Designing Sustainable Ethanol Oxidation Catalysts: The Role of Graphene Oxide in NiCuGO Composite Material
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
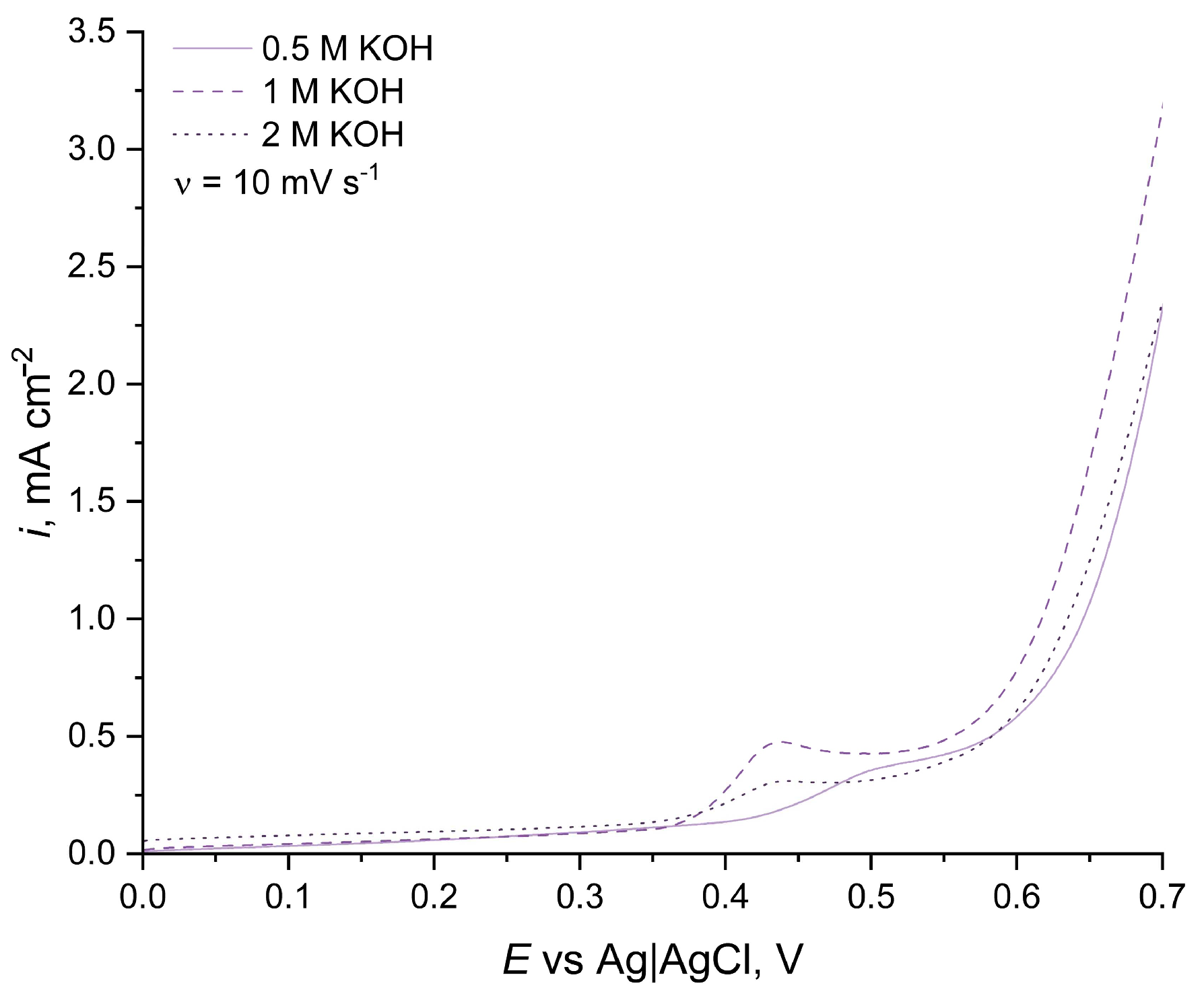
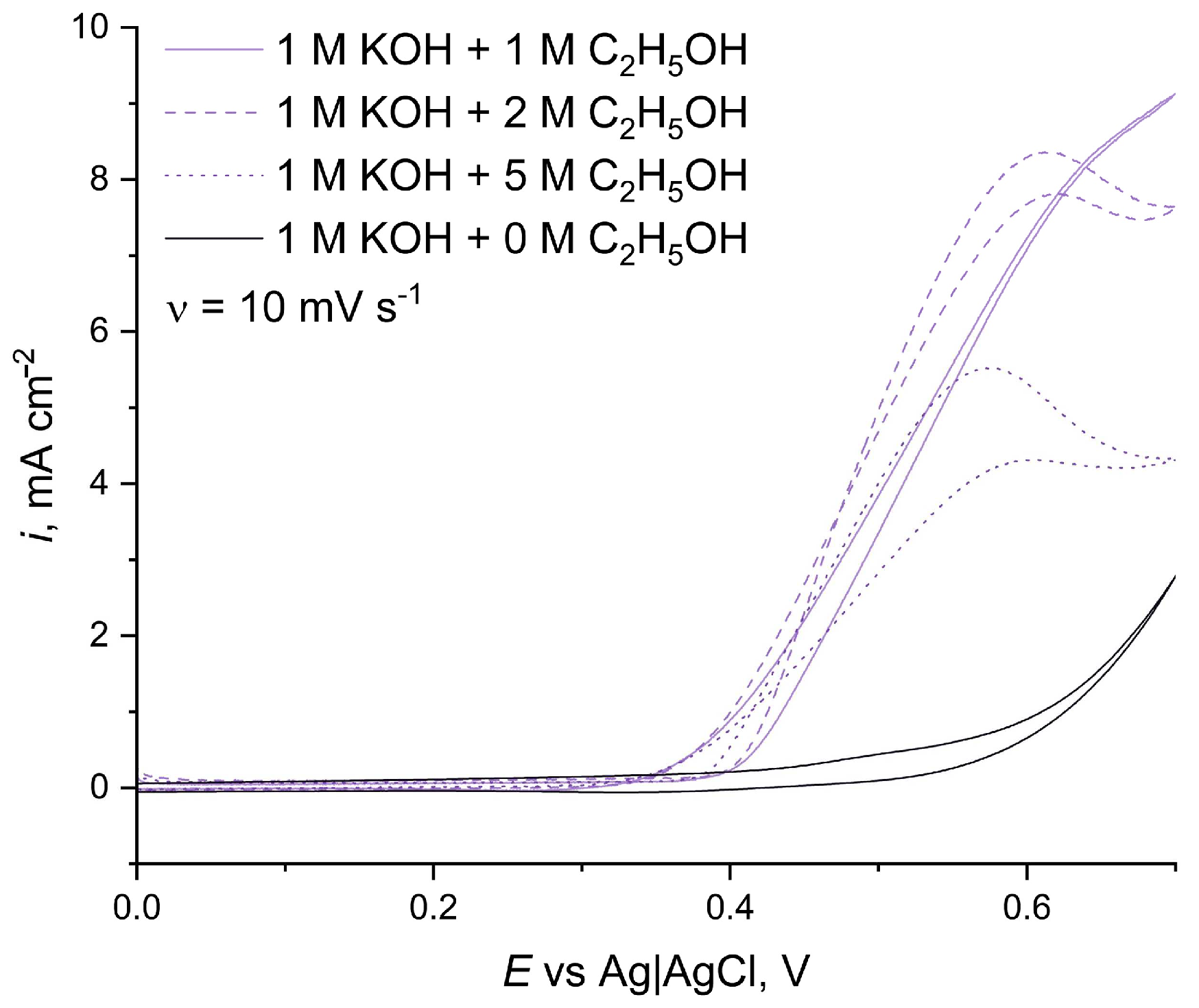
3.1. Influence of Ethanol Concentrations
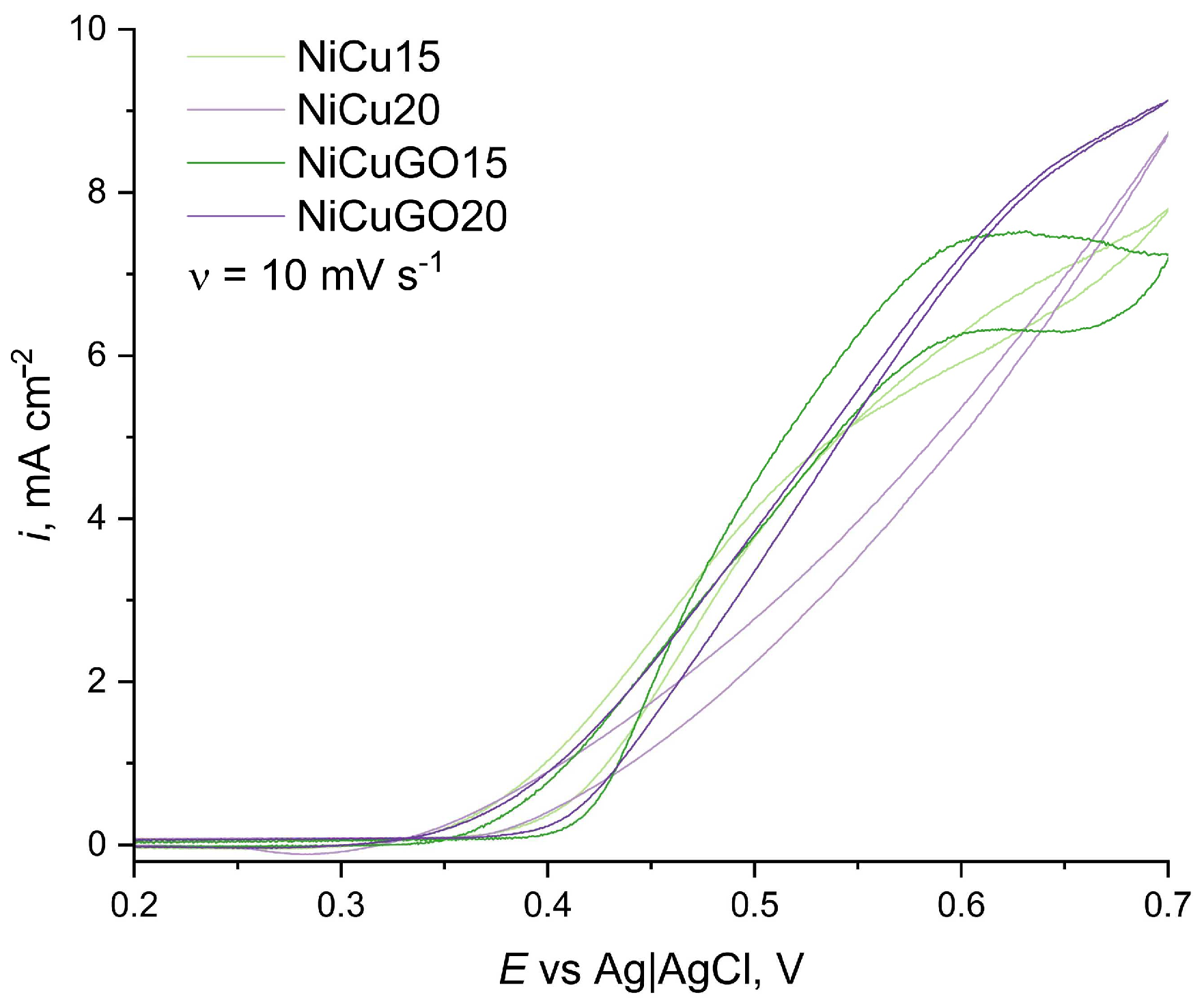
3.2. Activity towards Ethanol Oxidation
3.3. Activity towards Ethanol Oxidation
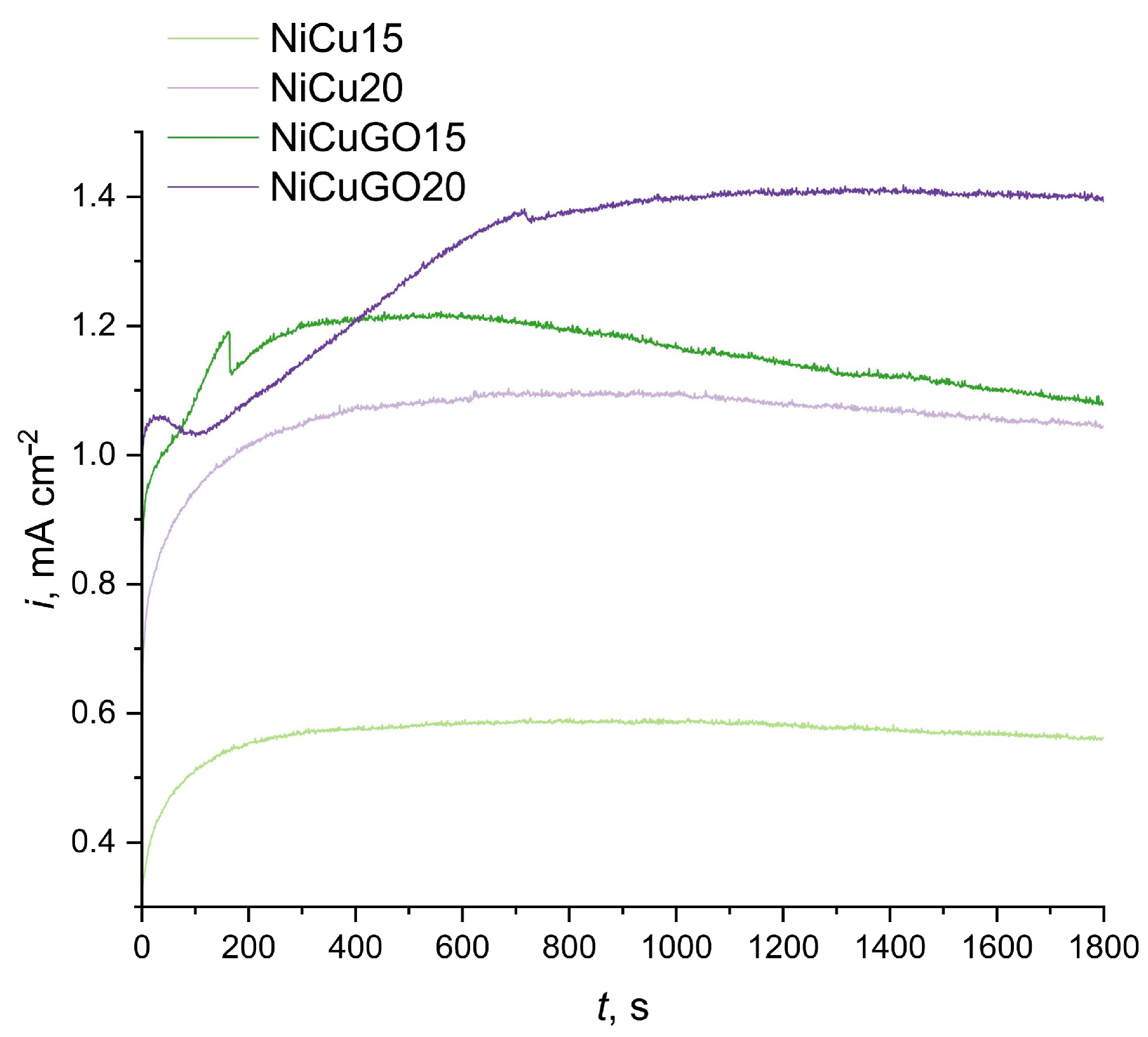
3.4. Stability during Ethanol Oxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sequeira, C.A.C.; Cardoso, D.S.P.; Martins, M.; Amaral, L. Novel materials for fuel cells operating on liquid fuels. AIMS Energy 2017, 5, 458–481. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Yang, J.; Chen, Y. Nanocatalysts for Electrocatalytic Oxidation of Ethanol. ChemSusChem 2019, 12, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.; Belgsir, E.M.; Léger, J.M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC). J. Appl. Electrochem. 2001, 31, 799–809. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Xie, D. A review of approaches for the design of high-performance electrocatalysts for ethanol electrooxidation. Surf. Interfaces 2022, 28, 101594. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Elsaid, K.; Abdelfatah, S.; Abdel Elabsir, A.M.; Hassiba, R.J.; Ghouri, Z.K.; Vechot, L. Direct alcohol fuel cells: Assessment of the fuel’s safety and health aspects. Int. J. Hydrogen Energy 2021, 46, 30658–30668. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.; Hacker, V.; Cobo, M. Impact of bioethanol impurities on steam reforming for hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 11923–11942. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, C.; Wang, Y.; Wang, K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts 2022, 12, 337. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hameed, R.M.A.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Holade, Y.; Tuleushova, N.; Tingry, S.; Servat, K.; Napporn, T.W.; Guesmi, H.; Cornu, D.; Kokoh, K.B. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production. Catal. Sci. Technol. 2020, 10, 3071–3112. [Google Scholar] [CrossRef]
