Abstract
A flue gas condenser (FGC) system recovers heat from exhaust flue gases in energy production and chemical plants, reducing air pollution due to dust, SOx, and HCl. An FGC system is divided into indirect contact condenser (ICC) and direct contact condenser (DCC) types. In an ICC, the exhaust gases do not mix with the working fluid, and a water film is formed during flue gas condensation for partial SOx removal. In a DCC, direct mixing of the exhaust flue gas with the cooling fluid (mainly water) occurs, with simultaneous absorption of SOx. In this study, we investigated the SO2 removal efficiency and heat recovery of an ICC, a DCC, and a DCC–ICC hybrid system, and compared the results of the hybrid system with those obtained for a single DCC type at the same liquid-to-gas (L/G) ratio. The SO2 removal characteristics of the hybrid system were examined based on the L/G ratio and absorbent-to-SO2 molar ratio. In the reference ICC-type FGC system, the exit temperature of the mixed gas was 28 °C, with the condensed water ratio and heat recovery efficiency being 80.9% and 93.4%, respectively. At an L/G ratio of 1.5–3.5, the SO2 removal efficiency of a single DCC was 31.5–65.9%, whereas that of the hybrid FGC system (with packing material) increased from 47.1% to 72.3%, which further increased to ~90% upon the addition of NaOH at a molar ratio of 0.7 and an L/G ratio of 1.5.
1. Introduction
Energy production plants and chemical plants based on the combustion of fossil fuels generate air pollution due to pollutants such as carbon monoxide, nitrogen oxide, volatile organic compounds, sulfur dioxide, and particulate matter. Various environmental facilities are used to control the generation of this air pollution. The exhaust gas temperature of an energy production plant is 70–150 °C, considering the risk of low-temperature corrosion of SOx and the diffusion of exhaust flue gases. The water vapor in the exhaust gases contains a significant amount of latent heat [1].
Waste incineration facilities produce various compounds owing to the composition of fuels, including dust, SOx, NOx, HCl, HF, dioxins, and heavy metals. Furthermore, the air pollution system consists of several processes. Electrostatic precipitators (ESPs) are commonly employed in waste incineration plants to eliminate dust, achieving a particle removal efficiency of >99.5% for even 10 µm particles [2]. NOx is removed by selective noncatalytic reduction (SNCR) within the high-temperature zone preceding the boiler, and selective catalytic reduction (SCR) is implemented in certain facilities [3]. Additionally, flue gas desulfurization (FGD) methods are adopted to eliminate residual acid gases like SOx and HCl.
FGD is classified into wet flue gas desulfurization (WFGD), semi-dry reactor (SDR), dry reactor (DR), etc. Despite having high water consumption and a high cost of wastewater treatment, WFGD achieves a significant desulfurization effect with low equipment costs. However, SDR is inexpensive because it does not require reheating energy or wastewater treatment, but its desulfurization efficiency is relatively lower than that of WFGD [4]. Dry FGD uses metal oxides and solid compounds such as copper oxide, magnesium oxide, and activated carbon as adsorbents. However, because they can adsorb SO2 on the surface, the SO2 removal rate is low, and consideration of solid waste disposal after reaction is crucial [4,5].
In exiting waste incineration facilities, the moisture content of exhaust gas is approximately 10% to 20% based on the fuel properties. If the sensible and latent heat released to the atmosphere is not recovered under 1 bar of exhaust gas and 15% of water vapor at 120 °C, then 644.4 kJ/kg of heat loss occurs. Therefore, the energy efficiency can be increased when the feed water of the process is preheated through waste heat recovery of the exhaust gas.
The flue gas condensation (FGC) system can simultaneously remove air pollution and recover waste heat from flue gases. The FGC system reduces the temperature of flue gases below the dew point, condensing water vapor within the flue gas. Furthermore, this system enhances overall energy efficiency by recovering the sensible and latent heat of the flue gas using cooling water [6,7,8,9]. FGC systems are typically divided into two types, depending on the heat transfer method between the cooling water and flue gas: indirect contact condensation (ICC) and direct contact condensation (DCC) types.
The ICC type consists of a general shell and tube-type heat exchanger, recovering the sensible and latent heat of flue gas through cooling water. In the ICC type, the flue gas and cooling water are separated by a tube wall. The cooling water is not contaminated during the heat recovery process; thus, the recovered heat can be used directly without additional processing. During the condensation process, a water film was generated on the surface of the heat exchanger. The fine particles [10,11], SOx [12,13,14], and heavy metals [15,16] in the flue gas contact the tube surface, reducing atmospheric pollution. The pollution removal efficiency of the ICC type can vary depending on the dew point temperature and pollution concentration of flue gas. However, the SO2 removal efficiency of the ICC type is too low to be used alone. In addition, low-temperature corrosion can occur on the tube surface [17,18,19]; therefore, to prevent corrosion, materials with high corrosion resistance, such as Teflon, stainless steel, and glass for heat exchanger tubes, must be used [20]. The DCC type utilizes a wet scrubber instead of the conventional indirect cooling heat exchanger to lower the temperature of the flue gas [21]. In this system, the flue gas directly contacts a low-temperature absorbent, providing higher cooling performance and preventing the corrosion of tubes [20]. The key strategy to improve SO2 removal efficiency in a DCC-type system is increasing the liquid-to-gas (L/G) ratio and using appropriate packing materials. However, this effectively increases the contact area between the exhaust gas and the absorbent [22]. A higher L/G ratio, while achieving effective desulfurization, results in increased power consumption within the system and generates a significant amount of wastewater. The large amounts of wastewater require an additional filtration process, which may not be economically advantageous [23,24,25]. When applying an FGC unit to an existing plant, the DCC type for complying with air pollution standards must be applied. Moreover, the efficiency of pollutant emission reduction in the DCC type must be enhanced while simultaneously recovering waste heat for direct use as a working fluid from exhaust gas.
Previous studies on FGC systems have typically assessed SOx removal and waste heat recovery separately for ICC-type and DCC-type systems. While research on hybrid systems combining ICC and DCC types exists, the primary focus has often been on heat recovery efficiency [26,27]. Some studies have also examined methods to enhance SO2 absorption by condensing wet flue gas downstream of WFGD, although heat recovery is not a primary focus in these cases [28]. In this study, a hybrid FGC system, integrating ICC and DCC types, was investigated, and its SO2 removal efficiency and heat recovery performance were simultaneously evaluated. The hybrid FGC system integrated an ICC stage at the exhaust flue gas inlet to improve heat recovery, while reducing flue gas temperatures to enhance SO2 absorption in the DCC section. Initially, the heat recovery and SOx removal characteristics of the ICC type were evaluated independently, followed by an analysis of the effects of varying feed water and additive conditions on the SOx removal characteristics of the hybrid system.
