Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process
Abstract
1. Introduction
2. Microplastic—A Modern Contaminant in SS
2.1. Characterization of MPs
2.2. Interactions and Health Effects between MPs and Organisms
2.3. Legal Status in EU
2.4. Sources of MPs in the Environment
3. SS Contamination by MPs
3.1. Land and Agricultural Use
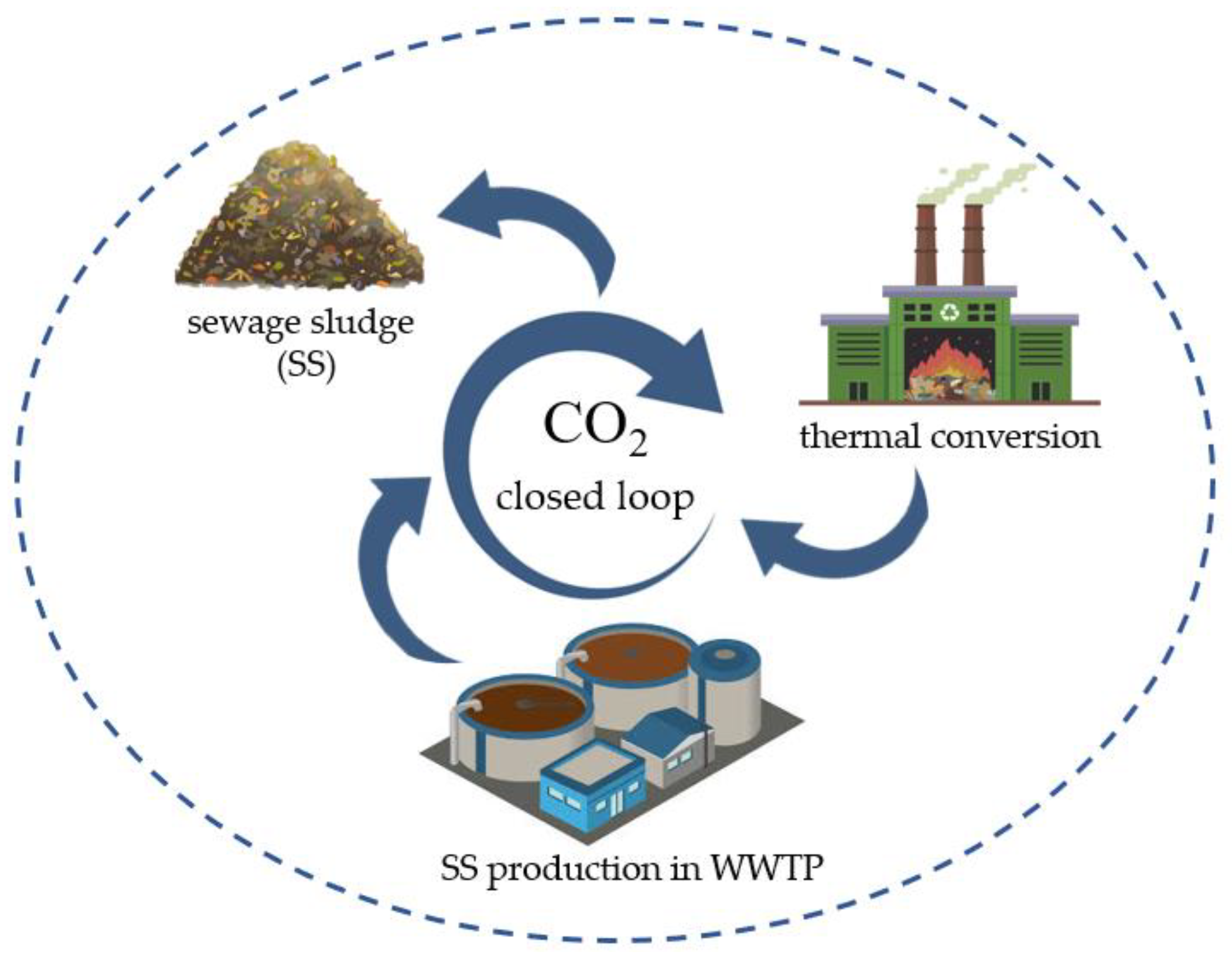
3.2. Energy Production
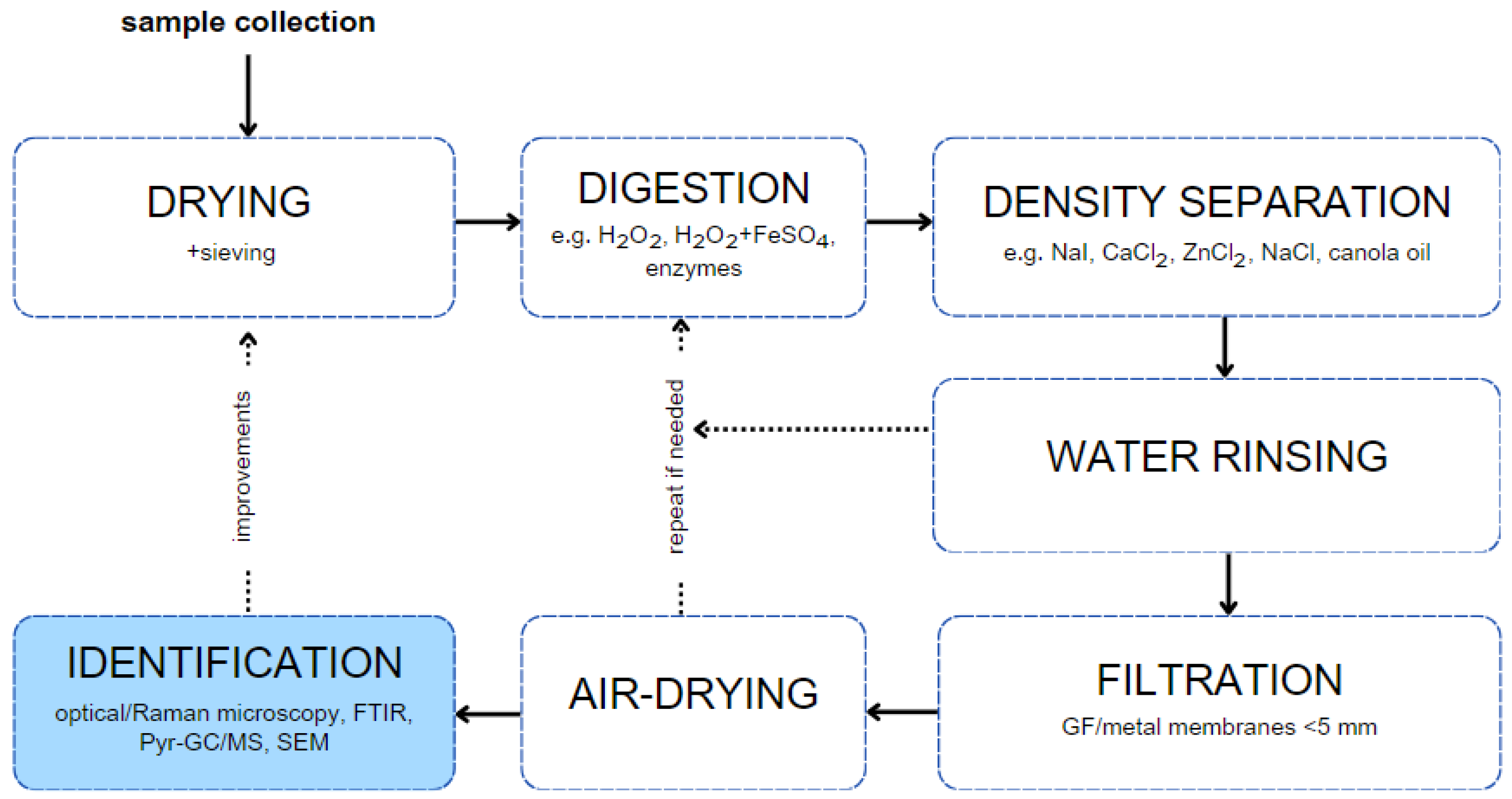
4. Separation and Identification of Sludge-Based MPs
4.1. Physical Analysis
4.2. Chemical Analysis
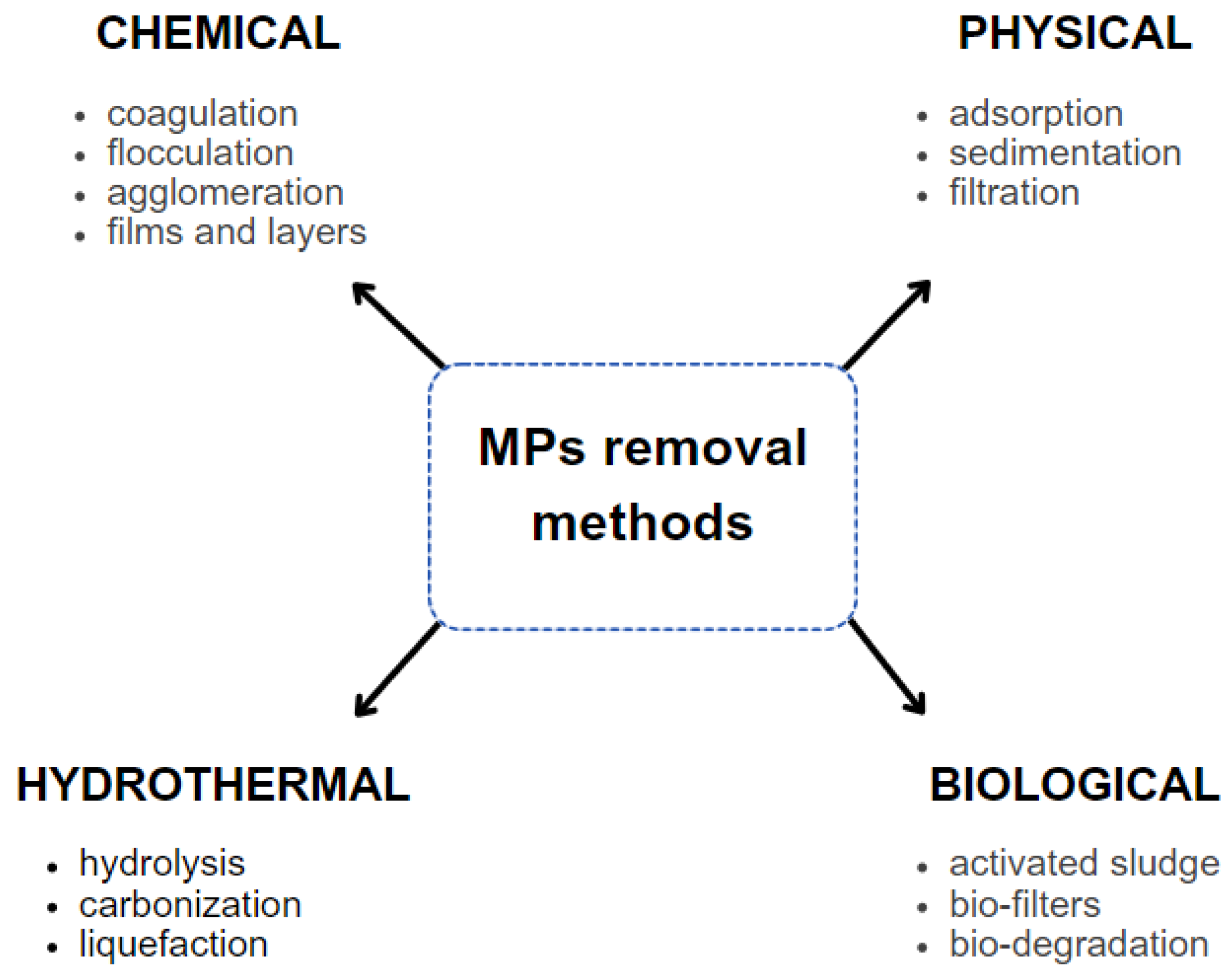
5. Removal of MPs from Sewage Systems
6. Hydrothermal Treatments of Sewage Sludge and the Effect on MPs
6.1. Thermal Hydrolysis Pretreatment (THP)
6.2. Hydrothermal Carbonization (HTC)
6.3. Hydrothermal Liquefaction (HTL)
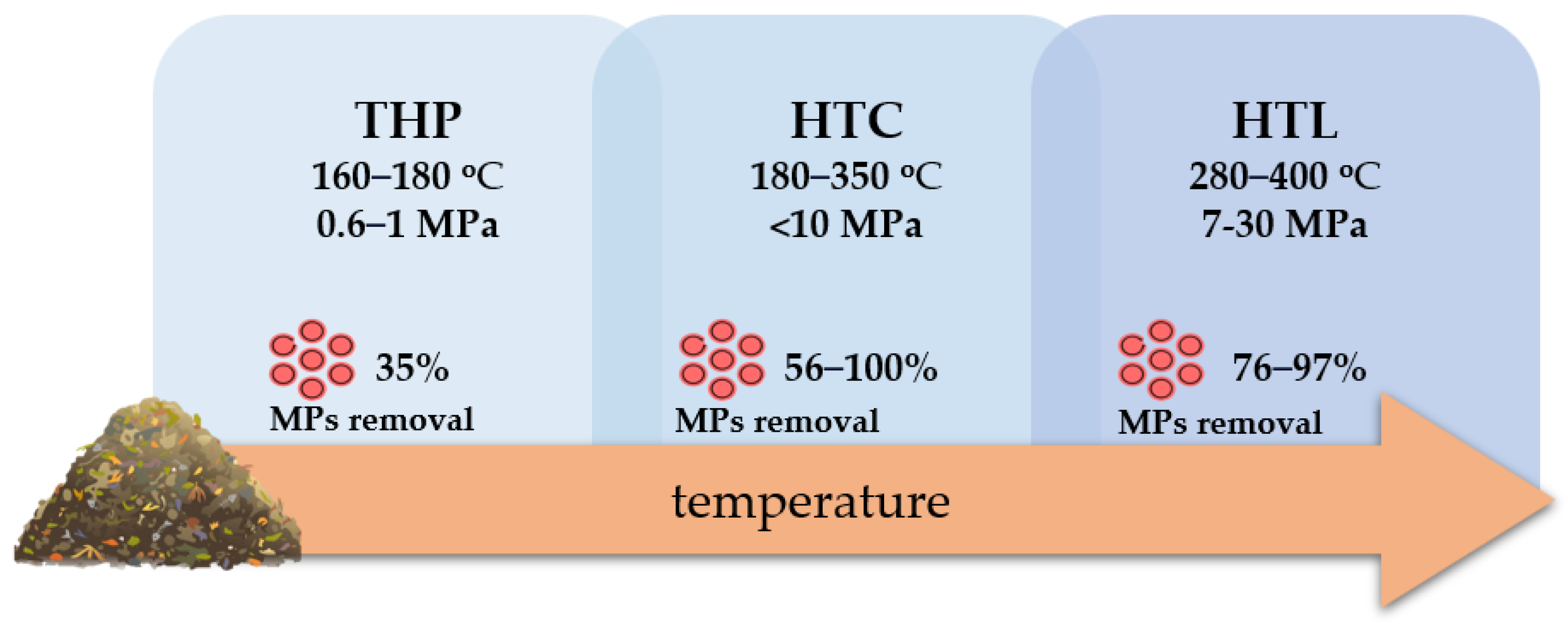
6.4. Comparison of Hydrothermal Methods Applied to Pretreat SS in Terms of MPs Reduction
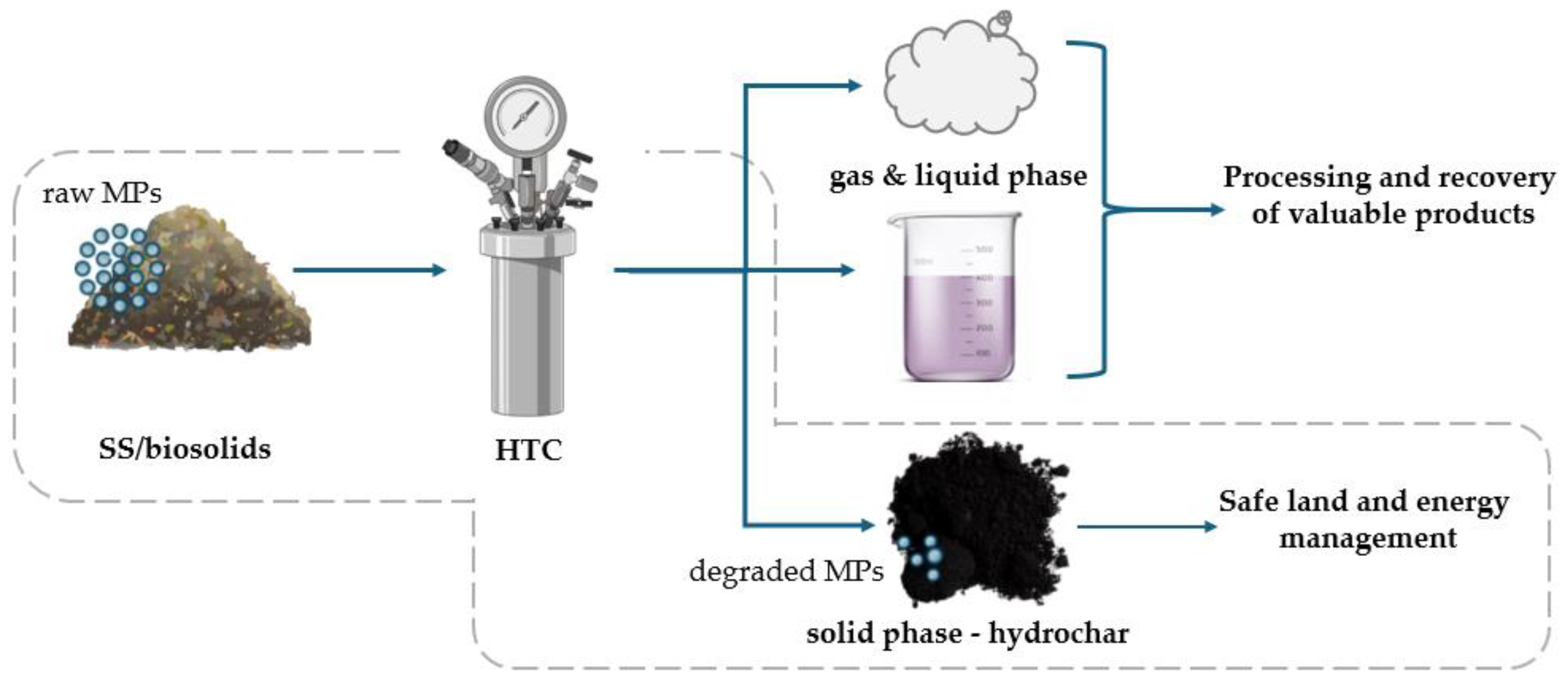
7. The Impact of HTC on Sewage-Derived MPs
7.1. MPs Quantity Reduction
7.2. Detection of MPs in HC
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| H2O2 | Hydrogen peroxide |
| CaCl2 | Calcium Chloride |
| ZnCl2 | Zinc Chloride |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ATR | Attenuated Total Reflection |
| FPA | Focal Plane Array |
| XPS | X-Ray Photoelectron Spectroscopy |
| Py-GC-MS | Pyrolysis-Gas Chromatography-Mass Spectrometry |
| TGA-DSC | Thermogravimetric Analysis With Differential Scanning Calorimetry |
| TED-GC/MS | Thermal Extraction-Desorption combined with Compressed Gas Chromatography Mass Spectrometer |
| VOCs | Volatile Organic Compounds |
| CO | Carbon monoxide |
| CH2O | Formaldehyde |
| PM | Particular Matter |
| H2 | Hydrogen |
| N2 | Nitrogen |
| P | Phosphorus |
| PMMA | Poly(Methyl Methacrylate] |
| PVDF | Polyvinylidene Fluoride |
| PO | Polyolefin |
| DOM | Dissolved Organic Matter |
| SS | Sewage Sludge |
| NCV | Net Calorific Value |
| WWTP | Wastewater Treatment Plant |
| MPs | Microplastics |
| HTC | Hydrothermal Carbonization |
| HC | Hydrochar |
| GHG | Greenhouse Gases |
| EU | European Union |
| CO2 | Carbon dioxide |
| CH4 | Methane |
| HHV | Heat Heating Value |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PE | Polyethylene |
| PP | Polypropylene |
| PVC | Polyvinyl Chloride |
| PET | Polyethylene Terephthalate |
| PS | Polystyrene |
| PC | Polycarbonate |
| PU | Polyurethane |
| PA | Polyamide |
| US | United States of America |
| UK | United Kingdom |
| REACH | Registration, Evaluation, Authorization and Restriction of Chemicals (European Regulation) |
| Av. | Average |
| a | mg g−1 |
| b | Particles g−1wet sewage sludge |
| c | Particles L−1 |
| nda | No data available |
References
