Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar
Abstract
1. Introduction
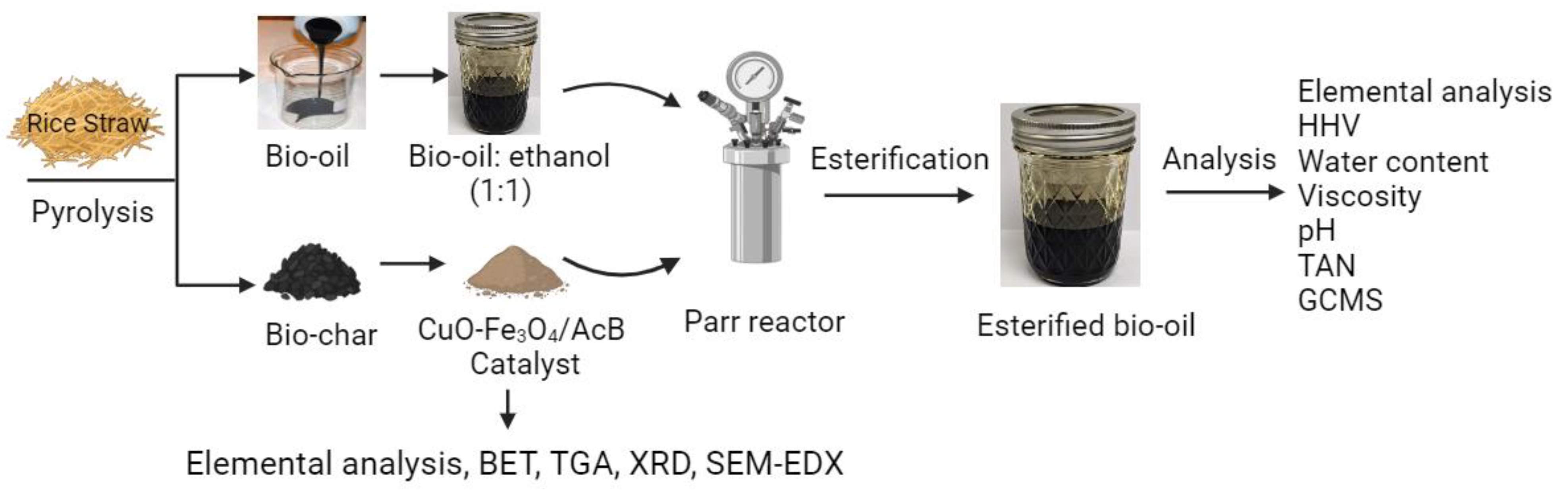
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis of Rice Straw
2.3. Preparation of Biochar-Based Catalysts
2.4. Preparation of CuO-Fe3O4/AcB
2.5. Catalyst Characterization
2.6. Bio-Oil Esterification Process
2.7. Physical and Chemical Characterization of Raw and Esterified Bio-Oil
2.8. Catalyst’s Stability
3. Results
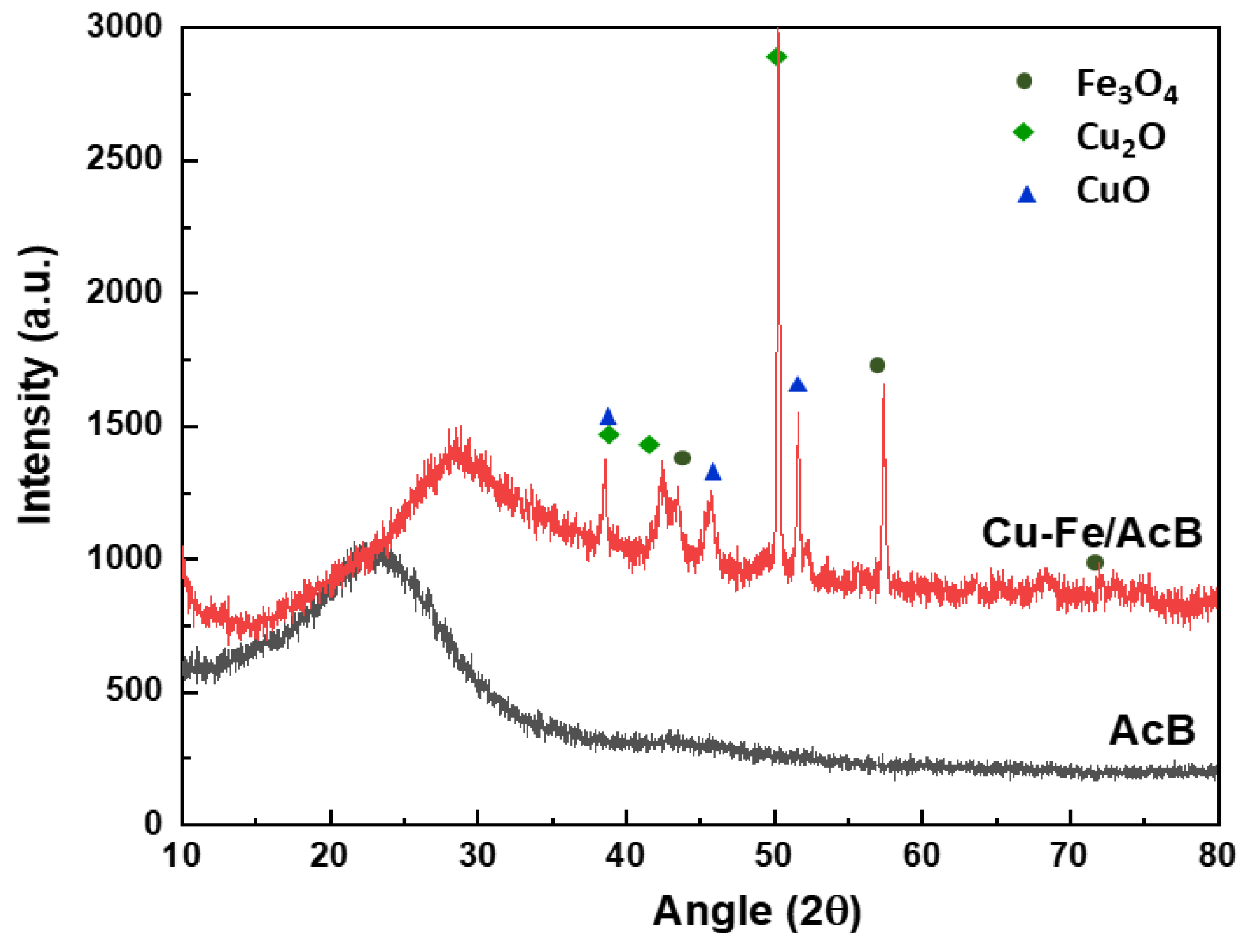
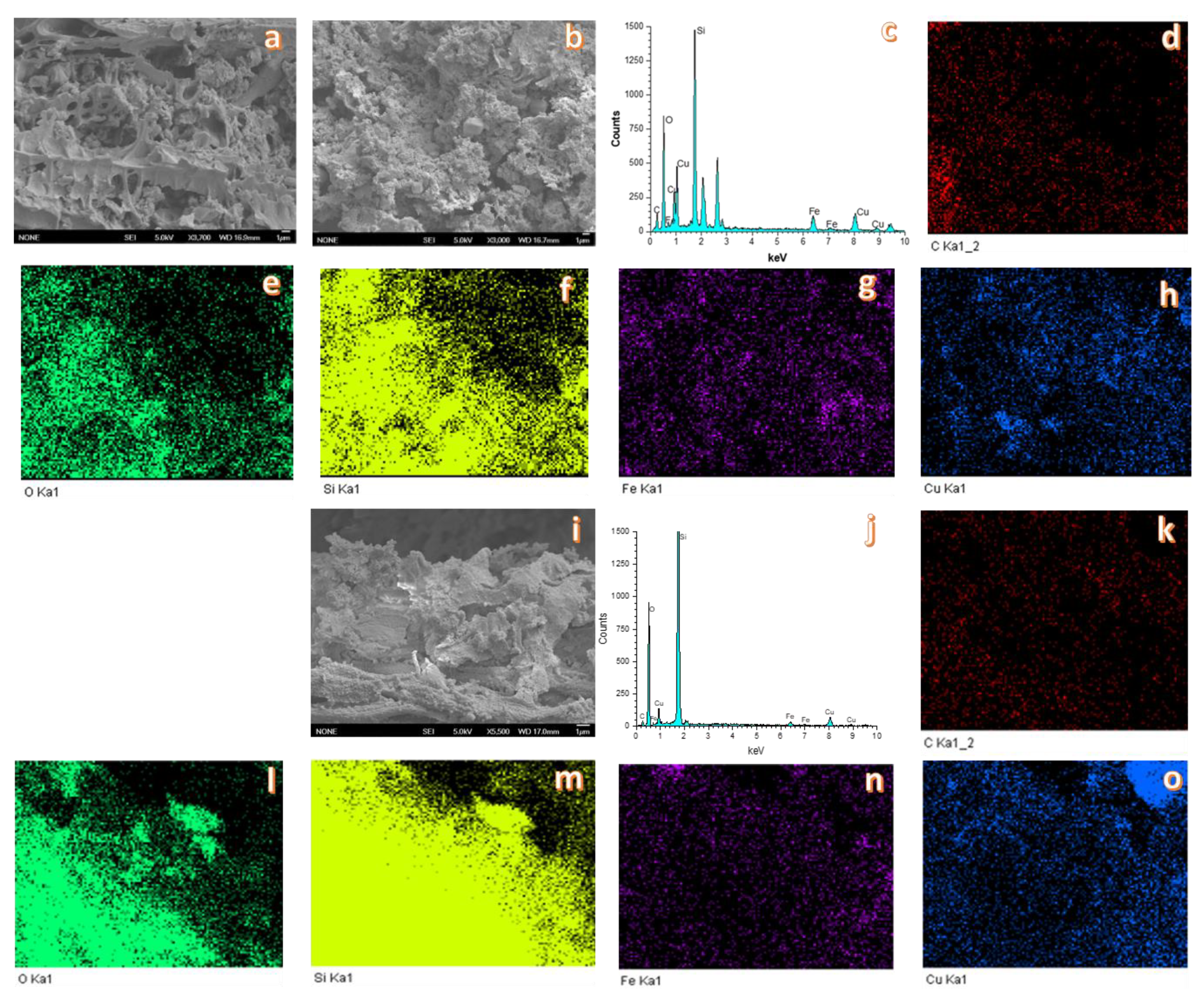
3.1. Catalyst Characterization
3.2. Product Yields, Elemental Analysis, and HHV
3.3. Physical Chractarization of Raw and Esterified Upgraded Bio-Oils
3.4. Chemical Chractarization of Raw and Esterified Upgraded Bio-Oils
3.5. Reaction Mechanism
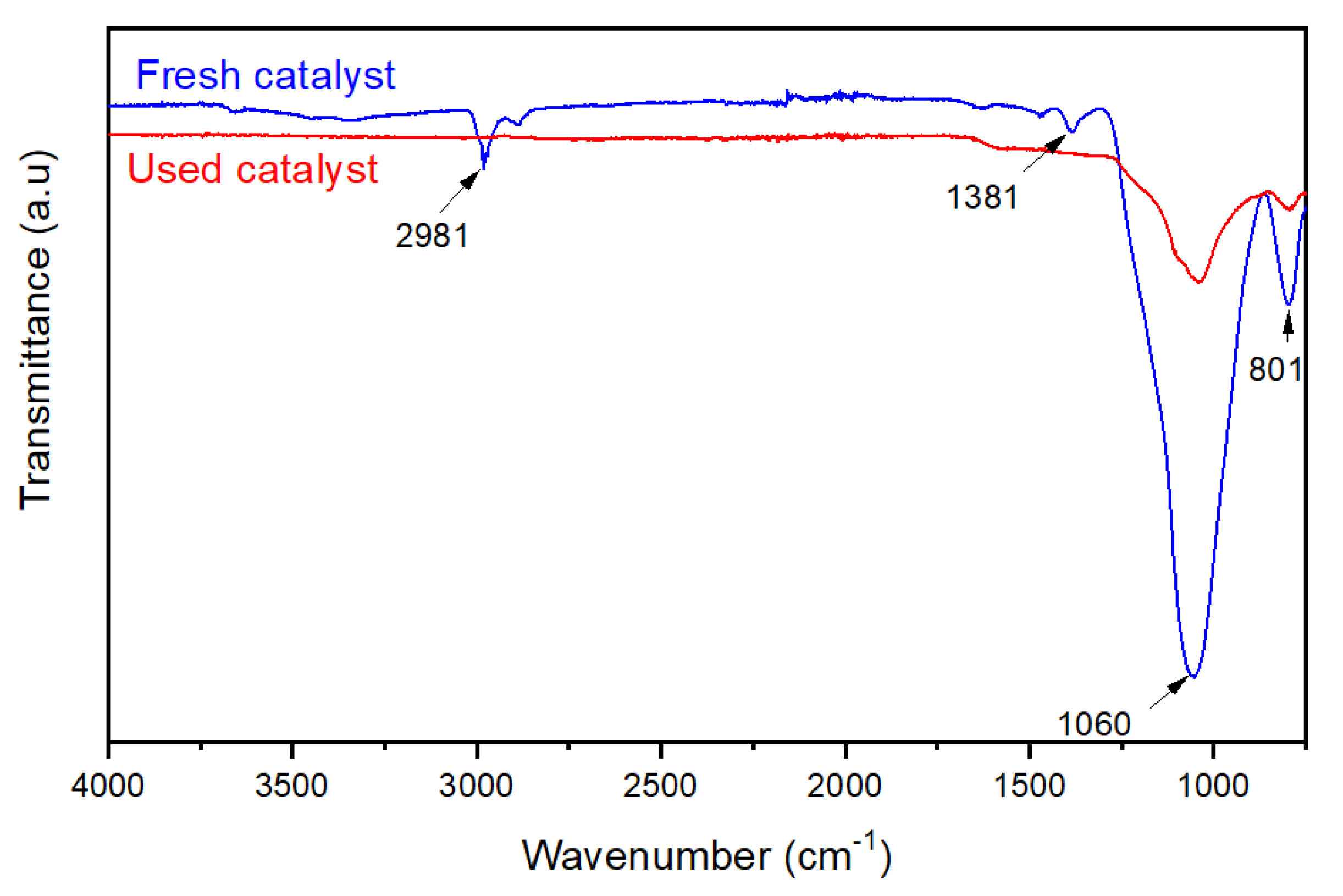
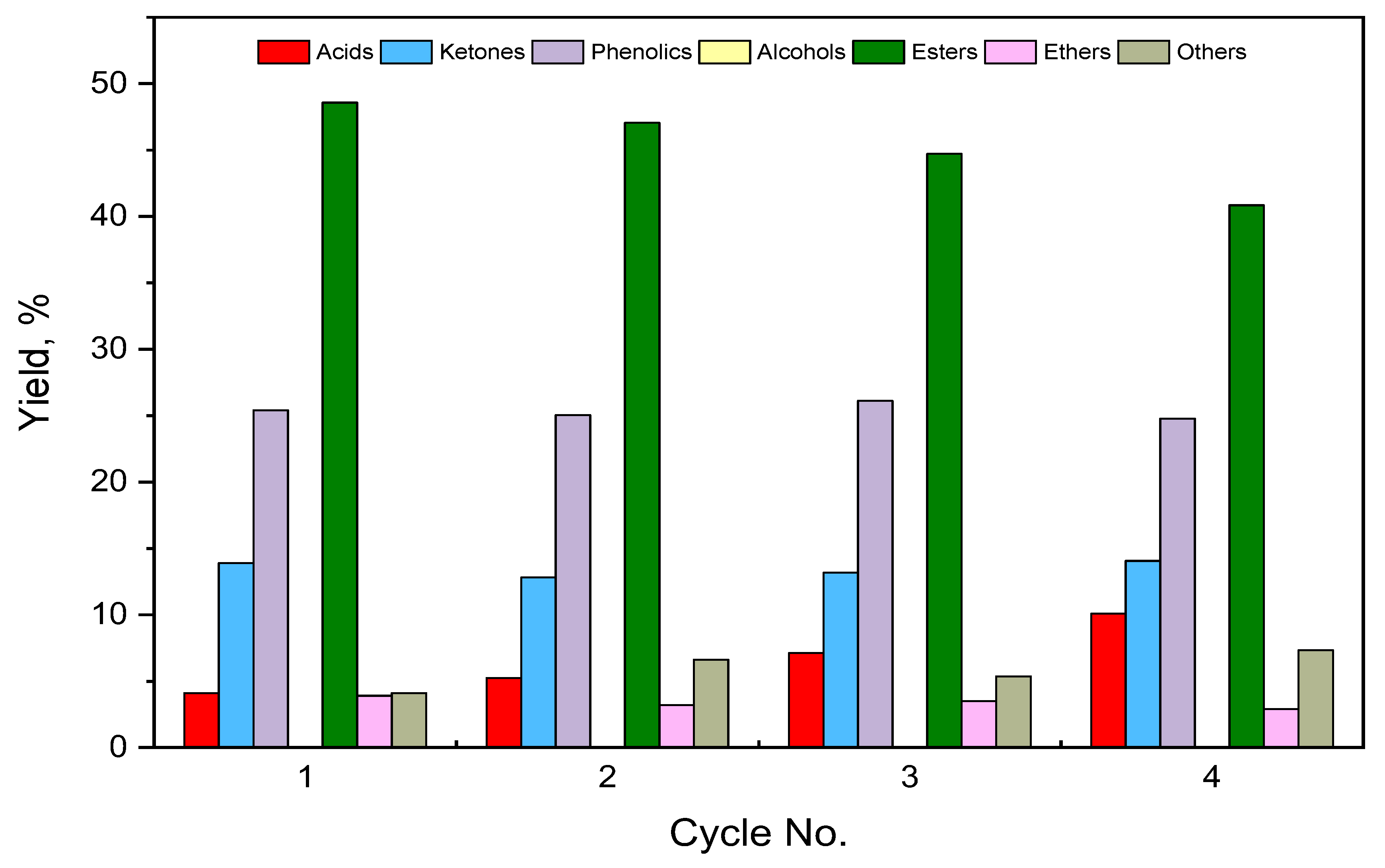
3.6. Catalyst’s Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Hu, S.; Zhang, Z.; Zhu, B.; Bai, D. The path to carbon neutrality in China: A paradigm shift in fossil resource utilization. Resour. Chem. Mater. 2022, 1, 129–135. [Google Scholar] [CrossRef]
- Shamoon, A.; Haleem, A.; Bahl, S.; Javaid, M.; Bala Garg, S.; Chandmal Sharma, R.; Garg, J. Environmental impact of energy production and extraction of materials—A review. Mater. Today Proc. 2022, 57, 936–941. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Shafie, S.M.; Masjuki, H.H.; Mahlia, T.M.I. Life cycle assessment of rice straw-based power generation in Malaysia. Energy 2014, 70, 401–410. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Shadangi, K.P.; Srivastava, R.K. Chapter 5—Agricultural waste to fuels and chemicals. Bioenergy Eng. Fundam. Methods Model. Appl. 2023, 87–98. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Singhal, A.; Tolvanen, H.; Valtonen, K.; Joronen, T.; Konttinen, J. Effect of pretreatment and biomass blending on bio-oil and biochar quality from two-step slow pyrolysis of rice straw. Waste Manag. 2022, 138, 298–307. [Google Scholar] [CrossRef]