2. Materials and Methods
2.1. Hybrid FGC System
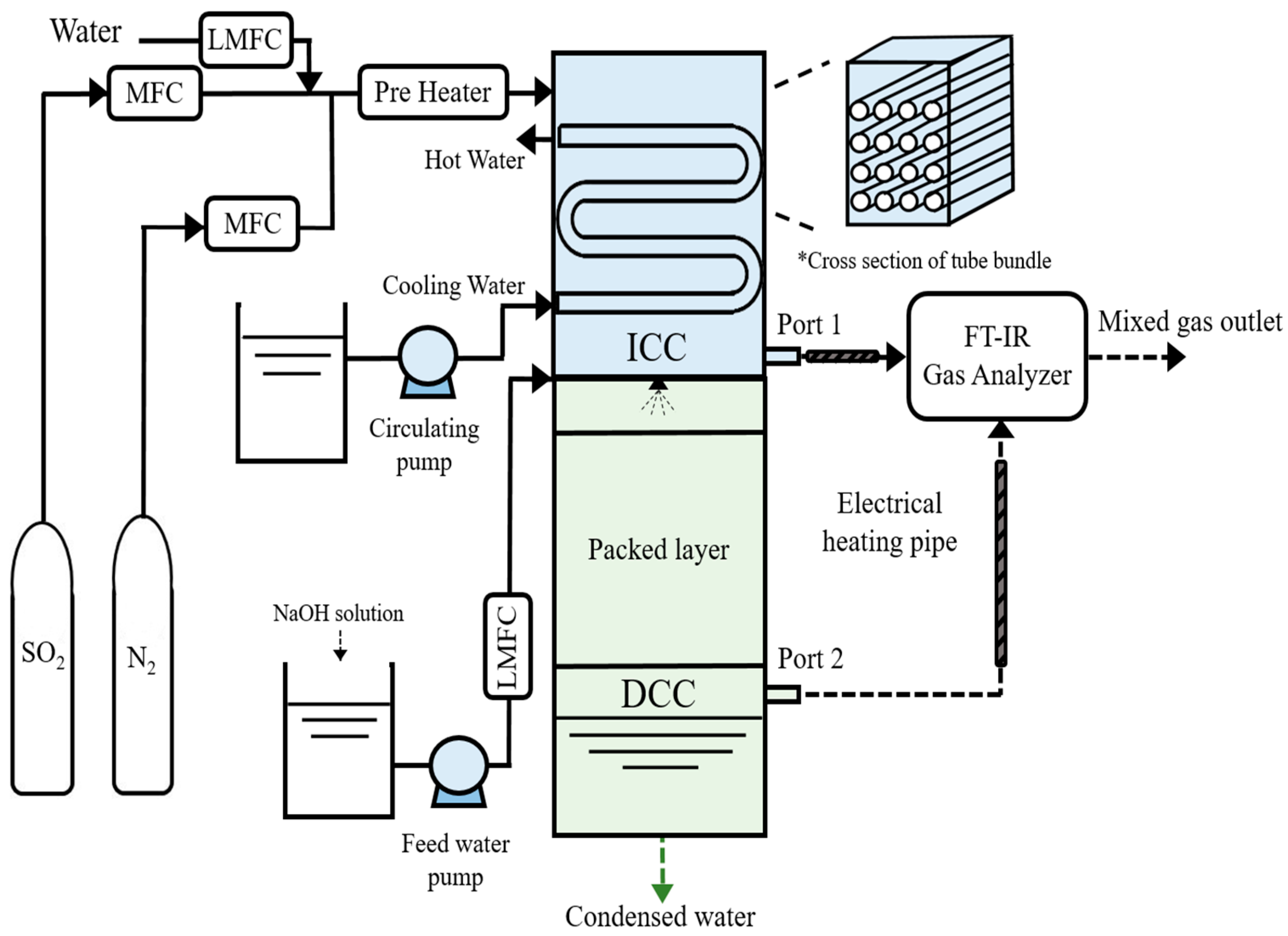
Figure 1 shows a schematic of the lab-scale FGC system, which consists of ICC type at the top and DCC type at the bottom. The ICC type is a rectangular duct-type heat exchanger. The housing has a width, length, and height of 131, 90, and 380 mm, respectively. The internal tube of the ICC type has an outer diameter of 15.5 mm, a length of 125 mm, and is composed of a fin-tube type. The tube bundle has four stages, including four tubes per stage, and the total tubes are 4 × 4. The exterior of the housing is made of refractory material for insulation. The housing size of the DCC type is similar to that of the ICC type, and the height is 500 mm. Packing materials, such as a 5 mm alumina ball, are used inside the DCC type, and water is supplied by the single spray nozzle of the full-cone type. The wastewater storage system is constructed at the bottom of the DCC type.
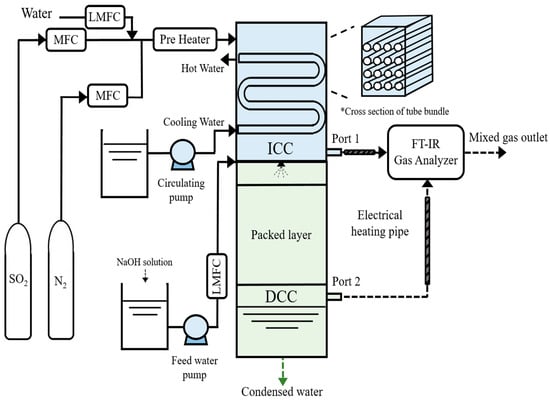
Figure 1.
Schematic of the lab-scale FGC system.
2.2. Experimental Method
N2, SO2, and water are controlled precisely using MFC (MKP-VIC220) and LMFC (Model Name) units. They are preheated to 125 °C using a preheater and introduced into the upper section of the ICC system. Water (H2Ol) undergoes a phase transition to vapor (H2Og) in the preheater. Mixed gases (N2, SO2, and H2Og) are introduced into the ICC upper section; moreover, they can be discharged to port 1 for ICC single experiments or port 2 for ICC and DCC integration experiments, based on the conditions. Cooling water for the ICC is maintained at 20 °C in a constant-temperature bath, introduced into the tubes, and recirculated after heat exchange with exhaust gases. Moisture condenses on the tube surface during heat transfer, forming a water film where SO2 reacts as the primary removal process. The mixed gas is introduced to the DCC section, directly contacting spray water for further SO2 removal. The spray water is supplied at room temperature. Moreover, measures are taken to prevent moisture condensation in the piping using electrical heating pipes, and SO2 and H2Og reduction characteristics are analyzed using an FT-IR gas analyzer (DX 4000, Finland) for the mixed gas discharged from the FGC system (port 1 and port 2).
