The Possibility of Using Waste from Dye Sorption for Methane Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Scheme
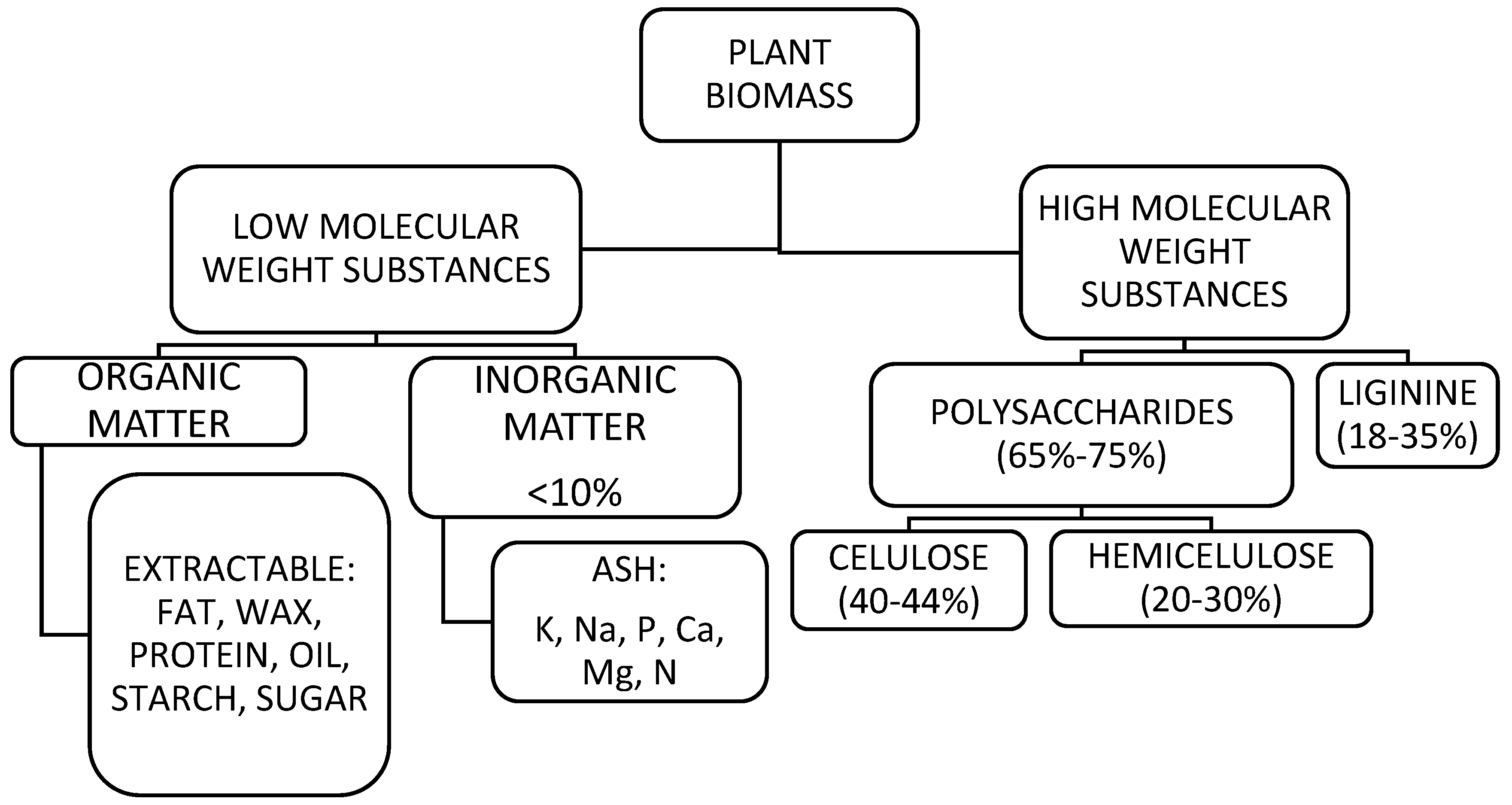
2.2. Raw Materials for the Production of Lignocellulosic Sorbents
2.3. Preparation of Sorbents
