductivity and high undercooling. Therefore, researchers usually use the addition of other auxiliary materials to improve the performance of phase change materials, making them have better application prospects. Among them, adding nano additives to phase change materials to form nano reinforced phase change materials can effectively improve the latent heat, undercooling, thermal conductivity and nucleation ability of a single-phase change material [
9]. This is because nanoparticles are small in size and have a large surface area. Nano-level additive is added into the phase change material so that the nano-level additive can combine with the phase change material and cooperate with the base liquid to change the original thermal properties of the base liquid and improve the performance of the phase change material [
10]. Nano oxides refer to oxides with particle sizes up to the nanometer level such as [
9] nano silica, nano titanium dioxide, nano copper, nano alumina, etc. These nano oxides have low costs and excellent thermal enhancement properties, making them the preferred nano additives. Nano titanium dioxide is widely used in photodegradation, battery electrodes, toothpaste preparation and silica gel extraction [
11,
12,
13,
14]. The average grain size of nano zinc oxide doped with inorganic substances will change [
15]. Li Jinping et al. [
16] added nano Cu to HFC134a hydrate and the results showed that nano Cu can effectively enhance the heat and mass transfer process of the hydrate. Said Samer et al. [
17] found that SiO
2 nanoparticles with a concentration of 0.3 wt% have a promoting effect on CO
2 gas consumption, while Ag nanoparticles have no effect on CO
2 gas consumption. In addition, Zhou Shidong et al. [
18] showed that the induction time for the formation of TBAB hydrates decreased with increasing concentration, with the concentrations being 0.00%, 0.04%, 0.06%, 0.08% and 0.1%.
This article develops a cold storage agent with a phase change temperature (onset temperature) of 273–286 K and a phase change latent heat of 135–168 kJ/kg by adjusting the proportion of tetrabutylammonium bromide added to water and different types of nanoparticles. In order to make PCMs more suitable for refrigeration, the performance of PCMs was optimized by adding nanoparticles. The effects of adding different types of nanoparticles on solution phase change temperature, phase change latent heat, undercooling and thermal stability were studied.
2. Materials and Methods
2.1. Experimental Materials
TBAB was chosen as the working fluid and purchased from BAISIYIJI, with purity ≥99.7%. Nanoparticles (SiO2, Al2O3, SiC and TiO2) of purity 99.9% and deionized water (Aladdin Reagent Co., Ltd., Shanghai, China) of resistivity 18 MV cm were used as nanofluid. TBAB hydrate was prepared by weighing different mass fractions of TBAB using a precision electronic balance (Shanghai Haosheng Scientific Instrument Co., Ltd., Shanghai, China, FA114, 0.1 mg, accuracy ± 0.02). The concentration of water solution shall be 10%, 20%, 30%, 40% and 50%, respectively, and they will be put in small beakers to make marks for later use and stirred with glass rods to fully mix and uniform them. An amount of 10 mL tetrabutylammonium bromide solution with a concentration of 40% was obtained. Four kinds of Nano-SiO2, nano-Al2O3, nano-SiC and nano-TiO2 with a concentration of 0.4% were added as additives and the solution was fully stirred to make the solution evenly mixed.
2.2. Experimental Apparatus
Two types of experiments were conducted: kinetic measurement of TBAB hydrate formation and visualization study of TBAB hydrate formation. The kinetic measurements were conducted using a constant temperature and humidity chamber (EXCAL 2213-HA Climats, Bordeaux, France) and a DSC (TA Q1000, −90~550 °C, ±0.1 °C) to investigate the effect of undercooling on TBAB hydrates and the process of crystal nucleation and growth. The experimental instrument is shown in
Figure 1. Visualization studies were observed using a low-temperature video microscope (Linkam FDCS196, −196~+125 °C, temperature stability < 0.01 °C)
2.3. Measurement Procedures
Test of cooling step cooling curve for cold storage materials. The experimental setup consists of an environmental chamber, a computer, a data recorder (MX100, Yokogawa Electric Corp., Tokyo, Japan) and T-type thermocouples. First, raise the temperature to 25 °C and keep it stable for 30 min, then lower the temperature at the rate of 1 °C/min to −5 °C, −8 °C, −10 °C, keep the temperature for 60 min, then raise the temperature at the rate of 1 °C/min to 25 °C and keep the temperature at 25 °C for 30 min. MX100 Data Acquisition Unit was used to record the temperature changes of phase change materials during the process. According to the measured data, the step-cooling curve of the phase change material was drawn and the T-X phase diagram of the composite phase change material system was drawn according to the determined phase change temperature. Repeat the experiment three times and take the average value.