2.3. Experimental Conditions
2.3.1. Single Experiment Based on ICC Type
Table 1 lists the conditions for the single experiment performed on the ICC type. In the single experiment, the SO2 removal efficiency and heat source recovery were characterized. The experimental conditions of the reference case (Case Ref.) are a mixed gas flow rate and temperature of 30 L/min and 125 °C, respectively. The mixed gas composition is 320 ppm of SO2 and 15% of moisture, and the residence time of the mixed gas in the ICC type is 7.8 s. Cases 1 to 4 are the experimental conditions for the characterization of SOx removal in the ICC-type reactor. Cases 5 and 6 are the conditions used for the analysis of the heat recovery characteristics of the heat exchanger. Cases 1 and 2 are the conditions in which the flow rate of the mixed gas is reduced, and the residence time is increased by 1.5 and 2 times, respectively, compared to that in the reference case. Case 3 represents a condition in which the moisture content in the exhaust gas increases to 45%, and Case 4 is a condition in which the residence time is twice that in the reference case, and the moisture content is 45% higher than that of the reference case. The removal efficiency of SO2 in the reactor is calculated based on its inlet and outlet SO2 concentrations and , respectively, where the subscript x denotes the type of the reactor. The same formula is used in the hybrid FGC experiment as well.
In Cases 5 and 6, we analyzed the heat recovery efficiency by increasing the coolant flow rate, respectively, compared to that in the reference case. The heat recovery characteristics of the mixed gas were analyzed using Equation (2).
where denotes the heat released by the flue gas, represents the mass flow rate of the dry flue gas, Tfg,in represents the initial temperature of the flue gas entering the FGC system, Tfg,out denotes the temperature of the flue gas exiting the FGC system, din represents the humidity ratio of the moist flue gas at the system inlet, dout is the humidity ratio of the moist flue gas at the system outlet, and r corresponds to the latent heat of water. The condensed water ratio is expressed as

Table 1.
Experimental conditions of the single ICC-type reactor.
The heat recovery efficiency of mixed gas is defined as the ratio of heat released because of heat transfer to the cooling water when it is cooled to the ambient condition (20 °C, 1.013 bar), as outlined in Equation (4).
Except for the experimental data, the remaining thermodynamic data were calculated based on REFPROP 10.0 [29].
2.3.2. Integrated Experiment on the ICC- and DCC-Type FGC System
Table 2 shows the experimental conditions for the characteristic analysis of SOx removal based on the FGC system, in which ICC and DCC types are integrated. In this experiment, the mixed gas flow rate is 60 L/min, owing to the minimum water flow rate of the spray nozzle. The removal characteristics of SO2 were analyzed based on the effects of the L/G ratio, with or without packing material, and absorbents such as NaOH. The L/G ratio is the mass fraction of water and gas, and the range of the L/G ratio is 1.5 to 3.5. The removal characteristics of SO2 were investigated with or without packing material based on an L/G ratio of 1.5 to 3.5. To evaluate the influence of the absorbent, the experiment was performed with an L/G ratio fixed at 1.5. The molar ratio of the additive was calculated based on a SO2 reduction of 320 ppm (SO2 input, zero emission).