- Yuan, L.S.; Zheng, Y.X.; Jia, M.L.; Zhang, S.J.; Wang, X.L.; Peng, C. Nanoporous nickel-copper-phosphorus amorphous alloy film for methanol electro-oxidation in alkaline medium. Electrochim. Acta 2015, 154, 54–62. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.H.; Eriksson, S.G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene—Metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Shul, Y.G.; Kundu, P.P. Progress in Developments of Inorganic Nanocatalysts for Application in Direct Methanol Fuel Cells. Crit. Rev. Solid State Mater. Sci. 2015, 40, 316–357. [Google Scholar] [CrossRef]
- Bong, S.; Kim, Y.-R.; Kim, I.; Woo, S.; Uhm, S.; Lee, J.; Kim, H. Graphene supported electrocatalysts for methanol oxidation. Electrochem. Commun. 2010, 12, 129–131. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Superior catalytic performance of NiCo2O4 nanorods loaded rGO towards methanol electro-oxidation and hydrogen evolution reaction. J. Mol. Liq. 2019, 291, 111306. [Google Scholar] [CrossRef]
- Noor, T.; Pervaiz, S.; Iqbal, N.; Nasir, H.; Zaman, N.; Sharif, M.; Pervaiz, E. Nanocomposites of NiO/CuO based MOF with rGO: An efficient and robust electrocatalyst for methanol oxidation reaction in DMFC. Nanomaterials 2020, 10, 1601. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Maciej, A.; Simka, W. Electrocatalytic properties of Co decorated graphene and graphene oxide for small organic molecules oxidation. Int. J. Hydrogen Energy 2020, 45, 1769–1783. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. Electrochemical deposition and characterization of NiCu coatings as cathode materials for hydrogen evolution reaction. Electrochem. Commun. 2008, 10, 1909–1911. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. NiCu mixed metal oxide catalyst for alkaline hydrogen evolution in anion exchange membrane water electrolysis. Electrochim. Acta 2021, 371, 137837. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Wang, J.; Geng, Y.; Zhang, B.; He, J.; Xue, J.; Frauenheim, T.; Li, M. Ni/Mo Bimetallic-Oxide-Derived Heterointerface-Rich Sulfide Nanosheets with Co-Doping for Efficient Alkaline Hydrogen Evolution by Boosting Volmer Reaction. Small 2021, 17, 2006730. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, P.; Wan, Y.; Zhang, H.; Xu, H.; Xiao, L.; Li, R.; Li, Y.; Zhang, J.; An, M. Active site engineering toward atomically dispersed M−N−C catalysts for oxygen reduction reaction. Coord. Chem. Rev. 2023, 495, 215400. [Google Scholar] [CrossRef]