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of microbial strains for biofuel production: Metabolic engineering, applications, and challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Greenhouse Gas Intensities of Transport Fuels in the EU in 2021; European Topic Centre on Climate change mitigation: Brussels, Belgium, 2023; Available online: www.eionet.europa.eu/etcs/etc-cm (accessed on 23 March 2024).
- Sivabalan, K.; Hassan, S.; Ya, H.; Pasupuleti, J. A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. J. Phys. Conf. Ser. 2021, 1831, 012033. [Google Scholar] [CrossRef]
- Ioelovich, M. Energy Potential of Natural, Synthetic Polymers and Waste Materials—A Review. Acad. J. Polym. Sci. 2018, 1, 555553. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability; Springer: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Dhurandhar, R.; Chakrabortty, S. Downstream process: Toward cost/energy effectiveness. Handb. Biofuels 2022, 249–260. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Khan, M.I.; Ben, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alexandria Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Król, K.; Iskra, K.; Ferens, W.; Miodoński, J.M. Testing properties of sewage sludge for energy use. Environ. Prot. Eng. 2019, 45, 61–73. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Eurostat. Data Browser. Sewage Sludge Production and Disposal from Urban Wastewater (in Dry Substance (d.s). 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/ten00030/settings_1/table?lang=en (accessed on 23 March 2024).
- Valchev, D.; Ribarova, I.; Borisov, B.; Radovanov, V.; Lyubomirova, V.; Kostova, I.; Dimova, G.; Karpuzova, O.; Lazarova, S. Valuable elements in sludge from eight municipal wastewater treatment plants in relation to their recovery potential. Environ. Sci. Eur. 2024, 36. [Google Scholar] [CrossRef]
- Vo, P.H.N.; Ky Le, G.; Huy, L.N.; Zheng, L.; Chaiwong, C.; Nguyen, N.N.; Nguyen, H.T.M.; Ralph, P.J.; Kuzhiumparambil, U.; Soroosh, D.; et al. Occurrence, spatiotemporal trends, fate, and treatment technologies for microplastics and organic contaminants in biosolids: A review. J. Hazard. Mater. 2024, 466, 133471. [Google Scholar] [CrossRef] [PubMed]
- Ben Mordechay, E.; Shenker, M.; Tarchitzky, J.; Mordehay, V.; Elisar, Y.; Maor, Y.; Ortega-Calvo, J.J.; Hennecke, D.; Polubesova, T.; Chefetz, B. Wastewater-derived contaminants of emerging concern: Concentrations in soil solution under simulated irrigation scenarios. Soil Environ. Health 2023, 1, 100036. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Asadi, H.; Chen, C.; Besalatpour, A.A. Fate of organic pollutants in sewage sludge during thermal treatments: Elimination of PCBs, PAHs, and PPCPs. Fuel 2022, 319, 123864. [Google Scholar] [CrossRef]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M.; Borgulat, A. The impact of sewage sludge processing on the safety of its use. Sci. Rep. 2022, 12, 1227. [Google Scholar] [CrossRef]