Table 2.
Experimental conditions of the hybrid FGC system.
NaOH aqueous solution was used as the additive, and the calculated molar ratio of the absorbent was 0.1 to 0.7 according to the following stoichiometric reaction equation:
3. Results and Discussion
3.1. Results of Single Experiment Based on the ICC-Type Reactor
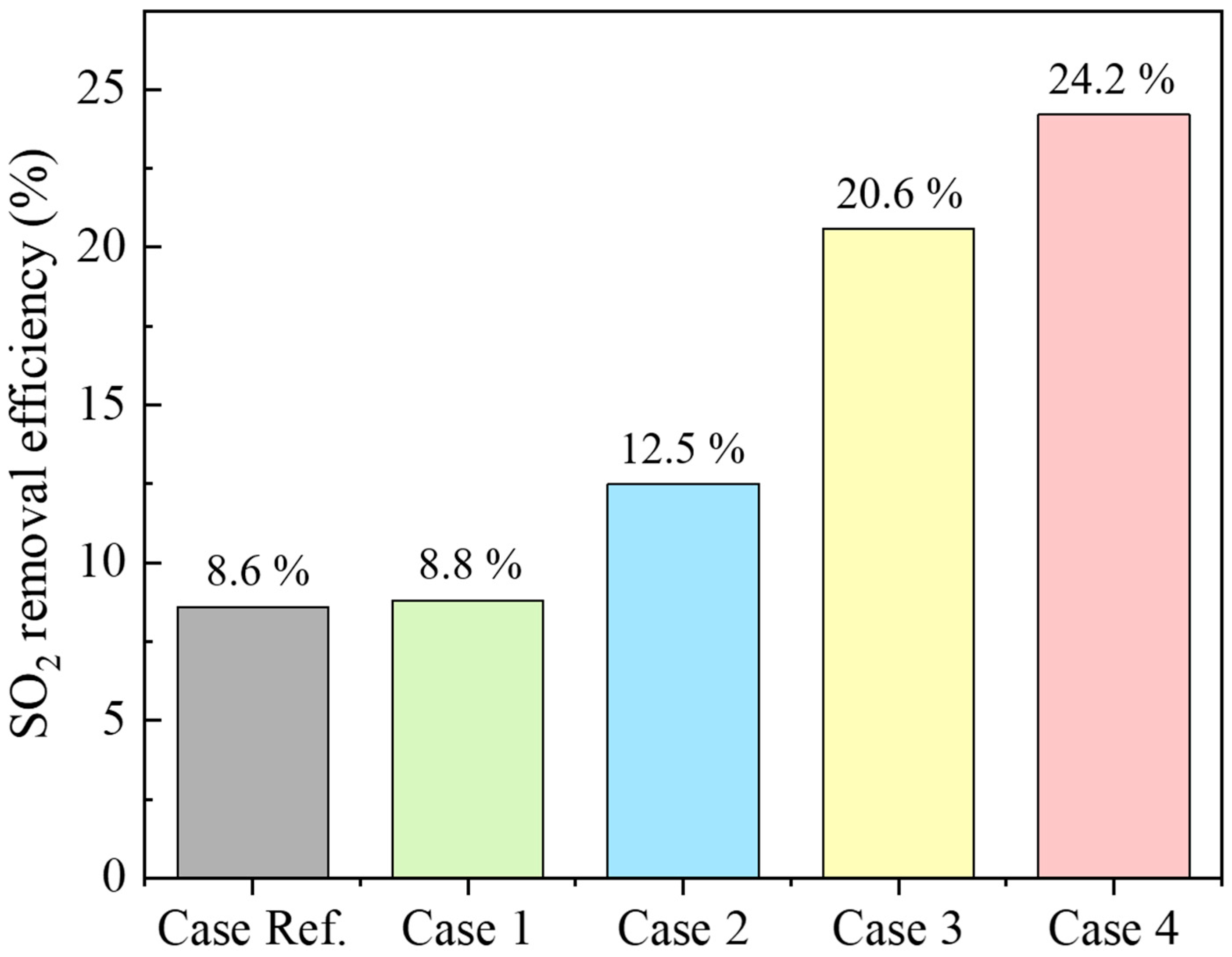
The FGC system, investigated in this study, recovers the sensible and latent heat of the mixed gas through the ICC-type and reduces SOx. Figure 2 shows the analysis results of the SO2 removal characteristics of the single ICC-type experiment. The main mechanism for SO2 removal in the ICC is the absorption process through the water film condensed on the heat exchanger surface. When the flue gas comes into contact with the cold tube surface, the vapor condenses, creating a concentration gradient of vapors in the radial direction of the tube, thus forming a Stefan flow toward the tube surface. Through this process, a condensation film is formed on the tube surface, providing a gas–liquid contact area for SO2 removal. The SO2 in the flue gas near the heat exchanger tube first dissolves into the water film on the tube surface. Similarly, SO2 farther from the tube diffuses toward the surface and is absorbed by the water film. In Case Ref., SO2 removal efficiency is 8.6%. Increasing residence time by 1.5 and 2 times enhances SO2 removal, reaching 8.8% for Case 1 and 12.5% for Case 2. This effect is attributable to increased contact time between H2Ol and SO2 on heat exchanger tube surfaces [12]. Case Ref., As the moisture content of the mixed gas increased to 45%, the SO2 removal efficiency was 20.6% compared to Case 3. In Case 4, where moisture content and residence time increased simultaneously, SO2 removal efficiency increased to approximately 24.2%. When the moisture content in the mixed gas increases, the dew point temperature increases, and the condensation start temperature of the mixed gas increases. When the moisture content in the mixed gas is 15%, the dew point is 55 °C, and when the moisture content is 45%, the dew point is 70 °C [26]. As the moisture content of the mixed gas increases, the moisture in the mixed gas condenses at the top of the reactor. Compared to the reference case, the water film formation area on the surface of the heat exchanger tube increases, thus increasing SO2 removal efficiency [12].
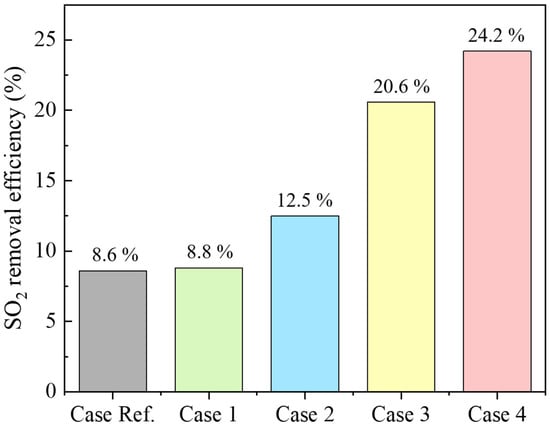
Figure 2.
SO2 removal efficiency by residence time and moisture content in mixed gas.