DSC analysis of thermodynamic properties. Differential scanning calorimeter (DSC) was used to investigate the thermal energy storage properties of the composite PCMs. The temperature obtained by intersecting the maximum slope of the endothermic peak with the baseline in the melting curve is the phase transition temperature (onset temperature). Although this temperature is not the initial melting temperature, it begins to absorb a large amount of heat after this temperature, so studying the initial temperature is more in line with the practical significance of refrigeration. Phase change latent heat refers to the heat absorbed or released by a substance from one phase to another under isothermal and isobaric conditions [
19]. The weighing sample is placed in an aluminum crucible and the mass is accurate to 0.01 mg. The same empty crucible as the sample crucible will be placed on the reference side of the DSC. In this process, all samples were heated and cooled repeatedly between −35 °C and 20 °C with a heating-cooling rate of 5 °C/min under a constant stream of nitrogen at a flow rate of 50 mL/min. Freezing curves and melting curves will be obtained. Repeat three times for each sample to obtain the average value and then use the analysis software TA (Advantage_v5.5.22) to obtain the phase change latent heat and phase change temperature of each sample.
Visualization of crystallization process. Linkam FDCS196 was used to observe the crystallization process of TBAB hydrate. A total of 2 μL TBAB solution was siphoned from the reagent bottle using an adjustable volume pipettor. Turn on the light source, adjust the position of the light source and then adjust the focal length of the lens so that the high-speed camera can take a clear outline of the liquid drop and fix the focal length. Use a dust-free cloth to erase the TBAB droplets that have been located. Start the high-speed camera for recording, absorb 2 μL TBAB solution and drop it in the form of liquid drops to the calibration position of the silicon wafer to ensure that the focal length of the imaging is the same as that of the initial debugging. When the TBAB hydrate crystal formation was observed, image recording was stopped. Repeat the above steps to film and record the nucleation and growth of TBAB hydrate crystals with different concentrations and the addition of nanoparticles.
3. Results and Discussion
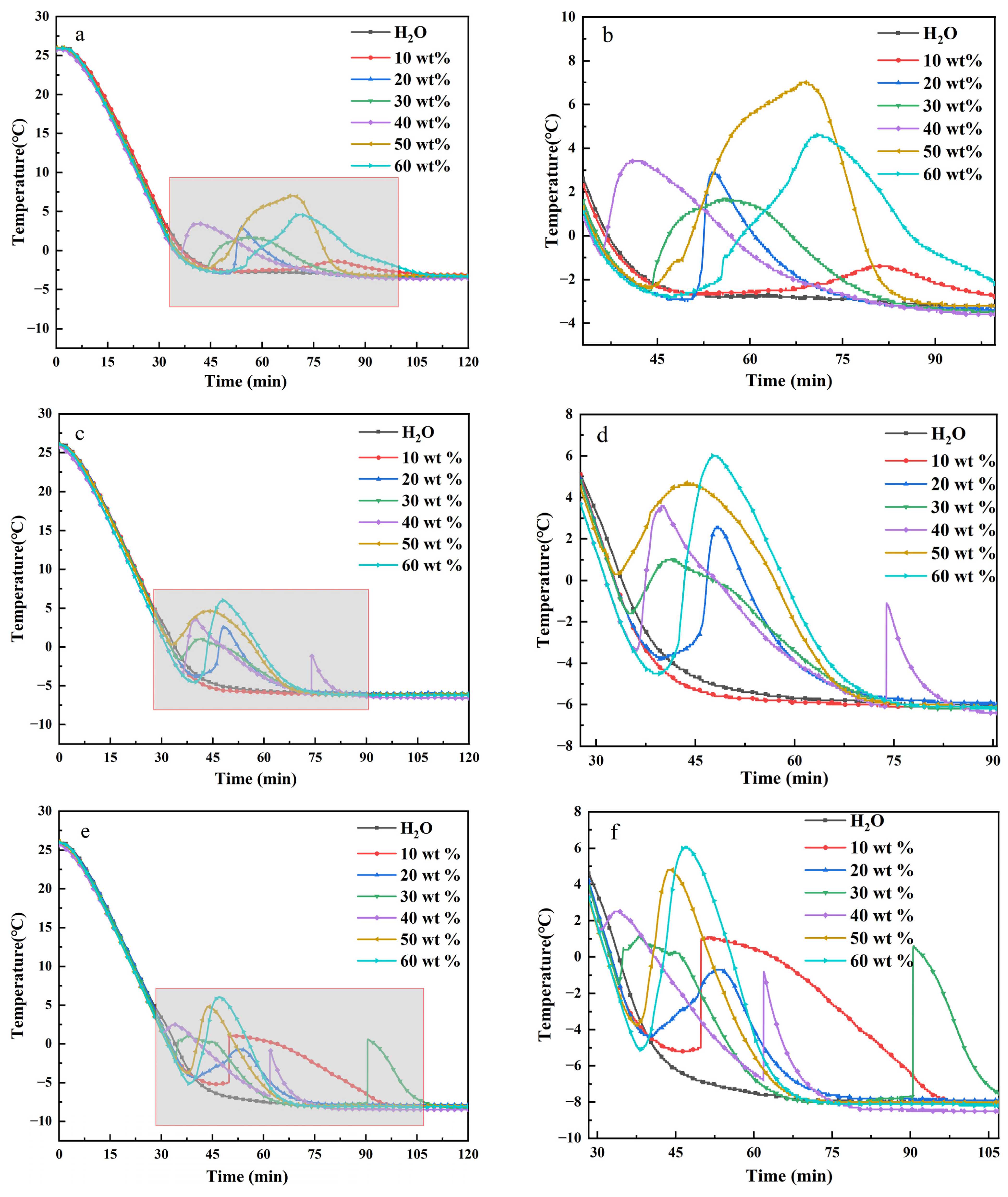
