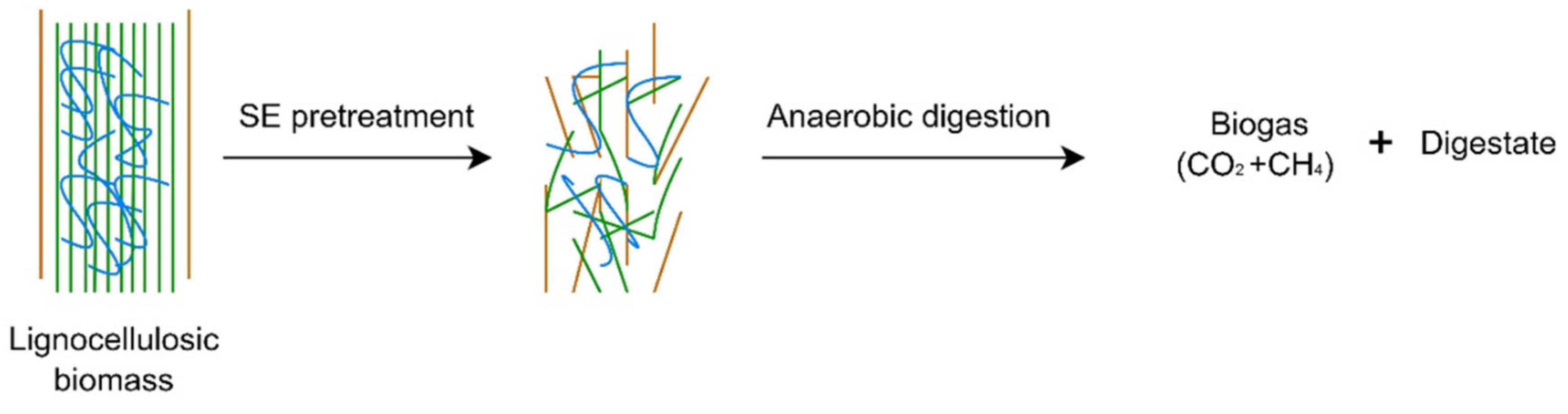
Lignocellulosic Biomass Valorisation by Coupling Steam Explosion Treatment and Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
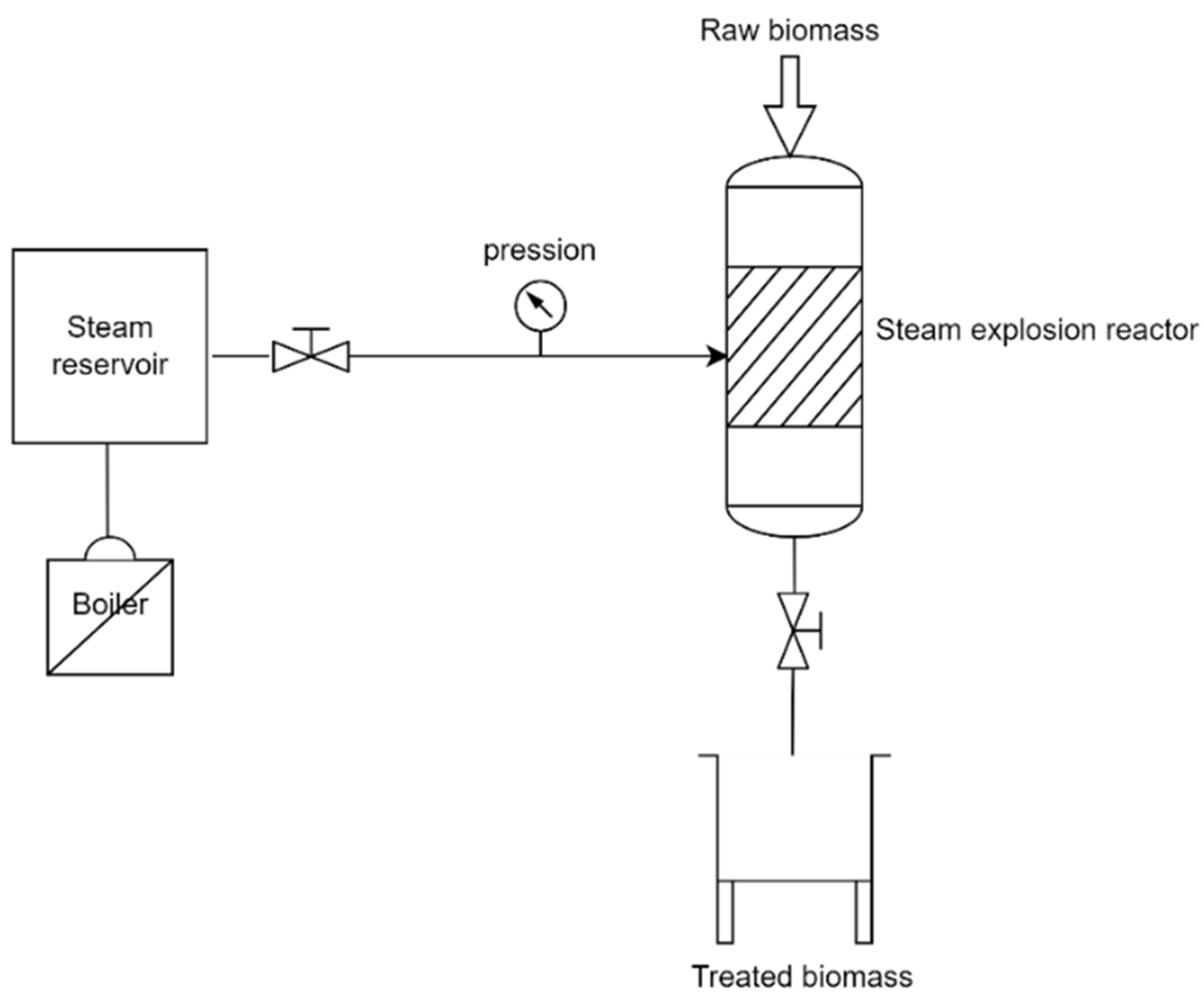
2.2. Steam Explosion Pretreatment
- Impregnation: The biomass was loaded into a beaker and hydrated with water. If necessary, the catalyst should be added at this step according to its required concentration (%w/w).
- Filtration and explosion reaction: the melange biomass/solvent was then filtered to remove the excess water and loaded into the steam explosion reactor. The temperature is maintained between 200–210 °C at the saturation vapor pressure. As described in the previous part, the SE is composed of two phases: steam cracking and explosive decompression. In the first phase, steam cracking consists of diffusing then condensing the steam at a high pressure inside the structure of the biomass. The water condensed at high temperatures will initiate the hydrolysis of the acetyl groups and induce the formation of organic acids. Depending on process’s conditions, the acids formed catalyse the hydrolysis of the hemicellulose fractions, modify the degree of crystallinity of the cellulose fraction and the structure of lignin. During the second phase, the explosive decompression causes a sudden drop in pressure, which will cause shear forces that modify the physical properties of the biomass. The treatment (two phases) takes between 3 and 6 min. Figure 2 illustrates the operating system (SE) process.
- At the end of the reaction, the valve of the reactor is opened to collect the biomass, which is then conserved at temperatures of 4 °C in order to avoid fungus formation at the top of the biomass.
2.3. Anaerobic Digestion Conditions
- The temperature of the process: The anaerobic digestion was performed either at a mesophilic temperature of 38 °C or at a psychrophilic temperature of 25 °C.
- The ratio of substrate to inoculum volatile solid: This parameter, denoted as SV (solids or volatile solids), determines the relative proportions of the organic biomass feedstock (substrate) and the microbial organic biomass (inoculum) used in the digestion process.