Table 3 presents the ICC-type exit temperature of the mixed gas, condensed water ratio, and heat recovery efficiency corresponding to varying cooling water flow rates. In Case Ref., the exit temperature of the mixed gas was 28 °C, with a condensed water ratio of 80.9% and a heat recovery efficiency of 93.4%. An increase in the cooling water flow rate increases the flow velocity of the cooling water inside the tubes, enhancing the heat transfer coefficient. As a result, the heat transfer rate between the flue gas and cooling water is enhanced. Consequently, in Case 6, the mixed gas exit temperature decreases to 24 °C, while the moisture condensation and heat recovery efficiency increase to 85.7% and 97.6%, respectively. By reducing the temperature of the exhaust flue gas, with a water content of 15%, from 120 °C to 24 °C, approximately 338.2 kJ/kg of enthalpy can be recovered per unit mass [29]. The high heat recovery efficiency of the mixed gas enhances the energy efficiency of FGC-based systems. Additionally, the reduced exit temperature of the mixed gas increases water vapor condensation, which contributes to the mitigation of white plume emissions [30].

Table 3.
Results of heat recovery efficiency according to cooling water flow.
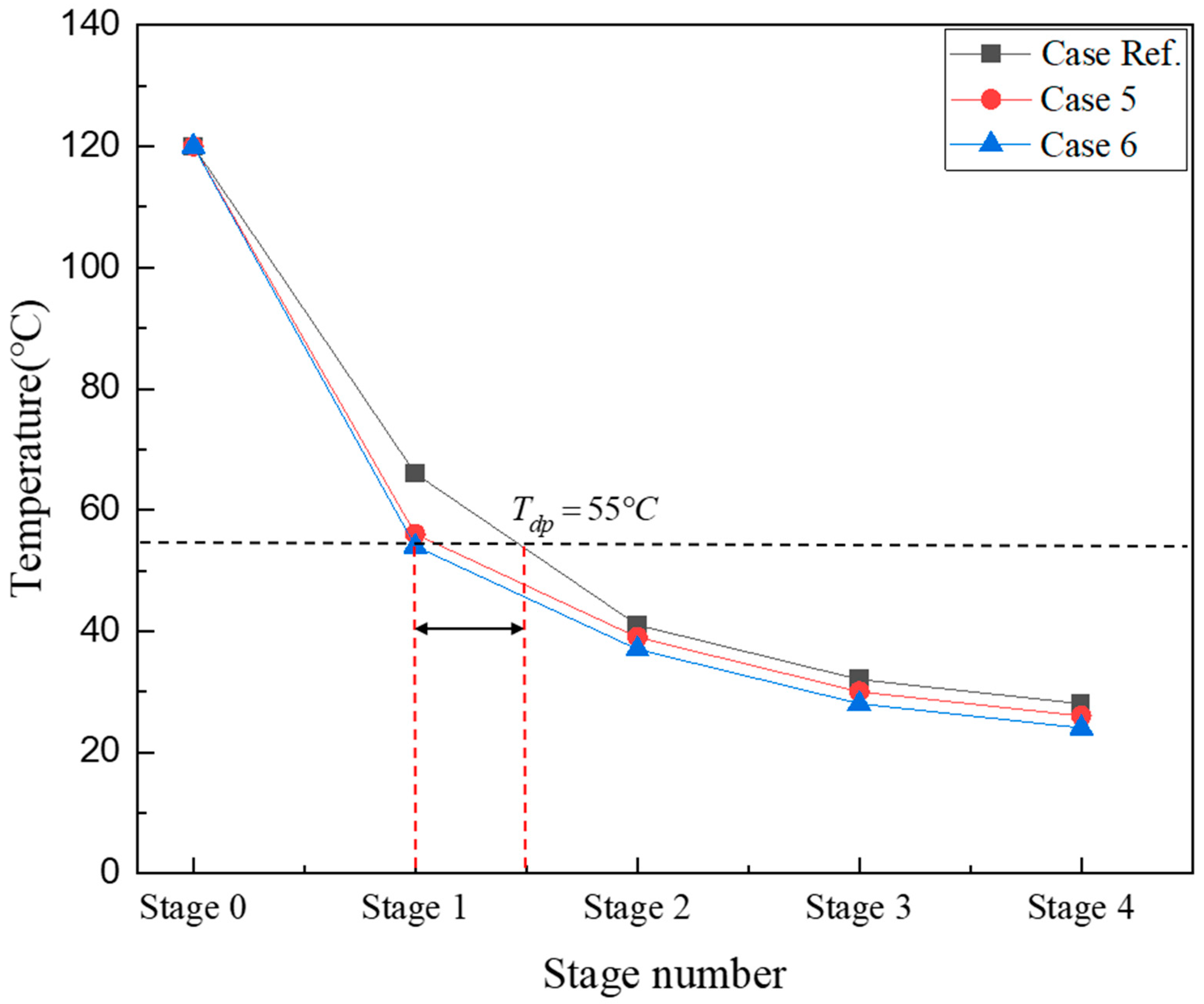
Figure 3 shows the temperature profile of flue gases in the ICC-type reactor. Stage 0 on the X-axis represents the top side of the ICC-type reactor where mixed gases are introduced, and the temperature is 120 °C. The temperature of the mixed gas is measured at the bottom of the heat exchanger tube in each stage. In the first stage, Case Ref. was 120 °C, and in the 4th stage, 28 °C. Cases 5 and 6, where the coolant flow rate increased, showed similar temperature trends. The mixed gas temperature of Case 6, which had the highest coolant flow rate, was the lowest in all regions. As indicated by the black dashed line, Tdp is 55 °C under the condition that the moisture content of the mixed gas is 15%. In all cases, if the temperature of the mixed gas in the first stage of the heat exchanger is above the dew point temperature, only sensible heat is recovered. In the second stage, latent and sensible heat are recovered simultaneously, and moisture condenses. In Cases 5 and 6, as the cooling water flow rate increases, the internal heat transfer coefficient increases; thus, the gas temperature drops to approximately 55 °C in the first stage, resulting in high condensation in the second stage. In Case Ref., sensible heat and latent heat were simultaneously recovered in the second stage, and the amount of condensate produced was lower than that in Cases 5 and 6. In Case Ref., the temperature of the mixed gas drops below the Tdp later than in other cases, as indicated by the red dashed lines. Furthermore, to realize efficient heat recovery, the size of the reactor can be reduced by shrinking the dimensions of the ICC stage by half; however, for SOx removal, the configured heat transfer area must be larger than that of a typical heat exchanger to secure a sufficiently large reaction area. To reduce the size of the ICC-type reactor, the amount of heat transfer at the top of the reactor must be increased to lower the temperature of the exhaust gas to below the dew point and secure the SOx reaction area.
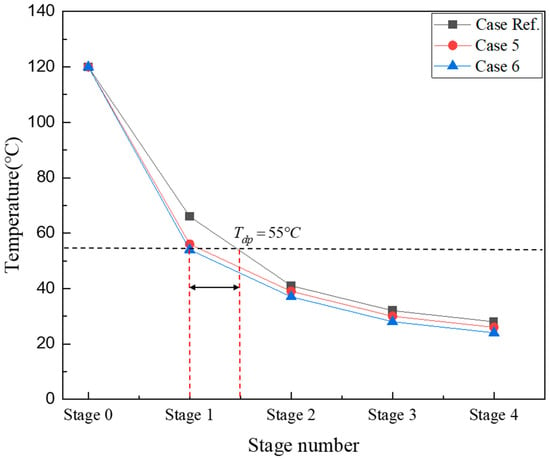
Figure 3.
Temperature profile of the single ICC type according to the cooling water flow rate.