Effect of different cooling storage conditions. The supercooling and storage time of phase change materials are greatly affected by the refrigeration environment, which is a key characteristic of them as energy storage materials TBAB hydrate step cooling test results are shown in
Figure 2. The image on the right is a partial enlargement. In the cold storage environment of −10 °C, the supercooling degree of TBAB hydrate with the concentration of 10, 20, 30, 40, 50 and 60 wt%, respectively, is 6.3, 3.5, 2.7, 1, 8.9 and 11.2 °C. Hydrates formed under a mass fraction of (40%) have a lower undercooling and shorter induction time. The low mass fraction (10%) makes it more difficult to form hydrate, the nucleation temperature is lower, the induction time is longer and the total growth time is shorter. In the cold storage environment of −8 °C, the supercooling degree of TBAB aqueous solution with the concentration of 10, 20, 30, 40, 50 and 60 wt% is 0, 6.4, 2.7, 7.2, 4.7 and 10.5 °C, respectively. When the final temperature rise of cold storage is −5 °C, the supercooling degrees of TBAB aqueous solution with the concentration of 10, 20, 30, 40, 50 and 60 wt% are 0, 5.7, 4.3, 4.4, 9.6 and 7.4 °C, respectively. Comprehensive analysis showed that within the mass fraction (40%), the induction time was shorter. When the mass fraction was greater than 40%, the induction time increased significantly with the increase of mass fraction, that is, the growth rate decreased.
Thermal properties of TBAB hydrate at different concentrations. Phase change temperature and latent enthalpy are two basic physical properties of phase change materials, which directly affect their application potential in air conditions. As the temperature increases, the TBAB molecules absorb latent heat and the phase changes to a liquid state as a result of the transfer of absorbed energy to kinetic energy, overcoming the intermolecular forces. As a result of this transfer, the measurement of the latent heat and melting temperature of the material is crucial. Considering that water is the substance with the highest latent heat of phase change in nature, water is selected as the solute carrier of phase change cold storage materials. In order to make the phase change materials have sufficient latent enthalpy, the mass fraction of H
2O in the primary ratio is above 40%. The thermal properties of pure TBAB solution at different concentrations was analyzed by DSC.