- Styszko, K.; Bolesta, W.; Worek, J.; Kaleta, D.; Nalepa, A.; Pyssa, J.; Cwynar, K.; Prus, Z.; Frydel, L. The occurrence of pharmaceuticals and other micropollutants in wastewater treatment plant in the aspect of interaction with microplastics. In Proceedings of the EGU General Assembly, Vienna, Austria, 14–19 April 2024; pp. 1–2. [Google Scholar]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5. [Google Scholar] [CrossRef]
- Wójcik, M.; Stachowicz, F.; Masłoń, A. Experimental Research of Sewage Sludge Conditioning with the Use of Selected Biomass Ashes. IOP Conf. Ser. Earth Environ. Sci. 2019, 214. [Google Scholar] [CrossRef]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Osorio Baquero, A.; Galloway, T. Variation in microplastic concentration, characteristics and distribution in sewage sludge & biosolids around the world. Sci. Total Environ. 2023, 891, 164068. [Google Scholar] [CrossRef] [PubMed]
- Wpływ Tworzyw Sztucznych na Zdrowie. Available online: https://edu.ekoagora.pl/mod/page/view.php?id=66 (accessed on 23 October 2023).
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Chen, L.; Huang, X.; Pan, F.; Liu, L.; Dong, B.; Liu, H.; Li, H.; Dai, X.; et al. Changes in physicochemical and leachate characteristics of microplastics during hydrothermal treatment of sewage sludge. Water Res. 2022, 222, 118876. [Google Scholar] [CrossRef]
- Kacprzak, M.; Wójcicka, K. Mikroplastiki. w oczyszczalni ścieków: źródła, Analityka Oznaczania, Toksyczność i Przemiany w Procesach Oczyszczania ścieków i Zagospodarowania Osadów; 17. In Proceedings of the Konferencja Metody zagospodarowania osadów ściekowych, Toruń, Poland, 26–28 September 2023. [Google Scholar]
- Klemmensen, N.D.R.; Chand, R.; Blanco, M.S.; Vollertsen, J. Microplastic abundance in sludge-treated fields: Variance and estimated half-life. Sci. Total Environ. 2024, 922, 171394. [Google Scholar] [CrossRef]
- Nayanathara Thathsarani Pilapitiya, P.G.C.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Tworzywa—Fakty w Pigułce 2023 [Internet]. Available online: https://plasticseurope.org/pl/knowledge-hub/tworzywa-fakty-w-pigulce-2023/ (accessed on 29 October 2023).
- Tworzywa Sztuczne w Obiegu Zamkniętym—Analiza Sytuacji w Europie [Internet]. Available online: https://plasticseurope.org/pl/wp-content/uploads/sites/7/2022/07/Circular-Economy-for-Plastics_raport_PL.pdf (accessed on 21 October 2023).
- W 2020 Roku Wzrosło Zapotrzebowanie na Plastik w Polsce [Internet]. Available online: https://klimat.rp.pl/tworzywa-sztuczne/art17077821-w-2020-roku-wzroslo-zapotrzebowanie-na-plastik-w-polsce (accessed on 29 October 2023).