2.4. Analysis Methods
3. Results and Discussion
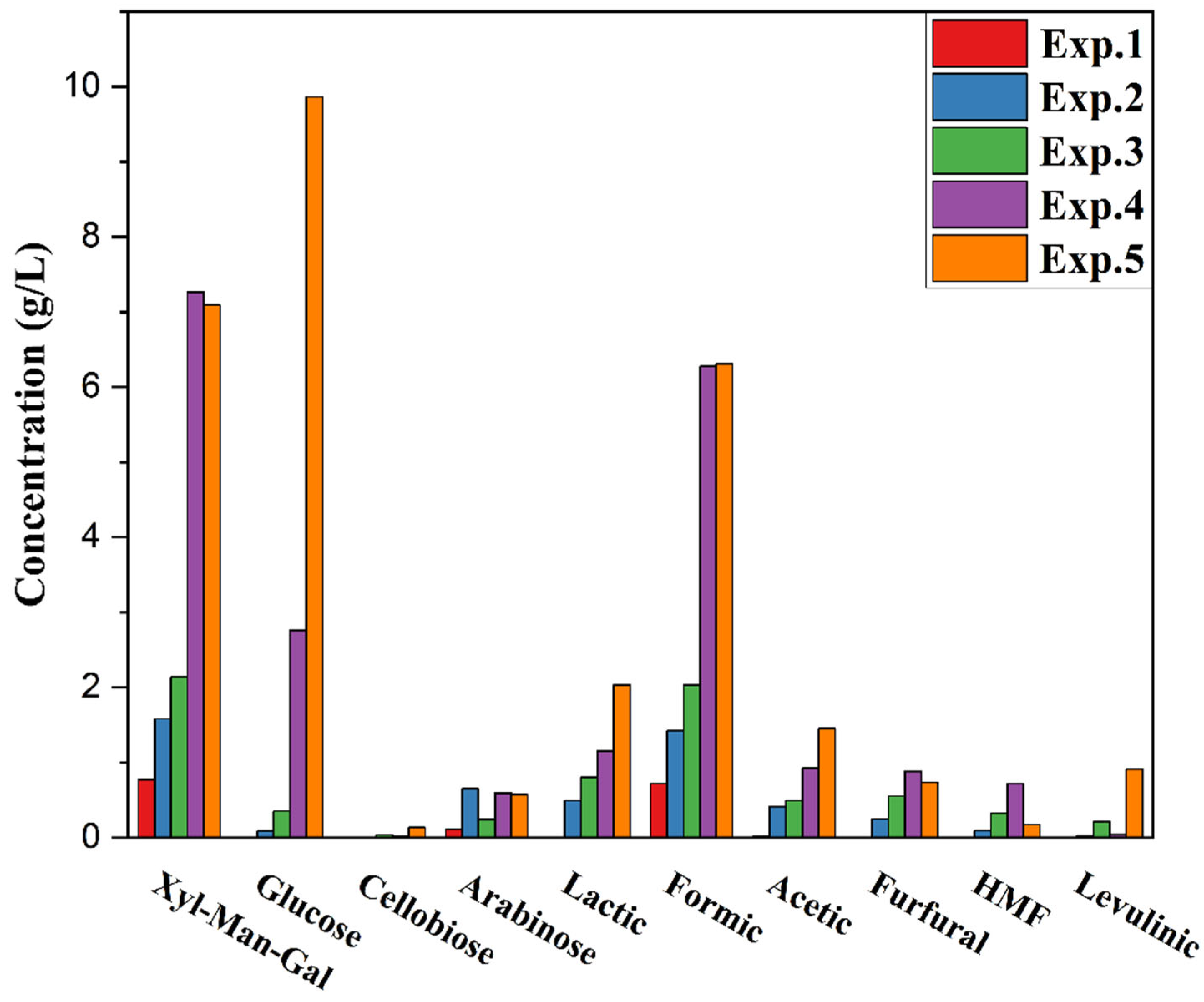
- The concentration of Xyl-Man-Gal and glucose is highly sensitive to all three factors.