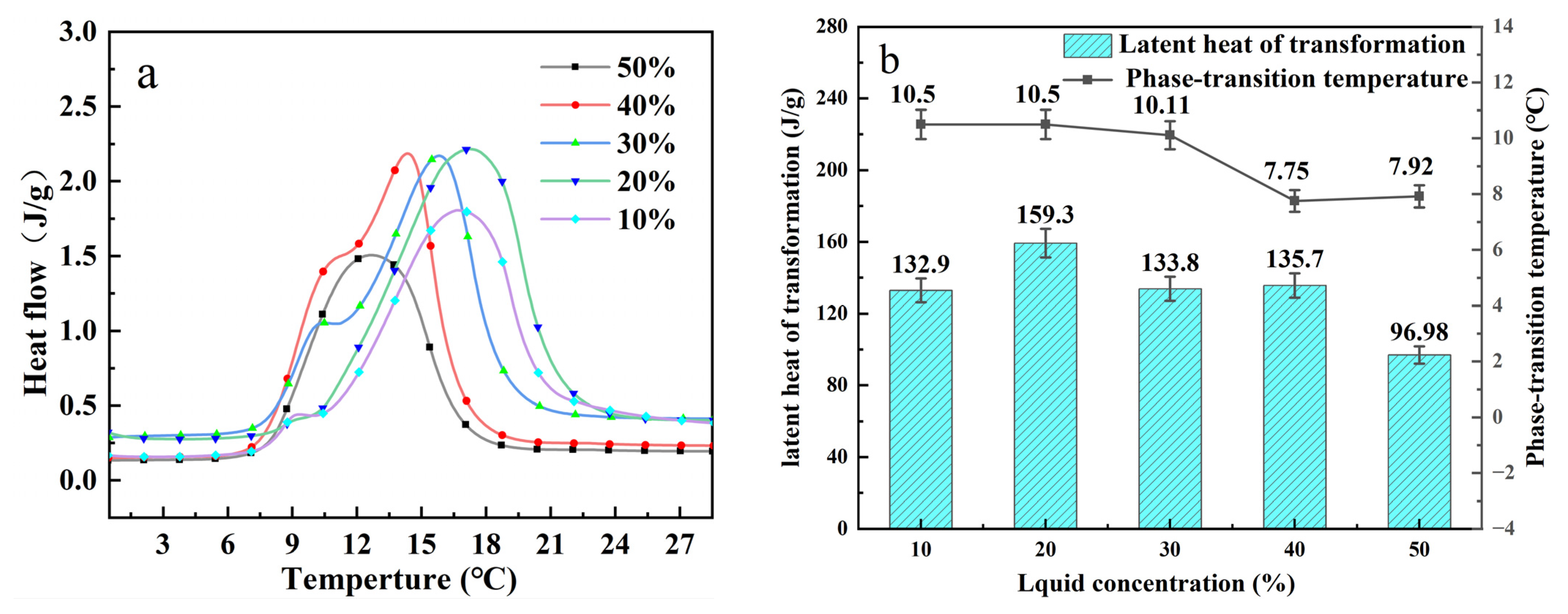
Figure 3a shows the DSC curves of TBAB solution at different concentrations. The onset temperature is estimated by the point at which the maximum slope of the endothermic peak in the melt curve meets the baseline. Although the temperature is not the initial melting temperature, it absorbs a large amount of energy after this temperature and therefore acts as the onset temperature. In temperature, substantial heat absorption begins after the transition temperature, therefore, it is more practical to examine the onset temperature. The latent heat of the phase transition is estimated by the figure. It can be clearly seen that the shape of DSC curves of TBAB solution with different concentrations is similar.
Through experimental calibration, the influence of different ratios of aqueous solution concentrations on the generation of phase change latent heat was determined. The latent heat of phase transition of deionized water was 340.01 J/g and the temperature of phase transition was about 0 °C. The measured values of phase transition temperature and latent heat are shown in
Figure 3b. The DSC curve peaks of TBAB solutions with different concentrations are roughly the same, with the larger peak being the solution with a concentration of 20%. The phase transition temperature measured by the cooling curve is different from that measured by the DSC curve. This is because there is a certain probability of solidification nucleation and it is related to the quality of the tested sample. The samples used in DSC are much smaller than those used in cooling curve experiments [
20].