2.4. BR46 Sorption
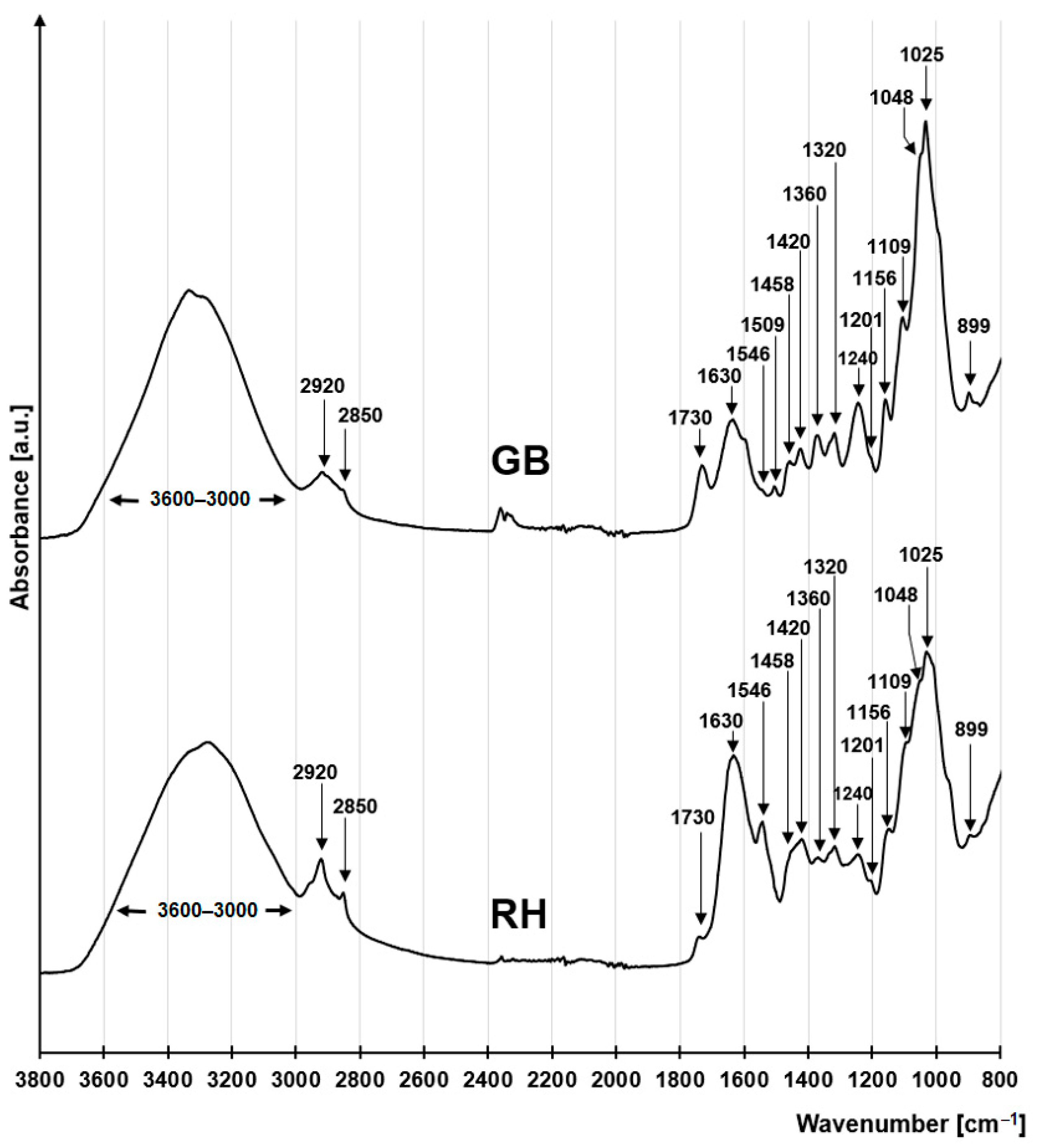
2.5. Physicochemical Analyses of Sorbents after Dye Sorption
2.6. Anaerobic Digestion
2.7. Calculation Methods
3. Results and Discussion
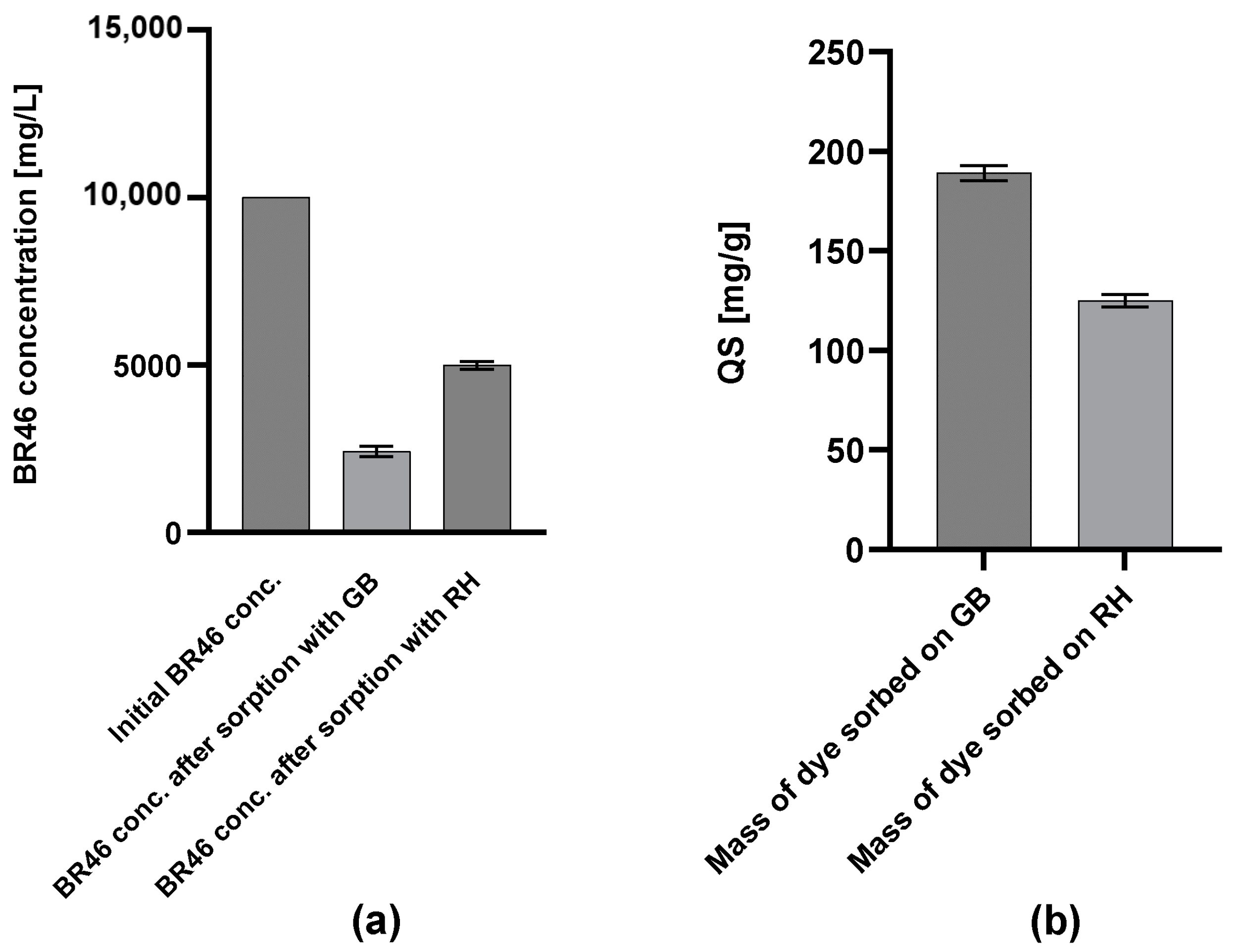