- Depending on the acid concentration, glucose is the most sensitive sugar, and it reaches its maximum at a higher acid concentration of 1%.
- 5-HMF, being a dehydration product of glucose (hexoses), increases in production with higher temperature, time, and acid concentration.
- The concentration of levulinic acid and formic acid is higher at 1% acid concentration, indicating the degradation of 5-HMF at this concentration.
- Furfural, as a degradation component of pentoses such as xylose, increases with increasing temperatures and reaction times. However, at a higher 1% acid concentration, its concentration decreases simultaneously with that of xylose.
- At a lower explosion temperature of 170 °C and a reaction time of three minutes, the conditions are not sufficient to degrade the lignocellulose, and the wood chips remained intact without any size reduction at the reactor outlet.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awadalla, O.A.; Atawy, W.A.; Bedaiwy, M.Y.; Ali, S.S.; Mahmoud, Y.A.-G. Anaerobic Digestion of Lignocellulosic Waste for Enhanced Methane Production and Biogas-Digestate Utilization. Ind. Crops Prod. 2023, 195, 116420. [Google Scholar] [CrossRef]
- Karrabi, M.; Ranjbar, F.M.; Shahnavaz, B.; Seyedi, S. A Comprehensive Review on Biogas Production from Lignocellulosic Wastes through Anaerobic Digestion: An Insight into Performance Improvement Strategies. Fuel 2023, 340, 127239. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Chatterjee, B.; Mazumder, D. Anaerobic Digestion for the Stabilization of the Organic Fraction of Municipal Solid Waste: A Review. Environ. Rev. 2016, 24, 426–459. [Google Scholar] [CrossRef]
- Rajagopal, R.; Bellavance, D.; Rahaman, M.d.S. Psychrophilic Anaerobic Digestion of Semi-Dry Mixed Municipal Food Waste: For North American Context. Process Saf. Environ. Prot. 2017, 105, 101–108. [Google Scholar] [CrossRef]
- Rajagopal, R.; Béline, F. Anaerobic Hydrolysis and Acidification of Organic Substrates: Determination of Anaerobic Hydrolytic Potential. Bioresour. Technol. 2011, 102, 5653–5658. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory Effect of Heavy Metals on Methane-Producing Anaerobic Granular Sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Selling, R.; Håkansson, T.; Björnsson, L. Two-Stage Anaerobic Digestion Enables Heavy Metal Removal. Water Sci. Technol. 2008, 57, 553–558. [Google Scholar] [CrossRef]