- Oasmaa, A.; Lehto, J.; Solantausta, Y.; Kallio, S. Historical Review on VTT Fast Pyrolysis Bio-oil Production and Upgrading. Energy Fuels 2021, 35, 5683–5695. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Fennell, P.; Jensen, A.D. A Review of Recent Research on Catalytic Biomass Pyrolysis and Low-Pressure Hydropyrolysis. Energy Fuels 2021, 35, 18333–18369. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kim, J.-Y.; Kim, K.-H.; Lee, S.; Choi, D.; Choi, I.-G.; Choi, J.W. The effect of storage duration on bio-oil properties. J. Anal. Appl. Pyrolysis 2012, 95, 118–125. [Google Scholar] [CrossRef]
- Maneechakr, P.; Karnjanakom, S. Improving the Bio-Oil Quality via Effective Pyrolysis/Deoxygenation of Palm Kernel Cake over a Metal (Cu, Ni, or Fe)-Doped Carbon Catalyst. ACS Omega 2021, 6, 20006–20014. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.B.M.; Steele, P.H.; Ingram, L. Characterization of fast pyrolysis bio-oils produced from pretreated pine wood. Appl. Biochem. Biotechnol. 2009, 154, 182–192. [Google Scholar] [CrossRef]
- Kazmi, W.W.; Park, J.-Y.; Amini, G.; Lee, I.-G. Upgrading of esterified bio-oil from waste coffee grounds over MgNiMo/activated charcoal in supercritical ethanol. Fuel Process. Technol. 2023, 250, 107915. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, I.-G.; Park, J.-Y.; Lee, K.-Y. Efficient upgrading of pyrolysis bio-oil over Ni-based catalysts in supercritical ethanol. Fuel 2019, 241, 207–217. [Google Scholar] [CrossRef]
- Shafaghat, H.; Kim, J.M.; Lee, I.-G.; Jae, J.; Jung, S.-C.; Park, Y.-K. Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts. Renew. Energy 2019, 144, 159–166. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Konstas, K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Catalytic upgradation of bio-oil over metal supported activated carbon catalysts in sub-supercritical ethanol. J. Environ. Chem. Eng. 2021, 9, 105059. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A.; Paone, E.; Catizzone, E. Recent catalytic advances in hydrotreatment processes of pyrolysis bio-oil. Catalysts 2021, 11, 157. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeon, W.; Lee, J.-H.; Nam, B.; Lee, I.-G. Effects of supercritical fluids in catalytic upgrading of biomass pyrolysis oil. Chem. Eng. J. 2019, 377, 120312. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, H.; Zhang, K. Supercritical fluids technology for clean biofuel production. Prog. Nat. Sci. 2009, 19, 273–284. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Wang, S.; Hasan, M.D.M.; Jiang, S.; Zhang, L.; Li, C.-Z. Reaction behaviour of light and heavy components of bio-oil in methanol and in water. Fuel 2018, 232, 645–652. [Google Scholar] [CrossRef]