- An, Y.; Ijaz, H.; Huang, M.; Qu, J.; Hu, S. The one-pot synthesis of CuNi nanoparticles with a Ni-rich surface for the electrocatalytic methanol oxidation reaction. Dalton Trans. 2020, 49, 1646–1651. [Google Scholar] [CrossRef]
- Abutaleb, A. Electrochemical oxidation of urea on NiCu alloy nanoparticles decorated carbon nanofibers. Catalysts 2019, 9, 397. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Synergistic effect of Cu-doped NiO for enhancing urea electrooxidation: Comparative electrochemical and DFT studies. J. Alloys Compd. 2022, 896, 162857. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, Z.; Yan, Z.; Zhu, L. Bimetallic nickel and copper supported pt catalyst for ethanol electro-oxidation in alkaline solution. Int. J. Electrochem. Sci. 2018, 13, 2164–2174. [Google Scholar] [CrossRef]
- Patel, D.A.; Giannakakis, G.; Yan, G.; Ngan, H.T.; Yu, P.; Hannagan, R.T.; Kress, P.L.; Shan, J.; Deshlahra, P.; Sautet, P.; et al. Mechanistic Insights into Nonoxidative Ethanol Dehydrogenation on NiCu Single-Atom Alloys. ACS Catal. 2023, 13, 4290–4303. [Google Scholar] [CrossRef]
- De Waele, J.; Galvita, V.V.; Poelman, H.; Gabrovska, M.; Nikolova, D.; Damyanova, S.; Thybaut, J.W. Ethanol dehydrogenation over Cu catalysts promoted with Ni: Stability control. Appl. Catal. A Gen. 2020, 591, 117401. [Google Scholar] [CrossRef]
- Jafarian, M.; Moghaddam, R.B.; Mahjani, M.G.; Gobal, F. Electro-catalytic oxidation of methanol on a Ni-Cu alloy in alkaline medium. J. Appl. Electrochem. 2006, 36, 913–918. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiao, J.; Yuan, J.; Shen, J.; Wang, A.-j.; Gong, P.; Weng, X.; Niu, L. Three-dimensional NiCu layered double hydroxide nanosheets array on carbon cloth for enhanced oxygen evolution. Electrochim. Acta 2018, 282, 735–742. [Google Scholar] [CrossRef]
- Roy, A.; Talarposhti, M.R.; Normile, S.J.; Zenyuk, I.V.; De Andrade, V.; Artyushkova, K.; Serov, A.; Atanassov, P. Nickel-Copper Supported on Carbon Black Hydrogen Oxidation Catalyst Integrated into Anion-Exchange Membrane Fuel Cell. Sustain. Energy Fuels 2018, 2, 2268–2275. [Google Scholar] [CrossRef]