3.1. Effectiveness of BR46 Sorption on GB and RHs
3.2. Physicochemical Analyses of Substrates Directed to Anaerobic Digestion
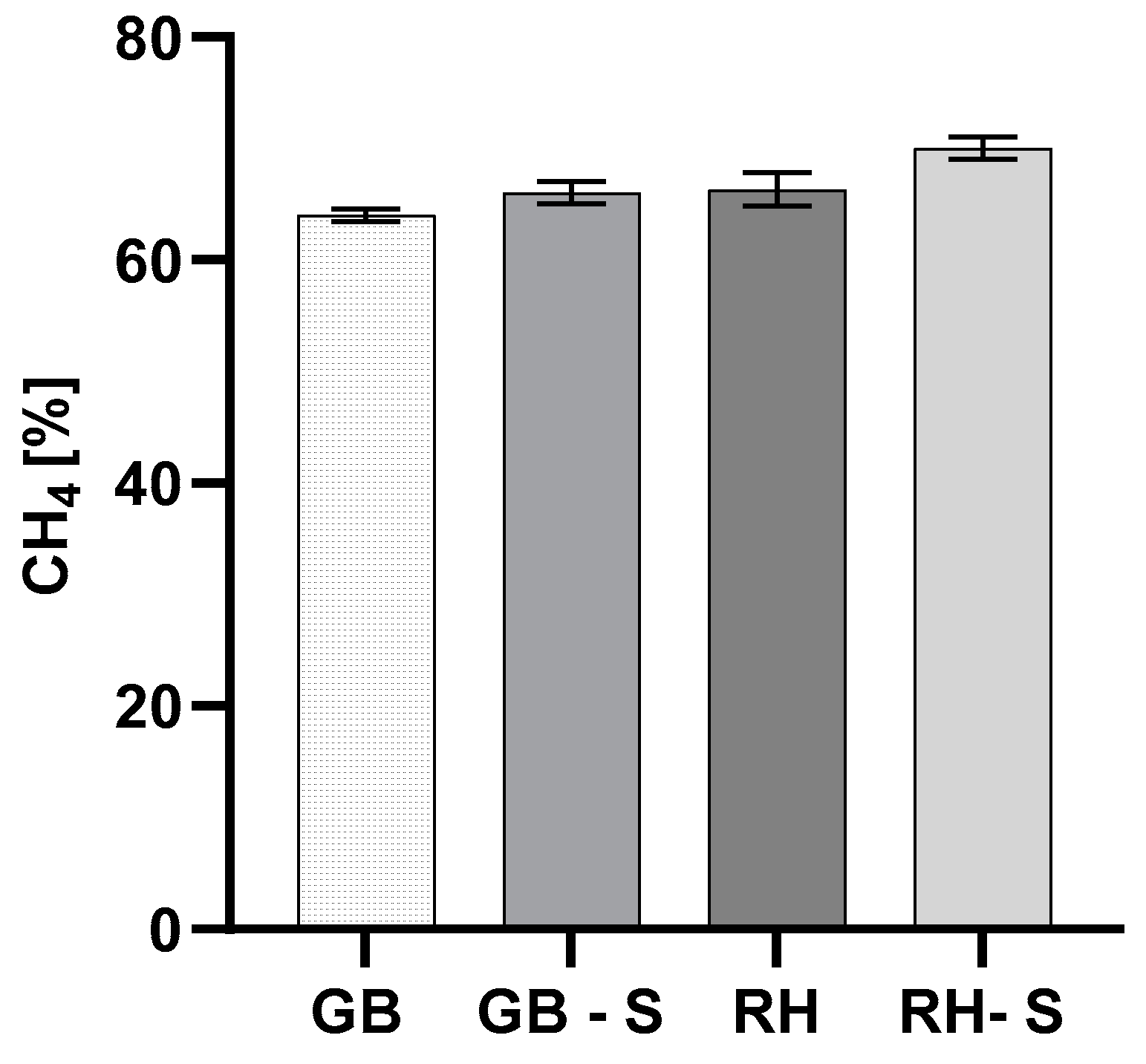
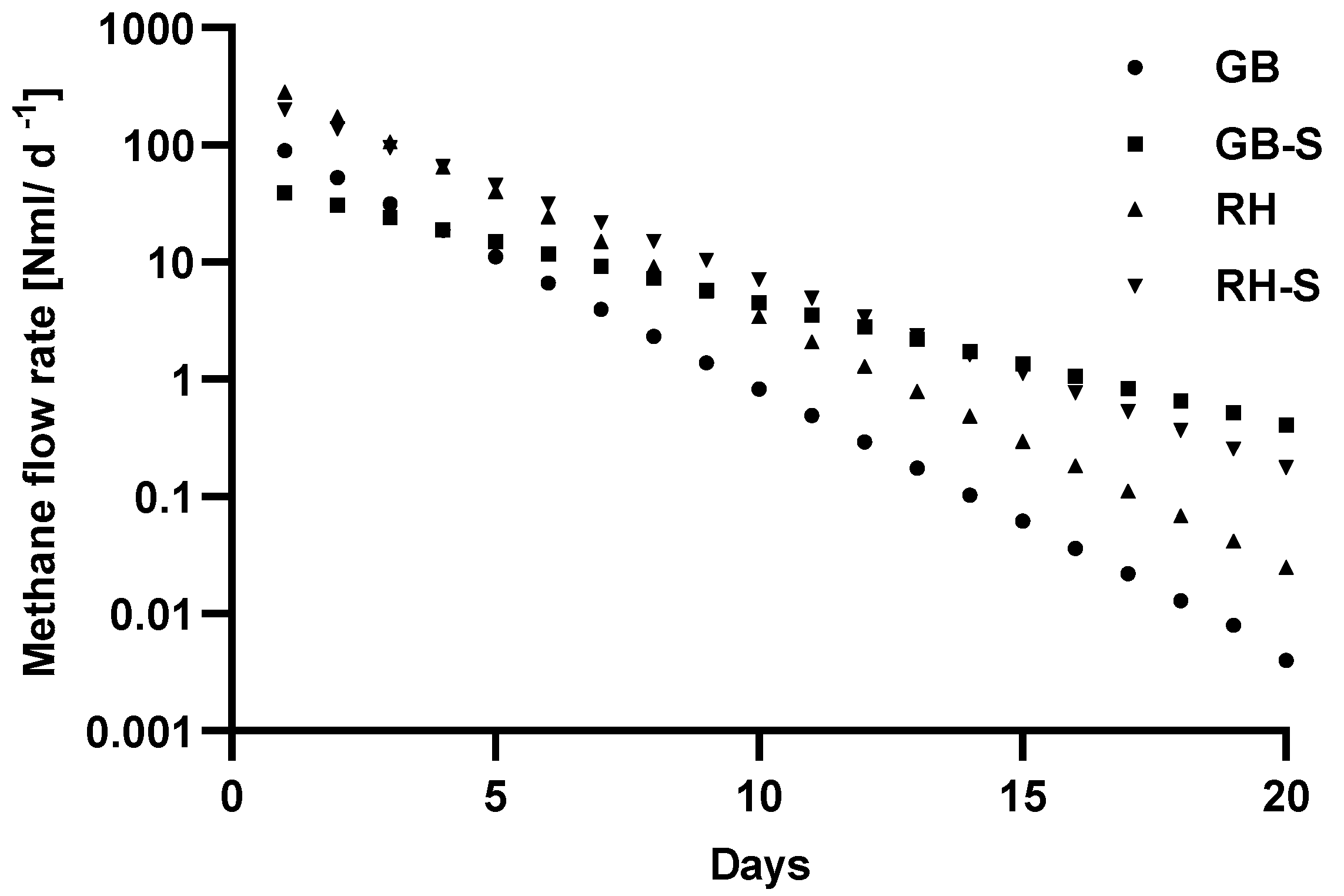
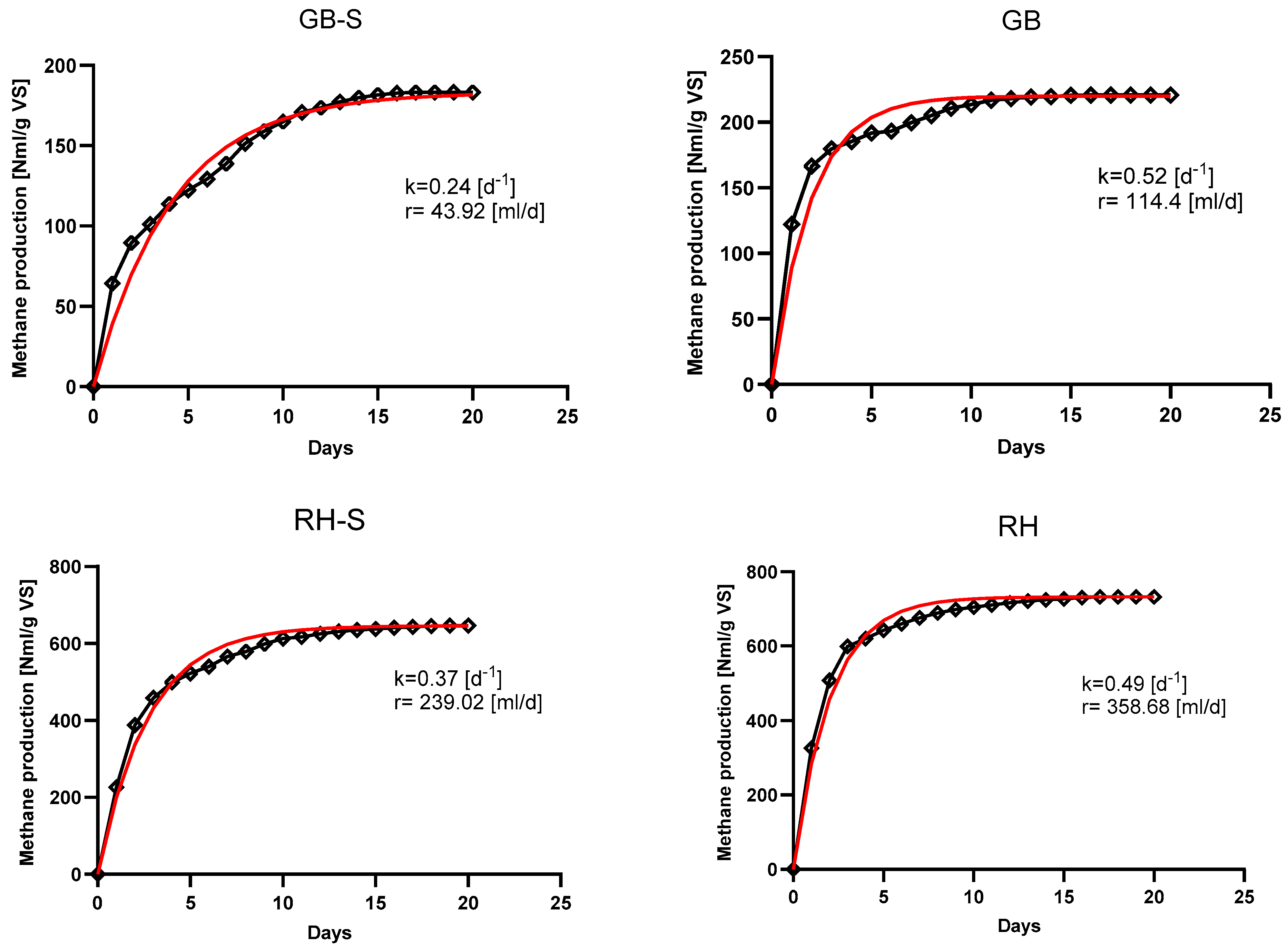
3.3. Efficiency of Anaerobic Digestion