- Hu, X.; Gunawan, R.; Mourant, D.; Hasan, M.D.M.; Wu, L.; Song, Y.; Lievens, C.; Li, C.-Z. Upgrading of bio-oil via acid-catalyzed reactions in alcohols—A mini review. Fuel Process. Technol. 2017, 155, 2–19. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Gutierrez, A.; Honkela, M.L.; Krause, A.O.I.; Heeres, H.J. Hydrotreatment of wood-based pyrolysis oil using zirconia-supported mono-and bimetallic (Pt, Pd, Rh) catalysts. Appl. Catal. A Gen. 2011, 407, 56–66. [Google Scholar] [CrossRef]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid mesoporous silica/noble-metal nanoparticle materials—Synthesis and catalytic applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- Setiabudi, H.; Aziz, M.; Abdullah, S.; Teh, L.; Jusoh, R. Hydrogen production from catalytic steam reforming of biomass pyrolysis oil or bio-oil derivatives: A review. Int. J. Hydrogen Energy 2020, 45, 18376–18397. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Cao, J.-P.; Zhao, X.-Y.; Yang, Z.; Liu, T.-L.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Catalytic upgrading of pyrolysis vapors from lignite over mono/bimetal-loaded mesoporous HZSM-5. Fuel 2018, 218, 33–40. [Google Scholar] [CrossRef]
- Nava, R.; Pawelec, B.; Castaño, P.; Álvarez-Galván, M.C.; Loricera, C.V.; Fierro, J.L.G. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B Environ. 2009, 92, 154–167. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Zacher, A.H.; Wang, L.; Ren, S.; Liang, J.; Wei, Y.; Liu, Y.; Tang, J.; Zhang, Q.; et al. A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis. Bioresour. Technol. 2012, 124, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kaewmeesri, R.; Nonkumwong, J.; Kiatkittipong, W.; Laosiripojana, N.; Faungnawakij, K. Deoxygenations of palm oil-derived methyl esters over mono- and bimetallic NiCo catalysts. J. Environ. Chem. Eng. 2021, 9, 105128. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar] [CrossRef]
- Sihombing, J.L.; Herlinawati, H.; Pulungan, A.N.; Simatupang, L.; Rahayu, R.; Wibowo, A.A. Effective hydrodeoxygenation bio-oil via natural zeolite supported transition metal oxide catalyst. Arab. J. Chem. 2023, 16, 104707. [Google Scholar] [CrossRef]