- Zhao, X.; Fuji, M.; Shirai, T.; Watanabe, H.; Takahashi, M.; Zuo, Y. Electrocatalytic evolution of hydrogen on the NiCu/Al2O3/nano-carbon network composite electrode. J. Mater. Sci. 2011, 46, 4630–4637. [Google Scholar] [CrossRef]
- Motlak, M.; Barakat, N.A.M.; El-Deen, A.G.; Hamza, A.M.; Obaid, M.; Yang, O.-B.; Akhtar, M.S.; Khalil, K.A. NiCu bimetallic nanoparticle-decorated graphene as novel and cost-effective counter electrode for dye-sensitized solar cells and electrocatalyst for methanol oxidation. Appl. Catal. A Gen. 2015, 501, 41–47. [Google Scholar] [CrossRef]
- Shoeb, M.; Mobin, M.; Rauf, M.A.; Adnan, S.M.; Ansari, M.Y. Graphene nickel[sbnd]copper nanocomposite (Gr@NiCu NCs) as a binder free electrode for high energy density supercapacitor and antimicrobial application. J. Mater. 2021, 7, 815–827. [Google Scholar] [CrossRef]
- Javan, H.; Asghari, E.; Ashassi-Sorkhabi, H. Design of new anodic bimetallic nanocatalyst composed of Ni–Cu supported by reduced carbon quantum dots for the methanol oxidation reaction. Diam. Relat. Mater. 2021, 115, 108348. [Google Scholar] [CrossRef]
- Rana, S.; Jonnalagadda, S.B. A facile synthesis of Cu-Ni bimetallic nanoparticle supported organo functionalized graphene oxide as a catalyst for selective hydrogenation of p-nitrophenol and cinnamaldehyde. RSC Adv. 2017, 7, 2869–2879. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, M.; Halder, A.; Chi, Q. Graphene directed architecture of fine engineered nanostructures with electrochemical applications. Electrochim. Acta 2017, 242, 202–218. [Google Scholar] [CrossRef]
- Vilana, J.; Gómez, E.; Vallés, E. Influence of the composition and crystalline phase of electrodeposited CoNi films in the preparation of CoNi oxidized surfaces as electrodes for urea electro-oxidation. Appl. Surf. Sci. 2016, 360, 816–825. [Google Scholar] [CrossRef]
- Maniam, K.K.; Chetty, R.; Thimmappa, R.; Paul, S. Progress in the Development of Electrodeposited Catalysts for Direct Liquid Fuel Cell Applications. Appl. Sci. 2022, 12, 501. [Google Scholar] [CrossRef]
- Wala, M.; Szewczyk, M.; Leśniak–Ziółkowska, K.; Kazek–Kęsik, A.; Simka, W. Preparation of NiCuGO composite and investigation of its electrocatalytic properties in methanol oxidation. Electrochim. Acta 2022, 425, 140743. [Google Scholar] [CrossRef]