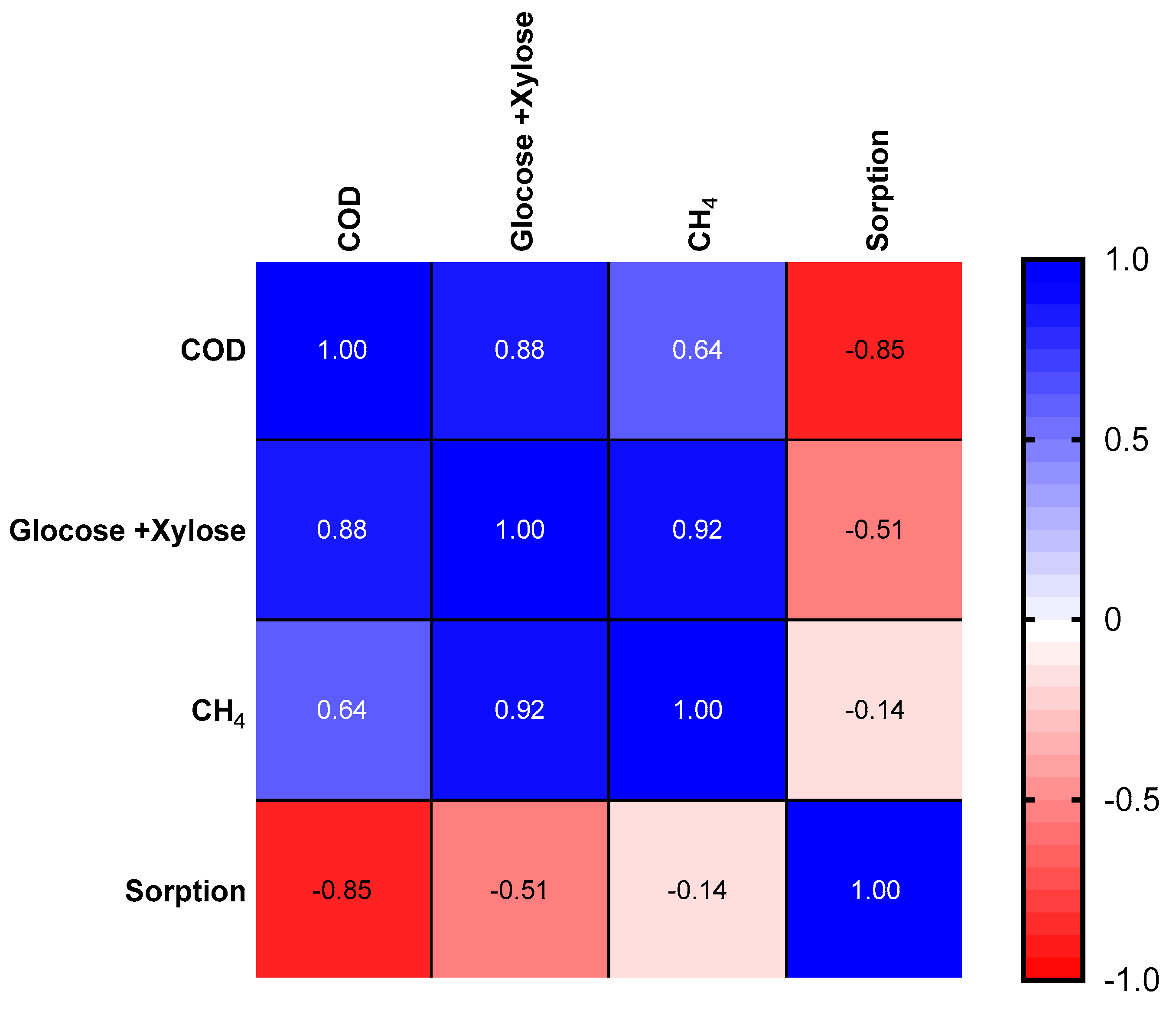
3.4. Analysis of the Digestate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gołąbeska, E.; Harasimowicz, A. Wybrane Problemy Związane z Realizacją Systemów Wykorzystujących Zieloną Energię; Oficyna Wydawnicza Politechniki Białostockiej: Białystok, Poland, 2023. [Google Scholar]
- Transformacja Energetyczna w Polsce-Klimatyczna Baza Wiedzy. Available online: https://klimatycznabazawiedzy.org/raport/transformacja-energetyczna-w-polsce/ (accessed on 1 August 2024).