- Ruan, C.; Akutsu, R.; Yang, K.; Zayan, N.M.; Dou, J.; Liu, J.; Bose, A.; Brody, L.; Lamb, H.H.; Li, F. Hydrogenation of bio-oil-derived oxygenates at ambient conditions via a two-step redox cycle. Cell Rep. Phys. Sci. 2023, 4, 101506. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Dang, Q.; Wang, J.; Chen, W. Upgrading of Bio-oil over Bifunctional Catalysts in Supercritical Monoalcohols. Energy Fuels 2012, 26, 2990–2995. [Google Scholar] [CrossRef]
- Chen, C.; Ling, H.; Wei, D.; Wei, Y.; Fan, D.; Zhao, J. Microwave catalytic co-pyrolysis of chlorella vulgaris and oily sludge by nickel-X (X = Cu, Fe) supported on activated carbon: Characteristic and bio-oil analysis. J. Energy Inst. 2023, 111, 101401. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Ge, Y.; Li, Z. Efficient depolymerization of alkali lignin to monophenols using one-step synthesized Cu–Ni bimetallic catalysts inlaid in homologous biochar. Biomass Bioenergy 2023, 175, 106873. [Google Scholar] [CrossRef]
- Zhang, M.; Han, X.; Wang, H.; Zeng, Y.; Xu, C.C. Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials. Sustainability 2023, 15, 7768. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, G.F. A rice mutant defective in Si uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, D.; Wei, L.; Shen, Q.; Ji, Z.; Chen, Y.; Zou, X.; Xu, C.; Zhou, J. Bio-oil production from hydrogenation liquefaction of rice straw over metal (Ni, Co, Cu)-modified CeO2 catalysts. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 200–206. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Q.; Wei, L.; Li, N.; Shen, Q.; Tian, W.; Zhou, J.; Long, J. Catalytic hydroliquefaction of rice straw for bio-oil production using Ni/CeO2 catalysts. J. Anal. Appl. Pyrolysis 2018, 130, 169–180. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.W.; Tsang, Y.F.; Kim, Y.T.; Lee, J. Engineered rice-straw biochar catalysts for the production of value-added chemicals from furan. Chem. Eng. J. 2020, 387, 124194. [Google Scholar] [CrossRef]
