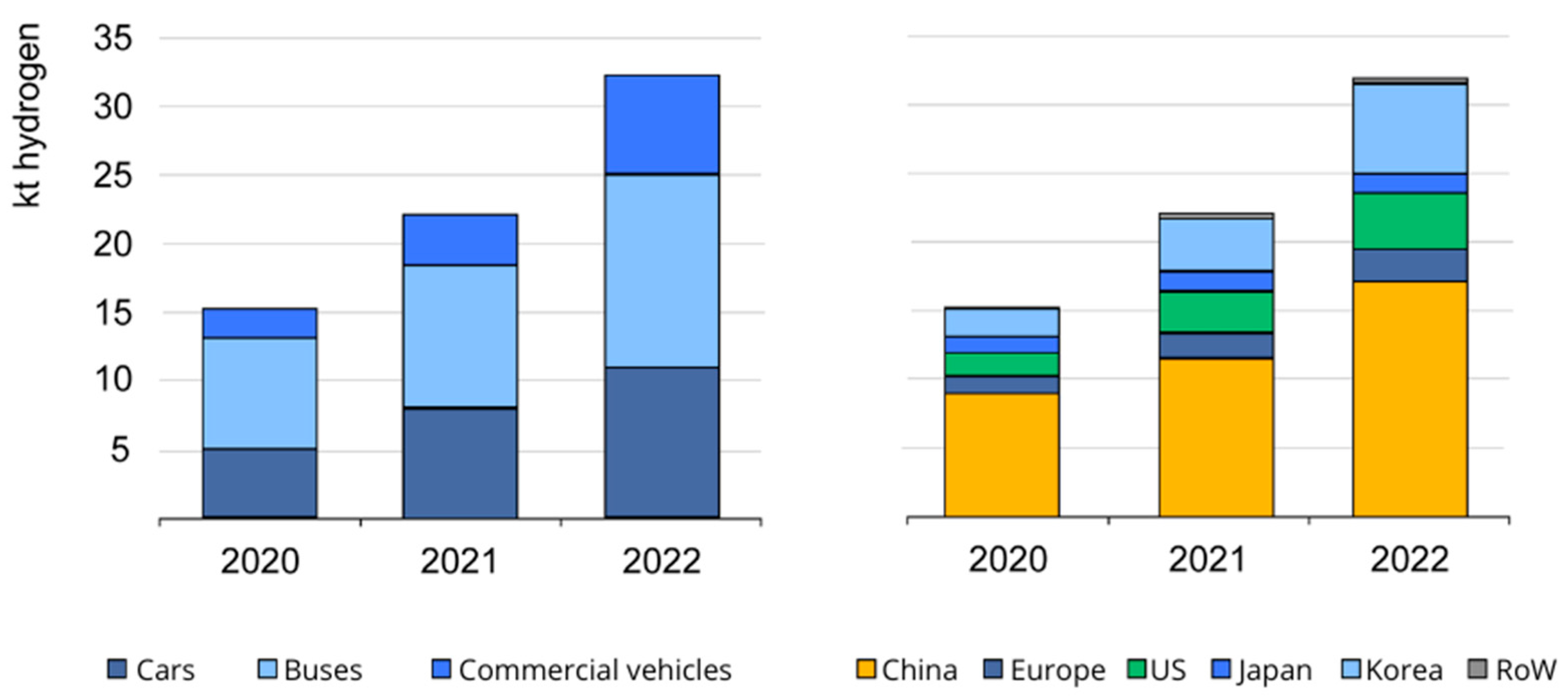
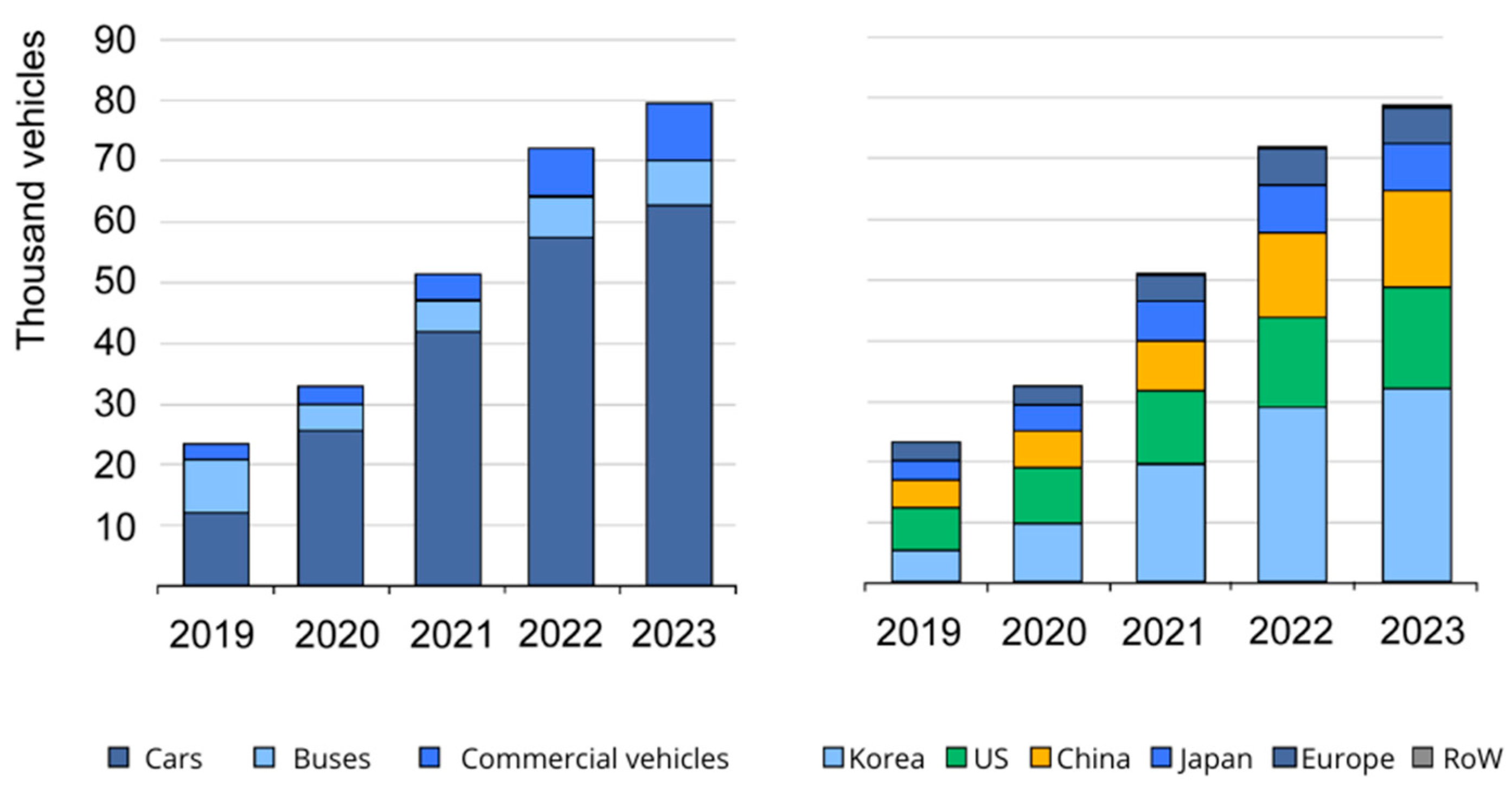
Hydrogen-Powered Vehicles: A Paradigm Shift in Sustainable Transportation
Abstract
1. Introduction
2. Hydrogen: The Ultimate Energy Carrier
3. Fuel Cells: Technological Challenges
3.1. General Comments
3.2. Alternative Catalysts for Hydrogen Production
3.2.1. Transition Metal Catalysts
3.2.2. Metal–Organic Frameworks (MOFs)
3.2.3. Single-Atom Catalysts (SACs)
3.2.4. Enzyme Mimetic Catalysts
3.2.5. Photocatalysts for Solar-Powered Hydrogen Production
3.3. Hydrogen Storage Solutions
3.3.1. Compressed Gas Storage
3.3.2. Liquid Hydrogen Storage
3.3.3. Metal Hydrides
3.3.4. Chemical Hydrogen Storage
3.3.5. Adsorption-Based Storage
3.3.6. Solid-State Hydrogen Storage
4. Preferred Criteria for Hydrogen Storage in Automotives and the Possibility of Using It in Microgrids
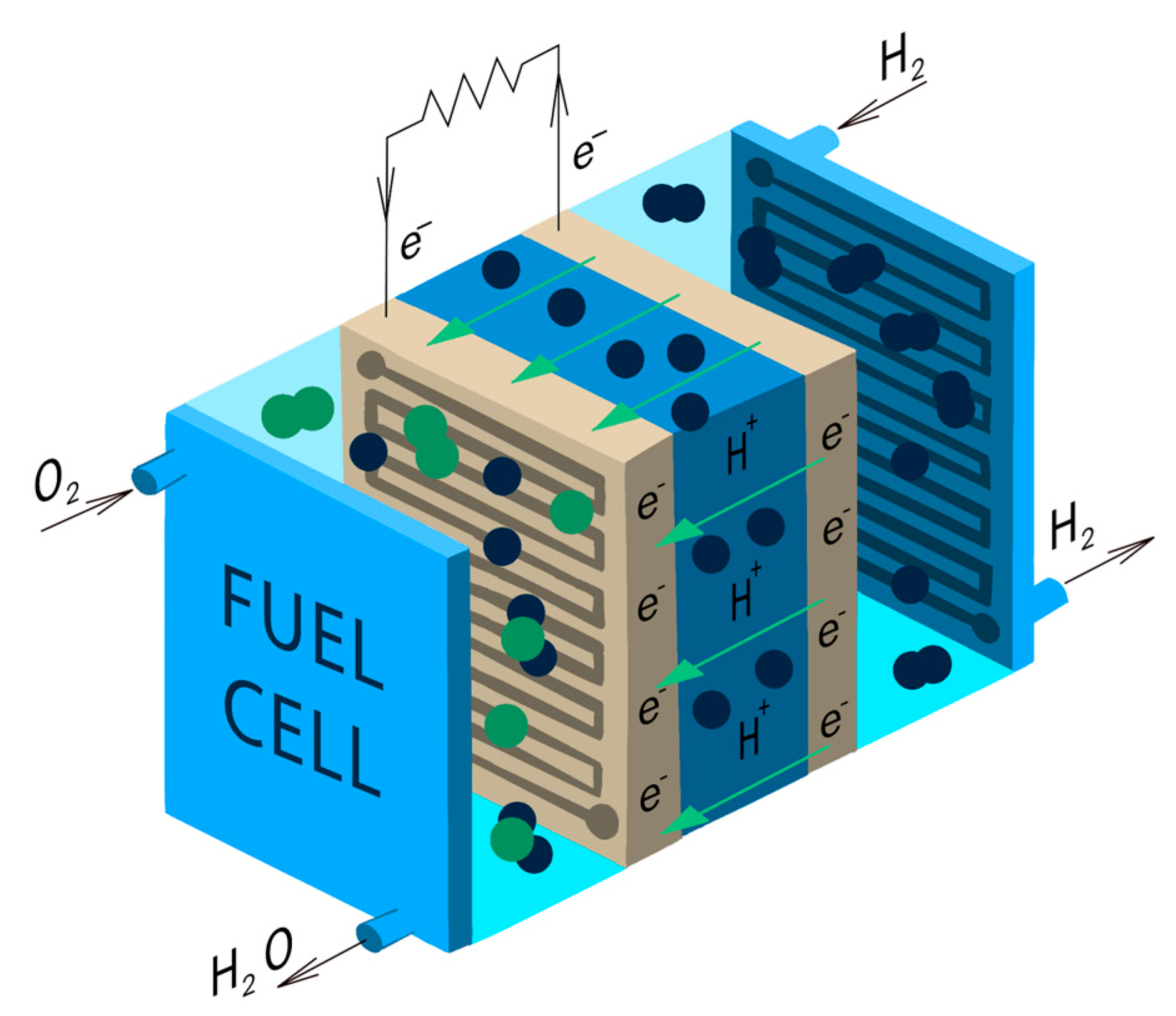
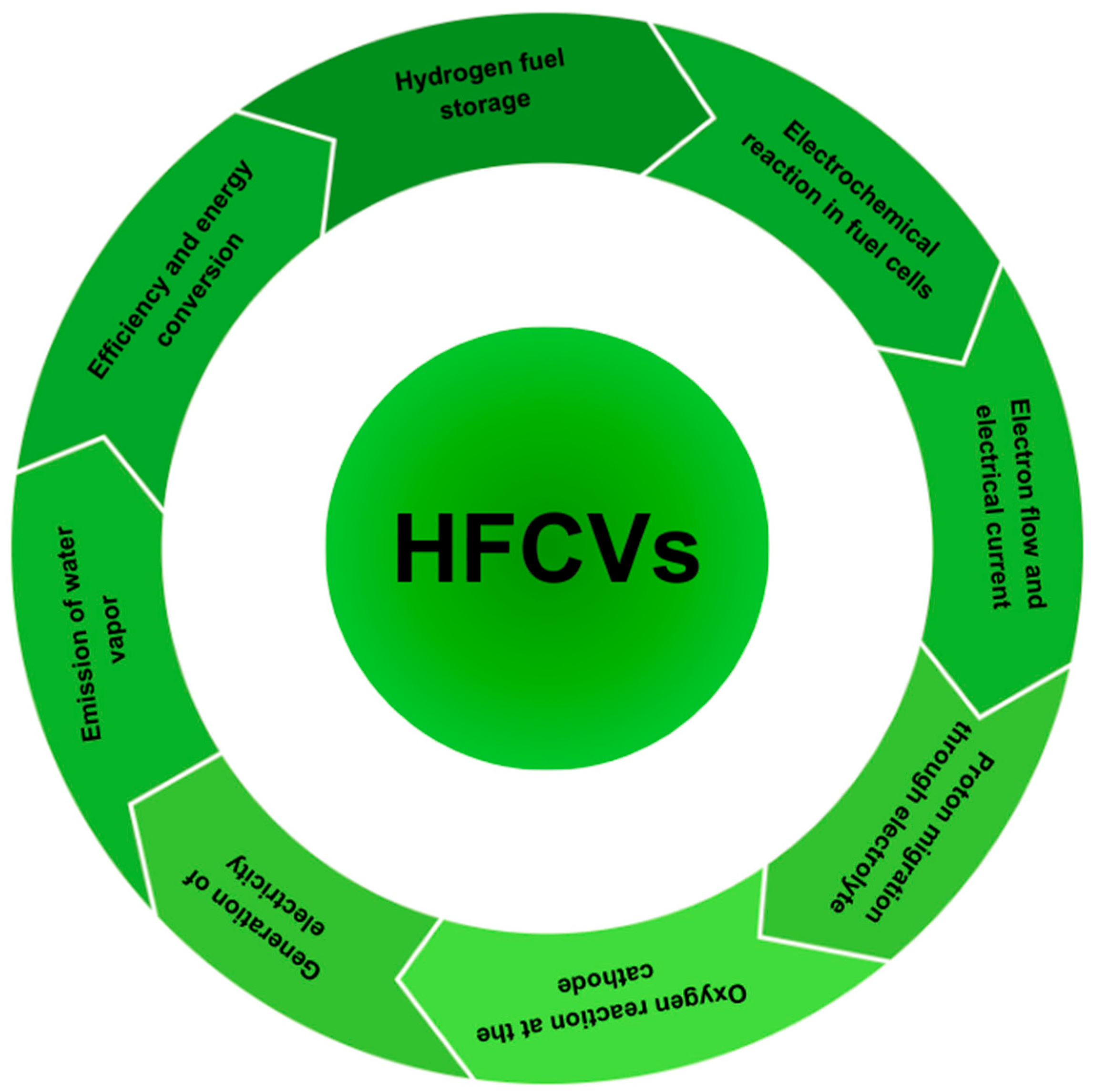
5. The Operational Sequence of HFCVs
- Hydrogen fuel storage: HFCVs store hydrogen gas in high-pressure tanks. The hydrogen can be produced through various methods, such as electrolysis or reforming of natural gas.
- Electrochemical reaction in fuel cells: the core component of a hydrogen fuel cell vehicle (HFCV) is the fuel cell stack, made up of numerous individual fuel cells. Each fuel cell comprises an anode and a cathode separated by an electrolyte membrane, often made of proton exchange membrane (PEM) or alkaline materials. Hydrogen gas is fed to the anode, where it undergoes a chemical reaction known as electrolysis. During this reaction, hydrogen molecules are divided into protons (H+) and electrons (e−).
- Electron flow and electrical current: the electrons produced in the anode side cannot traverse the electrolyte membrane, so they are forced to travel through an external circuit, generating an electric current that can be harnessed for electrical power.