- Lamnatou, C.; Cristofari, C.; Chemisana, D. Renewable Energy Sources as a Catalyst for Energy Transition: Technological Innovations and an Example of the Energy Transition in France. Renew Energy 2024, 221, 119600. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of Microwave Thermohydrolysis and Liquid Hot Water Pretreatment of Energy Crop Sida Hermaphrodita for Enhanced Methane Production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M.; Rusanowska, P. Progress in the Production of Biogas from Virginia Mallow after Alkaline-Heat Pretreatment. Biomass Bioenergy 2019, 126, 174–180. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane Production from Lignocellulosic Agricultural Crop Wastes: A Review in Context to Second Generation of Biofuel Production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Biomass Energy and the Environmental Impacts Associated with Its Production and Utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Kallel, F.; Chaari, F.; Bouaziz, F.; Bettaieb, F.; Ghorbel, R.; Chaabouni, S.E. Sorption and Desorption Characteristics for the Removal of a Toxic Dye, Methylene Blue from Aqueous Solution by a Low Cost Agricultural by-Product. J. Mol. Liq. 2016, 219, 279–288. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass Torrefaction as an Emerging Technology to Aid in Energy Production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef] [PubMed]
- Mahdizadeh, H.; Dadban Shahamat, Y.; Rodríguez-Couto, S. Discoloration and Mineralization of a Textile Azo Dye Using a Hybrid UV/O3/SBR Process. Appl. Water Sci. 2021, 11, 159. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Nadeem, A. Instantaneous Adsorption of Synthetic Dyes from an Aqueous Environment Using Kaolinite Nanotubes: Equilibrium and Thermodynamic Studies. ACS Omega 2021, 6, 845–856. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef] [PubMed]
- Jamee, R.; Siddique, R. Biodegradation of Synthetic Dyes of Textile Effluent by Microorganisms: An Environmentally and Economically Sustainable Approach. Eur. J. Microbiol. Immunol. (Bp) 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Deaconu, M.; Senin, R.; Stoica, R.; Athanasiu, A.; Crudu, M.; Oproiu, L.; Ruse, M.; Filipescu, C. Adsorption Decolorization Technique of Textile/Leather Dye Containing Effluents. Int. J. Waste Resour. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Yan, Z.; Yi, C.; Liu, T.; Yang, J.; Ma, H.; Sha, L.; Guo, D.; Zhao, H.; Zhang, X.; Wang, W. Effect of Lignin-Containing Highly Fibrillated Cellulose on the Adsorption Behavior of an Organic Dye. Bioresources 2021, 16, 6560–6576. [Google Scholar] [CrossRef]
- Szabó, A.K.; Bálint, J.; Molnár, A.; Aszalos, S.E.; Fora, C.G.; Loxdale, H.D.; Balog, A. Associational Susceptibility of Crop Plants Caused by the Invasive Weed Canadian Goldenrod, Solidago Canadensis, via Local Aphid Species. Front. Ecol. Evol. 2022, 10, 1080599. [Google Scholar] [CrossRef]
- Feng, Y.; Dionysiou, D.D.; Wu, Y.; Zhou, H.; Xue, L.; He, S.; Yang, L. Adsorption of Dyestuff from Aqueous Solutions through Oxalic Acid-Modified Swede Rape Straw: Adsorption Process and Disposal Methodology of Depleted Bioadsorbents. Bioresour. Technol. 2013, 138, 191–197. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.S. Valorisation of Post-Sorption Materials: Opportunities, Strategies, and Challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Ronda, A.; Della Zassa, M.; Martín-Lara, M.A.; Calero, M.; Canu, P. Combustion of a Pb(II)-Loaded Olive Tree Pruning Used as Biosorbent. J. Hazard. Mater. 2016, 308, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Standard Methods 1997: 2540 Solids. Available online: http://edgeanalytical.com/wp-content/uploads/Waste_SM2540.pdf (accessed on 15 September 2024).
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Sulieman, A.A.; Zhu, K.X.; Peng, W.; Hassan, H.A.; Obadi, M.; Ahmed, M.I.; Zhou, H.M. Effect of Agaricus Bisporus Polysaccharide Flour and Inulin on the Antioxidant and Structural Properties of Gluten-Free Breads. J. Food Meas. Charact. 2019, 13, 1884–1897. [Google Scholar] [CrossRef]