- Luo, Y.; Hassan, E.B.; Miao, P.; Xu, Q.; Steele, P.H. Effects of single-stage syngas hydrotreating on the physical and chemical properties of oxidized fractionated bio-oil. Fuel 2017, 209, 634–642. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y. Structural and adsorption characteristics of potassium carbonate activated biochar. RSC Adv. 2018, 8, 21012–21019. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Leahy, J.J.; Kwapinski, W. Catalytically upgrading bio-oil via esterification. Energy Fuels 2015, 29, 3691–3698. [Google Scholar] [CrossRef]
- Sapchenko, S.A.; Barsukova, M.O.; Belosludov, R.V.; Kovalenko, K.A.; Samsonenko, D.G.; Poryvaev, A.S.; Sheveleva, A.M.; Fedin, M.V.; Bogomyakov, A.S.; Dybtsev, D.N.; et al. Understanding Hysteresis in Carbon Dioxide Sorption in Porous Metal–Organic Frameworks. Inorg. Chem. 2019, 58, 6811–6820. [Google Scholar] [CrossRef]
- Serna, P.; Gates, B.C. Molecular Metal Catalysts on Supports: Organometallic Chemistry Meets Surface Science. Acc. Chem. Res. 2014, 47, 2612–2620. [Google Scholar] [CrossRef]
- Yuan, G.Q.; Jiang, H.F.; Lin, C.; Liao, S.J. Shape- and size-controlled electrochemical synthesis of cupric oxide nanocrystals. J. Cryst. Growth 2007, 303, 400–406. [Google Scholar] [CrossRef]
- Chandrappa, K.G.; Venkatesha, T.V. Generation of nanostructured CuO by electrochemical method and its Zn–Ni–CuO composite thin films for corrosion protection. Mater. Corros. 2013, 64, 831–839. [Google Scholar] [CrossRef]
- Elsayed, I.; Jackson, M.A.; Hassan, E.B. Hydrogen-Free Catalytic Reduction of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan Using Copper–Iron Oxides Bimetallic Nanocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 1774–1785. [Google Scholar] [CrossRef]
- Ferrara, F.; Orsini, A.; Plaisant, A.; Pettinau, A. Pyrolysis of coal, biomass and their blends: Performance assessment by thermogravimetric analysis. Bioresour. Technol. 2014, 171, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Héroguel, F.; Rozmysłowicz, B.; Luterbacher, J.S. Improving heterogeneous catalyst stability for liquid-phase biomass conversion and reforming. Chimia 2015, 69, 582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsayed, I.; Mashaly, M.; Eltaweel, F.; Jackson, M.A. Dehydration of glucose to 5-hydroxymethylfurfural by a core-shell Fe3O4@SiO2-SO3H magnetic nanoparticle catalyst. Fuel 2018, 221, 407–416. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.C.; Tanabe, E.H.; Bertuol, D.A. Review on Biomass Pyrolysis with a Focus on Bio-Oil Upgrading Techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Li, Y.; Ma, P.; Zhao, C. Stability evaluation of fast pyrolysis oil from rice straw. Chem. Eng. Sci. 2015, 135, 258–265. [Google Scholar] [CrossRef]
