3.1. Pre-Evaluation Based on Fuel Ash Composition
The evaluation criteria developed for the preliminary assessment of the potential of the biomass ash are summarised in
Table 1. The criteria have been adopted from the standard requirement for the use of silicate fly ash in concrete [
50]. SiO
2, Al
2O
3, and Fe
2O
3 are the pozzolanic oxides that may react in the presence of calcium to bound hydrate components [
51]. A high content of these elements is beneficial for the properties of SCM if they are in soluble states. Several species, such as MgO, Na
2O, P
2O
5, SO
3, and Cl, are limited to a certain value as they can impair concrete quality. For instance, a high Cl content increases the risk of corrosion [
52]. In addition to the limit values listed in DIN EN 450-1, the potassium content is also chosen as an evaluation parameter, as it often correlates with the risk of slagging [
52] and the risk of high particulate matter emissions during biomass combustion.
The results of the evaluation with respect to the selection criteria (
Table 1) are presented in
Table 2. The full results of the chemical analysis of the fuels are presented in
Table A2. Based on the results, several biomasses did not meet the selection criteria. OH and H were neglected due to the low AC (OH: 5.4 wt.% db, H: 5.6 wt.% db) and high potassium content (OH: 15.0 wt.% db, H: 45.3 wt.% db). Potassium is one of the main constituents that can induce slagging [
52]. In addition, D was also excluded from further analyses due to its high phosphorus content, which reached 21.6 wt.% db. As concluded by Al Pang et al., phosphorus content in the range of 10 wt.% db and 50 wt.% db may interfere with the setting time of the cement and thus induce micro-cracks [
53].
The biomass husk fuel ashes have a high content of silica. For instance, the silica content of the SH ash reaches 85.0 wt.% db, although it is still lower than the silica content of RH. This high silica content could be an indication that the SH ash is a highly pozzolanic material suitable as an SCM. However, it should be further investigated whether the silica content is in a reactive state. F does not meet all of the criteria (
Table 2), e.g., the SO
3 contents are above the limit values. However, it is not considered critical in the role of biomass as an SCM. Thus, SH and F were selected for further analysis, while RH was included as a reference.
3.2. Ash Composition
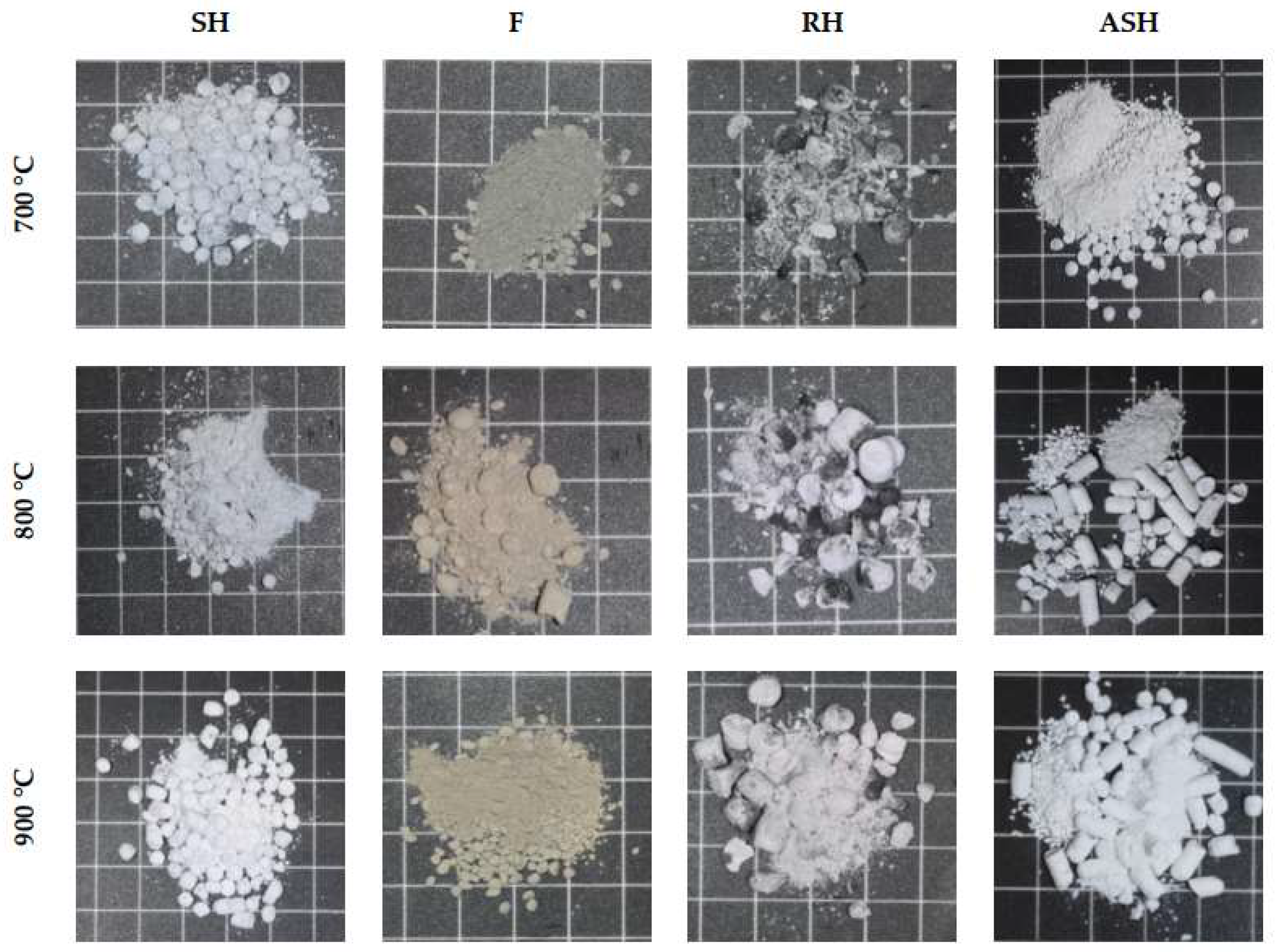
The visual images of the produced biomass ashes from the ashing process of the selected biomass (SH, F) at different ashing temperatures are shown in
Figure 2. For SH, additivated pellets with 1 wt.% kaolin (ASH) were also employed. RH ashes were used as a reference. The white colour in SH, RH, and ASH indicates that these ashes contain a high proportion of silica. In contrast, the F ashes have a slightly brownish colour. For all samples, especially the RH ashes, the higher ashing temperature increases the whiteness of the ashes. The high ashing temperature induces a more complete combustion process and thus removes carbon particles from the produced ash.