Crystallization process of TBAB solution at different concentrations. Visualization of hydrate formation is an important method in hydrate research. It is helpful to understand the law of hydrate formation and explore the growth kinetics of hydrate. The roughness of the surface of the hydrate crystal, the contact between the crystal surface and the solution and other factors will affect the instantaneous rate of crystal growth in the TBAB hydrate droplet. Therefore, the measured TBAB hydrate growth rate value is the average value of the period from nucleation to the growth of the crystal filled with droplets. The method is to select the hydrate crystal perpendicular to the plane of filming as the research object and take two images Δt = 10 s apart for calculation. Image J was used to mark the coordinate of the central point of the hydrate nucleus and the direction of crystal growth in each of the two diagrams. Comparing the crystal lengths between the two images, the difference in crystal lengths is ΔL. The growth rate of the 10 s crystal can be obtained by the formula v = ΔL/Δt. The average of the measured rates was taken to obtain the average crystal growth rate, and the results are shown in
Table 1. The growth morphology of TBAB at different concentrations is shown in
Figure 4.
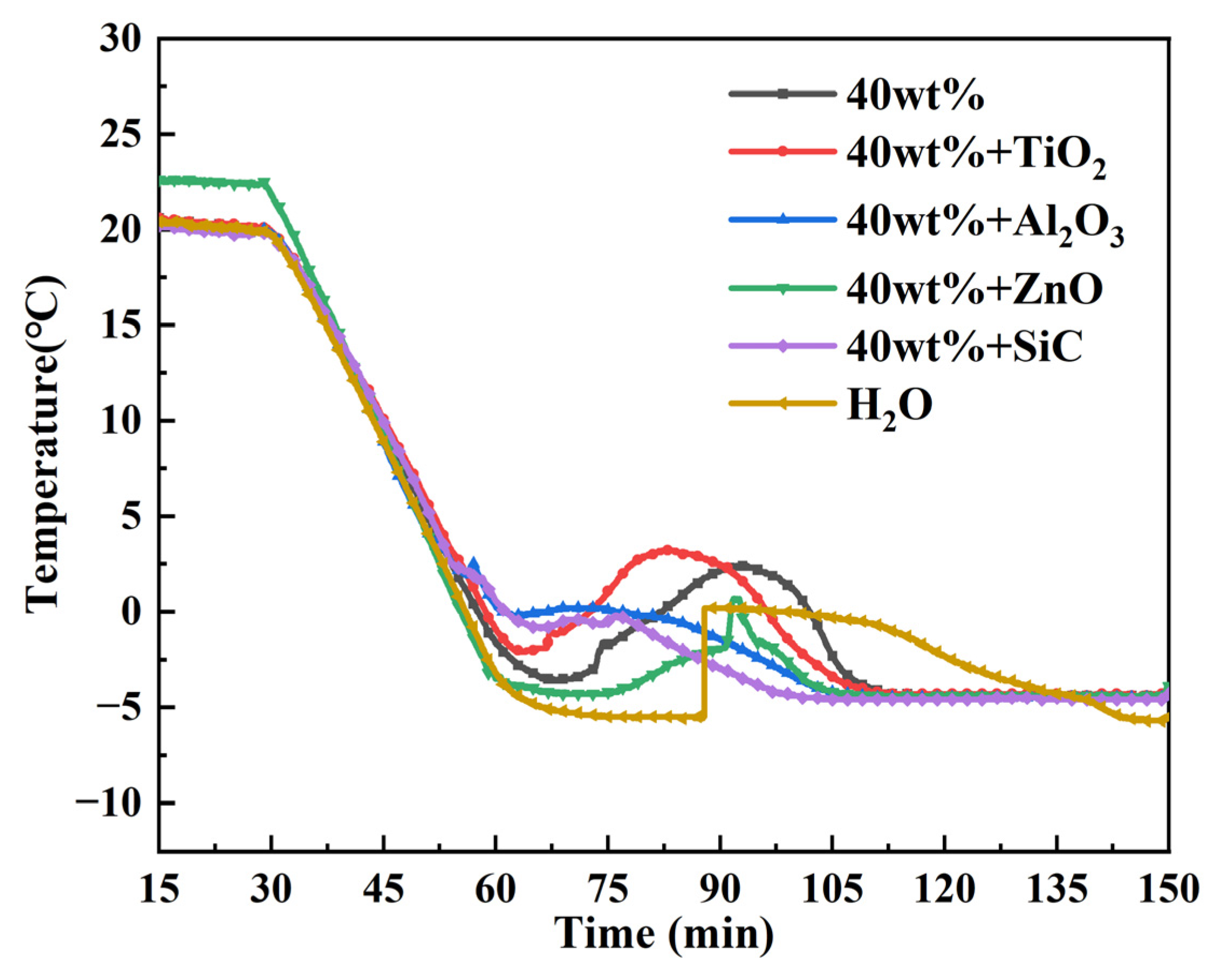
Effect of nanomaterials on the phase transition temperature of TBAB Solution. Nano-oxides include nano titanium dioxide, nano alumina and more. Adding these nano-oxides to PCMs to form nano reinforced phase change materials can improve the performance of pure PCMs. The influence of nano-oxides on the supercooling degree and phase transition point of TBAB aqueous solution is shown in
Figure 5. In the cold storage environment at −8 °C, the subcooling degree of TBAB aqueous solution with a concentration of 40 wt% is 7.2 °C. The addition of nanomaterials can significantly affect the subcooling degree of the solution. The subcooling degrees of four kinds of nanoparticles (TiO
2 Al
2O
3, ZnO and SiC) are 5.9 °C, 0.8 °C, 5.3 °C and 0 °C, respectively. This was expected as the addition of nanoparticles led to higher driving forces at the same experimental temperature. Nano TiO
2 is composed of uniform fine grains and has high chemical stability, thermal stability, superhydrophilicity, good dispersion, low cost and fire resistance [
21].