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, M.; Chen, X.; Hu, L.; Xu, Y.; Fu, W.; Li, C. A comparative review of microplastics in lake systems from different countries and regions. Chemosphere 2022, 286, 131806. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Anoopkumar, A.N.; Madhavan, A.; Binod, P.; Pandey, A.; Sindhu, R.; Awasthi, M.K. Degradation mechanism of microplastics and potential risks during sewage sludge co-composting: A comprehensive review. Environ. Pollut. 2023, 333, 122113. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, R.; Zhou, Q.; Li, L.; Li, Y.; Tu, C.; Zhao, X.; Xiong, K.; Christie, P.; Luo, Y. Abundance and morphology of microplastics in an agricultural soil following long-term repeated application of pig manure. Environ. Pollut. 2021, 272, 116028. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Tania, M.; Anand, V. The implementation of microbes in plastic biodegradation. J. Umm Al-Qura Univ. Appl. Sci. 2023. [Google Scholar] [CrossRef]
- Gigault, J.; Pedrono, B.; Maxit, B.; Ter Halle, A. Marine plastic litter: The unanalyzed nano-fraction. Environ. Sci. Nano 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Burrows, S.; Colwell, J.; Costanzo, S.; Kaserzon, S.; Okoffo, E.; Ribeiro, F.; Brien, S.O.; Toapanta, T.; Rauert, C.; Thomas, K.V.; et al. UV sources and plastic composition influence microplastic surface degradation: Implications for plastic weathering studies. J. Hazard. Mater. Adv. 2024, 14, 100428. [Google Scholar] [CrossRef]
- Chen, B.; He, B.; Wu, H.; Liu, A. Microplastic degradations in simulated UV light, natural light and natural water body: A comparison investigation. Emerg. Contam. 2024, 10, 100306. [Google Scholar] [CrossRef]
- Chowdhury, T.; Wang, Q. Study on Thermal Degradation Processes of Polyethylene Terephthalate Microplastics Using the Kinetics and Artificial Neural Networks Models. Processes 2023, 11, 496. [Google Scholar] [CrossRef]
- Adhikari, K.; Pearce, C.I.; Sanguinet, K.A.; Bary, A.I.; Chowdhury, I.; Eggleston, I.; Xing, B.; Flury, M. Accumulation of microplastics in soil after long-term application of biosolids and atmospheric deposition. Sci. Total Environ. 2024, 912, 168883. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Blazsó, M.; Jakab, E.; Miskolczi, N.; Bozi, J.; Czégény, Z. Thermo-catalytic studies on a mixture of plastic waste and biomass. J. Therm. Anal. Calorim. 2022, 147, 6259–6270. [Google Scholar] [CrossRef]
- Kefeli, A.A.; Razumovskii, S.D.; Zaikov, G.Y. Interaction of polyethylene with ozone. Polym. Sci. USSR 1971, 13, 904–911. [Google Scholar] [CrossRef]
- Teare, D.O.H.; Emmison, N.; Ton-That, C.; Bradley, R.H. Cellular attachment to ultraviolet ozone modified polystyrene surfaces. Langmuir 2000, 16, 2818–2824. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.H.; Ubando, A.T.; Naqvi, S.R.; Culaba, A.B. Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environmental effects: A state-of-the-art review. J. Hazard. Mater. 2021, 418, 126381. [Google Scholar] [CrossRef]
- Bandaru, S.; Ravipati, M.; Busi, K.B.; Phukan, P.; Bag, S.; Chandu, B.; Dalapati, G.K.; Biring, S.; Chakrabortty, S. A Review on the Fate of Microplastics: Their Degradation and Advanced Analytical Characterization. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Jiang, L.; Chen, X.; Zhao, Y.; Shi, W.; Xing, Z. From organic fertilizer to the soils: What happens to the microplastics? A critical review. Sci. Total Environ. 2024, 919, 170217. [Google Scholar] [CrossRef]
- Pan, K.; Chen, C.C.; Lin, L.; Xu, H.; Chen, F.; Li, Y.; Zhu, X.; Ma, J.; Lan, W. Adsorption of di(2-ethylhexyl)phthalate (DEHP) to microplastics in seawater: A comparison between pristine and aged particles. Bull. Environ. Contam. Toxicol. 2022, 109, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Y.; Jiang, L.; Han, W.; Zhao, Y.; Jiang, X.; Li, J.; Shi, W.; Zhang, X. Organic fertilizer facilitates the soil microplastic surface degradation and enriches the diversity of bacterial biofilm. J. Hazard. Mater. 2023, 459, 132139. [Google Scholar] [CrossRef]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. 2017, 24, 20360–20371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yin, L.; Wen, X.; Du, C.; Wu, L.; Long, Y.; Liu, Y.; Ma, Y.; Yin, Q.; Zhou, Z.; et al. Microplastics in sediment and surface water of west dongting lake and south dongting lake: Abundance, source and composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Maciąg, K.; Olszówka, M. Nauka w Służbie Przyrodzie—Wybrane Zagadnienia; Fundacja na Rzecz Promocji Nauki i Rozwoju TYGIEL: Lublin, Poland, 2015. [Google Scholar]
- Zhang, Y.; Wu, D.; Su, Y.; Xie, B. Occurrence, influence and removal strategies of mycotoxins, antibiotics and microplastics in anaerobic digestion treating food waste and co-digestive biosolids: A critical review. Bioresour. Technol. 2021, 330, 124987. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Giridharan, K.; Stalin, B.; Kumaran, S.; Kavimani, V.; Nagaprasad, N.; Tesfaye Jule, L.; Krishnaraj, R. Energy recovery of waste plastics into diesel fuel with ethanol and ethoxy ethyl acetate additives on circular economy strategy. Sci. Rep. 2022, 12, 5330. [Google Scholar] [CrossRef] [PubMed]
- Dessì, C.; Okoffo, E.D.; O’Brien, J.W.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Rauert, C.; Wang, X.; Thomas, K.V. Plastics contamination of store-bought rice. J. Hazard. Mater. 2021, 416, 125778. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Liberatori, G.; Ghilardi, A.; Del Giacco, L.; Puccini, M.; Ferraro, F.; Vitolo, S.; Corsi, I. The zebrafish (Danio rerio) embryo-larval contact assay combined with biochemical biomarkers and swimming performance in sewage sludge and hydrochar hazard assessment. Environ. Pollut. 2022, 302, 119053. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Hazarika, R.P.; Kumar, V.; Roy, A.; Pandit, S.; Prasad, R. Microplastics in marine and aquatic habitats: Sources, impact, and sustainable remediation approaches. Environ. Sustain. 2022, 5, 39–49. [Google Scholar] [CrossRef]
- El Morabet, R. Effects of Outdoor Air Pollution on Human Health [Internet]. Enc. Environ. Health 2019, 278–286. [Google Scholar] [CrossRef]
- Almaiman, L.; Aljomah, A.; Bineid, M.; Aljeldah, F.M.; Aldawsari, F.; Liebmann, B.; Lomako, I.; Sexlinger, K.; Alarfaj, R. The occurrence and dietary intake related to the presence of microplastics in drinking water in Saudi Arabia. Environ. Monit. Assess. 2021, 193. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Park, B.; Kim, S.K.; Joo, S.; Kim, J.S.; Jo, K.; Song, N.S.; Im, J.; Lee, H.J.; Kim, S.W.; Lee, S.B.; et al. Microplastics in large marine animals stranded in the Republic of Korea. Mar. Pollut. Bull. 2023, 189, 114734. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Li, J.; Tang, R.; Miu, Y.; Ma, X. Research on the influence of microplastics on marine life. In Proceedings of the 3rd International Conference on Air Pollution and Environmental Engineering, Penang, Malaysia, July 2021; IOP Publishing: Bristol, UK, 2021; Volume 631, p. 012006. [Google Scholar]
- Battaglia, F.M.; Beckingham, B.A.; McFee, W.E. First report from North America of microplastics in the gastrointestinal tract of stranded bottlenose dolphins (Tursiops truncatus). Mar. Pollut. Bull. 2020, 160, 111677. [Google Scholar] [CrossRef]
- Philipp, C.; Unger, B.; Ehlers, S.M.; Koop, J.H.E.; Siebert, U. First Evidence of Retrospective Findings of Microplastics in Harbour Porpoises (Phocoena phocoena) from German Waters. Front. Mar. Sci. 2021, 8, 682532. [Google Scholar] [CrossRef]
- Subaramaniyam, U.; Allimuthu, R.S.; Vappu, S.; Ramalingam, D.; Balan, R.; Paital, B.; Panda, N.; Rath, P.K.; Ramalingam, N.; Sahoo, D.K. Effects of microplastics, pesticides and nano-materials on fish health, oxidative stress and antioxidant defense mechanism. Front. Physiol. 2023, 14, 1217666. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tajbakhsh, S.; Darabi, A.H.; Spitz, J. Abundance, composition, and potential intake of microplastics in canned fish. Mar. Pollut. Bull. 2020, 160. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Fate and Impacts of Microplastics in the Environment: Hydrosphere, Pedosphere, and Atmosphere. Environments 2023, 10, 70. [Google Scholar] [CrossRef]
- Pothiraj, C.; Amutha Gokul, T.; Ramesh Kumar, K.; Ramasubramanian, A.; Palanichamy, A.; Venkatachalam, K.; Pastorino, P.; Barcelò, D.; Balaji, P.; Faggio, C. Vulnerability of microplastics on marine environment: A review. Ecol. Indic. 2023, 155, 111058. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/Nanoplastic Toxicity in Plants: An Imminent Concern. Environ. Monit. Assess. 2022, 195, 27. [Google Scholar] [CrossRef]
- Borah, S.J.; Gupta, A.K.; Gupta, A.; Bhawna; Kumar, S.; Sharma, R.; Kumar, R.; Kumar, P.; Dubey, K.K.; Kaushik, S.; et al. Grasping the supremacy of microplastic in the environment to understand its implications and eradication: A review. J. Mater. Sci. 2023, 58, 12899–12928. [Google Scholar] [CrossRef]
- Jia, L.; Liu, L.; Zhang, Y.; Fu, W.; Liu, X.; Wang, Q.; Tanveer, M.; Huang, L. Microplastic stress in plants: Effects on plant growth and their remediations. Front. Plant Sci. 2023, 14, 1226484. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- Radford, F.; Horton, A.; Hudson, M.; Shaw, P.; Williams, I. Agricultural soils and microplastics: Are biosolids the problem? Front. Soil Sci. 2022, 2, 941837. [Google Scholar] [CrossRef]
- Worek, J.; Rybczyński, A.; Styszko, K. Adsorption of micropollutants of pharmaceutical origin on microplastic particles. In Proceedings of the 4th International Conference Strategies toward Green Deal Implementation, online, 14–15 December 2023; Mineral and Energy Economy Research Institute Polish Academy of Sciences: Krakow, Poland, 2023; p. 171. [Google Scholar]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Chang, X.; Fang, Y.; Wang, Y.; Wang, F.; Shang, L.; Zhong, R. Microplastic pollution in soils, plants, and animals: A review of distributions, effects and potential mechanisms. Sci. Total Environ. 2022, 850, 157857. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Li, Y.; Tong, Y.W.; Ok, Y.S.; Wang, X. Multi-task prediction and optimization of hydrochar properties from high-moisture municipal solid waste: Application of machine learning on waste-to-resource. J. Clean. Prod. 2021, 278. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Komisja Europejska. EU Action against Microplastics [Internet]. Available online: http://www.europa.eu (accessed on 23 March 2024).