- Witowski, A.M.; Sawicka, M.; Fizyki, W.; Warszawskiego, U.; Girdwoyń, P.A.; Tomaszewski, T.J. Nowe Aspekty Spektroskopii Papieru w Podczerwieni i Obszarze Teraherców. Probl. Współczesnej Kryminal. 2023, 24, 153–164. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase Pretreatment of Wheat Straw: Effects of the Physicochemical Characteristics and the Kinetics of Enzymatic Hydrolysis. Biotechnol Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Grzybek, M.; Strachecka, A.; Jaworska, A.; Ludwiczuk, A. ATR-FTIR-Based Fingerprinting of Some Cucurbitaceae Extracts: A Preliminary Study. Acta Soc. Bot. Pol. 2018, 87, 3579. [Google Scholar] [CrossRef]
- Van Gulick, L.; Saby, C.; Morjani, H.; Beljebbar, A. Age-Related Changes in Molecular Organization of Type I Collagen in Tendon as Probed by Polarized SHG and Raman Microspectroscopy. Sci. Rep. 2019, 9, 7280. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U. Aminated Rapeseed Husks (Brassica Napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions. Molecules 2024, 29, 843. [Google Scholar] [CrossRef]
- Navarro, R.; Guzmán, J.; Saucedo, I.; Revilla, J.; Guibal, E. Recovery of Metal Ions by Chitosan: Sorption Mechanisms and Influence of Metal Speciation. Macromol. Biosci. 2003, 3, 552–561. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Gułkowski, S. Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain. Energies 2021, 14, 1150. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Chu, C.; Ni, J.; Neisiany, R.E.; You, Z. Biodegradable Elastomers and Gels for Elastic Electronics. Adv. Sci. 2022, 9, 2105146. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Edelman, P.G. An Overview of Biodegradable Polymers and Biodegradation of Polymers. In Degradable Polymers; Scott, G., Gilead, D., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 18–28. [Google Scholar] [CrossRef]
- Vorberg, S.; Tetko, I.V. Modeling the Biodegradability of Chemical Compounds Using the Online CHEmical Modeling Environment (OCHEM)-PMC. Mol. Inform. 2014, 33, 73–85. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5175213/ (accessed on 6 September 2024). [CrossRef] [PubMed]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of Biogas Production from Wild and Cultivated Varieties of Reed Canary Grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Petersson, A.; Thomsen, M.H.; Hauggaard-Nielsen, H.; Thomsen, A.B. Potential Bioethanol and Biogas Production Using Lignocellulosic Biomass from Winter Rye, Oilseed Rape and Faba Bean. Biomass Bioenergy 2007, 31, 812–819. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Bida, I.; Gladka, G.; Tymoshenko, A.; Kyrylov, S.; Mariychuk, R.; Tashyrev, O. Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria. Plants 2023, 12, 198. [Google Scholar] [CrossRef]
- Carre, P.; Citeau, M.; Robin, G.; Estorges, M. Hull Content and Chemical Composition of Whole Seeds, Hulls and Germs in Cultivars of Rapeseed (Brassica Napus). OCL-Oilseeds Fats Crops Lipids 2016, 23, A302. [Google Scholar] [CrossRef]
- Boucher, J.; Chabloz, C.; Lex, O.; Marison, I.W. Oleaginous Seeds, Press-Cake and Seed Husks for the Biosorption of Metals. J. Water Supply Res. Technol.-AQUA 2008, 57, 489–499. [Google Scholar] [CrossRef]
- Seppälä, M.; Laine, A.; Rintala, J. Screening of Novel Plants for Biogas Production in Northern Conditions. Bioresour. Technol. 2013, 139, 355–362. [Google Scholar] [CrossRef]
- Cvengroš, J.; Považanec, F. Production and Treatment of Rapeseed Oil Methyl Esters as Alternative Fuels for Diesel Engines. Bioresour. Technol. 1996, 55, 145–150. [Google Scholar] [CrossRef]
- Jahanshahi, A.; Lopes, M.; Brandão, M.; De Castro, E.A. Development of Bioenergy Technologies: A Scientometric Analysis. Heliyon 2023, 9, e20000. [Google Scholar] [CrossRef]
- Kougias, P.G.; Boe, K.; Einarsdottir, E.S.; Angelidaki, I. Counteracting Foaming Caused by Lipids or Proteins in Biogas Reactors Using Rapeseed Oil or Oleic Acid as Antifoaming Agents. Water Res. 2015, 79, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Spagni, A.; Casu, S.; Grilli, S. Decolourisation of Textile Wastewater in a Submerged Anaerobic Membrane Bioreactor. Bioresour. Technol. 2012, 117, 180–185. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pavlostathis, S.G. Decolorization and Toxicity of Reactive Anthraquinone Textile Dyes under Methanogenic Conditions. Water Res. 2004, 38, 1838–1852. [Google Scholar] [CrossRef]
- Mehrez, I.; Chandrasekhar, K.; Kim, W.; Kim, S.H.; Kumar, G. Comparison of Alkali and Ionic Liquid Pretreatment Methods on the Biochemical Methane Potential of Date Palm Waste Biomass. Bioresour. Technol. 2022, 360, 127505. [Google Scholar] [CrossRef]