Compared with the pure TBAB solution, the supercooling degree of composite PCMs decreased by 1.3 °C after adding nano titanium dioxide. The solidification and melting time can be significantly shortened by adding nano-Al
2O
3 to the phase change material and modifying the phase change material [
22]. Compared with pure TBAB solution, the supercooling degree (0.8 °C) and phase transition time (42 s) of the composite phase change material prepared by adding nano- Al
2O
3 are significantly reduced. Nano-ZnO is a cheap and environmentally-friendly material with high thermal conductivity of 1.160 W/mK [
23]. Compared to the pure TBAB solution, the supercooling degree of the composite phase change material prepared by adding nano-ZnO is reduced by 1.9 °C. Nano-SiC as a thermal conductivity promoter was added to modify the composite PCM [
24]. Compared to pure TBAB solution, composite phase change materials (with the addition of nano SiC) have no supercooling. Analysis shows that the addition of nano Al
2O
3 and SiC can significantly reduce the supercooling degree of PCMs and shorten the induction time of hydrate formation. This may be because for TBAB aqueous solution, nucleation is mainly heterogeneous and the addition of nanoparticles provides an additional surface for heterogeneous nucleation which may be larger than the critical size for solid crystal growth, making it easier for the crystal size on the nanoparticles to exceed the critical size. Therefore, nanoparticles provide the nucleus for TBAB aqueous solution molecules to aggregate and form solid crystals, thereby reducing undercooling [
25].
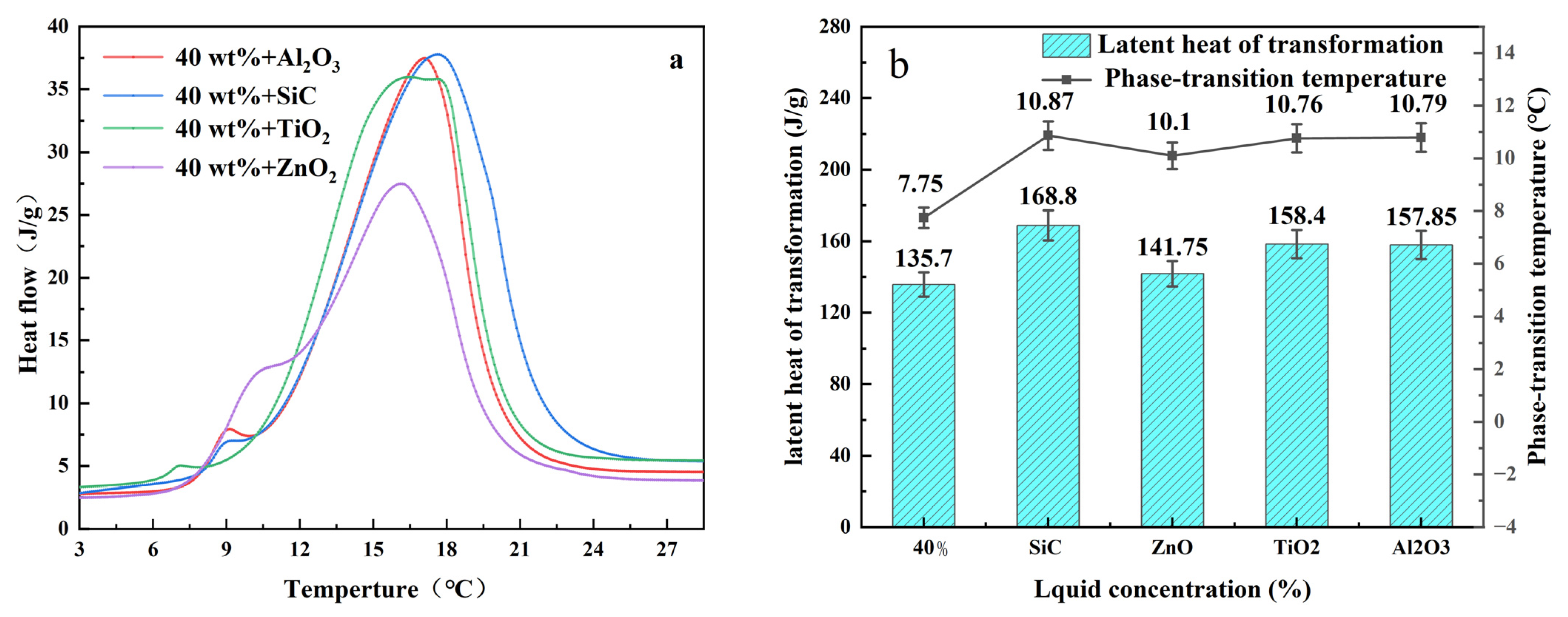
The thermal properties of phase transition materials adding nano-oxide were measured by DSC. The phase transition process is shown in
Figure 6a and the relevant data are listed in
Figure 6b. All DSC curves of PCMs were operated under the first heating/cooling. It can be seen from the table that the onset temperatures of 40% TBAB solution were 7.75 °C and 135.7 J/g. Compared with the pure TBAB solution, the phase transition temperature and latent heat of the composite PCM prepared by adding nanoparticles were significantly increased. The latent heat of phase transition increased by 24% and the temperature of phase transition increased by 3.12 °C after nano-SiC was added to the solution. Therefore, adding four different nanomaterials to TBAB solution can improve the thermal performance of phase change materials. After comprehensive analysis, adding nano-SiC into TBAB solution can result in better performance.