- Union, E. EUR-Lex [Internet]. Available online: https://eur-lex.europa.eu/EN/legal-content/glossary/regulation.html (accessed on 23 March 2024).
- Stany Zjednoczone Generują Więcej Plastikowych Śmieci niż Jakikolwiek Inny Kraj. Available online: https://irme.pl/7663-2/ (accessed on 23 March 2024).
- Shaaban, M.; Wang, X.; Song, P.; Hou, X.; Wei, Z. Microplastic pollution and e-waste: Unraveling sources, mechanisms, and impacts across environments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100891. [Google Scholar] [CrossRef]
- Schell, T.; Hurley, R.; Buenaventura, N.T.; Mauri, P.V.; Nizzetto, L.; Rico, A.; Vighi, M. Fate of microplastics in agricultural soils amended with sewage sludge: Is surface water runoff a relevant environmental pathway? Environ. Pollut. 2022, 293, 118520. [Google Scholar] [CrossRef] [PubMed]
- Worek, J.; Badura, X.; Białas, A.; Chwiej, J.; Kawoń, K.; Styszko, K. Pollution from Transport: Detection of Tyre Particles in Environmental Samples. Energies 2022, 15, 2816. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Z.; Lu, B.; Li, Z.; Zhang, S.; Liu, Y.; Luo, G. Hydrothermal pretreatment reduced microplastics in sewage sludge as revealed by the combined micro-Fourier transform infrared (FTIR) and Raman imaging analysis. Chem. Eng. J. 2022, 450, 138163. [Google Scholar] [CrossRef]
- Prus, Z.; Wilk, M. Microplastics occurrence in biosolids. In Plastics: Challenges and Biotechnological Solutions [Internet]; European Federation of Biotechnology: Barcelona, Spain, 2024; p. 39. Available online: https://www.efbiotechnology.org/plastics (accessed on 23 March 2024).
- Tran, T.K.A.; Raju, S.; Singh, A.; Senathirajah, K.; Bhagwat-Russell, G.; Daggubati, L.; Kandaiah, R.; Palanisami, T. Occurrence and distribution of microplastics in long-term biosolid-applied rehabilitation land: An overlooked pathway for microplastic entry into terrestrial ecosystems in Australia. Environ. Pollut. 2023, 336, 122464. [Google Scholar] [CrossRef]
- Chen, M.; Coleman, B.; Gaburici, L.; Prezgot, D.; Jakubek, Z.J.; Sivarajah, B.; Vermaire, J.C.; Lapen, D.R.; Velicogna, J.R.; Princz, J.I.; et al. Identification of microplastics extracted from field soils amended with municipal biosolids. Sci. Total Environ. 2024, 907, 168007. [Google Scholar] [CrossRef]
- Mohajerani, A.; Karabatak, B. Microplastics and pollutants in biosolids have contaminated agricultural soils: An analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks. Waste Manag. 2020, 107, 252–265. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, M.E.P.C.; Spyraki, M.E.P.M. Small particles, big concerns: Marine microplastics revisited. In Proceedings of the EP Intergroup Climate Change, Biodiversity & Sustainable Development Conference, Brussels, Belgium, 6 December 2023. [Google Scholar]
- Li, X.; Liu, L.; Zhang, X.; Yang, X.F.; Niu, S.; Zheng, Z.; Dong, B.; Hur, J.; Dai, X. Aging and mitigation of microplastics during sewage sludge treatments: An overview. Sci. Total Environ. 2024, 922, 171338. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Slynkova, N.; Dwyer, J.; Griffith, M.; Fernandes, M.; Jaeger, J.E.; Leusch, F.D.L. Comprehensive assessment of microplastics in Australian biosolids: Abundance, seasonal variation and potential transport to agroecosystems. Water Res. 2024, 250, 121071. [Google Scholar] [CrossRef]
- Worek, J.; Gawlak, E.; Kawoń, K.; Chwiej, J.; Bolesta, W.; Styszko, K. Risk of re-release of microplastics from sewage fertilisers into the environment. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. [Google Scholar]
- Singh, S.; Bhagwat, A. Microplastics: A potential threat to groundwater resources. Groundw. Sustain. Dev. 2022, 19, 100852. [Google Scholar] [CrossRef]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics contamination of groundwater: Current evidence and future perspectives. A review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef]
- Lee, J.; Cha, J.; Ha, K.; Viaroli, S. Microplastic pollution in groundwater: A systematic review. Environ. Pollut. Bioavailab. 2024, 36, 2299545. [Google Scholar] [CrossRef]
- Hooge, A.; Hauggaard-Nielsen, H.; Heinze, W.M.; Lyngsie, G.; Ramos, T.M.; Sandgaard, M.H.; Vollertsen, J.; Syberg, K. Fate of microplastics in sewage sludge and in agricultural soils. TrAC—Trends Anal. Chem. 2023, 166, 117184. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An Overlooked Entry Pathway of Microplastics into Agricultural Soils from Application of Sludge-Based Fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Lusher, A.; Bråte, I.; Munno, K.; Hurley, R.; Welden, N. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F.; Swedish, I.V.L. Screening of Microplastic Particles in and Downstream a Wastewater Treatment Plant; Swedish IVL: Stockholm, Sweden, 2014. [Google Scholar]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Hayany, B.E.; El Fels, L.; Quenea, K.; Dignac, M.-F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining—Image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Vollertsen, J.; Hansen, A.A. Microplastic in Danish Wastewater: Sources, Occurrences and Fate Fast DNA Sequencing for Optimization of Wastewater Treatment Plants; Environmental Project; Ministry of Environment and Food of Denmark, Environ Protect Agency: Odense, Denmark, 2017; Volume 1906, p. 55. Available online: https://vbn.aau.dk/en/publications/microplastic-in-danish-wastewater-sources-occurrences-and-fate (accessed on 17 April 2024).