- Proton migration through electrolyte: simultaneously, protons migrate through the electrolyte membrane to the cathode side.
- Oxygen reaction at the cathode: on the cathode side, oxygen from the air is introduced, and it reacts with electrons and protons that have traveled through the external circuit. This process produces water (H2O) as a waste product.
- Generation of electricity: the combination of electrons flowing through the external circuit and protons migrating through the electrolyte creates an electric current. This electrical energy can be utilized to power the electric motor of the vehicle, providing the necessary propulsion.
- Emission of water vapor: the only direct emission from the hydrogen fuel cell vehicle is water vapor, making HFCVs environmentally friendly. The overall reaction in the fuel cell can be represented as: 2H2 + O2 → 2H2O.
- Efficiency and energy conversion: hydrogen fuel cells are known for their high efficiency in converting chemical energy into electricity. The energy efficiency of HFCVs is generally higher compared to internal combustion engine vehicles, and they offer the advantage of zero tailpipe emissions.
- ΔH—the enthalpy change,
- T—the absolute temperature,
- ΔS—the entropy change.
- n—the number of electrons transferred per molecule of hydrogen (in this case, n = 2),
- F—the Faraday constant,
- E—the cell potential.
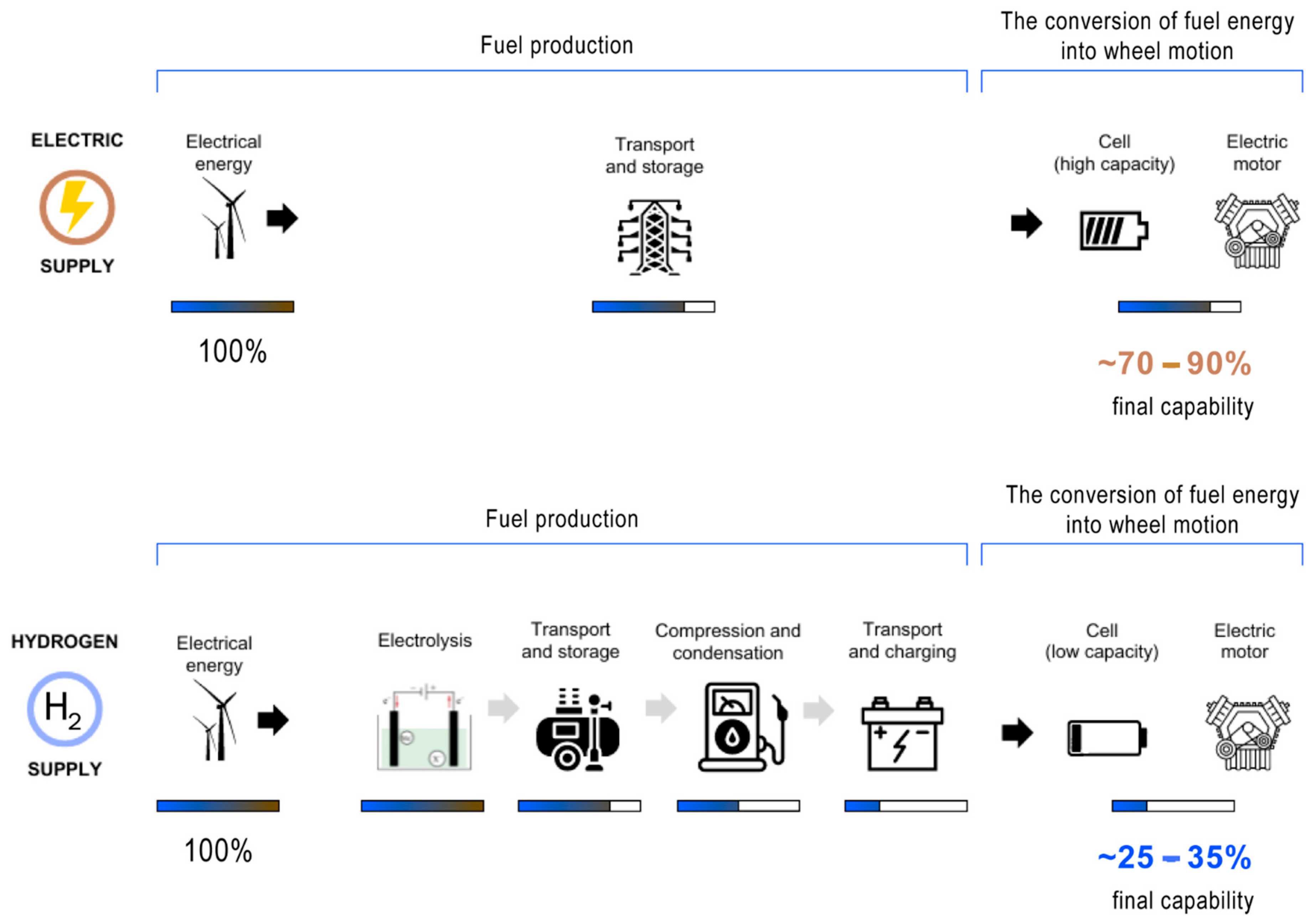
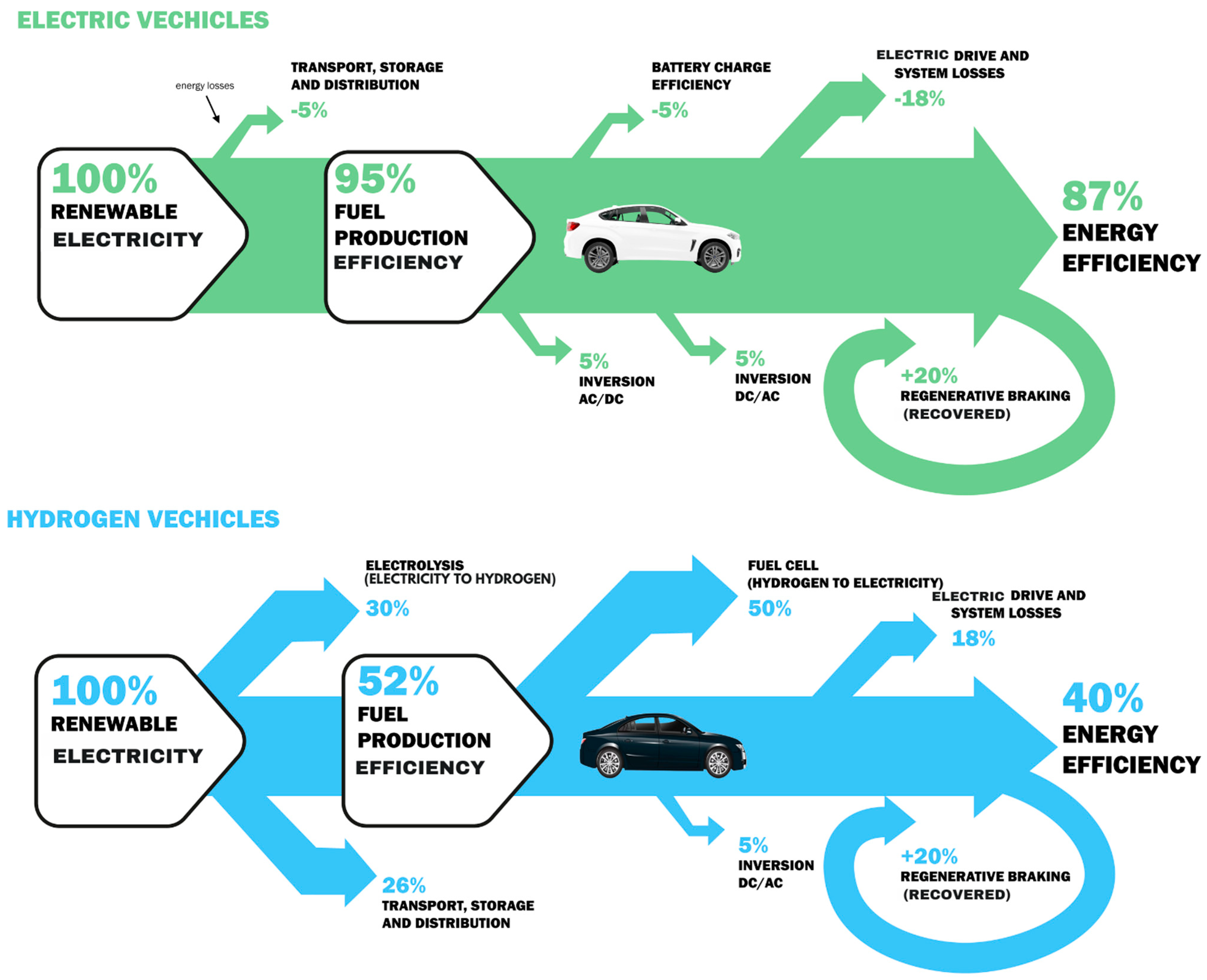
6. Distinguishing Electric and Hydrogen Cars
6.1. Energy Storage
- Electric cars: rely on batteries to store electrical energy,
- Hydrogen cars: utilize fuel cells to convert hydrogen into electrical energy.