- Wala, M.; Blacha–Grzechnik, A.; Stolarczyk, A.; Bajkacz, S.; Dydo, P.; Simka, W. Unexpected electrochemical oxidation of urea on a new NiCuGO composite catalyst. Int. J. Hydrogen Energy 2023, 48, 34229–34243. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Motlak, M. CoxNiy-decorated graphene as novel, stable and super effective non-precious electro-catalyst for methanol oxidation. Appl. Catal. B Environ. 2014, 154–155, 221–231. [Google Scholar] [CrossRef]
- Yang, J.; Shen, X.; Ji, Z.; Zhou, H.; Zhu, G.; Chen, K. In situ growth of hollow CuNi alloy nanoparticles on reduced graphene oxide nanosheets and their magnetic and catalytic properties. Appl. Surf. Sci. 2014, 316, 575–581. [Google Scholar] [CrossRef]
- Glass, D.E.; Galvan, V.; Prakash, G.K.S. The Effect of Annealing Temperature on Nickel on Reduced Graphene Oxide Catalysts on Urea Electrooxidation. Electrochim. Acta 2017, 253, 489–497. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-Metal Oxides/Graphene Nanocomposites as Multifunctional Electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Duan, D.; Gao, J.; Wang, Y.; Zhou, X.; Liu, S.; Wang, Y. Electrodeposition of copper-cobalt bimetallic phosphide on nickel foam as an efficient catalyst for overall water splitting. J. Electroanal. Chem. 2023, 939, 117478. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Wang, J.; Cao, B.; Yu, Y.; Zhou, C.; Zhang, G.; Wang, Z.; Zhao, C. Pulsed electrodeposition of well-ordered nanoporous Cu-doped Ni arrays promotes high-efficiency overall hydrazine splitting. J. Mater. Chem. A 2020, 8, 21084–21093. [Google Scholar] [CrossRef]
- Lotfi, N.; Shahrabi, T.; Yaghoubinezhad, Y.; Barati Darband, G. Electrodeposition of cedar leaf-like graphene Oxide@Ni–Cu@Ni foam electrode as a highly efficient and ultra-stable catalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 326, 134949. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Diaz, L.A.; Botte, G.G. Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim. Acta 2014, 134, 266–271. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Tsai, Y.C.; Wu, M.S. Honeycomb-like copper/cuprous oxide with supported nickel hydroxide layer as an electrode material for electrochemical oxidation of urea. J. Alloys Compd. 2020, 836, 155533. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.; Shen, R.; Ru, C.; Hu, Y. Effect of Bubble Behavior on the Morphology of Foamed Porous Copper Prepared via Electrodeposition. J. Electrochem. Soc. 2013, 160, D441–D445. [Google Scholar] [CrossRef]