- Omar, S.; Alsamaq, S.; Yang, Y.; Wang, J. Production of renewable fuels by blending bio-oil with alcohols and upgrading under supercritical conditions. Front. Chem. Sci. Eng. 2019, 13, 702–717. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Q.; Wang, X.; Zhang, L.; Wang, Y.; Luo, Z. Biogasoline Production from the Co-cracking of the Distilled Fraction of Bio-oil and Ethanol. Energy Fuels 2014, 28, 115–122. [Google Scholar] [CrossRef]
- Elsayed, I.; Jackson, M.A.; Hassan, E.B. Catalytic hydrogenation and etherification of 5-Hydroxymethylfurfural into 2-(alkoxymethyl)-5-methylfuran and 2,5-bis(alkoxymethyl)furan as potential biofuel additives. Fuel Process. Technol. 2021, 213, 106672. [Google Scholar] [CrossRef]


| EXP. | Catalyst | AcB (wt.%) | Cu and Fe (wt.%) | Cu (wt.%) | Fe (wt.%) | Cu:Fe Ratio |
|---|---|---|---|---|---|---|
| 1 | AcB support | 100 | 0 | 0 | 0 | - |
| 2 | 1 | 80 | 20 | 10 | 10 | 1:1 |
| 3 | 2 | 80 | 20 | 13.33 | 6.66 | 2:1 |
| 4 | 3 | 80 | 20 | 15 | 5 | 3:1 |
| 5 | 4 | 90 | 10 | 7.5 | 2.5 | 3:1 |
| 6 | 5 | 70 | 30 | 22.5 | 7.5 | 3:1 |
| EXP. No. | Catalyst | BET Surface Area (m2/g) | Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| 1 | AcB support | 203.75 | 0.389 | 3.71 |
| 2 | 1 | 58.32 | 0.253 | 14.75 |
| 3 | 2 | 56.41 | 0.234 | 15.39 |
| 4 | 3 | 53.19 | 0.213 | 16.03 |
| 5 | 4 | 74.81 | 0.299 | 10.64 |
| 6 | 5 | 23.89 | 0.176 | 36.78 |
| EXP. No. | Y (Liquid) | Y (Solid) | Y (Gas) | Elemental Analysis (wt.%) | HHV (MJ/kg) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | |||||
| Raw bio-oil | - | - | - | 54.2 | 7.7 | 1.8 | 36.3 | 21.3 |
| 1 | 56.3 | 4.3 | 39.4 | 60.7 | 7.2 | 2.1 | 28.0 | 24.3 |
| 2 | 37.4 | 17.6 | 45.0 | 62.3 | 7.6 | 2.2 | 27.9 | 27.8 |
| 3 | 38.4 | 17.3 | 44.3 | 62.9 | 6.6 | 3.1 | 27.4 | 28.9 |
| 4 | 36.0 | 19.1 | 44.9 | 65.4 | 9.1 | 2.8 | 22.7 | 30.9 |
| 5 | 37.4 | 19.3 | 43.3 | 66.9 | 9.1 | 2.2 | 21.8 | 32.1 |
| 6 | 36.9 | 19.6 | 43.5 | 67.0 | 8.8 | 3.3 | 20.9 | 31.9 |