4. Conclusions
In this paper, a cold storage agent with a phase change temperature (onset temperature) of 273–286 K was developed by adjusting the ratio. This article first used cooling step cooling curve, differential scanning calorimetry (DSC) and visualization analysis to conduct experimental studies on the supercooling temperature, phase transition temperature and latent heat of phase change materials with different concentrations. Comprehensive analysis showed that within the mass fraction (40%), the induction time was shorter. When the mass fraction was greater than 40%, the induction time increased significantly with the increase of mass fraction, that is, the growth rate decreased. This is because higher concentrations lead to agglomeration, which in some cases also reduces the latent heat capacity while increasing it. Considering the cold storage temperature, phase transformation temperature and latent heat, the cold storage effect is the best when 40% TBAB solution is used. Then, the effect of using different nanoparticles with the PCMs on the performance of a new technique used to enhance cold storage by investigating the thermal properties and thermal stability. When comparing additional nanoparticle PCMs with pure TBAB solution, the phase transition temperature and latent heat of the composite PCMs prepared by adding nanoparticles were significantly increased. The latent heat of phase transition increased by 24% and the temperature of phase transition increased by 3.12 °C after nano-SiC was added to the solution. Therefore, the thermal properties of phase change materials were enhanced by adding four different carbon nano additives to TBAB solution. After comprehensive analysis, adding nano-SiC into 40% TBAB solution can result inn better performance. The direct addition of nanomaterials can significantly enhance the properties of the PCMs, but the dispersion of nanomaterials is delicate. To achieve the desired effect, attention should be paid to the selection of nanomaterials and the concentration ratio used. From the literature review, 10 wt% and below are common. Smaller diameter nanoparticles and lighter nanoparticles have better results, but they tend to be more expensive.