- Shin, H.C.; Liu, M. Copper foam structures with highly porous nanostructured walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Zhang, H.; Zhang, Z.; Yan, P.; Zhang, J.; et al. Key Roles of Interfacial OH− ion Distribution on Proton Coupled Electron Transfer Kinetics toward Urea Oxidation Reaction. Small 2023, 19, 2302151. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yi, Q.; Zhang, Y.; Nie, H. NiCo/C-N/CNT composite catalysts for electro-catalytic oxidation of methanol and ethanol. J. Electroanal. Chem. 2017, 803, 95–103. [Google Scholar] [CrossRef]
- Miao, Z.; Xu, C.; Zhan, J.; Xu, Z. Morphology-control and template-free fabrication of bimetallic Cu–Ni alloy rods for ethanol electro-oxidation in alkaline media. J. Alloys Compd. 2021, 855, 157438. [Google Scholar] [CrossRef]
- Nassif, N.; Ghayad, I.; Hamid, Z.A. Review article: Direct ethanol fuel cells as a renewable source of energy. Egypt. J. Chem. 2021, 64, 2723–2729. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Henriksson, G.; Cornell, A. Insights on the ethanol oxidation reaction at electrodeposited PdNi catalysts under conditions of increased mass transport. Int. J. Hydrogen Energy 2021, 46, 1615–1626. [Google Scholar] [CrossRef]
- Akhairi, M.A.F.; Kamarudin, S.K. Catalysts in direct ethanol fuel cell (DEFC): An overview. Int. J. Hydrogen Energy 2016, 41, 4214–4228. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Tremiliosi-Filho, G.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Improved ethanol electro-oxidation at Ni@Pd/C and Ni@PdRh/C core–shell catalysts. J. Catal. 2020, 391, 175–189. [Google Scholar] [CrossRef]
- Altarawneh, R.M.; Brueckner, T.M.; Chen, B.; Pickup, P.G. Product distributions and efficiencies for ethanol oxidation at PtNi octahedra. J. Power Sources 2018, 400, 369–376. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Paul, S. Electrochemical development of Ni-Cu electrodes by direct and pulse current coating in ethanol electro-oxidation for DEFC. Port. Electrochim. Acta 2018, 36, 293–307. [Google Scholar] [CrossRef]