6.2. Dexterity
- Electric cars: efficient in converting stored energy into motion,
- Hydrogen cars: exhibit high efficiency, particularly in long-range applications.
6.3. Reception
- Electric cars: widely adopted globally, with increasing popularity,
- Hydrogen cars: still in the early stages of adoption, with limited availability.
6.4. Price and Operating Costs
- Electric cars: generally more affordable, with lower operating costs,
- Hydrogen cars: tend to have higher upfront costs, but operating costs may vary based on factors like fuel prices.
6.5. Refueling
- Electric cars: recharged through power outlets or charging stations,
- Hydrogen cars: refueled at specialized hydrogen refueling stations.
- Electric cars: benefit from a more established charging infrastructure, with widespread availability of charging stations,
- Hydrogen cars: face challenges in terms of the limited availability of hydrogen refueling stations.
7. Cars with Alternative Propulsion—Fuel Cells
8. Storage and Distribution Challenges
- Low energy density and storage pressures
- Material compatibility and embrittlement
- Cost and weight of storage systems
- Infrastructure investment
- Transportation logistics
- Safety concerns
- Hydrogen production proximity
- Scaling up production
- International standardization
9. Hydrogen Vehicle Infrastructure and Investment Strategies
10. Economic and Social Aspects of Using Hydrogen in Vehicles
- Manufacturing and development costs
- Fuel costs and availability
- Infrastructure costs
- Maintenance and operating costs
- Economic impact on the energy sector
- Environmental impact
- Energy security and geopolitical changes
- Job creation and economic opportunities
- Public health benefits
- Market adoption and consumer behavior
11. Key Startups and Patents in Hydrogen Fuel Cell Technology
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishimoto, Y.; Voldsund, M.; Nekså, P.; Roussanaly, S.; Berstad, D.; Gardarsdottir, S.O. Large-Scale Production and Transport of Hydrogen from Norway to Europe and Japan: Value Chain Analysis and Comparison of Liquid Hydrogen and Ammonia as Energy Carriers. Int. J. Hydrogen Energy 2020, 45, 32865–32883. [Google Scholar] [CrossRef]
- Merkisz, J.; Pielecha, J.; Lijewski, P.; Merkisz-Guranowska, A.; Nowak, M. Exhaust Emissions from Vehicles in Real Traffic Conditions in the Poznan Agglomeration. In Air Pollution XXI; Wit Press: Southampton, UK, 2013; Volume 174, pp. 27–38. [Google Scholar] [CrossRef]
- Merkisz, J.; Jacyna, M.; Merkisz-Guranowska, A.; Pielecha, J. The Parameters of Passenger Cars Engine in Terms of Real Drive Emission Test. Arch. Transp. 2014, 32, 43–50. [Google Scholar] [CrossRef][Green Version]
- Kamińska, M.; Rymaniak, Ł.; Lijewski, P.; Szymlet, N.; Daszkiewicz, P.; Grzeszczyk, R. Investigations of Exhaust Emissions from Rail Machinery during Track Maintenance Operations. Energies 2021, 14, 3141. [Google Scholar] [CrossRef]
- Szymlet, N.; Rymaniak, Ł.; Kurc, B. Chromatographic Analysis of the Chemical Composition of Exhaust Gas Samples from Urban Two-Wheeled Vehicles. Energies 2024, 17, 709. [Google Scholar] [CrossRef]
- Ziółkowski, A.; Fuć, P.; Jagielski, A.; Bednarek, M.; Konieczka, S. Comparison of the Energy Consumption and Exhaust Emissions between Hybrid and Conventional Vehicles, as Well as Electric Vehicles Fitted with a Range Extender. Energies 2023, 16, 4669. [Google Scholar] [CrossRef]
- Turoń, K. Hydrogen-Powered Vehicles in Urban Transport Systems–Current State and Development. Transp. Res. Procedia 2020, 45, 835–841. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel Cell Application in the Automotive Industry and Future Perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Ananthachar, V.; Duffy, J.J. Efficiencies of Hydrogen Storage Systems Onboard Fuel Cell Vehicles. Sol. Energy 2005, 78, 687–694. [Google Scholar] [CrossRef]
- Bairabathina, S.; Balamurugan, S. Review on Non-Isolated Multi-Input Step-up Converters for Grid-Independent Hybrid Electric Vehicles. Int. J. Hydrogen Energy 2020, 45, 21687–21713. [Google Scholar] [CrossRef]
- Cleveland, C.J.; Morris, C.G. Handbook of Energy: Diagrams, Charts, and Tables; Elsevier: Newnes, NSW, Australia, 2013; Volume 1, ISBN 0-08-091457-8. [Google Scholar]
- Balchin, J. Quantum Leaps: 100 Scientists Who Changed the World; Arcturus Publishing: London, UK, 2013; ISBN 1-78404-092-4. [Google Scholar]
- NASA. Available online: https://climate.nasa.gov/news/788/nasa-exploring-space-applications-of-hydrogen-and-fuel-cells (accessed on 25 May 2024).
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High Temperature (HT) Polymer Electrolyte Membrane Fuel Cells (PEMFC)—A Review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Polymers Application in Proton Exchange Membranes for Fuel Cells (PEMFCs). Phys. Sci. Rev. 2017, 2, 20170018. [Google Scholar] [CrossRef]
- Wu, D.; Peng, C.; Yin, C.; Tang, H. Review of System Integration and Control of Proton Exchange Membrane Fuel Cells. Electrochem. Energy Rev. 2020, 3, 466–505. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.; Brandon, N.P. Hydrogen and Fuel Cells: Towards a Sustainable Energy Future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Brimblecombe, P. Air Composition and Chemistry; Cambridge University Press: Cambridge, UK, 1996; ISBN 0-521-45972-9. [Google Scholar]
- Birol, F. The Future of Hydrogen: Seizing Today’s Opportunities; IEA Report Prepared for the G; OECD: Paris, France, 2019; Volume 20. [Google Scholar]
- Ni, Y.; Han, Z.; Chai, Y.; Wu, G.; Li, L. Catalytic Hydrogen Storage in Liquid Hydrogen Carriers. EES Catal. 2023, 1, 459–494. [Google Scholar] [CrossRef]
- Chen, L.; Verma, P.; Hou, K.; Qi, Z.; Zhang, S.; Liu, Y.-S.; Guo, J.; Stavila, V.; Allendorf, M.D.; Zheng, L. Reversible Dehydrogenation and Rehydrogenation of Cyclohexane and Methylcyclohexane by Single-Site Platinum Catalyst. Nat. Commun. 2022, 13, 1092. [Google Scholar] [CrossRef]
- Du, J.; Chang, S.; Song, J.; Zhao, J.; Zhu, Z. Cyclohexane Dehydrogenation over the Platinum Catalysts Supported on Carbon Nanomaterials. J. Fuel Chem. Technol. 2009, 37, 468–472. [Google Scholar] [CrossRef]
- Wang, B.; Froment, G.F.; Goodman, D.W. CO-Free Hydrogen Production via Dehydrogenation of a Jet A Hydrocarbon Mixture. J. Catal. 2008, 253, 239–243. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, Y.; Jia, Z.; Liu, J.-C.; Guo, J.; Cai, X.; Dong, C.; Wang, M.; Li, C.; Diao, J.; et al. Few-Atom Pt Ensembles Enable Efficient Catalytic Cyclohexane Dehydrogenation for Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 3535–3542. [Google Scholar] [CrossRef]
- Wang, B.; Goodman, D.W.; Froment, G.F. Kinetic Modeling of Pure Hydrogen Production from Decalin. J. Catal. 2008, 253, 229–238. [Google Scholar] [CrossRef]
- Peng, M.; Dong, C.; Gao, R.; Xiao, D.; Liu, H.; Ma, D. Fully Exposed Cluster Catalyst (FECC): Toward Rich Surface Sites and Full Atom Utilization Efficiency. ACS Cent. Sci. 2021, 7, 262–273. [Google Scholar] [CrossRef]
- Lu, S.; Yang, H.; Zhou, Z.; Zhong, L.; Li, S.; Gao, P.; Sun, Y. Effect of In2O3 Particle Size on CO2 Hydrogenation to Lower Olefins over Bifunctional Catalysts. Chin. J. Catal. 2021, 42, 2038–2048. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Djitcheu, X.; He, D.; Liu, H.; Zhang, Q. Techniques for the Characterization of Single Atom Catalysts. RSC Adv. 2022, 12, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Somekawa, S.; Watanabe, H.; Ono, Y.; Oaki, Y.; Imai, H. Preparation of Titania with Double Band Structure Derived from a Quantum Size Effect: Drastic Increase in the Photocatalytic Activity. Mater. Lett. 2021, 304, 130609. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Pan, Z.; Zhang, R.; Gao, Z.; Wang, G.; Huang, K.; Mu, X.; Bai, F.; Wang, Y. Visible-Light-Driven Non-Oxidative Dehydrogenation of Alkanes at Ambient Conditions. Nat. Energy 2022, 7, 1042–1051. [Google Scholar] [CrossRef]
- Kang, J.S.; Baek, J.Y.; Hwang, H.; Shin, H.S.; Yoon, C.W.; Shin, H.-J. Photocatalytic Dehydrogenation of Organic Hydrogen Carrier on Pd-TiO2 (110) Surfaces. J. Mater. Chem. A 2022, 10, 22701–22706. [Google Scholar] [CrossRef]
- Al-ShaikhAli, A.H.; Jedidi, A.; Cavallo, L.; Takanabe, K. Non-Precious Bimetallic Catalysts for Selective Dehydrogenation of an Organic Chemical Hydride System. Chem. Commun. 2015, 51, 12931–12934. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen Storage Methods: Review and Current Status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen Storage Technologies for Future Energy Systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The Survey of Key Technologies in Hydrogen Energy Storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of Hydrogen Storage Techniques for on Board Vehicle Applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Todorovic, R. Hydrogen Storage Technologies for Transportation Application. J. Undergrad. Res. 2015, 5, 56–59. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Jorgensen, S.W. Hydrogen Storage Tanks for Vehicles: Recent Progress and Current Status. Curr. Opin. Solid State Mater. Sci. 2011, 15, 39–43. [Google Scholar] [CrossRef]
- Hosgormez, H.; Etiope, G.; Yalçin, M. New Evidence for a Mixed Inorganic and Organic Origin of the Olympic Chimaera Fire (Turkey): A Large Onshore Seepage of Abiogenic Gas. Geofluids 2008, 8, 263–273. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Cissé, C.S.T.; Diallo, A.B. Discovery of a Large Accumulation of Natural Hydrogen in Bourakebougou (Mali). Int. J. Hydrogen Energy 2018, 43, 19315–19326. [Google Scholar] [CrossRef]
- Fuel Cells Works. Petroma Inc. Successfully produces electricity using natural hydrogen. Fuel Cells Works, 30 November 2019.