- Hidalgo, D.; Castro, J.; Díez, D.; Martín-Marroquín, J.M.; Gómez, M.; Pérez, E. Torrefaction at Low Temperature as a Promising Pretreatment of Lignocellulosic Biomass in Anaerobic Digestion. Energy 2023, 263, 125822. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.C.; Yang, B.; et al. Severity Factor Kinetic Model as a Strategic Parameter of Hydrothermal Processing (Steam Explosion and Liquid Hot Water) for Biomass Fractionation under Biorefinery Concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery Valorization of Autohydrolysis Wheat Straw Hemicellulose to Be Applied in a Polymer-Blend Film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Tompsett, G.A.; Guerra, P.; Timko, M.T.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Sugars and Char Formation on Subcritical Water Hydrolysis of Sugarcane Straw. Bioresour. Technol. 2017, 243, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Chornet, E.; Overend, R.P.; Heitz, M. Fractionnement de Deux Bois Tropicaux (Eucalyptus et Wapa) Par Traitement Thermomécanique En Phase Aqueuse. Partie I: Conversion et Profils de Solubilisation. Can. J. Chem. Eng. 1986, 64, 986–993. [Google Scholar] [CrossRef]
- Espirito Santo, M.C.; Fockink, D.H.; Pellegrini, V.O.A.; Guimaraes, F.E.G.; de Azevedo, E.R.; Ramos, L.P.; Polikarpov, I. Physical Techniques Shed Light on the Differences in Sugarcane Bagasse Structure Subjected to Steam Explosion Pretreatments at Equivalent Combined Severity Factors. Ind. Crops Prod. 2020, 158, 113003. [Google Scholar] [CrossRef]
- Bertuola, D.; Volpato, S.; Canu, P.; Santomaso, A.C. Prediction of Segregation in Funnel and Mass Flow Discharge. Chem. Eng. Sci. 2016, 150, 16–25. [Google Scholar] [CrossRef]
- Monschein, M.; Nidetzky, B. Effect of Pretreatment Severity in Continuous Steam Explosion on Enzymatic Conversion of Wheat Straw: Evidence from Kinetic Analysis of Hydrolysis Time Courses. Bioresour. Technol. 2016, 200, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Cara, C.; Manzanares, P.; Ballesteros, M.; Castro, E. Evaluation of Steam Explosion Pre-Treatment for Enzymatic Hydrolysis of Sunflower Stalks. Enzym. Microb. Technol. 2008, 42, 160–166. [Google Scholar] [CrossRef]
- Aguirre-Fierro, A.; Ruiz, H.A.; Cerqueira, M.A.; Ramos-González, R.; Rodríguez-Jasso, R.M.; Marques, S.; Lukasik, R.M. Sustainable Approach of High-Pressure Agave Bagasse Pretreatment for Ethanol Production. Renew. Energy 2020, 155, 1347–1354. [Google Scholar] [CrossRef]
- Chum, H.L.; Johnson, D.K.; Black, S.K. Organosolv Pretreatment for Enzymatic Hydrolysis of Poplars. 2. Catalyst Effects and the Combined Severity Parameter. Ind. Eng. Chem. Res. 1990, 29, 156–162. [Google Scholar] [CrossRef]