- Rahmani, K.; Habibi, B. NiCo alloy nanoparticles electrodeposited on an electrochemically reduced nitrogen-doped graphene oxide/carbon-ceramic electrode: A low cost electrocatalyst towards methanol and ethanol oxidation. RSC Adv. 2019, 9, 34050–34064. [Google Scholar] [CrossRef] [PubMed]
- Ghalkhani, M.; Mirzaie, R.A.; Banimostafa, A.; Sohouli, E.; Hashemi, E. Electrosynthesis of ternary nonprecious Ni, Cu, Fe oxide nanostructure as efficient electrocatalyst for ethanol electro-oxidation: Design strategy and electrochemical performance. Int. J. Hydrogen Energy 2023, 48, 21214–21223. [Google Scholar] [CrossRef]
- Yu, J.; Ni, Y.; Zhai, M. Simple solution-combustion synthesis of Ni-NiO@C nanocomposites with highly electrocatalytic activity for methanol oxidation. J. Phys. Chem. Solids 2018, 112, 119–126. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, Y.; Guo, Y. Copper@palladium-copper core-shell nanospheres as a highly effective electrocatalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2014, 270, 257–261. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, X.; Cheng, X.; Cheng, K.; Liu, Z.; Dai, Z. Advanced catalytic materials for ethanol oxidation in direct ethanol fuel cells. Catalysts 2020, 10, 166. [Google Scholar] [CrossRef]
- Hassan, H.B.; Hamid, Z.A. Electrodeposited Ni-Cr2O3 nanocomposite anodes for ethanol electrooxidation. Int. J. Hydrogen Energy 2011, 36, 5117–5127. [Google Scholar] [CrossRef]
- Lycke, D.R.; Gyenge, E.L. Electrochemically assisted organosol method for Pt-Sn nanoparticle synthesis and in situ deposition on graphite felt support: Extended reaction zone anodes for direct ethanol fuel cells. Electrochim. Acta 2007, 52, 4287–4298. [Google Scholar] [CrossRef]
- Bender, M.T.; Lam, Y.C.; Hammes-Schiffer, S.; Choi, K.S. Unraveling Two Pathways for Electrochemical Alcohol and Aldehyde Oxidation on NiOOH. J. Am. Chem. Soc. 2020, 142, 21538–21547. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Park, S.-M. Electrochemical Oxidation of Ethanol at Nickel Hydroxide Electrodes in Alkaline Media Studied by Electrochemical Impedance Spectroscopy. J. Korean Electrochem. Soc. 2005, 8, 117–124. [Google Scholar] [CrossRef]