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant—Macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef]
- Ren, X.; Sun, Y.; Wang, Z.; Barceló, D.; Wang, Q.; Zhang, Z.; Zhang, Y. Abundance and characteristics of microplastic in sewage sludge: A case study of Yangling, Shaanxi province, China. Case Stud. Chem. Environ. Eng. 2020, 2, 2–7. [Google Scholar] [CrossRef]
- Ren, P.J.; Dou, M.; Wang, C.; Li, G.Q.; Jia, R. Abundance and removal characteristics of microplastics at a wastewater treatment plant in Zhengzhou. Environ. Sci. Pollut. Res. 2020, 27, 36295–36305. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Telles Silveira, I.; Chua, A.; Leusch, F.D.L. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef]
- Okoffo, E.D.; O’Brien, S.; O’Brien, J.W.; Tscharke, B.J.; Rauert, C.; Rødland, E.S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Mueller, J.F.; et al. Does size matter? Quantification of plastics associated with size fractionated biosolids. Sci. Total Environ. 2022, 811, 152382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Wang, L.; Guo, H.; Zhang, J.; Gao, D. Effects of typical sludge treatment on microplastics in China—Characteristics, abundance and micro-morphological evidence. Sci. Total Environ. 2022, 826, 154206. [Google Scholar] [CrossRef] [PubMed]
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef] [PubMed]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhao, H.; Sun, H.; Sun, Y.; Zhao, J.; Xia, T. Investigation of microplastics in sludge from five wastewater treatment plants in Nanjing, China. J. Environ. Manage. 2022, 301, 113793. [Google Scholar] [CrossRef]
- Chand, R.; Kohansal, K.; Toor, S.; Pedersen, T.H.; Vollertsen, J. Microplastics degradation through hydrothermal liquefaction of wastewater treatment sludge. J. Clean. Prod. 2022, 335, 130383. [Google Scholar] [CrossRef]
- Patil, S.; Kamdi, P.; Chakraborty, S.; Das, S.; Bafana, A.; Krishnamurthi, K.; Sivanesan, S. Characterization and removal of microplastics in a sewage treatment plant from urban Nagpur, India. Environ. Monit. Assess. 2023, 195, 47. [Google Scholar] [CrossRef]
- Pittura, L.; Foglia, A.; Akyol, Ç.; Cipolletta, G.; Benedetti, M.; Regoli, F.; Eusebi, A.L.; Sabbatini, S.; Tseng, L.Y.; Katsou, E.; et al. Microplastics in real wastewater treatment schemes: Comparative assessment and relevant inhibition effects on anaerobic processes. Chemosphere 2021, 262, 128415. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xu, J.; Su, X.; Lu, M.; Wang, Z.; Zhang, Y. Occurrence and Characteristics of Microplastics in a Wastewater Treatment Plant. Bull. Environ. Contam. Toxicol. 2021, 107, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle balance and return loops for microplastics in a tertiary-level wastewater treatment plant. Water Sci. Technol. 2021, 84, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-automated analysis of microplastics in complex wastewater samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef]
- Chand, R.; Rasmussen, L.A.; Tumlin, S.; Vollertsen, J. The occurrence and fate of microplastics in a mesophilic anaerobic digester receiving sewage sludge, grease, and fatty slurries. Sci. Total Environ. 2021, 798, 149287. [Google Scholar] [CrossRef] [PubMed]
- Alavian Petroody, S.S.; Hashemi, S.H.; van Gestel, C.A.M. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef] [PubMed]
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Hartmann, N.B.; Rehne Jensen, P.; Nielsen, T.G.; Brinch, A. Microplastics Occurrence, Effects and Sources of Releases [Internet]; Danish Environmental Protection Agency: Odense, Denmark, 2015; pp. 1–6. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/118180844/Lassen_et_al._2015.pdf (accessed on 29 October 2023).
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Wijesekara, H.; Bolan, N.S.; Bradney, L.; Obadamudalige, N.; Seshadri, B.; Kunhikrishnan, A.; Dharmarajan, R.; Ok, Y.S.; Rinklebe, J.; Kirkham, M.B.; et al. Trace element dynamics of biosolids-derived microbeads. Chemosphere 2018, 199, 331–339. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Mikroplastik in Ausgewählten Kläranlagen des Oldenburgisch-Ostfriesischen; Wasserverbandes (OOWV) in Niedersachsen: Helgoland, Germany, 2014; Available online: https://www.muell-im-meer.de/sites/default/files/2020-08/Mintening%20et%20al%20%282014%29_Mikroplastik%20in%20ausgew%C3%A4hlten%20Kl%C3%A4ranlagen%20des%20OOWV%20in%20Niedersachsen.pdf (accessed on 29 October 2023).
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Giacomo, C.; Della, C.; Carla, C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Brandsma, S.; Nijssen, P.; van Velzen, M.; Leslie, H. Microplastics in River Suspended Particulate Matter and Sewage Treatment Plans. IVM Institute for Environ Studies. 2013. Available online: https://puc.overheid.nl/rijkswaterstaat/doc/PUC_147662_31/ (accessed on 12 December 2023).
- Wiśniowska, E.; Moraczewska-majkut, K.; Nocoń, W. Efficiency of microplastics removal in selected wastewater treatment plants—Preliminary studies. Des. Water Treat. 2018, 134, 23418. [Google Scholar] [CrossRef]
- Edo, C.; Gonzalez-Pleiter, M.; Leganes, F.; Fernandez-Pinas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
- Rezaei Rashti, M.; Hintz, J.; Esfandbod, M.; Bahadori, M.; Lan, Z.; Chen, C. Detecting microplastics in organic-rich materials and their potential risks to earthworms in agroecosystems. Waste Manag. 2023, 166, 96–103. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, Y.; Xu, L.; Shi, W.; Wang, F.; Leblanc, G.A.; Cui, S.; An, L.; Lei, K. Investigation of the microplastics profile in sludge from China’s largest Water reclamation plant using a feasible isolation device. J. Hazard. Mater. 2020, 388, 122067. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Loder, M.G.J.; Primpke, S.; Gergts, G. Identification of microplastic ineffluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Xu, Z.; Zhai, X.; Bai, X. Amplifiers of environmental risk of microplastics in sewage sludge: Thermal drying treatment. Sci. Total Environ. 2023, 905, 167029. [Google Scholar] [CrossRef]
- Ballice, L.; Reimert, R. Classification of volatile products from the temperature-programmed pyrolysis of polypropylene (PP), atactic-polypropylene (APP) and thermogravimetrically derived kinetics of pyrolysis. Chem. Eng. Process. 2002, 41, 289–296. [Google Scholar] [CrossRef]
- Cruz, J.N.; Ávila, J.J.L.; Martínez, K.D.; Hernández, I.P.; Zavariz, Á.D. Pyrolytic Liquid Fuel—An Alternative for Producing Electrical Energy in Mexico. J. Ecol. Engineer. 2022, 23, 227–232. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical review of microplastics removal from the environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Hadi, M.; Lin, C.; Nguyen, H.L.; Thai, V.B.; Hoang, H.G.; Vo, D.V.N.; Tran, H.T. Microplastics in sewage sludge: Distribution, toxicity, identification methods, and engineered technologies. Chemosphere 2022, 308, 136455. [Google Scholar] [CrossRef]
- Circelli, L.; Cheng, Z.; Garwood, E.; Yuksel, K.; Di Iorio, E.; Angelico, R.; Colombo, C. Comparison of ATR-FTIR and NIR spectroscopy for identification of microplastics in biosolids. Sci. Total Environ. 2024, 916, 170215. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Kutralam-Muniasamy, G. Metro station free drinking water fountain- A potential “microplastics hotspot” for human consumption. Environ. Pollut. 2020, 261. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Ribeiro, F.; O’Brien, J.W.; O’Brien, S.; Tscharke, B.J.; Gallen, M.; Samanipour, S.; Mueller, J.F.; Thomas, K.V. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography–mass spectrometry. Sci. Total Environ. 2020, 715, 136924. [Google Scholar] [CrossRef] [PubMed]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Perez-Martines, M.; Lozano-Castello, D.; Bueno-Lopez, A. Microplastics’ analysis in water: Easy handling of samples by a new Thermal Extraction Desorption-Gas Chromatography-Mass Spectrometry (TED-GC MS) methodology. Talanta 2023, 253, 123829. [Google Scholar] [CrossRef]
- Mansa, R.; Zou, S. Thermogravimetric analysis of microplastics: A mini review. Environ. Adv. 2021, 5, 100117. [Google Scholar] [CrossRef]
- Kossińska, N.; Krzyżyńska, R.; Ghazal, H.; Jouhara, H. Hydrothermal carbonisation of sewage sludge and resulting biofuels as a sustainable energy source. Energy 2023, 275, 127337. [Google Scholar] [CrossRef]
- Wilk, M.; Gajek, M.; Śliz, M.; Czerwińska, K.; Lombardi, L. Comparison of Fuels and Effluents Originating from Washing and Hydrothermal Carbonisation of Residual Biomass. Energies 2022, 15, 6499. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Han, W.; Jiao, J.; Ren, W.; Jia, G.; Huang, C.; Yang, Q. Migration and transformation modes of microplastics in reclaimed wastewater treatment plant and sludge treatment center with thermal hydrolysis and anaerobic digestion. Bioresour Technol. 2024, 400, 130649. [Google Scholar] [CrossRef]
- Crossley, O.P.; Thorpe, R.B.; Lee, J. Phosphorus recovery from process waste water made by the hydrothermal carbonisation of spent coffee grounds. Bioresour. Technol. 2020, 301, 122664. [Google Scholar] [CrossRef]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal carbonization as a strategy for sewage sludge management: Influence of process withdrawal point on hydrochar properties. Energies 2020, 13, 2890. [Google Scholar] [CrossRef]
- Czerwińska, K.; Wierońska-Wiśniewska, F.; Bytnar, K.; Mikusińska, J.; Śliz, M.; Wilk, M. The effect of an acidic environment during the hydrothermal carbonization of sewage sludge on solid and liquid products: The fate of heavy metals, phosphorus and other compounds. J. Environ. Manag. 2024, 365, 121637. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Huang, H.; Yu, C.; Fang, H.; Zhou, C.; Yin, X.; Chen, W.H.; Guo, X. chun Efficient conversion of sewage sludge into hydrochar by microwave-assisted hydrothermal carbonization. Sci. Total Environ. 2022, 803, 149864. [Google Scholar] [CrossRef]
- Pan, R. The Heavy Metals Transformation During The Pyrolysis And Hydrothermal Carbonation of Municipal Sewage Sludge. In Proceedings of the 5th International Conference on Environmental Prevention and Pollution Control Technologies (EPPCT 2023), EDP Sciences, Chengdu, China, 21–23 April 2023; Volume 393, p. 03001. [Google Scholar]
- Tasca, A.L.; Vitolo, S.; Gori, R.; Mannarino, G.; Raspolli Galletti, A.M.; Puccini, M. Hydrothermal carbonization of digested sewage sludge: The fate of heavy metals, PAHs, PCBs, dioxins and pesticides. Chemosphere 2022, 307, 135997. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Zhang, B.; Li, Y.; Liu, Z.; Jiao, W. Formation and toxicity of polycyclic aromatic hydrocarbons during CaO assisted hydrothermal carbonization of swine manure. Waste Manag. 2019, 100, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tian, L.; Liu, Z.; He, J.; Fu, H.; Huang, Q.; Xue, H.; Huang, Z. Distribution and toxicity of polycyclic aromatic hydrocarbons during CaO-assisted hydrothermal carbonization of sewage sludge. Waste Manag. 2021, 120, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Prus, Z.; Styszko, K.; Wilk, M. The Influence of Hydrothermal Carbonization Process on Reduction of Heavy Metals and Polycyclic Aromatic Hydrocarbons from Sewage Sludge—A Short Review. In Proceedings of the European Young Engineers Conference, Warsaw, Poland, 15–17 April 2024; Available online: https://www.eyec.ichip.pw.edu.pl/wp-content/uploads/12th_EYEC_Monograph_final.pdf (accessed on 12 June 2024).