- Lollar, B.S.; Onstott, T.C.; Lacrampe-Couloume, G.; Ballentine, C. The Contribution of the Precambrian Continental Lithosphere to Global H2 Production. Nature 2014, 516, 379–382. [Google Scholar] [CrossRef]
- Das, L. On-Board Hydrogen Storage Systems for Automotive Application. Int. J. Hydrogen Energy 1996, 21, 789–800. [Google Scholar] [CrossRef]
- Gomez, Y.A.; Oyarce, A.; Lindbergh, G.; Lagergren, C. Ammonia Contamination of a Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2018, 165, F189–F197. [Google Scholar] [CrossRef]
- Imamura, D.; Ebata, D.; Hshimasa, Y.; Akai, M.; Watanabe, S. Impact of Hydrogen Fuel Impurities on PEMFC Performance. SAE Trans. 2007, 116, 621–626. [Google Scholar]
- Zamel, N.; Li, X. Effect of Contaminants on Polymer Electrolyte Membrane Fuel Cells. Prog. Energy Combust. Sci. 2011, 37, 292–329. [Google Scholar] [CrossRef]
- KTVU FOX 2. Cause of Santa Clara hydrogen explosion and fire under investigation. KTVU FOX 2, 2 June 2019.
- Hazardex. Explosion at hydrogen fuel plant in US damages around 60 buildings. Hazardex, 8 April 2020.
- Lambert, F. Hydrogen station explodes, Toyota halts sales of fuel cell cars, is this the end. Electrek, 11 June 2019. [Google Scholar]
- Cunanan, C.; Tran, M.-K.; Lee, Y.; Kwok, S.; Leung, V.; Fowler, M. A Review of Heavy-Duty Vehicle Powertrain Technologies: Diesel Engine Vehicles, Battery Electric Vehicles, and Hydrogen Fuel Cell Electric Vehicles. Clean Technol. 2021, 3, 474–489. [Google Scholar] [CrossRef]
- Albatayneh, A.; Juaidi, A.; Jaradat, M.; Manzano-Agugliaro, F. Future of Electric and Hydrogen Cars and Trucks: An Overview. Energies 2023, 16, 3230. [Google Scholar] [CrossRef]
- Millo, F.; Caputo, S.; Piu, A. Analysis of a HT-PEMFC Range Extender for a Light Duty Full Electric Vehicle (LD-FEV). Int. J. Hydrogen Energy 2016, 41, 16489–16498. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chang, W.R. Life-Cycle Analysis of Greenhouse Gas Emission and Energy Efficiency of Hydrogen Fuel Cell Scooters. Int. J. Hydrogen Energy 2010, 35, 11947–11956. [Google Scholar] [CrossRef]
- Kim, H.; Hartmann, N.; Zeller, M.; Luise, R.; Soylu, T. Comparative Tco Analysis of Battery Electric and Hydrogen Fuel Cell Buses for Public Transport System in Small to Midsize Cities. Energies 2021, 14, 4384. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wu, F.-L.; Lai, W.-H.; Lai, M.-P. A Cost-Benefit Analysis of the Carbon Footprint with Hydrogen Scooters and Electric Scooters. Int. J. Hydrogen Energy 2016, 41, 13299–13307. [Google Scholar] [CrossRef]
- Hwang, J.J. Sustainable Transport Strategy for Promoting Zero-Emission Electric Scooters in Taiwan. Renew. Sustain. Energy Rev. 2010, 14, 1390–1399. [Google Scholar] [CrossRef]
- Halder, P.; Babaie, M.; Salek, F.; Haque, N.; Savage, R.; Stevanovic, S.; Bodisco, T.A.; Zare, A. Advancements in Hydrogen Production, Storage, Distribution and Refuelling for a Sustainable Transport Sector: Hydrogen Fuel Cell Vehicles. Int. J. Hydrogen Energy 2023, 52, 973–1004. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Li, Z.; Jiang, T.; Li, X. Two-Stage Robust Operation of Electricity-Gas-Heat Integrated Multi-Energy Microgrids Considering Heterogeneous Uncertainties. Appl. Energy 2024, 371, 123690. [Google Scholar] [CrossRef]
- Zheng, X.; Khodayar, M.E.; Wang, J.; Yue, M.; Zhou, A. Distributionally Robust Multistage Dispatch with Discrete Recourse of Energy Storage Systems. IEEE Trans. Power Syst. 2024, 2, 1–14. [Google Scholar] [CrossRef]
- Xia, W.; Ren, Z.; Qin, H.; Dong, Z. A Coordinated Operation Method for Networked Hydrogen-Power-Transportation System. Energy 2024, 296, 131026. [Google Scholar] [CrossRef]
- Plugpower. The Hydrogen Revolution: Reliability, Sustainability, and Grid Stability. Plug, 26 January 2024. Available online: https://www.plugpower.com/the-hydrogen-revolution-reliability-sustainability-and-grid-stability/ (accessed on 10 September 2024).
- Enatpter. The Lowdown on Microgrids. Enatpter, 29 November 2018. Available online: https://www.enapter.com/blog/the-lowdown-on-microgrids/ (accessed on 9 September 2024).
- Shahzad, S.; Abbasi, M.A.; Ali, H.; Iqbal, M.; Munir, R.; Kilic, H. Possibilities, Challenges, and Future Opportunities of Microgrids: A Review. Sustainability 2023, 15, 6366. [Google Scholar] [CrossRef]
- Uddin, M.; Mo, H.; Dong, D.; Elsawah, S.; Zhu, J.; Guerrero, J.M. Microgrids: A Review, Outstanding Issues and Future Trends. Energy Strategy Rev. 2023, 49, 101127. [Google Scholar] [CrossRef]
- Allwyn, R.G.; Al-Hinai, A.; Margaret, V. A Comprehensive Review on Energy Management Strategy of Microgrids. Energy Rep. 2023, 9, 5565–5591. [Google Scholar] [CrossRef]
- Szałek, A.; Pielecha, I.; Cieslik, W. Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC). Energies 2021, 14, 5018. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, D.; Wang, J.; Tu, W.; Wu, D.; Koh, S.W.; Gao, P.; Xu, Z.J.; Deng, S.; Zhou, Y. Raw Biomass Electroreforming Coupled to Green Hydrogen Generation. Nat. Commun. 2021, 12, 2008. [Google Scholar] [CrossRef]
- Changala, P.B.; Nguyen, T.L.; Baraban, J.H.; Ellison, G.B.; Stanton, J.F.; Bross, D.H.; Ruscic, B. Active Thermochemical Tables: The Adiabatic Ionization Energy of Hydrogen Peroxide. J. Phys. Chem. A 2017, 121, 8799–8806. [Google Scholar] [CrossRef]
- Deng, B.; Zhou, L.; Jiang, Z.; Jiang, Z.-J. High Catalytic Performance of Nickel Foam Supported Co2P-Ni2P for Overall Water Splitting and Its Structural Evolutions during Hydrogen/Oxygen Evolution Reactions in Alkaline Solutions. J. Catal. 2019, 373, 81–92. [Google Scholar] [CrossRef]
- Gurz, M.; Baltacioglu, E.; Hames, Y.; Kaya, K. The Meeting of Hydrogen and Automotive: A Review. Int. J. Hydrogen Energy 2017, 42, 23334–23346. [Google Scholar] [CrossRef]
- Liu, F.; Mauzerall, D.L.; Zhao, F.; Hao, H. Deployment of Fuel Cell Vehicles in China: Greenhouse Gas Emission Reductions from Converting the Heavy-Duty Truck Fleet from Diesel and Natural Gas to Hydrogen. Int. J. Hydrogen Energy 2021, 46, 17982–17997. [Google Scholar] [CrossRef]
- Berry, G.D.; Pasternak, A.D.; Rambach, G.D.; Smith, J.R.; Schock, R.N. Hydrogen as a Future Transportation Fuel. Energy 1996, 21, 289–303. [Google Scholar] [CrossRef]
- Pielecha, I.; Cieślik, W.; Szałek, A. Operation of Electric Hybrid Drive Systems in Varied Driving Conditions. Ekspolatacja Niezawodn. Maint. Reliab. 2018, 20, 16–23. [Google Scholar] [CrossRef]
- Buja, G.; Bertoluzzo, M.; Mude, K.N. Design and Experimentation of WPT Charger for Electric City Car. IEEE Trans. Ind. Electron. 2015, 62, 7436–7447. [Google Scholar] [CrossRef]
- Aminudin, M.; Kamarudin, S.; Lim, B.; Majilan, E.; Masdar, M.; Shaari, N. An Overview: Current Progress on Hydrogen Fuel Cell Vehicles. Int. J. Hydrogen Energy 2023, 48, 4371–4388. [Google Scholar] [CrossRef]