A significant difference in the granulometry between the F ashes and other ashes can be observed in
Figure 2. While the F ashes consist of more pulverised particles, more SH, RH, and ASH ashes retain their pelletised form after the thermal treatment. This effect may be due to the high silica content in SH, RH, and SH ashes. This is probably due to the relatively strong bonds between the silica units [
35].
The chemical composition of the ashes is summarised in
Table 3. Overall, the difference in the ashing temperature does not significantly affect the chemical composition of the ashes produced. The chemical composition of the ashes also deviates little from the results of the fuel ash analysis in
Table 2. This was also highlighted by Zeng et al. for bottom ashes from the combustion of blended biomass pellets in a small-scale combustion appliance [
23]. The addition of kaolin to SH increases the content of the pozzolanic oxides (SiO
2, Al
2O
3, Fe
2O
3) of ASH, as the major constituents of kaolin are Si and Al, as specified in
Table A1. Kaolin in the metastable form of metakaolin has been known for decades to be a highly efficient pozzolanic SCM [
54]. The addition of kaolin slightly reduces the silica content of ASH from around 88 wt.% db to around 86 wt.% db because the silica content of kaolin is still much lower than that of SH, which is 52.1 wt.% compared to 88 wt.%. With the addition of 1 wt.% kaolin, the pozzolanic oxide content of ASH is still lower than in the case of RH. From the chemical composition of the ashes, it can be concluded that the SH and ASH ashes are expected to have a pozzolanic higher reactivity than F ashes due to the significantly higher content of the pozzolanic oxide. However, CaO-rich ashes, such as F (see
Appendix A Table A2), may exhibit (latent) hydraulic properties.
LOI indicates the amount of unburnt carbon in the ash. Although it can also indicate the presence of carbonates and bound water, the major contribution of LOI is mainly residual carbon [
55,
56]. As shown in
Table 3, the LOI of all ashes produced is generally low. The LOI of all samples is less than 2%. The low LOI shows that the biomasses are completely burned during the combustion process. This could be a positive indication, as carbon content in SCMs may cause some disadvantages in the concrete mixture. For instance, the carbon content can reduce the fineness of the ash and, therefore, the reactivity of the cement mixture [
55]. Moreover, unburnt carbon has a significant impact on the workability of cement-based materials due to its high surface area.
Table 3 also shows that the pH of all ashes ranges between 9.1 and 12.9. F ashes have the highest pH at more than 12. The ashing temperature does not have a significant impact on the pH of the ashes. The addition of kaolin to SH reduces the pH of ASH ashes. ASH-700 possesses the lowest pH at 9.1. This is due to the addition of acidic compounds in the kaolin, such as SiO
2 and Al
2O
3. A high pH in concrete provides protection against destructive agents that can induce steel corrosion [
57]. The pH of the ashes is therefore a positive factor.
3.3. Mineral Phase Characterisation and Prediction
Figure 3 presents the transformation of the mineral phases in ashes as a function of ashing temperatures. XRD analyses indicate that the siliceous biomass ashes produced at 700 °C are characterised by a highly amorphous structure. At 700 °C, the proportion of amorphous structures in SH and RH is 94 wt.% and 96 wt.%, respectively. On the other hand, nearly half of the phases in F transform into crystalline structures at 700 °C. A notable reduction in the amorphous content at higher ashing temperatures can be observed for all non-additive ashes. For instance, the amorphous content decreases from 94 wt.% for SH-700 to 49% for SH-800 and 34% for SH-900, respectively. Crystallisation at higher ashing temperatures has also been reported in previous investigations, although the crystallisation temperature varies depending on the composition of the ash [
32,
35].
As shown in
Figure 3, the reduction in amorphous content in SH and RH at higher temperatures is mainly due to the formation of previously reactive amorphous SiO
2 into crystalline mineral phases of simple oxides, such as cristobalite and tridymite. In addition to siliceous crystalline phases, the presence of calcium potassium diphosphate is also detected in SH-800 and SH-900. The phosphorous content in SH ashes is relatively higher than in RH ashes, which, together with the alkali mineral content, contributes to the formation of calcium potassium diphosphate.