| Parameter [Unit] | GB | RH |
|---|---|---|
| Total solids [mg/g] | 977.5 ± 24.51 | 929.4 ± 31.90 |
| Mineral solids[mg/g] | 48.9 ± 3.23 | 48.7 ± 2.82 |
| Voiataile solids [mg/g] | 928.6 ± 36.17 | 880 ± 29.26 |
| Lignin [%] | 10.19 ± 0.78 | 8.52 ± 0.57 |
| Cellulose [%] | 37.09 ± 3.02 | 15.24 ± 1.19 |
| Hemicellulose [%] | 5.22 ± 0.43 | 2.91 ± 0.28 |
| Type of Sorbent (after BR46 Sorption) | Total Solids | Volatile Solids | Total Solids of Dye | Mineral Solids | Moisture of Sorbent |
|---|---|---|---|---|---|
| [mg/g] | [mg/g] | [mg/g] | [mg/g] | [%] | |
| GB | 236.3 ± 10.43 | 162.3 ± 8.90 | 37.6 ± 2.39 | 74.0 ± 5.73 | 76.4 ± 4.71 |
| RH | 240.5 ± 12.98 | 228.5 ± 9.12 | 26.8 ± 3.45 | 12.0 ± 1.20 | 76.0 ± 5.16 |
| Parameter | Shortcut | Unit | GB | GB–S | RH | RH–S |
|---|---|---|---|---|---|---|
| Total solids | TS | mg/g | 25.07 ± 1.05 | 26.70 ± 1.28 | 24.95 ± 0.97 | 24.55 ± 1.53 |
| Mineral solids | - | mg/g | 7.75 ± 0.43 | 9.41 ± 0.27 | 7.76 ± 0.56 | 7.64 ± 0.41 |
| Volatile solids | VS | mg/g | 17.32 ± 0.98 | 17.29 ± 0.42 | 17.19 ± 0.39 | 16.91 ± 0.85 |
| Chemical oxygen demand | COD | mg/L | 1872 ± 74 | 1463 ± 51 | 2105 ± 67 | 1722 ± 49 |
| Nitrogen | N | % | 2.71 ± 0.23 | 2.49 ± 0.19 | 2.37 ± 0.31 | 2.79 ± 0.17 |
| Carbon | C | % | 36.81 ± 1.23 | 36.39 ± 0.98 | 38.41 ± 1.09 | 38.89 ± 1.17 |
| Hydrogen | H | % | 4.92 ± 0.33 | 4.51 ± 0.27 | 5.17 ± 0.20 | 6.18 ± 0.19 |
| Total nitrogen | TN | mg/L | 32.00 ± 15.24 | 31.75 ± 12.65 | 51.13 ± 25.02 | 50,75 ± 20.90 |
| Total organic carbon | TOC | mg/L | 740.75 ± 14.78 | 725.94 ± 15.87 | 711.13 ± 20.8 | 599.88 ± 17.89 |
| Total carbon | TC | mg/L | 1125.63 ± 32.76 | 1158.44 ± 26.12 | 1191.25 ± 34.81 | 1031.25 ± 29.06 |
| Inorganic carbon | IC | mg/L | 384.88 ± 10.9 | 432.38 ± 12.4 | 479.88 ± 17.13 | 431.5 ± 15.74 |
| Glucose | - | mg/L | 34.53 ± 0.57 | 28.23 ± 0.87 | 66.44 ± 1.34 | 53.72 ± 1.90 |
| Xylose | - | mg/L | 250.23 ± 13.54 | 220.78 ± 10.82 | 318.40 ± 9.15 | 267.69 ± 14.98 |
| Parameter | Shortcut | Unit | GB | ⤓ * | GB–S | ⤓ | RH | ⤓ | RH–S | ⤓ |
|---|---|---|---|---|---|---|---|---|---|---|
| Total solids | TS | mg/g | 20.03 ± 0.95 | 20.1 | 18.20 ± 0.69 | 31.8 | 16.17 ± 0.75 | 35.2 | 15.7 ± 1.11 | 36.0 |
| Mineral solids | - | mg/g | 7.5 ± 0.39 | 3.2 | 6.7 ± 0.18 | 28.8 | 5.7 ± 0.46 | 26.5 | 6.2 ± 0.33 | 18.8 |
| Volatile solids | VS | mg/g | 12.8 ± 0.78 | 26.1 | 11.6 ± 0.55 | 32.9 | 10.9 ± 0.32 | 36.6 | 9.5 ± 0.81 | 43.8 |
| Chemical oxygen demand | COD | mg/L | 613 ± 21 | 67.3 | 574 ± 26 | 60.8 | 597 ± 30 | 71.6 | 458 ± 24 | 73.4 |
| Nitrogen | N | % | 2.64 ± 0.20 | 2.6 | 2.21 ± 0.17 | 11.2 | 2.09 ± 0.22 | 11.8 | 2.4 ± 0.25 | 14 |
| Carbon | C | % | 34.52 ± 1.33 | 6.2 | 34.27 ±1.21 | 5.8 | 34.82 ± 1.18 | 9.3 | 35.46 ± 1.54 | 8.8 |
| Hydrogen | H | % | 4.74 ± 0.36 | 3.7 | 4.34 ± 0.19 | 3.8 | 4.84 ± 0.20 | 6.4 | 4.84 ± 0.23 | 21.7 |
| Total nitrogen | TN | mg/L | 17.0 ± 6.21 | 46.9 | 17.75 ± 4.38 | 44.1 | 25.4 ± 5.6 | 50.3 | 28.25 ± 5.16 | 44.3 |
| Total organic carbon | TOC | mg/L | 297.2 ± 8.34 | 59.9 | 325.1 ± 7.21 | 55.2 | 244.7 ± 6.98 | 65.6 | 226.4 ± 7.81 | 62.3 |
| Total carbon | TC | mg/L | 467.25 ± 21.7 | 58.5 | 495.50 ± 16.17 | 57.2 | 408.45 ± 12.87 | 65.7 | 401.74 ± 17.42 | 61.0 |
| Inorganic carbon | IC | mg/L | 170.05 ± 6.42 | 55.8 | 170.4 ± 5.38 | 60.6 | 163.75 ± 7.17 | 65.9 | 175.34 ± 5.25 | 59.4 |
| Glucose | - | mg/L | 0.56 ± 0.03 | 98.4 | 0.24 ± 0.01 | 99.1 | 0.19 ± 0.02 | 99.7 | 0.21 ± 0.01 | 99.6 |
| Xylose | - | mg/L | 0.23 ± 0.02 | 99.9 | 0.33± 0.02 | 99.9 | 0.08 ± 0.01 | 100.0 | 0.19 ± 0.03 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka, A.; Jóźwiak, T.; Zieliński, M. The Possibility of Using Waste from Dye Sorption for Methane Production. Energies 2024, 17, 4756. https://doi.org/10.3390/en17194756
Nowicka A, Jóźwiak T, Zieliński M. The Possibility of Using Waste from Dye Sorption for Methane Production. Energies. 2024; 17(19):4756. https://doi.org/10.3390/en17194756
Chicago/Turabian StyleNowicka, Anna, Tomasz Jóźwiak, and Marcin Zieliński. 2024. "The Possibility of Using Waste from Dye Sorption for Methane Production" Energies 17, no. 19: 4756. https://doi.org/10.3390/en17194756
APA StyleNowicka, A., Jóźwiak, T., & Zieliński, M. (2024). The Possibility of Using Waste from Dye Sorption for Methane Production. Energies, 17(19), 4756. https://doi.org/10.3390/en17194756