| EXP. No. | Water Content | Viscosity at 40 °C Cst | Density at 40 °C (g/cm3) | pH | TAN (mg KOH/g) |
|---|---|---|---|---|---|
| Raw bio-oil | 38.5 | 1.88 | 0.92 | 4.3 | 83.9 |
| 1 | 35.2 | 1.78 | 0.87 | 4.6 | 75.7 |
| 2 | 34.4 | 1.68 | 0.84 | 4.8 | 74.5 |
| 3 | 34.0 | 1.75 | 0.84 | 4.8 | 72.6 |
| 4 | 33.9 | 1.79 | 0.85 | 4.9 | 70.8 |
| 5 | 32.1 | 1.63 | 0.78 | 5.6 | 60.3 |
| 6 | 32.7 | 1.77 | 0.86 | 5.1 | 71.1 |
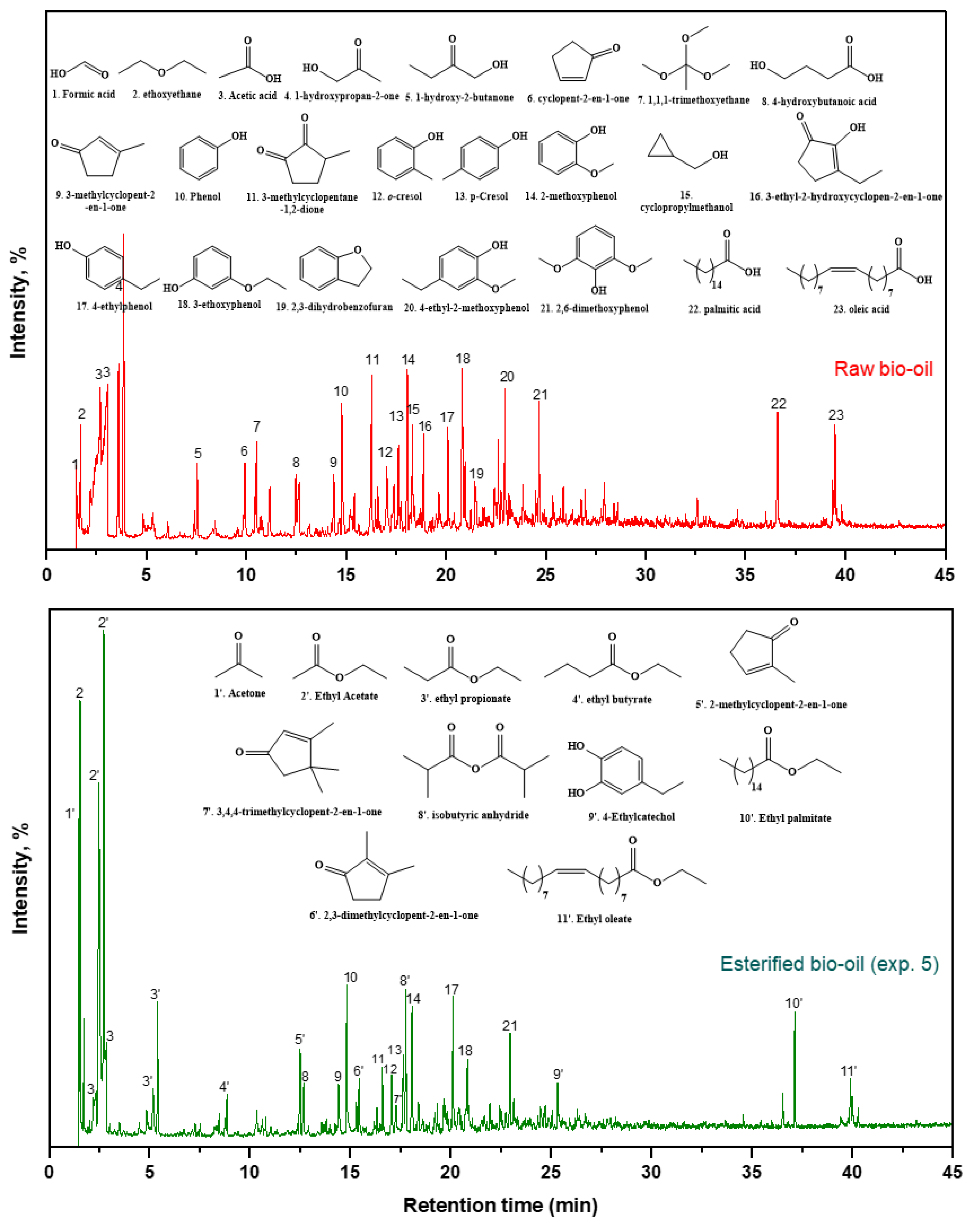
| RT (min) | Compound Name | (Area %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw Bio-Oil | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | ||
| Acids | ||||||||
| 1.57 | Formic acid | 2.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2.68 | Acetic acid | 32.32 | 11.02 | 6.62 | 10.29 | 6.27 | 2.08 | 6.27 |
| 12.66 | 4-hydroxybutanoic acid | 2.06 | 0.00 | 3.03 | 0.00 | 2.44 | 2.03 | 2.42 |
| 36.61 | Palmitic acid | 3.59 | 1.86 | 0.00 | 1.56 | 0.00 | 0.00 | 0.00 |
| 39.48 | Oleic acid | 2.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
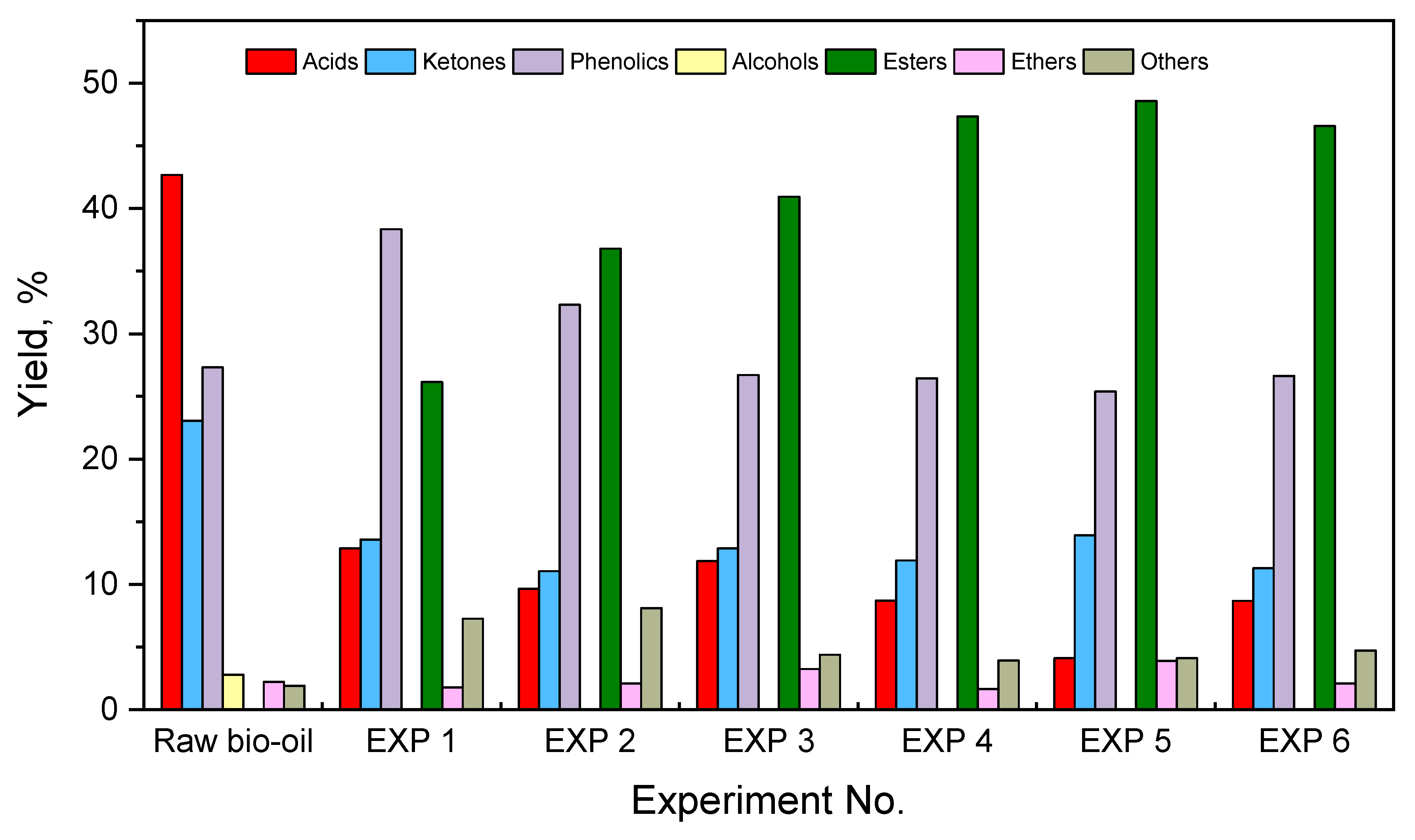
| Total | 42.68 | 12.88 | 9.65 | 11.85 | 8.71 | 4.11 | 8.69 | |
| Ketones | ||||||||
| 1.49 | Acetone | 0.00 | 1.90 | 0.00 | 1.87 | 1.98 | 4.12 | 2.01 |
| 3.87 | 1-hydroxypropan-2-one | 11.65 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 7.50 | 1-hydroxy-2-butanone | 1.82 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9.93 | Cyclopent-2-en-1-one | 2.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 12.50 | 2-methylcyclopent-2-en-1-one | 0.00 | 3.68 | 3.18 | 3.01 | 2.84 | 2.80 | 3.19 |
| 14.41 | 3-methylcyclopent-2-en-1-one | 1.99 | 3.45 | 2.31 | 2.48 | 2.21 | 2.15 | 2.26 |
| 16.30 | 3-methylcyclopentane-1,2-dione | 5.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 16.60 | 2,3-dimethylcyclopent-2-en-1-one | 0.00 | 2.06 | 3.46 | 3.70 | 3.52 | 3.35 | 3.84 |
| 17.29 | 3,4,4-trimethylcyclopent-2-en-1-one | 0.00 | 2.49 | 2.11 | 1.81 | 1.36 | 1.49 | 0.00 |
| Total | 23.06 | 13.58 | 11.06 | 12.87 | 11.91 | 13.91 | 11.3 | |
| Phenolics | ||||||||
| 14.79 | Phenol | 3.30 | 5.97 | 4.19 | 4.52 | 4.64 | 4.73 | 4.54 |
| 17.03 | o-cresol | 2.76 | 3.52 | 2.71 | 2.79 | 2.90 | 2.48 | 5.57 |
| 17.63 | p-Cresol | 2.23 | 2.81 | 2.27 | 2.41 | 3.02 | 2.53 | 0.00 |
| 18.04 | 2-methoxyphenol | 4.07 | 8.17 | 5.86 | 5.08 | 5.24 | 4.75 | 5.17 |