As shown in
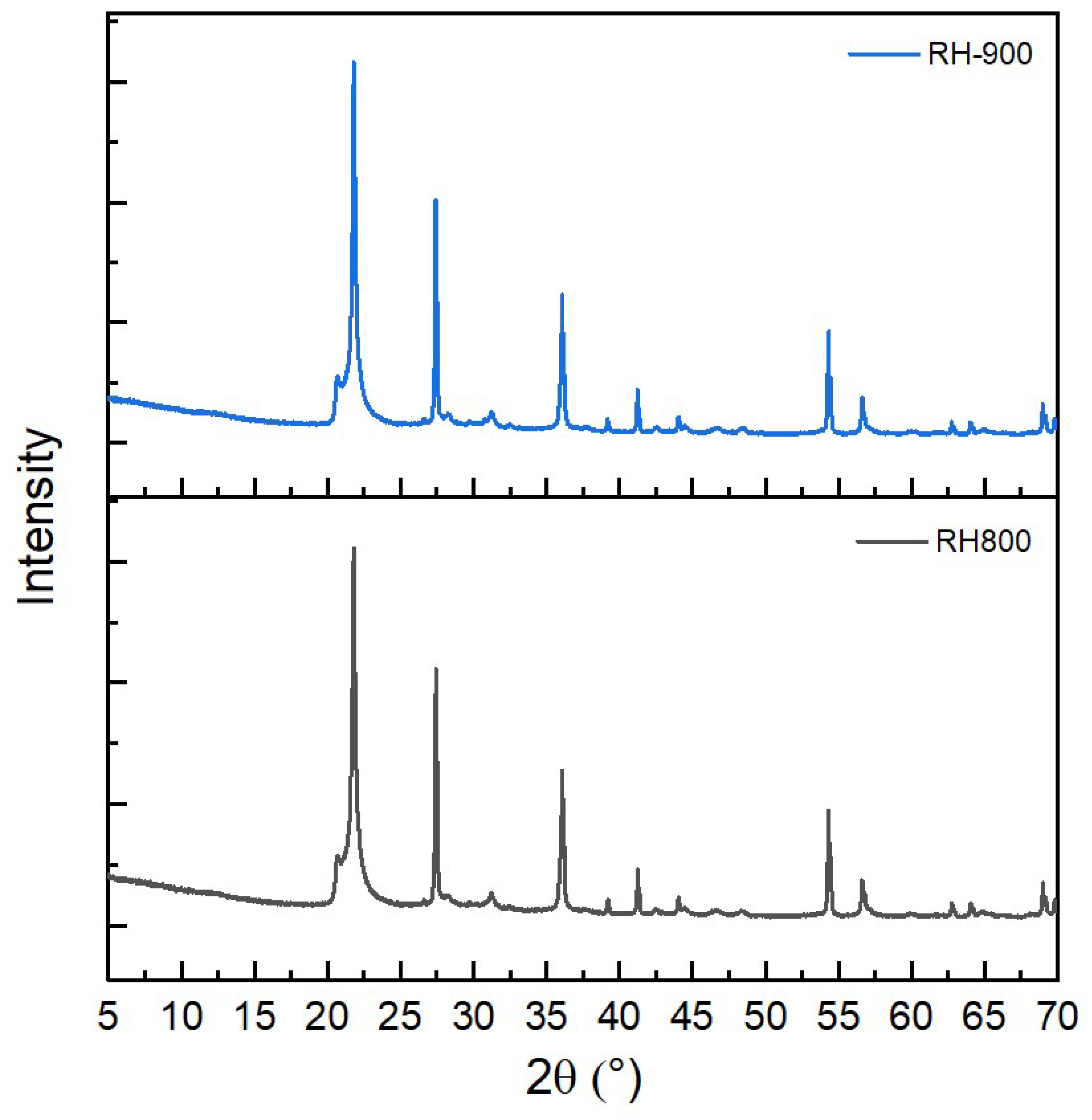
Figure 3c, the amorphous content of RH-900 is 8 wt.% higher than that of RH-800, although the higher ashing temperature should reduce the amorphous content of the ash, as shown clearly in SH. This effect could be due to the inaccuracy of the quantification methods. Some phases sometimes show broadened peaks, making it difficult to distinguish between crystalline and amorphous content, especially in systems with multi-phase and low crystallinity. In principle, the diffraction patterns of RH-800 and RH-900 are very similar, as shown in
Figure A1. From a qualitative perspective, the amorphous content of RH-800 and RH-900 should not vary.
Unlike the siliceous ashes, the phase transformation of F ashes is relatively more complex. As shown in
Table A2, the major elements of F are CaO (37.6 wt.%) and SiO
2 (37.3 wt.%). Therefore, the mineral phases in F ashes are dominated by the phases in the CaO-SiO
2 phase diagram. At 700 °C, the SiO
2 in the form of quartz accounts for about 10 wt.% of the total phases. The content of quartz decreases at higher temperatures mainly due to the reaction with calcium to form Ca-silicates, like wollastonite (CaSiO
3). Moreover, a fair amount of mayenite (Ca
12Al
14O
33) is present in F ashes, which is formed by the interaction between CaO and Al
2O
3. The amount of mayenite increases with rising ashing temperatures, reaching more than 11 wt.% at 900 °C. The presence of mayenite in Ca-rich ashes has also been reported in some of the literature [
58,
59,
60]. The complete results of the XRD analysis of the ashes are presented in
Table A3 and
Table A4.