- Martins, L.S.; Guimarães, L.F.; Junior, A.B.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric Car Battery: An Overview on Global Demand, Recycling and Future Approaches towards Sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef]
- Patil, H.; Kalkhambkar, V.N. Grid Integration of Electric Vehicles for Economic Benefits: A Review. J. Mod. Power Syst. Clean Energy 2020, 9, 13–26. [Google Scholar] [CrossRef]
- Ahmadi, P.; Khoshnevisan, A. Dynamic Simulation and Lifecycle Assessment of Hydrogen Fuel Cell Electric Vehicles Considering Various Hydrogen Production Methods. Int. J. Hydrogen Energy 2022, 47, 26758–26769. [Google Scholar] [CrossRef]
- Brenna, M.; Foiadelli, F.; Leone, C.; Longo, M. Electric Vehicles Charging Technology Review and Optimal Size Estimation. J. Electr. Eng. Technol. 2020, 15, 2539–2552. [Google Scholar] [CrossRef]
- Sendek-Matysiak, E.; Pyza, D. Prospects for the Development of Electric Vehicle Charging Infrastructure in Poland in the Light of the Regulations in Force. Arch. Transp. 2021, 57, 43–58. [Google Scholar]
- Kast, J.; Morrison, G.; Gangloff, J.J., Jr.; Vijayagopal, R.; Marcinkoski, J. Designing Hydrogen Fuel Cell Electric Trucks in a Diverse Medium and Heavy Duty Market. Res. Transp. Econ. 2018, 70, 139–147. [Google Scholar] [CrossRef]
- Williams, S.E.; Davis, S.C.; Boundy, R.G. Transportation Energy Data Book: Edition 36; Oak Ridge National Lab (ORNL): Oak Ridge, TN, USA, 2017. [Google Scholar]
- Pielecha, I.; Dimitrov, R.; Mihaylov, V. Energy Flow Analysis Based on a Simulated Drive of a Hybrid Locomotive Powered by Fuel Cells. Rail Veh. Pojazdy Szyn. 2022, 68. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Fuel Cell Power Systems for Maritime Applications: Progress and Perspectives. Sustainability 2021, 13, 1213. [Google Scholar] [CrossRef]
- Kast, J.; Vijayagopal, R.; Gangloff, J.J., Jr.; Marcinkoski, J. Clean Commercial Transportation: Medium and Heavy Duty Fuel Cell Electric Trucks. Int. J. Hydrogen Energy 2017, 42, 4508–4517. [Google Scholar] [CrossRef]
- Smith, J.R.; Aceves, S.M.; Johnson, N.L.; Amsden, A.A. Progress toward an Optimized Hydrogen Series Hybrid Engine; Technical Report; USDOE: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal Hydride Materials for Solid Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F.; Wagner, F.W. Challenges for Fuel Cells in Transport Applications. J. Power Sources 2000, 86, 40–51. [Google Scholar] [CrossRef]
- Zaetta, R.; Madden, B. Hydrogen Fuel Cell Bus Technology State of the Art Review; NextHyLights: Ottobrunn, Germany, 2013. [Google Scholar]
- Eudy, L.; Post, M.; Jeffers, M. Zero Emission Bay Area (ZEBA) Fuel Cell Bus Demonstration Results: Fifth Report; National Renewable Energy Lab (NREL): Golden, CO, USA, 2016. [Google Scholar]
- Folkesson, A.; Andersson, C.; Alvfors, P.; Alaküla, M.; Overgaard, L. Real Life Testing of a Hybrid PEM Fuel Cell Bus. J. Power Sources 2003, 118, 349–357. [Google Scholar] [CrossRef]
- Pramuanjaroenkij, A.; Kakaç, S. The Fuel Cell Electric Vehicles: The Highlight Review. Int. J. Hydrogen Energy 2023, 48, 9401–9425. [Google Scholar] [CrossRef]
- Genovese, M.; Schlüter, A.; Scionti, E.; Piraino, F.; Corigliano, O.; Fragiacomo, P. Power-to-Hydrogen and Hydrogen-to-X Energy Systems for the Industry of the Future in Europe. Int. J. Hydrogen Energy 2023, 48, 16545–16568. [Google Scholar] [CrossRef]
- Gallas, D.; Stobnicki, P. Adoption of Modern Hydrogen Technologies in Rail Transport. J. Ecol. Eng. 2022, 23, 84–91. [Google Scholar] [CrossRef]
- Cavaliere, P. Hydrogen Applications. In Water Electrolysis for Hydrogen Production; Springer: Berlin/Heidelberg, Germany, 2023; pp. 653–727. [Google Scholar]
- Brazzola, N.; Patt, A.; Wohland, J. Definitions and Implications of Climate-Neutral Aviation. Nat. Clim. Change 2022, 12, 761–767. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2023; International Energy Agency: Paris, France, 2023. [Google Scholar]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Pavlyuk, V.; Dmytriv, G.; Chumak, I.; Gutfleisch, O.; Lindemann, I.; Ehrenberg, H. High Hydrogen Content Super-Lightweight Intermetallics from the Li–Mg–Si System. Int. J. Hydrogen Energy 2013, 38, 5724–5737. [Google Scholar] [CrossRef]
- Burch, R.; Buss, R. Absorption of Hydrogen by Palladium–Copper Alloys. Part 1.—Experimental Measurements. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 913–921. [Google Scholar] [CrossRef]
- Baba, K.; Sakamoto, Y.; Flanagan, T.B.; Kuji, T.; Craft, A. Electrical Resistance Anomalies and Hydrogen Solubilities in the Disorder-Order System Pd/Sub 3/Mn. Scr. Metall. 1987, 21, 299–303. [Google Scholar] [CrossRef]
- Richardson, T.J.; Slack, J.L.; Farangis, B.; Rubin, M.D. Mixed Metal Films with Switchable Optical Properties. Appl. Phys. Lett. 2002, 80, 1349–1351. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y. Palladium–Silver Thin Film for Hydrogen Sensing. Sens. Actuators B Chem. 2007, 123, 101–106. [Google Scholar] [CrossRef]
- Hara, M.; Sakurai, J.; Akamaru, S.; Hashizume, K.; Nishimura, K.; Mori, K.; Okabe, T.; Watanabe, K.; Matsuyama, M. Thermodynamic and Magnetic Properties of Pd0. 93Ag0. 07 Hydride. Mater. Trans. 2007, 48, 3154–3159. [Google Scholar] [CrossRef]
- Vocaturo, R.; Tresca, C.; Ghiringhelli, G.; Profeta, G. Prediction of Ambient-Pressure Superconductivity in Ternary Hydride PdCuHx. J. Appl. Phys. 2022, 131, 033903. [Google Scholar] [CrossRef]
- Sivasamy, R.; Venugopal, P.; Kumar, K.V.; Espinoza-González, R. Synthesis and Characterizations of Pd/Mn (Mn1. 36Pd0. 64) O4 Nanocomposite: An Experimental and Theoretical Approach. Vacuum 2020, 182, 109683. [Google Scholar] [CrossRef]
- Gryaznov, V.; Serebryannikova, O.; Serov, Y.M.; Ermilova, M.; Karavanov, A.; Mischenko, A.; Orekhova, N. Preparation and Catalysis over Palladium Composite Membranes. Appl. Catal. A Gen. 1993, 96, 15–23. [Google Scholar] [CrossRef]
- Padama, A.A.B.; Kasai, H.; Budhi, Y.W. Hydrogen Absorption and Hydrogen-Induced Reverse Segregation in Palladium–Silver Surface. Int. J. Hydrogen Energy 2013, 38, 14715–14724. [Google Scholar] [CrossRef]
- Dillon, E.; Jimenez, G.; Davie, A.; Bulak, J.; Nesbit, S.; Craft, A. Factors Influencing the Tensile Strength, Hardness, and Ductility of Hydrogen-Cycled Palladium. Mater. Sci. Eng. A 2009, 524, 89–97. [Google Scholar] [CrossRef]
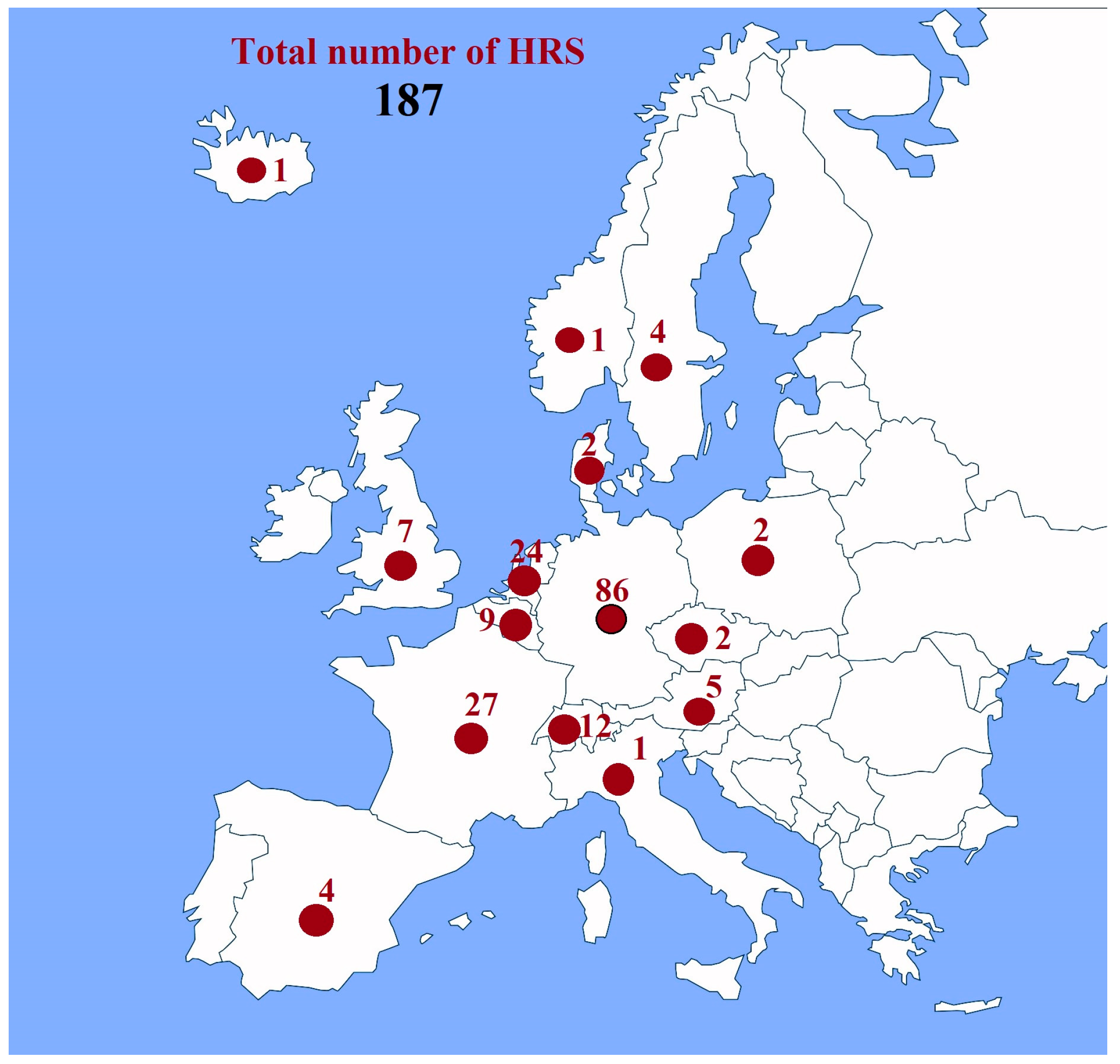
- Apostolou, D.; Xydis, G. A Literature Review on Hydrogen Refuelling Stations and Infrastructure. Curr. Status Future Prospects. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Di Micco, S.; Jannelli, E. Design and Costs Analysis of Hydrogen Refuelling Stations Based on Different Hydrogen Sources and Plant Configurations. Energies 2022, 15, 541. [Google Scholar] [CrossRef]
- European Hydrogen Observatory Observatory. Hydrogen Refuelling Stations. Available online: https://observatory.clean-hydrogen.europa.eu/hydrogen-landscape/distribution-and-storage/hydrogen-refuelling-stations (accessed on 9 September 2024).