3.2. Results of the Hybrid FGC System
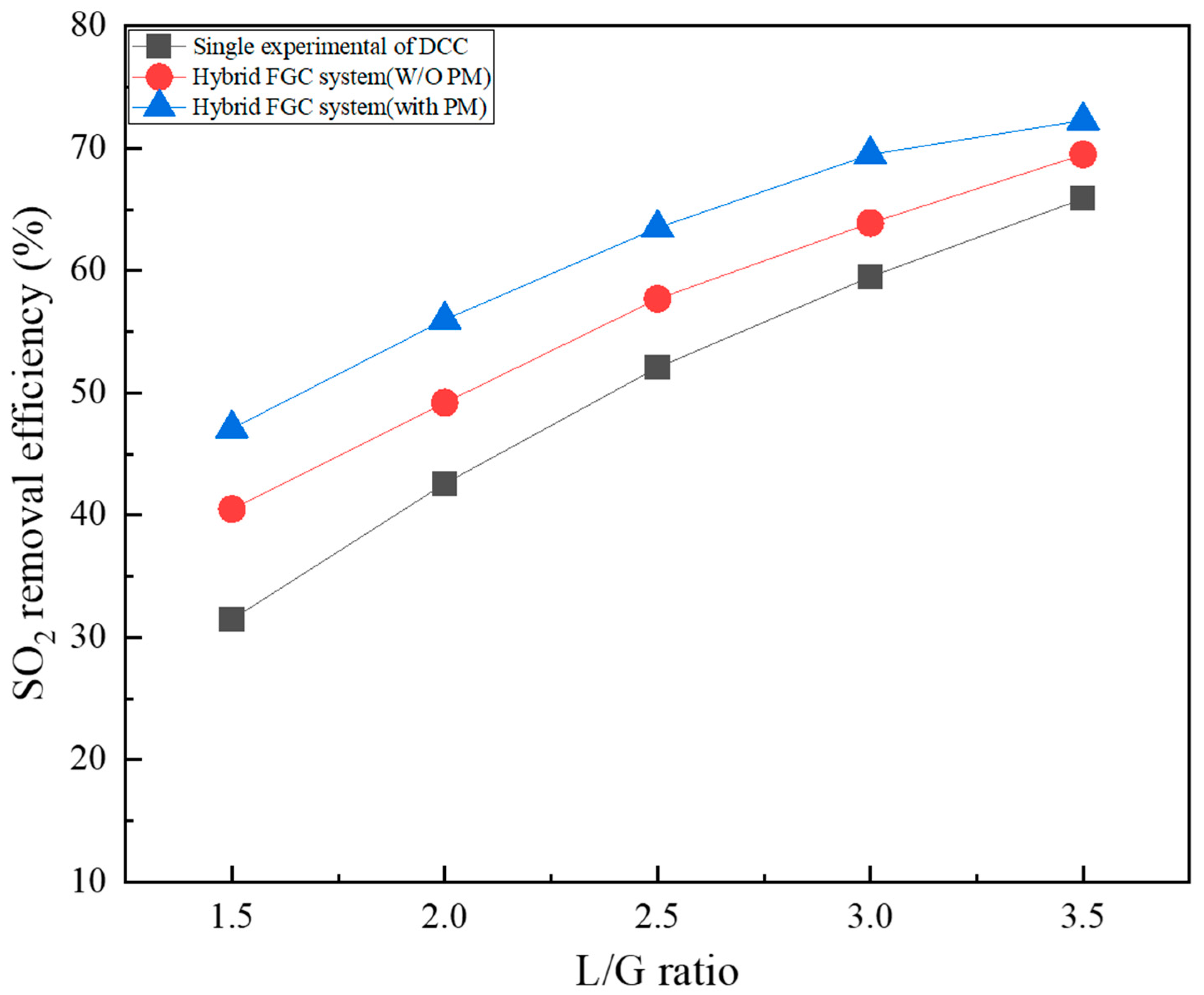
Figure 4 shows a comparison of the SO2 removal characteristics of a single DCC-type reactor and a hybrid FGC system (an integrated FGC system of ICC and DCC). The DCC part of the hybrid FGC system investigated SO2 removal characteristics depending on with/without packing material. At the 1.5 L/G ratio, the SO2 removal efficiency was 31.5% for a single DCC-type reactor, and the W/O and packing material of the novel FGC system were 40.5% and 47.1%, respectively. The SO2 removal efficiency of the W/O packing material case of the hybrid FGC system was approximately 9% higher than that of a single DCC-type reactor, owing to the first removal in ICC. In addition, the SO2 removal efficiency of the case containing packing material (packing material W) increased by 6.6% compared to the W/O case. At a relatively low 1.5 and 2.0 L/G ratio, there is a low mass transfer area between the mixed gas and spray water. Therefore, additional reaction area and reaction time are increased through the packing material, thus increasing SO2 removal efficiency. Under all conditions, SO2 removal efficiency increased as the L/G ratio increased. At the 3.5 L/G ratio, the SO2 removal efficiency was 65.9% for a single DCC-type reactor, and the W/O and W packing material of the hybrid FGC system were 69.5% and 72.3%, respectively. At the 3.5 L/G ratio, the difference in SO2 removal effect by condition compared to the 1.5 L/G ratio was reduced to approximately 3% to 4%. At low L/G ratios, the amount of water injected is low; thus, the influence of the ICC-type reactor and packing material appears significantly; however, at high L/G ratios, sufficient amounts of water and SO2 are removed; therefore, the difference in the SO2 removal efficiency is not significant. At a high L/G ratio, the amount of wastewater produced increases proportionally; therefore, selecting an appropriate L/G ratio is crucial for efficient FGC operation. The SO2 removal effect of the W/O packing material case increased linearly up to an L/G ratio of 3.0, and the removal rate decreased. In the other two cases, the increase in SO2 removal efficiency decreases as the L/G ratio increases. Furthermore, there is a limit to highly efficient SO2 removal (>90%) using only water in the FGC system.
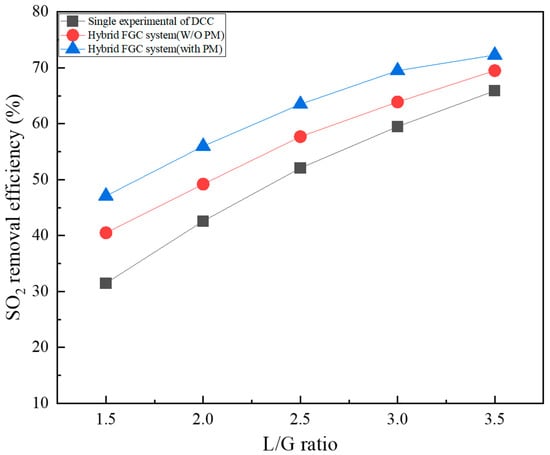
Figure 4.
SO2 removal efficiency of the hybrid FGC system.
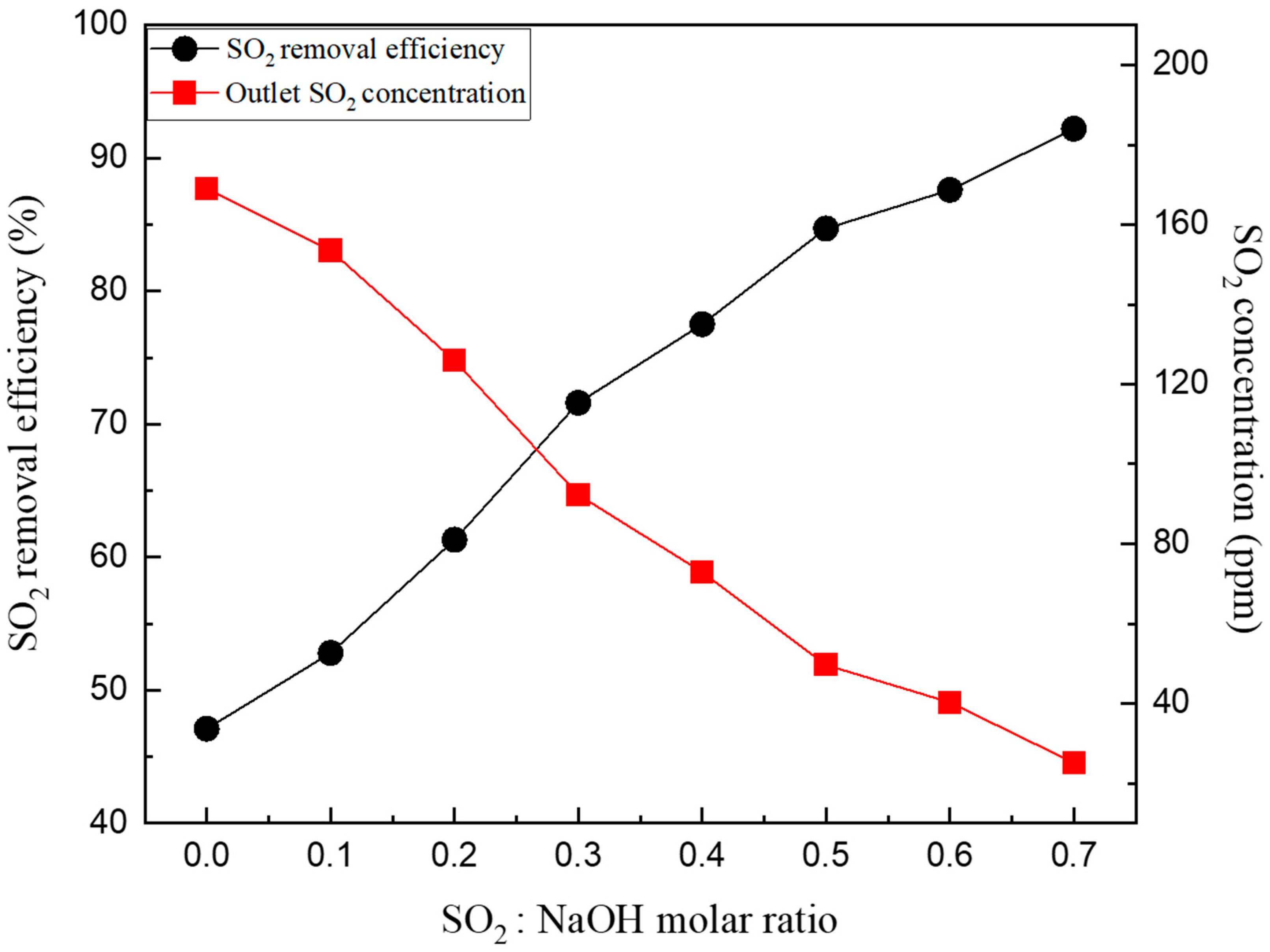
Figure 5 shows the results of SO2 removal characteristics according to the influence of the molar ratio of the additive under an L/G ratio of 1.5. The molar ratio of the additive of 0 is the result of an L/G ratio of packing material W of 1.5 in Figure 4. As the molar ratio of the additive increased from 0.1 to 0.7, the SO2 removal efficiency increased from 40% to 92%. Under the 0.1 molar ratio condition, the SO2 removal efficiency increased by 5% compared to the 0 molar ratio condition where pure water was added; however, when increased to 0.3, the SO2 reduction efficiency was 71.6%. At a molar ratio of 0.5 or higher, a SOx removal effect of approximately 80% was observed.