- Peterson, A.A.; Vogel, F.; Lachance, P.; Froling, M.; Antal, J.M. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X. Microplastic Degradation in Sewage Sludge by Hydrothermal Carbonization: Efficiency and Mechanisms. Chemosphere 2022, 297, 134203. [Google Scholar] [CrossRef]
- Ni, B.J.; Zhu, Z.R.; Li, W.H.; Yan, X.; Wei, W.; Xu, Q.; Xia, Z.; Dai, X.; Sun, J. Microplastics Mitigation in Sewage Sludge through Pyrolysis: The Role of Pyrolysis Temperature. Environ. Sci. Technol. Lett. 2020, 7, 961–967. [Google Scholar] [CrossRef]
- Jun, C.; Na, W.; Dongsheng, W.; Zhang, W. Molecular properties and biotoxicity of dissolved organic matter leached from microplastic (MP-DOM) during typical hydrothermal treatment of sewage sludge. Sci. Total Environ. 2023, 892, 164548. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef]
- Zhao, P.; Lin, C.; Li, Y.; Zhang, J.; Huang, N.; Cui, X.; Liu, F.; Guo, Q. Combustion and slagging characteristics of hydrochar derived from the co-hydrothermal carbonization of PVC and alkali coal. Energy 2022, 244, 122653. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Xu, F.; Wang, Z.; Zhang, G. Insights into the evolution of chemical structures in hydrochars from hydrothermal carbonization of PVC. J. Energy Inst. 2022, 105, 323–333. [Google Scholar] [CrossRef]

| Study Number | Country | Size (If Stated), µm | Type of SS | Av. Concentration, Particles g−1 dw | Identification Method | Reference |
|---|---|---|---|---|---|---|
| 1 | China | >200 <200 | Dewatered sludge | 2.533 5.16 | stereomicroscopy, FTIR | [106] |
| 2 | Norway | >50 | Stabilized and dewatered sludge Dewatered sludge | 19.898 8.237 2.475 2.78 7.966 1.695 | nda | [107] |
| 3 | Sweden | >300 | Dewatered sludge | 16.7 | FTIR | [108] |
| 4 | China | >37 | Dewatered sludge | 13.787 15.08 37.463 | stereomicroscopy, FTIR | [109] |
| 5 | Chile | >8 | Dewatered and dried sludge | 34 | stereomicroscopy | [110] |
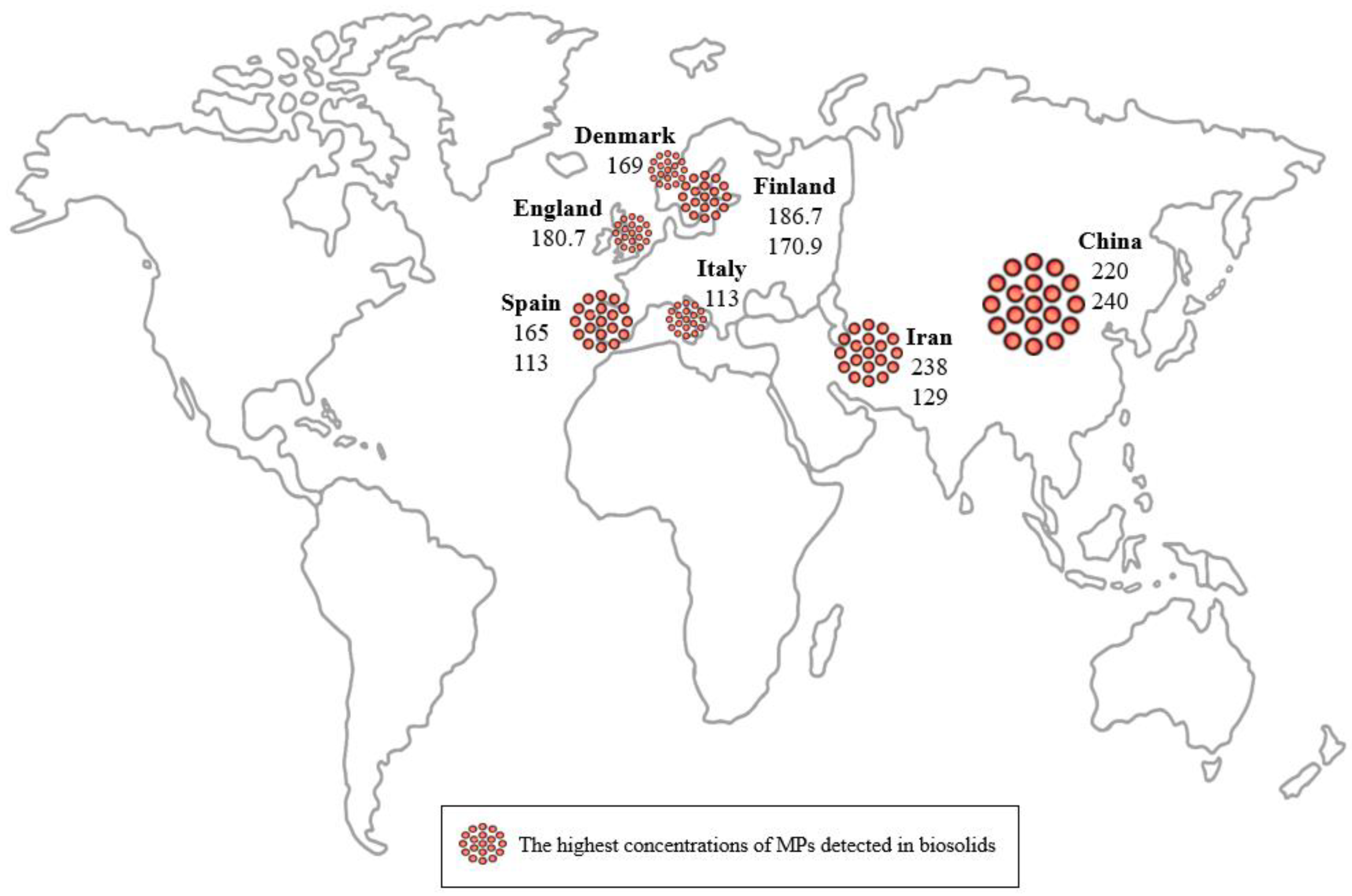
| 6 | Finland | >20 | Dewatered and dried sludge Dewatered, dried, anaerobically digested, and stabilized sludge | 186.7 76.3 | stereomicroscopy, FTIR | [111] |
| 7 | Morocco | <500 >2000 | Dewatered sludge | 36 | stereo- and Raman microscopy, Py-GC/MS + staining | [112] |
| 8 | China | >37 | Dewatered sludge | 1.57–56.4 22.7 | FTIR | [113] |
| 9 | Canada | >1.6 | Final biosolid Digested sludge | 14.1 11.469 14.407 | stereomicroscopy, FTIR | [114] |
| 10 | USA | 20–400 | Final biosolid | 1 | optical microscopy, FTIR | [115] |
| 11 | Denmark | 20–500 | Aerobically digested sludge | 169 | FTIR | [116] |
| 12 | Finland | <250 >500 | Dewatered sludge Digested sludge | 23 170.9 | optical microscopy, FTIR | [117] |
| 13 | Korea | <106 <306 | Dewatered sludge | 14.895 9.475 13.2 | digital microscopy, FTIR | [118] |
| 14 | Sweden | >10 | Digested sludge | 14.13 | stereomicroscopy, FTIR | [119] |
| 15 | China | 8–1000 | Dewatered sludge | 220 | stereomicroscopy, FTIR | [120] |
| 16 | China | 80–1700 | Dewatered sludge | 2.92 | optical microscopy | [121] |
| 17 | Australia | >25 | Digested sludge | 52.1 | stereomicroscopy, FTIR | [122] |
| 18 | China | >1 | Dewatered sludge | 1.02 | metallurgic microscopy, FTIR | [123] |
| 19 | Australia | <5000 | Final biosolid | 75.3 a | Py-GC/MS | [124] |
| 20 | China | - | Anaerobically digested sludge | 7.5 | FTIR, SEM | [125] |
| 21 | Turkey | <2000 | Final biosolid | 32 | stereo- and Ramanmicroscopy | [126] |
| 22 | Spain | >50 | Final biosolid | 7 | stereomicroscopy, FTIR | [90] |
| 23 | England | >50 | Anaerobically digested sludge Dewatered sludge | 180.7 97.2 74.7 | stereomicroscopy, FTIR | [127] |
| 24 | China | - | Dehydrated sludge | 14 22.36 21.25 29.66 13.06 | optical microscopy, FTIR | [128] |
| 25 | Denmark | >10 | Dewatered sludge * | 0.810 | stereomicroscopy, FTIR | [129] |
| 26 | India | 21–294 | Dewatered sludge | 0.830 | stereo-and SEM microscopy, FTIR | [130] |
| 27 | Italy | >300 | Anaerobically digested sludge | 4.7 | stereomicroscopy, FTIR | [131] |
| 28 | China | >68 ≥900 | Dewatered sludge | 12.73 | stereomicroscopy, FTIR | [132] |
| 29 | China | - | Dehydrated dewatered sludge | 6.91 2.19 0.23 | digital microscopy, FTIR | [133] |
| 30 | Finland | >20 | Dewatered and anaerobically digested sludge Anaerobically digested sludge | 9.379 102 c | stereo- and Raman microscopy | [134] |
| 31 | England | 25–178 | Digested sludge | 7.652 0.5 26 2.0628 | FTIR | [135] |
| 32 | Sweden | >100 | Digested sludge | 6.36 | stereomicroscopy, FTIR | [136] |
| 33 | Iran | >37 | Digested sludge Dewatered sludge | 238 129 | stereo- and optical microscopy, Raman spectroscopy | [137] |
| 34 | China | >20 | Dewatered sludge | 240± 31 | optical microscopy, Raman spectroscopy | [35] |
| 35 | Germany | ≥10 | Final biosolid | 1–24 | nda | [138] |
| 36 | Ireland | 250–4000 | Final biosolid | 4.196–15.385 | stereo- and SEM microscopy, FTIR | [139] |
| 37 | Australia | <1000 | Digested sludge | 0.996 | stereo- and SEM microscopy, FTIR | [140] |
| 38 | Canada | >1 | Digested sludge Digested and dewatered sludge | 14.9 4.4 | stereomicroscopy, FTIR | [141] |
| 39 | Chile | >8 | Digested and dewatered sludge | 18–41 | stereomicroscopy | [110] |
| 40 | England | - | Dewatered sludge | 2 | stereomicroscopy, FTIR | [142] |
| 41 | Germany | >10 | Dewatered sludge | 1–24 | optical microscopy, FTIR | [143] |
| 42 | China | >25 | Dewatered sludge | 1.6 0.7 | stereomicroscopy, FTIR | [144] |
| 43 | Italy | >10 | Dewatered and dry sludge | 113 | stereomicroscopy, FTIR | [145] |