In contrast to the other ash samples, the reduction of the amorphous content in ASH is not notably detected, as shown in
Figure 3. At 900 °C, the proportion of the amorphous phases in ASH still reaches more than 90 wt.%. The addition of kaolin is proven to stabilise the amorphous structure in ASH at high ashing temperatures. The formation of silica-based crystalline structures, like cristobalite and tridymite, which are highly present in SH-800 and SH-900, can be reduced. Kaolinite (Al
2Si
2O
5(OH)
4) is the most dominant mineral phase in kaolin [
25]. During the thermochemical process, kaolinite releases water and forms meta-kaolinite (Al
2O
3.2SiO
2), a highly amorphous mixture of alumina and silica. The meta-kaolinite is supposed to bind potassium to form K-Al-silicates, like kalsilite and leucite. As observed in
Figure 3, kalsilite is present in ASH ashes in a small amount, which is less than 3%. The potassium content in ASH ashes is much lower than the alumina content. Thus, the absence of potassium might cause the amorphous meta-kaolinite to still be present in ASH ashes.
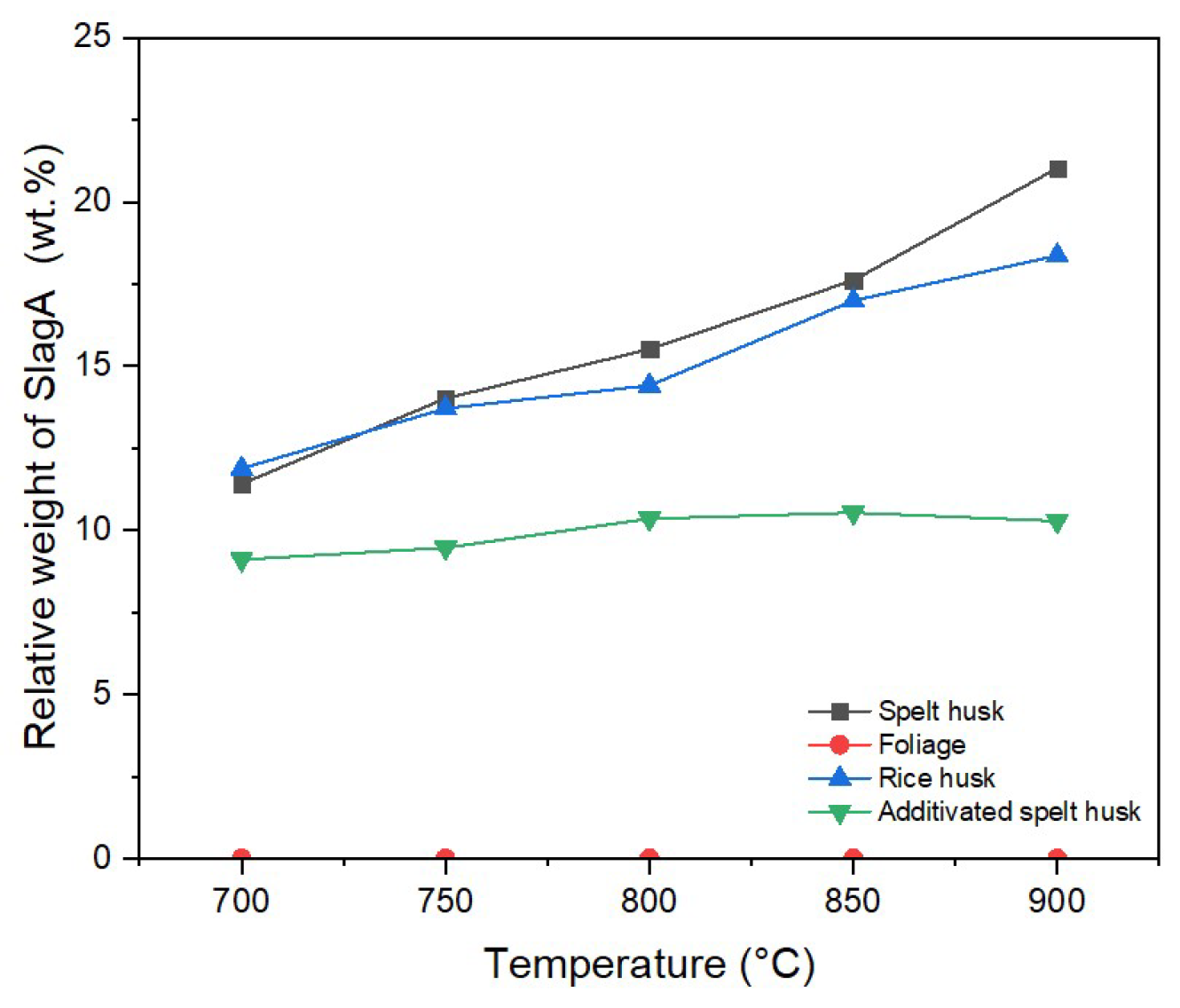
The slagging tendency of the biomass ashes predicted with the thermodynamic equilibrium calculation is presented in
Figure 4. The calculation is based on the chemical composition of the ash samples. As shown in
Figure 4, the ashing temperature plays a significant role in the slag formation of SH and RH, because the slag formation increases with the increasing temperature. For instance, the share of slag in SH at 700 °C is around 10%, but it reaches more than 20% at 900 °C. The slag formation at higher temperatures was also indicated by our previous simulation results [
35]. This could be due to the melting of the phases with a low melting point, such as K-silicates. On the other hand, the slag formation in F is not detected in the monitored temperature range. The ashes with high CaO content have a higher ash-melting temperature, as reported in [
61]. The observation of the slag formation in ASH reveals that the addition of kaolin can inhibit the slagging tendency. At 900 °C, the proportion of slag in ASH remains less than 10%. As mentioned in
Section 3.3, the Al in kaolin binds potassium to form K-Al-silicates, such as kalsilite, as presented in
Figure 3. Kalsilite is known to have a higher melting temperature than K-silicates. Therefore, the slag formation in ASH can be mitigated.