| 20.11 | 4-ethylphenol | 2.49 | 5.43 | 4.27 | 3.89 | 4.49 | 3.84 | 4.04 |
| 20.82 | 3-ethoxyphenol | 5.02 | 5.44 | 5.03 | 3.68 | 3.17 | 2.90 | 3.13 |
| 22.96 | 4-ethyl-2-methoxyphenol | 2.50 | 4.48 | 3.52 | 2.62 | 2.99 | 2.13 | 2.68 |
| 24.64 | 2,6-dimethoxyphenol | 2.70 | 0.00 | 2.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| 25.32 | 4-Ethylcatechol | 2.25 | 2.53 | 2.41 | 1.71 | 0.00 | 2.04 | 1.50 |
| Total | 27.32 | 38.35 | 32.3 | 26.7 | 26.45 | 25.4 | 26.63 | |
| Alcohols | ||||||||
| 18.33 | Cyclopropylmethanol | 2.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 2.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Esters | ||||||||
| 2.71 | Ethyl acetate | 0.00 | 16.67 | 24.83 | 27.69 | 33.89 | 36.55 | 32.55 |
| 5.41 | Ethyl propionate | 0.00 | 2.76 | 5.98 | 7.04 | 6.99 | 7.59 | 7.46 |
| 8.86 | Ethyl butyrate | 0.00 | 0.00 | 1.02 | 1.20 | 1.49 | 1.52 | 1.40 |
| 37.13 | Ethyl palmitate | 0.00 | 4.24 | 3.34 | 3.19 | 3.22 | 3.04 | 3.15 |
| 39.92 | Ethyl oleate | 0.00 | 2.48 | 1.62 | 1.82 | 1.75 | 1.39 | 2.01 |
| Total | 0.00 | 26.15 | 36.79 | 40.94 | 47.34 | 48.57 | 46.57 | |
| Ethers | ||||||||
| 1.54 | Ethoxyethane | 2.22 | 1.77 | 2.10 | 3.25 | 1.67 | 3.91 | 2.09 |
| Total | 2.22 | 1.77 | 2.10 | 3.25 | 1.67 | 3.91 | 2.09 | |
| Others | ||||||||
| 21.44 | 2,3-dihydrobenzofuran | 1.91 | 2.19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 17.76 | Isobutyric anhydride | 0.00 | 5.08 | 8.10 | 4.39 | 3.92 | 4.10 | 4.72 |
| Total | 1.91 | 7.27 | 8.1 | 4.39 | 3.92 | 4.1 | 4.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.; Elsayed, I.; Hassan, E.B. Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar. Energies 2024, 17, 407. https://doi.org/10.3390/en17020407
Ibrahim A, Elsayed I, Hassan EB. Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar. Energies. 2024; 17(2):407. https://doi.org/10.3390/en17020407
Chicago/Turabian StyleIbrahim, Alhassan, Islam Elsayed, and El Barbary Hassan. 2024. "Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar" Energies 17, no. 2: 407. https://doi.org/10.3390/en17020407
APA StyleIbrahim, A., Elsayed, I., & Hassan, E. B. (2024). Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar. Energies, 17(2), 407. https://doi.org/10.3390/en17020407