- Agudelo, R.A.; García-Aparicio, M.P.; Görgens, J.F. Steam Explosion Pretreatment of Triticale (× Triticosecale Wittmack) Straw for Sugar Production. New Biotechnol. 2016, 33, 153–163. [Google Scholar] [CrossRef]
- Brownell, H.H.; Yu, E.K.C.; Saddler, J.N. Steam-Explosion Pretreatment of Wood: Effect of Chip Size, Acid, Moisture Content and Pressure Drop. Biotechnol. Bioeng. 1986, 28, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Pielhop Thomas, J.A.R.R.H.S. Steam Explosion Pretreatment of Softwood: The Effect of the Explosive Decomposition on Enzymatic Digestibility. Biotechnol. Biofuels 2016, 9, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Foston, M.; Leisen, J.; DeMartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of Porosity of Lignocellulosic Biomass before and after Pretreatment by Using Simons’ Stain and NMR Techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef]
- Muzamal, M.; Gamstedt, E.K.; Rasmuson, A. Mechanistic Study of Microstructural Deformation and Stress in Steam-Exploded Softwood. Wood Sci. Technol. 2017, 51, 447–462. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Molaverdi, M. Structural Features Influential to Enzymatic Hydrolysis of Cellulose-Solvent-Based Pretreated Pinewood and Elmwood for Ethanol Production. Bioprocess Biosyst. Eng. 2018, 41, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biomethane Potential of Wheat Straw: Influence of Particle Size, Water Impregnation and Thermal Hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, B.H.; Wang, Y.; Yong, X.Y.; Yang, Z.H.; Jia, H.H.; Jiang, M.; Wei, P. Effect of Steam Explosion Pretreatment on the Anaerobic Digestion of Rice Straw. RSC Adv. 2016, 6, 88417–88425. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam Explosion Pretreatment of Wheat Straw to Improve Methane Yields: Investigation of the Degradation Kinetics of Structural Compounds during Anaerobic Digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining Sugars and Natural Antioxidants from Olive Leaves by Steam-Explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of Oligosaccharides and Biofuels from Miscanthus Using Combinatorial Steam Explosion and Ionic Liquid Pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-Step Pretreatment of Oil Palm Trunk for Ethanol Production by Thermotolerent Saccharomyces Cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Vaidya, A.A.; Donaldson, L.A.; Singh, A.P. Improvement in the Enzymatic Hydrolysis of Biofuel Substrate by a Combined Thermochemical and Fungal Pretreatment. Wood Sci. Technol. 2016, 50, 1003–1014. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Han, G.; Zhang, Y.; Jiang, W. Comparison of Different Methods to Produce Pineapple Leaf Fibers with Steam Explosion. J. Nat. Fibers 2019, 18, 12–20. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy Metals Interact with the Microbial Community and Affect Biogas Production in Anaerobic Digestion: A Review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Ni, B.J.; Han, X.; Fan, L.; Yuan, Z. Dissecting Microbial Community Structure and Methane-Producing Pathways of a Full-Scale Anaerobic Reactor Digesting Activated Sludge from Wastewater Treatment by Metagenomic Sequencing. Microb. Cell Factories 2015, 14, 1–11. [Google Scholar] [CrossRef]

| Experiment | Conditions |
|---|---|
| 1 | Lower conditions of T° and time |
| 2 | Medium conditions of T° and time |
| 3 | Higher conditions of T° and time |
| 4 | Medium conditions, lower concentration of an acid catalyst |
| 5 | Medium conditions, higher concentration of an acid catalyst |
| Sample | Ca | Fe | K | Mg | Na | Al | Cd | Co | B |
|---|---|---|---|---|---|---|---|---|---|
| SE without a catalyst | 726.7 | 196.9 | 132.8 | 115.7 | 120.3 | 71.1 | 1.7 | 26.4 | 14.6 |
| SE with a lower concentration of the catalyst | 446.6 | 194 | 125.4 | 84.9 | 177.1 | 131.5 | 1.6 | 26.5 | 16.4 |
| SE with a higher concentration of the catalyst | 462.4 | 318.9 | 122.9 | 62.3 | 268.9 | 159.1 | 1.9 | 26.3 | 31.4 |
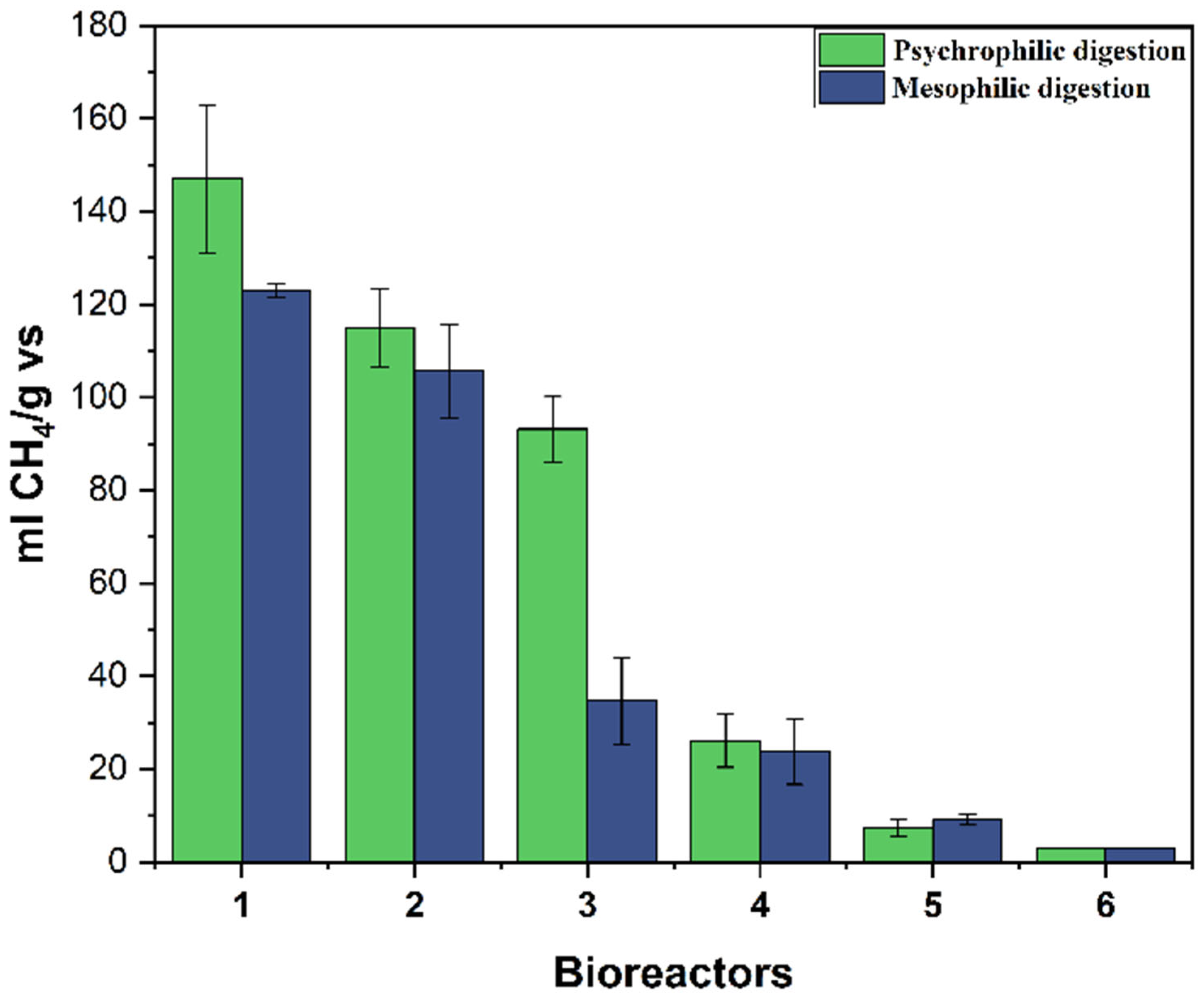
| Bioreactor | Conditions | Conversion (%) |
|---|---|---|
| Bioreactor 1 | SE with a lower concentration of the acid catalyst, S/I = 1/2 | 72.64 ± 0.59 |
| Bioreactor 2 | SE with higher SE conditions, S/I = 1/2 | 36.71 ± 0.82 |
| Bioreactor 3 | SE with a higher concentration of the acid catalyst, S/I = 1/2 | 1.22 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaib, O.; Abatzoglou, N.; Achouri, I.E. Lignocellulosic Biomass Valorisation by Coupling Steam Explosion Treatment and Anaerobic Digestion. Energies 2024, 17, 677. https://doi.org/10.3390/en17030677
Chaib O, Abatzoglou N, Achouri IE. Lignocellulosic Biomass Valorisation by Coupling Steam Explosion Treatment and Anaerobic Digestion. Energies. 2024; 17(3):677. https://doi.org/10.3390/en17030677
Chicago/Turabian StyleChaib, Oumaima, Nicolas Abatzoglou, and Inès Esma Achouri. 2024. "Lignocellulosic Biomass Valorisation by Coupling Steam Explosion Treatment and Anaerobic Digestion" Energies 17, no. 3: 677. https://doi.org/10.3390/en17030677
APA StyleChaib, O., Abatzoglou, N., & Achouri, I. E. (2024). Lignocellulosic Biomass Valorisation by Coupling Steam Explosion Treatment and Anaerobic Digestion. Energies, 17(3), 677. https://doi.org/10.3390/en17030677