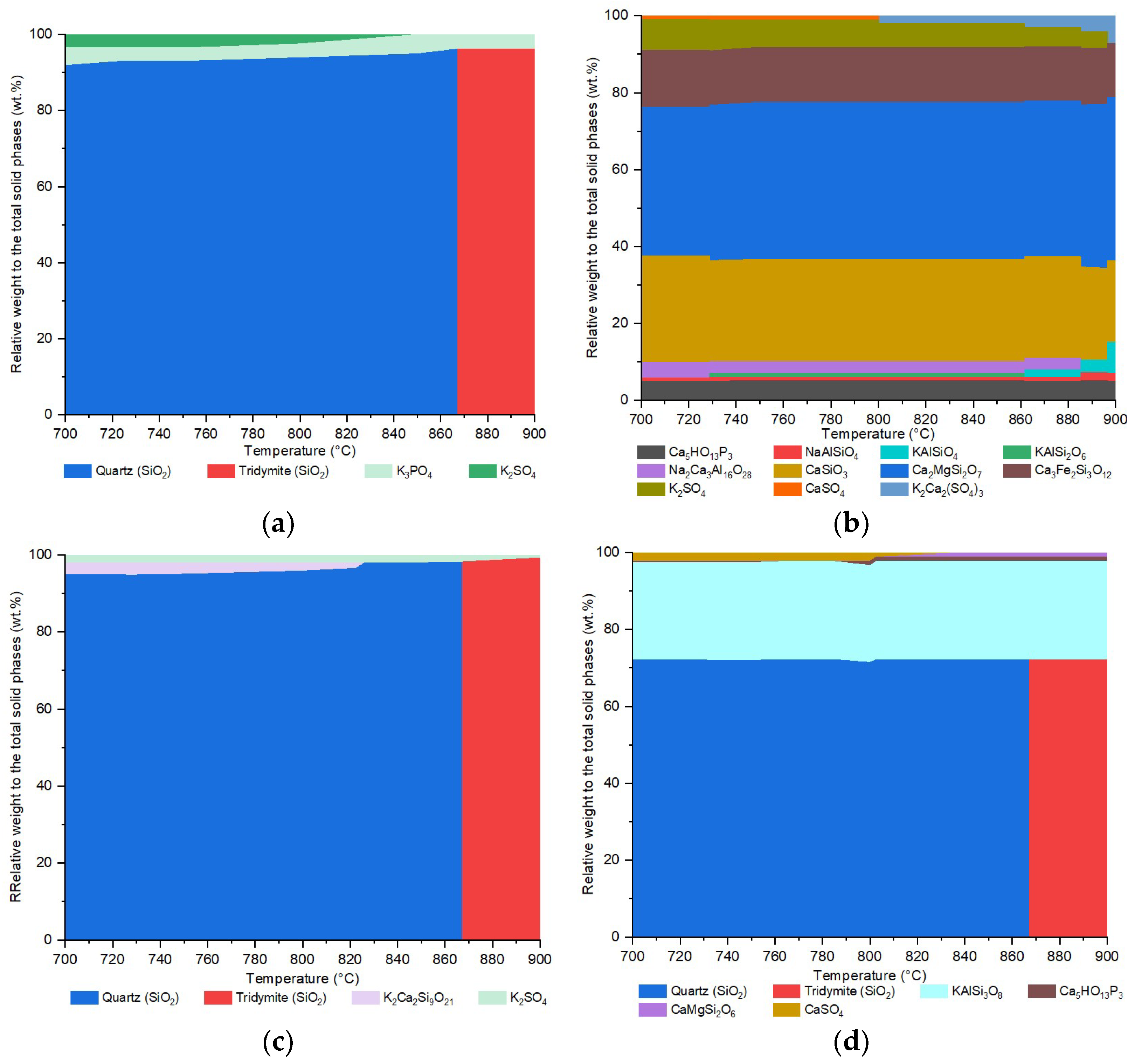
The mineral phase transformations calculated using FactSage during the ashing between 700 °C and 900 °C are presented in
Figure 5. In general, the simulation using FactSage can predict the presence of similar mineral groups but fails to determine the identical mineral phases detected through the XRD analysis. As shown in
Figure 5, SiO
2-phases are calculated as the most dominant species in SH, RH, and ASH. Nevertheless, the simulation fails to predict the formation of cristobalite, as illustrated in
Figure 3. Instead, quartz is formed and completely transformed to tridymite at around 860 °C. Thermodynamically, cristobalite is formed at temperatures above 1400 °C [
62]. However, the presence of alkali metals in biomass ash can significantly reduce the formation temperature of cristobalite. Furthermore, at 700 °C, the proportion of quartz is significantly overestimated because the metastable phases, such as amorphous silica, do not thermodynamically exist [
63].
The simulation can predict the existence of some similar but not identical mineral phases. For instance, the calculation predicts the minor presence of potassium phosphate (K3PO4) in SH instead of calcium potassium diphosphate, as detected through XRD. Moreover, instead of kalsilite, a more stable K-Al-silicate in the form of KAlSi3O8 is predicted as the second major phase in ASH. The simulation fails to predict silicates, like wollastonite and gehlenite, in RH. Instead, the Ca-silicates are predicted to bind the potassium to form K2Ca2Si9O21. For sulphates, instead of gehlenite, the result shows the formation of K2SO4 in RH-700.