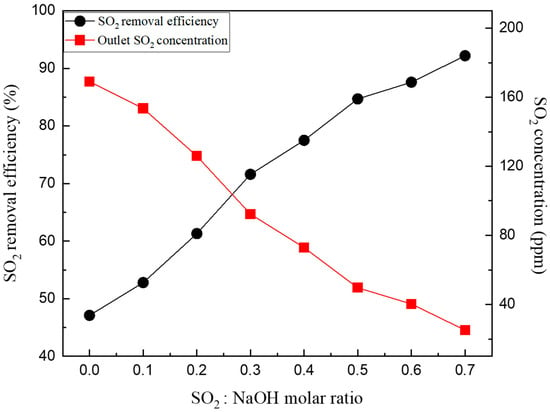
Figure 5.
SO2 removal characteristics according to the molar ratio of NaOH.
Table 4 presents a comparative analysis of desulfurization efficiency between the single DCC-type system and the hybrid FGC system based on the W or W/O of packing material and the molar ratio of additives. At an L/G ratio of 3.5, the single DCC-type system achieved a 65.9% reduction in SOx emissions. In contrast, the ICC–DCC hybrid FGC system demonstrated approximately 65% SOx removal efficiency at L/G ratios of 3 and 2.5, depending on the presence of packing material. The hybrid system reduced the L/G ratio by more than 1 compared to the single DCC-type system, thereby improving operational efficiency and reducing the consumption of additives and wastewater production. For instance, when alkaline absorbents were used, the hybrid system achieved ~65% SOx removal at an L/G ratio of 1.5 with a molar ratio between 0.2 and 0.3, and 92.2% removal at a molar ratio of 0.7. Notably, the ICC type facilitates the primary absorption of SO2 from the flue gases, which reduces the demand on the DCC type. As a result, high SOx removal efficiency can be achieved even at lower L/G ratios. The optimized supply of water and additives in the hybrid system not only reduces operating costs but also maximizes efficiency. This system improves both the SO2 removal and heat recovery efficiency, significantly enhancing the overall system performance.

Table 4.
SO2 removal efficiency of the hybrid FGC system under different experimental conditions.
4. Conclusions
To address the need for improving SO2 removal efficiency, along with the sensible and latent heat recovery characteristics, we investigated a system integrating ICC and DCC types of flue gas condensers. The experimental results showed that the SO2 removal efficiency increased during the ICC-type latent heat recovery process, where SO2 was absorbed by the water film on the tube surface. The key factors influencing the efficiency of the ICC type included the flue gas residence time, water vapor content, and cooling water flow rate, with increased flow improving both heat recovery and vapor condensation. In the hybrid FGC system, SO2 removal was achieved at a low L/G ratio compared to that in the single DCC type. When NaOH was used as an absorbent, the SO2 removal efficiency exceeded 90% at an L/G ratio of 1.5 and a NaOH-to-SO2 molar ratio >0.7. Despite the removal of SO2 in the ICC type, chemical absorbents were still required to achieve high-efficiency desulfurization.
The hybrid FGC system demonstrated an effective SO2 removal and heat recovery performance, particularly at low L/G ratios. However, the condensation process in the ICC type presents a risk of low-temperature corrosion on heat exchanger surfaces due to the formation of acidic compounds such as sulfuric acid. To ensure long-term operational stability and heat exchanger durability, manufacturing these components from corrosion-resistant materials (e.g., AL-6XN, stainless steel alloys, Teflon coatings, glass-lined tubes) is necessary. Furthermore, for long-term reliability, appropriate selection of these corrosion-resistant materials is essential to mitigate material degradation, reduce maintenance requirements, and lower overall operational costs.