- Almeida, R.; Cassang, A.; Lin, D.; Abe, M. Public-Private Partnership Systems and Sustainable Development in Asia and the Pacific; ESCAP: Bangkok, Thailand, 2020. [Google Scholar]
- U. S. Government Publishing Office. Infrastructure Investment and Jobs Act; U.S. Government Publishing Office: Washington, DC, USA, 2021.
- Fakhreddine, O.; Gharbia, Y.; Derakhshandeh, J.F.; Amer, A. Challenges and Solutions of Hydrogen Fuel Cells in Transportation Systems: A Review and Prospects. World Electr. Veh. J. 2023, 14, 156. [Google Scholar] [CrossRef]
- Cihlar, J.; Lejarreta, A.V.; Wang, A.; Melgar, F.; Jens, J.; Rio, P. Hydrogen Generation in Europe: Overview of Costs and Key Benefits; EU Publications: Luxembourg, 2021. [Google Scholar]
- Sens, L.; Piguel, Y.; Neuling, U.; Timmerberg, S.; Wilbrand, K.; Kaltschmitt, M. Cost Minimized Hydrogen from Solar and Wind–Production and Supply in the European Catchment Area. Energy Convers. Manag. 2022, 265, 115742. [Google Scholar] [CrossRef]
- Díaz, M.T.M.; Oróstica, H.C.; Guajardo, J. Economic Analysis: Green Hydrogen Production Systems. Processes 2023, 11, 1390. [Google Scholar] [CrossRef]
- Janssen, J.L.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-Specific Cost Projections for Renewable Hydrogen Production through off-Grid Electricity Systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]
- Radner, F.; Strobl, N.; Köberl, M.; Winkler, F.; Esser, K.; Trattner, A. Off-Grid Hydrogen Production: Analysing Hydrogen Production and Supply Costs Considering Country-Specifics and Transport to Europe. Int. J. Hydrogen Energy 2024, 80, 1197–1209. [Google Scholar] [CrossRef]
- European Hydrogen Observatory Observatory. Cost of Hydrogen Production. Available online: https://observatory.clean-hydrogen.europa.eu/index.php/hydrogen-landscape/production-trade-and-cost/cost-hydrogen-production (accessed on 9 September 2024).

| 2020 | 2025 | Target | |
|---|---|---|---|
| Gravimetric capacity [g/kg] | 45 | 55 | 65 |
| Volumetric capacity [g/dm3] | 30 | 40 | 50 |
| Storage cost [$/kg] | 333 | 300 | 266 |
| Ambient temperature [°C] | −40 to +60 | −40 to +60 | −40 to +60 |
| Delivered hydrogen temperature [°C] | −40 to +85 | −40 to +85 | −40 to +85 |
| Released hydrogen pressure [bar] | 5 to 12 | 5 to 12 | 5 to 12 |
| Charging time [min] | 3 to 5 | 3 to 5 | 3 to 5 |
| Capability [%] | 90 | 90 | 90 |
| Toyota and MIT Collaboration | |
|---|---|
| Project overview: Toyota partnered with the Massachusetts Institute of Technology (MIT) to develop new catalyst materials that reduce platinum loading while maintaining high performance. | Outcomes: This collaboration has led to significant advancements in catalyst technology, contributing to the development of more cost-effective fuel cell vehicles. |
| Ballard Power Systems and University of British Columbia (UBC) | |
| Project overview: Ballard Power Systems collaborates with UBC on several projects aimed at improving fuel cell performance and durability. | Outcomes: Joint research has resulted in the commercialization of new fuel cell technologies, helping Ballard maintain its position as a leader in the industry. |
| Hydrogen Europe and Imperial College London | |
| Project overview: Hydrogen Europe, a coalition of industry stakeholders, collaborates with Imperial College London on research to enhance hydrogen production and storage technologies. | Outcomes: This partnership has produced innovative solutions for hydrogen infrastructure, facilitating the broader adoption of fuel cell technologies. |
| Shell and ITM Power | |
| Project overview: Shell, a global energy company, collaborated with ITM Power, a specialist in hydrogen energy solutions. | Outcomes: Joint ventures to develop large-scale hydrogen refueling stations and electrolysis projects. Developed Europe’s largest hydrogen electrolysis plant in Germany. Expanded the hydrogen refueling network across the UK and Europe. Increased hydrogen production capacity from renewable sources. Improved infrastructure for hydrogen refueling, supporting fuel cell vehicles. Accelerated the adoption of hydrogen as a clean energy carrier. |
| Steam Methane Reforming (SMR) | |
|---|---|
| For 2022, the levelized production costs of hydrogen by SMR in Europe were, on average, on approximately 6.23 EUR/kg of hydrogen. | 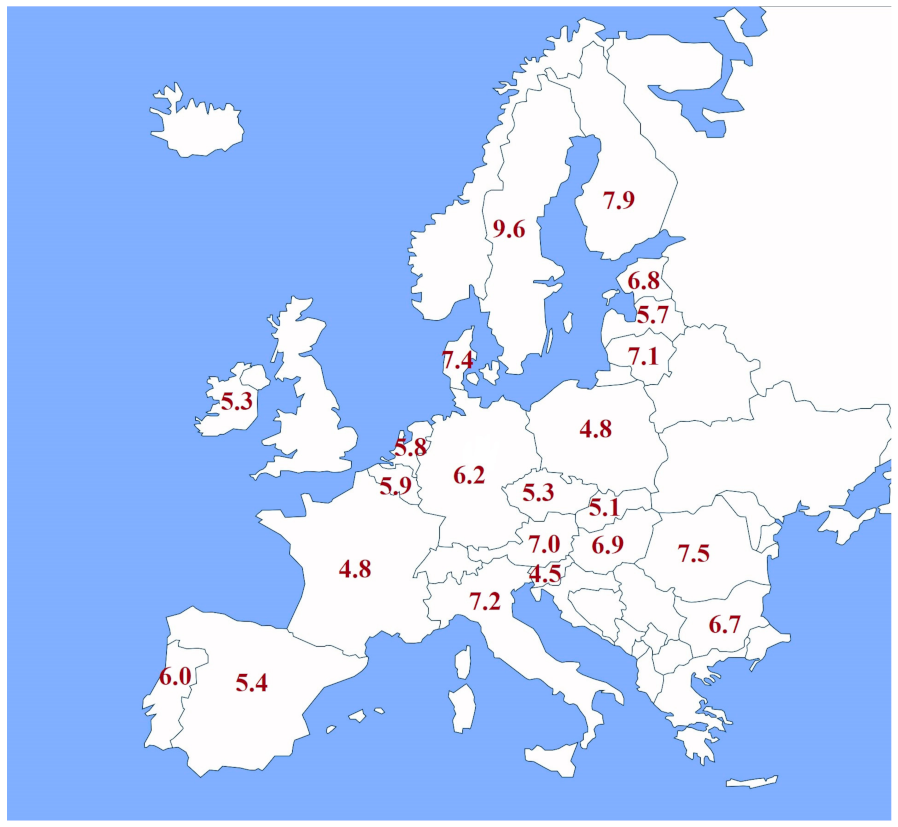 |
| Grid-connected electrolysis | |
| The hydrogen production costs using grid electricity in Europe were estimated in range of 3.89–16.44 EUR/kg of hydrogen, with the average for all countries being 9.85 EUR/kg. |  |
| Water electrolysis with a direct connection to a renewable energy source (renewable hydrogen) | |
| Hydrogen production costs via electrolysis with direct connection to a renewable energy source in Europe vary from 4.18 to 9.60 EUR/kg of hydrogen, with the average for all countries being 6.86 EUR/kg. Even though hydrogen production via electrolysis with a direct connection to a renewable energy source avoids electricity costs like network costs and taxes, the electrolyzer capacity factor is limited by the capacity factor of the renewable source it is connected to. | 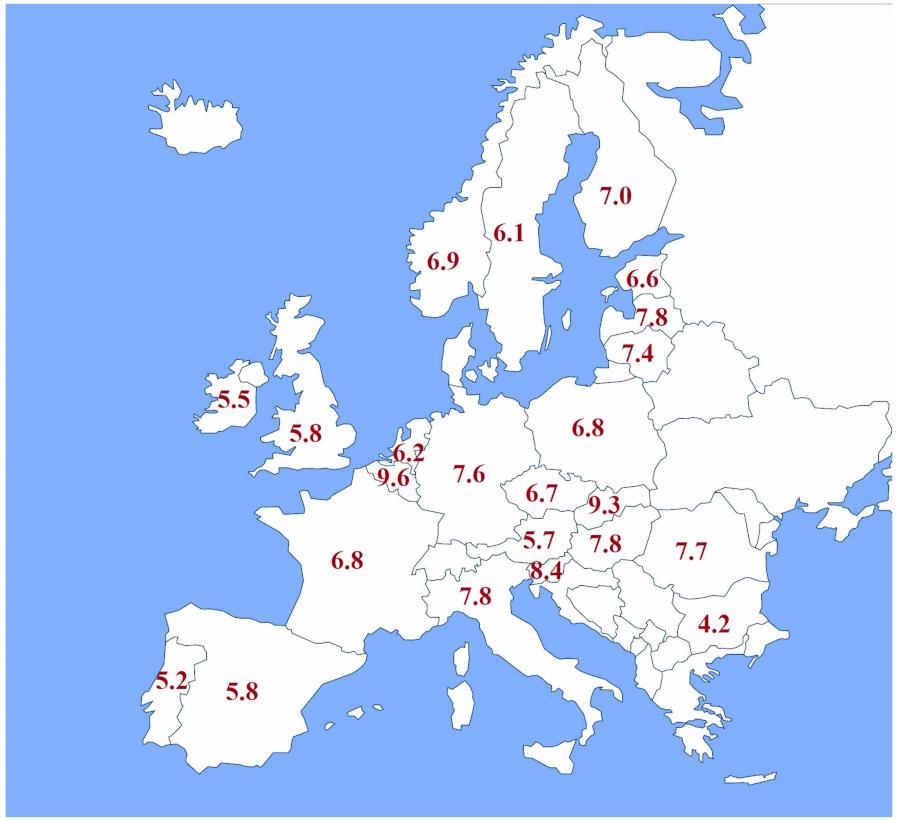 |
| Riversimple | |
|---|---|
| Location: United Kingdom | Riversimple is a British car manufacturer that specializes in hydrogen fuel cell electric vehicles (FCEVs). Its flagship model, the Rasa, is a lightweight, two-seater car designed for maximum efficiency and minimal environmental impact. The Rasa has a range of about 300 miles on a full tank of hydrogen and emits only water. |
| Hyundai Hydrogen Mobility (HHM) | |
| Location: Switzerland (partnership between Hyundai and H2 Energy) | HHM is a joint venture between Hyundai and H2 Energy that focuses on deploying hydrogen fuel cell trucks in Switzerland. Although not a startup in the traditional sense, this partnership aims to provide hydrogen fuel cell solutions for heavy-duty transport, reducing carbon emissions in the logistics sector. |
| Loop Energy | |
| Location: Canada | Loop Energy is a Canadian company that provides hydrogen fuel cell systems for commercial vehicles, including buses and trucks. Its technology aims to offer efficient and reliable fuel cell solutions to reduce greenhouse gas emissions in transportation. |
| Nikola Motor Company | |
| Location: United States | Nikola is an American startup that develops hydrogen fuel cell trucks for long-haul transportation. Its vehicles, such as the Nikola Tre and Nikola Two, are designed to offer zero-emission solutions for the trucking industry. Nikola is also involved in building a hydrogen refueling infrastructure to support their vehicles. |
| Hyperion Motors | |
| Location: United States | Hyperion Motors is a startup focused on producing high-performance hydrogen fuel cell vehicles. Its first vehicle, the XP-1, is a supercar that showcases the potential of hydrogen fuel cells in delivering high power and long range with zero emissions. The XP-1 aims to demonstrate the feasibility and advantages of hydrogen as a fuel for performance vehicles. |
| Hyzon Motors | |
| Location: United States | Hyzon Motors specializes in hydrogen fuel cell-powered commercial vehicles, such as trucks and buses. Hyzon’s goal is to accelerate the adoption of hydrogen fuel cell technology in the commercial vehicle market, providing zero-emission solutions for logistics and public transportation. |
| Proterra | |
| Location: United States | Proterra is known for its electric buses, but it has also been exploring hydrogen fuel cell technology. Its mission is to provide clean, quiet transportation options for urban environments, and its developments in hydrogen fuel cell buses aim to extend the range and efficiency of their electric vehicle offerings. |
| Wrightbus | |
| Location: United Kingdom | Wrightbus is a UK-based company that has developed the world’s first hydrogen double-decker bus. It aims to provide zero-emission public transportation solutions, and its hydrogen buses are designed to offer the same range and performance as traditional diesel buses while producing only water vapor as an emission. |
| New Flyer Industries | |
| Location: Canada/United States | New Flyer is a leading manufacturer of transit buses and has been developing hydrogen fuel cell buses as part of its Xcelsior CHARGE H2™ line. Its hydrogen buses are designed for long-range, zero-emission transit operations, providing a sustainable alternative for public transportation networks. |
| Van Hool | |
| Location: Belgium | Van Hool is a Belgian manufacturer that produces hydrogen fuel cell buses. Its A330 and Exqui. City models are designed to offer efficient and environmentally friendly public transportation options. Van Hool’s hydrogen buses are already in operation in several cities across Europe. |
| Caetanobus | |
| Location: Portugal | Caetanobus is a Portuguese bus manufacturer that has developed the H2. City Gold, a hydrogen fuel cell bus. This bus is designed for urban transit and aims to provide a zero-emission solution with the same reliability and comfort as traditional buses. The H2. City Gold is part of Caetanobus’s effort to lead in sustainable public transportation solutions. |
| Fuel Cell Design Innovations | |
|---|---|
| Advanced catalyst structures: Platinum and platinum-based alloy nanotubes as electrocatalysts for fuel cells | US Patent No. 2009/0220835 A1: This patent focuses on the development of efficient catalysts for proton exchange membrane (PEM) fuel cells. It describes the use of nanostructured platinum alloys that improve catalytic activity and reduce platinum loading, enhancing overall fuel cell efficiency and reducing costs. |
| Improved membrane technology: Development of novel proton-conductive polymers for proton exchange membrane fuel cell (PEMFC) technology | US Patent No. 7,615,300 B2: This patent details a novel proton exchange membrane with enhanced conductivity and durability. The membrane incorporates advanced materials such as sulfonated polyaryl ether ketones, which improve the performance and longevity of PEM fuel cells. |
| High-performance bipolar plates: Composite bipolar plate for a fuel cell and method | DE Patent No. 102004043513 A1: This patent introduces a new design for bipolar plates used in fuel cells. The plates are made from lightweight composite materials with optimized flow field patterns, which enhance gas distribution and reduce pressure drops, leading to higher fuel cell efficiency and performance. |
| Hydrogen production methods | |