The phase transformation of F between 700 °C and 900 °C is dominated by the formation of calcium-bearing silicates, such as CaMgSi
2O
7, CaSiO
3, and Ca
2Fe
2Si
3O
12, with a total share of around 80%. The group of K-Ca-sulphates is also predicted, which constitutes around 10 wt.% of the total solid mass. The discrepancy between the XRD analysis and the simulation results of F is the failure of the simulation to predict the formation of mayenite, which is one of the most dominant phases in F ashes, according to the XRD results. The simulation predicts only a very small fraction of Ca-aluminate in the form of Na
2Ca
3Al
16O
28. Furthermore, the thermochemical equilibrium calculation fails to predict the formation of simple oxides, such as quartz and lime, as it neglects the kinetic limitations of the process and thus favours the formation of silicates [
64].
3.4. Analysis of the Specific Surface Area
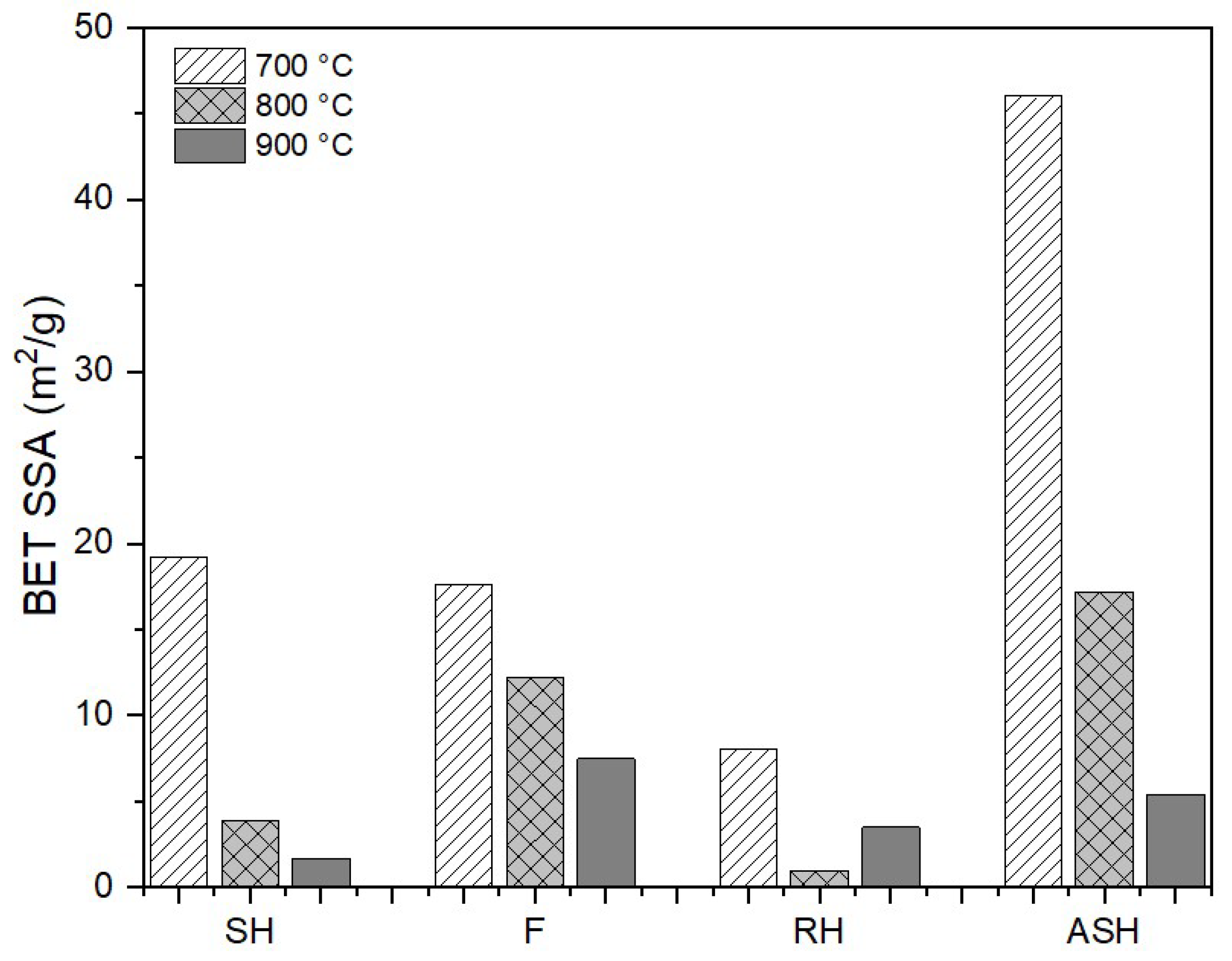
In order to analyse the structural characteristics of the ashes, the BET specific surface area (SSA) of the ashes was measured using the gas sorption method. As shown in
Figure 6, increasing combustion temperatures result in ashes with lower BET SSA. For instance, the BET of F-900 is 7.5 m
2/g, while the BET SSA of F-700 reaches 17.6 m
2/g. The decreasing BET SSA of the biomass ashes may be caused by the slag formation process [
35,
36]. As also shown in the FactSage results in
Figure 4, the slag formation tends to increase at higher temperatures, which is in agreement with our previous results [
35]. BET SSA is known to have an inverse relationship with the particle size [
65,
66]. As biomass slag is usually associated with a higher particle size than ash (>3.15 mm) [
24,
67,
68], the BET SSA of biomass slag should be lower.