- Chemchoub, S.; El Attar, A.; Oularbi, L.; Younssi, S.A.; Bentiss, F.; Jama, C.; El Rhazi, M. Electrosynthesis of eco-friendly electrocatalyst based nickel-copper bimetallic nanoparticles supported on poly-phenylenediamine with highest current density and early ethanol oxidation onset potential. Int. J. Hydrogen Energy 2022, 47, 39081–39096. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Jadali, S. Rational design of PdCu nanoparticles supported on a templated Ni foam: The cooperation effect of morphology and composition for electrocatalytic oxidation of ethanol. Int. J. Hydrogen Energy 2021, 46, 39387–39403. [Google Scholar] [CrossRef]

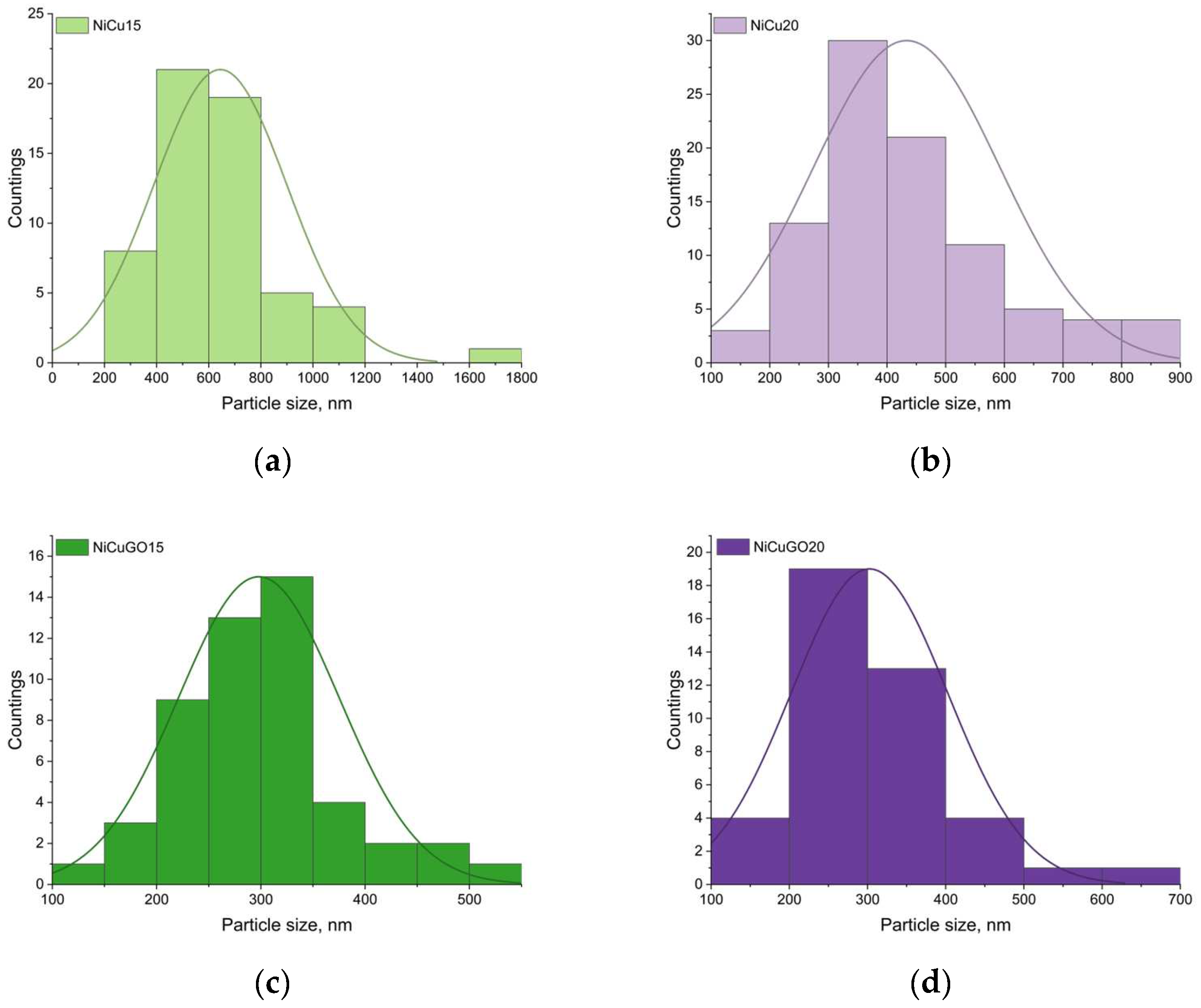
| Sample Name | GO Addition, g L−1 | i, A dm−2 |
|---|---|---|
| NiCu15 | - | 15.6 |
| NiCu20 | 20.8 | |
| NiCuGO15 | 0.5 | 15.6 |
| NiCuGO20 | 20.8 |
| Sample | EpA, V | ipA, mA cm−2 | i@Erev, mA cm−2 |
|---|---|---|---|
| NiCu15 | 0.65 | 7.05 | 7.75 |
| NiCu20 | - | - | 8.70 |
| NiCuGO15 | 0.63 | 7.5 | 7.25 |
| NiCuGO20 | 0.66 | 8.6 | 9.10 |
| Material | ipA, mA cm−2 | Reference |
|---|---|---|
| NiCuGO20 | 8.6 | This article |
| NiCo | 180 | [57] |
| NiCu NR | 86.1 | [58] |
| Ni20@Pd60Rh20/C | 4.0 | [62] |
| PtNi/C | 5.6 | [63] |
| NiCu | 12.4 | [64] |
| NiCo@ErN-GO | 64.2 | [65] |
| NiCuFe2O3 | 101 | [66] |
| Ni-NiO | 120 | [67] |
| PdCu | 166 | [68] |
| PdCdrGO | 179 | [69] |
| Ni-Cr2O3 | 327 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wala-Kapica, M.; Szewczyk, M.; Simka, W. Designing Sustainable Ethanol Oxidation Catalysts: The Role of Graphene Oxide in NiCuGO Composite Material. Energies 2024, 17, 288. https://doi.org/10.3390/en17020288
Wala-Kapica M, Szewczyk M, Simka W. Designing Sustainable Ethanol Oxidation Catalysts: The Role of Graphene Oxide in NiCuGO Composite Material. Energies. 2024; 17(2):288. https://doi.org/10.3390/en17020288
Chicago/Turabian StyleWala-Kapica, Marta, Magdalena Szewczyk, and Wojciech Simka. 2024. "Designing Sustainable Ethanol Oxidation Catalysts: The Role of Graphene Oxide in NiCuGO Composite Material" Energies 17, no. 2: 288. https://doi.org/10.3390/en17020288
APA StyleWala-Kapica, M., Szewczyk, M., & Simka, W. (2024). Designing Sustainable Ethanol Oxidation Catalysts: The Role of Graphene Oxide in NiCuGO Composite Material. Energies, 17(2), 288. https://doi.org/10.3390/en17020288