| Efficient electrolysis systems and others: Power dispatch system for electrolytic production of hydrogen from wind power | Patent No. WO2010/048706 A1: This patent describes an advanced electrolyzer system that uses renewable energy sources such as solar or wind power to produce hydrogen. The system incorporates high-efficiency electrolyzer stacks and innovative power management techniques to maximize hydrogen output while minimizing energy consumption. |
| Hydrogen-producing fuel cell systems and methods of operation | US Patent No. 2024/0063411 A1: The methods include initiating supply of a stored hydrogen stream, which includes stored hydrogen gas, to a fuel cell stack. Prior to initiating, the stored hydrogen gas is stored in a low-pressure hydrogen storage tank at a hydrogen storage pressure. The methods also include generating an electrical power output from the stored hydrogen gas with the fuel cell stack. The methods further include, during a supply time interval that is subsequent to initiating, monitoring a hydrogen supply variable that is indicative of flow of the stored hydrogen stream to the fuel cell stack. The methods also include detecting changes in the hydrogen supply variable and responding to them. |
| Method for producing hydrogen | US Patent No. 6,506360 B1: The hydrogen production method described involves reacting aluminum with water in the presence of sodium hydroxide as a catalyst. The equipment used for this process regulates the reaction’s intensity and duration by adjusting the pressure and temperature, which in turn controls the extent to which a fuel cartridge is submerged in water. |
| Photocatalytic hydrogen production: Photocatalytic hydrogen production from water over mixed-phase titanium dioxide nanoparticles | Patent No. WO2016/005855 A1: This patent covers a method for producing hydrogen using photocatalysts that harness sunlight. The process involves the use of nanostructured titanium dioxide (TiO2) combined with other semiconductors to enhance the efficiency of water splitting under solar illumination. |
| Photocatalytic hydrogen production from water over Ag-Pd-Au deposited on titanium dioxide materials | Patent No. WO2015/118424 A1: Photocatalysts and methods for generating hydrogen from water are described. The photocatalysts include photoactive titanium dioxide particles with an anatase-to-rutile ratio of at least 2:1, combined with silver, palladium, and gold metals deposited on the titanium dioxide surface. The molar ratio of gold to palladium ranges from 0.1 to 5, and the ratio of gold to silver ranges from 0.1 to 3. |
| Biological hydrogen production: Microorganism having a gene for improved hydrogen-generating capability, and process for producing hydrogen | US Patent No. 2007/0202585 A1: This patent details a method for producing hydrogen using genetically engineered microorganisms. The microorganisms are modified to enhance their hydrogenase activity, allowing them to efficiently convert organic substrates into hydrogen gas. |
| Hydrogen storage solutions | |
| High-capacity metal hydrides: Metal hydrides and their use in hydrogen storage applications | US Patent No. 9,376,316 B2: This patent describes the use of advanced metal hydrides for hydrogen storage. The metal hydrides have high hydrogen absorption capacities and can release hydrogen at moderate temperatures, making them suitable for onboard storage in hydrogen-powered vehicles. |
| Nanotube storage: Apparatus with large-surface-area nanostructures for hydrogen storage, and methods of storing hydrogen | US Patent No. 2010/0276304 A1: Method and apparatus for storing hydrogen. In one embodiment, the method involves using a storage device that includes a substrate with a nanostructure mat applied to at least one side. This nanostructure mat consists of multiple nanostructures with a surface ionization state that enables the adsorption of multiple hydrogen layers. The process also includes exposing the nanostructure mat to hydrogen, allowing it to adsorb more than one layer of hydrogen onto the nanostructures. |
| Liquid organic hydrogen carriers: Hydrogen storage by means of organic liquid compounds | FR Patent No. 3115031 A1: This patent details a system for storing and transporting hydrogen using liquid organic hydrogen carriers. LOHCs can absorb and release hydrogen through chemical reactions, offering a safe and efficient way to handle hydrogen fuel. |
| Integrated systems and applications | |
| Fuel cell integration in vehicles: Integrated fuel cell system | US Patent No. 6,376,113 B1: This patent covers an integrated fuel cell system for automotive applications. The system includes a compact fuel cell stack, onboard hydrogen storage, and power management electronics, optimized for use in passenger vehicles to achieve high efficiency and performance. |
| Hydrogen fuel cell hybrid locomotives | US Patent No. 8,117,969 B1: A hydrogen hybrid locomotive features a battery system that powers multiple electric traction motors for moving the locomotive along railroad tracks, along with a fuel cell power plant that charges the batteries and drives the electric motors. The fuel cell power plant includes one or more fuel cell modules that generate electrical current by reacting hydrogen fuel with oxygen from the intake air, with the amount of current being proportional to the air mass flow. An air system provides the necessary air mass flow to the fuel cell module to produce the required electrical current for the locomotive’s operating conditions. Additionally, a cooling system manages the temperature of the fuel cell power modules based on the current being produced. |
| Portable fuel cell systems: Portable hydrogen generator and fuel cell system * | US Patent No. 2006/0112635 A1: This patent describes a portable fuel cell system designed for use in remote or off-grid applications. The system is lightweight, compact, and includes integrated hydrogen storage and power conditioning units, making it ideal for mobile power generation. |
| Station: Hydrogen fueling station | US Patent No. 6,510,925 B2: This patent details the design of an advanced hydrogen refueling station. The station includes high-efficiency compressors, cryogenic storage tanks, and automated dispensing systems to ensure fast and safe refueling of hydrogen-powered vehicles. |
| High-efficiency hydrogen tanks: Hydrogen storage tank | US Patent No. 8,628,609 B2: This patent focuses on high-pressure hydrogen storage tanks that use advanced composite materials to achieve high storage densities while maintaining safety standards. The tanks are designed to fit into existing vehicle frameworks without significant modifications, facilitating easier integration into new and retrofitted vehicles. |
| Onboard hydrogen generation methods and system for hydrogen production by water electrolysis | US Patent No. 10,487,408 B2: This patent covers a system for onboard hydrogen generation using water electrolysis powered by renewable energy sources. The system is designed to produce hydrogen as needed, reducing the dependency on hydrogen refueling infrastructure and enabling longer travel distances between refueling stops. |
| System and method for generating hydrogen gas | US Patent No. 2007/0138006 A1: A hydrogen gas generation system is designed for use in various mobile vehicles, such as cars, trucks, balloons, dirigibles, airships, ships, or boats. This system includes an onboard hydrogen generator that produces hydrogen gas, preferably through an electrolysis process. The generated hydrogen is stored in an onboard storage tank. The stored hydrogen is then supplied to the vehicle’s propulsion system, where it is used to generate power for moving the vehicle. Additionally, an onboard electrical generation system provides some of the electricity needed for the electrolysis process. For instance, the vehicle may be equipped with an onboard electrical generator that supplies the necessary electricity for hydrogen production. |
| Power electronics and control systems | |
| Integrated power management system: Fuel cell power system and method of controlling a fuel cell power system ** | US Patent No. 6,743,536 B2: This patent describes an integrated power management system that optimizes the flow of electricity between the fuel cell stack, battery, and electric motor. The system includes advanced algorithms that manage power distribution in real time, improving overall vehicle efficiency and performance. |
| High-efficiency inverters: Hybrid fuel cell vehicle with multi-power source and multi-drive system and method of control | US Patent No. 8,016,061 B2: This patent focuses on a new design for inverters used in fuel cell vehicles. The inverters are more efficient and compact, enabling better integration into vehicle electrical systems and improving the conversion of DC power from the fuel cell to AC power for the electric motor. |
| Thermal management solutions | |
| Advanced cooling systems: Cooling system for a fuel cell stack *** | US Patent No. 6,866,955 B2: This patent introduces an advanced cooling system that uses a combination of liquid cooling and phase-change materials to manage the heat generated by the fuel cell stack. The system is designed to maintain optimal operating temperatures under various driving conditions, enhancing the durability and performance of the fuel cell. |
| Heat recovery systems: Waste heat recovery means for fuel cell power system | US Patent No. 6,926,979 B2: This patent describes a heat recovery system that captures and utilizes the waste heat from the fuel cell stack to improve vehicle efficiency. The recovered heat can be used for cabin heating or to pre-heat the fuel cell stack, reducing energy consumption and improving cold-start performance. |
| Vehicle integration techniques | |
| Compact integration framework: Vehicle mounting structure for fuel cell | US Patent No. 7,533,748 B2: This patent covers a compact integration framework for fuel cell systems in vehicles. The framework includes a specially designed chassis and mounting system that accommodates the fuel cell stack, hydrogen tanks, and associated components without compromising vehicle design and functionality. |
| Structural integration: Structural fuel cells and components thereof | US Patent No. 8,057,938 B1: This patent details a method for integrating fuel cell components into the structural elements of the vehicle, such as the frame and body panels. This approach not only saves space but also enhances the vehicle’s structural integrity and safety. |
| ISO Standards for Fuel Cells | |