The results of the BET SSA measurement show that RH ashes have a different trend compared to other ashes. While the BET SSA of other ashes decreases with the increasing ashing temperatures, the BET SSA of RH-800 is lower than RH-900. There are some factors that could be the cause of the low BET SSA of RH-800, such as the amorphous content and the particle size. Firstly, the amorphous content of RH ashes shows the same trend as the BET SSA, as shown in
Figure 3. At first sight, amorphous content could be directly correlated with BET SSA. However, the results of ASH ashes show that this is not always the case. Secondly, as mentioned earlier, particle size has a direct correlation with BET SSA. RH-800 might have a slightly larger particle size than RH-900. However, as the particle size measurement of ash particles is quite challenging and was not conducted in this study, the actual cause of the low BET SSA value of RH-800 cannot be verified.
The addition of kaolin to the SH significantly improves the BET SSA. At 700 °C, the BET SSA of the SH ash is less than 20 m2/g, while the BET SSA of the ASH ash is more than 46 m2/g. However, the addition of kaolin does not prevent the reduction in the BET SSA in ASH ashes. The BET SSA of ASH ashes significantly decreases to 17.2 m2/g and 5.4 m2/g at 800 °C and 900 °C, respectively. This may be due to the agglomeration of the amorphous structures.
3.5. Evaluation of Pozzolanic Reactivity of the Ashes
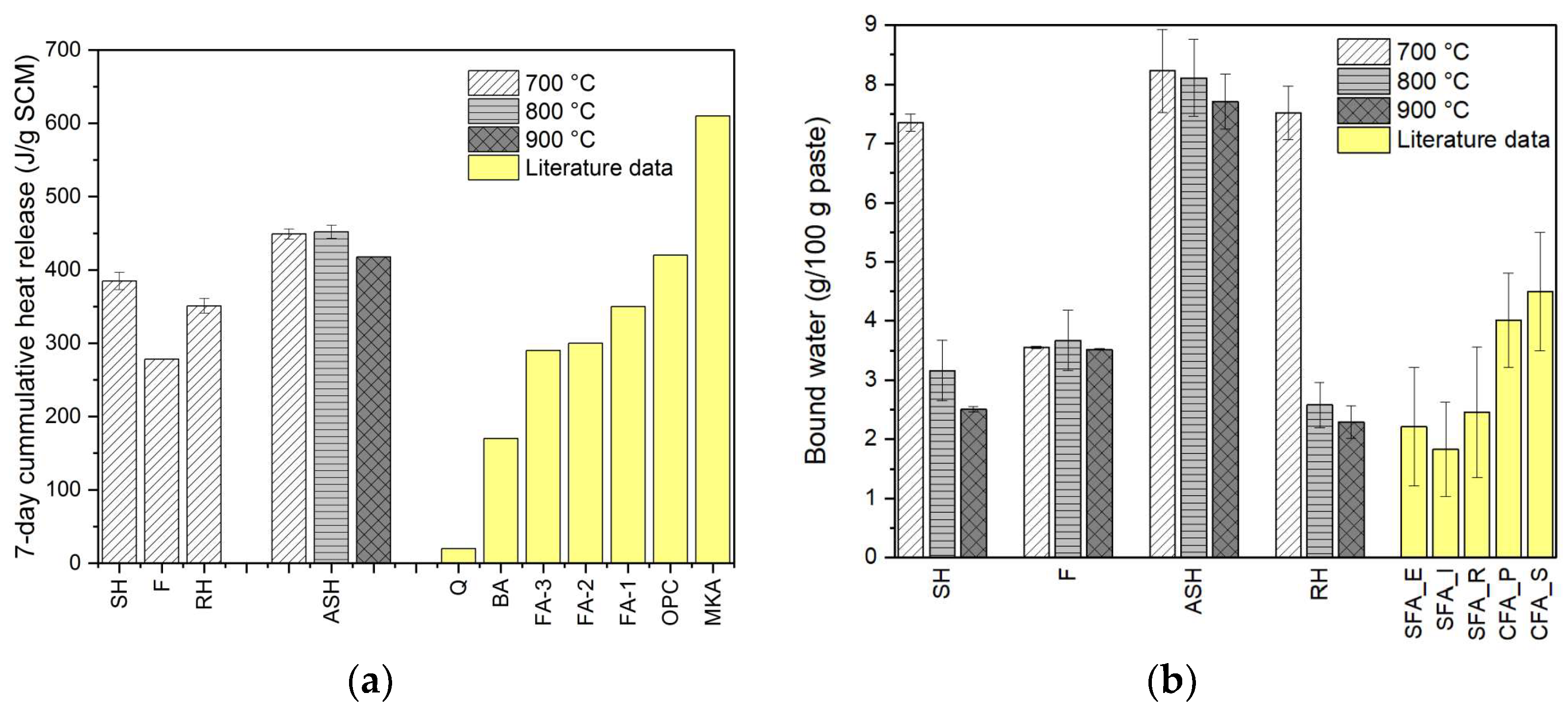
The pozzolanic reactivity of the ashes was analysed using the isothermal calorimetry and the bound water measurement.
Figure 7 presents the cumulative heat release during the 7-day curing process and the bound water measurement of the cementitious paste made from biomass ashes. Except for ASH, the heat release measurements were only carried out for the biomass ashes produced at 700 °C. As illustrated in
Figure 7, the SH ashes show a comparable reactivity to the RH ashes. SH-700 even has a higher cumulative heat release compared to RH-700. Among the non-additive samples, the highest pozzolanic reactivity was recorded for SH-700 with a bound water of 7.3 g/100 g paste and a cumulative heat release of 385 J/g SCM. The measurement of heat release was conducted to verify the reactivity test according to the bound water measurement.