Author Contributions
Conceptualization, W.Y. and Y.L.; methodology, Y.L.; investigation, H.C.; resources, Y.L.; data curation, H.C.; writing—original draft preparation, H.C.; writing—review and editing, Y.L.; visualization, H.C.; supervision, C.R.; project administration, W.Y.; funding acquisition, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant number [20193410100050].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This study was supported by a Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean Government (MOTIE) (20193410100050, development of new process for low temperature denitrification from coal combustion exhaust gas treatment).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Abbreviations | ||
| L/G | liquid-to-gas ratio | |
| PM | packing material | |
| W | with | |
| W/O | with out | |
| Symbols | ||
| cp | specific heat capacity | [kJ/kg/K] |
| C | SO2 concentration | [ppm] |
| D | humidity ratio | |
| H | specific enthalpy | [kJ/kg] |
| Q | heat transfer rate | [kW] |
| r | latent heat of water vaporization | [kJ/kg] |
| T | temperature | [°C] |
| Greek | ||
| SO2 removal efficiency | [%] | |
| Subscripts | ||
| 0 | reference state | |
| a | dry flue gas | |
| D | DCC | |
| dp | dew point | |
| fg | flue gas | |
| g | gas | |
| H | hybrid | |
| in | inlet | |
| I | ICC | |
| l | liquid | |
| out | outlet | |
| v | vapor |
References
- Gao, D.; Li, Z.H.; Zhang, H.; Chen, H.; Cheng, C.; Liang, K. Moisture and latent heat recovery from flue gas by nonporous organic membranes. J. Clean. Prod. 2019, 225, 1065–1078. [Google Scholar] [CrossRef]
- Bacon, G.H.; Li, R.; Liang, K.Y. Control particulate and metal HAPs. Chem. Eng. Prog. 1997, 93, 59–67. [Google Scholar]
- Vehlow, J. Air pollution control systems in WtE units: An overview. Waste Manag. 2015, 37, 58–74. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jozewicz, W. Flue gas desulfurization: The state of the art. J. Air Waste Manag. Assoc. 2001, 51, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Riffat, S.; Bai, H.; Zheng, X.; Reay, D. Recent progress in liquid desiccant dehumidification and air conditioning: A review. Energy Built. Environ. 2020, 1, 106–130. [Google Scholar] [CrossRef]
- Osakabe, M.; Yagi, K.; Itoh, T.; Ohmasa, K. Condensation heat transfer on tubes in actual flue gas (Parametric study for condensation behavior). Heat Transf. —Asian Res. Co-Spons. By Soc. Chem. Eng. Jpn. Heat Transf. Div. ASME 2003, 32, 153–166. [Google Scholar] [CrossRef]
- Osakabe, M.; Itoh, T.; Yagi, K. Condensation heat transfer of actual flue gas on horizontal tubes. Heat Transf. Asian Res. 1999, 32, 153–166. [Google Scholar] [CrossRef]
- Osakabe, M.; Itoh, T.; Ohmasa, K. Condensation heat transfer on spirally finned tubes in actual flue gas. J. Mar. Eng. Soc. Jpn 2000, 35, 260–267. [Google Scholar] [CrossRef][Green Version]
- Cui, L.; Song, X.; Li, Y.; Wang, Y.; Feng, Y.; Yan, L.; Dong, Y. Synergistic capture of fine particles in wet flue gas through cooling and condensation. Appl. Energy 2018, 225, 656–667. [Google Scholar] [CrossRef]
- Grigonyte, J.; Nuutinen, I.; Koponen, T.; Lamberg, H.; Tissari, J.; Jokiniemi, J.; Sippula, O. Evaluation of a heat exchanger designed for efficient fine particle precipitation in small-scale wood combustion. Energy Fuels 2014, 28, 6058–6065. [Google Scholar] [CrossRef]
- Jia, L.; Peng, X.F.; Sun, J.D.; Chen, T.B. An experimental study on vapor condensation of wet flue gas in a plastic heat exchanger. Heat Transf.—Asian Res. Co-Spons. By Soc. Chem. Eng. Jpn. Heat Transf. Div. ASME 2001, 30, 571–580. [Google Scholar] [CrossRef]
- Jeong, K.; Kessen, M.J.; Bilirgen, H.; Levy, E.K. Analytical modeling of water condensation in condensing heat exchanger. Int. J. Heat Mass Transf. 2010, 53, 2361–2368. [Google Scholar] [CrossRef]
- Jeong, K.; Levy, E.K. Theoretical prediction of sulfuric acid condensation rates in boiler flue gas. Int. J. Heat Mass Transf. 2012, 55, 8010–8019. [Google Scholar] [CrossRef]
- Jiao, F.; Cheng, Y.; Zhang, L.; Yamada, N.; Sato, A.; Ninomiya, Y. Effects of HCl, SO2 and H2O in flue gas on the condensation behavior of Pb and Cd vapors in the cooling section of municipal solid waste incineration. Proc. Combust. Inst. 2011, 33, 2787–2793. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Yamada, N.; Sato, A.; Ninomiya, Y. Effect of HCl, SO2 and H2O on the condensation of heavy metal vapors in flue gas cooling section. Fuel Process. Technol. 2013, 105, 181–187. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, X.; Li, Y. Review of flue gas acid dew-point and related low temperature corrosion. J. Energy Inst. 2020, 93, 1666–1677. [Google Scholar] [CrossRef]
- Butcher, T.A.; Litzke, W. Condensing Economizers for Small Coal-Fired Boilers and Furnaces; Brookhaven National Lab. (BNL): Upton, NY, USA, 1994. [Google Scholar]
- Li, Z.; Sun, F.; Shi, Y.; Li, F.; Ma, L. Experimental study and mechanism analysis on low temperature corrosion of coal fired boiler heating surface. Appl. Therm. Eng. 2015, 80, 355–361. [Google Scholar] [CrossRef]
- Zhong, W.; Ji, W.; Cao, X.; Yuan, Y. Flue gas water recovery by indirect cooling technology for large-scale applications: A review. J. Therm. Sci. 2020, 29, 1223–1241. [Google Scholar] [CrossRef]
- Hou, J.; Che, D.; Liu, Y.; Jiang, Q. A new system of absorption heat pump vs. boiler for recovering heat and water vapor in flue gas. Energy Procedia 2018, 152, 1266–1271. [Google Scholar] [CrossRef]
- Hrastel, I.; Gerbec, M.; Stergařsek, A. Technology optimization of wet flue gas desulphurization process. Chem. Eng. Technol. 2007, 30, 220–233. [Google Scholar] [CrossRef]
- Dunning, S.; Katz, L.S. Energy Calculations & Problem Solving Sourcebook: A Practical Guide for the Certified Energy Manager Exam, 1st ed.; The Fairmont Press, Inc.: Lilburn, Georgia, 2017. [Google Scholar]
- Gellings, C.W. Efficient Use and Conservation of Energy; Eolss Publishers, Co. Ltd.: Oxford, UK, 2009; Volume I. [Google Scholar]
- Rahmani, F.; Mowla, D.; Karimi, G.; Golkhar, A.; Rahmatmand, B. SO2 removal from simulated flue gas using various aqueous solutions: Absorption equilibria and operational data in a packed column. Sep. Purif. Technol. 2015, 153, 162–169. [Google Scholar] [CrossRef]
- Maalouf, S.; Ksayer, E.B.; Clodic, D. Investigation of direct contact condensation for wet flue-gas waste heat recovery using Organic Rankine Cycle. Energy Convers. Manag. 2016, 107, 96–102. [Google Scholar] [CrossRef]
- Zhang, L.; Zhai, H.; He, J.; Yang, F.; Wang, S. Application of exergy analysis in flue gas condensation waste heat recovery system evaluation. Energies 2022, 15, 7525. [Google Scholar] [CrossRef]
- Cui, L.; Lu, J.; Song, X.; Tang, L.; Li, Y.; Dong, Y. Energy conservation and efficiency improvement by coupling wet flue gas desulfurization with condensation desulfurization. Fuel 2021, 285, 119209. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Shuangchen, M.; Jin, C.; Kunling, J.; Lan, M.; Sijie, Z.; Kai, W. Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renew. Sustain. Energy Rev. 2017, 73, 225–235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).