|---|---|
| ISO 14687:2019—Hydrogen fuel quality | This document outlines the quality characteristics required for hydrogen fuel used in proton exchange membrane (PEM) fuel cell applications in road vehicles. It defines the minimum quality standards for hydrogen fuel to ensure its suitability for both vehicular and stationary applications. |
| ISO 16111:2008 (new ISO 16111:2018)—Transportable gas storage devices—Hydrogen absorbed in reversible metal hydride | Provides the specifications and testing methods for transportable hydrogen storage devices using metal hydrides. Defines the requirements applicable to the material, design, construction, and testing of transportable hydrogen gas storage systems, referred to as “metal hydride assemblies” (MH assemblies) which utilize shells not exceeding 150 l internal volume and having a maximum developed pressure (MDP) not exceeding 25 MPa (250 bar). |
| ISO 22734:2019—Hydrogen generators using water electrolysis | Specifies the safety requirements for hydrogen generators that use water electrolysis. This document specifies the construction, safety, and performance standards for modular or factory-matched hydrogen gas generation systems, referred to as hydrogen generators. These systems use electrochemical reactions to electrolyze water and produce hydrogen. |
| ISO 14687-2:2012 (new ISO 14687:2019)—Hydrogen fuel—Product specification—Part 2: Proton exchange membrane (PEM) fuel cell applications for road vehicles | Defines the product specification for hydrogen fuel in PEM fuel cell applications, specifically for road vehicles. The document details the quality characteristics of hydrogen fuel to ensure consistency in the hydrogen product used for proton exchange membrane (PEM) fuel cell systems in road vehicles. |
| ISO 26142:2010—Hydrogen detection apparatus | Specifies the specifications for hydrogen detection devices designed to improve safety. This international standard outlines the performance criteria and testing for hydrogen detection equipment intended to measure and monitor hydrogen concentrations in stationary applications. |
| ISO standards for hydrogen use in the automotive industry | |
| ISO 19880-1:2020—Gaseous hydrogen—Fueling stations—Part 1: General requirements | Establishes the general requirements for the design, construction, operation, and maintenance of hydrogen fueling stations. This document specifies the minimum requirements for the design, installation, commissioning, operation, inspection, and maintenance of both public and private hydrogen fueling stations that provide gaseous hydrogen to light-duty road vehicles, such as fuel cell electric vehicles. It does not apply to the dispensing of cryogenic hydrogen or hydrogen to metal hydride applications. |
| ISO 17268:2012 (new ISO 17268:2020)—Gaseous hydrogen land vehicle refueling connection devices | Defines the specifications for connectors used in refueling hydrogen land vehicles. This document defines the design, safety, and operation characteristics of gaseous hydrogen land vehicle (GHLV) refueling connectors. This includes details on the receptacle and protective cap (mounted on the vehicle) and the nozzle. |
| ISO 23828:2013 (new ISO 23828:2022)—Fuel cell road vehicles—Energy consumption measurement—Vehicles fueled with compressed hydrogen | This document outlines the methods for measuring the energy consumption of fuel cell road vehicles powered by compressed hydrogen. It details the procedures for assessing the energy use of fuel cell passenger cars and light-duty trucks that rely on compressed hydrogen and are not capable of external charging. |
| ISO 14687-1:1999 (new ISO 14687:2019)—Hydrogen fuel—Product specification—Part 1: All applications except proton exchange membrane (PEM) fuel cell for road vehicles | Provides the product specifications for hydrogen fuel for all applications except PEM fuel cells for road vehicles. This international standard defines the quality characteristics of hydrogen fuel to ensure consistency and uniformity in the hydrogen product as it is produced and distributed for use in vehicles, appliances, or other fueling applications. |
| ISO 12619-3:2014 series—Road vehicles—Compressed hydrogen gas (CGH2) and hydrogen/natural gas blend fuel system components | A series of standards that specify the requirements for components used in compressed hydrogen gas and hydrogen/natural gas blend fuel systems for road vehicles. This document specifies general requirements and definitions for fuel system components designed for compressed gaseous hydrogen (CGH2) and hydrogen/natural gas blends, intended for use in motor vehicles as defined in ISO 3833. |
| Other relevant standards | |
| ISO/TR 15916:2015—Basic considerations for the safety of hydrogen systems | Provides guidance on the safety considerations for the design and operation of hydrogen systems. This document offers guidelines for the use of hydrogen in both its gaseous and liquid states, as well as for its storage in these or other forms such as hydrides. It outlines fundamental safety concerns, hazards, and risks and details the properties of hydrogen that are pertinent to safety considerations. |
| ISO 13984:1999—Liquid hydrogen—Land vehicle fuel tanks | Specifies the requirements for fuel tanks designed to hold liquid hydrogen for land vehicles. This international standard applies to the design and installation of liquid hydrogen (LH2) fueling and dispensing systems. It covers the system designed for dispensing liquid hydrogen to a vehicle, including that portion of the system components that manage cold gaseous hydrogen from the vehicle’s tank, specifically addressing the system components situated between the land vehicle and the storage tank. |
| GB/T 29126-2012—Fuel cell electric vehicles—Onboard hydrogen system—Test methods | This standard outlines the test methods for the onboard hydrogen system of fuel cell electric vehicles. It applies to fuel cell electric vehicles that use compressed hydrogen as fuel, where the operating pressure does not exceed 35 MPa at an ambient temperature of 15 °C. |
| Startup | Patent |
|---|---|
| QuantumScape (solid-state batteries) | |
| QuantumScape, a Silicon Valley startup, is focused on developing lithium-metal solid-state batteries that promise to revolutionize the electric vehicle industry with higher energy density, faster charging capabilities, and improved safety compared to traditional lithium-ion batteries. | QuantumScape has a strong portfolio of patents related to solid-state battery technology. These patents cover innovations such as ceramic separators, electrolyte compositions, and methods for stabilizing lithium-metal anodes. The company’s patents are key to securing its technological edge and have been a key factor in attracting major investors such as Volkswagen. |
| Solid Power (solid-electrolyte batteries) | |
| Solid Power is another notable startup working on solid-state batteries, focusing on using sulfide-based solid electrolytes to improve energy density and safety. The company aims to replace liquid electrolytes in lithium-ion batteries with a solid electrolyte that prevents the formation of dendrites that can cause short circuits. | Solid Power holds patents related to solid-state electrolyte materials, battery cell architecture, and manufacturing processes. Its patent portfolio helps it protect its unique approach to battery technology and has been instrumental in forging partnerships with industry giants like BMW and Ford. |
| Form Energy (iron–air batteries) | |
| Form Energy is a pioneer in the development of iron–air batteries, a novel energy storage solution designed for long-term grid storage. These batteries use abundant and cheap iron to store energy, making them an attractive option for large-scale energy storage, especially to offset intermittent renewable energy sources such as wind and solar. | The company’s patent strategy focuses on the unique chemistry and design of iron–air batteries, including methods for managing oxygen flow, electrode materials, and cell configurations. These patents protect its core technology and facilitate entry into the energy storage market. |
| Sila Nanotechnologies (silicon anode batteries) | |
| Sila Nanotechnologies is working on next-generation batteries, replacing traditional graphite anodes with silicon-based anodes that offer higher energy density and longer battery life. This innovation is particularly relevant for consumer electronics and electric vehicles, where there is a high demand for improved battery performance. | Sila Nanotechnologies has secured numerous patents covering silicon-dominant anode compositions, nanostructured materials, and manufacturing techniques. These patents provide a competitive advantage in a market with high demand for high-energy density and long-life batteries and have attracted partnerships with companies such as Daimler and BMW. |
| Ambri (liquid metal batteries) | |
| Ambri develops liquid metal batteries for grid-scale energy storage. Its technology uses a liquid calcium alloy anode, a molten salt electrolyte, and a solid antimony cathode. These batteries are designed to offer low-cost, long-lasting, and safe energy storage for renewable energy integration. | Ambri holds several patents on liquid metal battery technology, including unique cell designs, electrode materials, and manufacturing processes. These patents are essential to securing financing and partnerships with utilities and grid operators who are increasingly interested in scalable, cost-effective energy storage solutions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurc, B.; Gross, X.; Szymlet, N.; Rymaniak, Ł.; Woźniak, K.; Pigłowska, M. Hydrogen-Powered Vehicles: A Paradigm Shift in Sustainable Transportation. Energies 2024, 17, 4768. https://doi.org/10.3390/en17194768
Kurc B, Gross X, Szymlet N, Rymaniak Ł, Woźniak K, Pigłowska M. Hydrogen-Powered Vehicles: A Paradigm Shift in Sustainable Transportation. Energies. 2024; 17(19):4768. https://doi.org/10.3390/en17194768
Chicago/Turabian StyleKurc, Beata, Xymena Gross, Natalia Szymlet, Łukasz Rymaniak, Krystian Woźniak, and Marita Pigłowska. 2024. "Hydrogen-Powered Vehicles: A Paradigm Shift in Sustainable Transportation" Energies 17, no. 19: 4768. https://doi.org/10.3390/en17194768
APA StyleKurc, B., Gross, X., Szymlet, N., Rymaniak, Ł., Woźniak, K., & Pigłowska, M. (2024). Hydrogen-Powered Vehicles: A Paradigm Shift in Sustainable Transportation. Energies, 17(19), 4768. https://doi.org/10.3390/en17194768