The bound water of SH decreases significantly at higher ashing temperatures. For instance, the bound water of SH-900 decreases to 2.5 g/100 g paste. This trend is also shown in the RH ashes. The declining reactivity at high ashing temperatures is less pronounced for ASH. This is again due to the stabilised amorphous phase even at high temperatures. Kaolin can also prevent the formation of crystalline structures. In addition, as shown in
Figure 7, kaolinite-containing material like metakaolin possesses a very high pozzolanic reactivity. Metakaolin releases about 600 J/g of SCM heat during the seven-day curing process. This could be the reason why the reactivity of ASH is significantly higher than that of SH. Although the addition of kaolin to the biomass pellets was only 1 wt.% of the total mass of the pellets, the proportion of kaolin in ASH increases significantly due to the devolatilization of organic matter during the ashing process. Based on the calculation considering the AC of the SH and the LOI of the kaolin, the proportion of kaolin in the produced ash is about 10 wt.%.
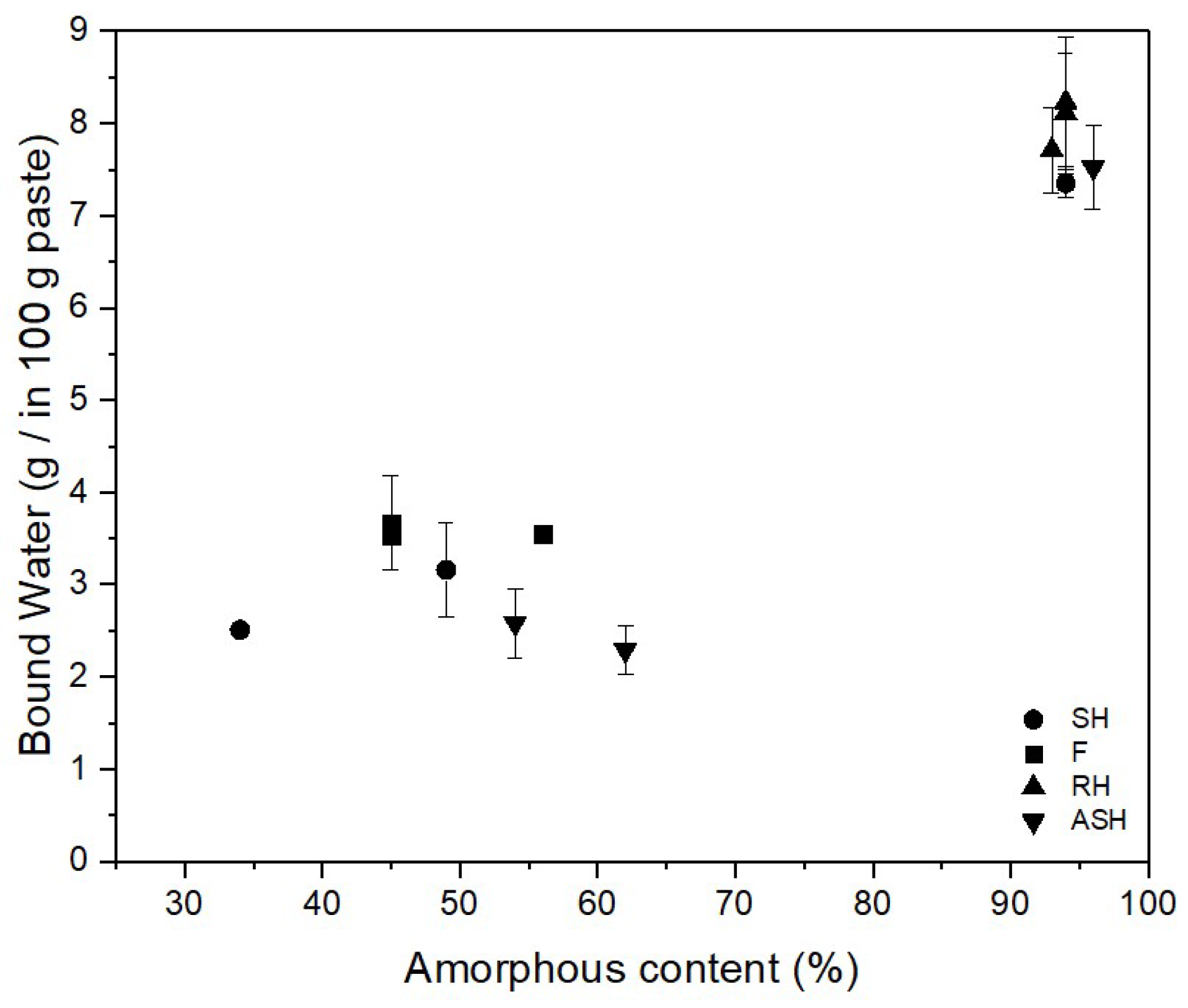
As shown in
Figure 8, a close relationship can be observed between the amorphous content and the reactivity of the ash. A highly amorphous structure seems to result in a high reactivity of ash and vice versa. This is mainly because only the active or amorph fraction participates in the pozzolanic reaction [
69]. Thus, the amorphousness of SCMs is an important factor in determining reactivity.
On the other hand, the relationship between the BET SSA and the reactivity of the ash is more complicated. A higher specific surface area provides more sites for the hydration reaction to happen simultaneously. Nevertheless, if the whole grains dissolve over time, the total reaction measured will not be affected. Thus, the BET SSA of the biomass-based SCM only affects the rate of reaction and has a limited effect on the total reactivity measured after seven days.