| 44 | Netherlands | >300 | Dewatered sludge | 0.37 0.95 b 0.51 b 0.76 b | nda | [146] |
| 45 | Poland | >0.109 | Dewatered sludge Digested sludge | 15 28 21 6.7 51 62.6 | nda | [147] |
| 46 | Spain | >25 36–4720 29–2220 | Digested sludge Dried and dewatered sludge | 165 101 113 | stereomicroscopy, FTIR | [148] |
| 47 | US | - | Dewatered sludge | 4 (only fibres) | optical microscopy | [149] |
| 48 | Australia | >20 | Dewatered sludge | 55.4 73.8 62.2 | nda | [150] |
| 49 | US | >1.2 | Dewatered sludge | 12.2 | optical microscopy, LDIR | [47] |
| 50 | China | - | Dewatered sludge | 4.04 | stereomicroscopy, FTIR | [151] |
| 51 | France | ≥2000 | Dewatered sludge | 36 | stereo- and Raman microscopy, Py-GC/MS | [112] |
| 52 | Germany | <500 | Dewatered sludge | 1–24 | stereo- and optical microscopy, FTIR | [152] |
| 53 | China | >0.45 | Dewatered sludge Dried sludge | 4.8 2.7 5.4 2.2 | optical and SEM microscopy, FTIR | [153] |
| Aspect/Technology | THP | HTC | HTL |
|---|---|---|---|
| Process Conditions | 160–180 °C, 0.6–1 MPa | 180–350 °C, <10 MPa | 280–400 °C, 7–30 MPa |
| Main feature | effective reduction and disinfection of SS | ||
| Main product | biogas | solid hydrochar | liquid bio-oil (bio-crude) |
| Requirements of SS pretreatment | mixing and thickening | dewatering, thickening, digestion, or stabilization | |
| Nutrient recovery | recovery of P, N, K from solid phase | recovery of P, N, K from aqueous phase | |
| Energy and moisture content | increase in CH4 yield in subsequent anaerobic digestion | hydrochar with lower energy content compared to HTL bio-crude, characterized by significant moisture content, may need additional drying for fuel purposes, but 5 times less energy required for drying in comparison to SS | upgrading and moisture reduction in bio-crude to meet fuel properties |
| Operational costs | lower than for HTC | lower than for HTL | the highest energy input due to the highest temperature and pressure applications |
| Complexity | high-pressure reactors and control systems | high-pressure reactors and control systems with more developed installation for bio-crude refining | |
| Impact on MPs | minor reduction | significant reduction | |
| MPs/Identification Method | SS-Derived MPs | HC-Derived MPs | Reference |
|---|---|---|---|
| PC, PMMA, PS, PU, PVDF, PA, PE, PET, PO, PP, PVC | Identification of MPs > 50 μm: µ-FTIR (Spotlight 200i with Spectrum Two System, PerkinElmer, Inc., Waltham, MA, USA), eight scans in the reflectance mode. Spectra wavelength range: 600–4000 cm−1; magnification: 50×; resolution: 4 cm−1. Results compared with commercial FTIR library in Spectrum IR software (PerkinElmer, Inc., Waltham, MA, USA). Identification of MPs < 50 μm: confocal Raman spectroscopy (inVia, Renishaw, PLC, Wotton-under-Edge, UK). Wavelength of red laser: 785 nm; grating 10 × 10 μm; magnification: 50×; spectra wavelength range: 100–3200 cm−1; 10% of laser power with 0.5 s exposure. Results baseline-corrected and compared with a standard Raman library (WiRE 5.3, Renishaw, PLC). | Examination of morphology and comparative purposes: SEM (Ultra55, Zeiss, Jena, Germany), Secondary Electron mode. Resolution: 2 nm @1.0 kV. MPs coated in gold. Examination of functional groups: µ-FTIR (Spotlight 200i with Spectrum Two System, PerkinElmer, Inc., Waltham, MA, USA). Grating: 35 × 35 μm; 32 scans; spectra wavelength range: 600–4000 cm−1; magnification: 50×; resolution: 4 cm−1. Verification of surface elements and primary element component analysis: XPS (AXIS Ultra DLD, Kratos Analytical Ltd., Tokyo, Japan), Al Kα X-ray source (1486.7 eV). Resolution: 100 eV and 1 eV, and 50.0 eV and 0.1 eV); spectra fitted using Advantage 5.9 (Thermo Fisher Scientific, Waltham, MA USA), binding energies calibration using containment carbon: C1s ¼ 284.50 eV. | Jiang C. et al. (2022) [92] |
| PET, PA, PP, PE, PS, PU | Identification of MPs > 30 μm: optical microscope (Leica DM2500 and DM2500 LED, Leica Corporation, Wetzlar, Germany). Camera: 5 MPSHD (Leica MC170 HD, Leica Corporation, Wetzlar, Germany). Identification of MPs > 30 μm: µ-FTIR (Spotlight 200i, PerkinElmer, Inc., Waltham, MA, USA), 24 scans. Spectra wavelength range: 600–4000 cm−1; resolution: 4 cm−1. | Verification of surface elements: XPS (PHI 5000 VersaProbe, ULVAC-PHI, Kanagawa, Japan). Band energy range: 0–1200 eV. Examination of functional groups: µ-FTIR (Spotlight 200i, PerkinElmer, Inc., Waltham, MA, USA), 24 scans. Spectra wavelength range: 600–4000 cm−1, resolution: 4 cm−1. | Xu Z. and Bai X. (2022) [179] |
| PE, PP, PA | Examination of surface morphology: SEM, HITACHI, SU8010. Examination of chemical compositions changes: XPS (ESCALAB 250Xi, Thermo Fisher). Al Kα X-ray source (1486.6 eV); 12.5 eV voltage; energy step: 0.05 eV; dwelling time: 40–50 ms. | Jun C. et al. (2023) [181] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prus, Z.; Wilk, M. Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process. Energies 2024, 17, 4219. https://doi.org/10.3390/en17174219
Prus Z, Wilk M. Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process. Energies. 2024; 17(17):4219. https://doi.org/10.3390/en17174219
Chicago/Turabian StylePrus, Zuzanna, and Małgorzata Wilk. 2024. "Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process" Energies 17, no. 17: 4219. https://doi.org/10.3390/en17174219
APA StylePrus, Z., & Wilk, M. (2024). Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process. Energies, 17(17), 4219. https://doi.org/10.3390/en17174219








